Abstract
The neurobehavioral effects of 24 hr of total sleep deprivation (SD) on working memory in young healthy adults was studied using functional magnetic resonance imaging. Two tasks, one testing maintenance and the other manipulation and maintenance, were used. After SD, response times for both tasks were significantly slower. Performance was better preserved in the more complex task. Both tasks activated a bilateral, left hemisphere-dominant frontal-parietal network of brain regions reflecting the engagement of verbal working memory. In both states, manipulation elicited more extensive and bilateral (L>R) frontal, parietal, and thalamic activation. After SD, there was reduced blood oxygenation level-dependent signal response in the medial parietal region with both tasks. Reduced deactivation of the anterior medial frontal and posterior cingulate regions was observed with both tasks. Finally, there was disproportionately greater activation of the left dorsolateral prefrontal cortex and bilateral thalamus when manipulation was required. This pattern of changes in activation and deactivation bears similarity to that observed when healthy elderly adults perform similar tasks. Our data suggest that reduced activation and reduced deactivation could underlie cognitive impairment after SD and that increased prefrontal and thalamic activation may represent compensatory adaptations. The additional left frontal activation elicited after SD is postulated to be task dependent and contingent on task complexity. Our findings provide neural correlates to explain why task performance in relatively more complex tasks is better preserved relative to simpler ones after SD.
Keywords: working memory, prefrontal cortex, cortical deactivation, sleep deprivation, functional imaging, BOLD fMRI
Introduction
Sleep deprivation (SD), even for one night, can result in diminished alertness and cognitive performance. Although the behavioral changes accompanying SD have been studied extensively, the underlying neural correlates of these changes have been less well characterized, not the least because of the need to account for the contributions of cognitive domain tested, task complexity, arousal, and duration of SD (Kjellberg, 1975; Wilkinson, 1992). In this study, we focused on how task complexity interacts with state to modulate cortical activation as healthy young adults performed working memory tasks.
We chose to study working memory because neuropsychological (Horne, 1988; Wimmer et al., 1992; Harrison and Horne, 1998) and EEG studies (Werth et al., 1997; Cajochen et al., 1999) suggest that physiological changes taking place in the frontal lobes after SD contribute significantly to cognitive decline. However, frontal lobe dysfunction alone cannot account for why performance is relatively preserved with moderately complex tasks (Wilkinson, 1965; Hockey et al., 1998; Linde et al., 1999; Harrison and Horne, 2000) but is degraded with simpler tasks (Kjellberg, 1975; Gillberg and Akerstedt, 1998). Given that SD is accompanied by a lowering of arousal (Babkoff et al., 1991), higher task complexity is thought to minimize performance decline by temporarily increasing arousal or sustained attention (Wilkinson, 1965). Top-down increase in thalamic activation (Portas et al., 1998) may be one means toward this because a reduction in thalamic activation after SD has been associated with performance decline (Thomas et al., 2000).
We chose to evaluate a single cognitive domain because divergent results have been obtained from existing functional imaging studies relating to SD, depending on the cognitive domain tested. For example, frontal and parietal activation after SD has been shown to increase in experiments involving verbal learning (Drummond et al., 2000, 2001), decrease in experiments involving serial subtraction (Drummond et al., 1999; Thomas et al., 2000), or show no change in an experiment testing attention (Portas et al., 1998).
Even within a particular cognitive domain, frontal activation may increase with task difficulty up to a point and then decrease (Callicott et al., 1999), reflecting an overwhelming of processing capacity, a loss of motivation (Jaeggi et al., 2003), or both. [Here, the term “load” refers to the number of items that have to be maintained in working memory (e.g., in a Sternberg-type maintenance task or n-back tasks). “Task complexity” refers to the increase in types of cognitive process required to perform the task (e.g., manipulation vs maintenance). “Difficulty” is used when either or both of these conditions are fulfilled when similar cognitive domains are tested; when comparing tasks tapping different domains, subjective rating or response time (RT) is used to gauge difficulty.] Subjective difficulty may have been excluded as a potential source of divergent imaging results (Drummond and Brown, 2001), but this assertion has not been tested explicitly.
To examine the interaction between state and task complexity, we used a two-by-two experimental design to test working memory after 24 hr of total SD and rested wakefulness (RW). The less demanding task engaged maintenance of information, whereas the more demanding task required manipulation in addition to maintenance. Short-term total SD, although artificial (chronic SD is more common and has greater general relevance), affords better experimental control. The experiments were relatively short because the benefit of task complexity is often temporary. Furthermore, the loss of interest with prolonged testing results in a decline in arousal and performance (Wilkinson, 1965).
On the basis of previous work, we predicted that in both states, manipulation would increase prefrontal (Smith et al., 1998; D'Esposito et al., 1999) and parietal (Veltman et al., 2003) activation to a greater extent than maintenance. Furthermore, in light of behavioral data showing that moderately complex tasks are less affected than simpler tasks, we also expected that manipulation would result in better preserved performance and elicit disproportionately greater frontal lobe activation. Because several parallels have been drawn between decline in prefrontal function after SD and aging (Harrison et al., 2000), we used the extensive work on aging and cognition (Reuter-Lorenz, 2002; Cabeza et al., 2004) as a framework to interpret our observations concerning the modulation of activation after SD.
Materials and Methods
Subject characteristics. Fourteen right-handed, healthy undergraduate volunteers (five women; mean age, 23 years; range, 19-24) participated in the study after giving informed consent. They were selected from a wider pool of candidates who answered a questionnaire on their sleeping habits and who kept a sleep diary for 1 week. Only volunteers with habitual good sleep, who slept no later than 1 A.M. and woke up no later than 9 A.M., were studied. The participants of this study slept an average of 7.2 ± 0.9 hr per night in the week preceding SD. Volunteers were screened for a history of excessive daytime sleepiness and insomnia. None of the volunteers had a history of psychiatric illness, obstructive sleep apnea, narcolepsy, or periodic leg movements in sleep, as ascertained by a physician (W.C.C). None of the volunteers was on medication. Alcohol and recreational drug use were excluded.
Experimental protocol. Subjects were scanned twice, once during RW and once after SD. The two scanning sessions were conducted 1 week apart to minimize the possibility of residual effects of SD affecting cognition of volunteers who underwent a SD scan before a RW scan (Van Dongen et al., 2003). The order of scanning was counterbalanced across subjects to reduce the potential influence of practice, learning, and order effects on cortical activation. Subjects abstained from smoking, caffeine, and other stimulants for 24 hr before being scanned. Alcohol was similarly disallowed. While undergoing SD, subjects were monitored in the laboratory from 9 P.M. onward. They were allowed to engage in non-strenuous activities such as watching videos and conversing. They did not interact with persons outside the laboratory. Every hour throughout the study night and under supervision, subjects rated their sleepiness using the Epworth sleepiness scale (ESS) and performed a simple reaction time task (SRT). The SRT required that subjects respond by pressing the appropriate key, depending on whether they saw a left- or right-pointing arrow. Arrows appeared at random (1.0-5.0 sec) after the start of each trial. One hundred eighty trials were executed during each testing session. Scanning took place after 22.9 ± 0.8 hr of wakefulness.
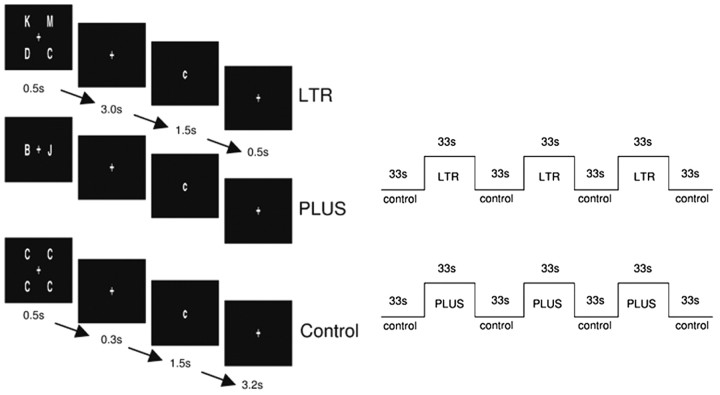
Experimental tasks. Two working memory tasks were used (Fig. 1). LTR evaluated maintenance and was adapted from previous work on verbal working memory (Reuter-Lorenz et al., 2000). Four different uppercase letters were presented for 0.5 sec, followed by a delay period of 3.0 sec, during which a fixation cross was displayed. A lowercase probe letter was then presented for 1.5 sec, and this was followed by fixation for an additional 0.5 sec. Subjects signaled a match or a nonmatch by pressing one of two response buttons. Half the probes matched the target letters. Response omissions were reported as a proportion of total possible responses.
Figure 1.
Schematic showing exemplars of stimuli used in LTR and PLUS and presentation timings. The control condition was identical for both tasks.
The control condition was designed to match for perceptual and motor responses. Four identical uppercase letters appeared for 0.5 sec. This was followed by a shorter 0.3 sec delay period before the appearance of a lowercase probe that matched the target in half the trials. Subjects signaled a match or nonmatch using one of two response buttons.
PLUS was designed to engage manipulation of items retained in verbal working memory. Two different letters were presented, and subjects were instructed to shift each letter forward alphabetically and to keep in mind the results. For example, if “B” and “J” were presented, subjects had to remember “c” and “k” to be matched with the probe. Matches comprised half the trials. Stimulus presentation sequence, timing, and control condition were identical to that used in LTR.
Before scanning, each subject performed a practice run. Task and control blocks each lasted 33 sec. Each block consisted of six trials (5.5 sec per trial). Each experimental run consisted of four control blocks alternating with three task blocks. Each subject was presented with three runs of LTR and three runs of PLUS during each of the two sessions. The order of LTR and PLUS were counterbalanced across subjects. Session order was also counterbalanced.
Imaging procedure. Stimuli were projected onto a screen using a liquid crystal display projector and viewed by subjects through a rearview mirror. Subjects responded by pressing buttons on a hand-held response box with the right hand. A bite-bar was used to reduce head motion. Images were acquired on a 3T Allegra magnetic resonance imaging system (Siemens, Erlangen, Germany). A gradient echoplanar imaging sequence was used with a repetition time of 3000 msec, field of view of 192 × 192 mm, and a 64 × 64 mm pixel matrix. Thirty-two oblique axial slices with thickness 3 mm (0.3 mm gap) approximately parallel to the AC-PC (anterior commissure-posterior commissure) line were acquired. High-resolution coplanar T2-weighted anatomical images were also obtained. An additional high-resolution image was acquired using a T1-weighted three-dimensional-MPRAGE sequence for the purpose of image display in Talairach space (Talairach and Tournoux, 1988).
Image analysis. Motion correction was performed in-scanner using PACE (Siemens). Functional images were processed with Brain Voyager 2000 version 4.9 (Brain Innovation, Maastricht, Holland). Mean intensity normalization was performed to obtain the same average intensity for each slice across scans. Interslice timing differences attributable to slice acquisition order were adjusted using sinc interpolation. Gaussian filtering was applied in the spatial domain using a smoothing kernel of 4 mm full-width at half-maximum (FWHM) for individual activation maps and 8 mm FWHM for group level activation maps. Intrasession image alignment to correct for motion across runs was performed using the first image of the functional run that was acquired immediately before a coplanar T2-weighted image, as the reference image. The T2 images were used to register the functional data set to the volunteers' own three-dimensional image. The resulting aligned dataset was then transformed into Talairach space. The group level anatomical image was an arithmetical average of the individuals' structural images.
Functional analysis was performed using a general linear model with four predictors of interest (LTRRW, LTRSD, PLUSRW, PLUSSD) and a confound predictor for each run. Parameter estimates obtained for each predictor and for each subject were used in a random-effects analysis. For contrasts of interest, a threshold of p < 0.005 (uncorrected) was used (see note in Results regarding this). The cluster threshold used was nine contiguous voxels. Whole-brain voxel-by-voxel analyses for state-dependent effects were then performed for each task using the contrasts LTRSD>LTRRW and PLUSSD>PLUSRW. The analysis tool used discriminated between LTRSD>LTRRW, where LTRSD>LTRcontrol, and LTRSD>LTRRW, where LTRSD<LTRcontrol. The former was termed “activation” and the latter “deactivation.”
The term “reduced activation” was used when LTRSD<LTRRW and LTRSD>LTRcontrol or if PLUSSD<PLUSRW and PLUSSD> PLUScontrol. The time course of the blood oxygenation level-dependent (BOLD) response in each activated or deactivated region was inspected manually to verify the classification of activity change.
Region of interest (ROI)-based analysis was used to supplement whole-brain voxel-by-voxel analysis to provide information on state by task interaction in regions showing significant activation or deactivation. To minimize selection bias, the ROI analysis involved voxels that were activated jointly in all four experimental conditions and that were revealed in the group level activation maps. For each ROI, parameter estimates were obtained from significantly activated voxels within a 15 × 15 × 15 mm cube centered on the activation peak. This method of ROI analysis was applied to the midline frontal regions, parietal region, and thalamus.
Whereas it is trivial to demonstrate prefrontal activation in working memory tasks, previous work has shown that there are frequently inter-individual differences in the spatial location of activation (Miller et al., 2002; Wei et al., 2004). This could dilute observed effects in this region with multi-subject voxel-by-voxel analysis. To verify that the task by state interaction observed in the left prefrontal region at a slightly lenient threshold of p < 0.005 was not spurious, we performed a functional ROI analysis of activation in this region by obtaining parameter estimates of the individual subject's activation within the region of the left dorsolateral prefrontal cortex (corresponding to the middle frontal gyrus).
To further evaluate the relationship between BOLD signal and task performance after SD, we examined the linear correlation between signal change and behavioral performance (RT only, because accuracy results were range limited) in the left prefrontal, midline frontal (anterior medial frontal and posterior cingulate were analyzed separately), and parietal regions and thalamus.
Results
Behavioral results
Behavioral data for 14 subjects were analyzed, but one subject's in-scanner data were lost as a result of a technical error. Subjects reported a greater subjective sense of sleepiness after SD, reflected by the increase in ESS (t(13) = 9.4; p < 0.001) (Table 1). The variability of RTs during SRT was greater after SD (t(13) = 2.2; p < 0.05), although there was no difference in mean RTs across states. Subjects omitted more responses after SD for LTR (t(12) = 2.4; p < 0.05) but not for PLUS. After SD, RTs to the common control condition did not increase as a function of time-on-task, suggesting that interest was maintained. The RT was slower for both LTR (t(12) = 2.2; p < 0.05) and PLUS (t(12) = 2.5; p < 0.05) after SD. There was a significant decline in performance accuracy after SD for LTR (t(12) = 2.7; p < 0.05), but not for PLUS.
Table 1.
Behavioral data recorded during rested wakefulness and after sleep deprivation (SD in parentheses)
|
|
Rested wakefulness |
Sleep deprived |
|---|---|---|
| Measures of sleepiness | ||
| ESS | 4.1 (4.1) | 17.1 (4.0)*** |
| Simple RT (msec) | 378 (58) | 394 (82) |
| LTR | ||
| Omitted responses (%) | 0.2 (0.5) | 4.0 (6.3)* |
| Accuracy | 0.959 (0.049) | 0.902 (0.097)** |
| RT (msec) | 825 (80) | 883 (110)** |
| PLUS | ||
| Omitted responses (%) | 0.4 (1.1) | 2.3 (3.8) |
| Accuracy | 0.957 (0.055) | 0.926 (0.086) |
| RT (msec) |
786 (119) |
860 (144)**
|
Significant differences across states using paired t test are indicated: *p < 0.05; **p < 0.005; ***p < 0.001.
Activation during RW
During RW, LTR elicited bilateral, left hemisphere-dominant activation in the prefrontal [Brodmann's area (BA) 9/46] and precentral (BA 6) regions, insula (BA 13), and thalamus (Fig. 2.). In both parietal lobes, activation in the inferior parietal (BA 40) region extended medially into the precuneus (BA 7). PLUS elicited more extensive activation of the same set of areas activated by LTR. Areas that were activated to a greater extent in PLUS compared with LTR were the left prefrontal region around the middle frontal gyrus (BA 9/46), left inferior parietal lobule (BA 39/40), left insula (BA 13), and bilateral thalamus (Fig. 2; Table 2). No region was more active in LTR compared with PLUS. During RW, cortical deactivation was greater for PLUS compared with LTR (Fig. 3).
Figure 2.
Statistical activation maps of BOLD signal change for LTR and PLUS in RW and SD. Activations are projected onto the unfolded cortical surface of an individual volunteer's brain. Regions showing greater activation for PLUS than LTR for each state appear in the bottom panels.
Table 2.
Regions where PLUS elicited greater activation than LTR in each state
|
|
|
PLUS>LTR (RW) |
PLUS>LTR (SD) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region |
BA |
x
|
y
|
z
|
t
|
x
|
y
|
z
|
t
|
||||||
| Frontal | |||||||||||||||
| Left middle frontal gyrus | 9/46 | −40 | 24 | 24 | 6.2 | −38 | 28 | 24 | 6.3 | ||||||
| Right middle frontal gyrus | 9/46 | 28 | 48 | 15 | 4.2 | 46 | 10 | 33 | 5.3 | ||||||
| Left precentral gyrus | 6 | −47 | 4 | 34 | 4.9 | −43 | 5 | 24 | 4.6 | ||||||
| Parietal | |||||||||||||||
| Left inferior parietal lobule | 39/40 | −43 | −45 | 38 | 8.0 | −40 | −50 | 45 | 5.2 | ||||||
| Right angular gyrus | 39 | 32 | −61 | 37 | 6.5 | ||||||||||
| Temporal | |||||||||||||||
| Left middle temporal gyrus | 37 | −48 | −50 | −1 | 4.3 | −45 | −43 | −8 | 3.3 | ||||||
| Subcortical | |||||||||||||||
| Left thalamus | −11 | 11 | 13 | 5.5 | −14 | −12 | 18 | 4.0 | |||||||
| Right thalamus |
|
10 |
−11 |
18 |
4.6 |
10 |
−11 |
17 |
7.3 |
||||||
Figure 3.
Reduced task-related deactivation in the anterior medial frontal (a) and posterior cingulate (b) regions after SD. Error bars denote SE (c) The correlation between RTs and BOLD signal change in the anterior medial frontal region jointly activated in both states and tasks. d, ROI from which the extent of deactivation was determined.
Activation after SD in comparison with RW
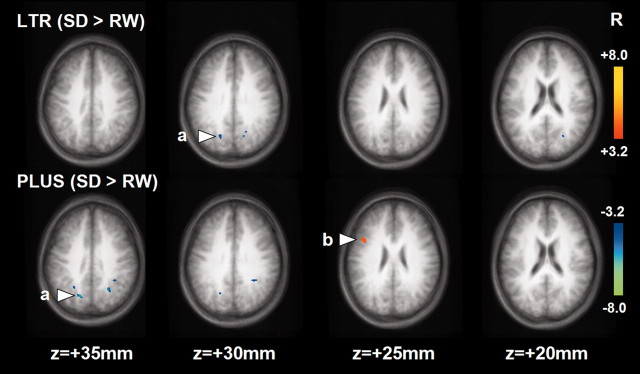
After SD, LTR and PLUS elicited activation in regions overlapping with those during RW (Fig. 2; Table 2). Voxel-by-voxel analysis showed that after SD, both LTR and PLUS elicited a smaller task-related BOLD signal in the parietal region (bilateral BA 7; inclusive of the precuneus) than during RW (Fig. 4).
Figure 4.
Statistical activation maps showing differences in activation elicited by each task during SD and RW. a, Parietal region that showed reduced activation after SD; b, left prefrontal region that showed increased activation after SD. The deactivated areas are not shown.
A larger post-SD increase in BOLD signal was observed in the left dorsolateral prefrontal cortex (around the middle frontal gyrus; BA 9), relative to that obtained during RW. Prefrontal activation in response to LTR was not significantly different after SD (Fig. 4). The anticipated state-by-task interaction was observed in a random-effects analysis, at a less stringent threshold of p < 0.005. To confirm that these results were not spurious, the results of the ROI analysis based on the individual subject's data showed significant state-by-task interaction (F(1,12) = 17.4; p < 0.001) in addition to main effects of state (F(1,12) = 6.6; p < 0.05) and task (F(1,12) = 38.0; p < 0.001) (Fig. 5).
Figure 5.
Parameter estimates obtained from individual subjects' ROI in the left dorsolateral prefrontal cortex. A main effect of task on activation and disproportionately higher activation in response to PLUS during SD are illustrated. Error bars denote ± 1 SE.
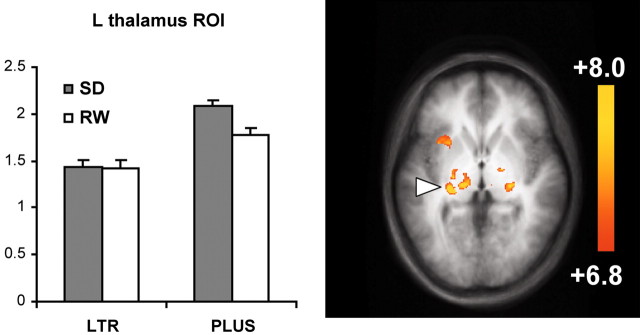
ROI analysis of activation in the thalamus showed main effects of state (F(1,13) = 6.8; p < 0.05) and task (F(1,13) = 11.6; p < 0.005). Although there was a trend suggestive of a state-by-task interaction, this did not reach statistical significance (F(1,13) = 2.3; p < 0.15) (Fig. 6).
Figure 6.
Thalamic regions jointly activated in both tasks and both states. Parameter estimates from the thalamic region indicated (peak coordinate, -16, -21, 2) are shown. Error bars denote ± 1 SE.
Reduced deactivation after SD was observed in the anterior medial frontal (BA 10) and left posterior cingulate (BA 31) regions in voxel-by-voxel contrasts (Fig. 3; Table 3). In addition, ROI-based analyses showed significant main effects of task and state in the anterior medial frontal (task: F(1,13) = 36.3, p < 0.001; state: F(1,13) = 12.8, p < 0.005) and posterior cingulate (task: F(1,13) = 116.3, p < 0.001; state: F(1,13) = 4.6, p < 0.05) regions. Neither of these areas showed a significant state-by-task interaction.
Table 3.
Regions showing differences in activation and deactivation between SD and RW for each task
|
|
Region |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Activation |
BA |
x
|
y
|
z
|
t
|
||||
| LTRSD<LTRRW | |||||||||
| Left precuneus | 7 | −23 | −64 | 26 | 3.6 | ||||
| Right precuneus | 31 | 24 | −64 | 19 | 3.5 | ||||
| PLUSSD<PLUSRW | |||||||||
| Left precuneus | 7 | −25 | −53 | 33 | 3.5 | ||||
| Left precuneus | 7 | −17 | −65 | 35 | 5.7 | ||||
| Right precuneus | 7 | 23 | −58 | 35 | 5.1 | ||||
| PLUSSD>PLUSRW | |||||||||
| Left middle frontal gyrus | 9 | −41 | 12 | 25 | 3.5 | ||||
| Deactivation | |||||||||
| PLUSSD>PLUSRW | |||||||||
| Left anterior medial frontal cortex | 10 | −2 | 54 | 14 | 3.9 | ||||
| Left posterior cingulate gyrus |
31 |
−8 |
−45 |
30 |
4.1 |
||||
The RT was inversely correlated with deactivation (r = 0.57; p < 0.05) in the anterior medial frontal region (Fig. 3). The RT did not correlate with BOLD signal change in the left dorsolateral prefrontal, posterior cingulate, and parietal regions or thalamus.
Discussion
The present study showed that after SD, increased as well as decreased cortical activation and decreased deactivation occurred relative to activation elicited during RW. These state-related changes in activation involved several brain regions in addition to the frontal lobes. We explained the observed changes in the context of SD-related performance decline or conservation. Consistent with the findings of previous behavioral studies, we found that higher task complexity results in better preserved performance. We posit that reduced activation of the parietal region and reduced task-related deactivation of the medial frontal regions related to performance decline after SD. We further suggested that the disproportionate (relative to RW) increase in frontal activation in the PLUS task performed during SD is compensatory in nature. We found that task complexity and state interact to modulate brain activation in a manner resembling comparisons between task-related cortical activation in healthy young adults and healthy elderly volunteers. In turn, this suggests that some adaptive mechanisms for cognitive processing under conditions of diminished processing resources (Craik, 1986) may be common between SD and aging (Harrison et al., 2000).
Task-related differences in activation and deactivation
As expected, PLUS elicited greater prefrontal and parietal activation than LTR in both states in accordance with the notion that manipulation engages additional processing resources relative to maintenance when working memory load is not excessive (Owen et al., 1996; D'Esposito et al., 1999; Postle et al., 1999; Veltman et al., 2003).
Additionally, we observed greater left dorsomedial thalamus activation with PLUS. Prior working-memory experiments have demonstrated thalamic activation in association with increased working memory demand (Barch et al., 1997; Callicott et al., 1999; Manoach et al., 2003). The increase in thalamic activation in concert with prefrontal activation is consonant with the existence of extensive reciprocal connections between the dorsomedial thalamic nucleus and the prefrontal cortex. Thalamic activation is also modulated by an increase in sustained attention (Kinomura et al., 1996; Coull, 1998), and it is conceivable that either or both these mechanisms could contribute to the task-related difference in thalamic activation observed here.
Deactivation, referring to a reduction of BOLD signal during task performance relative to the baseline (Gusnard and Raichle, 2001), was more pronounced with the more complex task in both states. The deactivated posterior cingulate and anterior medial frontal regions we observed are part of a “default network” (Gusnard et al., 2001; Gusnard and Raichle, 2001) that is more active during passive (baseline) than active (task) conditions in a wide variety of experiments (Mazoyer et al., 2001; McKiernan et al., 2003). The present findings are consistent with the notion that these regions are disengaged during the performance of cognitive tasks and that the magnitude of deactivation may increase in accordance with processing demands (McKiernan et al., 2003). Additionally, the inverse relationship between magnitude of deactivation and RT suggests that at a particular level of task complexity, greater deactivation may be related to more efficient task performance.
Relative reduction in parietal activation after SD
Although there are some divergent results across functional imaging studies of SD, there appears to be a consistent association between reduced activation and a decline in behavioral performance (Drummond et al., 1999, 2000; Thomas et al., 2000; Habeck et al., 2004).
In the context of SD, less impaired word recall correlated with greater activation in the parietal lobes (Drummond et al., 2000; Drummond and Brown, 2001). Conversely, the reduced activation of the parietal regions might have contributed to reduced performance in serial subtraction (Drummond et al., 1999; Thomas et al., 2000). In the present study, SD resulted in reduced activation of bilateral parietal regions slightly medial to the mid-portion of the intraparietal sulcus. This region has been activated in several previous experiments examining working memory (Schumacher et al., 1996; Cabeza et al., 2002) and may be involved in retrieval operations specific to working memory (Cabeza et al., 2002). Healthy volunteers with a higher memory span showed greater activation in this region relative to those with a lower memory span (Mecklinger et al., 2003). It is, therefore, reasonable to suggest that reduced activation in this region may relate to performance decline in SD.
An interaction between state and task effects may result in modulation of frontal and thalamic activation
The dynamic nature of cognitive dysfunction after SD is highlighted by the observation that performance was relatively better preserved with the more complex task, whereas in the case of a structural lesion of the frontal lobes, greater task complexity would be expected to accentuate cognitive deficits.
Thus, the additional engagement of the frontal lobes after SD may be contingent on sufficient task complexity. This notion is concordant with studies showing that moderately complex tasks are relatively unaffected by SD, perhaps because they can engage attention better than simpler tasks (Harrison and Horne, 2000).
In this regard, the frontal lobes may exercise top-down effects on thalamic activation (Coull, 1998). We note that thalamic activation was greater for PLUS compared with LTR during wakefulness. Additionally, thalamic activation for both tasks was further increased after SD. This pair of findings suggests that increased sustained attention, reflected by increased thalamic activity, may contribute to maintaining cognitive performance when arousal is low, such as is the case after SD (Kinomura et al., 1996; Portas et al., 1998).
Relative reduction of deactivation in midline frontal regions after SD
Accompanying the modest performance decline in both tasks after SD, there was a reduction in task-related deactivation relative to that elicited during RW for both tasks (Fig. 3). It is important to point out that unless the control task is considered, reduced deactivation can be construed as increased activation. This might, in fact, have been observed in previous studies (Drummond and Brown, 2001; Drummond et al., 2001) but was not reported appropriately.
Given previous observations concerning the significance of task-related cortical deactivation, we posit that the present findings signify that after SD, there is diminished capacity to recruit cognitive resources required to engage in goal-directed behavior. In support of this postulate, the magnitude of task-related mid-line frontal deactivation was reduced in healthy elderly volunteers in whom performance in word classification was poorer than in young, healthy controls (Lustig et al., 2003). It may well be that midline deactivation is a generic indicator of capacity to direct cognitive resources to the task at hand. However, in view of the partially compensated performance in PLUS after SD, this statement awaits confirmation by the results of a study in which task complexity and load (or both) are parametrically manipulated (McKiernan et al., 2003) after SD.
State-related changes in activation: synthesis
The multiple changes in cortical activation and deactivation that accompany task performance after SD indicate that the basis for performance impairment (and possibly its conservation) is more complex than was inferred from behavioral studies that pointed to the frontal lobes as the major source of performance decline (Harrison et al., 2000). A reasonable approach to interpreting the present data are to conceive of regions showing relatively reduced levels of activation (medial parietal, occipital) and deactivation (anterior medial frontal, posterior cingulate) as those contributing to dysfunction. Areas showing increased task-related activation after SD may be considered to be regions that might play a compensatory role. This framework of interpreting functional imaging results has been applied to studies on healthy aging (Reuter-Lorenz, 2002; Cabeza et al., 2004). In using this framework, we are cognizant that, under different contexts, increases in task-related regional cortical activation may relate to more (Gray et al., 2003; Mecklinger et al., 2003) or less (Reuter-Lorenz, 2002; Cabeza et al., 2004) efficient processing.
As a rule, the results of these and other functional imaging studies are interpreted in the context of obtained behavioral results. Because the present behavioral results and imaging findings in relation to PLUS correspond to compensated, SD-related performance decline, the use of the “compensatory view” framework (Cabeza, 2002) to interpret our findings is justified.
Comparison with patterns of cortical activation and deactivation in healthy elderly adults
Both SD and healthy aging result in a decline in working memory (Harrison et al., 2000). In addition, the three types of activity modulation observed in young SD individuals have parallels in healthy elderly adults, speaking to the possibility that common mechanisms may underlie cognitive decline in both conditions
In tasks that the elderly can perform (Grady et al., 1994; Esposito et al., 1999; Madden et al., 1999; Rypma and D'Esposito, 2000; Cabeza et al., 2004), frontal activation is often increased and frequently bilateral. After SD, such bilateral increases in frontal activation have been observed with verbal learning and divided attention tasks (Drummond et al., 2000, 2001).
That some tasks decrease frontal activation (Drummond et al., 1999; Thomas et al., 2000) whereas some increase it after SD (the present experiment) bears similarity to the differences in frontal activation that result from task manipulations in the elderly, underscoring the need to explore a gamut of different tasks before attempting to explain the neural basis for cognitive decline in these states (Cabeza et al., 2004). An informative illustration: when encoding words without specific instruction, elderly volunteers exhibited reduced frontal activation and poor memory retrieval. However, when provided with environmental support to facilitate encoding, the elderly volunteers showed a nonselective (bilateral) increase in frontal activation and correspondingly improved mnemonic performance relative to their younger counterparts (Logan et al., 2002). This suggests that the engagement of compensatory neural responses may be contingent on the use of specific mental operations or strategies.
A relative reduction of occipital lobe activation (Grady et al., 1994; Madden et al., 1996; Cabeza et al., 1997) has been observed in the elderly, and this finding appears to be task independent (Cabeza et al., 2004). The basis for this reduction in activation is unclear, although it has been suggested that sensory processing might be impaired in the elderly (Li and Lindenberger, 2002). We observed relatively trivial reduction in occipital deactivation after SD in the present study. However, such reduction in occipital activation has been observed (but not highlighted) in at least three previous studies (Drummond et al., 1999, 2001; Habeck et al., 2004).
Footnotes
This work was supported by National Medical Research Council Grant 2000/0477, Biomedical Research Council Grant 014, and The Shaw Foundation (4K/SIS/TFTM).
Correspondence should be addressed to Dr. Michael W. L. Chee, Cognitive Neuroscience Laboratory, 7 Hospital Drive, #01-11, Singapore 169611, Singapore. E-mail: mchee@pacific.net.sg.
Copyright © 2004 Society for Neuroscience 0270-6474/04/244560-08$15.00/0
References
- Babkoff H, Caspy T, Mikulincer M (1991) Subjective sleepiness ratings: the effects of sleep deprivation, circadian rhythmicity and cognitive performance. Sleep 14: 534-539. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD (1997) Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia 35: 1373-1380. [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002) Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17: 85-100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI (1997) Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci 17: 391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L (2002) Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage 16: 317-330. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar M, Dolcos F, Prince SE, Budde M, Nyberg L (2004) Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364-375. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Foy R, Dijk DJ (1999) Frontal predominance of a relative increase in sleep delta and theta EEG activity after sleep loss in humans. Sleep Res Online 2: 65-69. [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, Goldberg TE, Weinberger DR (1999) Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex 9: 20-26. [DOI] [PubMed] [Google Scholar]
- Coull JT (1998) Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55: 343-361. [DOI] [PubMed] [Google Scholar]
- Craik FIM (1986) A functional account of age differences in memory. In: Human memory and cognitive capabilities, mechanisms and performances (Klix F, Hagendorf H, eds), pp 409-422: Amsterdam: Elsevier.
- D'Esposito M, Postle BR, Ballard D, Lease J (1999) Maintenance versus manipulation of information held in working memory: an event-related fMRI study. Brain Cogn 41: 66-86. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG (2001) The effects of total sleep deprivation on cerebral responses to cognitive performance. Neuropsychopharmacology 25: S68-S73. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC (1999) Sleep deprivation-induced reduction in cortical functional response to serial subtraction. NeuroReport 10: 3745-3748. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB (2000) Altered brain response to verbal learning following sleep deprivation. Nature 403: 655-657. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Brown GG (2001) Increased cerebral response during a divided attention task following sleep deprivation. J Sleep Res 10: 85-92. [DOI] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF (1999) Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain 122: 963-979. [DOI] [PubMed] [Google Scholar]
- Gillberg M, Akerstedt T (1998) Sleep loss and performance: no “safe” duration of a monotonous task. Physiol Behav 64: 599-604. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV (1994) Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci 14: 1450-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS (2003) Neural mechanisms of general fluid intelligence. Nat Neurosci 6: 316-322. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685-694. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001) Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98: 4259-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y (2004) An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Brain Res Cogn Brain Res 18: 306-321. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA (1998) Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res 7: 95-100. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA (2000) The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl 6: 236-249. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA, Rothwell A (2000) Prefrontal neuropsychological effects of sleep deprivation in young adults-a model for healthy aging? Sleep 23: 1067-1073. [PubMed] [Google Scholar]
- Hockey GR, Wastell DG, Sauer J (1998) Effects of sleep deprivation and user interface on complex performance: a multilevel analysis of compensatory control. Hum Factors 40: 233-253. [DOI] [PubMed] [Google Scholar]
- Horne JA (1988) Sleep loss and “divergent” thinking ability. Sleep 11: 528-536. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrod K (2003) Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. NeuroImage 19: 210-225. [DOI] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE (1996) Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 271: 512-515. [DOI] [PubMed] [Google Scholar]
- Kjellberg A (1975) Effects of sleep deprivation on performance of a problem-solving task. Psychol Rep 37: 479-485. [DOI] [PubMed] [Google Scholar]
- Li KZ, Lindenberger U (2002) Relations between aging sensory/sensorimotor and cognitive functions. Neurosci Biobehav Rev 26: 777-783. [DOI] [PubMed] [Google Scholar]
- Linde L, Edland A, Bergstrom M (1999) Auditory attention and multiattribute decision-making during a 33 h sleep-deprivation period: mean performance and between-subject dispersions. Ergonomics 42: 696-713. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL (2002) Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 33: 827-840. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL (2003) Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA 100: 14504-14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Coleman RE, Provenzale JM, DeGrado TR, Hoffman JM (1996) Adult age differences in regional cerebral blood flow during visual world identification: evidence from H215O PET. NeuroImage 3: 127-142. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE (1999) Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp 7: 115-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Greve DN, Lindgren KA, Dale AM (2003) Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. NeuroImage 20: 1670-1684. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N (2001) Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54: 287-298. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR (2003) A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394-408. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Weber K, Gunter TC, Engle RW (2003) Dissociable brain mechanisms for inhibitory control: effects of interference content and working memory capacity. Brain Res Cogn Brain Res 18: 26-38. [DOI] [PubMed] [Google Scholar]
- Miller MB, Van Horn JD, Wolford GL, Handy TC, Valsangkar-Smyth M, Inati S, Grafton S, Gazzaniga MS (2002) Extensive individual differences in brain activations associated with episodic retrieval are reliable over time. J Cogn Neurosci 14: 1200-1214. [DOI] [PubMed] [Google Scholar]
- Owen AM, Evans AC, Petrides M (1996) Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex 6: 31-38. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD (1998) A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18: 8979-8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Berger JS, D'Esposito M (1999) Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc Natl Acad Sci USA 96: 12959-12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P (2002) New visions of the aging mind and brain. Trends Cogn Sci 6: 394-400. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA (2000) Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 12: 174-187. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M (2000) Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci 3: 509-515. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA (1996) PET evidence for an amodal verbal working memory system. NeuroImage 3: 79-88. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA (1998) Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA 95: 876-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers.
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D (2000) Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 9: 335-352. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF (2003) The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26: 117-126. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SA, Dolan RJ (2003) Maintenance versus manipulation in verbal working memory revisited: an fMRI study. NeuroImage 18: 247-256. [DOI] [PubMed] [Google Scholar]
- Wei X, Yoo SS, Dickey CC, Zou KH, Guttmann CR, Panych LP (2004) Functional MRI of auditory verbal working memory: long-term reproducibility analysis. NeuroImage 21: 1000-1008. [DOI] [PubMed] [Google Scholar]
- Werth E, Achermann P, Borbely AA (1997) Fronto-occipital EEG power gradients in human sleep. J Sleep Res 6: 102-112. [DOI] [PubMed] [Google Scholar]
- Wilkinson RT (1965) Sleep deprivation. In: Physiology of human survival (Edholm R, Bacharach A, eds), pp 399-430. London: Academic.
- Wilkinson RT (1992) Sleep, arousal and performance. In: The measurement of sleepiness (Broughton RJ, Ogilvie RD, eds), pp 254-265. Boston: Birkhauser.
- Wimmer F, Hoffmann RF, Bonato RA, Moffitt AR (1992) The effects of sleep deprivation on divergent thinking and attention processes. J Sleep Res 1: 223-230. [DOI] [PubMed] [Google Scholar]