Abstract
The NMDA receptor complex represents a key molecular element in the pathogenesis of long-term synaptic changes and motor abnormalities in Parkinson's disease (PD). Here we show that NMDA receptor 1 (NR1) subunit and postsynaptic density (PSD)-95 protein levels are selectively reduced in the PSD of dopamine (DA)-denervated striata. These effects are accompanied by an increase in striatal levels of αCa2+-calmodulin-dependent protein kinase II (αCaMKII) autophosphorylation, along with a higher recruitment of activated αCaMKII to the regulatory NMDA receptor NR2A-NR2B subunits. Acute treatment of striatal slices with R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride, but not with l-sulpiride, mimicked the effect of DA denervation on both αCaMKII autophosphorylation and corticostriatal synaptic plasticity. In addition to normalizing αCaMKII autophosphorylation levels as well as assembly and anchoring of the kinase to the NMDA receptor complex, intrastriatal administration of the CaMKII inhibitors KN-93 (N-[2-[[[3-(4-chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide) and antennapedia autocamtide-related inhibitory peptide II is able to reverse both the alterations in corticostriatal synaptic plasticity and the deficits in spontaneous motor behavior that are found in an animal model of PD. The same beneficial effects are produced by a regimen of l-3,4-dihydroxyphenylalanine (l-DOPA) treatment, which is able to normalize αCaMKII autophosphorylation. These data indicate that abnormal αCaMKII autophosphorylation plays a causal role in the alterations of striatal plasticity and motor behavior that follow DA denervation. Normalization of CaMKII activity may be an important underlying mechanism of the therapeutic action of l-DOPA in PD.
Keywords: 6-OHDA, Parkinson's disease, CaMKII, LTP, rat, striatum
Introduction
The main pathological feature of Parkinson's disease (PD) is the degeneration of dopamine (DA)-containing nigrostriatal neurons leading to the motor symptoms observed in this disorder. Accordingly, pharmacological DA replacement with l-3,4-dihydroxyphenylalanine (l-DOPA) represents the most effective treatment of PD. In addition to its DAergic projection, the striatum also receives a massive glutamatergic innervation arising from most cortical areas, conveying sensorimotor, limbic, and cognitive information (Graybiel, 1990; Smith and Bolam, 1990; Calabresi et al., 1996). The NMDA receptor complex has been shown to be altered in both experimental parkinsonism and PD (Calabresi et al., 2000a; Dunah and Standaert, 2001). NMDA receptor antagonists exert a beneficial effect in this disorder (Papa and Chase, 1996; Vila et al., 1999).
At the molecular level, it has become increasingly evident that the NMDA receptor complex is a dynamic structure that is intimately involved in the regulation of corticostriatal long-term potentiation (LTP) (Calabresi et al., 1996), which is altered in experimental parkinsonism (Menegoz et al., 1995; Ulas and Cotman, 1996; Ingham et al., 1998; Dunah et al., 2000).
NMDA receptors are oligomeric complexes formed by the coassembly of members of three receptor subunit families: NMDA receptor 1 (NR1), NR2A-NR2D (Hollmann and Heinemann, 1994), and NR3A-NR3B (Das et al., 1998; Nishi et al., 2001). In the CNS synapses, NMDA receptors are clustered in a highly organized subcellular fraction, the postsynaptic density (PSD), where clustering of ionotropic glutamate receptors to scaffolding proteins and signaling elements can be dynamically regulated (Kennedy, 2000; Gardoni et al., 2001). It is known, in fact, that the physiological properties of NMDA receptors are determined not only by their subunit composition and cellular localization but also by the composition of the complex formed by interacting proteins governing the response of the signaling cascade, downstream of receptor activation. Among these, the α subunit of calcium-calmodulin-dependent protein kinase II (CaMKII) is directly linked to the C-terminal region of the NR2A and NR2B subunits of the NMDA receptor complex (Gardoni et al., 1998; Strack et al., 2000) and competes in NR2A binding with PSD-95 (Gardoni et al., 2001).
CaMKII- and tyrosine-dependent phosphorylation of NMDA receptors is altered after nigrostriatal denervation (Menegoz et al., 1995; Oh et al., 1999). However, at present there is no information about the association of PSD proteins (i.e., αCaMKII and PSD-95) with NMDA receptors in experimental parkinsonism and their link to DA-dependent plasticity and motor behavior.
Thus, in the present study, biochemical, electrophysiological, and behavioral approaches have been used to unravel the role of CaMKII in the rat striatum after 6-hydroxydopamine (6-OHDA) lesioning of the nigrostriatal pathway. In particular, treatment with either the specific kinase inhibitors N-[2-[[[3-(4-chlorophenyl)-2-propenyl]methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide (KN-93) and antennapedia autocamtide-related inhibitory peptide II (Ant-AIP-II) or l-DOPA has been used to address the functional significance of the αCaMKII autophosphorylation level in the formation of LTP and motor response alterations in experimental parkinsonism.
Materials and Methods
Subjects. Adult male Wistar rats (150-250 gm) were used for all of the experiments.
6-OHDA lesions. Deeply anesthetized rats were injected with 6-OHDA (8 μg/4 μl of saline containing 0.1% ascorbic acid) into the substantia nigra at a rate of 0.38 μl/min (Paxinos and Watson, 1986). Sham-operated rats were injected with vehicle at the same coordinates. Fifteen days later, the rats were tested with 0.05 mg/kg subcutaneous apomorphine, and contralateral turns to the lesion were counted for 40 min. Only those rats that made at least 200 contralateral turns were used for biochemical, electrophysiological, and behavioral experiments. It has been demonstrated previously that rats meeting this screening criterion have >95% depletion of striatal dopamine (Schwarting and Huston, 1996). The effects of the CaMKII inhibitors KN-93 (Sigma, St. Louis, MO) and Ant-AIP-II (Inalco, Milan, Italy) and the inactive analog KN-92 (Sigma) on the electrophysiological and behavioral response was assessed 1 month after 6-OHDA lesions. 6-OHDA dopamine-lesioned and sham-operated rats were injected with KN-93 (1.0 μg/μl), KN-92 (1.0 μg/μl), or Ant-AIP-II (500 μm) and vehicle, respectively, into the striatum ipsilateral to the lesion at a rate of 0.5 μl/min (total volume, 2 μl), using the following stereotaxic coordinates: anteroposterior (AP), +0.7; lateral (L), +4; ventrodorsal (VD), -4.6. R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH 23390) (1 μg/1 μl; Tocris Cookson, Bristol, UK) and vehicle were unilaterally injected in the striatum of control rats using the following stereotaxic coordinates: AP, +0.7; L, +4; VD, -4.6.
Triton-insoluble fraction preparation, endogenous phosphorylation, and immunoprecipitation studies. The striatum was rapidly homogenized in cold 0.32 m sucrose containing (in mm): 1 HEPES, 1 MgCl2, 1 NaHCO3, and 0.1 PMSF, pH 7.4, in the presence of a complete set of protease inhibitors (Complete; Roche Diagnostics, Mannheim, Germany) and phosphatase inhibitors. The homogenized tissue was centrifuged at 1000 × g for 10 min. The resulting supernatant was centrifuged at 3000 × g for 15 min to obtain a fraction of mitochondria and synaptosomes. The pellet was resuspended in hypotonic buffer in a glass-glass potter and centrifuged at 100,000 × g for 1 hr. The pellet was resuspended in 1 ml of buffer containing 75 mm KCl and 1% Triton X-100 and centrifuged at 100,000 × g for 1 hr. The final pellet was homogenized by 10 strokes in a glass-glass potter in 20 mm HEPES. An equal volume of glycerol was added and stored at -80°C. This fraction is referred to as the “Triton-insoluble fraction” (TIF). The TIF was used instead of the classical PSD preparation because the amount of the starting material was very limited. However, the protein composition of this preparation was carefully tested for the absence of presynaptic markers (i.e., synaptophysin and synaptotagmin) and for the enrichment in the PSD proteins (αCaMKII, PSD-95, NMDA, and AMPA receptor subunits) (Gardoni et al., 2001).
Ten micrograms of purified striatal TIF were incubated in a 20 mm HEPES buffer, pH 7.4, containing 10 mm MgCl2, 10-5 m okadaic acid, 100 μm PMSF, and 20 mm DTT with 100 μm [γ-32P]ATP (1 μCi/tube; 5000 Ci/mmol; Amersham Biosciences, Little Chalfont, UK) in the presence of 1 mm CaCl2 and 40 μg/ml calmodulin. Reactions were performed at 37°C for 5 min and were stopped by liquid nitrogen.
Aliquots of 10 μg of phosphorylated or native TIFs were incubated overnight at 4°C in buffer A, containing: 200 mm NaCl, 10 mm EDTA, 10 mm Na2HPO4, 0.5% NP-40, and 0.1% SDS in a final volume of 200 μl with antibodies against NR2A-NR2B or αCaMKII. Protein A-agarose beads (5 mg/tube), washed in the same buffer, were added, and incubation continued for 2 hr. The beads were collected by centrifugation and washed three times with buffer A, sample buffer for SDS-PAGE was added, and the mixture was boiled for 3 min. Beads were pelleted again by centrifugation, and supernatants were applied to 6% SDS-PAGE.
CaMKII assay. CaMKII activity was performed using the SignaTECT CaMKII assay system (Promega, Madison, WI).
Antibodies. Monoclonal αCaMKII antibody and polyclonal glutamate receptor 1 (GluR1), NR1, NR2A-NR2B, and NR2A antibodies were purchased from Chemicon (Temecula, CA); polyclonal antibody against p286-anti-active αCaMKII was purchased from Promega, and monoclonal antibody against PSD-95 was purchased from Affinity BioReagents (Golden, CO).
Data analysis and statistical evaluation. Quantitation of Western blot analysis was performed using computer-assisted imaging (Quantity-One System; Bio-Rad, Hercules, CA), and statistical evaluations were performed using ANOVA, followed by Bonferroni as a post hoc comparison test.
Electrophysiological experiments. Animals were killed by cervical translocation 24 and 72 hr after the injection of CaMKII inhibitor to obtain corticostriatal slices for electrophysiological recordings. Briefly, vibratome-cut coronal slices (200-300 μm) were transferred to a recording chamber and submerged in a continuously flowing Krebs' solution (35°C, 2-3 ml/min) gassed with 95% O2-5% CO2. Intracellular recording electrodes were filled with 2 m KCl (30-60 MΩ). Signals were recorded with an Axoclamp 2A amplifier, displayed on a separate oscilloscope, and stored and analyzed on a digital system (pClamp 8; Axon Instruments, Foster City, CA). For synaptic stimulation, bipolar electrodes, located in the white matter between the cortex and the striatum, were used to activate corticostriatal fibers. Magnesium ions were omitted from the medium to better disclose the NMDA-mediated component of the EPSP. Under this experimental condition, high-frequency stimulation (HFS) of corticostriatal fibers (three trains, 3 sec duration, 100 Hz frequency, 20 sec interval) was used as an LTP-inducing protocol (Calabresi et al., 1992; Centonze et al., 1999). Quantitative data on modifications of EPSPs are expressed as a percentage of the controls, the latter representing the mean of responses recorded during a stable period (15-30 min) before the repetitive HFS synaptic stimulation. Values represent mean ± SEM of changes in the respective cell populations. Student's t test (for unpaired observations) was used to compare the means, and ANOVA was used when multiple comparisons were made against a single control group. For the molecular experiments shown in Figure 2, we incubated corticostriatal slices from normal rats for 15 min in one or more of the following drugs at the given concentration: 10 μm SCH 23390, 3 μm l-sulpiride, 30 μm forskolin (Tocris Cookson), or 10 μm H89 (Sigma).
Figure 2.
Distinct effects of DA receptor antagonists on αCaMKII autophosphorylation and corticostriatal LTP formation. A, Western blot analysis of total αCaMKII (bottom) and p286-αCaMKII (top) in TIFs obtained from control, 3 μm l-sulpiride-treated, or 10 μm SCH 23390-treated corticostriatal slices; the same amount of protein was loaded per lane (p < 0.01, l-sulpiride vs control; p < 0.05, SCH 23390 vs control). *p < 0.01, l-sulpiride versus control; **p < 0.05, SCH 23390 versus control. B, TIF proteins from treated corticostriatal slices were immunoprecipitated with an NR2A-NR2B polyclonal antibody. Western blot analysis was performed in the immunoprecipitated (i.p.) material with αCaMKII and NR2A-NR2B antibodies (+73.4 ± 17.5%, p < 0.05, SCH 23390 vs control; -79.5 ± 10.2%, p < 0.01, l-sulpiride vs control). C, Changes in corticostriatal EPSP amplitude after tetanic stimulation under control conditions, in the presence of 3 μm l-sulpiride-treated slices (p < 0.05; control vs l-sulpiride measured 30 min after the induction; n = 23), in 10 μm SCH 23390-treated slices (p < 0.001; control vs SCH 23390 measured 30 min after the induction; n = 20), and after in vivo intrastriatal administration of 3 mm SCH 23390 (p < 0.001; control vs SCH 23390 intrastriatally at 30 min after induction; n = 13). D, Limb-use asymmetry induced in control rats 24 hr after the injection of 3 mm SCH 23390 (**p < 0.001; SCH 23390 intrastriatally vs sham; n = 8) ipsi, Ipsilateral; contra, contralateral. E, Western blot analysis of active p286-αCaMKII and total αCaMKII in TIFs obtained from treated corticostriatal slices. F, TIF proteins from treated corticostriatal slices were immunoprecipitated with an NR2A-NR2B polyclonal antibody. Western blot analysis was performed in the immunoprecipitated material with αCaMKII and NR2A-NR2B antibodies. WB, Western blot. L-sul, l-sulpiride; SCH, SCH 23390; i.s., intrastriatal; Forsk, forskolin.
Behavioral testing. Limb-use asymmetry was assessed using the cylinder test of Schallert et al. (2000). Each rat was individually introduced in a transparent cylinder and videotaped for 5 min (Lundblad et al., 2002). The number of supporting wall contacts, executed independently with the right or the left forelimb, was counted. The percentage of wall contacts executed by the impaired forelimb (contralateral to the lesion) was then subtracted from the percentage of contacts of the nonimpaired forelimb to obtain a limb-use asymmetry score. A rotarod system of constant and accelerating treadmills (TSE Technical & Scientific Equipment, Homburg, Germany) was used to coordinate motor activity and general motor disability. All animals were trained for at least one session before the 6-OHDA lesion. Each experimental group (sham, 6-OHDA, and 6-OHDA plus either KN-93 or Ant-AIP-II) was tested at different times after injection of either CaMKII inhibitors or the inactive analog KN-92. The rats were placed on the rod and sequentially tested at 4, 12, 20, 28, 36, and 40 rpm for a maximum of 300 sec at each speed. Overall rod performance was expressed as the integral of time spent on the rod versus turning speed (Rozas et al., 1997).
L-DOPA treatment. At 1 month after the 6-OHDA lesion, rats started to receive twice-daily intraperitoneal injections of 10 mg/kg l-DOPA plus 7.5 mg/kg DOPA-decarboxylase inhibitor benserazide (Cenci et al., 1998) or physiological saline (“lesion-only”) for acute and chronic treatment (1 and 4 d, respectively). l-DOPA-treated rats were used for electrophysiological, biochemical, and behavioral experiments. Rat performances were tested in the cylinder and the rotarod 15-20 min after the injection of l-DOPA.
Results
DA denervation disrupts motor behavior, alters NMDA-mediated synaptic plasticity, and induces abnormal αCaMKII autophosphorylation
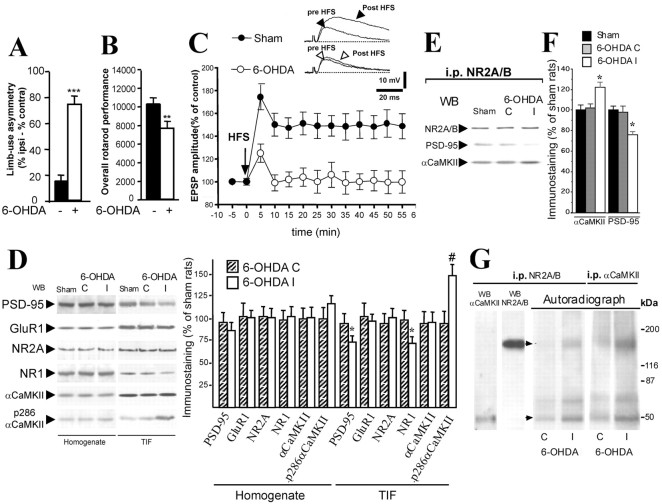
An extensively used rat model of PD, obtained by unilateral injections of 6-OHDA in the ascending nigrostriatal pathway, was used (Rozas et al., 1997; Schallert et al., 2000; Picconi et al., 2003). As reported previously, sham-operated rats had a low level of limb-use asymmetry compared with DA-denervated animals (Fig. 1A). Furthermore, 6-OHDA-lesioned animals show a worsened rotarod performance compared with controls (Fig. 1B). These abnormalities in spontaneous motor performance produced by DA denervation are paralleled by an altered synaptic plasticity in the corticostriatal pathway (Calabresi et al., 1997, 2000a). Accordingly, repetitive HFS of glutamatergic corticostriatal afferents induced a normal LTP in sham-lesioned controls but not in the DA-denervated subjects (Fig. 1C).
Figure 1.
Motor performances, synaptic plasticity, and alteration of αCaMKII-PSD-95 binding to the NMDA receptor complex in sham- and 6-OHDA-lesioned striatum. A, A limb-use asymmetry test was performed in sham-operated and 6-OHDA denervated rats (n = 8 in each group). 6-OHDA-lesioned rats preferentially use the limb ipsilateral to the lesion (***p < 0.0001; 6-OHDA vs sham). ipsi, Ipsilateral; contra, contralateral. B, Coordinated locomotor activity on a rotarod is significantly impaired after dopamine denervation (**p < 0.001; 6-OHDA vs sham). C, HFS of corticostriatal fibers induced LTP in sham-operated rats (filled circles; p < 0.01; EPSP amplitude after vs before HFS; n = 25) but not in 6-OHDA-lesioned animals (open circles; p > 0.05; EPSP amplitude after vs before HFS; n = 20). D, Effects of 6-OHDA lesioning on the NMDA receptor complex in the rat striatum. Striatal homogenates (left) and TIFs (right) from sham- and 6-OHDA-lesioned animals were analyzed by Western blot analysis with PSD-95, GluR1, NR2A, NR1,αCaMKII, and active p286-αCaMKII antibodies. The same amount of protein was loaded per lane (*p < 0.05; #p < 0.01; 6-OHDA I vs 6-OHDA C; n = 8 for each group). E, TIF proteins from sham- and 6-OHDA-lesioned animals were immunoprecipitated with an NR2A-NR2B polyclonal antibody. Western blot analysis was performed in the immunoprecipitated (i.p.) material with αCaMKII and PSD-95 antibodies. F, Quantitative analysis of Western blot performed on coimmunoprecipitated material. *p < 0.01 (6-OHDA I vs sham). G, TIF proteins were phosphorylated under conditions known to maximally activate CaMKII and then immunoprecipitated with anti-NR2A-NR2B (left) or anti-CaMKIIα (right). Autoradiography obtained after immunoprecipitation shows two major phosphorylated protein bands: a 50 kDa band (bottom arrow) pointing to autophosphorylated αCaMKII and a 170 kDa band (top arrow) indicating phosphorylated NR2A-NR2B. Left lanes, Identification by Western blot of NR2A-NR2B and αCaMKII in the immunocomplex. WB, Western blot; I, striatum ipsilateral to the lesion; C, striatum contralateral to the lesion.
Because LTP requires activation of the NMDA receptors (Calabresi et al., 1992; Bortolotto and Collingridge, 1998; Giese et al., 1998) in the striatum as well as in other brain areas, the absence of LTP after denervation is likely to reflect an alteration of NMDA receptor-associated signaling elements. Because the NMDA receptor complex is enriched in the PSD fraction, we first measured protein levels of NMDA receptor subunits and other PSD-associated signaling proteins in both striatal homogenates and purified TIFs from sham- and 6-OHDA-lesioned rats (the TIF preparation is particularly enriched in PSD proteins) (Gardoni et al., 2001) by Western blot analysis. Levels of the AMPA receptor subunit GluR1, the NMDA receptor subunit NR2A, and αCaMKII were not altered in striatal TIFs from 6-OHDA-lesioned rats (Fig. 1D), suggesting that the gross composition of the TIF was not affected by DA denervation. In contrast, the levels of both PSD-95 and NR1 showed a reduction in the denervated striata (Fig. 1D). The levels of p286-autophosphorylated (active) αCaMKII were increased (p < 0.01; 6-OHDA vs sham) without a concomitant increase in total αCaMKII immunoreactivity (Fig. 1D). Accordingly, a kinase assay revealed that Ca2+-calmodulin-dependent CaMKII activity was elevated in the striatum ipsilateral to the 6-OHDA lesion compared with the contralateral side (+54.1 ± 9.9%; p < 0.01; n = 8). No differences in NR1 and PSD-95 protein level were found in the crude membrane (P2) fraction (data not shown), suggesting a specific alteration in the TIF. No difference was found in the expression level of any tested proteins in the homogenate fractions (Fig. 1D).
Altered composition of NMDA receptor complex in experimental parkinsonism
A large number of studies have identified the NMDA receptor NR2A-NR2B subunits in PSD as a nucleation site for scaffolding proteins (i.e., PSD-95) (Kornau et al., 1995) and for specific enzymes (i.e., αCaMKII) (Gardoni et al., 1998; Strack and Colbran, 1998). It is known that NMDA receptor-αCaMKII interaction occurs with unphosphorylated kinase but it is strengthened by kinase autophosphorylation (Gardoni et al., 1998, 2001). αCaMKII coimmunoprecipitation with the NMDA receptor NR2A-NR2B subunits has been demonstrated previously both in in vitro experiments (Gardoni et al., 1998, 2001) and in animal models (Gardoni et al., 2003; Kamphuis et al., 2003). In particular, αCaMKII coprecipitation has been observed only with NR2A-NR2B but not with other ionotropic glutamate receptor subunits present in PSD (Gardoni et al., 1998).
Coimmunoprecipitation experiments performed with a polyclonal NR2A-NR2B antibody showed an altered assembly of PSD-95 and αCaMKII to NR2A-NR2B subunits of the NMDA receptor in the striatum ipsilateral to the 6-OHDA lesion compared with the contralateral side. Indeed, increased αCaMKII binding to NR2A-NR2B (+23.5 ± 3.9%; p < 0.01; 6-OHDA I vs sham), paralleled by a reduced association of PSD-95 (-26.1 ± 2.7%; p < 0.01; 6-OHDA I vs sham) was found (Fig. 1E,F). This effect was accompanied by increased levels of CaMKII-dependent phosphorylation of the NR2A-NR2B subunits. As shown in Figure 1G, the side of the striatum ipsilateral to the 6-OHDA lesion showed increased 32P phosphate incorporation in a 170 kDa protein band (+112.4 ± 17.9%; p < 0.01; 6-OHDA vs sham) and a 50 kDa protein band (+93.4 ± 13.5%; p < 0.01; 6-OHDA vs sham), corresponding to NR2A-NR2B and αCaMKII, respectively (Gardoni et al., 1998, 2001; Kamphuis et al., 2003), as revealed by Western blot analysis performed on the immunocomplex (Fig. 1G, left lanes). In addition, immunoprecipitation experiments were performed, incubating phosphorylated samples with anti-CaMKIIα antibody (Fig. 1G, right). If the two proteins are indeed associated, then immunoprecipitation with anti-CaMKIIα antibody should yield similar results. Figure 1G shows an identical pattern of phosphorylated proteins in anti-CaMKIIα and anti-NR2A-NR2B immunoprecipitates, thus confirming that the immunoprecipitation of either protein entails the coprecipitation of the other.
The effects of DA denervation are mimicked by pharmacological antagonism of D1 DA receptors
The next step in our study was to investigate whether the effect of DA denervation on CaMKII autophosphorylation and synaptic plasticity could be mimicked by acute pharmacological antagonism of DA receptors. Therefore, we incubated corticostriatal slices from normal rats for 15 min with either 10 μm SCH 23390, an antagonist of D1-like DA receptors, or 3 μm l-sulpiride, an antagonist of D2-like DA receptors. SCH 23390 increased p286-αCaMKII immunostaining, whereas l-sulpiride reduced it (Fig. 2A). This effect was paralleled by an altered assembly of αCaMKII to NR2A-NR2B regulatory subunits of the NMDA receptor in coimmunoprecipitation experiments (Fig. 2B) (+73.4 ± 17.5%, p < 0.05, SCH 23390 vs control; -79.5 ± 10.2%, p < 0.01, l-sulpiride vs control). The two DA receptor antagonists also had opposite effects on corticostriatal LTP. In fact, whereas SCH 23390 blocked this form of synaptic plasticity, as shown previously in mice (Calabresi et al., 2000b) (Fig. 2C), l-sulpiride increased it (Fig. 2C). The intrastriatal injection of 3 mm SCH 23390 was able to block corticostriatal synaptic plasticity (Fig. 2C) and to induce limb-use asymmetry (Fig. 2D) in normal rats.
Interestingly, incubation of corticostriatal slices from normal rats with the protein kinase A inhibitor H89 mimicked the effects of SCH 23390 on p286-αCaMKII immunostaining (Fig. 2E) (p < 0.005; H89 vs control); direct stimulation of adenylyl cyclase with forskolin resulted in reduced phosphorylation of Thr286-αCaMKII (p < 0.05; forskolin vs control) without any effect on total αCaMKII immunostaining. Coincubation of H89 with l-sulpiride still results in an increased Thr286 phosphorylation (p < 0.01; H89 plus l-sulpiride vs control). As expected, these data were paralleled by similar modifications of αCaMKII binding of the NMDA receptor subunits NR2A-NR2B (Fig. 2F) (p < 0.01, H89 vs control; p < 0.05, forskolin vs control; p < 0.01, H89 plus l-sulpiride vs control).
l-DOPA restores motor performances and synaptic plasticity via a normalization of CaMKII activity
To show that the behavioral, electrophysiological, and biochemical changes secondary to 6-OHDA lesions were causally linked to the loss of endogenous DA, we tested whether l-DOPA treatment could normalize the observed changes. 6-OHDA-lesioned rats received either acute (1 d) or chronic (4 d) treatment with therapeutic doses of l-DOPA (10 mg/kg methyl l-DOPA combined with 7.5 mg/kg benserazide, i.p., twice daily). This treatment regimen produced a significant anti-akinetic effect (see below) without inducing dyskinetic behavior. Four hours after the last injection of l-DOPA, the animals were used for behavioral, molecular, and electrophysiological experiments. Interestingly, 1 d of l-DOPA treatment only partially reversed the deficits in spontaneous forelimb use and rotarod performance induced by the 6-OHDA lesions, and failed to restore LTP (Fig. 3A-C). In contrast, 4 d of treatment had significant beneficial effects on the animals' motor behavior and fully restored normal synaptic plasticity (Fig. 3A-C). In parallel, 4 d but not 1 d of l-DOPA treatment was able to reduce to control levels both p286-αCaMKII immunostaining (Fig. 3D,E) (p < 0.01; 6-OHDA plus 4 d l-DOPA vs 6-OHDA) and CaMKII activity (Fig. 3F) (p < 0.01; 6-OHDA plus 4 d l-DOPA vs 6-OHDA) without affecting the total αCaMKII immunostaining in the TIF.
Figure 3.
Motor performances, synaptic plasticity, and NMDA receptor complex in sham-operated rats, 6-OHDA-lesioned rats, and lesioned rats after l-DOPA treatment. A, A limb-use asymmetry test was performed on sham- and 6-OHDA-lesioned rats after 1 or 4 d of l-DOPA treatment (n = 8 in each group). Only 4 d of l-DOPA treatment were able to restore the normal limb-use symmetry in 6-OHDA-lesioned animals (**p < 0.001, 6-OHDA vs sham; *p < 0.01, 6-OHDA plus 4 d l-DOPA vs 6-OHDA; not significant, p > 0.05, 6-OHDA plus 4 d l-DOPA vs sham). ipsi, Ipsilateral; contra, contralateral. B, Coordinated locomotor activity on a rotarod is significantly recovered after 4 d of l-DOPA administration (***p < 0.0001, 6-OHDA vs sham; **p < 0.01, 6-OHDA plus 4 d l-DOPA vs 6-OHDA; not significant, p > 0.05, 6-OHDA plus 1 d l-DOPA vs 6-OHDA). C, HFS of corticostriatal fibers induced LTP in chronically l-DOPA-treated rats (filled circles; p < 0.01; EPSP amplitude after vs before HFS; n = 20) but not in 6-OHDA-lesioned animals or in acute l-DOPA-treated rats (open circles and filled triangles, respectively; p > 0.05; EPSP amplitude after vs before HFS; n = 23). D, E, Western (WB) blot analysis of total and p286-αCaMKII in striatal TIFs obtained from sham- and 6-OHDA-lesioned animals in the absence or presence of 1 and 4 d l-DOPA treatment (*p < 0.01, 6-OHDA vs sham; §p < 0.05, 6-OHDA plus 4 d l-DOPA vs 6-OHDA). F, CaMKII activity in striatal TIFs obtained from sham- and 6-OHDA-lesioned animals in the absence or presence of 1 and 4 d l-DOPA treatment (*p < 0.01, 6-OHDA vs sham; §p < 0.01, 6-OHDA plus 4 d l-DOPA vs 6-OHDA).
The effects of l-DOPA are mimicked by intrastriatal CaMKII inhibition
Previous findings have suggested that αCaMKII autophosphorylation may be a critical step in the regulation of both physiological and pathological forms of synaptic plasticity (Giese et al., 1998). To assess the role of this kinase in the pathogenesis of parkinsonian symptoms and in the therapeutic effect of l-DOPA, we intrastriatally injected KN-93, a competitive and selective inhibitor of CaMKII, on the side of the striatum ipsilateral to the 6-OHDA lesion. Six, 24, and 72 hr after injection, the animals were used for molecular, electrophysiological, and behavioral experiments. By 24 hr, the administration of KN-93 had completely reversed the lesion-induced deficits in spontaneous forelimb use and rotarod performance (Fig. 4A,B). In addition, striatal LTP (measured on denervated slices obtained from the same animals) was completely rescued (Fig. 4C). We also used the inactive analog KN-92 and failed to observe both the behavioral and electrophysiological (n = 4) effects produced by the active compound (data not shown). At 72 hr, the effects of the intrastriatal injection of KN-93 were completely lost on either behavioral abnormalities or synaptic plasticity (Fig. 4A-C).
Figure 4.
Effect of the CaMKII inhibitor KN-93 on motor performances, synaptic plasticity, and the NMDA receptor complex of DA-denervated animals. A, A limb-use asymmetry test was performed in sham-operated, 6-OHDA denervated, and 6-OHDA plus KN-93 animals (n = 8 in each group). 6-OHDA-lesioned rats preferentially use the limb ipsilateral to the lesion (***p < 0.0001; 6-OHDA vs sham). CaMKII inhibitor reversed the parkinsonian-like motor features in the lesioned animals 24 hr after the injection, although the effect was lost 72 hr after the injection (not significant, p > 0.05, 6-OHDA plus 24 hr KN-93 vs sham; ***p < 0.0001, 6-OHDA plus 72 hr KN-93 vs sham). ipsi, Ipsilateral; contra, contralateral. B, Coordinated locomotor activity on a rotarod is significantly impaired after dopamine denervation but recovers to normal levels (i.e., sham group) in KN-93-injected 6-OHDA rats 24 hr but not 72 hr after the injection (**p < 0.001, 6-OHDA vs sham; not significant, p > 0.05, 6-OHDA plus 24 hr KN-93 vs sham; **p < 0.001, 6-OHDA plus 72 hr KN-93 vs sham). C, HFS of corticostriatal fibers induced LTP in sham-operated rats (filled circles; p < 0.01; EPSP amplitude after vs before HFS; n = 25) but not in 6-OHDA-lesioned animals (open circles; not significant, p > 0.05; EPSP amplitude after vs before HFS; n = 20). Intrastriatal injection of CaMKII inhibitor was able to restore normal LTP 24 hr (open diamonds) but not 72 hr (filled diamonds) after the injection (24 hr KN-93, p < 0.01, EPSP amplitude after vs before HFS, n = 20; 72 hr KN-93, not significant, p > 0.05, EPSP amplitude after vs before HFS, n = 15). D, Western blot (WB) analysis of αCaMKII, active p286-αCaMKII, NR1, and PSD-95 in striatal TIFs from sham- and 6-OHDA-lesioned animals in the absence or presence of 24 or 72 hr KN-93 treatments (*p < 0.001, 6-OHDA vs sham; **p < 0.01, 6-OHDA plus 24 hr KN-93 vs 6-OHDA; ***p < 0.01, 6-OHDA plus 72 hr KN-93 vs sham). E, TIF proteins from sham- and 6-OHDA-lesioned animals in the absence or presence of 24 or 72 hr KN-93 treatments were phosphorylated under conditions known to maximally activate CaMKII and then immunoprecipitated with anti-NR2A-NR2B. Autoradiography obtained after immunoprecipitation shows a phosphorylated 50 kDa protein band pointing to autophosphorylated αCaMKII. Three independent experiments were performed on different TIF preparations and replicated three times in each TIF preparation (p < 0.01, 6-OHDA vs sham; not significant, p > 0.05, 6-OHDA plus 24 hr KN-93 vs sham; p < 0.01, 6-OHDA plus 24 hr KN-93 vs 6-OHDA; p < 0.01, 6-OHDA plus 72 KN-93 vs sham). i.s., Intrastriatal; i.p., immunoprecipitated.
Twenty-four hours of KN-93 treatment restored Thr286-αCaMKII auto-phosphorylation to control levels (Fig. 4D) (p < 0.001, 6-OHDA vs sham; p < 0.01, 6-OHDA plus 24 hr KN-93 vs 6-OHDA) without affecting the total αCaMKII immunostaining in the TIF (Fig. 4D, top). However, 72 hr after KN-93 intrastriatal injection, p286-αCaMKII immunostaining was again increased up to 6-OHDA levels (Fig. 4D). Similar results were obtained when measuring Ca2+-calmodulin-dependent CaMKII activity (data not shown). In contrast, both 24 and 72 hr KN-93 treatment did not produce any significant effect on the reduced PSD-95 and NR1 protein levels in the TIFs of denervated striata (Fig. 4D, bottom). As shown in Figure 4E, the relative amounts of 32P-autophosphorylated αCaMKII bound to NR2A-NR2B subunits in the immunocomplex varied according to KN-93 inhibition treatment; these data indicate that this pool of αCaMKII is also strictly modulated by the treatment with kinase antagonist.
At the 6 hr postinjection interval, KN-93 treatment had produced a dramatic decrease in both CaMKII activity and Thr286-αCaMKII autophosphorylation compared with sham (data not shown). In accordance with the well known effect of CaMKII inhibition on synaptic plasticity, no LTP induction was observed at this time point (data not shown). In line with these findings, no beneficial effect of KN-93 on rotarod and limb-use performance was measured at 6 hr (data not shown). These data strongly suggest that a fine tuning of αCaMKII autophosphorylation is necessary for both physiological striatal plasticity and normal motor activity.
We used a novel strategy, targeting CaMKII inhibition in vivo, to confirm the role of abnormal CaMKII function in experimental parkinsonism as revealed by KN-93 experiments. The AIP-II synthetic peptide, a highly specific and potent inhibitor of CaMKII, fused to the antennapedia homeodomain peptide to facilitate internalization into living cells, was injected intrastriatally on the side ipsilateral to the 6-OHDA lesion. Twenty-four hours after injection, the animals were used for molecular, electrophysiological, and behavioral experiments. The injection of Ant-AIP-II confirmed the results obtained with KN-93. Ant-AIP-II reversed the lesion-induced deficits in asymmetry limb-use test and rotarod performance (Fig. 5A,B). Moreover, striatal LTP was also completely rescued in these animals (Fig. 5C).
Figure 5.
Effect of Ant-AIP-II on motor performances, synaptic plasticity, and the NMDA receptor complex of DA-denervated animals. A, A limb-use asymmetry test was performed in sham-operated rats, 6-OHDA denervated animals, and 6-OHDA-lesioned rats receiving a striatal unilateral injection of Ant-AIP-II (n = 8 in each group). 6-OHDA-lesioned rats preferentially use the limb ipsilateral to the lesion (**p < 0.001; 6-OHDA vs sham). Ant-AIP-II peptide reversed the parkinsonian-like motor features in the lesioned animals (not significant, p > 0.05; 6-OHDA plus Ant-AIP-II vs sham). ipsi, Ipsilateral; contra, contralateral. B, Coordinated locomotor activity on a rotarod is significantly impaired after dopamine denervation but recovers to normal levels (i.e., sham group) after unilateral AIP-2 injection (*p < 0.01, 6-OHDA vs sham; not significant, p > 0.05, 6-OHDA plus Ant-AIP-II vs sham). C, Intrastriatal injection of AIP-2 was able to restore normal LTP in 6-OHDA denervated rats (sham, filled circles, p < 0.01, EPSP amplitude after vs before HFS, n = 25; 6-OHDA, open circles, not significant, p > 0.05, EPSP amplitude after vs before HFS, n = 20; 6-OHDA plus Ant-AIP-II, open diamonds, p < 0.01, EPSP amplitude after vs before HFS, n = 20). D, Western blot analysis of active p286-αCaMKII in striatal TIFs from sham- and 6-OHDA-lesioned animals in the absence or presence of 24 hr of Ant-AIP-II treatment (*p < 0.01, 6-OHDA vs sham; **p < 0.01, 6-OHDA plus Ant-AIP-II 24 hr vs 6-OHDA). E, TIF proteins from sham- and 6-OHDA-lesioned animals in the absence or presence of 24 hr of Ant-AIP-II treatment were phosphorylated under conditions known to maximally activate CaMKII and then immunoprecipitated with anti-NR2A-NR2B. Representative autoradiography obtained after immunoprecipitation shows two major phosphorylated protein bands: a 50 kDa band (bottom arrow) pointing to autophosphorylated αCaMKII and a 170 kDa band (top arrow) indicating phosphorylated NR2A-NR2B (*p < 0.005, 6-OHDA vs sham; **p < 0.005, 6-OHDA plus Ant-AIP-II vs 6-OHDA). i.s., Intrastriatal.
Twenty-four hours of Ant-AIP-II treatment restored p286-αCaMKII immunostaining to control levels (Fig. 5D) (p < 0.01; 6-OHDA plus Ant-AIP-II 24 hr vs 6-OHDA) without affecting the total αCaMKII immunostaining in the TIF (Fig. 5D, top). As expected, similar results were obtained by measuring Ca2+-calmodulin-dependent CaMKII activity (data not shown).
Coimmunoprecipitation experiments performed with NR2A-NR2B antibody show that 24 hr of Ant-AIP-II treatment was able to reduce to control levels 32P phosphate incorporation in the NR2A-NR2B phospho-band (170 kDa; p < 0.005, 6-OHDA vs sham; p < 0.005, 6-OHDA plus 24 hr Ant-AIP-II vs 6-OHDA) and in the αCaMKII protein band (50 kDa) in the immunocomplex (Fig. 5E), demonstrating that 24 hr of kinase inhibition rescues all of the modification induced by 6-OHDA on the NMDA receptor-αCaMKII complex.
Discussion
The signs and symptoms of PD have been critically linked to a loss of striatal DA and to a secondary overactivity of corticostriatal glutamatergic transmission (Calabresi et al., 1993, 2000a). l-DOPA pharmacotherapy is able to produce a significant symptomatic improvement, and NMDA receptor antagonists may also ameliorate parkinsonian deficits (Vila et al., 1999).
Despite their clinical relevance, the molecular mechanisms underlying the interactions between DA and NMDA receptors in the control of striatal plasticity and motor behavior have remained elusive. Although it has been speculated that intracellular signal transduction pathways can establish a cross-talk between these two receptor systems, the key elements governing such a cross-talk have remained unknown. The present data show that CaMKII can be considered a critical player in this process, functioning as a signal integrator downstream of DA and glutamate receptors in the PSD. This conclusion is supported by several lines of evidence involving behavioral testing, studies of striatal-specific synaptic plasticity, and biochemical analyses of the structural organization of the postsynaptic compartment in 6-OHDA-lesioned rats. Although multiple alterations of subunit composition and phosphorylation of NMDA receptors have been reported previously in this animal model (Menegoz et al., 1995; Oh et al., 1998; Dunah et al., 2000), our results are the first to demonstrate the pathogenic role of a signaling protein downstream of NMDA receptor activation. In this line, the defective plasticity of corticostriatal synapses (which parallels the development of motor abnormalities) in the denervated striatum was accompanied by an increase in αCaMKII autophosphorylation along with a higher recruitment of activated αCaMKII to the regulatory NR2A-NR2B NMDA receptor subunits. A normalization of αCaMKII autophosphorylation and activity was both necessary and sufficient to rescue LTP and to produce a recovery of physiological motor performances in 6-OHDA-lesioned rats. These results, obtained using two different pharmacological approaches (i.e., l-DOPA and specific CaMKII inhibitors), strongly support a central role for CaMKII in the modulation of synaptic events in DA-denervated striatal spiny neurons.
In the last decade, overwhelming evidence has indicated that CaMKII activity is crucial for synaptic plasticity events (Pettit et al., 1994; Liu et al., 1999; Lisman et al., 2002). The increased levels of αCaMKII autophosphorylation found in the 6-OHDA striatum are likely to disturb the biochemical machinery underlying LTP formation. Indeed, it is known that postsynaptically increased CaMKII activity enhances synaptic transmission, and therefore prevents any additional induction of LTP (Pettit et al., 1994). Accordingly, we found that the denervation-induced deficit in LTP formation was reversed by treatments that restored normal levels of CaMKII activity and autophosphorylation in the PSD.
In the last few years, different groups have identified NMDA receptor subunits NR2A-NR2B as a target for αCaMKII in the PSD, although the exact nature and the pathophysiological role of these complex interactions have yet to be elucidated (Gardoni et al., 1998, 2001; Strack and Colbran, 1998). Here we show that a balanced level of αCaMKII autophosphorylation and activity as well as correct assembly and anchoring of the kinase to the NMDA receptor complex in the PSD are required to reverse both the alterations in corticostriatal synaptic plasticity and the deficits in spontaneous motor behavior that are found in the 6-OHDA model of PD. In addition, the results of the present study, showing a decreased PSD-95 protein level in the TIF and an increased αCaMKII/PSD-95 protein ratio bound to the NMDA receptor in the DA-denervated striatum, provide support for a pathophysiological role of disturbed interactions between NMDA-associated proteins in neurological disorders (Takagi et al., 2000; Gardoni et al., 2001).
Previous studies have reported alterations in both the composition and the phosphorylation state of NMDA receptors in 6-OHDA-lesioned rats (Menegoz et al., 1995; Oh et al., 1999; Dunah et al., 2000). Our results extend these data, demonstrating not only an altered compartmentalization of the NR1 subunit in 6-OHDA rats but also a complex alteration of NMDA receptor-associated proteins, such as αCaMKII and PSD-95.
The present study is the first to use validated measures of akinesia and parkinsonian-like disability in rats to demonstrate the therapeutic action of treatments that normalize CaMKII activity in the DA-denervated striatum. Apparent discrepancies with previous reports can be related to the different lesioning paradigms and subcellular fractionation-solubilization techniques used (Menegoz et al., 1995; Dunah et al., 2000). In fact, although our biochemical experiments are performed in a PSD-like fraction (Gardoni et al., 2001), none of the previous studies used such a highly purified membrane fraction in which the NMDA signaling complex is enriched.
DA denervation precludes activation of both D1- and D2-like receptors from endogenous DA. Interestingly, we found that acute treatment of striatal slices with SCH 23390 but not l-sulpiride mimicked the effect of DA denervation on both αCaMKII autophosphorylation and corticostriatal synaptic plasticity. These findings suggest that D1-like but not D2-like receptors are specifically involved in the upregulation of αCaMKII autophosphorylation levels. Accordingly, we found that by targeting adenylate cyclase as well as PKA activity, we were able to mimic the effects of DA denervation and D1 receptor inhibition.
In summary, the present findings provide the first demonstration that an αCaMKII hyperphosphorylated state plays a causal role in the pathophysiology of parkinsonian motor disability and in the maladaptive striatal plasticity secondary to DA denervation. Indeed, the 6-OHDA lesion-induced deficits in spontaneous forelimb use and rotarod performance were completely reversed 24 hr after an intrastriatal injection of KN-93 (i.e., at the same time point at which αCaMKII autophosphorylation and activity had returned to control levels), but not at different time points. Similarly, LTP was completely rescued at this postinjection interval. This initial pharmacological approach describing the role of CaMKII in the pathogenesis of PD would be greatly enhanced by performing experiments in CaMKII knock-out mice. Unfortunately, these animals show a profound malfunctioning of glutamatergic transmission leading to epilepsy and learning deficits (Butler et al., 1995), thus rendering their use infeasible in terms of our goals. To overcome this problem, we set up a novel method to inhibit in vivo CaMKII, making use of injection of the CaMKII autoinhibitory peptide fused to the antennapedia peptide. The results obtained with this method fully confirmed our observations obtained with KN-93.
Interestingly, a significant recovery of motor function was also obtained by a regimen of l-DOPA administration that was effective in reversing the lesion-induced increase in CaMKII activity and in restoring corticostriatal LTP. The present data therefore suggest that the therapeutic action of l-DOPA may be mediated by a normalization of CaMKII activity in the striatum. Thus, our data identify CaMKII as the gateway of striatal NMDA- and DA-dependent functions (Calabresi et al., 2000a; Dunah et al., 2000).
Footnotes
This work was supported by a Fondo Integrativo Speciale Ricerca from Ministero dell'Istruzione, Università e Ricerca (Neurobiotecnologie, M.D.), Telethon Grant GGP02035 (P.C.), Fondo per gli Investimenti della Ricerca di Base 2001 (G.B.), and The Kocks' Foundation and The Swedish Association of the Neurologically Disabled (M.A.C.). We thank M. Tolu for his technical assistance.
Correspondence should be addressed to Dr. Paolo Calabresi, Clinica Neurologica, Dipartimento di Neuroscienze, Università di Roma Tor Vergata, via Montpellier 1, 00133 Rome, Italy. E-mail: calabre@uniroma2.it.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245283-09$15.00/0
B.P. and F.G. contributed equally to this work.
References
- Bortolotto ZA, Collingridge GL (1998) Involvement of calcium/calmodulin-dependent protein kinases in the setting of a molecular switch involved in hippocampal LTP. Neuropharmacology 37: 535-544. [DOI] [PubMed] [Google Scholar]
- Butler LS, Silva AJ, Abeliovich A, Watanabe Y, Tonegawa S, McNamara JO (1995) Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc Natl Acad Sci USA 92: 6852-6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G (1992) Long term potentiation in the striatum unmasked by removing the voltage-dependent magnesium block of NMDA receptor channels. Eur J Neurosci 4: 929-935. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Sancesario G, Bernardi G (1993) Electrophysiology of dopamine-denervated striatal neurons: implications for Parkinson's disease. Brain 116: 433-452. [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G (1996) The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci 19: 19-24. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E (1997) Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci 17: 4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Bernardi G (2000a) Electrophysiology of dopamine in normal and denervated striatal neurons. Trends Neurosci 23: S57-S63. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P (2000b) Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci 20: 8443-8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Björklund A (1998) l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10: 2694-2706. [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Picconi B, Calabresi P, Giacomini P, Bernardi G (1999) Unilateral dopamine denervation blocks corticostriatal LTP. J Neurophysiol 82: 3575-3589. [DOI] [PubMed] [Google Scholar]
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N (1998) Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature 393: 377-381. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG (2001) Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci 21: 5546-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG (2000) Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-d-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Mol Pharmacol 57: 342-352. [PubMed] [Google Scholar]
- Gardoni F, Caputi A, Cimino M, Pastorino L, Cattabeni F, Di Luca M (1998) Calcium/calmodulin-dependent protein kinase II is associated with NR2A/B subunits of NMDA receptor in postsynaptic densities. J Neurochem 71: 1733-1741. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Schrama LH, Kamal A, Gispen WH, Cattabeni F, Di Luca M (2001) Hippocampal synaptic plasticity involves competition between αCaMKII and PSD-95 for binding to the NR2A subunit of the NMDA receptor. J Neurosci 21: 1501-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F, Pagliardini S, Setola V, Cattabeni F, Battaglia G, Di Luca M (2003) The NMDA receptor complex is altered in an animal model of human cerebral heterotopia. J Neuropathol Exp Neurol 62: 662-675. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870-873. [DOI] [PubMed] [Google Scholar]
- Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13: 133-154. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17: 31-108. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW (1998) Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci 18: 4732-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis PJ, Gardoni F, Kamal A, Croiset G, Bakker JM, Cattabeni F, Gispen WH, van Bel F, Di Luca M, Wiegant VM (2003) Long lasting effects of neonatal dexamethasone treatment on spatial learning and hippocampal synaptic plasticity. Involvement of the NMDA receptor complex. FASEB J 17: 911-991. [DOI] [PubMed] [Google Scholar]
- Kennedy MB (2000) Signal-processing machines at the postsynaptic density. Science 290: 750-754. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269: 1737-1740. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175-190. [DOI] [PubMed] [Google Scholar]
- Liu J, Fukunaga K, Yamamoto H, Nishi K, Miyamoto E (1999) Differential roles of Ca2+/calmodulin-dependent protein kinase II and mitogen-activated protein kinase activation in hippocampal long-term potentiation. J Neurosci 19: 8292-8299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci 15: 120-132. [DOI] [PubMed] [Google Scholar]
- Menegoz M, Lau LF, Herve D, Huganir RL, Girault JA (1995) Tyrosine phosphorylation of NMDA receptor in rat striatum: effects of 6-OH-dopamine lesions. NeuroReport 7: 125-128. [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y (2001) Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci 21: RC185(1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JD, Russell D, Vaughan CL, Chase TN (1998) Enhanced tyrosine phosphorylation of striatal NMDA receptor subunits: effect of dopaminergic denervation and l-DOPA administration. Brain Res 813: 150-159. [DOI] [PubMed] [Google Scholar]
- Oh JD, Vaughan CL, Chase TN (1999) Effect of dopamine denervation and dopamine agonist administration on serine phosphorylation of striatal NMDA receptor subunits. Brain Res 821: 433-442. [DOI] [PubMed] [Google Scholar]
- Papa SM, Chase TN (1996) Levodopa-induced dyskinesias improved by a glutamate antagonist in parkinsonian monkeys. Ann Neurol 39: 574-578. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Orlando, FL: Academic.
- Pettit DL, Perlman S, Malinow R (1994) Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science 266: 1881-1885. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci 6: 501-506. [DOI] [PubMed] [Google Scholar]
- Rozas G, Guerra MJ, Labandeira-Garcia JL (1997) An automated rotarod method for quantitative drug-free evaluation of overall motor deficits in rat models of parkinsonism. Brain Res Brain Res Protoc 2: 75-84. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39: 777-787. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP (1996) The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 50: 275-331. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP (1990) The neuronal network of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends Neurosci 13: 259-265. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ (1998) Autophosphorylation-dependent targeting of calcium calmodulin kinase II by the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 273: 20689-20692. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ (2000) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 275: 23798-23806. [DOI] [PubMed] [Google Scholar]
- Takagi N, Logan R, Teves L, Wallace MC, Gurd JW (2000) Altered interaction between PSD-95 and the NMDA receptor following transient global ischemia. J Neurochem 74: 169-178. [DOI] [PubMed] [Google Scholar]
- Ulas J, Cotman C (1996) Dopaminergic denervation of striatum results in elevated expression of NR2A subunit. NeuroReport 7: 1789-1793. [DOI] [PubMed] [Google Scholar]
- Vila M, Marin C, Ruberg M, Jimenezs A, Raisman-Vozari R, Agid Y, Tolosa E, Hirsch EC (1999) Systemic administration of NMDA and AMPA receptor antagonists reverses the neurochemical changes induced by nigrostriatal denervation in basal ganglia. J Neurochem 73: 344-352. [DOI] [PubMed] [Google Scholar]