Abstract
Unexpected novel events generate an orienting response that plays an important role in some forms of learning and memory. The orienting response involuntarily captures attention and rapidly habituates as events become familiarized. Although evidence from patients with focal lesions and scalp and intracranial event-related brain potential recordings supports the involvement of a distributed neural network involving association cortex and the limbic system in novelty detection, the key neural substrates and temporal dynamics have not been defined. While subjects performed a bi-field visual-selective attention task with random novel stimuli embedded in either attended or unattended visual fields, we measured rapid changes of regional blood oxygenation level-dependent (BOLD) signal to target and novel stimuli using single-trial analysis of event-related functional magnetic resonance imaging with a 4T scanner. Habituation was quantified by serial BOLD signal changes during the first 10 novel stimuli for each subject. Novel stimuli activated the bilateral superior/middle frontal gyrus, temporal-parietal junction, superior parietal lobe, cingulate gyrus, hippocampus, and fusiform gyrus. The superior/middle frontal gyrus and hippocampus showed significant reduction of BOLD signal during the first few novel stimuli, whereas the signals in the fusiform and cingulate gyrus were constant. Prefrontal and hippocampal responses to attended and unattended novel stimuli were comparably habituated. These results, and previous data from lesion studies, support the view that prefrontal and hippocampal regions are involved in rapid automatic detection and habituation to unexpected environmental events and are key elements of the orienting response in humans.
Keywords: orienting response, novelty detection, event-related fMRI, single trial analysis, visual attention, limbic system
Introduction
Novelty detection is critical to both avoiding dangers and adapting to environmental changes. A novel stimulus provokes an orienting response that declines or habituates as the stimulus becomes familiar (Sokolov, 1963). Neural representation of new events is one of the most elementary forms of learning and memory (Parker et al., 1998; Ranganath and Rainer, 2003). Rodent studies demonstrate that the frontal cortex and hippocampus (Hip) are critical for detecting a mismatch between novel events and familiar environment (Bunsey and Eichenbaum, 1996). Event-related brain potential (ERP) evidence from patients with focal lesions or implanted electrodes suggests involvement of distributed prefrontal-hippocampal and multimodal posterior association cortices in novelty detection (Courchesne et al., 1975; Knight, 1984; Yamaguchi and Knight, 1991b; Friedman and Simpson, 1994; Knight, 1996; Halgren et al., 1998; Knight and Scabini, 1998). Recent functional magnetic resonance imaging (fMRI) studies have explored more precise cortical and subcortical networks, which include the prefrontal cortex in addition to the posterior association cortex and the hippocampal region (Stern et al., 1996; Opitz et al., 1999; Clark et al., 2000; Downar et al., 2001; Kiehl et al., 2001; Strange and Dolan, 2001; Hunkin et al., 2002).
The temporal dynamics of fMRI blood oxygenation level-dependent (BOLD) signal to novel stimuli has been investigated (Clark et al., 2000; Strange and Dolan, 2001), but the results have varied among studies, likely because of differences in the hemodynamic response of brain regions of interest as well as to signal-to-noise limitations of lower-field scanners. Conventional event-related fMRI requires averaging of multiple trials in an individual subject and may lose unique information associated with learning and habituation to repeated single events (Ugurbil et al., 1999). High-field 4T MRI permits measurement of the BOLD signal evolution with sensitivity enough to detect changes within a single trial without averaging over many trials (Richter et al., 1997). In this approach, a single trial can be averaged over a group of subjects, permitting a study of rapid habituation to novel stimuli. The present study is the first exploration of neural activity change to novel stimuli associated with stimulus repetition using a single-trial analysis in a high magnetic field environment.
Another unexplored issue in previous fMRI studies is the relationship between attentional state and neural responses to novel events (Friedman et al., 1998). In most ERP and fMRI studies, novel stimuli have been presented while subjects sustained attention to a train of ongoing events. However, biologically significant novel events often occur out of the attentional focus in daily life. Because the orienting response is evoked automatically, it is predicted that the key neural substrates for novelty detection should respond comparably with either attended or unattended novel events.
Materials and Methods
Subjects. Ten right-handed healthy subjects [nine females and one male; mean age, 19.3 ± 1.6 (SD)] participated in the study according to the guidelines of the University of California. All subjects gave written informed consent before participation and were screened against medical, neurological, and psychiatric illnesses and use of prescribed medications.
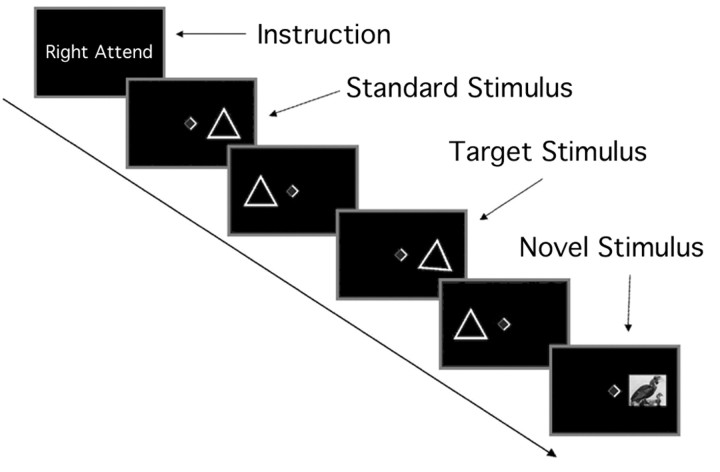
Behavioral task. Visual stimuli were displayed on a liquid crystal display projector and back-projected onto a translucent Plexiglas screen mounted on the head coil. The screen was placed at the distance of 50 cm from the angled mirror in the head radiofrequency (RF) coil. We used a bi-field visual-selective attention paradigm allowing examination of the response to attended versus unattended novel events (Fig. 1). Visual stimuli consisted of three categories [i.e., standard (82%), target (9%), and novel stimuli (9%)]. Standard and target stimuli were presented as a triangle (7.3 × 5.3°). The target triangle was tilted 10° clockwise relative to the upright triangle standard stimulus. Novel stimuli consisted of 240 different colored images (7.3 × 5.3°) such as pictures of animals, buildings, or landscapes, which were selected randomly from the picture database (Suwazono et al., 2000). Emotional pictures were not included. All stimuli were presented in a random sequence in either the right or left visual field with an eccentricity of 7.5° to the center of the screen. Subjects fixated a central diamond (0.5 × 0.5°) and attended to a sequence of visual stimuli in one visual field. The brighter side of the diamond indicates the visual field to be attended. Stimulus duration was 150 msec with a 517 msec interstimulus interval for standard and target stimuli and 200 msec duration with a 467 msec interstimulus interval for novel stimuli. The longer stimulus duration for novel stimuli was adopted so that the peripherally presented complex visual novels would be more likely to capture attention. These short presentation times for both target and novel stimuli prevented subjects from making a saccade to a stimulus in each visual field.
Figure 1.
Stimulus paradigm in a bi-field visual-selective attention study.
The subject's task was to make a speeded button-press response on each target presentation only in the attended visual field, while stimuli in the opposite field were to be ignored. Previous bi-field attention experiments, in which pictures similar to the current study were used as novel stimuli, have demonstrated that unpredictable novel pictures generated behavioral and physiological changes associated with the orienting response (Suwazono et al., 2000). The experiment was a mixed design (both blocked and event related) allowing for analysis of blocks of trials as well as single trials. The direction of attention alternated every 36 sec between the right and left visual field. Each block repeated alternatively five times for one experimental session, and the whole experiment consisted of five sessions. The stimulus sequence was identical across subjects, and the first block was always attend-to-right condition. We obtained 60 event-related responses to target and novel stimuli for each visual field in the attended and unattended condition, respectively. Each categorized event occurred with varied interstimulus intervals of 4-26 sec, and this randomized stimulus interval was suitable for event-related fMRI analysis. The subjects had a short training session without novel stimuli before entry into the scanner. Their eye movements were monitored carefully in the scanner, and central fixation was emphasized during the session. The subjects were forced to sustain selective attention to one visual field because of the fast rate of stimulus presentation and the difficult discriminability of the target stimulus.
fMRI data acquisition and analysis. Functional images were acquired with a 4 Tesla Varian INOVA scanner and a transmission electromagnetic microscopy send-and-receive RF head coil using a two-shot gradient echo-echoplanar imaging sequence (22.4 cm2 field of view; 64 × 64 matrix size; in-plane resolution, 3.5 × 3.5 mm; 18 5 mm axial slice with 0.5 mm interslice gap; repetition time, 1 sec per half of k-space; echo time, 28 msec; flip angle, 20°). High-resolution magnetization-prepared (MP)-Flash three-dimensional T1-weighted scans were acquired for anatomical normalization. The functional data were sinc interpolated in time to correct the fMRI acquisition sequence, and then motion correction was performed using a six-parameter automated algorithm. A hemodynamic response function (HRF) was derived empirically from the sensorimotor cortex for all subjects (Aguirre et al., 1998) who performed a task requiring manual responses to flickering checkerboards (20 Hz) briefly resented (200 msec) at the central vision. We modeled fMRI BOLD signal changes evoked by each stimulus category by means of covariates composed of subject-specific HRF estimates and entered the results into the modified general linear model (Worsley and Friston, 1995). Contrasts of parameter estimates across sessions comparing novel versus standard stimuli and target versus standard stimuli separately for each attentional condition were calculated in a voxel-wise manner for each subject. A direct comparison of novel versus target activation was also performed in each attentional condition. In addition to event-related analysis, to confirm the subjects' allocation of sustained attention to one visual field in each block, functional activation was analyzed by convolving a boxcar reference function with the time series of HRF representing the periodic alteration of conditions (attend-right and attend-left) in the blocked design. The significance threshold was determined at P < 0.05 by Bonferroni correction for multiple comparisons and statistical data were subjected to multi-subjects analysis with a random effect model. All functional data coregistered with the three-dimensional anatomical image were spatially normalized to the Montreal Neurologic Institute (MNI) template and smoothed using a 8mm isotropic Gaussian kernel. The MNI coordinates were then converted to the Talairach coordinates (Talairach and Tournoux, 1988).
We examined attention effects on the neural activity to novel stimuli by combining an interaction analysis of attention by stimulus type with a subtraction analysis of stimulus type. First, regions were determined by the effect of stimulus type (i.e., novel versus standard) for each attentional condition. These regions were masked by regions that showed a significant interaction between attentional condition and stimulus type (uncorrected P = 0.05). The regions activated by attended novel stimuli with inclusive interaction masking were considered as attention sensitive, whereas the regions activated by ignored novel stimuli with exclusive interaction masking were defined as attention insensitive. We also plotted the time course of changes in BOLD signal response to attended and unattended novel stimuli in the representative regions to confirm attention effects on BOLD signals.
To study the effect of stimulus repetition on BOLD signal changes, we analyzed signal intensity changes in response to the first 10 novel and target stimuli in the first experimental session and in subsequent sessions. After the region of interest (ROI) was determined by the main effect of stimulus type in the group level analysis, we identified 10 regional voxels that showed maximal responses by novel and target stimuli compared with standard stimuli in each subject within the ROI. The time series (∼16 sec after stimulus onset) of percentage mean BOLD signal changes were obtained from those voxels in unaveraged 10 individual trials. The maximal value in the time series for each single trial was subjected to repeated-measures ANOVA using a within-subject factor of sequence number (first to tenth) with adjustment by the Greenhouse-Geisser correction. Trend analyses were also performed on the time series of mean values across the subjects.
Behavioral data analysis. In addition to a simple measurement of mean reaction time to the target stimuli, we analyzed the effect of novel stimuli on the reaction time to targets. Three types of target trials were identified according to the interval between target and preceding novel stimulus: target stimuli presented >5 sec after a preceding novel stimulus; target stimuli presented within 2 sec after the preceding attended novel stimuli; and target stimuli presented within 2 sec after the preceding unattended novel stimuli. The mean values of each target categories were subjected to repeated-measures ANOVA associated with the Dunn post hoc analysis.
Results
Spatial attention effects on visual cortices
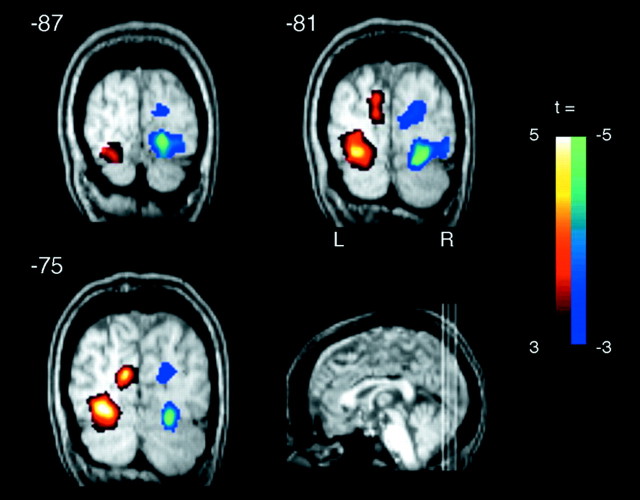
First, we confirmed that subjects allocated spatial attention to one visual field during each block. Figure 2 shows activation patterns of the occipital cortices as a function of attentional state, which was demonstrated by contrasting attend-to-right and attend-to-left conditions using a block design analysis. A significant increase in BOLD signal was obtained in primary (p < 0.01) and association (p < 0.001) visual cortices in the hemisphere contralateral to the attended visual field. This indicates that subjects allocated spatial attention to one visual field during each selective attention block (Martinez et al., 1999; Somers et al., 1999).
Figure 2.
Attention-related activations in the occipital cortex. A two-tailed t test with the contrast of right-attend block versus left-attend block demonstrated that sustained attention to one visual field activated the striate and lingual gyrus contralateral to the attended visual field, indicating that subjects surely paid attention to one visual field for each block. The activation data were overlaid on a normalized structural MRI of a single subject. The location of the three coronal slices are given in Talairach coordinates (Y values). The left hemisphere appears on the left side of image.
Brain areas activated by novel stimuli
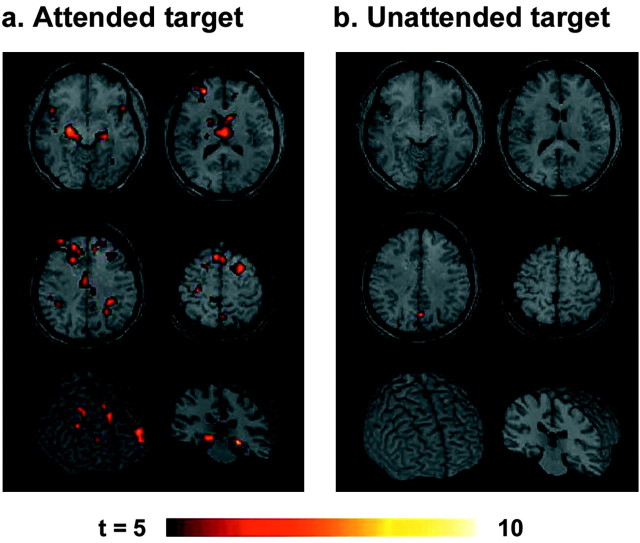
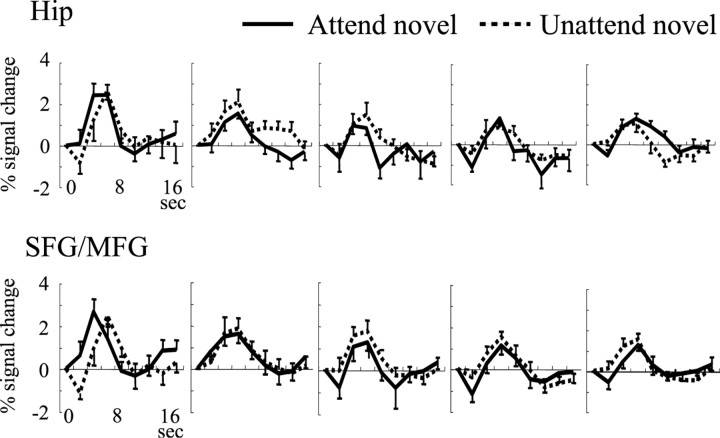
Novel stimuli in the attended field generated increased BOLD signal in distributed cortical regions, involving the border between the superior and middle frontal gyrus (SFG/MFG), cingulate gyrus (CG), precuneus (PCu), superior and inferior parietal lobules (SPL and IPL), middle temporal gyrus (MTG), cuneus (Cu), fusiform gyrus (FG), and hippocampus (Hip) in contrast to standard stimuli (Fig. 3a; Table 1). Several regions were also activated by the novel stimuli presented in the unattended visual field, including the SFG/MFG, CG, PCu, FG, and Hip (Fig. 3b; Table 1). The analysis using interaction masking showed abolished or reduced activations in the SPL, IPL, MTG, CG, Pcu, and FG (Fig. 3c), indicating activation of these regions are dependent on voluntary attention. In contrast, activations in the SFG/MFG and Hip were insensitive to attentional allocation (Fig. 3d). The time course of the signal changes illustrated differential activation levels by attended and unattended novel stimuli in the FG but similar patterns in the SFG/MFG and Hip (Fig. 3e). When the activity to novel events was compared directly to target-related activity, both attended and unattended novel stimuli also activated the right MFG (maximum t = 3.55) and left Hip (maximum t = 4.84) in addition to the bilateral FG (maximum t = 5.39).
Figure 3.
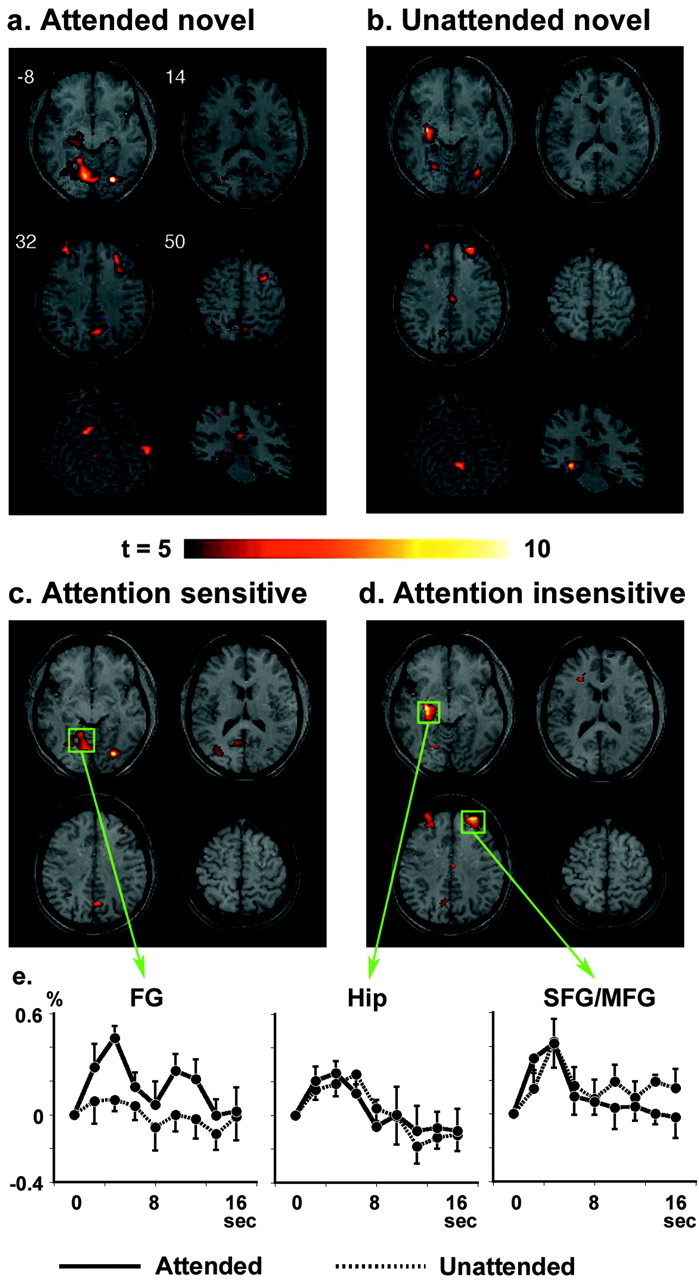
Brain regions activated by attended novel stimuli (a) and unattended novel stimuli (b). The middle panels represent the regions that are sensitive (c) and insensitive (d) to attentional state, resulting from the interaction masking analysis of main stimulus type effect. e, Time course plots of signal changes are shown for attended (solid line) and unattended (dashed line) novel stimuli in the left FG, left Hip, and right SFG/MFG. Solid values represent average ± SEM across 10 subjects. The activation map was overlaid on a normalized structural MRI, which was obtained by transforming high-resolution MP-Flash three-dimensional structural images into standard stereotactic space (Talairach and Tournoux, 1988). The locations of the four axial slices are given in Talairach coordinates (Z values).
Table 1.
Significantly activated regions for each stimulus condition and stimulus type in contrast to standard stimuli
|
Region |
BA |
L/R |
X |
Y |
Z |
Maximum t value |
|---|---|---|---|---|---|---|
| Attended novel stimuli | ||||||
| SFG | 9 | L | −38 | 46 | 30 | 8.05 |
| MFG | 9 | R | 32 | 34 | 34 | 7.07 |
| 6 | R | 32 | 12 | 54 | 7.70 | |
| Posterior CG | 23 | L | −4 | −30 | 24 | 7.12 |
| 30 | R | 24 | −68 | 6 | 8.32 | |
| PCu | 31 | L | −8 | −50 | 30 | 8.21 |
| 31 | R | 6 | −62 | 28 | 10.8 | |
| SPL | 7 | R | 30 | −52 | 46 | 8.64 |
| IPL | 40 | L | −32 | −54 | 44 | 9.02 |
| MTG | 39 | R | 45 | −73 | 18 | 8.16 |
| Cu | 18 | L | −10 | −76 | 16 | 6.74 |
| Middle occipital gyrus | 18 | R | 26 | −80 | −4 | 18.8 |
| FG | 37 | L | −42 | −48 | −18 | 10.4 |
| 37 | R | 42 | −56 | −18 | 7.60 | |
| Hip | L | −28 | −22 | −12 | 5.93 | |
| R | 28 | −20 | −14 | 5.49 | ||
| Unattended novel stimuli | ||||||
| SFG | 9 | L | −28 | 48 | 32 | 6.49 |
| 9 | R | 30 | 48 | 34 | 8.34 | |
| CG | 23 | R | 6 | −18 | 34 | 6.27 |
| PCu | 7 | L | −6 | −62 | 40 | 6.05 |
| FG | 19 | L | −22 | −68 | −8 | 6.20 |
| 19 | R | 36 | −78 | −12 | 6.94 | |
| Hip | L | −30 | −14 | −14 | 14.2 | |
| Attended target stimuli | ||||||
| SFG/MFG | 10 | L | −26 | 52 | 16 | 9.68 |
| 9 | R | 12 | 56 | 26 | 7.95 | |
| Middle frontal gyrus | 8 | R | 36 | 22 | 42 | 9.30 |
| 8 | L | −36 | 24 | 44 | 10.8 | |
| Precentral gyrus | 4 | L | −44 | −14 | 38 | 8.35 |
| Anterior CG | 32 | R | 10 | 36 | 20 | 9.51 |
| CG | 24 | L | −2 | −8 | 34 | 6.58 |
| PCu | 7 | R | 30 | −44 | 44 | 8.83 |
| Supramarginal gyrus | 40 | L | −40 | −42 | 32 | 6.73 |
| 40 | R | 52 | −48 | 28 | 6.65 | |
| Thamalus (anterior nucleus) | L | −6 | −4 | 6 | 9.94 | |
| R | 6 | −4 | 8 | 10.7 | ||
| Caudate (body) | L | −14 | 8 | 14 | 6.23 | |
| R | 12 | 12 | 14 | 8.17 | ||
| Lentiform nucleus (putamen) | L | −28 | −12 | 0 | 9.04 | |
| Insula | 13 | L | −36 | 14 | −4 | 7.27 |
| 13 | R | 38 | 16 | −6 | 9.58 | |
| FG | 37 | R | 42 | −46 | −18 | 6.09 |
| Hip | L | −28 | −18 | −14 | 8.69 | |
| R | 30 | −20 | −12 | 7.08 | ||
| Unattended target stimuli | ||||||
| PCu |
7 |
R |
4 |
−70 |
34 |
7.76 |
Locations are given as Talairach coordinates. BA, Brodmann's area; L, left; R, right.
Furthermore, stimulus laterality affected the brain activation pattern. For example, the occipital cortex showed larger activity over the hemisphere contralateral to stimulus presentation (visual field times hemisphere interaction; F(1,9) = 12.1, P < 0.01 in FG for attended and unattended novel stimuli). In contrast, the stimulus side did not affect activities in the SFG/MFG and Hip for attended and unattended novel stimuli (no main effect of visual field and no interaction of visual field times hemisphere in both the SFG/MFG and Hip).
Brain areas activated by target stimuli
Subjects detected target stimuli with 94.3 ± 7.7% accuracy (range, 80.8-99.3%; false alarm rate, 2.3 ± 1.5 per whole session; reaction time, 663 ± 20 msec). There was a significant difference among reaction times to three types of target stimuli (626 ± 32 msec for targets without preceding novels, 657 ± 32 msec for targets preceded by unattended novels, and 704 ± 52 msec for targets preceded by attended novels; F(2,18) = 10.4; P < 0.001). Planned comparisons revealed that the reaction time to target stimuli with attended novel stimuli preceding within 2 sec was significantly longer than that to the other two types of target stimuli (p < 0.02) and that the reaction time to target stimuli with unattended novel stimuli were marginally delayed compared with that to target stimuli without preceding novel stimuli (p < 0.1).
Targets generated a distinct activation pattern from novels, although some areas were commonly activated by target and novel stimuli (Fig. 4a; Table 1). Several regions including motor response-related areas (precentral gyrus, supplementary motor cortex, putamen), supramarginal gyrus, thalamus, caudate nucleus, and insula were activated preferentially by target stimuli. These activations were also confirmed by calculation of the contrast between target-related versus novelty-related activity. The CG, PCu, and IPL were activated by target stimuli as much as in novel stimuli. Thus, these activities disappeared in a comparison of target-related versus novelty-related activation. Although the SFG/MFG and Hip were more activated by novel stimuli than target stimuli as mentioned above, they were also activated by target stimuli. However, voxel-based analysis revealed that target and novel stimuli activated distinct areas within each region (i.e., only 0.4% of pixels in SFG/MFG and 4.1% in Hip were commonly activated by both target and novel stimuli). Task-irrelevant targets in the unattended visual field elicited activation only in the PCu (Fig. 4b; Table 1).
Figure 4.
Brain regions activated by attended target stimuli (a) and unattended target stimuli (b). The format is the same as Figure 3.
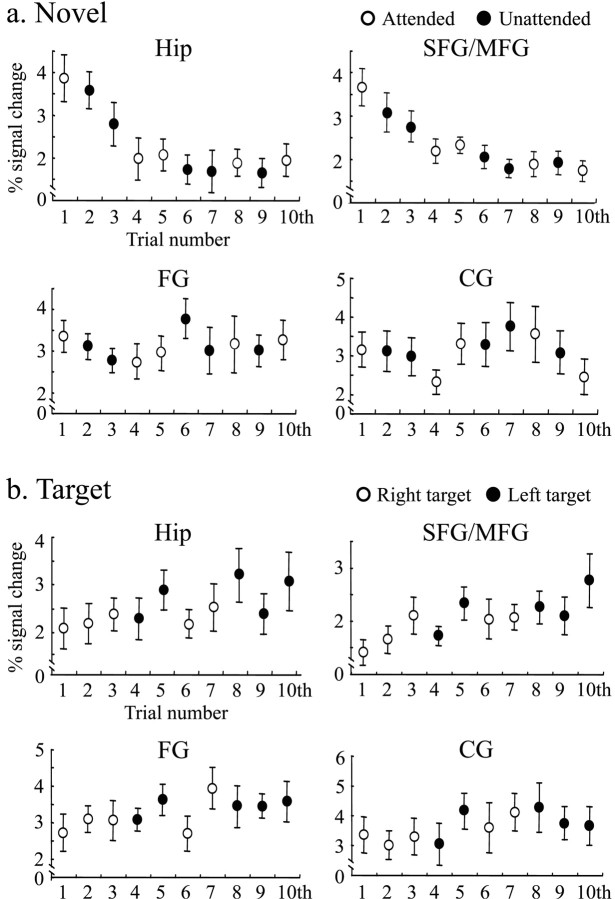
Temporal changes of BOLD signal to novel stimuli
We analyzed temporal changes of BOLD signal associated with repetitions of novel stimuli in the first experimental session by applying a single-trial analysis for each subject. Because Hip and SFG/MFG were activated by both attended and unattended novel stimuli, we measured signal changes in these regions. BOLD signal in both regions decreased rapidly during the presentation of the first four novel stimuli (stimulus sequence effect through the first to tenth stimuli; F(9,81) = 9.02, ϵ = 0.31, P < 0.0005 for Hip; F(9.81) = 9.00, ϵ = 0.47, P < 0.0001 for SFG/MGF) (Fig. 5a). The time series of BOLD signal changes were fitted best to a quadratic regression function for both regions (F(2,7) = 64.2, p < 0.0001 for Hip; F(2,7) = 10.0, p < 0.01 for SFG/MFG). There was no difference in the degree and speed of the habituation in Hip and SFG/MFG (interaction of region site and stimulus sequence; F(9,81) = 0.94; ϵ = 0.44; p = 0.49). The fMRI signals remained constant after the presentation of the fourth novel stimuli at approximately half of the initial response (stimulus sequence effect through the fourth to tenth stimuli; F(6,54) = 0.58, ϵ = 0.61, p = 0.74 for Hip; F(6,54) = 1.87, ϵ = 0.14, P = 0.1 for SFG/MGF). To examine whether the habituation effects in Hip and SFG/MFG are modulated by attentional allocation, we divided 10 novel stimuli into two groups according to the attentional state (stimuli were presented in the attended visual field for the first, fourth, fifth, eighth, and tenth trials, and other stimuli were in the unattended visual field). Responses to the first five stimuli demonstrated similar habituation for attended and unattended conditions (F(4,36) = 15.1, ϵ = 0.60, P < 0.0001 for Hip and F(4,36) = 15.4, ϵ = 0.57, P < 0.0001 for SFG/MFG in attended condition; F(4,36) = 11.1, ϵ = 0.57, P < 0.005 for Hip and F(4,36) = 3.71, ϵ = 0.67, P < 0.05 for SFG/MFG in unattended condition; no interactions of either region site times stimulus sequence or region site times stimulus sequence times attentional condition) (Fig. 5a). The hemodynamic response curves to each novel stimulus were also plotted in Figure 6, showing signal reduction as a function of stimulus repetition.
Figure 5.
Effects of stimulus repetition on BOLD signal to novel (a) and target (b) stimuli at the Hip, SFG/MFG, FG, and CG. The maximal signal intensity value in the hemodynamic response curve within 10 sec after stimulus on set was obtained from each subject. Time duration after the first standard stimulus to each novel stimulus was 8, 10, 18, 24, 44, 48, 50, 54, 66, and 70 sec, respectively.
Figure 6.
Changes of the time series of hemodynamic response curve for first five trials in response to attended and unattended novel stimuli in the Hip and SFG/MFG. Values represent average ± SEM across 10 subjects.
To examine whether habituation is selective to the Hip and SFG/MFG regions, we compared the signal changes with those in the FG and CG that showed significant activity in response to novelty stimuli. Neither of these regions showed a habituated response with any significant fitted regression functions (p > 0.3 for both regions, Fig. 5a). Because the brain regions used in single-trial analysis were selected based on the main novelty effect, there was a possibility of missing brain areas that might show habituation without the main novelty effect. If such regions were to exist, they would show activation only in the first session. However, the activated brain regions in the first session were comparable with those obtained in the whole-session analysis. We compared the response habituation to novel stimuli with that to target stimuli (Fig. 5b). In contrast to the response to novel stimuli, the signal-to-target stimuli did not show habituation patterns in any of the four regions. Rather, the responses in the CG and Hip showed a trend of increased response as a function of stimulus number, fitted best with a quadratic regression function during the first 10 target stimuli in the first session (F(2,7) = 10.7, p < 0.01 for CG; F2(2,7) = 6.4, p < 0.05 for Hip). There were no such trends of responses to targets in the remaining sessions.
We also analyzed temporal changes of BOLD signal to novel stimuli within a session for other remaining sessions and found no habituation effects in any these sessions. To examine a long-term change of hemodynamic response across the whole experiment, we compared mean signal changes from the first through fifth experimental session. There were no significant trends of mean signal changes as a function of experimental session in all four regions.
Finally, we performed a correlation analysis for coactivations of the prefrontal cortex and Hip to compare the correlated activity of these two regions between novel and target events. The correlation coefficient was calculated across the first 10 novel and 10 target stimuli, respectively, for each subject, using the highest signal values that were obtained in the time series analysis at the SFG/MFG and Hip. The correlation of prefrontal and hippocampal activity was significantly higher for the novel events (0.51 ± 0.19; range, 0.18-0.75) than the target events (0.22 ± 0.35; range, -0.22 to 0.75) (t = 2.34; p < 0.05).
Discussion
The principal findings of this study were: (1) prefrontal cortex and Hip were activated by novel stimuli, regardless of whether the stimuli were attended or unattended; (2) prefrontal and hippocampal novelty-related activity habituates rapidly; and (3) neither the response to novel stimuli in other brain regions nor the response to target stimuli show habituation. Thus, the current study provides compelling evidence that a prefrontal-hippocampal neural system is involved in generating automatic orienting response to novel events.
In humans, the processing of attended and unattended information has been investigated by the ERP technique. The auditory mismatch negativity and P3a component index changes in brain activity related to involuntary detection and processing of stimulus deviance. Both prefrontal and hippocampal lesions abolish the normal P3a increase to the first few novel events. Hippocampal lesions disrupt the P3a and sympathetic skin response (Knight, 1996), and prefrontal lesions reduce the frontal P3a component (Knight, 1984) and the autonomic response to novel stimuli (Yokoyama et al., 1987). Thus, this lesion data and the fMRI results in normals provide converging data on the habituation properties of these regions to novel events. However, other lesions such as those involving the temporal-parietal junction also reduce the P3a (Yamaguchi and Knight, 1991b), suggesting that a distributed neural systems is involved in novelty processing. Consistent with these ERP data, the current study demonstrated widely distributed cortical activations by novel stimuli when they are presented in the attended visual field. This is also consistent with recent fMRI studies using a novelty oddball task (Opitz et al., 1999; Kiehl et al., 2001).
However, when novel events occurred outside of attentional focus, the activated regions were confined to the prefrontal cortex and Hip. It is unlikely that these activities are residuals after the general activity is reduced by the removal of spatial attention effects, because the activity levels in these regions were comparable between the attended and unattended conditions. Activity in the parietal and occipital regions is dependent on the voluntary attentional state. Positron emission tomographic studies indicate that hippocampal activation by novel information occurs at an early stage of a long-term memory encoding process with little attentional modulation (Tulving et al., 1996; Iidaka et al., 2000). Because involuntary capture of attention is the central mechanism of the orienting response (Sokolov, 1963), the comparable prefrontal-hippocampal activations to both attended and unattended novel stimuli indicates that these structures are critical regions for the orienting response. A number of studies in rodents have demonstrated that the prefrontal cortex and Hip are activated by behavioral arousal and salient sensory stimuli. Specifically, the Hip plays a critical role in mnemonic processes by detecting mismatches between novel events and familiar environment (Squire, 1992; Bunsey and Eichenbaum, 1996), whereas the functional role of right prefrontal cortex in episodic memory is still controversial (Brewer et al., 1998; Wagner et al., 1998). Thus, the present study seems to fit with evidence that a prefrontal-hippocampal neural system plays an important role in some forms of episodic memory for novel events.
The neural network for target detection was distinct from that for novelty processing, although some areas were commonly activated by target and novel stimuli. Several regions including motor response-related areas (precentral gyrus, supplementary motor cortex, putamen), supramarginal gyrus, thalamus, caudate nucleus, and insula were activated preferentially by target stimuli. These brain areas activated by target detection are consistent with other fMRI studies (McCarthy et al., 1997; Menon et al., 1997). They were not activated by low-frequency task-irrelevant stimuli, typically novel stimuli, which were embedded in trains of standard and oddball targets. Thus, these brain areas are more involved in detecting task-defined stimulus relevance. ERP studies in lesioned patients have suggested that the temporal-parietal junction is critical for generation of both the P3b and P3a (Knight et al., 1989; Yamaguchi and Knight, 1991b). Consistent with this proposal, the present study demonstrated the activation of the supramarginal gyrus in target detection and inferior parietal lobule in novelty detection. However, this region does not seem to be involved in involuntary novelty detection because of the lack of activation by unattended novel stimuli. The temporal-parietal junction appears to be involved in the network that represents the voluntary orienting system to salient stimuli.
The unattended target stimuli generated activation of a circumscribed area in the PCu. This region might be involved in automatic detection of small changes in the perceptual content of visual stimuli, regardless of attention. Recent fMRI studies suggested the involvement of this region in shifting attention (Simon et al., 2002; Astafiev et al., 2003). However, this is not likely in the present study because the PCu activation was also observed in the attended condition, which required no need for shifting attention. Alternatively, the posterior cingulate and adjacent PCu are implicated in monitoring and interpreting events occurring in our environment, especially in the peripheral visual space (Raichle, 2000).
The orienting response is crucial for preparing to respond to novel events, and repetition of a novel event rapidly diminishes its biological salience. ERP and intracranial studies have demonstrated rapidly habituated response to stimulus repetition within the first several trials of a recording session (Knight, 1984; Yamaguchi and Knight, 1991a; Friedman and Simpson, 1994; Knight and Scabini, 1998). However, ERPs cannot precisely localize the neural sources of these effects, and intracranial recordings are not typically performed simultaneously in cortical and limbic regions. Thus, no prior evidence exists with both temporal and spatial information regarding habituation of the orienting response. The present study provides direct evidence of rapidly habituating responses to novel events in prefrontal and hippocampal regions. Interestingly, by modeling neural sources for the P3a habituated response, Friedman et al. (2001) found that only the prefrontal dipole activity showed reduction over time, fitting with habituated hemodynamic response in the prefrontal cortex.
The effect of stimulus repetition has also been studied in previous fMRI experiments. Clark et al. (2000) reported repetition-related changes in response amplitude to distracter stimuli embedded in a detection task. Prefrontal activity linearly increased as a function of stimulus repetition, which was inconsistent with ERP studies using the same paradigm. In contrast, other fMRI studies demonstrated habituating responses in the hippocampal region associated with familiarity to stimulus novelty (Strange et al., 1999), in which the hemodynamic response was measured by a block design, so the time scale of response change was larger than the current study. Because subjects encountered a unique picture for each novelty trial, perceptual aspects of stimulus novelty were kept intact across the experiment. However, because the appearance of picture stimuli was not informed to subjects beforehand, contextual novelty was strongly engaged at the experimental onset. In this regard, most previous fMRI studies have informed their subjects about occurrence of novel events before the start of the experiment, decreasing the initial surprising value of the novel events. Because the stimulus sequence of novel stimuli was the same across subjects, there is also a possibility that the fixed stimulus order may have affected the habituation effect. In contrast to the habituation effect to novel stimuli, habituation effects are not expected for voluntary detection of target stimuli because contextual novelty is not associated with the feature of those stimuli. Responses to target stimuli showed an augmentation tendency in the CG and Hip. The distinct time courses of neural response to target and novel stimuli may be attributable to changes in either response strategy or confidence level of discrimination associated with repeated target presentation (Clark et al., 2000).
Finally, additional evidence for the involvement of the prefrontal-hippocampal neural system in novelty processing can be obtained from neurochemical studies. Rodent experiments have revealed a neurochemical basis for prefrontal-hippocampal activation to novel stimuli and their habituated response. It was demonstrated that basal forebrain cholinergic neurons projecting to the prefrontal cortex and Hip are activated by salient stimuli, and they are involved in arousal and/or attentional processes (Acquas et al., 1996). It was also reported that the prefrontal cortical and hippocampal ACh release was reduced by habituation and the reduction pattern was almost identical in both regions. Thus, the cholinergic transmitter system may underlie the prefrontal-hippocampal novelty detection system demonstrated in the current study. The differential role of the prefrontal cortex and Hip on novelty processing is undetermined. However, evidence from lesion and electrophysiological recordings indicates that the prefrontal cortex initiates the orienting response within 150 msec with subsequent rapid engagement of hippocampal cortices within the ensuing 150 msec (Alain et al., 1998).
In summary, the present study demonstrated that (1) the prefrontal cortex and Hip are automatically activated by novel stimuli, regardless of the locus of attention, and (2) only prefrontal-hippocampal novelty-related activity habituates rapidly. Thus, the current study and previous data from lesion studies support the view that prefrontal-hippocampal regions are involved in rapid automatic detection and habituation to unexpected environmental events and are key elements of the orienting response in humans.
Footnotes
This work was supported by grants from the National Institutes of Health (NS21135, PO NS40813, and MH63548) and the Japanese Ministry of Education, Science, Sports and Culture. We thank B. Inglis for technical assistance.
Correspondence should be addressed to Dr. Shuhei Yamaguchi, Department of Neurology, Shimane University School of Medicine, 89-1 Enya-cho, Izumo 693-8501, Japan. E-mail: yamagu3n@med.shimane-u.ac.jp.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245356-08$15.00/0
References
- Acquas E, Wilson C, Fibiger HC (1996) Conditioned and unconditioned stimuli increase frontal cortical and hippocampal acetylcholine release: effects of novelty, habituation, and fear. J Neurosci 16: 3089-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M (1998) The variability of human, BOLD hemodynamic responses. NeuroImage 8: 360-369. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL, Knight RT (1998) A distributed cortical network for auditory sensory memory in humans. Brain Res 812: 23-37. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M (2003) Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci 23: 4689-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD (1998) Making memories: brain activity that predicts how well visual experience will be remembered. Science 281: 1185-1187. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H (1996) Conservation of hippocampal memory function in rats and humans. Nature 379: 255-257. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L (2000) Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol 83: 3133-3139. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R (1975) Stimulus novelty, task relevance, and the visual evoked potential in man. Electroencephalogr Clin Neurophysiol 39: 131-143. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2001) The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. NeuroImage 14: 1256-1267. [DOI] [PubMed] [Google Scholar]
- Friedman D, Simpson GV (1994) Amplitude and scalp distribution of target and novel events: effects of temporal order in young, middle-aged and older adults. Cognit Brain Res 2: 49-63. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski VA, Cycowicz YM (1998) Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology 35: 508-520. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H (2001) The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neurosci Biobehav Rev 25: 355-373. [DOI] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P (1998) Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalogr Clin Neurophysiol 106: 156-164. [DOI] [PubMed] [Google Scholar]
- Hunkin NM, Mayes AR, Gregory LJ, Nicholas AK, Nunn JA, Brammer MJ, Bullmore ET, Williams SCR (2002) Novelty-related activation within the medial temporal lobes. Neuropsychologia 40: 1456-1464. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FI (2000) The effect of divided attention on encoding and retrieval in episodic memory revealed by positron emission tomography. J Cogn Neurosci 12: 267-280. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF (2001) Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology 38: 133-142. [PubMed] [Google Scholar]
- Knight RT (1984) Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol 59: 9-20. [DOI] [PubMed] [Google Scholar]
- Knight RT (1996) Contribution of human hippocampal region to novelty detection. Nature 383: 256-259. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D (1998) Anatomical bases of event-related potentials and their relationship to novelty detection in humans. J Clin Neurophysiol 15: 3-13. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC (1989) Contribution of the temporal-parietal juntion to the auditory P3. Brain Res 502: 109-116. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA (1999) Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364-369. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P (1997) Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol 77: 1630-1634. [DOI] [PubMed] [Google Scholar]
- Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A (1997) Combined event-related fMRI and EEG evidence for temporal-parietal cortex activation during target detection. NeuroReport 8: 3029-3037. [DOI] [PubMed] [Google Scholar]
- Opitz B, Mecklinger A, von Cramon DY, Kruggel F (1999) Combined electrophysiological and hemodynamic measures of the auditory oddball. Psychophysiology 36: 142-147. [DOI] [PubMed] [Google Scholar]
- Parker A, Wilding E, Akerman C (1998) The von Restorff effect in visual object recognition memory in human and monkeys: the role of frontal/perirhinal interaction. J Cogn Neurosci 10: 691-703. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2000) The neural correlates of consciousness: an analysis of cognitive skill learning. In: The new cognitive neuroscience (Gazzaniga MS, ed), pp 1305-1318. Cambridge, MA: MIT. [DOI] [PMC free article] [PubMed]
- Ranganath C, Rainer G (2003) Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci 4: 193-202. [DOI] [PubMed] [Google Scholar]
- Richter W, Ugurbil K, Georgopoulos A, Kim SG (1997) Time-resolved fMRI of mental rotation. NeuroReport 8: 3697-3702. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S (2002) Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron 33: 475-487. [DOI] [PubMed] [Google Scholar]
- Sokolov EN (1963) Higher nervous functions: the orienting reflex. Annu Rev Physiol 25: 545-580. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RBH (1999) Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci USA 96: 1663-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR (1992) Memory and the hippocampus: a synthesis from findings with rats, monkeys and humans. Psychol Rev 99: 195-231. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Suigura RM, Vedantham V, Rosen BR (1996) The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA 93: 8660-8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ (2001) Adaptive anterior hippocampal responses to oddball stimuli. Hippocampus 11: 690-698. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RNA, Friston KJ, Dolan RJ (1999) Segregating the functions of human hippocampus. Proc Natl Acad Sci USA 96: 4034-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwazono S, Machado L, Knight RT (2000) Predictive value of novel stimuli modifies visual event-related potentials and behavior. Clin Neurophysiol 111: 29-39. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. New York: Thieme.
- Tulving E, Markowitsch MJ, Craik FIM, Habib R, Houle S (1996) Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex 6: 71-79. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Hu X, Chen W, Zhu XH, Kim SG, Georgopoulos A (1999) Functional mapping in the human brain using high magnetic fields. Philos Trans R Soc Lond B Biol Sci 354: 1195-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD (1998) Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. NeuroReport 9: 3711-3717. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ (1995) Analysis of fMRI time-series revisited—again. NeuroImage 2: 173-181. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT (1991a) P300 generation by novel somatosensory stimuli. Electroencephalogr Clin Neurophysiol 78: 50-55. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT (1991b) Anterior and posterior association cortex contributions to the somatosensory P300. J Neurosci 11: 2039-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K, Jennings R, Ackles P, Hood P, Boller F (1987) Lack of heart rate changes during an attention-demanding task after right hemisphere lesions. Neurology 37: 624-630. [DOI] [PubMed] [Google Scholar]