Abstract
Myelin-reactive T-cells are activated by traumatic spinal cord injury (SCI) in rodents and humans. Despite the historical association of these cells with experimental and clinical neuropathology, recent data suggest a neuroprotective role for myelin-reactive T-cells. Because of the biological and therapeutic implications of these findings, we attempted to reproduce the original neuroprotective vaccine protocols in a model of rat SCI. Specifically, MBP-reactive T-cell function was enhanced in SCI rats via passive or active immunization. Locomotor function was assessed using a standardized locomotor rating scale (Basso–Beattie–Bresnahan scale) and was correlated with myelin and axon sparing. The functional and anatomical integrity of the rubrospinal pathway also was analyzed using the inclined plane test and anatomical tract tracing. MBP-immunized rats exhibited varying degrees of functional impairment, exacerbated lesion pathology, greater rubrospinal neuron loss, increased intraspinal T-cell accumulation, and enhanced macrophage activation relative to SCI control groups. These data are consistent with the conventional view of myelin-reactive T-cells as pathological effector cells.
Keywords: inflammation, autoimmunity, neuroprotection, vaccine, myelin basic protein, EAE
Introduction
Myelin-reactive T-lymphocytes are found in all humans (Pette et al., 1990; Wucherpfennig et al., 1994). Normally, these cells are maintained in an unactivated/nonpathogenic state by mechanisms of peripheral tolerance. In multiple sclerosis, activation of myelin-reactive T-cells causes CNS inflammation that promotes demyelination and axonal transection with ensuing neurological dysfunction (Steinman, 1996; Trapp et al., 1998; Conlon et al., 1999). Spinal cord injury (SCI), traumatic brain injury, stroke, and peripheral nerve trauma also activate CNS autoreactive T-cells capable of causing neuropathology (Prochazka et al., 1971; Olsson et al., 1992, 1993; Popovich et al., 1996; Becker et al., 1997; Kil et al., 1999; Jones et al., 2002). For example, unilateral penetrating ocular trauma can elicit sympathetic ophthalmia, a suspected autoimmune disease in which T-cells activated by eye trauma attack the undamaged (sympathizing) eye, causing bilateral ocular inflammation and blindness (Chan and Mochizuki, 1999). A similar phenomenon can occur after traumatic SCI.
Using a clinically relevant model of spinal contusion injury, we have shown that MBP-reactive T-cells are activated by SCI. If isolated within 7 d of injury, then injected into naive rats, these cells cause mild and transient paralysis and neuroinflammation (Popovich et al., 1996). Similar results were obtained using transgenic (Tg) mice with a T-cell repertoire biased toward recognition of MBP (Jones et al., 2002). Indeed, we observed impaired locomotor recovery and reflex function in SCI-Tg mice compared with SCI littermate controls. Functional impairment was accompanied by exacerbated secondary degeneration and demyelination with increased intraspinal expression of proinflammatory cytokine mRNA (Jones et al., 2002). Collectively, these data support the long-held belief that myelin-reactive T-cells provoke pathology and loss of function in the CNS.
Recently, a hypothesis was proposed suggesting that MBP-reactive T-cell activation is a physiological response to CNS disease/injury and is required for protection and repair; however, the frequency of activated T-cells is too low for biologically significant neuroprotection to be observed (Schwartz et al., 1999; Schwartz and Kipnis, 2001; Schwartz and Moalem, 2001). Consequently, this response must be boosted to yield a therapeutic benefit. Support for this novel concept of “protective autoimmunity” was originally provided in Lewis rat models of optic nerve injury and spinal contusion injury (Moalem et al., 1999; Hauben et al., 2000a). In both cases, expansion of MBP-reactive T-cells via active (i.e., injection of MBP) or passive (injection of MBP-reactive cells) immunization improved functional recovery and reduced neuronal cell loss.
To date, the neuroprotective potential of myelin-reactive T-cells has not been independently confirmed. However, therapeutic vaccination has been proposed as a clinical therapy for several neurodegenerative conditions including SCI (Schwartz et al., 1999; Hauben et al., 2000b), Alzheimer's disease (Morgan et al., 2000), glaucoma (Fisher et al., 2001), and amyotrophic lateral sclerosis (Angelov et al., 2003).
To extend our original observations in SCI rats and mice and the proof-of-principle experiments on which the concept of protective autoimmunity was founded (Moalem et al., 1999; Hauben et al., 2000a), we completed a comprehensive anatomical and functional analysis of acute therapeutic vaccination in a controlled model of rat spinal contusion injury using the original and updated immunization protocols described by Hauben et al. (2000a, 2001a). The current data fail to support a neuroprotective role for myelin-reactive T-cells. Instead, myelin-reactive T-cells precipitate varying degrees of intraspinal inflammation that are associated with functional impairment and exacerbation of post-traumatic lesion pathology.
Materials and Methods
Generating MBP-reactive T-cell lines (TMBP cells). TMBP cells were generated by immunizing female Lewis rats with 25 μg of guinea pig MBP (GP-MBP), prepared by the method of Swanborg and Shulman (1970) emulsified in an equal volume of complete Freund's adjuvant (CFA; 4 mg/ml Mycobacterium tuberculosis). Lymph node cells were isolated from the popliteal lymph nodes (draining site of injections) of immunized rats and placed in culture with 2% autologous serum and 200 μgof GP-MBP. After 3 d in culture, lymphoblasts were separated on a Ficoll-Paque Plus gradient (Amersham Biosciences, Piscataway, NJ) and placed in propagation medium containing RPMI medium supplemented with 10% FCS and T-cell growth factors (i.e., supernatant of polyclonal T-cells activated with concanavalin A). After 5–7 d of propagation, cells were restimulated with 200 μg of GP-MBP using irradiated thymocytes as antigen-presenting cells. T-cell lines were expanded by at least three cycles of propagation and restimulation before being transferred to spinal-injured rats. Antigen specificity was confirmed after each propagation cycle by testing the ability of the cells to proliferate in response to MBP stimulation.
Spinal contusion and transection injury. Adult female Lewis rats (8–10 weeks) were used for both active and passive immunization protocols. All surgical and postoperative care procedures were performed in accordance with the Ohio State University Institutional Animal Care and Use Committee. Under ketamine (80 mg/kg)/xylazine (50 mg/kg) anesthesia, rats received a partial laminectomy of the eighth thoracic vertebrae. For contusion injuries, animals were placed in a spinal frame, and the exposed spinal cord was displaced 1.1 mm (moderate injury) or 1.3 mm (severe injury) using an electromagnetic SCI device (Popovich et al., 1997). A separate group of animals was subjected to complete spinal cord transection (n = 16). Briefly, a complete laminectomy was performed, and the periosteum was removed. After cutting the dura, visible blood vessels on the dorsal spinal surface were cauterized using a small battery-powered cautery tool. Using irridectomy scissors together with gentle aspiration, the spinal cord was cut from the dorsal to the ventral surface until clear separation was noted between the rostral and caudal stumps of the spinal cord and the ventral dura was visible. Care was taken to maintain the integrity of the ventral dura. To ensure complete transection, the rostral and caudal stumps were gently lifted from the spinal canal using a blunt probe and gentle aspiration. All surgical animals received prophylactic antibiotics (Gentocin; 1 mg/kg) before surgery and daily thereafter. Bladders were voided manually two to three times daily until voluntary expression returned. In transected and immunized animals that developed experimental autoimmune encephalomyelitis (EAE), body weight was maintained via oral Nutrical supplementation until the animal recovered the ability to move and eat on its own. Regardless of these precautions, two rats that received TMBP cells developed severe EAE and died. Animals in which the injury biomechanics (i.e., force or impulse/momentum) exceeded 3 SDs from the mean were excluded from all analyses [n = 2 (active) and n = 1 (passive) immunizations].
Passive or active immunization. Passively immunized rats were injected (intraperitoneally) 1 hr after spinal contusion injury or complete spinal transection with 1 ml of sterile PBS or 107 TMBP cells (see above) suspended in 1 ml of sterile PBS (n = 8/group). Actively immunized rats were randomized into one of four groups, then received intradermal injections, at the base of the tail, of (1) GP-MBP (100 μg) emulsified in incomplete Freund's adjuvant (IFA), (2) GP-MBP plus CFA (containing Mycobacterium tuberculosis at 0.5 mg/ml), (3) PBS plus IFA, or (4) PBS plus CFA (n = 8/group). These concentrations of antigen and Mycobacterium tuberculosis were described by Hauben et al. (2000a, 2001a) to be non-encephalitogenic (i.e., do not cause EAE) and neuroprotective in SCI rats. Uninjured control animals were immunized to test the effector potential of activated T-cells (n = 2/group). All animals injected with TMBP cells developed transient ascending paralysis characterized by a drooping tail and hind limb paraparesis. The encephalitogenic potential of these cells was apparent in SCI rats as flaccid paralysis in the open field 3–5 d after immunization (Fig. 1 B, C). In contrast, the low concentration of Mycobacterium tuberculosis used for active immunization was insufficient to elicit clinical signs of EAE. Of four actively immunized control rats, only one rat injected with MBP/CFA exhibited tail weakness. No other deficits were observed in actively immunized rats.
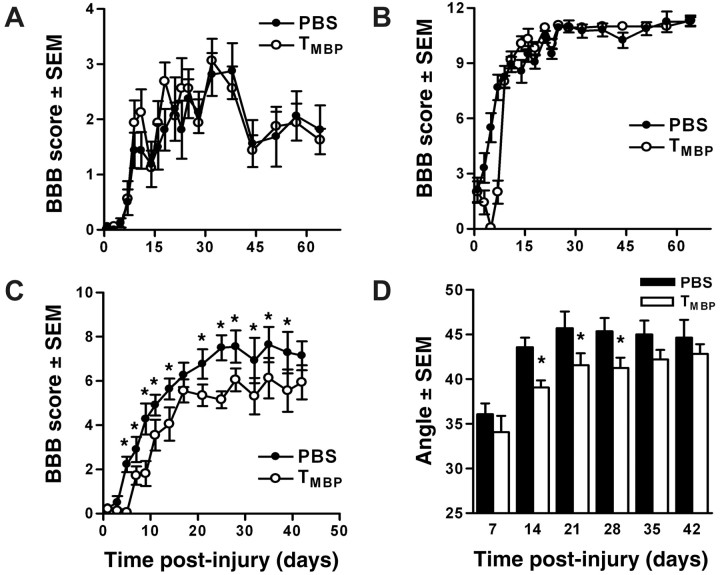
Figure 1.
Passive immunization with TMBP cells impairs functional recovery after severe, but not moderate, spinal contusion injury. Female Lewis rats receiving 107 TMBP cells after complete transection (A) or moderate (1.1 mm displacement) contusion injury (B) achieved similar levels of spontaneous locomotor recovery relative to PBS control rats. Conversely, after severe contusion injury (1.3 mm displacement), consistently impaired overground locomotion was evident in rats injected with 107 TMBP cells relative to PBS control rats (BBB = 5.9 ± 2.2 vs 7.1 ± 1.7; p < 0.05 via repeated measures ANOVA; *p < 0.05) (C). Inclined plane performance also was significantly impaired in rats receiving TMBP cells after severe SCI (D; p < 0.05 via repeated measures ANOVA; *p < 0.05). n = 7–8/group in A–D.
Behavioral analyses. Gross locomotor recovery after SCI was assessed using the Basso–Beattie–Bresnahan (BBB) locomotor rating scale (Basso et al., 1995). For 1 week before surgery, rats were acclimated to the testing environment (90 cm diameter plastic wading pool; 4 cm height). At 1, 3, 5, 7, and 10 d post injury (dpi), and twice weekly thereafter for a total of 6–9 weeks, rats were placed in this environment and observed for 4 min. Each hind limb was scored by two investigators blinded to the treatment protocol. Individual hind limb scores were averaged to provide a single score for each animal per session. Once a plateau in locomotor recovery was evident (≥28 dpi), a frequency analysis of weight support and plantar stepping was performed across all groups.
A subset of animals was analyzed using the inclined plane task (Rivlin and Tator, 1977). This task evaluates the animal's ability to maintain body position on a board that is incrementally raised to increasing angles. Performance on the inclined plane correlates with the integrity of the rubrospinal tract (and other nonpyramidal pathways) after SCI (Midha et al., 1987; Fehlings and Tator, 1995). Before surgery, animals were acclimated to the testing apparatus (i.e., a board covered with a rubber mat containing horizontal ridges spaced 3 mm apart). After injury, animals were tested in two positions (right side or left side up) once a week for 6 weeks. Rats were tested in each position, after which the angle was increased incrementally by 5°. The maximum angle at which the animal could maintain a stationary position on the board for 10 sec was recorded for each position and averaged to obtain a single score for each animal.
Tissue processing. At designated times after injury, animals were anesthetized and perfused intracardially with 100 ml of sterile PBS, pH 7.4, followed by 300 ml of 4% paraformaldehyde. Spinal cords were removed and postfixed for 2 hr before being rinsed and immersed in phosphate buffer (0.2 m) overnight. The following day, tissues were immersed in 30% sucrose for 48 hr then were blocked into 2 cm segments centered on the injury site before embedding in optimal cutting temperature compound (Thermo Shandon, Pittsburgh, PA). Embedded spinal cords were stored at –80°C until they were sectioned.
Immunohistochemical staining of injured spinal cord. Spinal cords were serially sectioned in the coronal plane at 10 μm and collected on Super-Frost Plus slides (Fisher Scientific, Houston, TX). Inflammatory cells and axon profiles were identified on adjacent sections using the following antibodies (specificity; dilution): OX-19 (T-cells; 1:1000; Serotec, Indianapolis, IN), ED-1 (activated macrophages/microglia; 1:8000; Serotec), and RT97 (200 kDa neurofilament; 1:2000; Chemicon, Temecula, CA). Slides were overlaid with serum (4–10%) for 60 min before incubating with primary antisera. After incubating overnight at 4°C, sections were rinsed three times with buffer before overnight incubation (4°C) with biotinylated secondary antibody (1:400). Slides were rinsed three times, and endogenous peroxidase activity was quenched by applying 6% MeOH/H2O2 for 15 min. Bound antibody was visualized by applying Elite ABC (Vector Laboratories, Burlingame, CA) for 1 hr at room temperature, followed by DAB (OX-19, ED-1) or DAB–nickel (neurofilament) substrate (Vector Laboratories). Sections were dehydrated, cleared in xylene, and coverslipped with Permount (Fisher Scientific).
Analyses of myelin sparing. A complete series of sections spanning the rostrocaudal extent of the contusion lesion was stained with eriochrome cyanine (EC) to reveal spared myelin. For each animal, the epicenter was defined qualitatively as the section containing the least amount of intact tissue. That section and sections located 100 μm rostral and caudal to it were used to quantify the area of spared myelin at the epicenter. The area of spared myelin was estimated using the Cavalieri method (Gundersen et al., 1988a). Briefly, high-power light microscopy was used to delineate spared white matter as defined by EC staining. Digital images were printed with a calibrated scale bar, then were enlarged. Uniform grids of equally spaced points were placed randomly on each printout such that the grid covered the entire section. Points located within regions of spared white matter were tallied. The spinal cord cross-sectional area was estimated in the same manner. Point tallies were converted into area estimates using the formula: area = a/p ×ΣP, where a/p equals the area represented by each point and ΣP equals the number of points counted in each image.
Quantitation of T-cells, neurofilament, and macrophages. Using an Axioplan microscope (Zeiss, Oberkochen, Germany) fitted with a monocular counting reticule, all positively stained T-cells within the spinal cord parenchyma and with uniform membrane staining were counted in equidistant sections cut from an 8 mm segment centered on the impact site, as described previously (Jones et al., 2002). In sections adjacent to those used to quantify T-cells, activated macrophages/microglia and neurofilament-positive area were quantified using image analysis, as described previously (Popovich et al., 1997). Those data are expressed as proportional area (area of ED-1+ labeling divided by the total cross-sectional area) (Popovich et al., 1997).
Retrograde labeling of rubrospinal neurons. At times corresponding with a plateau in functional recovery (42 dpi), a T13 laminectomy was performed in a subset of uninjured control, PBS-injected, and MBP-immunized (active and passive; see above) SCI rats (n = 4/group). Those rats were suspended in a stereotaxic frame, and a glass micropipette (outer tip diameter, 30 μm) was positioned 0.7 mm lateral to the dorsal spinal artery and inserted to a depth of 1 mm. Bilateral injections of 1% Fluorogold (FG; Fluorochrome, Denver, CO) (500 nl/side per rat) were made into the dorsolateral white matter over a 10 min period using pressurized microinjection. The pipette was left in place for 2 min, then was slowly withdrawn over 4 min. One week after FG injection, animals were anesthetized and killed via intracardiac perfusion with fixative (see above). The spinal cord and brain were removed, and serial sections (50 μm) were cut through the entire red nucleus (RN). Sections were mounted on gelatin-coated slides, coverslipped in Immu-mount (Thermo Shandon), and stored in the dark (4°C) until they were analyzed. To verify that FG had not diffused into the injury site, which would increase the likelihood of spurious labeling rostral to the impact site, longitudinal sections spanning the distance from the caudal pole of the contusion lesion to the injection site were cut for each animal. Transverse sections cut through the thoracic and cervical spinal cord above the lesion were used to verify that FG+ axons distributed in the dorosolateral spinal cord and that ependymal cells did not label with FG (indicative of leakage into the CSF).
Quantitation of RN neurons. Under UV fluorescence on a Axioplan 2 Imaging microscope (Zeiss) equipped with a motorized z-axis and X-Y stage (Ludl Electronics, Hawthorne, NY), FG-labeled rubrospinal neurons (RSNs) were counted using the optical fractionator technique (stereology module of an MCID 6.0 Elite; Imaging Research, St. Catharines, Ontario, Canada). For each animal, the rostral and caudal poles of the RN were identified, and the mean section height was determined by averaging the measured section thickness of five random visual fields at the rostral pole (Sterio, 1984; Gundersen et al., 1988b). Equidistant sections spanning the RN were analyzed. Using a random start in the x-y plane, a counting frame measuring 15,000 μm2 was applied to optical sections that encompassed the section thickness (excluding 1 μm “guard volumes” at the top and bottom of the section). FG+ neurons were counted by focusing through these optical sections. Neurons were tallied only if they did not intersect the “forbidden” line and had an in-focus nucleus within the allowed sampling volume (see Fig. 5 A, B). FG+ RSN tallies were converted into estimates of total (bilateral) RSN number (N) using the formula: N = Q × 1/hsf × 1/asf × 1/ssf, where Q equals the total number of appropriately sampled FG+ RSNs and hsf, asf, and ssf equal the tissue height, area, and section sampling fractions, respectively. FG-labeled neurons in the RN were easily identified because of their bilateral location in the ventral midbrain tegmentum and the absence of labeling in adjacent brainstem nuclei. FG+ cells were ∼10–40 μm in diameter and possessed a dim but uniformly fluorescent nucleus, often with visible nucleoli and intensely positive neuritic processes. Based on size and morphology, all FG+ cells were neurons. Select tissue sections were counter-stained with a fluorescent Nissl stain (Neurotrace Green; Molecular Probes, Eugene, OR) to demonstrate distribution of labeled neurons relative to all cells in the RN.
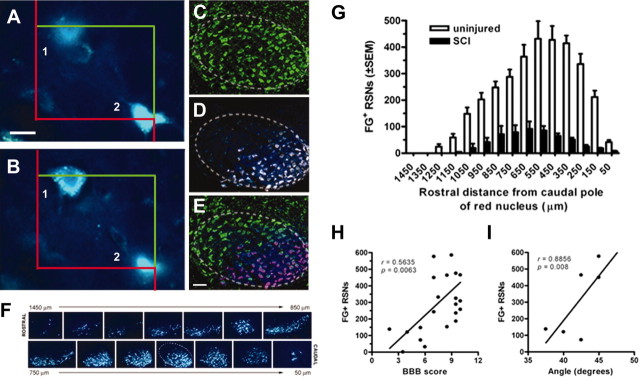
Figure 5.
Unbiased stereological quantitation of retrogradely labeled FG+ RSNs in uninjured and SCI Lewis rats. Digital images illustrate components of the optical fractionator technique used for estimating FG+ RSN numbers (A, B). The images are “optical sections” separated by 7 μm and illustrate a cell (1) with a clearly visible nucleus that lies within the counting frame (superimposed red and green boxes) or is bisected by a green, but not red, line. In contrast, another cell (2) is bisected by the red (“forbidden”) line and is not in focus. Based on these criteria, only cell 1 is counted. Using this approach, RSNs were counted in 100 μm intervals spanning the rostrocaudal extent (∼1500 μm) of both red nuclei of uninjured control rats after FG injections into the upper lumbar spinal cord. The somatotopic organization of the RN is illustrated by the ventrolateral restriction of retrogradely labeled neurons (D, F) predominantly within the caudal pole of the nucleus (F, G). Neurotrace Green (C) and FG (D) images were merged (E) to illustrate the ventrolateral restriction of FG+ RSNs relative to all neurons in RN. Evenly spaced sections through the RN illustrate the rostrocaudal organization of FG+ RSNs in the right RN of an uninjured Lewis rat (F). Scale bar, 20 or 200 μm (in A and C, respectively). The dotted line in C–E and caudal row of F delineates approximate boundaries of RN. After SCI, the number of FG-labeled RSNs in SCI rats correlates with functional recovery as determined by open-field locomotor analysis (H; BBB score; r = 0.56; p < 0.01) or performance on the inclined plane (I; r = 0.89; p < 0.01). n = 4/group in G.
Statistical analyses. Behavioral scores from BBB analysis and inclined plane tests were analyzed by repeated measures ANOVA (time vs treatment). Frequency analyses were analyzed using a χ2 test. Cross-sectional area, myelin, and neurofilament sparing at the epicenter were analyzed by one-way ANOVA. Two-way ANOVA was used to analyze rostrocaudal differences in myelin, ED-1 immunoreactivity, and T-cell number (treatment vs distance from the epicenter). Post hoc analyses consisted of Newman–Keuls or Bonferroni's pairwise comparisons. Anatomical and functional correlations were performed using Pearson's test. All differences were considered significant at p < 0.05. All tests were performed using GraphPad Prism version 4.00 (GraphPad, San Diego, CA).
Results
Passive immunization with TMBP cells has no effect on spontaneous locomotor recovery after spinal cord transection or moderate spinal contusion injury
Functional recovery was evaluated for 6–9 weeks after a complete spinal transection or contusion injury using the BBB open-field locomotor rating scale (Basso et al., 1995). Spinal transected rats recovered limited hind limb movements by 7 dpi with no apparent differences between rats that received injections of PBS or TMBP cells (Fig. 1A). Similarly, all rats receiving a moderate (1.1 mm displacement) contusion injury recovered plantar stepping, but without fore limb–hind limb coordination (mean BBB score, 10–11) (Fig. 1B). Performance on the inclined plane in transected or moderately contused rats was unaffected by TMBP cells (data not shown).
Passive immunization with TMBP cells impairs spontaneous locomotor recovery after severe spinal contusion injury
Hauben et al. (2000a) originally described the neuroprotective effects of myelin-reactive T-cells using a more severe contusion injury than described above (Fig. 1B). Thus, we hypothesized that failure to observe neuroprotection was related to differences in injury severity and that a more severe injury would reduce the threshold needed to detect a treatment effect. In subsequent experiments, BBB scores for PBS-treated rats receiving a 1.3 mm displacement injury were 7.1 ± 1.7 (Fig. 1C) and were consistent with the 7.3 ± 0.8 scores reported by Hauben et al. (2000a) for PBS control animals. These scores indicate extensive movement of hip, knee, and ankle joints with half the animals (6 of 11) achieving weight-supported plantar placement on at least one side by 28 dpi. In contrast, severely injured rats injected with TMBP cells exhibited significant functional impairment (p < 0.05 vs PBS controls) over most of the 6 week evaluation period (Fig. 1C). Indeed, only one TMBP-injected rat (1 of 10) achieved weight support by 28 dpi (p = 0.031; χ2 test). Performance on the inclined plane also was significantly impaired in TMBP-treated rats (Fig. 1D)(p < 0.05).
Active immunization with MBP impairs acute recovery from severe SCI and exacerbates chronic locomotor deficits
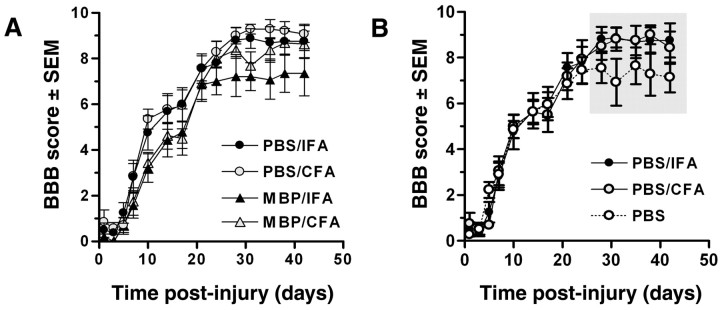
Clinically, active immunization may have greater therapeutic use than passive immunization for treating neurodegeneration and CNS trauma. Consequently, the efficacy of active immunization protocols previously shown to enhance recovery after SCI were evaluated (Hauben et al., 2000a, 2001a). Unlike the acute flaccid paralysis caused by TMBP cells between 1 and 5 dpi (evident as BBB scores of 0 in Fig. 1B,C), neither MBP/IFA nor MBP/CFA injections evoked paralytic changes (i.e., EAE). However, by 1 week after injury, gross functional impairment was consistently evident in MBP-immunized groups relative to PBS/adjuvant controls (Fig. 2A). BBB scores for MBP-immunized rats ranged from 1.7 ± 1.6 at 7 dpi to 4.6 ± 1.9 at 17 dpi compared with scores of 2.9 ± 1.8 at 7 dpi to 6.0 ± 2.0 at 17 dpi in PBS-treated rats. Notably, by 7 dpi, only isolated movements of a single hind limb joint (BBB score, 1) were observed in 10 of 15 MBP-immunized rats. Conversely, 12 of 16 PBS/adjuvant-treated rats exceeded this threshold within 1 week. Of these 12 rats, 7 continued to improve over the next 10 d showing extensive movements of the hip, knee, and/or ankle joints with some weight-supported plantar placement. Only 1 of 15 MBP-immunized rats recovered any weight support during this time interval (0–17 dpi). Despite these changes, chronic BBB scores (>28 dpi) were not significantly different between groups.
Figure 2.
Acute functional recovery after SCI is impaired by active immunization with MBP but is enhanced chronically by immunological adjuvants (CFA and IFA). Injection of MBP/CFA or MBP/IFA consistently delayed spontaneous recovery during the first 2–3 weeks after injury (A). These deficits were not maintained at later post-injury periods. In contrast, delayed improvements in weight support (WS) and plantar stepping (PS) were evident in rats injected with PBS/CFA or PBS/IFA emulsions (B; shaded regions indicate points at which BBB scores and/or frequency analysis of WS and PS were found to be statistically different via two-way ANOVA (BBB: p = 0.0017; p < 0.05 vs PBS) orχ2 test for frequency analyses (p < 0.01 or p < 0.001 for WS and PS, respectively). n = 7–8/group in A and B.
In general, expansion of MBP-reactive T-cells (via active or passive immunization) impaired spontaneous recovery of locomotor function (Table 1). Indeed, only 4% (1 of 25) of MBP-immunized rats recovered the ability to plantar step by 4 weeks after injury, with 6 additional rats achieving this milestone by 6 weeks. In contrast, plantar stepping was observed in 45% (10 of 22) of PBS- and PBS/adjuvant-treated animals over this same period (Table 1).
Table 1.
MBP immunization impairs recovery of weight-supported (WS) plantar stepping (STEP) after SCI
|
|
28 dpi |
35 dpi |
42 dpi |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
WS |
STEP |
WS |
STEP |
WS |
STEP |
|||
| PBS controls | 69% (18/26)*** | 38% (10/26)** | 82% (18/22)* | 41% (9/22) | 73% (16/22)*** | 45% (10/22) | |||
| MBP immunized |
44% (11/25) |
4% (1/25) |
52% (12/23) |
22% (5/23) |
48% (11/23) |
30% (7/23) |
|||
Frequency analysis of rats achieving WS STEP on one or both sides between 4 and 6 weeks after injury (total achieving a BBB score of 9 or 10/total number of rats). Four PBS-injected and two MPB-immunized rats were killed at 28 dpi for use in an assay unrelated to this experiment. This accounts for the drop in animal numbers between 28 and 35 dpi. *p < 0.05 versus MBP immunized; **p < 0.01 versus MBP immunized; ***p = 0.07 or ***p = 0.09 versus MBP immunized.
Immunological adjuvants improve chronic locomotor recovery after SCI
Retrospective analysis of the control data described in Table 1 revealed functionally significant differences in recovery between animals injected with PBS alone or emulsions of PBS and adjuvant. Despite identical patterns of recovery during the first 3 weeks after injury, final BBB scores (28–42 dpi) were different (p < 0.05), and the frequency of weight support (p < 0.001) and plantar stepping (p < 0.01) was greater in rats injected with adjuvant (CFA or IFA) (Fig. 2B). No differences were noted between CFA- and IFA-injected rats (Fig. 2B).
MBP immunization promotes secondary injury and augments T-cell infiltration and macrophage activation after SCI
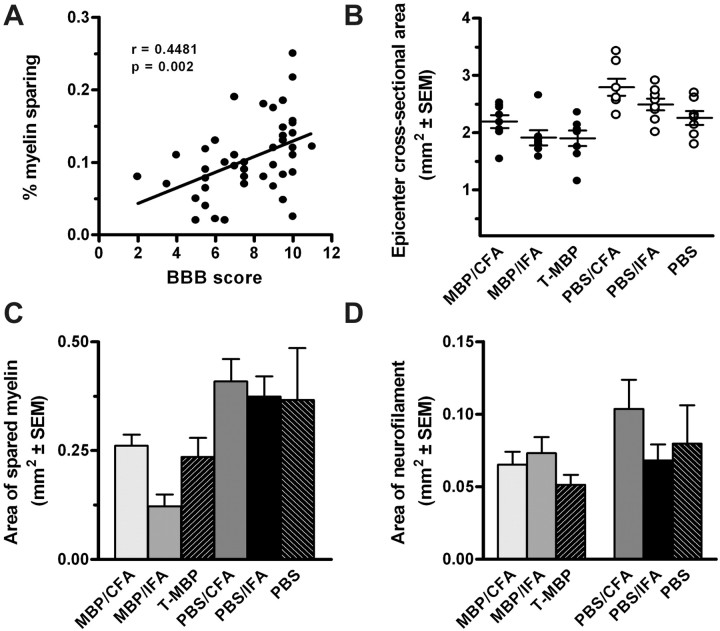
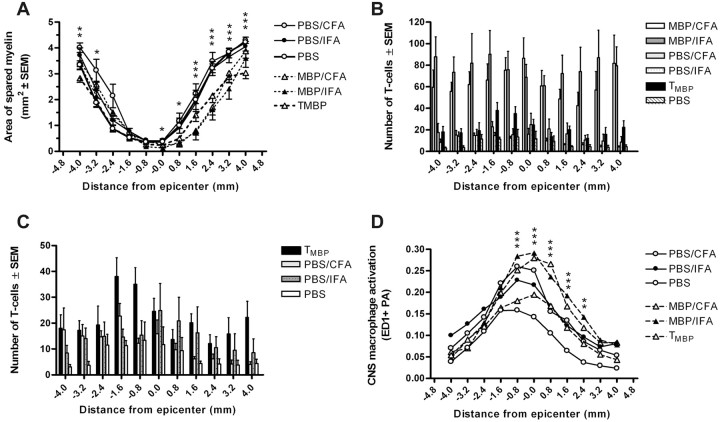
In passively and actively immunized rats, final BBB scores correlated with percentage of myelin sparing at the epicenter (i.e., less myelin was measured in animals with the greatest functional impairment) (Fig. 3A). Between-group analyses revealed a marked reduction (p < 0.0001) in the spinal cord cross-sectional area at the injury site in MBP-immunized rats (Fig. 3B). This corresponded with a reduction in the area of spared myelin (p < 0.05) and a trend toward decreased neurofilament immunoreactivity at the epicenter of MBP-immunized animals compared with control groups (Fig. 3C,D). Because our previous data showed that MBP-reactive T-cells expanded lesion pathology beyond the epicenter (Jones et al., 2002), we extended our analyses over the rostrocaudal extent of the contusion lesion. Those analyses revealed marked changes in demyelination in MBP-immunized rats (Fig. 4A). This pathology was most notable caudal to the lesion epicenter (p < 0.0001).
Figure 3.
MBP immunization exacerbates myelin loss after SCI. In all experimental groups, final BBB scores correlated with the percentage of myelin sparing at the injury site (area of stained myelin/cross-sectional area) (A; r = 0.45; p < 0.01; Pearson's correlation). MBP immunization also reduced the spinal cord cross-sectional area (B; p < 0.0001; ANOVA) and the area of myelin sparing relative to control groups at the injury site (C; p < 0.05; ANOVA). Neurofilament immunoreactivity at the epicenter was not significantly different between groups (D; p = 0.2). n = 45 in A; n = 7–8/group in B–D.
Figure 4.
MBP immunization enhances myelin loss and intraspinal inflammation at and beyond the site of injury. Myelin sparing was reduced over the rostrocaudal axis of the injured spinal cord in rats injected with TMBP cells, MBP/CFA, or MBP/IFA (A; p < 0.01). MBP immunization also enhanced intraspinal T-cell accumulation throughout the injured spinal cord (B, C; p < 0.0001). Note that the data in C is identical to that in B but without MBP/CFA or MBP/IFA data. This illustrates the reduced magnitude but significant increase in T-cell infiltration in rats immunized with TMBP cells and adjuvant (CFA or IFA in PBS) relative to PBS controls (C; p < 0.0001). Increased macrophage/microglia activation (ED1-labeling; D; p < 0.0001) was associated with areas of prominent T-cell accumulation and enhanced myelin loss (compare Fig. 4 A–D). All comparisons used two-way ANOVA (n = 6–8/group in A–D). Asterisks in A and D indicate differences between one or more of the MBP-immunized groups relative to PBS control groups: *p < 0.05, **p < 0.01, or ***p < 0.0001. Although statistical significance was found throughout the graphs in B and C, asterisks were excluded to simplify viewing of data sets.
Active immunization with MBP/CFA or MBP/IFA dramatically enhanced intraspinal T-cell accumulation in the chronically injured spinal cord (p < 0.0001) (Fig. 4B). T-cell infiltration also was increased in TMBP and PBS/adjuvant-injected rats, but only slightly above numbers found in PBS control animals (Fig. 4C). Noted differences between actively and passively immunized rats are consistent with previous reports in the EAE model, in which large numbers of T-cells persisted in the spinal cord of actively, but not passively, immunized rats (Hickey et al., 1987). Regardless of the mode of immunization, in MBP-immunized rats (but not in PBS or PBS/adjuvant rats) T-cells were found throughout rostral/caudal spinal segments colocalized to regions of increased myelin loss and enhanced macrophage/microglia activation (p < 0.0001) (Fig. 4 D).
Retrograde labeling of RSNs after SCI correlates with locomotor recovery and is reduced by expansion of MBP-reactive T-cells
Spontaneous recovery of locomotor function in SCI rats is supported in part by rubrospinal axons that survive the primary mechanical insult and secondary neurodegenerative cascades initiated at the site of injury (Fehlings and Tator, 1995; Basso et al., 2002). To supplement the morphometric analyses (Figs. 3, 4) and to confirm previous reports of myelin-reactive T-cell-mediated neuroprotection of rubrospinal pathways after SCI (Hauben et al., 2000a), we quantified neurons throughout the RN in SCI rats with and without MBP immunizations. Stereological quantitation revealed 3782.0 ± 293 retrogradely labeled RSNs throughout the ventral and ventrolateral RN in uninjured rats (Fig. 5A–G). No labeling of RSNs was detected in the dorsomedial RN, where neurons projecting to the cervical spinal cord originate (Shieh et al., 1983; Strominger et al., 1987). After SCI, an identical labeling pattern was evident; however, the total number of labeled RSNs was markedly reduced (Fig. 5G). The total number of labeled RSNs correlated with final BBB scores (r = 0.56; p < 0.01) (Fig. 5H) and performance on the inclined plane (r = 0.89; p < 0.01) (Fig. 5I).
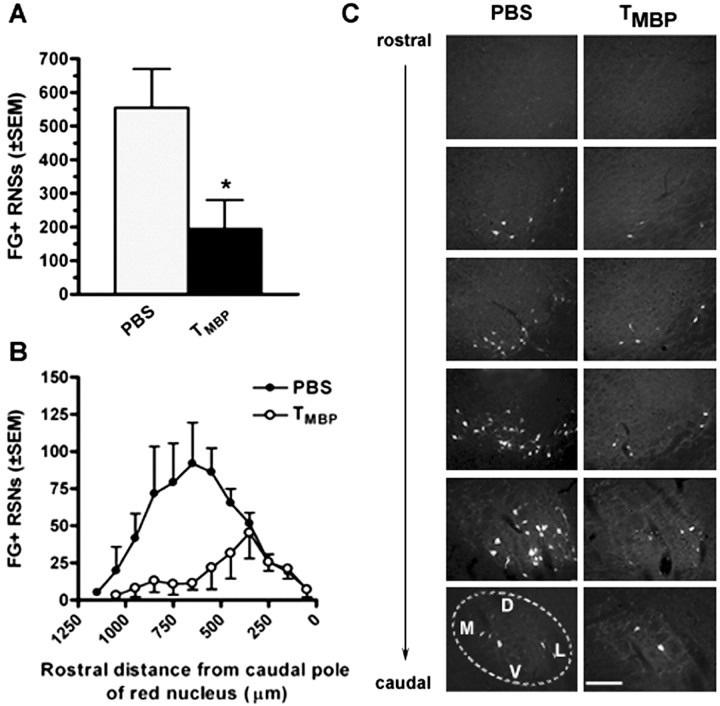
Passive immunization dramatically reduced the total number of labeled RSNs (n = 194.5 ± 86 in TMBP-injected rats vs 555.0 ± 114 RSNs in PBS-injected rats) (Fig. 6). RSN cell loss in TMBP-injected rats was most evident in the rostral RN (Fig. 6B). Active immunization did not affect RSN survival after SCI (data not shown; p = 0.5733 via ANOVA for PBS/IFA-, PBS/CFA-, MBP/IFA-, and MBP/CFA-injected rats). Stereological cell counts were compared with counts obtained using more conventional biased profile counts. Using this approach, identical patterns of cell loss were observed; however, the total cell number was overestimated by an average of 30% for each group (data not shown).
Figure 6.
TMBP cells reduce retrograde labeling of RSN in SCI rats. The total number of labeled RSNs is decreased in SCI rats injected with TMBP cells versus PBS injected rats (A; n = 194.5 ± 86 and 555.0 ± 114 FG+ RSNs for TMBP and PBS-injected rats, respectively; *p < 0.05; unpaired t test). Analysis across the rostrocaudal extent of the nucleus reveals preferential loss of labeling in neurons located ∼300 μm rostral to the caudal pole in rats injected with TMBP cells (B). Fluorescent micrographs represent equidistant sections (separated by 150 μm from the rostral to caudal pole) from the right RN of a rat injected with PBS or TMBPcells (C). The dotted line in the caudalmost PBS section delineates approximate boundaries of RN with dorsal (D), medial (M), lateral (L), and ventral (V) orientation provided. Scale bar, 200 μm. n = 4/group in A and B.
Discussion
Myelin-reactive T-cells: pathological effector cells in the CNS
For >70 years, scientists have immunized animals with myelin proteins or myelin-reactive T-cells to induce EAE, a model of CNS inflammation and demyelination (Rivers et al., 1933; Steinman, 2003). Our data support this historical role of myelin-reactive T-cells as effectors of CNS pathology. Indeed, MBP immunizations exacerbated neurological deficits, demyelination, and/or neuronal survival/function in animals subjected to a clinically relevant model of SCI. These deficits were associated with increased numbers of infiltrating T-cells and enhanced macrophage/microglial activation in areas of secondary injury. These observations illustrate the destructive potential of enhancing adaptive and innate immune reactions as an acute therapy for SCI.
Namely, MBP-reactive T-cells produce or elicit the production of cytokines like interferon (IFN) γ, tumor necrosis factor (TNF) α, interleukin (IL)-1β, and IL-12. Exposure to TNFα, IL-1β, or IFNγ inhibits neurotransmission and can enhance pain transmission by increasing spinal cord dorsal horn neuron excitability (Koller et al., 1997; Vikman et al., 2003). These cytokines have been implicated in the onset and progression of demyelinating pathology and the reciprocal activation of macrophages and T-cells (Balashov et al., 1997; Akassoglou et al., 1998; Chang et al., 1999; Bitsch et al., 2000; Ahmed et al., 2003). Indeed, MBP-reactive T-cells promote demyelination and axon damage via cytokine-mediated activation of microglia and macrophages (Huitinga et al., 1990; Gimsa et al., 2000). Data in Figure 4 illustrate this relationship after SCI (i.e., marked T-cell infiltration and macrophage activation are colocalized to areas of secondary myelin loss only in MBP-immunized rats). Although we and others have shown that these (and other) cytokines are increased within the CNS after experimental and human SCI (Kil et al., 1999; Hayes et al., 2002; Jones et al., 2002), their contribution to post-traumatic SCI lesion pathology and neurological dysfunction is unknown.
Other T-cells: pathological or physiological effector cells in the CNS?
Despite overwhelming evidence that myelin-reactive T-cells cause neuropathology and loss of function, to categorically define all T-cells as “destructive” would be misleading. Indeed, numerous studies support a protective, reparative, or immune-regulatory role for T-cells, macrophages, and cytokines in models of neuronal injury and CNS disease (Batchelor et al., 1999; Serpe et al., 1999; Mason et al., 2001; Bieber et al., 2003; Hofstetter et al., 2003). In the present study, T-cell infiltration and macrophage/microglia activation were enhanced in CFA/PBS-immunized rats (Figs. 2, 3, 4). However, in contrast to MBP-immunized rats, these changes occurred in animals exhibiting improved functional recovery and increased myelin/axon sparing. This suggests that an undefined constituent of CFA caused activation of endogenous T-cells but without adverse effects on spinal cord structure or function.
Bacterial heat shock proteins (HSPs) (e.g., HSP65, HSP70) are a principal component of CFA and could suppress inflammatory-mediated pathology at the injury site. Indeed, HSP-reactive T-cells exist in all individuals and are activated by injury/inflammation (Anderton et al., 1993; Basu et al., 2000). When this activation is augmented via injection of CFA or purified HSP, HSP-reactive T-cells release regulatory cytokines (e.g., IL-10, IL-4, TGF-β) that suppress inflammatory-mediated injury in rheumatoid arthritis and EAE (Birnbaum et al., 1998; Puga Yung et al., 2003). IFA/PBS-injected rats may also benefit from a similar mechanism of protection. However, instead of delivering HSP in the inoculum, the adjuvant effects of IFA might increase the efficiency of endogenous HSP-reactive T-cell activation in response to HSPs synthesized and released after SCI (Mautes and Noble, 2000).
Still, given the complex and poorly understood duality of neuroinflammation, it is debatable whether the function of any T-cell should be augmented after acute SCI. Indeed, Ibarra et al. (2003) have shown that pharmacological inhibition of T-cell responses to spinal cord antigens dramatically improves anatomical and functional recovery from SCI. Using a dorsal spinal hemisection lesion model, Gonzales et al. (2003) have shown enhanced functional recovery and neuroprotection when post-traumatic T-cell infiltration is inhibited. Similarly, traumatic and excitotoxic brain pathology are attenuated in T-cell-deficient mice (Fee et al., 2003; Chen et al., 2004). In each case, a direct cytotoxic effect of T-cells on neurons (or glia) is inferred because polyclonal activation of purified T-cells causes axonal transection, lysis of oligodendrocytes, and neurotoxicity in human and murine culture models (Antel et al., 1994; Medana et al., 2001; Giuliani et al., 2003).
Protective autoimmunity revisited
To replicate the proof-of-principle experiments that defined protective autoimmunity, we evaluated MBP immunization protocols in age-matched female Lewis rats subjected to SCI. Unless noted otherwise, all experimental parameters including antigen concentration, adjuvant type, route of administration, and time of administration relative to injury matched those of Hauben et al. (2000a, 2001a). Moreover, primary outcome measures (i.e., locomotor recovery and retrograde labeling of RSNs) were identical, and additional tools were used to improve sensitivity for observing a treatment effect (e.g., different lesion severities, stereological analyses, inclined plane test). Despite these efforts, we never observed neuroprotection or functional improvements in MBP-immunized rats.
Initially, we suspected that differences in injury severity could explain our inability to observe autoimmune neuroprotection. Indeed, TMBP cells did not adversely affect chronic locomotor recovery in rats subjected to moderate SCI (Fig. 1B). However, locomotor recovery was impaired in TMBP-treated rats after severe SCI (Fig. 1C,D) despite the fact that PBS control rats achieved levels of recovery (BBB score, 7.1 ± 1.7) identical to the PBS control rats reported by Hauben et al. (2000a) (BBB score, 7.3 ± 0.8). It is possible that the extreme pathogenicity of our TMBP cells interfered with their ability to elicit a neuroprotective response (Fisher et al., 2001). Indeed, TMBP-injected rats developed complete hind limb paralysis (i.e., EAE) within 3–5 d of injection (Fig. 1B,C), whereas Hauben et al. (2000a) described only “mild” EAE symptoms. Still, when clinical signs of EAE were negligible or absent (i.e., MBP/CFA- or MBP/IFA-injected rats in Fig. 2), we still did not observe neuroprotection. Thus, neither injury severity nor encephalitogenicity of the immunization protocol can account for the disparity between our studies and those of Hauben et al. (2000a). However, our data suggest that the pathogenicity of myelin-reactive T-cells is enhanced by severe SCI.
After SCI, the therapeutic value of myelin-reactive T-cells has been defined mainly by changes in BBB locomotor rating scores. Given our experience in developing, using, and training others to use this scale, we predict that the magnitude of “protection” previously attributed to enhanced autoimmune T-cell activity is attributable in part to inconsistent interpretation of acute hind limb recovery as defined by the BBB scale. Specifically, most rats that benefit from protective autoimmunity achieve BBB scores of 5–8 (i.e., varying degrees of hind limb flexion recovery, but without weight support or plantar stepping) (Hauben et al., 2000a, 2001a,b; Yoles et al., 2001; Kipnis et al., 2002). Because these rats negotiate their environment mostly by their unaffected forelimbs, reports describing this level of recovery as an improvement in “non-coordinated walking ability” have overstated the magnitude and perhaps the functional significance of a therapeutic effect (Kipnis et al., 2002).
Typically, the validity of BBB scores are confirmed by morphometric analysis of myelin sparing at the lesion site (Fig. 3) (Behrmann et al., 1992; Basso et al., 1996). Unfortunately, no quantitative myelin sparing data are available for studies that have reported improved BBB scores after myelin-reactive T-cell therapy. Instead, quantitation of retrogradely labeled RN neurons has been used as an anatomical correlate of functional recovery (Hauben et al., 2000a, 2001a). Although valid, this approach is technically more difficult and can produce misleading data if injections are not restricted to the spinal cord below the level of injury. For example, intraspinal injection of a retrograde anatomical tracer at vertebral level T13 (upper lumbar spinal cord) should predominantly label neurons in the ventrolateral RN (Strominger et al., 1987). Diffuse labeling of dorsomedial neurons and/or glia indicates that dye was applied above thoracic levels or that it leaked into the injury site. Either would produce spurious labeling and would not predict the magnitude of rubrospinal axon “sparing” below the level of injury. Hauben et al. (2000a, 2001a) previously reported increased survival of RSNs in SCI rats injected with TMBP cells. However, labeled RSNs were diffusely distributed and were concentrated in the dorsomedial, rather than the ventrolateral, RN (Hauben et al., 2000a). This suggests that increased RSN labeling could have occurred because of dye leaking into and rostral to the injury site rather than as a result of T-cell-mediated neuroprotection.
In the present study, labeled RSNs were quantified over the rostrocaudal extent of both red nuclei in MBP-immunized and control rats. The data show that TMBP cells reduced RSN labeling throughout the ventrolateral RN of SCI rats compared with PBS control rats (Fig. 6). This implies that TMBP cells exacerbate neuronal injury within the RN, impair axonal transport (required for retrograde transport of tracer), or exacerbate axonal injury at the site of injury. However, a similar reduction in labeling of RSN was absent in actively immunized rats, suggesting that the pathogenic potential of TMBP cells is greater than that of the heterogeneous T-cells activated by MBP/CFA or MBP/IFA injections. This hypothesis has been supported by comparative studies in EAE (Held et al., 1993).
In summary, we were unable to demonstrate a therapeutic benefit of autoimmune vaccination in SCI rats. Instead, myelin-reactive T-cells impaired spontaneous functional recovery and exacerbated tissue injury at and beyond the site of trauma. Based on these data and suspended vaccine trials in multiple sclerosis and Alzheimer's disease (Bielekova et al., 1999; Kappos et al., 2000; Check, 2002), we question the safety of intentionally activating CNS-reactive T-cells as a therapy for SCI. Given the plasticity of anatomical pathways preserved after injury, restoration of useful function through rehabilitation or drug therapy could be thwarted by enhancing the activity of an inherently pathogenic T-cell population.
Footnotes
This work was supported by the National Institute for Neurological Disorders and Stroke, the National Institute on Aging, and an Integrative Immunobiology training program from the National Institute of Allergy and Infectious Diseases. We thank Drs. Dana McTigue and Michael Beattie for critical evaluation of this manuscript and Pat Walters, Qin Yin, Ming Wang, and Ingrid Gienapp for technical contributions to this work.
Correspondence should be addressed to Dr. Phillip Popovich, Department of Molecular Virology, Immunology and Medical Genetics, 2078 Graves Hall, The Ohio State University College of Medicine and Public Health, 333 West Tenth Avenue, Columbus, OH 43210. E-mail: Popovich.2@osu.edu.
DOI:10.1523/JNEUROSCI.0406-04.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/243752-10$15.00/0
References
- Ahmed Z, Baker D, Cuzner ML (2003) Interleukin-12 induces mild experimental allergic encephalomyelitis following local central nervous system injury in the Lewis rat. J Neuroimmunol 140: 109–117. [DOI] [PubMed] [Google Scholar]
- Akassoglou K, Bauer J, Kassiotis G, Pasparakis M, Lassman H, Kollias G, Probert L (1998) Oligodendrocyte apoptosis and primary demyelination induced by local TNF/p55TNF receptor signaling in the central nervous system of transgenic mice. Am J Pathol 153: 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderton SM, van der ZR, Goodacre JA (1993) Inflammation activates self hsp60-specific T cells. Eur J Immunol 23: 33–38. [DOI] [PubMed] [Google Scholar]
- Angelov DN, Waibel S, Guntinas-Lichius O, Lenzen M, Neiss WF, Tomov TL, Yoles E, Kipnis J, Schori H, Reuter A, Ludolph A, Schwartz M (2003) Therapeutic vaccine for acute and chronic motor neuron diseases: Implications for amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 100: 4790–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antel JP, Williams K, Blain M, McRea E, McLaurin J (1994) Oligodendrocyte lysis by CD4+ T cells independent of tumor necrosis factor. Ann Neurol 35: 341–348. [DOI] [PubMed] [Google Scholar]
- Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL (1997) Increased interleukin 12 production in progressive multiple sclerosis: induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci USA 94: 599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139: 244–256. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC (2002) Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: experimental evidence and a review of literature. Restor Neurol Neurosci 20: 189–218. [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12: 1539–1546. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JYF, Porritt MJ, Frerichs F, Donnan GA, Howells DW (1999) Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci 19: 1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, Hallenbeck JM (1997) Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci USA 94: 10873–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann DL, Bresnahan JC, Beattie MS, Shah BR (1992) Spinal cord injury produced by consistent displacement of the cord in rats: behavioral and histologic analysis. J Neurotrauma 9: 197–217. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Kerr S, Rodriguez M (2003) Efficient central nervous system remyelination requires T cells. Ann Neurol 53: 680–684. [DOI] [PubMed] [Google Scholar]
- Bielekova B, Muraro PA, Golestaneh L, Pascal J, McFarland HF, Martin R (1999) Preferential expansion of autoreactive T lymphocytes from the memory T-cell pool by IL-7. J Neuroimmunol 100: 115–123. [DOI] [PubMed] [Google Scholar]
- Birnbaum G, Kotilinek L, Miller SD, Raine CS, Gao YL, Lehmann PV, Gupta RS (1998) Heat shock proteins and experimental autoimmune encephalomyelitis. II: environmental infection and extra-neuraxial inflammation alter the course of chronic relapsing encephalomyelitis. J Neuroimmunol 90: 149–161. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Kuhlmann T, Da Costa C, Bunkowski S, Polak T, Bruck W (2000) Tumour necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlation with demyelinating activity and oligodendrocyte pathology. Glia 29: 366–375. [DOI] [PubMed] [Google Scholar]
- Chan CC, Mochizuki M (1999) Sympathetic ophthalmia: an autoimmune ocular inflammatory disease. Springer Semin Immunopathol 21: 125–134. [DOI] [PubMed] [Google Scholar]
- Chang JT, Shevach EM, Segal BM (1999) Regulation of interleukin (IL)-12 receptor beta2 subunit expression by endogenous IL-12: a critical step in the differentiation of pathogenic autoreactive T cells. J Exp Med 189: 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Check E (2002) Nerve inflammation halts trial for Alzheimer's drug. Nature 415: 462. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ljunggren HG, Zhu SW, Winblad B, Zhu J (2004) Reduced susceptibility to kainic acid-induced excitoxicity in T-cell deficient CD4/CD8(–/–) and middle-aged C57BL/6 mice. J Neuroimmunol 146: 33–38. [DOI] [PubMed] [Google Scholar]
- Conlon P, Oksenberg JR, Zhang J, Steinman L (1999) The immunobiology of multiple sclerosis: an autoimmune disease of the central nervous system. Neurobiol Dis 6: 149–166. [DOI] [PubMed] [Google Scholar]
- Fee D, Crumbaugh A, Jacques T, Herdrich B, Sewell D, Auerbach D, Piaskowski S, Hart MN, Sandor M, Fabry Z (2003) Activated/effector CD4+ T cells exacerbate acute damage in the central nervous system following traumatic injury. J Neuroimmunol 136: 54–66. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH (1995) The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol 132: 220–228. [DOI] [PubMed] [Google Scholar]
- Fisher J, Levkovitch-Verbin H, Schori H, Yoles E, Butovsky O, Kaye JF, Ben Nun A, Schwartz M (2001) Vaccination for neuroprotection in the mouse optic nerve: implications for optic neuropathies. J Neurosci 21: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa U, Peter SV, Lehmann K, Bechmann I, Nitsch R (2000) Axonal damage induced by invading T cells in organotypic central nervous system tissue in vitro: involvement of microglial cells. Brain Pathol 10: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani F, Goodyer CG, Antel JP, Yong VW (2003) Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol 171: 368–379. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS (2003) Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 184: 456–463. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ (1988a) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96: 379–394. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorenson FB, Vesterby A, West MJ (1988b) The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96: 857–881. [DOI] [PubMed] [Google Scholar]
- Hauben E, Butovsky O, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Leibowitz-Amit R, Pevsner E, Akselrod S, Neeman M, Cohen IR, Schwartz M (2000a) Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci 20: 6421–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M (2000b) Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet 355: 286–287. [DOI] [PubMed] [Google Scholar]
- Hauben E, Agranov E, Gothilf A, Nevo U, Cohen A, Smirnov I, Steinman L, Schwartz M (2001a) Posttraumatic therapeutic vaccination with modified myelin self-antigen prevents complete paralysis while avoiding autoimmune disease. J Clin Invest 108: 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Ibarra A, Mizrahi T, Barouch R, Agranov E, Schwartz M (2001b) Vaccination with a Nogo-A-derived peptide after incomplete spinal-cord injury promotes recovery via a T-cell-mediated neuroprotective response: comparison with other myelin antigens. Proc Natl Acad Sci USA 98: 15173–15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KC, Hull TC, Delaney GA, Potter PJ, Sequeira KA, Campbell K, Popovich PG (2002) Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J Neurotrauma 19: 753–761. [DOI] [PubMed] [Google Scholar]
- Held W, Meyermann R, Qin Y, Mueller C (1993) Perforin and tumor necrosis factor alpha in the pathogenesis of experimental allergic encephalomyelitis: comparison of autoantigen induced and transferred disease in Lewis rats. J Autoimmun 6: 311–322. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Cohen JA, Burns JB (1987) A quantitative immunohistochemical comparison of actively versus adoptively induced experimental allergic encephalomyelitis in the Lewis rat. Cell Immunol 109: 272–281. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Sewell DL, Liu F, Sandor M, Forsthuber T, Lehmann PV, Fabry Z (2003) Autoreactive T cells promote post-traumatic healing in the central nervous system. J Neuroimmunol 134: 25–34. [DOI] [PubMed] [Google Scholar]
- Huitinga I, van Rooijen N, de Groot CJ, Uitdehaag BM, Dijkstra CD (1990) Suppression of experimental allergic encephalomyelitis in Lewis rats after elimination of macrophages. J Exp Med 172: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra A, Correa D, Willms K, Merchant MT, Gui, zar-Sahagun G, Grijalva I, Madrazo I (2003) Effects of cyclosporin-A on immune response, tissue protection and motor function of rats subjected to spinal cord injury. Brain Res 979: 165–178. [DOI] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan JZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG (2002) Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci 22: 2690–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Comi G, Panitch H, Oger J, Antel J, Conlon P, Steinman L (2000) Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 6: 1176–1182. [DOI] [PubMed] [Google Scholar]
- Kil K, Zang YCQ, Yang D, Markowski J, Fuoco GS, Vendetti GC, Rivera VM, Zhang JZ (1999) T-cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol 98: 201–207. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M (2002) Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci USA 99: 15620–15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller H, Siebler M, Hartung H-P (1997) Immunologically induced electrophysiological dysfunction: implications for inflammatory diseases of the CNS and PNS. Prog Neurobiol 52: 1–26. [DOI] [PubMed] [Google Scholar]
- Mason JL, Suzuki K, Chaplin DD, Matsushima GK (2001) Interleukin-1beta promotes repair of the CNS. J Neurosci 21: 7046–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautes AE, Noble LJ (2000) Co-induction of HSP70 and heme oxygenase-1 in macrophages and glia after spinal cord contusion in the rat. Brain Res 883: 233–237. [DOI] [PubMed] [Google Scholar]
- Medana I, Martinic MA, Wekerle H, Neumann H (2001) Transection of major histocompatibility complex class I-induced neurites by cytotoxic T lymphocytes. Am J Pathol 159: 809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midha R, Fehlings MG, Tator CH, Saint-Cyr JA, Guha A (1987) Assessment of spinal cord injury by counting corticospinal and rubrospinal neurons. Brain Res 410: 299–308. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M (1999) Autoimmune T-cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med 5: 49–55. [DOI] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW (2000) A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408: 982–985. [DOI] [PubMed] [Google Scholar]
- Olsson T, Diener P, Ljungdahl A, Hojeberg B, van der Meide PH, Kristensson K (1992) Facial nerve transection causes expansion of myelin autoreactive T cells in regional lymph nodes and T cell homing to the facial nucleus. Autoimmun 13: 117–126. [DOI] [PubMed] [Google Scholar]
- Olsson T, Sun JB, Solders G, Xiao BG, Hojeberg B, Ekre HP, Link H (1993) Autoreactive T and B cell responses to myelin antigens after diagnostic sural nerve biopsy. J Neurol Sci 117: 130–139. [DOI] [PubMed] [Google Scholar]
- Pette M, Fujita K, Kitze B, Whitaker JN, Albert E, Kappos L, Wekerle H (1990) Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology 40: 1770–1776. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Stokes BT, Whitacre CC (1996) Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res 45: 349–363. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT (1997) The cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol 377: 443–464. [DOI] [PubMed] [Google Scholar]
- Prochazka M, Voltnerova M, Stefan J (1971) Studies of immunological reactions after brain injury. II. Antibodies against brain tissue lipids after blunt head injury in man. Int Surg 55: 322–326. [PubMed] [Google Scholar]
- Puga Yung GL, Le TD, Roord S, Prakken B, Albani S (2003) Heat shock proteins (HSP) for immunotherapy of rheumatoid arthritis (RA). Inflamm Res 52: 443–451. [DOI] [PubMed] [Google Scholar]
- Rivers TM, Sprunt DH, Berry GP (1933) Observations on attempts to produce disseminated encephalomyelitis in monkeys. J Exp Med 58: 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH (1977) Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg 47: 577–581. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J (2001) Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med 7: 252–258. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Moalem G (2001) Beneficial immune activity after CNS injury: prospects for vaccination. J Neuroimmunol 113: 185–192. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Cohen I, Lazarov-Spiegler O, Moalem G, Yoles E (1999) The remedy may lie in ourselves: prospects for immune cell therapy in central nervous system protection and repair. J Mol Med 77: 713–717. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ (1999) Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodefiecient (scid) mice. J Neurosci 19: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh JY, Leong SK, Wong WC (1983) Origin of the rubrospinal tract in neonatal, developing, and mature rats. J Comp Neurol 214: 79–86. [DOI] [PubMed] [Google Scholar]
- Steinman L (1996) Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85: 299–302. [DOI] [PubMed] [Google Scholar]
- Steinman L (2003) Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J Exp Med 197: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterio DC (1984) The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc 134: 127–136. [DOI] [PubMed] [Google Scholar]
- Strominger RN, McGiffen JE, Strominger NL (1987) Morphometric and experimental studies of the red nucleus in the albino rat. Anat Rec 219: 420–428. [DOI] [PubMed] [Google Scholar]
- Swanborg RH, Shulman S (1970) Purification of an organ-specific thermo-stable antigen found in bovine brain. Immunology 19: 31–40. [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L (1998) Axonal transection in the lesions of multiple sclerosis. N Eng J Med 338: 278–285. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Hill RH, Backstrom E, Robertson B, Kristensson K (2003) Interferon-gamma induces characteristics of central sensitization in spinal dorsal horn neurons in vitro. Pain 106: 241–251. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Zhang J, Witek C, Matsui M, Modabber Y, Ota K, Hafler DA (1994) Clonal expansion and persistence of human T cells specific for an immunodominant myelin basic protein peptide. J Immunol 152: 5581–5592. [PubMed] [Google Scholar]
- Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M (2001) Protective autoimmunity is a physiological response to CNS trauma. J Neurosci 21: 3740–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]