Abstract
In the actively foraging rat, hippocampal pyramidal cells have strong spatial correlates. Each “place cell” fires rapidly only when the rat enters a particular delimited portion of its environment, called the “place field” of that cell. Hippocampal pyramidal cells also exhibit spatial selectivity during a physiological state that occurs during sleep, termed “small irregular activity” (SIA), because of the appearance of the hippocampal EEG. It is not known whether rats determine their current location in space during SIA using current visual information or whether they recall the location in which they fell asleep. To address this question, we recorded spikes from ensembles of CA1 pyramidal cells and hippocampal EEG while rats slept along the edge of a large circular recording arena with minimal local features in a room with prominent distal visual cues. To move the rats to a new location in the room while they were sleeping, we slowly rotated the recording arena on which they slept to a new orientation in the room. Hippocampal place cell activity in subsequent SIA episodes reflected the location in the room in which the rats fell asleep, rather than the location to which they were moved, although the alignment of the rats' spatial map was governed by the room cues in the subsequent active foraging session. Thus, the hippocampal population activity during SIA does not result from the processing of current visual information but instead probably reflects a memory for the location in which the rat fell asleep.
Keywords: sleep, EEG, hippocampus, memory, place cells, theta
Introduction
In the actively foraging rat, hippocampal pyramidal cells have strong spatial correlates. Each “place cell” fires rapidly only when the rat enters a particular delimited portion of its environment, called the “place field” of that cell (O'Keefe and Dostrovsky, 1971; O'Keefe, 1976; O'Keefe and Nadel, 1978). Thus, the spiking activity across a population of simultaneously recorded place cells can be used to estimate the current location of the rat (Wilson and McNaughton, 1993; Zhang et al., 1998). Place fields are controlled by an interaction of visual and self-motion cues. If the visual cues in an environment are rotated, place fields rotate by an equal amount (O'Keefe and Conway, 1978; Muller and Kubie, 1987; Bostock et al., 1991), but only if the rat has learned previously that the cues are stable (Knierim et al., 1995; Jeffery and O'Keefe, 1999). The activity across the hippocampal population is thought to reflect the rat's internal representation of its location in space, because errors in the rat's behavior correspond to misalignments in place cell firing (O'Keefe and Speakman, 1987; Lenck-Santini et al., 2002; Rosenweig et al., 2003).
As shown by Jarosiewicz et al. (2002), hippocampal pyramidal cells also exhibit place-specific firing during a physiological state that occurs during sleep, termed “small irregular activity” (SIA), after the appearance of the hippocampal EEG (Vanderwolf, 1971; Whishaw, 1972). Each SIA episode, lasting on the order of a few seconds and occurring approximately one to two times per minute, is characterized by an abrupt flattening of the hippocampal EEG and a sudden change in the hippocampal population activity: most CA1 pyramidal cells fall silent, except for a small subset of cells that become very active. These active cells generally correspond to place cells whose place fields are in the location in which the rat is sleeping (Jarosiewicz et al., 2002), suggesting that SIA is a state of heightened arousal during which the rat's current location in space is represented in the brain. Consistent with this interpretation, SIA is accompanied by neocortical EEG desynchronization and can be elicited in a sleeping rat by playing an auditory stimulus (Jarosiewicz and Skaggs, 2004).
These findings raise the question of whether the place-related activity during SIA results from the processing of current sensory information or from the reactivation of a memory for the location in which the rat fell asleep. To address this question, rats were allowed to fall asleep along the edge of a circular recording arena with minimal local features in a room with prominent distal visual cues. While the rat slept, the arena was slowly rotated clockwise ∼90°, moving the rat to a new location in the room without waking it. The population activity during SIA episodes after the rotation was found to reflect the location in the room in which the rat fell asleep, not the location to which it was moved, even though the alignment of the spatial map was governed by the room cues during active foraging before and after the sleep session. Thus, the place-related activity in SIA is likely to reflect a memory for the location in which the rat fell asleep, rather than the processing of current visual information.
Materials and Methods
Subjects. Data were collected from six male Sprague Dawley rats, weighing between 350 and 500 gm at the time of surgery. Each rat was housed individually in a 12 hr light/dark cycle in a temperature-controlled room with food and water available ad libitum. For 1-2 weeks before surgery, each rat was handled and placed in the recording arena for several hours per day to sleep and to forage for randomly scattered sweetened food pellets. Rats were food deprived to ∼95% of their ad libitum weight to motivate them to forage for food pellets when they were available. All handling and recording were done during the light phase of the cycle.
Surgery. All surgery was performed under sterile conditions. Rats were anesthetized with ketamine (60 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.), and boosts of ketamine and xylazine were given during surgery as necessary. Once deeply anesthetized, rats were secured in ear bars in a Kopf stereotaxic frame (David Kopf Instruments, Tujunga, CA). A small (∼1 cm) incision was made along the midline of the scalp to expose the cranium. Skin and connective tissue were retracted, and seven small holes were drilled into the cranium to accommodate jeweler's screws, one of which was later connected to a ground channel.
A small hole was drilled over the frontal cortex (∼1 mm diameter, centered on 2 mm anterior, 2 mm lateral from bregma) to accommodate a bipolar neocortical EEG recording electrode, consisting of a twisted pair of 0.0045 inch (coated) stainless steel wires with ends spaced 2 mm apart vertically. An EMG electrode, consisting of a twisted pair of 0.0045 inch (coated) stainless steel wires, each with 1 mm exposed at the tip and bent 2 mm back to form a hook, was inserted into the dorsal neck musculature by routing it under the skin from the incision. A few square 10 mA pulses of 1 msec duration were passed through the EMG wires at 1 Hz using a stimulus isolation unit and a Grass S88 stimulator to check for muscle twitch to ensure proper placement of the wires. Another larger hole was drilled over the right hippocampus (∼2 mm diameter, centered on 3.5 mm posterior, 2-3 mm lateral from bregma). The dura was retracted, and the exposed cortex was covered with sterilized petroleum jelly. The base of a Kopf “hyperdrive,” which contained 12 individually drivable tetrodes (McNaughton et al., 1983b; Recce and O'Keefe, 1989) and two single-channel reference/EEG electrodes all bundled to ∼1.5 mm diameter at the base, was lowered toward the exposed cortex. In addition, a small hole was drilled at 0.5 mm anterior, 4.0 mm lateral from bregma to accommodate a 26 gauge guide cannula (Plastics One, Roanoke, VA), which entered the brain at a 30° angle mediolaterally, its tip inserted to within 1 mm of the medial septum/diagonal band of Broca. These cannulas were used for microinfusion studies not reported in this paper; no animal received microinfusions before or during data collection for the studies reported here. All implants were cemented in place with dental acrylic, which was anchored to the cranium by jeweler's screws.
Just after surgery, the tetrodes and reference electrodes were lowered ∼680 μm toward the hippocampus, and the wound was covered with antibiotic ointment and a mild local anesthetic ointment. Over the next few days, the wound was cleaned, and ointment was reapplied daily until the animal recovered. Tetrodes were gradually lowered over a few hours each day until they arrived at the hippocampal CA1 pyramidal cell layer (∼2 mm deep), which was identified by its well characterized EEG and spike waveform characteristics (Ranck, 1973; Fox and Ranck, 1975, 1981; O'Keefe, 1976; O'Keefe and Nadel, 1978; McNaughton et al., 1983a; Buzsáki et al., 1992; Skaggs et al., 1996).
Behavioral apparatus. Once stable cells and good EEG signals were obtained, recordings were performed while rats foraged for randomly scattered food pellets (“run 1”), slept, and then foraged again (“run 2”) on a circular elevated arena (∼1.5 m diameter) with a 40-cm-high transparent border around the edge. To minimize local cues and maximize distal cues, uniformly colored and textured vinyl flooring was installed in the recording arena, and the arena was placed in a soundproof room with large visual cues on the walls. Before each pretraining and recording session, the arena was cleaned and rotated to a new random orientation, but the distal cues always remained stable. Any local cues that the rat deposited before the rotation were promptly removed.
Run sessions began when a ceiling-mounted feeder was turned on, dropping sweetened food pellets onto the arena every 28 sec. After ∼20 min of run 1, the feeder was turned off, and the rats usually fell asleep spontaneously somewhere along the wall of the arena. The rat was allowed to sleep in this location for ∼10-15 min, and then the arena was rotated ∼90° for ∼10-15 min via a silent motor-driven wheel abutting the edge of the arena, controlled from the adjoining room, moving the rat to a new location in the room. The rat was allowed to continue sleeping in this new location for another ∼10-15 min. The food pellets were then turned back on, and the rat was allowed to forage for another ∼20 min (run 2). Arena rotations during which the rat woke up and started walking around were terminated; if the rat subsequently went back to sleep, another arena rotation was attempted. Each rotation was carefully documented, and the total amount of rotation could be deduced from the position records.
Electrophysiology and recording. For data acquisition, the top of the hyperdrive was connected to a headstage containing preamplifiers and a ring of light-emitting diodes used for position tracking by a camera mounted on the ceiling over the recording chamber. The headstage was attached to a pair of soft, flexible cables, partially suspended by a counterweight system to help ease the load on the rat's head. The cables ascended through the ceiling of the recording chamber into the adjoining room, where they connected to the Cheetah recording system (Neuralynx, Tucson, AZ), consisting of eight eight-channel amplifiers with software-configurable high- and low-pass filters, feeding their output to a custom-made controller and analog-to-digital processor. During recording, signals from each channel of each tetrode were filtered to 600-6000 Hz, sampled at 32 kHz per channel, formatted, and fed to a Windows NT system (Neuralynx) running custom-written acquisition and control software. Each time the signal on any one of the tetrode channels crossed a specified threshold, a 1 msec sample of data from all four channels of that tetrode was written to disk, beginning 0.25 msec before the threshold was crossed, capturing the spike waveform on each channel along with its timestamp. Continuous recordings were also obtained of EEG signals from one channel on each tetrode, from an EEG electrode near the hippocampal fissure, from an EEG electrode in the prefrontal cortex, and from an EMG electrode in the dorsal neck musculature, at a bandwidth of 1-475 Hz (EEGs) or 100-475 Hz (EMG) and a sampling rate of 999 Hz. At the same time, position records containing information about the distribution of light across the video image were acquired at 60 Hz and written to disk. The rat's velocity was estimated as the change in position two timestamps before and two timestamps after the current timestamp, divided by the elapsed time. The error of the tracker was approximately one-half the width of the ring of light-emitting diodes on the headstage, or 2.5 cm.
Once an adequate number of stable CA1 complex-spike cells were obtained and robust theta activity was visible on the hippocampal EEG electrode during locomotion, a recording session was performed. EEG and EMG signals, spike waveforms, and the position of the rat were recorded simultaneously while the rat slept and ran for randomly scattered food pellets. Before the rotation experiments began, each rat underwent one to three recording sessions, each on separate days, in which auditory stimuli were played during sleep (this was part of a different experiment, the results of which are not presented here). The rotation experiment began after these experiments. Rotation experiments were performed as often as possible for each rat until good cells could no longer be obtained, the rat stopped sleeping well, or the rat otherwise became unusable, at which point it was killed and its hyperdrive was removed for reuse.
Cell isolation. Spike waveforms, EEG and EMG signals, and the rat's position data, along with the respective timestamps, were stored onto disk during the recording session for off-line analysis. Spikes were assigned to individual units by automated cluster-cutting software (Klustakwik, K. D. Harris, Rutgers University, Newark, NJ), and clusters were then manually verified and cleaned using Mclust (A. D. Redish, University of Minnesota, Minneapolis, MN). Isolated units were judged to be pyramidal cells or interneurons (Fox and Ranck, 1981) or artifact according to their average waveforms, autocorrelograms, interspike interval histograms, etc.; only those units judged to be relatively clean, well isolated pyramidal cells were included in further analysis.
Data analysis. SIA episodes were delineated as follows: the hippocampal EEG from the entire sleep session was divided into 500 msec bins, and if the hippocampal EEG amplitude was lower than a specified threshold, the bin was classified as SIA. This threshold was determined separately for each data set by calculating the 20th percentile of the hippocampal EEG amplitude distribution from the sleep session and visually finding a local minimum near that value in the amplitude distribution, because SIA was previously found to occupy ∼20% of sleep (Jarosiewicz et al., 2002).
To construct “correlation maps,” a mean SIA population activity vector was constructed by calculating the mean firing rate of each cell during all SIA episodes occurring before the arena rotation (“pre-rotation SIA”), and another mean SIA population vector was calculated for the group of SIA episodes occurring after the arena rotation (“post-rotation SIA”). A mean population activity vector was also calculated for each pixel of the environment by determining the mean firing rate of each cell across the entire run session in that pixel, using the adaptive binning method of Skaggs et al. (1996). One such set of vectors was made for run 1, and one set was made for run 2. Then, for each pixel in the environment, the correlation coefficient was calculated between the SIA population activity vector and the mean run population activity vector in that pixel, and the result was a correlation map reflecting the similarity between the population activity during SIA and the population activity in each location in the environment. Thus, four correlation maps were created for each data set, one for each combination of pre-rotation SIA and post-rotation SIA with run 1 and run 2. Peaks in these maps represent a reconstruction of the rat's internal representation of its current location in space during SIA, as determined by the relative activity of its hippocampal place cells.
Bayesian reconstruction was used to determine when the rat's internal representation of its location in space, as reflected by the firing of its hippocampal place cell population, reverted to room coordinates after the rat awakened in the new location. The rat's internal representation of its position x = (x,y) given the numbers of spikes fired by the recorded cells n = (n1, n2,..., nN) in each time bin τ was determined using the standard formula of conditional probability:
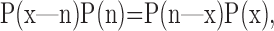 |
1 |
where P(x), the probability for the rat's internal spatial representation to be in position x, was assumed to be uniform over the arena; P(n), the probability for the numbers of spikes n to occur, was determined by normalizing P(x|n) over x, and P(n|x), the probability for the numbers of spikes n to occur given that the animal is at location x, was determined by the firing rate maps of those cells from the preceding waking period (run 1). Assuming that the spike trains are Poisson distributed and that place cells are statistically independent of one another (Zhang et al., 1998):
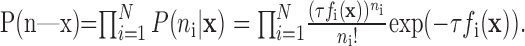 |
2 |
P(x|n) was then calculated by inserting these expressions into Equation 1, and the peak of this probability distribution:
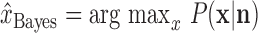 |
3 |
was taken as the rat's internal representation of its current location.
Results
Of 19 recording sessions from the six rats, 10 were successful in that the rat remained asleep during the rotation and for at least 10 min afterward, and robustly SIA-active cells were present both before and after the rotation. It was difficult to obtain multiple successful experiments from a single rat, because rats were less likely to sleep soundly on the arena on days subsequent to a successful rotation. Only one successful data set was obtained from each of four of the rats (p158-05, p163-14, p169-08, and p175-08); however, five successful data sets were obtained from one particularly sound sleeper (p181-02, -06, -07, -10, and -13). Results did not differ between p181 and the other four rats, or across data sets from rat p181, so results from all nine of the above data sets were included as independent samples in the analysis. In one data set (p159-09), the cell activity profile during run 2 was distinctly different from that of the other data sets: the place fields rotated with the arena rather than remaining aligned with the room cues. Although the results from this data set are consistent with the other data sets, the fact that its control condition was not satisfied precluded the rest of its analysis. Thus, its case is discussed separately below.
SIA population activity reflects memory reactivation
Correlation maps peak in old location of rat
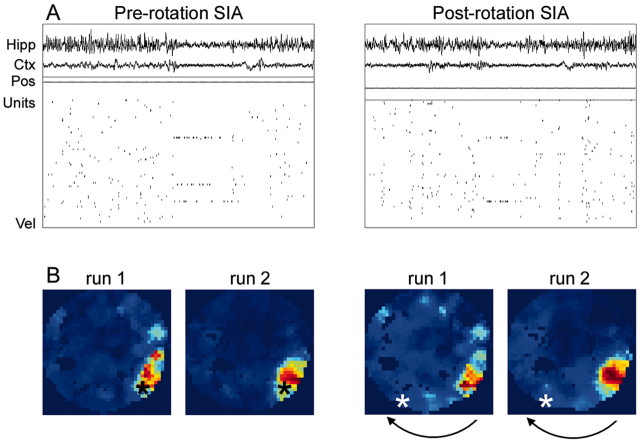
Figure 1A shows an example of EEG and cell ensemble activity during SIA before and after the rotation during sleep for one data set (p181-10). In this example, two of the three cells that were active during SIA before the rotation were also active during SIA after the rotation. Changes in the SIA population activity sometimes occurred when the rat made slight shifts in its position; thus, correlation maps were constructed to show that even when the SIA population activity changed during a sleep epoch, it still represented the location in which the rat fell asleep and not the location to which it was rotated. Figure 1B shows the set of correlation maps from this data set. Consistent with previous findings (Jarosiewicz et al., 2002), during SIA before the arena rotation, the rat's representation of its current location closely matched its actual location (Fig. 1B, left two panels). After the arena was rotated, moving the rat to a new location in the room, however, the population activity during subsequent SIA episodes continued to reflect the location in which the rat fell asleep, rather than its new location (Fig. 1B, right two panels). There was a strong correlation between the entries in the pre-rotation and post-rotation correlation maps whether the run population activity vectors were taken from run 1 (r = 0.906) or run 2 (r = 0.965), verifying that the distal room cues governed the hippocampal place fields during the run sessions. Thus, this rat did not update its current spatial representation on the basis of current visual input during SIA.
Figure 1.
Effect of arena rotation on place representation during SIA: results from one recording session (p181-10). A, Each panel contains a 20 sec sample of hippocampal EEG (Hipp), neocortical EEG (Ctx), the rat's x and y coordinates (Pos; arbitrary scale), spikes from 73 simultaneously recorded CA1 pyramidal cells (Units), and the rat's velocity (Vel; arbitrary scale, with zero aligned at the bottom of the panel) from a single sleep epoch. During SIA, the hippocampal and neocortical EEG abruptly flatten, and most hippocampal CA1 pyramidal cells become quiet, except for a small subset of cells having place fields that generally encompass the location in which the rat is sleeping. The left panel shows an SIA episode occurring before the rotation (Pre-rotation SIA). The right panel shows an SIA episode occurring after the rotation (Post-rotation SIA). In this example, two of the three cells that were active in pre-rotation SIA were also active in post-rotation SIA. B, In each correlation map, the correlation coefficient between the mean population activity vector across all pre-rotation SIA episodes (left two panels) or all post-rotation SIA episodes (right two panels) and the mean population activity vector during run before the sleep session (run 1) or during run after the sleep session (run 2) were plotted for each pixel of the arena. The direction of the arena rotation is indicated by the black arrows. Blue areas in the map correspond to low correlation values, and red areas correspond to high correlation values. Peaks in the correlation maps correspond to areas in the arena where the population activity during run most strongly resembles the population activity during SIA, and thus presumably reflects the rat's internal representation of its location. The asterisk represents the rat's actual location (the color of the asterisk is varied solely for visibility). The rat's representation of its current location during SIA before the rotation closely matched its actual location, but after the arena was rotated, moving the rat to a new location, the population activity during subsequent SIA episodes continued to reflect the rat's original location in the room rather than its new location.
To show that this finding was consistent across data sets, the correlation maps from all data sets were rotationally aligned on the actual location of the rat and averaged together (Fig. 2). The peak in the mean aligned correlation map occurred in the location in which the rats fell asleep (Fig. 2, left two panels) and not in the location to which they were rotated (Fig. 2, right two panels), supporting the hypothesis that the population activity during SIA reflects a memory for the location in which the rat fell asleep rather than an updated spatial representation based on current sensory input. All of the data sets in this analysis showed a strong correlation between the pre-rotation and post-rotation correlation maps whether the run population activity vectors being compared with the mean SIA population activity vectors were taken from run 1 (mean and SEM across data sets: r = 0.744 ± 0.096) or run 2 (r = 0.808 ± 0.068), verifying that the distal cues governed the rat's spatial representation during active foraging.
Figure 2.
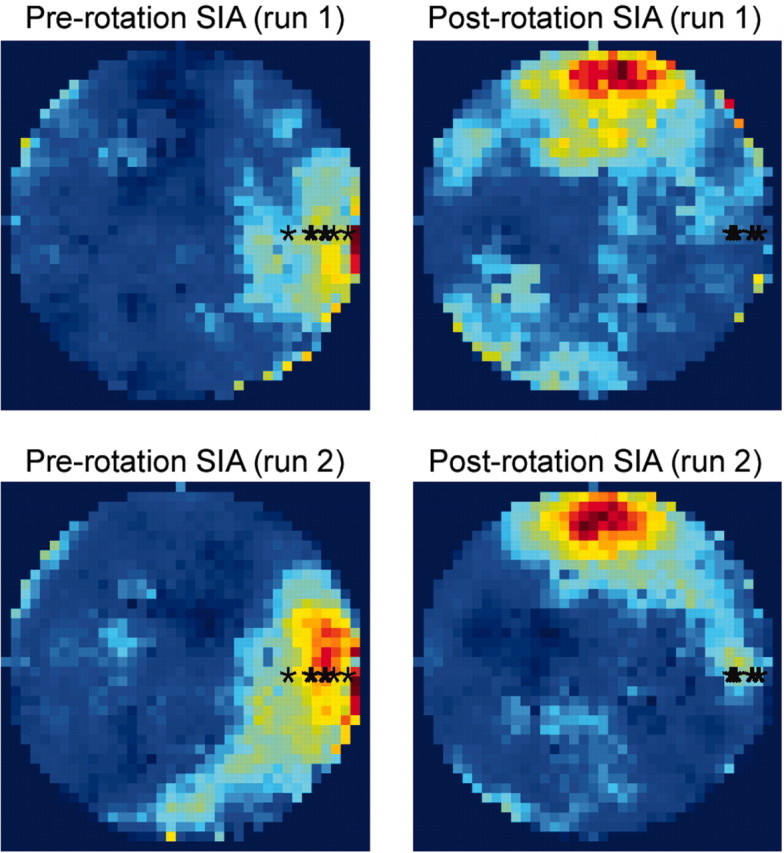
Effect of arena rotation on place representation during SIA: average results across data sets. Correlation maps from nine data sets from five different rats were rotationally aligned on the actual location of the rat (denoted by the black asterisks) and averaged together. Consistent with the data from the example recording session shown in Figure 1, on average, the peak in the mean aligned correlation map occurred in the location in which the rat fell asleep (left two panels) and not in the location to which the rat was rotated (right two panels).
Population activity is similar in pre-rotation and post-rotation SIA
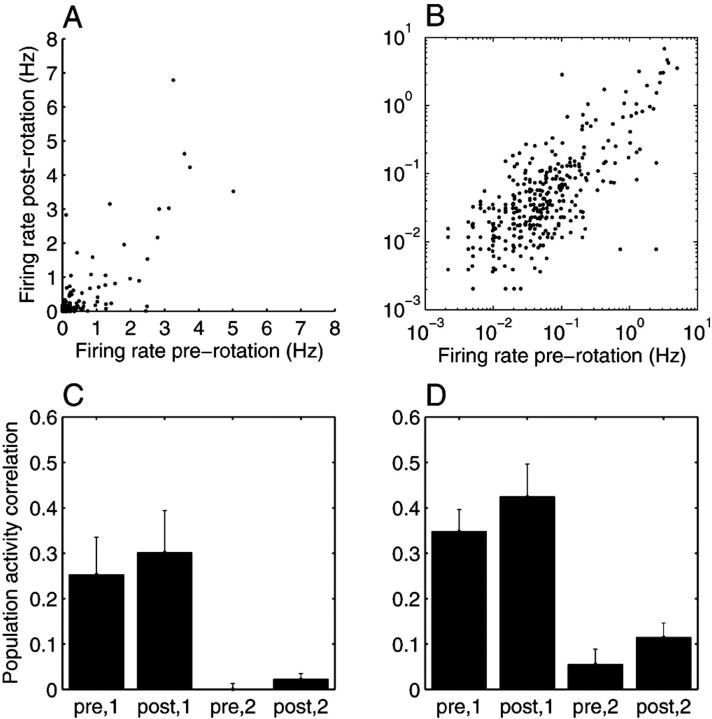
Figure 3, A and B, shows the mean firing rate of each cell during SIA before the rotation against its mean firing rate during SIA after the rotation, using all 631 cells from the nine data sets. In general, cells that were active in SIA before the rotation were also active in SIA after the rotation (mean and SEM across data sets: r = 0.747 ± 0.095).
Figure 3.
Quantification of results. A, B, Hippocampal population activity during SIA before and after the arena rotation. A, The mean firing rate of each cell during SIA before the rotation is plotted against its mean firing rate during SIA after the rotation, using all 631 cells from the nine data sets. In general, cells that were active in SIA before the rotation were also active in SIA after the rotation. B, The same data as in A are plotted on a log-log scale to show that even the firing rates of cells that were not very active during SIA (less than ∼1 Hz) were similar in pre-rotation and post-rotation SIA. Grouping together all cells from all data sets, the correlation between the mean pre-rotation SIA and the mean post-rotation SIA population activity vectors was 0.808. The mean ± SEM of the correlation for each individual data set was 0.747 ± 0.095. C, D, For each data set, mean population activity vectors were constructed for pre-rotation SIA, post-rotation SIA, active foraging in the pixel of the arena in which the rat fell asleep, and active foraging in the pixel of the arena to which the rat was rotated, and the correlation coefficient was calculated for each combination of vectors from SIA and active foraging. The means and SEMs of these correlations are plotted for run 1 (C) and run 2 (D). The correlation between the post-rotation SIA vector and the population activity in the location in which the rat fell asleep (post,1) was significantly higher than the correlation between post-rotation SIA and the population activity in the location to which the rat was rotated (post,2), using run population activity vectors from either run 1 (p = 0.01) or run 2 (p = 0.001).
Post-rotation SIA population activity is more similar to that of the rat's old location than its new location
Because SIA-active cells sometimes change over time during a single sleep session, and because place cells can have multiple place fields, a more decisive analysis than the correlation between the pre-rotation and post-rotation SIA population activity vectors is the correlation between the post-rotation SIA population activity vector on the one hand and the run population activity vector in the rat's original or new location on the other (Fig. 3C,D). If the population activity during SIA reflects a memory for the location in which the rat fell asleep, then the correlation between the post-rotation SIA population activity vector and the run population activity in the spot in which the rat fell asleep (“post,1”) should be higher than the correlation between post-rotation SIA vector and the run population activity vector in the spot to which the rat was moved (“post,2”). Indeed, post,1 (grand mean ± SEM = 0.301 ± 0.093 for run 1; 0.424 ± 0.072 for run 2) was significantly higher than post,2 (0.023 ± 0.012 for run 1; 0.115 ± 0.032 for run 2) when using the population activity vectors from either run 1 (p = 0.01) (Fig. 3C) or run 2 (p = 0.001) (Fig. 3D).
Awakening and reorientation to the distal visual cues
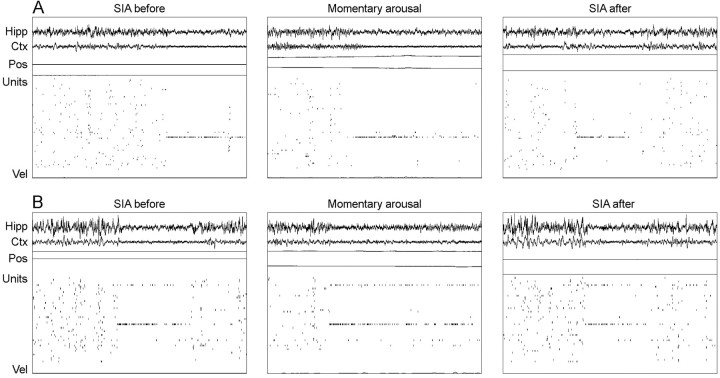
Rats occasionally made small head movements and postural shifts during a sleep session, usually returning to sleep within a few seconds. When these momentary arousals occurred during an arena rotation, the population activity during subsequent SIA episodes was not affected. The same population activity occurred as before the arousal (Fig. 4), indicating that brief arousals without perambulation were not sufficient for the rat to reorient its spatial map to the distal visual cues; however, if the arousals were followed by perambulation, as they were at the end of post-rotation sleep sessions, hippocampal population activity realigned with the distal visual room cues shortly after the rat began walking around (Fig. 5).
Figure 4.
Examples of momentary arousals during arena rotations. Each panel contains a 20 sec sample of hippocampal EEG (Hipp), neocortical EEG (Ctx), the rat's x and y coordinates (Pos; arbitrary scale), spikes from an ensemble of simultaneously recorded CA1 pyramidal cells (Units), and the rat's velocity (Vel; arbitrary scale, with zero aligned at the bottom of the panel). Each horizontal set of three panels shows an SIA episode before the rotation (SIA before), the population activity during a momentary arousal occurring during the arena rotation (Momentary arousal), and an SIA episode after the momentary arousal (SIA after) from a single recording session. A, From p169-08 (77 U). The arena had been rotated ∼75° at the time of the momentary arousal. B, From p181-02 (48 U). The arena had been rotated ∼40° at the time of the momentary arousal. Momentary arousals did not affect the population activity during SIA, indicating that they were insufficient to cause rats to reorient their internal spatial map to the distal visual cues.
Figure 5.
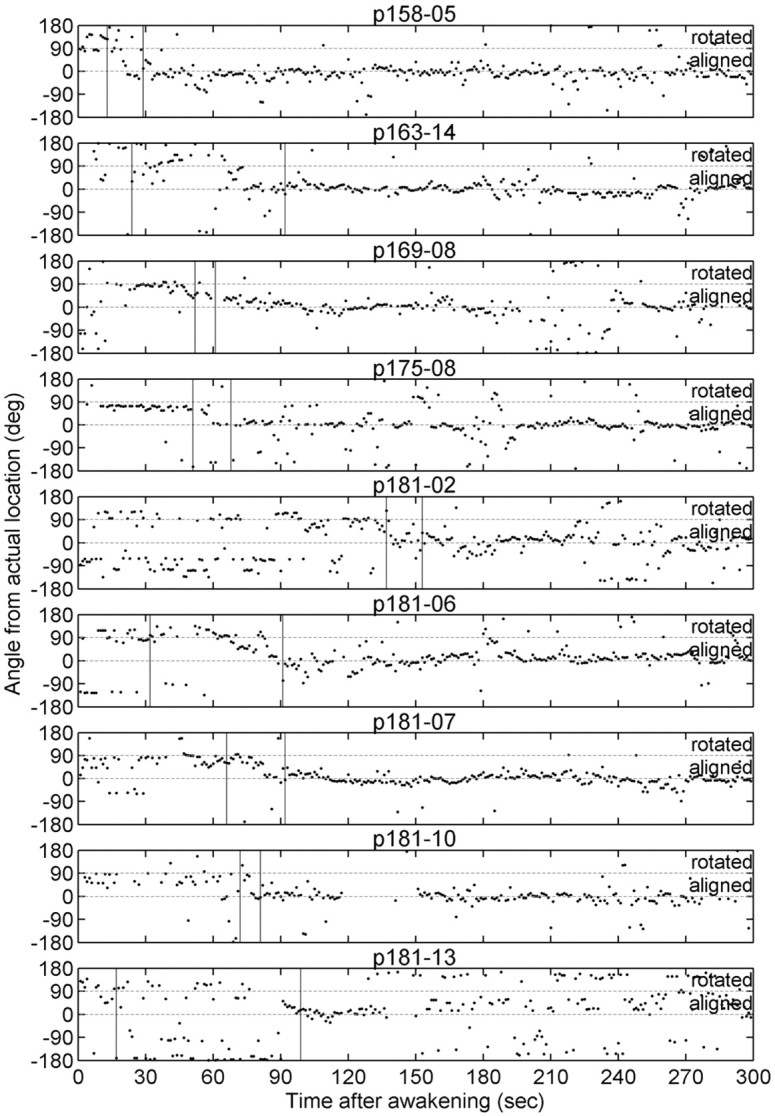
Reorientation to the distal room cues after full awakening. To determine at what point the rat's spatial map realigned with the distal room cues after full awakening (i.e., the last arousal before the rat began walking around), Bayesian reconstruction was used to determine the rat's internal representation of its current location in space, as described in Materials and Methods, and the angle on the arena between the rat's actual and reconstructed position was calculated and plotted for each data set as a function of time after awakening (bin size = 1 sec; only bins in which at least 10 spikes occurred and at least 3 cells fired were used in the reconstruction). In each of these data sets, the arena had been rotated 90° clockwise during sleep; thus, the moment that the rat realigned its spatial representation with the distal visual cues is marked by a change in the angle between its real and reconstructed position from +90 to 0°. The first vertical line in each panel marks the moment the rat walked at least 10 cm away from the location in which it awoke, and the second vertical line represents the moment the rat walked at least 50 cm away; in most data sets, the rat realigned its spatial map within this time interval. Thus, the realignment appears to occur shortly after the rat begins walking through its environment.
Case of the anomalous rat whose spatial map remained rotated with the arena in run 2
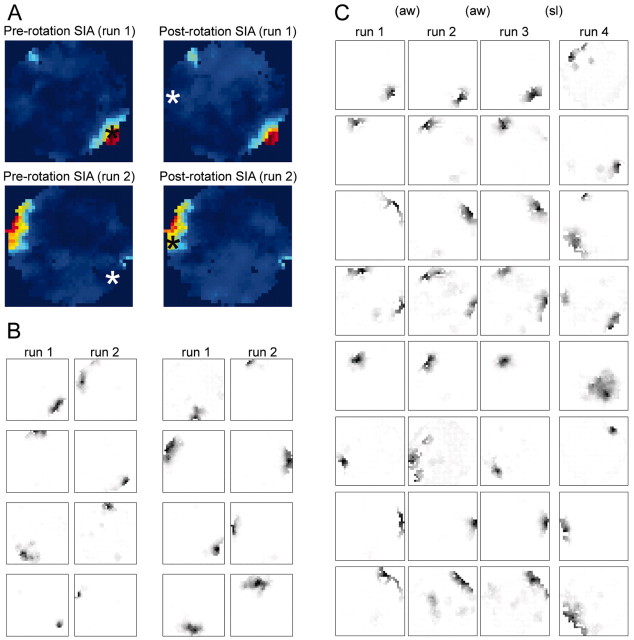
In one anomalous data set (p159-09), not included in the rest of the analysis, the place fields never realigned with the distal visual cues during run 2. That is, the place fields obtained from the entire run 2 session were rotated ∼150° from their locations in run 1 (Fig. 6A,B), corresponding to the amount by which the arena had been rotated during sleep. Thus, the main comparison in the data analysis, between the population activity during SIA and the place-related population activity at the location in which the rat fell asleep versus at the location to which it was rotated, was meaningless for this rat, because these two places were equivalent. This rat was consistent with the other rats in that its population activity pattern during SIA did not change when the arena was rotated, but the conclusion that could be drawn from this finding in the other rats, that different factors control place-related activity during active waking versus during SIA, could not be drawn for this rat. Moreover, the observation that the external cues were not sufficient to govern this rat's spatial map forces us to consider the possibility that other factors, such as local cues, may have been relevant for the other rats as well.
Figure 6.
Results from an anomalous rat whose place fields remained rotated with the arena in run 2. A, Correlation maps from data set p159-09; same format as Figure 1 B, except that the arena in this data set was rotated ∼150° instead of 90°. B, Representative place field maps from eight of the simultaneously recorded CA1 pyramidal cells from run 1 (20 min) and run 2 (23 min). Consistent with the other rats, the post-rotation SIA population activity continued to reflect the rat's original location; however, unlike all other rats, this rat's place fields remained aligned with the arena coordinates during run 2, rather than realigning with the distal room cues. C, To test whether this rat always aligned its spatial map with the arena frame rather than the room frame, we rotated the arena while this rat ran and slept in a single recording session (data set p 159-10). Representative place field maps from eight of the simultaneously recorded cells are shown for each of the stable run periods, run 1-run 4, each lasting ∼5 min. Two 180° rotations occurred while the rat was awake and actively foraging for food pellets (aw): one between run 1 and run 2 and the other between run 2 and run 3. A third 180° rotation occurred while the rat was sleeping (sl), between run 3 and run 4. When the arena was rotated during active waking, the place fields remained aligned with the room coordinates, but when the arena was rotated during sleep, the place fields in the subsequent run period remained rotated with the arena as they did in data set p159-09. Data from this anomalous rat were not used in the other analyses.
To give more insight into why this rat's place fields did not revert to the room cue frame, it was subjected to an additional manipulation in which three rotations were performed during a single recording session, two during run and one during sleep. The rat was allowed to run on the motionless arena for at least 5 min before and after each rotation so that place fields could be mapped. Figure 6C shows eight representative place field maps from each of the inter-rotation periods. When the arena was rotated during run, the place fields remained aligned with the room coordinates, but when the arena was rotated during sleep, the place fields rotated with the arena. Thus, this rat did not always align its spatial map with the arena; it only did so when the arena was rotated during sleep. The possible implications of these results are considered in Discussion.
Discussion
When rats were moved to a new location in the room during sleep, the population activity in subsequent SIA episodes was found to reflect the location in which the rats fell asleep, rather than the location to which they were moved, even though the place fields were controlled by visual room cues during active waking before and after the sleep session. The simplest interpretation is that the place-related activity in SIA does not arise from the processing of current sensory information but from a reactivation of the memory for where the rat fell asleep.
Another possibility is that rats used current sensory information to determine their location during SIA, but that instead of using the prominent distal visual cues, the rats used whatever minimal local arena cues were still available to them despite our efforts to eliminate them. It is likely that some local arena cues were available to the rats, because one rat was able to keep its spatial map aligned with the rotated arena for 23 min in run 2, despite the conflict between the orientation of the arena and the orientation of the distal visual room cues. The appearance and texture of the arena were rotationally uniform, so it is unlikely that this rat was able to use visual or texture cues to maintain the alignment of its spatial map with the arena, but it might have been able to deposit and use local olfactory cues. In support of this possibility, there is evidence that rats can use local olfactory cues to guide navigation when visual information is absent (Lavenex and Schenk, 1998; Maaswinkel and Whishaw, 1999; Wallace et al., 2002), and rats are more impaired at navigating in the dark when local olfactory cues are shuffled than when they are stable (Stuchlik et al., 2001; Stuchlik and Bures, 2002); however, rats are more likely to use visual rather than olfactory information if both are available and the two sets of cues are in conflict (Lavenex and Schenk, 1995; Maaswinkel and Whishaw, 1999), as was the case for five of the six rats in the present study. In a follow-up experiment, the anomalous rat was found to align its spatial map with the arena in subsequent run periods when the arena had been rotated during sleep, but with the room when the arena had been rotated during active foraging. It is not clear why this rat used local cues to align its spatial map in the subsequent run session after the arena had been rotated during sleep, but its results are consistent with the other rats in that its hippocampal population activity during SIA did not reflect the processing of current visual information.
Nevertheless, if rats are able to use local olfactory cues to determine their current location during active waking, it is possible that they are able to use local olfactory cues to determine their current location during SIA. In line with this concept, some aspect of the rats' perception, or perceptual attention, would have been altered during SIA, such that nearby cues became more salient than distal cues, and they aligned their maps with local cues in SIA but distal cues (in most cases) during active waking. This possibility would still require the reactivation of a memory during SIA for the association between the deposited olfactory cues and the location in which they were deposited; thus, even if rats do use local olfactory cues to determine their current location in space during SIA, they must also be using memory. The issue that distinguishes this possibility from the original memory reactivation interpretation, then, is whether the cues used to reactivate the memory for the location in which the rat fell asleep are external (e.g., distal visual cues or local olfactory cues) or internal. The present study has eliminated the possibility that rats use distal visual information as external cues to determine their location during SIA. One way to eliminate the possibility that the rats use local olfactory cues would be by repeating this study in anosmic rats. If the SIA-active cells after the rotation still reflect the location in which the rats fell asleep rather than the location to which they were rotated, then the rats cannot be using olfactory cues to determine their current location in space during SIA. This finding would provide strong evidence that the population activity during SIA reflects an internally activated memory for the location in which the rats fell asleep.
If sleeping rats maintain a memory for their current location in space, where is this memory stored? The hippocampal population activity does not contain any obvious information about the rat's sleeping location during rapid eye movement (REM) and slow-wave sleep (SWS); however, mean firing rates of hippocampal cells tend to covary across physiological states (Csicsvari et al., 1999; Hirase et al., 2001), suggesting that context information might be preserved between waking and sleep. Patterns of hippocampal activity during REM and SWS resemble patterns present during the immediately preceding waking period more than expected by chance (Wilson and McNaughton, 1994; Skaggs and McNaughton, 1996; Louie and Wilson, 2001); thus, it is possible that the information about the rat's spatial history contained in these reactivated patterns is enough to reconstruct its current location at SIA onset. This hypothesis would predict that if the path represented by the hippocampal population activity just before SIA onset were reconstructed with a small enough temporal grain, it would reflect a trajectory to the rat's current location. Another possibility is that the rat's current spatial location is stored within the hippocampus as a “latent attractor” (Doboli et al., 2000), not evident in the patterns of neural activity but available in the short-term synaptic structure of the network, that can be reactivated when the animal is aroused. This memory reactivation could be aided by local sensory cues that had been associated with that location before the rat fell asleep, such as the rat's sleeping position and the resulting pressure of the floor on various parts of its body, local olfactory cues, etc.
It is important for a sleeping animal to maintain in memory the context in which it is sleeping. For example, people need to sleep differently on the top bunk of a narrow bunk bed than on their king-sized bed at home, and rats need to remember their location relative to nearby passages through which potential predators can enter or through which they can escape from danger if necessary. SIA might provide the neural substrate for such context sensitivity during sleep.
Footnotes
This work was supported by National Science Foundation Grant IRI-9720350 (W.E.S.), the University of Pittsburgh, the Center for the Neural Basis of Cognition, and an Andrew Mellon Predoctoral Fellowship (B.J.). We thank Bruce McNaughton for his comments on a previous draft of this manuscript.
Correspondence should be addressed to Beata Jarosiewicz, Department of Neurobiology and Center for the Neural Basis of Cognition, University of Pittsburgh, 245 McGowan Center, 3025 East Carson Street, Pittsburgh, PA 15203. E-mail: beata@cnbc.cmu.edu.
W. E. Skaggs's present address: California Regional Primate Research Center, Road 98 and Hutchison Drive, Davis, CA 95616.
Copyright © 2004 Society for Neuroscience 0270-6474/04/245070-08$15.00/0
References
- Bostock E, Muller RU, Kubie JL (1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193-206. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horvath Z, Urioste R, Hetke J, Wise K (1992) High-frequency network oscillation in the hippocampus. Science 256: 1025-1027. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G (1999) Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci 19: 274-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doboli S, Minai A, Best PJ (2000) Latent attractors: a model for context-dependent place representations in the hippocampus. Neural Comput 12: 1009-1043. [DOI] [PubMed] [Google Scholar]
- Fox SE, Ranck Jr JB (1975) Localization and anatomical identification of theta and complex spike cells in dorsal hippocampal formation of rats. Exp Neurol 49: 299-313. [DOI] [PubMed] [Google Scholar]
- Fox SE, Ranck Jr JB (1981) Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res 41: 399-410. [DOI] [PubMed] [Google Scholar]
- Hirase H, Leinekugel X, Czurkó A, Csicsvari J, Buzsáki G (2001) Firing rates of hippocampal neurons are preserved during subsequent sleep episodes and modified by novel awake experience. Proc Natl Acad Sci USA 98: 9386-9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosiewicz B, Skaggs WE (2004) Level of arousal during the small irregular activity state in the rat hippocampal EEG. J Neurophysiol 92: 2649-2657. [DOI] [PubMed] [Google Scholar]
- Jarosiewicz B, McNaughton BL, Skaggs WE (2002) Hippocampal population activity during the small-amplitude irregular activity state in the rat. J Neurosci 22: 1373-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery KJ, O'Keefe JM (1999) Learned interaction of visual and idiothetic cues in the control of place field orientation. Exp Brain Res 127: 151-161. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL (1995) Place cells, head direction cells, and the learning of landmark stability. J Neurosci 15: 1648-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Schenk F (1995) Influence of local environmental olfactory cues on place learning in rats. Physiol Behav 58: 1059-1066. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Schenk F (1998) Olfactory traces and spatial learning in rats. Anim Behav 56: 1129-1136. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini P, Muller RU, Save E, Poucet B (2002) Relationships between place cell firing fields and navigational decisions by rats. J Neurosci 22: 9035-9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Wilson MA (2001) Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29: 145-156. [DOI] [PubMed] [Google Scholar]
- Maaswinkel H, Whishaw IQ (1999) Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res 99: 143-152. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J (1983a) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52: 41-49. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, O'Keefe J, Barnes CA (1983b) The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit recordings. J Neurosci Methods 8: 391-397. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL (1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7: 1951-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51: 78-109. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Conway D (1978) Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res 31: 573-590. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: preliminary evidence from unit activity in the freely moving rat. Brain Res 34: 171-175. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford: Clarendon.
- O'Keefe J, Speakman A (1987) Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res 68: 1-27. [DOI] [PubMed] [Google Scholar]
- Ranck Jr JB (1973) Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats, part I: behavioral correlates and firing properties. Exp Neurol 41: 461-531. [DOI] [PubMed] [Google Scholar]
- Recce ML, O'Keefe J (1989) The tetrode: a new technique for multiunit extracellular recording. Soc Neurosci Abstr 15: 1250. [Google Scholar]
- Rosenweig ES, Redish AD, McNaughton BL, Barnes CA (2003) Hippocampal map realignment and spatial learning. Nat Neurosci 6: 609-615. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL (1996) Replay of neuronal firing sequences in the rat hippocampus during sleep following spatial experience. Science 271: 1870-1873. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA (1996) Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6: 149-172. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Bures J (2002) Relative contribution of allothetic and idiothetic navigation to place avoidance on stable and rotating arenas in darkness. Behav Brain Res 128: 179-188. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Fenton AA, Bures J (2001) Substratal idiothetic navigation of rats is impaired by removal or devaluation of extramaze and intramaze cues. Proc Natl Acad Sci USA 98: 3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH (1971) Limbic-diencephalic mechanisms of voluntary movement. Psychol Rev 78: 83-113. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Gorny B, Whishaw IQ (2002) Rats can track odors, other rats, and themselves: implications for the study of spatial behavior. Behav Brain Res 131: 185-192. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ (1972) Hippocampal electroencephalographic activity in the Mongolian gerbil during natural behaviours and wheel running and in the rat during wheel running and conditioned immobility. Can J Psychol 26: 219-239. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1993) Dynamics of hippocampal ensemble code for space. Science 261: 1055-1058. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 265: 676-679. [DOI] [PubMed] [Google Scholar]
- Zhang K, Ginzburg I, McNaughton BL, Sejnowski TJ (1998) Interpreting neuronal population activity by reconstruction: unified framework with application to hippocampal place cells. J Neurophysiol 79: 1017-1044. [DOI] [PubMed] [Google Scholar]