Abstract
Hypocretinergic (orexinergic) neurons in the lateral hypothalamus project to motor columns in the lumbar spinal cord. Consequently, we sought to determine whether the hypocretinergic system modulates the electrical activity of motoneurons. Using in vivo intracellular recording techniques, we examined the response of spinal motoneurons in the cat to electrical stimulation of the lateral hypothalamus. In addition, we examined the membrane potential response to orthodromic stimulation and intracellular current injection before and after both hypothalamic stimulation and the juxtacellular application of hypocretin-1. It was found that (1) hypothalamic stimulation produced a complex sequence of depolarizing- hyperpolarizing potentials in spinal motoneurons; (2) the depolarizing potentials decreased in amplitude after the application of SB-334867, a hypocretin type 1 receptor antagonist; (3) the EPSP induced by dorsal root stimulation was not affected by the application of SB-334867; (4) subthreshold stimulation of dorsal roots and intracellular depolarizing current steps produced spike potentials when applied in concert to stimulation of the hypothalamus or after the local application of hypocretin-1; (5) the juxtacellular application of hypocretin-1 induced motoneuron depolarization and, frequently, high-frequency discharge; (6) hypocretin-1 produced a significant decrease in rheobase (36%), membrane time constant (16.4%), and the equalizing time constant (23.3%); (7) in a small number of motoneurons, hypocretin-1 produced an increase in the synaptic noise; and (8) the input resistance was not affected after hypocretin-1. The juxtacellular application of vehicle (saline) and denatured hypocretin-1 did not produce changes in the preceding electrophysiological properties.
We conclude that hypothalamic hypocretinergic neurons are capable of modulating the activity of lumbar motoneurons through presynaptic and postsynaptic mechanisms. The lack of hypocretin-induced facilitation of motoneurons may be a critical component of the pathophysiology of cataplexy.
Keywords: hypothalamus, motor, sleep, orexin, cataplexy, narcolepsy
Introduction
A specific population of neurons that is located in the lateral and dorsomedial hypothalamus synthesizes two neuropeptides, hypocretin (Hcrt)-1 and Hcrt-2 (also called orexin A and orexin B) (de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998; Nambu et al., 1999; Sakurai, 1999). The actions of these peptides are mediated by two membrane receptors, Hcrtr-1 and Hcrtr-2, that are coupled with G-proteins (Sakurai et al., 1998; Trivedi et al., 1998).
The projections of hypocretinergic neurons exhibit extensive divergence as demonstrated by the presence of Hcrt-containing terminals at all levels of the neuraxis (Peyron et al., 1998; Chen et al., 1999; Date et al., 1999; Nambu et al., 1999; van den Pol, 1999; Zhang et al., 2002). Hcrt-containing terminals are present in forebrain, brainstem, and spinal cord structures that are involved in the regulation of posture and movement, sleep and wakefulness, sensory input, autonomic activities, as well as other behaviors (Peyron et al., 1998; Chemelli et al., 1999; Chen et al., 1999). For example, hypocretinergic neurons innervate the dorsal horn as well as the ventral horn of the spinal cord (van den Pol, 1999). Recent work from our laboratory has confirmed the preceding data vis-à-vis suprasegmental and segmental projection sites of hypocretinergic neurons within the CNS of the cat (Yamuy et al., 2000; Fung et al., 2001; Zhang et al., 2001a,b, 2002).
Consequently, we were interested in examining the manner in which Hcrt affects the activity of spinal motoneurons. We determined that the Hcrt system facilitates the response of spinal motoneurons and produces specific changes in their electrophysiological properties that result in an increase in their excitability. Preliminary results have been reported previously (Yamuy et al., 2000, 2001).
Materials and Methods
Experiments were performed in 11 adult male cats (3.5-4.5 kg). All animals were in good health as determined by veterinarians in the Department of Laboratory Medicine of the University of California Los Angeles School of Medicine. The experimental procedures that were used were in accord with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals, National Research Council (1996).
Surgical procedures
Details of the various surgical procedures that were performed to record intracellularly from lumbar motoneurons have been described previously (Morales et al., 1987; Xi et al., 1997). Briefly, under isofluorane anesthesia, the trachea was cannulated and the right carotid artery and jugular vein were catheterized. A laminectomy was performed to expose the lumbosacral cord (L4-S1). In one group of cats (n = 9), the dorsal roots L5-S1 were cut distally, the ventral roots were left intact, and the following hindlimb nerves were cut distally and placed on silver hook electrodes for electrical stimulation: the trunks of the nerves that innervate the hamstrings muscles, triceps surae, common peroneal, and quadriceps. The trunk of the sciatic nerve, before its division in the popliteal fossa, was also placed on a stimulating electrode. In another group of animals (n = 2), both the dorsal and ventral roots were cut distally, and one ventral root (L7) was placed on a silver hook stimulating electrode. In all cases, the dorsal root of the spinal segment in which recording was being conducted was placed on a silver hook stimulating electrode. A hole in the cranium, ∼6 mm in diameter, provided access for an electrode used for electrical stimulation of the lateral hypothalamus (see below). After the completion of surgery, α-chloralose (60 mg/kg, i.v.; 10 cats) or Nembutal (40 mg/kg, i.v.; 1 cat) was substituted for isofluorane. The data obtained were similar when either anesthetic was used (see below).
Stimulation and recording procedures
Stainless steel electrodes (∼5 MΩ tip resistance) were used for electrical stimulation of the hypothalamus, which was performed ipsilaterally to the recorded spinal motoneurons (0.5 Hz; single pulses or trains of four pulses at 300 Hz; 500-1000 μA, 0.8 msec). The tip of the hypothalamic stimulating electrode was directed to anterior 10, lateral 1.5, height -2, according to Berman's atlas of the feline forebrain. A high concentration of hypocretinergic neurons is present in this hypothalamic area in the cat (Zhang et al., 2001b).
Motoneurons were identified by antidromic stimulation of their axons in the ventral roots or nerves. The severance of dorsal roots L5-S1 in cats with intact ventral roots eliminated the possibility of contaminating the antidromic response of α-motoneurons with orthodromically induced responses. Dorsal root stimulation allowed for the recording of reflex responses from motoneurons.
Intracellular recordings from motoneurons were performed using broken-tip micropipettes filled with 3 m KCl or 2 m K-citrate (tip resistances were 5-10 and 15-30 MΩ, respectively); these micropipettes were aligned and glued, under microscopic visualization, to a multibarreled electrode that was used for the pressure ejection of various substances (see below). The tip of the recording micropipette protruded 75-150 μm from the tip of the multibarrel assembly.
The recording electrodes were connected to a high-input impedance preamplifier with negative capacitance compensation (Axoclamp 2A; Axon Instruments, Union City, CA). Current pulses were injected into the motoneurons through the built-in bridge circuit of the preamplifier. Intracellular DC records were amplified (10× and 100×) and recorded on VHS tape using a pulse code modulation module (model 4000; Vetter, Rebersburg, PA). The ejecting micropipettes were connected to a two-channel picoinjector (model P100; Harvard Apparatus, Holliston, MA). Pressures of varying intensity and duration were used (2-50 psi, 0.5-40 sec) to juxtacellularly apply Hcrt-1 [100 μm diluted in saline; Hcrt-1 (orexin A); American Peptide, Sunnyvale, CA], Hcrt-1 that was denatured by boiling for a period of 5 min (100 μm in saline), and vehicle (saline). Hcrt-1 was chosen instead of Hcrt-2 because it acts on Hcrt receptor types 1 and 2 and both Hcrts are colocalized in the axonal projections of hypocretinergic neurons (Date et al., 1999). Henceforth, unless specified otherwise and for the sake of simplicity, we will refer to Hcrt-1 as Hcrt. SB-334867, a Hcrt type 1 receptor blocker (Smart et al., 2001) (provided by GlaxoSmithKline, Essex, UK), was applied juxtacellularly (100 μm) onto spinal motoneurons.
The arterial blood pressure of the cats was constantly monitored; the systolic pressure was kept between 110 and 140 mmHg. Rectal temperature was maintained at 38.5 ± 0.5°C; pCO2 was kept at 4-5%. At the completion of the experiments, DC anodal current (100 μA, 30 sec) was injected at the site of hypothalamic stimulation for subsequent anatomical identification. Animals were then given injections of an overdose of Nembutal (80-100 mg/kg, i.v.) and perfused through the aorta with saline, followed by a solution of 10% formalin that contained 2% of potassium ferrocyanide.
Analysis of data
Data were analyzed from motoneurons that exhibited antidromic action potential amplitudes that were ≥55 mV. The following parameters were measured of the depolarizing potentials that were evoked by hypothalamic and dorsal root stimulation: peak amplitude, measured from baseline to peak; latency to the onset, measured from the initiation of the stimulation artifact to the foot of the EPSP; latency to peak, measured from the initiation of the stimulation artifact to the peak of the EPSP; half-width duration, which is the duration of the EPSP at half amplitude; half-decay time, measured from the peak of the EPSP to the point where it repolarized to its half amplitude; and total duration, measured from the foot of the EPSP to the point where it returned to baseline. The peak amplitude of the IPSP that was evoked in a portion of the recorded motoneurons after hypothalamic stimulation was measured from baseline to the peak of the potential. In addition, the following electrophysiological properties of motoneurons were determined before and after the juxtacellular application of Hcrt: changes in resting membrane potential (RP), rheobase (Rh), input resistance (Rin), membrane time constant (τb), and equalizing time contant (τc). Membrane depolarization was measured from baseline before Hcrt ejection to the point of maximum depolarization after Hcrt ejection. The Rh was determined as the minimum intensity of a 50 msec depolarizing current pulse that was necessary to elicit a full-sized action potential. The Rin was calculated, using the “direct method” (Zengel et al., 1985; Engelhardt et al., 1989), from the averaged voltage change that was produced by subthreshold, depolarizing, or hyperpolarizing current pulses of 50 msec duration. Time constant values were obtained from the decay transient of the voltage response that followed the cessation of a depolarizing or hyperpolarizing current pulse; this method, which has been described previously in detail, consists of successively “peeling” exponential terms with the longest time constant from semilogarithmic plots of voltage versus time (Engelhardt et al., 1989, 1995).
The statistical level of significance for the difference between the mean values of each variable obtained during the control condition (before drug application) compared with those obtained after drug application was determined using the two-tailed paired Student's t test. The level of significance was set at p < 0.05. Because more than one ejection of Hcrt or vehicle was applied to some motoneurons, the sample numbers for the mean (±SEM) of each variable represent, unless indicated otherwise, the number of experimental trials.
Results
Motoneuron response to electrical stimulation of the hypothalamus
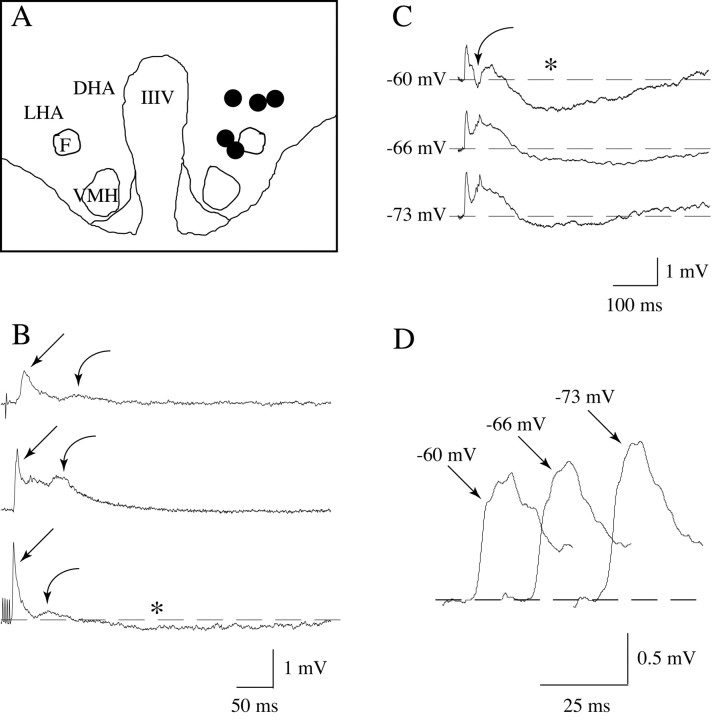
Stimulation of the perifornical area of the hypothalamus (Fig. 1A, filled circles; stimulation sites in five cats) with a single pulse or train pulses (Fig. 1B,C) produced a complex depolarizing response in spinal motoneurons that consisted of an early, fast depolarization that reached its peak at a mean latency of 18.5 msec (±0.17 msec; n = 12), followed by a trough and a second depolarizing potential that reached its maximum amplitude at ∼30-60 msec (Fig. 1B, bottom trace, C). The mean latency to the onset of the motoneuron response was 14.25 msec (±0.64 msec; n = 12), and the full duration of the hypothalmus-induced depolarizing potentials sequence lasted ∼100 msec (Fig. 1B). A late hyperpolarization, which lasted up to 500 msec, followed these depolarizing potentials in most, but not all, of the recorded motoneurons (Fig. 1B, bottom trace, C). It should be noted that this IPSP was recorded with both K-citrate- and KCl-filled micropipettes (see Figs. 1 and 3).
Figure 1.
Motoneuron response to the electrical stimulation of the perifornical region of the hypothalamus. The schematic illustrates the region of the hypothalamus that was electrically stimulated (A); each filled circle indicates the site of stimulation for each of five cats. Single (B, top trace), paired (B, middle trace), or trains of four pulses (B, bottom trace) delivered to the hypothalamus induced a complex response in lumbar motoneurons, i.e., an early depolarizing potential (straight arrows), followed by a trough, and a subsequent depolarizing potential (curved arrows) that exhibited a long-duration time course and a variable latency to peak. A late, long-lasting hyperpolarizing potential was frequently present (indicated by an asterisk in the bottom trace in B and top trace in C). Clamping the resting potential at different levels by intracellular current injection produced changes in the amplitude and waveform of these potentials that were consistent with the typical behavior of postsynaptic potentials (C); for example, the amplitude of the early depolarizing potential decreased after depolarization, whereas it increased when the resting potential was maintained at hyperpolarized levels (D). The recordings in B were obtained from different triceps surae motoneurons using K-citrate-filled micropipettes, whereas those in C and D were obtained from a sciatic motoneuron using a KCl-filled micropipette; the amplitude of their spikes was >65 mV. Each trace is an average of 8-12 sweeps. IIIV, Third ventricle; DHA, dorsal hypothalamic area; F, descending column of the fornix; LHA, lateral hypothalamic area; VMH, ventromedial hypothalamic nucleus.
Figure 3.
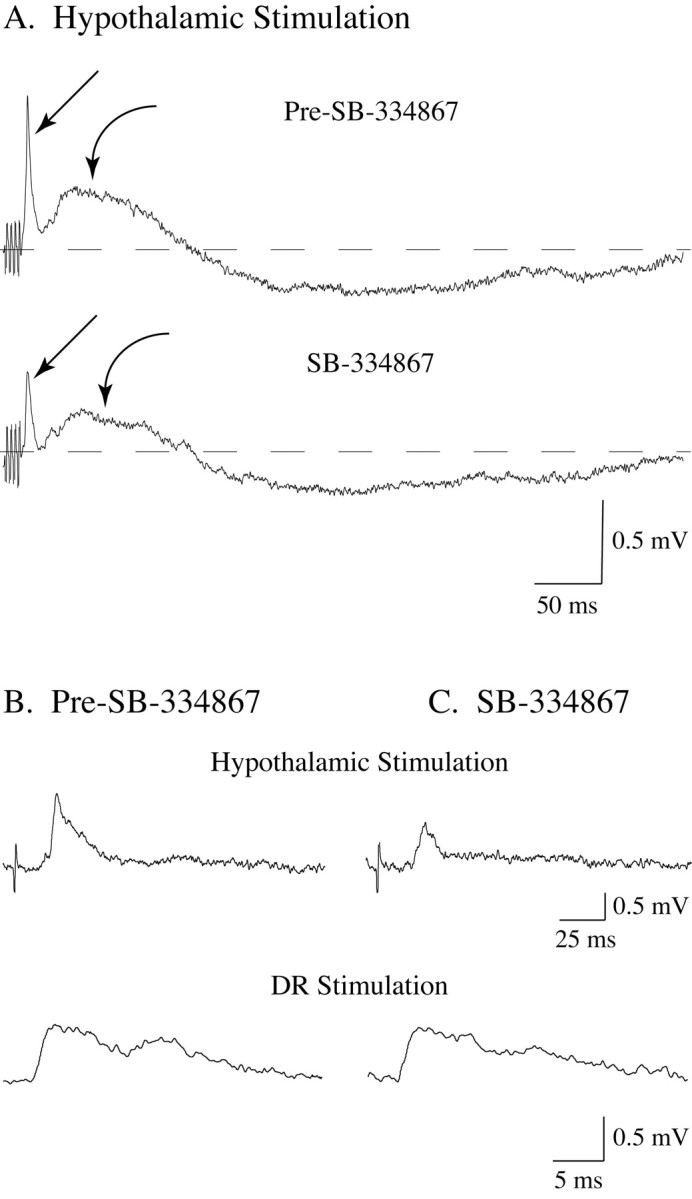
The depolarizing responses of lumbar motoneurons to hypothalamic stimulation decrease in amplitude after the juxtacellular application of SB-334867, an antagonist of Hcrt type 1 receptors. The stimulation of the perifornical hypothalamus induced a depolarizing response that consisted of early and late EPSPs (A, straight and curved arrows, respectively). After the application of SB-334867 onto the recorded motoneuron, the early and late hypothalamically induced EPSPs decreased by 46.8 and 37.1%, respectively. No evident change occurred in the late IPSP after the application of SB-334867. Whereas SB-334867 decreased the synaptic response to hypothalamic stimulation (A and top traces in B and C), it did not affect the reflex EPSP (bottom traces in B and C). The records in A were obtained from a hamstrings motoneuron using a K-citrate-filled micropipette; those in B and C were obtained from a triceps surae cell using a KCl-filled micropipette. Each trace is an average of 10-20 sweeps.
The motoneuron depolarizing and hyperpolarizing responses to hypothalamic stimulation exhibited a behavior typical of postsynaptic potentials (EPSPs and IPSPs) (i.e., the amplitude of the depolarizing potentials decreased by depolarizing the motoneuron, and it increased when the cell was hyperpolarized whereas the opposite occurred for the late hyperpolarizing potential) (Fig. 1C,D). Interestingly, a discrete IPSP was observed at the initial portion of the second EPSP when the motoneuron was depolarized (Fig. 1C, curved arrow, top trace).
Hypothalamic stimulation-induced depolarization of spinal motoneurons facilitated the direct response of these cells to subthreshold intracellular depolarizing current pulses and their reflex response to stimulation of Ia afferents in the dorsal roots. Figure 2 shows the responses of a spinal motoneuron to stimulation of the hypothalamus (Fig. 2A1) and a depolarizing current pulse (Fig. 2A2). When delivered separately, each of these stimuli was ineffective (i.e., subthreshold) in producing a discharge in the motoneuron. However, when hypothalamic stimulation was delivered during the depolarizing current step, the motoneuron responded with an action potential (Fig. 2A3). Similar results were obtained when hypothalamic stimulation interacted with Ia afferent stimulation. As shown in Figure 2B, stimulation of the hypothalamus and dorsal root that were subthreshold when delivered separately (Fig. 2B1,B2) were effective in eliciting an action potential in the motoneuron when they were delivered with interstimulus intervals sufficient to promote the temporal summation of their responses (Fig. 2B3). The facilitated state that was exerted by hypothalamic stimulation lasted several tens of milliseconds. As shown in Figure 2C, intervals between hypothalamic and dorsal root stimulation that were equal to or shorter than 65 msec (Fig. 2C2) were effective in producing motoneuron discharge.
Figure 2.
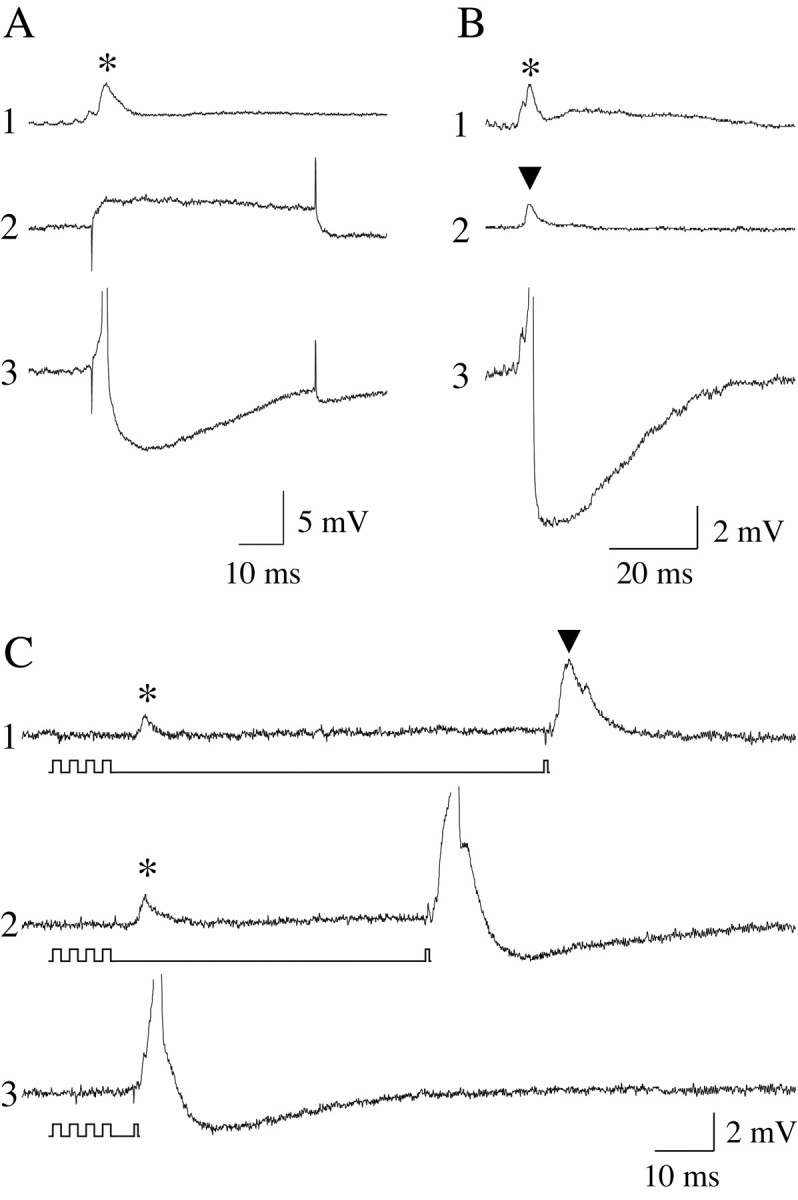
Hypothalamic stimulation facilitates the discharge of lumbar motoneurons after subthreshold depolarizing current and orthodromic stimuli. Independent stimulation of the hypothalamus and the application of intracellular current steps (1.6 nA, 50 msec) did not elicit an active response in motoneurons (A1 and A2, respectively). However, when the same subthreshold, intracellularly injected depolarizing current pulse was conditioned with hypothalamic stimuli, the motoneuron was driven to threshold and discharged an action potential (A3). Similarly, whereas independent hypothalamic (B1) and dorsal root stimulation (B2) did not excite motoneurons, conditioning the dorsal root stimulus with that delivered to the hypothalamus produced orthodromic firing of the motoneuron (B3). Hypothalamic stimulation facilitated the discharge of motoneurons to dorsal root stimulation for interstimulus periods ≤65 msec (C2). *, Hypothalamic stimulation-induced response; ▾, reflex response.
To determine whether a component of the hypothalamically induced response was mediated by the local release of Hcrt, the Hcrt type 1 receptor antagonist SB-334867 was juxtacellularly applied during hypothalamic stimulation. After the application of SB-334867, there was a statistically significant decrease in the mean amplitude of the hypothalamus stimulation-induced early EPSP (1.85 ± 0.17 mV vs 1.47 ± 0.21 mV; n = 12; p < 0.001; paired Student's t test) (Fig. 3A, straight arrows). The mean amplitude of the second EPSP was also reduced by the application of SB-334867 (0.37 ± 0.06 mV vs 0.3 ± 0.07 mV; n = 12; p < 0.003; paired Student's t test) (Fig. 3A, curved arrows). In contrast to the EPSPs, the mean amplitude of the hypothalamically induced late IPSP did not change after the application of the Hcrt-1 receptor antagonist (0.35 ± 0.08 mV vs 0.34 ± 0.07 mV; n = 12; p = 0.567). To test whether SB-334867 actions were specific on the hypothalamically induced EPSPs, this compound was applied after dorsal root stimulation. SB-334867 produced no change in the mean amplitude of the reflex EPSP (1.0 ± 0.16 mV vs 1.0 ± 0.22 mV; n = 6; p = 0.42; Student's t test). The top traces in Figure 3, B and C, illustrate that the application of SB-334867 onto a triceps motoneuron produced a 38% decrease in the amplitude of the hypothalamically induced EPSP; in contrast, the reflex response remained virtually unchanged (bottom traces in Fig. 3B,C). It is important to note that no statistically significant changes were observed in the mean RP after the application of SB-334867.
Motoneuron responses to juxtacellular ejection of hypocretin
Records from 32 motoneurons before and after the juxtacellular ejection of Hcrt (n = 44) were included in the present study. In selected experiments, we determined whether the juxtacellular application of solutions of vehicle (n = 6) and Hcrt that was previously boiled for 5 min (100 μm; n = 2) exerted any effect on motoneurons. In contrast to the application of Hcrt, the ejection of either vehicle or a boiled (denatured) Hcrt solution did not induce changes in the electrophysiological properties of spinal motoneurons.
Reflex response
Stimulation of dorsal roots at intensities that varied between threshold (T) and 2T elicited the typical EPSP monosynaptic reflex response that was, at the higher intensities, accompanied by an inhibitory component attributable to the recruitment of Ia and Ib inhibitory interneurons (Edgley and Jankowska, 1987; Jankowska, 1992). Whereas Hcrt produced motoneuron depolarization in all cases, and frequently motoneuron discharge, the mean amplitude of the reflex EPSP did not significantly change after the application of this peptide (2.1 ± 0.37 mV vs 2.2 ± 0.39 mV, control and Hcrt, respectively; n = 13; p = 0.484; paired Student's t test). However, Hcrt induced statistically significant changes in the time course of the reflex responses that were recorded during the period in which motoneurons were depolarized. The mean half-width decreased by 10.6% (4.96 ± 0.44 msec vs 4.43 ± 0.43 msec, control and Hcrt, respectively; n = 13; p < 0.001; paired Student's t test), the half-decay time decreased by 12.3% (3.9 ± 0.29 msec vs 3.42 ± 0.32 msec, control and Hcrt, respectively; n = 13; p < 0.02; paired Student's t test), and the full duration of the EPSP decreased by 10.6% (18.6 ± 1.76 msec vs 16.3 ± 1.43 msec, control and Hcrt, respectively; n = 13; p < 0.005; paired Student's t test).
Motoneuron depolarization
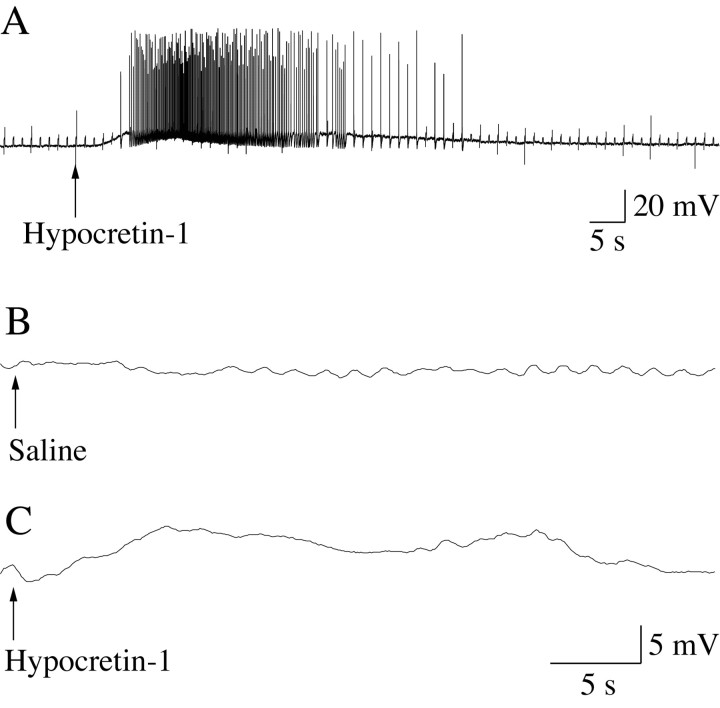
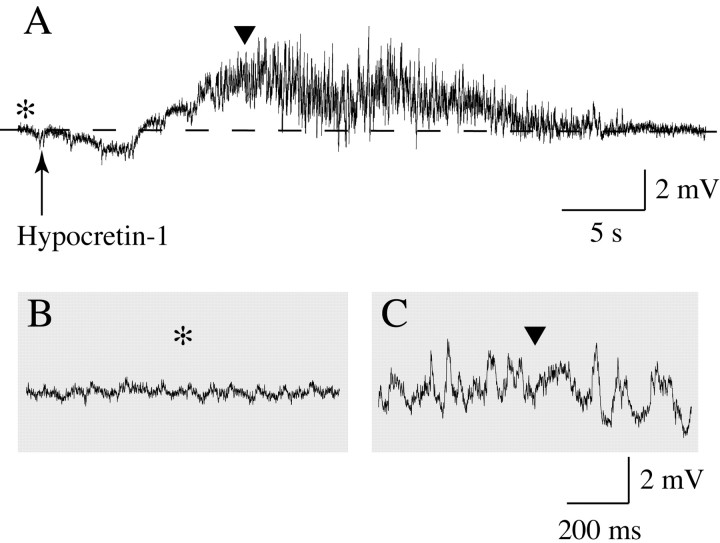
Motoneuron depolarization occurred in all motoneurons after the application of Hcrt (5.8 ± 1.05 mV; n = 32). These Hcrt-induced changes in RP are illustrated in Figure 4 for two representative lumbar motoneurons. The recording shown in Figure 4A was obtained from a motoneuron in a cat that was anesthetized with Nembutal. This motoneuron depolarized after Hcrt application (indicated by the arrow), and depolarization was followed by high-frequency bursts of action potentials. Figure 4, B and C, depict the response of a motoneuron to saline (vehicle) and Hcrt application in a cat that was anesthetized with α-chloralose. Whereas saline did not evoke changes in the RP, Hcrt produced a 7 mV depolarization that lasted ∼30 sec.
Figure 4.
The juxtacellular application of Hcrt-1 produced membrane depolarization and the discharge of lumbar spinal cord motoneurons. A representative motoneuron that was recorded from a cat anesthetized with Nembutal (A) and orthodromically stimulated (L7 dorsal root; 0.5 Hz) began to depolarize ∼1.6 sec after the onset of the ejection of Hcrt-1 from the tip of a pipette adjacent to the recorded cell (A, arrow) (3 psi, 5 sec). After a plateau of depolarization, during which time the motoneuron discharged at a high frequency, the resting potential slowly returned to baseline. The duration of Hcrt-1-induced depolarization was ∼1 min. A motoneuron that was recorded from a cat during α-chloralose anesthesia (B, C) did not exhibit a significant change in its resting potential after the application of saline (B, arrow) (15 psi, 10 sec). However, after the same ejection pressure was applied through a second ejecting micropipette that contained Hcrt-1 (C, arrow), the motoneuron responded with a long-lasting depolarization. The motoneuron in A was not identified, whereas that in B and C was a hamstrings motoneuron. Hcrt-1 was injected at a concentration of 100 μm, diluted in saline.
Rheobase
The application of Hcrt produced a 36% decrease in Rh (Table 1). As shown in Figure 5 for a representative motoneuron, the decrease in Rh was associated with membrane depolarization. Another experimental paradigm that was used to determine changes in the responsiveness of lumbar motoneurons induced by Hcrt application is shown in Figure 5A, wherein rheobasic current pulses, 150-500 msec in duration, were applied to motoneurons. After the ejection of Hcrt, motoneurons responded to an identical current pulse with trains of action potentials (Fig. 5B).
Table 1.
Changes in the electrophysiological properties of motoneurons after the application of Hcrt-1
|
|
Pre-hypocretin |
Hypocretin |
|---|---|---|
| Rheobase (nA)* | 6.4 ± 0.89 (7) | 4.1 ± 0.85 (7) |
| Input resistance (MΩ) | 2.4 ± 0.22 (24) | 2.5 ± 0.23 (24) |
| Threshold voltage (mV)* | 16.0 ± 2.3 (5) | 9.4 ± 2.3 (5) |
| τa (msec) | 44.2 ± 7.4 (21) | 50.8 ± 7.34 (21) |
| τb (msec)** | 6.1 ± 0.43 (24) | 5.1 ± 0.38 (24) |
| τc (msec)**
|
0.90 ± 0.08 (24) |
0.69 ± 0.05 (24) |
The data were obtained from 13 motoneurons that were recorded from four adult cats. Values are means ± SE. The figures in parentheses are the number of injections of Hcrt-1.
*p<0.05;** p<0.0002.
Figure 5.
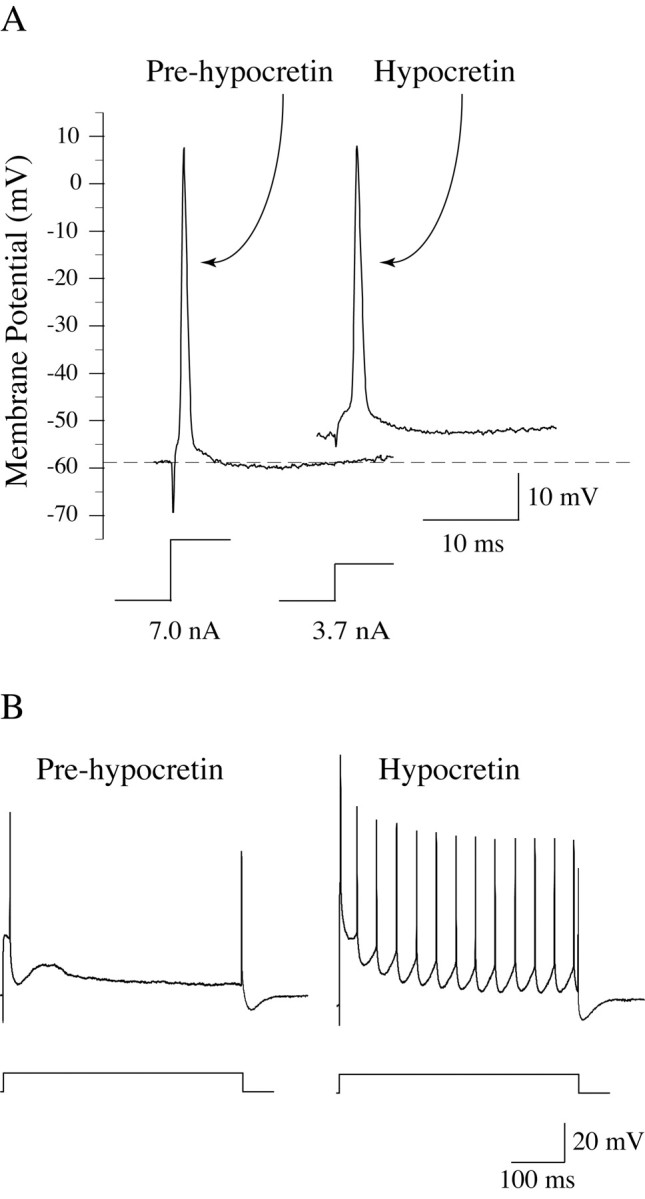
Hcrt-1 produced a decrease in the Rh of lumbar spinal cord motoneurons. A triceps surae motoneuron with a resting potential of -58 mV (A, left spike, Pre-hypocretin) responded with a single action potential to a rheobasic current step of 7.0 nA (indicated beneath the action potential in A). After the ejection of Hcrt-1, the motoneuron depolarized ∼5 mV and its rheobasic current decreased to 3.7 nA (A, right spike, Hypocretin). Hcrt-1 induced an increase in the responsiveness of lumbar spinal cord motoneurons. Before the ejection of Hcrt-1, a sciatic motoneuron responded with a single action potential to a rheobasic current pulse (2.0 nA, 500 msec) (B, left trace). After Hcrt-1 application, the same cell responded to an identical stimulus with a train of action potentials (B, right trace).
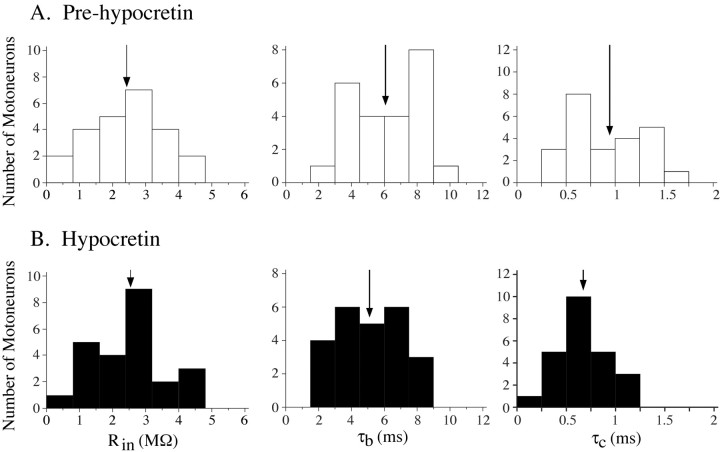
Input resistance
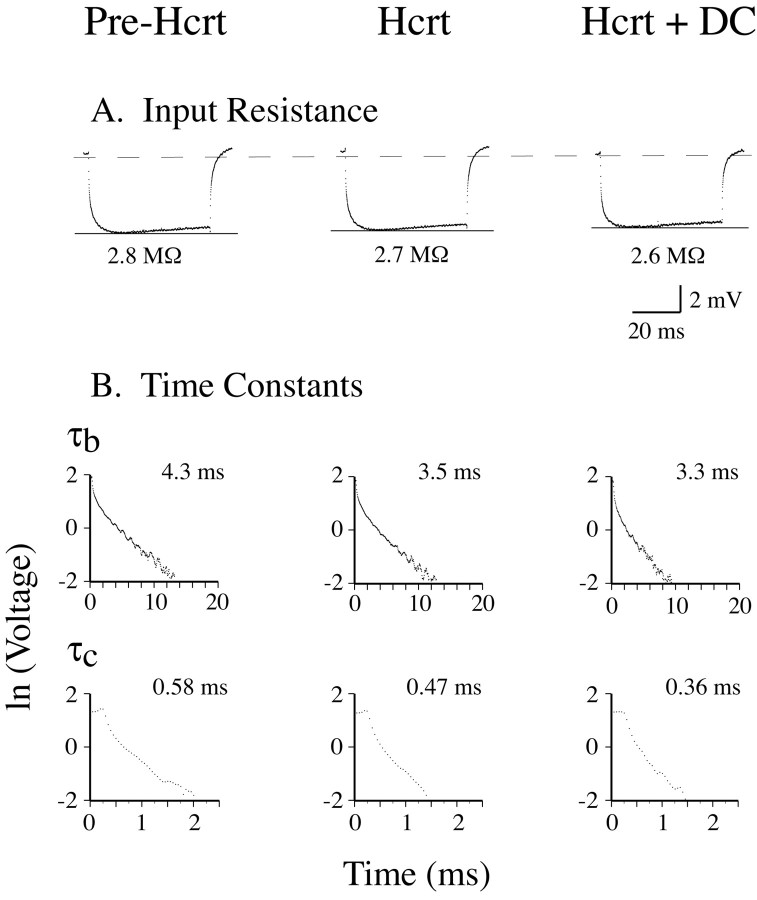
Changes in the Rin of neurons reflect changes in the conductance of the cell membrane (Engelhardt et al., 1989). Therefore, we determined whether Hcrt was capable of affecting this electrophysiological property of motoneurons. We found that the mean Rin of motoneurons did not change after the application of Hcrt (Figs. 6A,7A,B, left histograms; Table 1). It should be noted that Rin, as indicated by its measurement using the direct method, remained at similar values during Hcrt-induced motoneuron depolarization as well as when the motoneuron RP was kept approximately at the control (pre-Hcrt) level by passing hyperpolarizing DC current after the application of Hcrt (Fig. 6A, middle and right trace, respectively).
Figure 6.
Hcrt-induced effects on the input resistance and time constants of lumbar motoneurons. The averaged response of a hamstrings motoneuron to a -3 nA current pulse was recorded during three different experimental conditions: (1) before Hcrt-1 ejection (i.e., control conditions) (A, left trace); (2) after Hcrt-1 ejection while the motoneuron was depolarized by ∼5mV (A, middle trace); and (3) during a period in which the Hcrt-induced depolarization was blocked by repolarizing its resting potential to its control, pre-Hcrt level by applying constant hyperpolarizing current (A, right trace). Compared with pre-Hcrt conditions, the input resistance (measured by the direct method) did not substantially change after Hcrt-1 application either during the depolarized state or when the resting potential was clamped to the pre-Hcrt level. The analysis of time constants during each experimental condition for this motoneuron was performed using the peeling method (B). After successive peelings, the membrane time constant (τb) and the first equalizing time constant (τc) were determined (B, left traces). Both τb and τc decreased during Hcrt-1-induced depolarization (B, middle traces), indicating that the peptide produced an increase in the membrane conductance. This increase in membrane conductance was still present when the resting potential of the motoneuron was artificially maintained at control levels, indicating that it was not caused by the depolarization induced by Hcrt-1 (B, right traces).
Figure 7.
A, B, The mean input resistance of motoneurons was not affected (left graphs) whereas the mean membrane and equalizing time constants changed significantly after the application of Hcrt-1 (middle and right graphs). The arrows indicate the mean values of input resistance, τb, and τc.
In the present study, the threshold voltage (i.e., the product of Rh and the Rin) (Gustafsson and Pinter, 1984; Yamuy et al., 1992b) calculated for five motoneurons in which both Rh and Rin were quantified showed a statistically significant decrease (41%) after Hcrt application (Table 1).
Time constants
The existence of changes in membrane conductance can be determined by calculating the membrane time constant (τb) (Engelhardt et al., 1989, 1995). In addition, changes in the first equalizing time constant (τc) are also related to changes in membrane conductance (Rall, 1977; Yamuy et al., 1992b; Liu et al., 1996). Figure 6B shows changes in the time constants of a hamstring motoneuron that occurred after the application of Hcrt. Initially, the time constant of the slow process described by Ito and Oshima (1965) (τa) was determined to correct the raw data for an accurate measurement of τb (Yamuy et al., 1992b). After successive peelings, τb and τc were obtained. After Hcrt pressure ejection, τb decreased from 4.3 to 3.5 msec (18.6%) (Fig. 6B, top scattergram in the second column). In addition, τc decreased from 0.58 to 0.47 msec after Hcrt application (19%) (Fig. 6B, bottom scattergram in the second column). Note, as indicated by the scattergrams in the right column in Figure 6B, that the Hcrt-induced decrease in both τb and τc continued to develop during the period of time when the RP was held at control values by passing hyperpolarizing DC current through the membrane. When the RP returned to control values and no hyperpolarizing current was injected, both τb and τc reverted to baseline values (data not shown). Overall, Hcrt application produced a statistically significant decrease in the mean τb and τc (16.4 and 23.3%, respectively) (Fig. 7A,B, middle and right histograms; Table 1).
Membrane noise
In a small number of motoneurons (2 of 16 neurons in which Rin was assessed before and after Hcrt ejection), there was an increase in the amplitude of the membrane synaptic noise after Hcrt was applied. Figure 8A shows a motoneuron that depolarized by ∼4 mV after Hcrt ejection (indicated by the arrow). In portions of the recording of Figure 8A (which are shown at a faster time base in Fig. 8,B and C, before and after Hcrt ejection, respectively), it is possible to view that large-amplitude depolarizing potentials, which resemble EPSPs, occurred only after the application of Hcrt (Fig. 8C).
Figure 8.
Increase in membrane synaptic activity (noise) after Hcrt-1 application. A, Approximately 5 sec after Hcrt-1 was ejected (arrow), this motoneuron depolarized and large depolarizing potentials appeared. The portions of the recording in A that are indicated by an asterisk and a solid triangle are depicted, at a faster time base, in B and C, respectively. Note that the depolarizing potentials, which resemble EPSPs, are present only after the application of Hcrt-1 (C).
Discussion
In the present study, we have obtained direct in vivo evidence that the hypothalamic hypocretinergic system is capable of inducing changes in the synaptic responses and electrical properties of lumbar motoneurons that result in the facilitation of their discharge.
Hypothalamic stimulation-induced response of lumbar motoneurons
During the first 100 msec after stimulation of the perifornical region of the hypothalamus, a complex, predominantly excitatory synaptic response was recorded in motoneurons. These data indicate a facilitatory physiological action of the hypocretinergic system on spinal motoneurons. In addition, these EPSPs were frequently followed by a late, long-lasting IPSP. The application of SB-334867 reduced the amplitude of the hypothalamically induced EPSPs but not that of the hypothalamically induced IPSP. This indicates that, at least in part, only the excitatory synaptic drive was mediated by the action of Hcrt-1 on receptors located in the vicinity of the injecting micropipette. Interestingly, this effect of SB-334867 was selective on hypothalamically induced EPSPs; the early component of the reflex reponse (i.e., the Ia EPSP), known to be mediated by ionotropic glutamatergic mechanisms (Walmsley and Bolton, 1994; Rekling, 2000), was not affected by the Hcrt-1 antagonist. These results indicate that Hcrt does not act presynaptically on Ia afferents to facilitate the activity of motoneurons (see below).
Because SB-334867 did not abolish the hypothalamically induced response, a portion of this response was likely evoked by either one or both of the following: (1) a Hcrt-induced facilitation of excitatory and inhibitory inputs to motoneurons that are located at the suprasegmental (i.e., brainstem) or spinal cord level (i.e., spinal interneurons or presynaptic terminals of descending projections), or (2) stimulation of other hypothalamic descending systems in which somas or fibers are intermingled with Hcrt-containing cells in the perifornical area. It should also be considered that the applied SB-334867 may have not reached all of the Hcrt type 1 receptors in the recorded motoneuron and that part of the hypothalamically induced EPSP was mediated by Hcrt type 2 receptors that were not likely blocked by SB-334867 at the concentration used in our study.
Hcrt-induced effects on the motoneuron reflex response
The juxtacellular application of Hcrt facilitated the discharge of motoneurons after subthreshold stimulation of dorsal roots in a manner similar to that which followed the electrical stimulation of the hypothalamus in concert with subthreshold stimulation of dorsal roots. In addition, Hcrt produced a small but statistically significant decrease in the mean half-width, half-decay time, and duration of the reflex EPSP. The reduced time course of this EPSP suggests that Hcrt produced an increase in the input conductance of motoneurons (Rekling et al., 2000).
Hcrt-induced effects on the resting potential of motoneurons
Membrane depolarization, which was frequently accompanied by motoneuron discharge, occurred in lumbar motoneurons after the application of Hcrt. The Hcrt-induced increase in the firing rate and excitability of spinal motoneurons agrees with previously reported studies that have shown that Hcrt excites neurons in the locus coeruleus (de Lecea et al., 1998; Bourgin et al., 2000). Based on the large change that was induced in the mean RP, it is likely that Hcrt exerts a potent physiological excitatory effect on motoneurons. In addition, the data indicate that Hcrt acts on motoneurons that innervate different hindlimb muscles. Therefore, we proposed that the activation or suppression of the discharge of hypocretinergic neurons is accompanied by a general increase or decrease, respectively, in the responsiveness of lumbar motoneurons.
Rheobase
To determine whether Hcrt affects motoneuron excitability, we examined whether this peptide induced changes in the rheobasic current of lumbar motoneurons (Fleshman et al., 1981; Yamuy et al., 1992a). Hcrt was found to induce a significant decrease in the mean Rh (i.e., it increased neuronal excitability). Because a decrease in Rh can also be produced by an increase in the Rin (Engelhardt et al., 1989), we examined whether the application of Hcrt was followed by changes in Rin.
Input resistance
The Rin was not affected after the application of Hcrt. Similar results were reported by Hagan et al. (1999) for neurons recorded in the locus coeruleus. The lack of change in Rin suggests either that the direct method of determining this electrical property was not sensitive enough to detect small modifications in the resistance of the cell membrane, or that Hcrt produced changes in two or more ionic conductances that cancelled each other (Ivanov and Aston-Jones, 2000), thereby promoting membrane depolarization without alterations in Rin. In fact, after Hcrt, motoneurons depolarized by ∼5.8 mV; therefore, the decrease in threshold voltage attributable to depolarization (Gustafsson and Pinter, 1984; Yamuy et al., 1992a,b) may be entirely responsible for the decrease in Rh.
Time constants
τb, which has been shown to be a sensitive indicator of modifications in membrane resistance (Ito and Oshima, 1965; Zengel et al., 1985; Engelhardt et al., 1989, 1995), decreased after the application of Hcrt. Because it is assumed that membrane capacitance remains constant in the present experimental paradigm, Hcrt-induced changes in τb are expected to reflect a decrease in membrane resistance. τc has been proposed to be caused by the passive soma-dendritic redistribution of the charge injected through the membrane (Rall, 1969, 1977). The decrease in τc that followed the application of Hcrt provides further supports the concept that this peptide induces alterations in specific resistances of the motoneuron membrane (Rall, 1977; Engelhardt et al., 1989).
The preceding changes in τb and τc, as well as those in the time course of the dorsal root-induced EPSP, indicate that one or more ionic conductances of spinal motoneurons are activated after the application of Hcrt. The question then arises of why the Hcrt-induced increase in membrane conductance was not reflected by similar changes in Rin. It is possible that this discrepancy is attributable to the activation of cationic currents accompanied by the decrease of a different cationic current. Data obtained from in vitro studies indicate that Hcrt acts on some postsynaptic neurons by activating inward currents (i.e., Ca2+ and the electrogenic Na+/Ca2+ exchanger currents) (van den Pol et al., 1998; Eriksson et al., 2001; Burlet et al., 2002). In contrast, a Hcrt-induced decrease in the K+ current has been documented for neurons in the locus coeruleus (Hagan et al., 1999; Horvath, 1999; Ivanov and Aston-Jones, 2000). These actions of Hcrt on different cationic currents would elicit depolarization without significant changes in the Rin on the postsynaptic cell (van den Pol et al., 1998; Burlet et al., 2002). In addition, changes in τb have been proposed to be more sensitive than those of Rin to both proximal and distal alterations in membrane conductance (Carlen and Durand, 1981). Redman et al. (1987) have proposed that the disparity between changes in τb and Rin may be a result of modifications in the resting potassium conductance at distal dendritic sites. In previous studies, we have reported that disproportionate changes of τb, compared with those of Rin, occur in hypoglossal motoneurons during postsynaptic inhibitory processes (Fung et al., 2000).
The preceding results are in agreement with those obtained from in vitro studies that have reported that Hcrt produces neuronal depolarization (Horvath et al., 1999; Bayer et al., 2001; Eggermann et al., 2001; Eriksson et al., 2001; Liu et al., 2001, 2002; Brown et al., 2002; Soffin et al., 2002) and changes in membrane conductance (Eriksson et al. 2001; Burlet et al. 2002). However, in neurons of the locus coeruleus, the application of Hcrt promotes depolarization accompanied by a decrease or no change in membrane conductance (Hagan et al., 1999; Ivanov and Aston-Jones, 2000). Thus, it is conceivable that Hcrt acts differentially according to the intrinsic properties of the target cell to produce an increase in its excitability.
Mode of action of Hcrt on motoneurons
Hcrt-induced effects on lumbar motoneurons may be attributable to presynaptic or postsynaptic mechanisms. The examination of this question using in vitro techniques has indicated that both mechanisms of action exist for Hcrt in other cells (van den Pol et al., 1998; Bayer et al., 2001; Eriksson et al., 2001; Burlet et al., 2002, Grudt et al., 2002). Although the present experimental design cannot provide definitive evidence, we proposed that the Hcrt system normally acts on spinal motoneurons both presynaptically and postsynaptically for the following reasons. We found that after Hcrt application there was an increase in membrane depolarizing noise in a small number of motoneurons. This indicates that Hcrt is capable of acting presynaptically by increasing the release of neurotransmitter, putatively glutamate, from a population of axon terminals that impinge on spinal motoneurons (van den Pol, 1998; John et al., 2003; Peever et al., 2003). In favor of a postsynaptic action of Hcrt, (1) applied Hcrt produced large-amplitude, long-lasting membrane depolarization, which would not be expected if the mechanism of action were purely presynaptic (Del Negro and Chandler, 1998; Parker et al., 1998), and (2) preliminary anatomical data indicate that Hcrt-containing fibers are apposed to lumbar motoneurons and that these cells contain Hcrt membrane receptors (Yamuy et al., 2000). A physiological postsynaptic effect induced by Hcrt is peculiar, because most peptides are colocalized with and act to alter the release of classic neurotransmitters (Lohof et al., 1993; Li et al., 1998). There are, however, reports that indicate a postsynaptic action for Hcrt and other neuropeptides such as substance P (Li and Zhuo, 2001; Burlet et al., 2002; Katayama et al., 2003).
Considerations of the physiological significance of Hcrt actions on spinal motoneurons
The hypocretinergic system has been suggested to play a role in a variety of physiological processes, most of which involve activation of the somatomotor system (Pu et al., 1998; van den Pol et al., 1998; Chemelli et al., 1999; Haynes et al., 1999; Lin et al., 1999; Siegel, 1999; Tamura et al., 1999; Kilduff and Peyron, 2000; Sutcliffe and de Lecea, 2000; Lin et al., 2002; Wu et al., 2002). Hcrt-containing neurons project to regions that are involved in the generation of somatomotor activity (Peyron et al., 1998; Fung et al., 2001; Zhang et al., 2002) that provides a foundation for the concept that Hcrt is involved in the control of motor processes. Functional data that support this concept have been reported (Sakurai, 1999). Hypocretin has been shown to excite neurons in areas involved in the control of motoneuron activity such as the ventral tegmentum, locus coeruleus noradrenergic neurons, cholinergic and non-cholinergic neurons in the dorsolateral pontine tegmentum, and neurons in the nucleus pontis oralis (Hagan et al., 1999; Horvath et al., 1999; Ivanov and Aston-Jones, 2000; Nakamura et al., 2000; Burlet et al., 2002; Soffin et al., 2002; Xi et al., 2002). In addition, data recently provided by Kiyashchenco et al. (2002) and Wu et al. (2002) indicate that an increased release of Hcrt occurs during periods of wakefulness accompanied by movement. Furthermore, Torterolo et al. (2003) found that Hcrt-containing neurons are activated only during behaviors that entail somatomotor activity [i.e., active wakefulness as well as carbachol-induced rapid eye movement (REM) sleep with its ancillary motor activity such as REMs and muscular twitches], whereas these neurons are not activated during wakefulness in the absence of movement or during quiet (non-REM) sleep.
The present results indicate that Hcrt enhances motoneuron excitability and promotes motoneuron discharge. Hcrt-induced excitation of motoneurons, mediated by presynaptic and postsynaptic mechanisms, provides hypocretinergic neurons with the capability of modulating the level of activity of the final common pathway (i.e., somatic motoneurons). We propose that this action of Hcrt on motor output is important in the physiological regulation of motor activity in situations that involve certain hypothalamus-driven behaviors and that its deficit is a critical element in the pathophysiology of cataplexy.
Footnotes
This work was supported by United States Public Health Service Grants MH 43362, NS 09999, NS 23426, AG 04307, and HL 60296. We are grateful to Drs. John K. Engelhardt and Francisco R. Morales for critique of this manuscript and Michael Kase, Mathew Fruzza, Oscar Ramos, Andrui Nazarian, and Reza Khorsan for technical assistance. We also thank GlaxoSmithKline for generously providing SB-334867.
Correspondence should be addressed to Dr. Jack Yamuy, Department of Physiology, University of California Los Angeles School of Medicine, Center for the Health Sciences, Los Angeles, CA 90095. E-mail: jyamuy@ucla.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245336-10$15.00/0
References
- Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, Mü-hlethaler M (2001) Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci 14: 1571-1575. [DOI] [PubMed] [Google Scholar]
- Berman AL (1968) The brainstem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. Madison, WI: University of Wisconsin Press.
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L (2000) Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci 20: 7760-7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL (2002) Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci 22: 8850-8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlet S, Tyler CJ, Leonard CS (2002) Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: implications for wakefulness and narcolepsy. J Neurosci 22: 2862-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen PL, Durand D (1981) Modelling the postsynaptic location and magnitude of tonic conductance changes resulting from neurotransmitters or drugs. Neuroscience 6: 839-846. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437-451. [DOI] [PubMed] [Google Scholar]
- Chen C-T, Dun SL, Kwok EH, Dun NJ, Chang J-K (1999) Orexin A-like immunoreactivity in the rat brain. Neurosci Lett 260: 161-164. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M (1999) Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA 96: 748-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Chandler SH (1998) Regulation of intrinsic and synaptic properties of neonatal rat trigeminal motoneurons by metabotropic glutamate receptors. J Neurosci 18: 9216-9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E (1987) An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol (Lond) 389: 647-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Serafin M, Bayer D, Machard D, Saint-Mleux B, Jones BE, Mühlethaler M (2001) Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience 108: 177-181. [DOI] [PubMed] [Google Scholar]
- Engelhardt JK, Morales FR, Yamuy J, Chase MH (1989) Cable properties of spinal cord motoneurons in adult and aged cats. J Neurophysiol 61: 194-201. [DOI] [PubMed] [Google Scholar]
- Engelhardt JK, Morales FR, Castillo PE, Pedroarena C, Pose I, Chase MH (1995) Experimental analysis of the method of “peeling” exponentials for measuring passive electrical properties of mammalian motoneurons. Brain Res 675: 241-248. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE, Haas HL (2001) Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21: 9273-9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman JW, Munson JB, Sypert GW, Friedman WA (1981) Rh, Rin, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol 46: 1326-1338. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Xi M-C, Engelhardt JK, Morales FR, Chase MH (2000) Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res 885: 262-272. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Sampogna S, Morales FR, Chase MH (2001) Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res 903: 257-262. [DOI] [PubMed] [Google Scholar]
- Grudt TJ, van den Pol AN, Perl ER (2002) Hypocretin-2 (orexin-B) modulation of superficial dorsal horn activity in rat. J Physiol (Lond) 538: 517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Pinter MJ (1984) An investigation of threshold properties among cat spinal α-motoneurones. J Physiol (Lond) 357: 453-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DNC, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N (1999) Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911-10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JRS (1999) Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20: 1099-1105. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van den Pol AN (1999) Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415: 145-159. [PubMed] [Google Scholar]
- Ito M, Oshima T (1965) Electrical behavior of the motoneurone membrane during intracellularly applied current steps. J Physiol (Lond) 180: 607-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G (2000) Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. NeuroReport 11: 1755-1758. [DOI] [PubMed] [Google Scholar]
- Jankowska E (1992) Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335-378. [DOI] [PubMed] [Google Scholar]
- John J, Wu M, Kodama T, Siegel JM (2003) Intravenously administered hypocretin-1 alters brain amino acid release: an in vivo microdialysis study in rats. J Physiol (Lond) 548: 557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Homma T, Honda K, Hirai K (2003) Actions of orexin-A in the myenteric plexus of the guinea-pig small intestine. NeuroReport 14: 1515-1518. [DOI] [PubMed] [Google Scholar]
- Kilduff TS, Peyon C (2000) The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci 23: 359-365. [DOI] [PubMed] [Google Scholar]
- Kiyashchenco LI, Mileykovskiy BY, Maidment N, Lam HA, Wu M-F, John J, Peever J, Siegel JM (2002) Release of hypocretin (orexin) during waking and sleep states. J Neurosci 22: 5282-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Zhuo M (2001) Substance P and neurokinin A mediate sensory synaptic transmission in young rat dorsal horn neurons. Brain Res Bull 55: 521-531. [DOI] [PubMed] [Google Scholar]
- Li Y-X, Zhang Y, Lester HA, Schuman EM, Davidson N (1998) Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci 18: 10231-10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E (1999) The sleep disorder canine narcolepsy is caused by a mutation in the Hcrt (orexin) receptor 2 gene. Cell 98: 365-376. [DOI] [PubMed] [Google Scholar]
- Lin Y, Matsumura K, Tsuchihashi T, Abe I, Iida M (2002) Chronic central infusion of orexin-A increases arterial blood pressure in rats. Brain Res Bull 57: 619-622. [DOI] [PubMed] [Google Scholar]
- Liu RH, Yamuy J, Engelhardt JK, Xi MC, Morales FR, Chase MH (1996) Cell size and geometry of spinal cord motoneurons in the adult cat following the intramuscular injection of adriamycin: comparison with data from aged cats. Brain Res 738: 121-130. [DOI] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK (2002) Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci 22: 9453-9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XH, Morris R, Spiller D, White M, Williams G (2001) Orexin A preferentially excites glucose-sensitive neurons in the lateral hypothalamus of the rat in vitro. Diabetes 50: 2431-2437. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Mu-ming P (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363: 350-353. [DOI] [PubMed] [Google Scholar]
- Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH (1987) Motoneurons properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol 57: 1118-1129. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T (2000) Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res 873: 181-187. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K (1999) Distribution of orexin neurons in the adult rat brain. Brain Res 827: 243-260. [DOI] [PubMed] [Google Scholar]
- Parker D, Soderberg C, Zotova E, Shupliakov O, Bartfai T, Larhammar D, Brodin L, Grillner S (1998) Co-localized neuropeptide Y and GABA have complementary presynaptic effects on sensory synaptic transmission. Eur J Neurosci 10: 2856-2870. [DOI] [PubMed] [Google Scholar]
- Peever JH, Lai YY, Siegel JM (2003) Excitatory effects of hypocretin-1 (orexin-A) in the trigeminal motor nucleus are reversed by NMDA antagonism. J Neurophysiol 89: 2591-2600. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing Hcrt (orexin) project to multiple neuronal systems. J Neurosci 18: 9996-10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu S, Jain MR, Kalra PS, Kalra SP (1998) Orexins, a novel family of hypothalamic neuropeptides, modulate pituitary luteinizing hormone secretion in an ovarian steroid-dependent manner. Regul Pept 78: 133-136. [DOI] [PubMed] [Google Scholar]
- Rall W (1969) Time constants and electrotonic length of membrane cylinders and neurons. Biophys J 9: 1483-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W (1977) Core conductor theory and cable properties of neurons. In: Handbook of physiology. The nervous system. Cellular biology of neurons, Vol 1, Pt 1 (Kandel ER, ed), pp 39-97. Bethesda, MD: American Physiological Society. [Google Scholar]
- Redman SJ, McLachlan GD, Hirst GD (1987) Nonuniform passive membrane properties of rat lumbar sympathetic ganglion cells. J Neurophysiol 57: 633-644. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL (2000) Synaptic control of motoneuronal excitability. Physiol Rev 80: 767-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T (1999) Orexins and orexin receptors: implication in feeding behavior. Regul Pept 85: 25-30. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu W-S, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptor that regulate feeding behavior. Cell 92: 573-585. [DOI] [PubMed] [Google Scholar]
- Siegel JM (1999) Narcolepsy: a key role for Hcrts (orexins). Cell 98: 409-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC (2001) SB-334867-A: the first selective orexin-1 receptor antagonist. Br J Pharmacol 132: 1179-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffin EM, Evans ML, Gill CH, Harries MH, Benham CD, Davies CH (2002) SB-334867-A antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology 42: 127-133. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L (2000) The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res 62: 161-168. [DOI] [PubMed] [Google Scholar]
- Tamura T, Irahara M, Tezuka M, Kiyokawa M, Aono T (1999) Orexins, orexigenic hypothalamic neuropeptides, suppress the pulsatile secretion of luteinizing hormone in ovariectomized female rats. Biochem Biophys Res Commun 264: 759-762. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH (2003) Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep 26: 25-28. [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LHT, Guan X-M (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438: 71-75. [DOI] [PubMed] [Google Scholar]
- van den Pol AN (1999) Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J Neurosci 19: 3171-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao X-B, Obrietan K, Kilduff TS, Belousov AB (1998) Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci 18: 7962-7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Bolton PS (1994) An in vivo pharmacological study of single group Ia fibre contacts with motoneurons in the cat spinal cord. J Physiol (Lond) 481: 731-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, John J, Maidment N, Lam HA, Siegel JM (2002) Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol 283: R1079-R1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi M-C, Liu R-H, Yamuy J, Morales FR, Chase MH (1997) Electrophysiological properties of lumbar motoneurons in the α-chloralose anesthetized cat during carbachol-induced motor inhibition. J Neurophysiol 78: 129-136. [DOI] [PubMed] [Google Scholar]
- Xi M-C, Fung SJ, Yamuy J, Morales FR, Chase MH (2002) Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol 87: 2880-2888. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Engelhardt JK, Morales FR, Chase MH (1992a) Active electrophysiological properties of spinal motoneurons in aged cats following axotomy. Neurobiol Aging 13: 231-238. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Engelhardt JK, Morales FR, Chase MH (1992b) Passive electrical properties of motoneurons in aged cats following axotomy. Brain Res 570: 300-306. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Sampogna S, Morales FR, Chase MH (2000) Orexin (hypocretin)-A and orexin type 1 receptor immunoreactivity in the ventral horn of the cat spinal cord. Soc Neurosci Abstr 26: 761.2. [Google Scholar]
- Yamuy J, Fung SJ, Xi M-C, Wilson CL, Morales FR, Chase MH (2001) Hypocretin-1-induced effects on spinal cord motoneurons of the cat. Soc Neurosci Abstr 27: 411.9. [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB (1985) (1985) Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol 53: 1323-1344. [DOI] [PubMed] [Google Scholar]
- Zhang J-H, Sampogna S, Morales FR, Chase MH (2001a) Hypocretin (orexin)-like immunoreactivity in the cat brainstem. Soc Neurosci Abstr 27: 908.7. [Google Scholar]
- Zhang J-H, Sampogna S, Morales FR, Chase MH (2001b) Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study. Sleep 24: 67-76. [DOI] [PubMed] [Google Scholar]
- Zhang J-H, Sampogna S, Morales FR, Chase MH (2002) Age-related changes in hypocretin (orexin) immunoreactivity in the cat brainstem. Brain Res 930: 206-211. [DOI] [PubMed] [Google Scholar]