Abstract
The central nucleus of the inferior colliculus (ICC) is a major site of synaptic interaction in the central auditory system. To understand how ICC neurons integrate excitatory and inhibitory inputs for processing temporal information, we examined postsynaptic responses of ICC neurons to repetitive stimulation of the lateral lemniscus at 10-100 Hz in rat brain slices. The excitatory synaptic currents mediated by AMPA and NMDA receptors and the inhibitory current mediated by GABAA receptors were pharmacologically isolated and recorded by whole-cell patch-clamp techniques. The response kinetics of AMPA receptor-mediated EPSCs and GABAA receptor-mediated IPSCs were similar and much faster than those of NMDA receptor-mediated EPSCs. AMPA EPSCs could follow each pulse of stimulation at a rate of 10-100 Hz but showed response depression during the course of repetitive stimulation. GABAA IPSCs could also follow stimulus pulses over this frequency range but showed depression at low rates and facilitation at higher rates. NMDA EPSCs showed facilitation and temporal summation in response to repetitive stimulation, which was most pronounced at higher rates of stimulation. GABAA inhibition suppressed activation of NMDA receptors and reduced both the degree of AMPA EPSC depression and the extent of temporal summation of NMDA EPSCs. The results indicate that GABAA receptor-mediated inhibition plays a crucial role in maintaining the balance of excitation and inhibition and in allowing ICC neurons to process temporal information more precisely.
Keywords: auditory system, patch-clamp recording, AMPA receptor, NMDA receptor, GABAA receptor, temporal processing
Introduction
The central nucleus of the inferior colliculus (ICC) is a major synaptic integration center in the auditory system. The ICC receives afferent inputs that include monaural and binaural projections from a number of nuclei in the lower auditory brainstem. Some of these inputs are excitatory whereas others are inhibitory, using either GABA or glycine as the neurotransmitter (Glendenning and Baker, 1988; Saint Marie et al., 1989; Glendenning et al., 1992; González-Hernández et al., 1996; Zhang et al., 1998).
Many ICC neurons receive convergent excitatory and inhibitory projections (Torterolo et al., 1995; Covey et al., 1996; Kuwada et al., 1997; Pedemonte et al., 1997). The physiological responses of ICC neurons depend on the interaction between excitatory and inhibitory action, which provides a mechanism for processing auditory temporal information (Casseday and Covey, 1995). For example, some neurons in the ICC of echolocating bats are tuned for sound duration. These neurons respond preferentially with offset spikes at favorable durations of the tone. The selectivity of responses to tone duration is probably attributable to a summation of the rebound membrane depolarization after synaptic inhibition and delayed synaptic excitation. Blocking GABAergic or glycinergic inhibition can eliminate duration tuning, suggesting that the neural code for sound duration is the result of convergence of excitatory and inhibitory inputs (Casseday et al., 2000).
Intracellular studies in rat, mouse, and gerbil brain slices have shown that excitatory and inhibitory postsynaptic responses can be elicited in the same ICC neuron by stimulation of the ascending afferent fiber tract, the lateral lemniscus (Wagner, 1996; Li et al., 1998; Moore et al., 1998; Reetz and Ehret, 1999; Ma et al., 2002a). The excitatory response consists of two components. An early rapid component is mediated by AMPA receptors, whereas a later and slower component is mediated by NMDA receptors (Ma et al., 2002a). The inhibitory response is mediated mainly by GABAA receptors (Ma et al., 2002a,b). With a single pulse of current stimulation, the AMPA, NMDA, and GABAA receptor-mediated responses overlap each other temporally. The overall postsynaptic response represents the interaction of excitatory and inhibitory postsynaptic responses (Ma et al., 2002a).
Although the types of synaptic receptors that mediate excitatory and inhibitory synaptic transmission in the ICC have been identified by immunocytochemical labeling, receptor binding, physiological recording, and the use of pharmacological agents (Sanes et al., 1987; Petralia and Wenthold, 1992; Winer et al., 1995; Fubara et al., 1996; Wagner, 1996; Gaza and Ribak, 1997; Moore et al., 1998; Caicedo and Eybalin, 1999; Ma et al., 2002a,b), little is known about how activation of receptors influences temporal processing in ICC neurons. In this study, we recorded the postsynaptic responses to repetitive stimulation of the lateral lemniscus and examined the effects of AMPA, NMDA, and GABAA receptor antagonists. The purpose of this study was to determine the contribution of each of these synaptic receptors to temporal integration in the ICC.
Materials and Methods
Brain slice preparation. Wistar albino rats (Charles River, St. Constant, Quebec, Canada) between 10 and 13 d of age were used for intracellular recordings. The animals were decapitated rapidly, and their brains were dissected in 24-26°C oxygenated artificial CSF (ACSF). Brain slices were cut at 400-500 μm in the frontal plane through the auditory midbrain with a tissue slicer. Slices containing the ICC were stored in the oxygenated ACSF at room temperature for at least 1 hr before any physiological recordings were made. A slice was then transferred to a recording chamber and completely submerged in ACSF, which circulated through the chamber at a flow rate of 10 ml/min. The ACSF consisted of (in mm) 129 NaCl, 3 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgSO4, 20 NaHCO3, 3 HEPES, and 10 glucose and had an osmolarity of 290-310 mOsm/kg. The ACSF was continuously oxygenated with 95% O2-5% CO2 and had a pH of 7.4. The temperature of the solution in the recording chamber was maintained at 29-30°C. Using a multiple valve system, the slice was perfused with normal ACSF or ACSF containing antagonists of excitatory and/or inhibitory synaptic receptors [i.e., CNQX (10-15 μm), dl-APV (100 μm), kynurenic acid (4 mm), bicuculline (10 μm), and strychnine (0.5-1 μm)]. All these drugs were purchased from Sigma-Aldrich (Oakville, Ontario, Canada).
Whole-cell patch-clamp recording. Patch pipettes were made from thin-walled glass tubing (1.1 mm outer diameter, 0.8 mm inner diameter; Kimax-51; Kimble, Vineland, NJ) with a two-stage vertical puller (Narishige, Tokyo, Japan). The patch recording pipettes were filled with a solution containing the following composition (in mm): 110 CsF, 20 TEA, 10 EGTA, 10 HEPES, 2 MgCl2, 4 NaCl, and 0.5 QX-314. The pH was 7.3, and the osmolarity was 290 mOsm/kg. These electrodes were used for studying AMPA and NMDA receptor-mediated responses. In experiments for studying GABAergic responses, and the interaction of synaptic excitation and inhibition, patch pipettes were filled with a solution containing the following chemicals (in mm): 130 K-gluconate, 0.6 EGTA, 10 HEPES, 2 MgCl2, 5 KCl, 2 ATP, 0.3 GTP, and 0.5 QX-314. The pH was 7.25, and the osmolarity was 280 mOsm/kg. The resistance of the patch electrode filled with either of the above solutions was 4-7 MΩ. Series resistances were usually <20 MΩ and were compensated by 70-90%. The junction potential for the CsF internal solution was 8.5 mV and for the K-gluconate internal solution was 15.1 mV. Holding potentials, unless indicated otherwise, are cited in the text without correcting for the junction potential.
A bipolar-stimulating electrode constructed from two insulated tungsten wires with a tip separation of 50 μm was placed on the ipsilateral lateral lemniscus just below the ICC or on the lateral-ventral part of the laminar region of the ICC. Synaptic responses were evoked by a single, unrepeated pulse of a 0.1 msec square wave and 10 pulses of the same square wave at frequencies of 10-100 Hz produced by a stimulator (S-8800; Grass, West Warwick, RI) and delivered through an isolation unit. The cell membrane potential was clamped at -60 mV in most cases, unless indicated otherwise, while the synaptic responses were recorded.
Whole-cell patch-clamp recordings from ICC neurons were made by an Axopatch 200A amplifier (Axon Instruments, Union City, CA). The data were filtered at 5 kHz, digitized at 2-10 kHz by Digidata 1320A interface, acquired by pClamp8, and analyzed off-line by pClamp. Numerical averages are presented as means ± SEMs. The significant difference was evaluated by either Student's t test or χ2 test.
All procedures in these experiments were consistent with the guidelines of the Canadian Council on Animal Care and were approved by the Carleton University Animal Care Committee.
Results
Excitatory responses mediated by AMPA and NMDA receptors
Electrical stimulation of synaptic inputs from the lateral lemniscus usually elicited inward postsynaptic currents (PSCs) while the neuron was held at -60 mV. Figure 1A shows PSCs elicited from an ICC neuron by a single, unrepeated current pulse. The response recorded in normal ACSF had a fast rise time and a slow decay time (Fig. 1A, top trace). A previous study in our laboratory has shown that synaptic responses of ICC neurons to stimulation of lemniscal inputs contain excitatory and inhibitory components (Ma et al., 2002a). The inhibitory component consists of GABAA and glycine receptor-mediated responses, and the excitatory component consists of AMPA and NMDA receptor-mediated responses. Therefore, in the present study, to investigate AMPA and NMDA EPSCs separately, we applied specific synaptic receptor antagonists to isolate each of these components. EPSCs mediated by AMPA receptors were dissected by application of the NMDA, glycine, and GABAA receptor antagonists (i.e., APV, strychnine, and bicuculline, respectively) (Fig. 1A, middle trace). EPSCs mediated by NMDA receptors were isolated by application of the AMPA, glycine, and GABA receptor antagonists (i.e., CNQX, strychnine, and bicuculline, respectively) (Fig. 1A, bottom trace). Comparison of these two components showed that the AMPA EPSCs had faster kinetics than the NMDA EPSCs. The 10-90% rise time and decay time, time to peak, and half-width of the AMPA EPSCs were all shorter than those of NMDA EPSCs (t test; p < 0.01) (Table 1).
Figure 1.
Synaptic responses of an ICC neuron to single pulse stimulation of the lateral lemniscus consisted of AMPA and NMDA EPSCs. A, Control response (top trace) was recorded with the brain slice perfused in normal ACSF. After application of APV, bicuculline, and strychnine, the late part of the EPSC was suppressed, revealing an early response with a short time course (middle trace). With the application of CNQX, bicuculline, and strychnine, the early part of the EPSC was blocked, and a later part with a longer time course appeared (bottom trace). The early and fast component was mediated by AMPA receptors, and the later and slower one was mediated by NMDA receptors. B, C, Pharmacologically isolated AMPA and NMDA EPSCs recorded from two separate ICC neurons were graded as the stimulus strength increased. The vertical line indicates an artifact produced by a 0.1 msec current pulse stimulus.
Table 1.
Kinetic properties of pharmacologically isolated PSCs mediated by AMPA, NMDA, and GABAA receptors in ICC neurons
|
|
AMPA (n = 26) |
NMDA (n = 37) |
GABAA (n = 12) |
|---|---|---|---|
| Rise time (msec) | 4.4 ± 0.5 | 27.3 ± 3.3 | 3.9 ± 0.5 |
| Decay time (msec) | 40.3 ± 6.3 | 137.7 ± 12.5 | 48.4 ± 5.0 |
| Time to peak (msec) | 6.8 ± 0.7 | 48.6 ± 5.6 | 6.7 ± 0.5 |
| Half-width (msec) |
13.7 ± 1.5 |
117.3 ± 12.9 |
22.9 ± 2.0 |
All of the measurements were made when the amplitude of the PSCs was ~ 100 pA with an appropriate stimulus intensity.
The amplitudes of both AMPA and NMDA EPSCs increased progressively as the strength of stimulation increased. Figure 1, B and C, shows graded AMPA (top traces) and NMDA (bottom traces) EPSCs recorded from two separate ICC neurons. The increase in the amplitude of the EPSCs was likely attributable to spatial summation as more presynaptic fibers were activated by the stimulus.
AMPA and NMDA receptor-mediated EPSCs to repetitive stimulation
To investigate how AMPA and NMDA receptors are involved in response to repetitive stimulation, we examined pharmacologically isolated AMPA (n = 23) and NMDA (n = 10) EPSCs, respectively, at stimulation rates of 10-100 Hz. At these frequencies, there was depression of responses mediated by AMPA receptors, but facilitation of ones mediated by NMDA receptors.
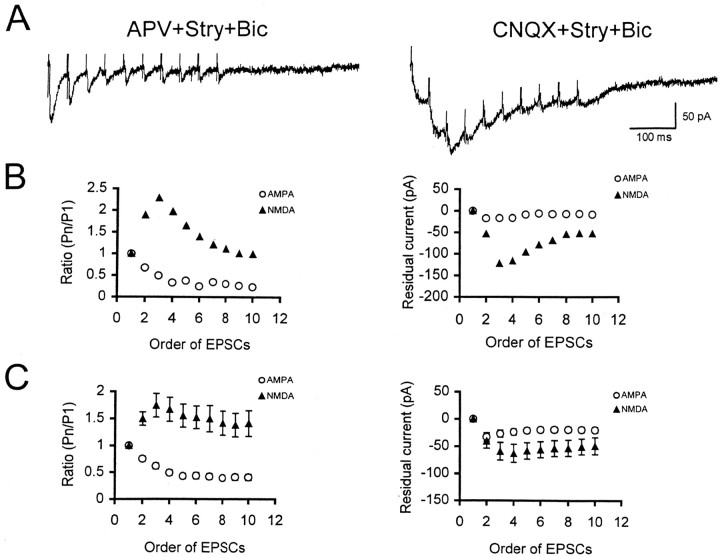
Figure 2A shows AMPA (left trace) and NMDA (right trace) EPSCs recorded from the same ICC neuron to 10 pulses of repetitive stimulation at 25 Hz. The amplitude of AMPA EPSCs progressively decreased over the period of 10 stimulus pulses. In contrast, there was an increase in the amplitude of NMDA EPSCs in responses to stimulus pulses 2-8 with a return toward the initial baseline level for pulses 9 and 10. We made a measurement of the amplitude of each EPSC (i.e., the peak current relative to the current level at the time of the first stimulus artifact) and calculated the ratio of each EPSC to the first one to estimate the extent of response depression or facilitation. The ratios (Pn/P1) of the amplitude of each EPSC were then plotted as a function of the order of stimulus pulse presentation for AMPA and NMDA EPSCs, respectively (Fig. 2B, left). A summary of the average ratios of AMPA (n = 19) and NMDA (n = 9) EPSCs at a stimulation rate of 25 Hz is shown in Figure 2C (left). The data indicate that AMPA EPSCs were depressed but NMDA EPSCs were facilitated and showed temporal summation over the 10 pulses of stimulation.
Figure 2.
Characteristics of AMPA and NMDA EPSCs elicited by 10 pulses of stimulation at 25 Hz. A, Left trace, AMPA EPSCs were isolated after application of APV, strychnine, and bicuculline. Right trace, NMDA EPSCs were isolated after application of CNQX, strychnine, and bicuculline. The vertical line indicates a stimulus artifact. B, Left, Ratios of the amplitude (measured from the peak of EPSCs to the baseline) of Pn relative to the first EPSC (P1) in the series of responses mediated by AMPA and NMDA receptors were plotted separately as a function of the order of stimulus pulses. Right, RCs (measured from the end of each response to the baseline) of each AMPA and NMDA EPSCs were plotted as a function of the order of stimulus pulses. C, Left, Average ratios of AMPA (n = 19) and NMDA (n = 9) EPSCs were plotted as a function of the order of stimulus pulse. Right, Average RCs of AMPA and NMDA EPSCs recorded from the same group of neurons were plotted as a function of the order of stimulus pulses. Note that SEs were very small and the error bars for AMPA responses shown in C (left and right) are hard to discern.
We also noted that each EPSC, especially the NMDA one, did not return to the baseline level. We measured this current as a residual current (RC) (von Gersdorff et al. 1997). The RCs of each AMPA and NMDA EPSC for the neuron shown in Figure 2A (Fig. 2B, right) and the average RCs of AMPA (n = 19) and NMDA (n = 9) EPSCs at a stimulation rate of 25 Hz (Fig. 2C, right) were plotted as a function of the stimulus order. AMPA and NMDA EPSCs exhibited different levels of RCs. For the neuron shown in Figure 2A, the RC for AMPA EPSCs was very small (<20 pA), whereas the RC for NMDA EPSCs was much greater (>50 pA). The average maximal RC for the AMPA and NMDA EPSCs was 33.2 ± 7.8 and 63.0 ± 16.7 pA, respectively. Over the period of repetitive stimulation, the change in the amplitude of NMDA EPSCs was consistent with a change in the RC, suggesting that the large RC accounts for facilitation and temporal summation of the NMDA EPSCs.
We then examined whether the extent of synaptic depression of AMPA EPSCs and facilitation of NMDA EPSCs were dependent on stimulus rate. The results for a representative neuron and averages for all cells are given in Figures 3 and 4 (sample sizes for each stimulus rate are indicated in the figure legend). Figure 3A shows pharmacologically isolated AMPA EPSCs recorded from an ICC neuron to repetitive stimulation at 12.5, 25, 50, and 100 Hz. Ten pulses of repetitive stimulation elicited 10 discrete responses at each of the four stimulus frequencies. The EPSCs progressively decreased and tended to stabilize after the fourth response (Fig. 3A). Comparing response ratios during the later, relatively stable part of the sequence (i.e., the responses to pulses 4-10), one can see that EPSC amplitudes underwent a greater reduction at 25 Hz than 12.5 Hz. (Fig. 3B). At higher stimulus frequencies (50 and 100 Hz), the ratios were slightly larger than those at low stimulus frequencies, which might be attributable to the larger RC produced at higher stimulus rates (Fig. 3B). Indeed, the RC was progressively larger as the stimulus rate was increased (Fig. 3C). However, at high rates, unlike NMDA EPSCs, AMPA EPSCs did not show facilitation, although the degree of AMPA EPSC depression was reduced.
Figure 3.
AMPA EPSCs in response to repetitive stimulation were rate dependent. A, AMPA EPSCs recorded from an ICC neuron were elicited by repetitive stimulation at 12.5, 25, 50, and 100 Hz after blocking NMDA, GABAA, and glycine receptors with APV, bicuculline, and strychnine, respectively. The vertical line indicates a stimulus artifact. B, Ratios of EPSCs (Pn) relative to the first EPSC (P1) at each of the four rates of stimulation were plotted as a function of the order of stimulus pulses. C, RCs for each EPSC elicited by stimulation at these four rates were plotted as a function of the order of the stimulus pulses. D, Average ratios for stimulus rates of 12.5 Hz (n = 13), 25 Hz (n = 13), 50 Hz (n = 13), and 100 Hz (n = 8). E, Average RCs for 12.5 Hz (n = 16), 25 Hz (n = 17), 50 Hz (n = 16), and 100 Hz (n = 8).
Figure 4.
NMDA EPSCs in response to repetitive stimulation were rate dependent. A, NMDA EPSCs recorded from an ICC neuron were elicited by repetitive stimulation at 12.5, 25, and 50 Hz after blocking AMPA, GABAA, and glycine receptors with CNQX, bicuculline, and strychnine, respectively. The vertical line indicates a stimulus artifact. B, Ratios of EPSCs (Pn) to the first EPSC (P1) at each of these three rates were plotted as a function of the order of stimulus pulses. C, RCs for each EPSC elicited at these three rates were plotted as a function of the order of stimulus pulses. D, Average ratios for stimulus rates of 12.5 Hz (n = 9), 25 Hz (n = 10), 50 Hz (n = 8), and 100 Hz (n = 5). E, Average RCs for 12.5 Hz (n = 10), 25 Hz (n = 10), 50 Hz (n = 7), and 100 Hz (n = 5).
The average ratios and RCs are shown in Figure 3, D and E, respectively. Similar results were obtained in each of the neurons investigated. The average curves were consistent with those found in individual neurons. The absolute values of ratios and RCs varied somewhat among neurons, but the general relationship between response magnitude and stimulus rate was the same for all cells.
Figure 4A shows pharmacologically isolated NMDA EPSCs recorded from an ICC neuron at stimulus repetitive rates of 12.5, 25, and 50 Hz. At 12.5 Hz, individual EPSCs were seen for each pulse of stimulus current. At 25 and 50 Hz, however, the subsequent EPSCs were obscured and summed to create a large inward current, which outlasted the stimulus train for several hundred milliseconds. The ratios of the EPSC amplitude over the period of 10 stimulus pulses were larger for higher stimulus rates (Fig. 4B), indicating a rate dependence of facilitation with temporal summation of the NMDA EPSCs. The RC also increased more with high stimulus rates (Fig. 4C). The larger RC seems to account for the larger extent of temporal summation of the NMDA EPSCs.
The average data are shown in Figure 4, D and E, respectively. The results for individual neurons were consistent with each other and similar to those shown in the average curves.
NMDA EPSCs to repetitive stimulation at rates of 20 Hz and higher exhibited a considerable temporal summation in every ICC neuron tested (n = 10). Therefore, we further analyzed the characteristics of temporal summation of NMDA EPSCs. Figure 5A shows pharmacologically isolated NMDA EPSCs to repetitive stimulation at 20 and 50 Hz in three ICC neurons (top, middle, and bottom traces). Individual NMDA EPSCs to each stimulus pulse were hardly discernible. At the higher rate (50 Hz), 10 EPSCs merged to become an even larger inward current, which reached its peak quicker than that elicited by the lower rate of stimulation. The range of rise times (10-90%) for the 10 summed NMDA EPSCs in all six cells was 31-114 msec at 20 Hz and 21-49 msec at 100 Hz (Fig. 5B). However, the summed inward current decayed more slowly at higher than at lower rates (Fig. 5A). The half-width of summed NMDA EPSCs varied between 200 and 550 msec for different neurons but was relatively constant across different stimulus rates for each individual neuron (Fig. 5C). Figure 5D shows the maximum RC during repetitive stimulation at 10, 20, 25, 50, and 100 Hz for seven neurons. Usually, higher rates resulted in larger RCs.
Figure 5.
Characteristics of summed NMDA EPSCs. A, NMDA EPSCs recorded from three ICC neurons (top, middle, and bottom traces) were elicited by repetitive stimulation at 20 Hz (left traces) and 50 Hz (right traces) after blocking AMPA, GABAA, and glycine receptors with CNQX, bicuculline, and strychnine, respectively. The vertical line indicates a stimulus artifact. B, Rise time (10-90%) of the summed NMDA EPSCs recorded from six neurons was plotted as a function of stimulus rate. Each curve represents one neuron. C, Half-widths (time course at half amplitude) of summated NMDA EPSCs recorded from six neurons were plotted as a function of stimulus rate. D, Maximum RCs of NMDA EPSCs recorded from seven neurons were plotted as a function of stimulus rate. The data shown for cells 1, 2, and 3 in A are represented by •, ○, and ▴, respectively.
Inhibitory response mediated by GABAA receptors
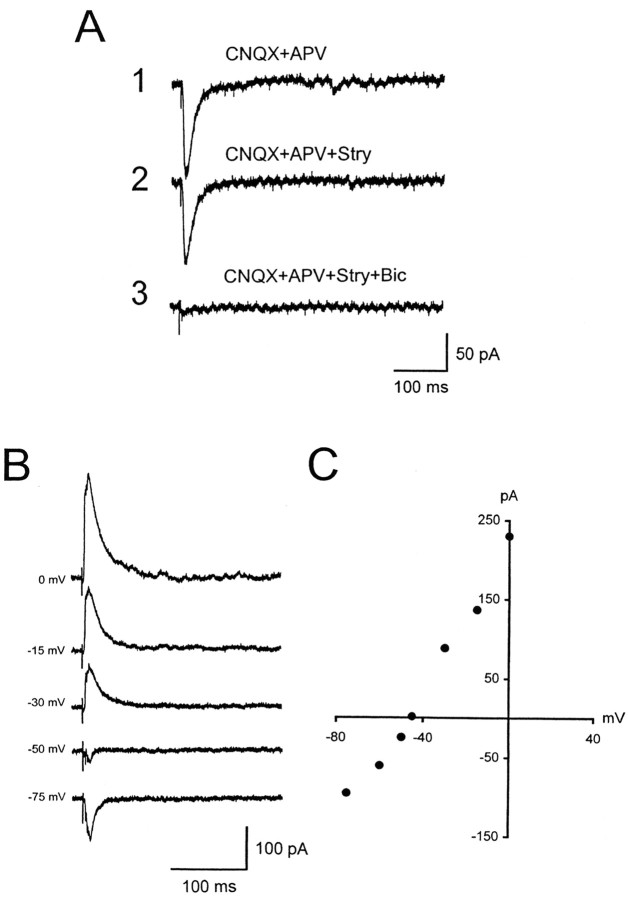
IPSCs were recorded after blocking AMPA and NMDA receptors with CNQX and APV in 36 neurons (Fig. 6A, trace 1). As shown previously (Ma et al., 2002b), the IPSCs were GABAergic and were largely blocked by the GABAA receptor antagonist bicuculline (Fig. 6A, trace 3). They were not greatly affected by the glycine receptor antagonist strychnine (Fig. 6A, trace 2). Pharmacologically isolated GABAergic IPSCs to a single pulse of stimulation were recorded with the slice in ACSF containing CNQX, APV, and strychnine. Figure 6B shows GABAergic IPSCs recorded from an ICC neuron held at various membrane potentials. The IPSCs were inward at membrane potentials of -50 mV or lower and outward at membrane potentials of -40 mV or higher. The reversal potential was -45 mV (Fig. 6C). The average reversal potential of the IPSCs for all neurons tested (n = 11) was -50.0 ± 1.5 mV. Because the junction potential was -15 mV, the corrected reversal potential was -65 mV, which is close to the theoretical equilibrium potential (-68.5 mV) of chloride ions with the extracellular and intracellular concentrations used in this study.
Figure 6.
Inhibition in the ICC was mediated by GABAA receptors. A1, An inward current recorded from an ICC neuron was elicited by a single pulse of stimulation after blocking AMPA and NMDA receptors with CNQX and APV. A2, Adding strychnine had little effect on the inward current. A3, After adding bicuculline, the inward current was completely blocked, indicating that this current was mediated by GABAA receptors. B, Pharmacologically isolated GABAergic IPSCs (in the presence of CNQX, APV, and strychnine) were recorded from an ICC neuron at membrane potentials of 0, -15, -30, -50, and -75 mV. The vertical line indicates a stimulus artifact. C, The current-voltage relationship of the GABAergic IPSCs shown in B.
The kinetics of the GABAergic IPSCs were closer to those of the AMPA EPSCs than to those of the NMDA EPSCs (Table 1). The rise time and time to peak for the AMPA and GABAA receptor-mediated responses were very close to each other. The decay time was only slightly longer, but the half-width was nearly twice as long as that of the AMPA EPSCs (t test; p < 0.05). However, the rise time and decay time, time to peak, and half-width of the GABAergic IPSCs were all much shorter than those of the NMDA EPSCs (t test; p < 0.01).
We next examined GABAergic IPSCs to repetitive stimulation at 10-100 Hz (n = 12). Figure 7A shows an example. GABAergic IPSCs were recorded from an ICC neuron held at -30 mV. At 10, 20, 50, and 100 Hz, individual GABAergic IPSCs to each pulse of stimulation were discernible. The amplitude of the individual GABAergic IPSCs progressively decreased over the 10 current pulses at 10-20 Hz (Fig. 7A). At 50 and 100 Hz, the IPSCs to pulses 2-5 showed facilitation and subsequent IPSCs showed a gradual decline in amplitude (Fig. 7A). The higher the rate, the larger was the degree of facilitation (Fig. 7B). These results demonstrate that GABAA IPSCs show a response depression at lower rates of stimulation but facilitation during the first few stimulus pulses at higher rates. Like AMPA and NMDA EPSCs, GABAA IPSCs also had RCs that were rate dependent. The higher the rate, the larger the RC became. For the neuron shown in Figure 7A, the RC was <20 pA at 10-20 Hz but increased to 50-150 pA at 50-100 Hz (Fig. 7C). The RC promoted facilitation with temporal summation of the GABAA IPSCs.
Figure 7.
Characteristics of GABAergic IPSCs in response to repetitive stimulation. A, Pharmacologically isolated GABAergic IPSCs recorded from an ICC neuron were elicited by repetitive stimulation at 10, 20, 50, and 100 Hz after application of kynurenic acid and strychnine. Kynurenic acid and strychnine were used to block glutamatergic excitatory responses and glycinergic inhibitory responses, respectively. The vertical line indicates a stimulus artifact. B, Ratios of IPSCs (Pn) relative to the first IPSC (P1) were plotted as a function of the order of stimulus pulses at each of the four stimulus rates. C, RCs for the IPSCs were plotted as a function of the order of stimulus pulses at the four stimulus rates. D, Average ratios for stimulus rates of 12.5 Hz (n = 11), 25 Hz (n = 12), 50 Hz (n = 12), and 100 Hz (n = 9). E, Average RCs for 12.5 Hz (n = 12), 25 Hz (n = 12), 50 Hz (n = 12), and 100 Hz (n = 9).
Average data for ratios and RCs are shown in Figure 7, D and E, respectively (see figure legend for sample sizes). The results obtained from individual neurons were consistent with those shown in the average curves. The incidence of facilitation at 50 Hz and higher was much greater for GABAA than AMPA receptor-mediated responses (χ2 = 14.75; df = 1; p < 0.001).
Role of GABAA receptors in response to repetitive stimulation
The effect of activation of GABAA receptors on temporal processing in ICC neurons was investigated by examining responses to 10 pulses of repetitive stimulation before and after blocking GABAA receptors with bicuculline (n = 3). As shown in Figure 8, synaptic responses were recorded from an ICC neuron under two conditions: normal ACSF (left) and ACSF containing bicuculline (right). With the slice in normal ACSF, individual responses to each pulse of repetitive stimulation were discernible at the four rates of stimulation tested (20, 25, 50, and 100 Hz), but the amplitude of the responses progressively decreased over the stimulus period. In contrast, after blocking GABAA receptors, individual responses after the first pulse were barely discernible, especially at higher stimulation rates. The 10 responses merged and became a large and long-lasting (several hundreds of milliseconds) inward current, a pattern that was very similar to that of summed NMDA EPSCs described above. Similar results were obtained from all three cells. The results suggest that blocking GABAA receptors led to uninhibited activation of NMDA receptors, therefore rendering the neuron incapable of producing discrete responses to each pulse of repetitive stimulation.
Figure 8.
Effect of bicuculline on responses to repetitive stimulation. Inward currents recorded from an ICC neuron were elicited by repetitive stimulation at rates of 20, 25, 50, and 100 Hz with the brain slice in normal ACSF (left traces) and in ACSF containing bicuculline (right traces). The vertical line indicates a stimulus artifact.
Effect of GABAergic inhibition on AMPA and NMDA receptor-mediated EPSCs
To investigate further how activation of GABAA receptors affects AMPA and NMDA receptor-mediated responses to repetitive stimulation, we examined isolated AMPA EPSCs (n = 3) and NMDA EPSCs (n = 3) with or without GABAergic activity. Figure 9A shows an example of the interaction between GABAergic activity and AMPA EPSCs to 50 Hz stimulation in an ICC neuron. After blocking NMDA and glycine receptors with APV and strychnine, AMPA EPSCs were recorded without blocking GABAA receptors (Fig. 9A1). Then, the AMPA EPSCs were recorded alone after blocking NMDA, glycine, and GABAA receptors with APV, strychnine, and bicuculline, respectively (Fig. 9A2). The neuron responded to each pulse of stimulation with a clear discrete inward current. In the presence of GABAergic activity, the AMPA EPSCs were not depressed but showed a slight facilitation (i.e., the ratios of the EPSCs were larger than 1). Without GABAergic activity, however, the AMPA EPSCs underwent a depression (i.e., the ratios were smaller than 1) (Fig. 9A3). Similar results were obtained from all three neurons. These results indicate that GABAergic activity led to a lesser degree of synaptic depression compared with that seen for AMPA EPSCs alone.
Figure 9.
Effect of bicuculline on AMPA and NMDA EPSCs. A, Inward currents recorded from an ICC neuron were elicited by repetitive stimulation at 50 Hz with the brain slice in APV and strychnine (A1), and in APV, strychnine, and bicuculline (A2). The responses in A1 contained both AMPA and GABAA receptor-mediated components. The responses in A2 contained AMPA EPSCs only. Ratios of the EPSCs (Pn) relative to the first EPSC (P1) were plotted as a function of the order of stimulus pulses (A3). B, Inward currents recorded from an ICC neuron for repetitive stimulation at 50 Hz with the brain slice in CNQX and strychnine (B1) and in CNQX, strychnine, and bicuculline (B2). The responses in B1 contained NMDA and GABAA receptor-mediated components. The responses in B2 contained NMDA EPSCs only. Ratios of EPSCs (Pn) relative to the first EPSC (P1) were plotted as a function of the order of stimulus pulses (B3). The vertical line indicates a stimulus artifact.
Figure 9B shows an example of the interaction between NMDA EPSCs and GABAA IPSCs to repetitive stimulation of 50 Hz. NMDA EPSCs in the presence of a GABAergic activity were recorded after blocking AMPA and glycine receptors with CNQX and strychnine (Fig. 9B1). The NMDA EPSCs were recorded alone after blocking AMPA, glycine, and GABAA receptors with CNQX, strychnine, and bicuculline, respectively (Fig. 9B2). Under neither condition was the neuron able to respond to each pulse of repetitive stimulation with a discrete inward current. Rather, the EPSCs showed substantial temporal summation. The NMDA EPSCs alone exhibited more summation than the NMDA EPSCs with GABAergic activity (Fig. 9B3). Similar results were obtained from all three cells. These results suggest that activation of GABAA receptors decreased the extent of temporal summation of the NMDA EPSCs.
Role of inhibition in synaptic integration
To examine further the contribution of synaptic inhibition to temporal processing in ICC neurons, we compared normal synaptic responses and responses with blocked GABAergic and glycinergic inhibition at the membrane potential of the cell at -40 mV (n = 8). Figure 10 shows an example. This neuron responded to a single pulse of stimulation in normal ACSF with an initial inward PSC, followed by a small outward current (Fig. 10A, left trace). After blocking glycine and GABAA receptors with strychnine and bicuculline, respectively, the response became exclusively inward, consisting of a large, fast current with a short duration, followed by a slower, longer current (Fig. 10A, right trace). Similar results were found in all eight neurons. The early part of the inward response was likely mediated by AMPA receptors, and the late component was likely mediated by NMDA receptors (Ma et al., 2002a).
Figure 10.
Effect of synaptic inhibition on responses to single and repetitive stimulation. A, An inward current, followed by an outward current, recorded from an ICC neuron with the membrane potential held at -40 mV was elicited by a single pulse of stimulation with the brain slice in normal ACSF (left trace) and in ACSF containing strychnine and bicuculline (right trace). B-D, Responses to repetitive stimulation at 25, 50, and 100 Hz with the brain slice in normal ACSF (left traces) and in ACSF containing strychnine and bicuculline (right traces). The vertical line indicates a stimulus artifact.
This neuron responded to 10 pulses of repetitive stimulation at 25-100 Hz with 10 discrete PSCs in normal ACSF (Fig. 10B-D, left traces). Once the synaptic inhibition was blocked, however, the neuron could not follow each stimulus pulse after the first few current pulses. The later responses merged and became one large inward current, which generated several small spikes unsynchronized to the repetitive stimulus. At 25 Hz, the neuron continued to respond to later pulses with discrete EPSCs of smaller amplitude and longer time course (Fig. 10B, right trace). At higher stimulus rates (50-100 Hz), the subsequent EPSCs became a single large inward current after the first one or two EPSCs. The large inward current outlasted the stimulus period (Fig. 10C,D, right traces) and was very similar to the summed NMDA EPSC. These results suggest that synaptic inhibition, most likely GABAergic, exerts a profound effect on the temporal response pattern to repetitive stimulation of synaptic inputs in ICC neurons.
Discussion
Receptor kinetics
We examined temporal characteristics of AMPA, NMDA, and GABAA receptor-mediated responses in the ICC and determined how the kinetics of these receptors contribute to temporal features of responses to repetitive stimulation. We found that the AMPA receptors had the fastest kinetics. AMPA EPSCs could follow separate pulses of repetitive stimulation over the range of 10-100 Hz, although the responses were depressed at higher rates. There was no evidence of response facilitation at any rate of stimulation. The IPSCs mediated by GABAA receptors could follow repetitive current pulse stimulation up to 100 Hz. The GABAA receptors and AMPA receptors had similar rise times and times to peak for channel opening, but GABAA receptors had longer half-widths for channel closing compared with AMPA receptors. This difference probably accounts for the slight facilitation at 50 Hz or higher found with GABAA receptor-mediated responses.
The NMDA receptors had the slowest kinetics. The NMDA EPSCs were unable to follow pulses of repetitive stimulation at rates above 25 Hz and exhibited facilitation at rates between 20 and 100 Hz. Similar results have been reported in cerebellar granule cells (D'Angelo et al., 1995). Both rise and decay times of the NMDA EPSCs in ICC neurons were around three to six times longer than either AMPA EPSCs or GABAA IPSCs. The slow rise and decay times of the NMDA EPSCs are attributed to the kinetics of NMDA receptor channels (Lester et al., 1994; Johnston and Wu, 1995). Because the average decay time (∼137 msec) was longer than the interval between stimulus pulses at 10 Hz or higher, single NMDA EPSCs could not return to baseline before the next pulse of stimulation, resulting in RCs and leading to temporal summation of EPSCs.
Response depression
Both AMPA and GABAA responses showed depression during repetitive stimulation. This reduction in response amplitude resembled short-term synaptic plasticity of responses in other neural systems (Malenka and Siegelbaum, 2001). The depression of AMPA EPSCs and GABAA IPSCs is probably attributable to a presynaptic mechanism. Several hypotheses have been proposed to explain how a presynaptic mechanism could account for synaptic depression. These include presynaptic calcium current inactivation (Wu and Saggau, 1997), regulation of transmitter release by metabotropic receptors (Trussell, 2002), or depletion of the pool of releasable synaptic vesicles (Liu and Tsien, 1995). Also, at the calyceal synapse of neurons in the medial nucleus of the trapezoid body in the auditory brainstem, glutamate was found to feed back onto presynaptic terminals, inhibiting calcium channels and transmitter release through presynaptic metabotropic glutamate receptors (Barnes-Davies and Forsythe, 1995; Takahashi et al., 1996). Evidence from our own previous studies suggests that activation of presynaptic GABAB receptors can reduce the strength of GABAergic inhibition (Ma et al., 2002b) and glutamatergic excitation (Wu et al., 2004) by reducing transmitter release in the ICC. In the present study, AMPA and GABAA receptor-mediated responses were elicited by stimulation of the lemniscal pathway, which releases both glutamate and GABA at terminals in the ICC. Thus, GABA molecules were probably released while recording EPSCs, although the GABAA receptors were blocked. If GABAergic and glutamatergic terminals were located in close proximity to each other on the neurons from which recordings were made, it is possible that GABA molecules reached glutamate terminals to activate GABAB receptors, thus reducing glutamate release. Also, during recordings of GABAergic IPSCs, GABA molecules could have affected presynaptic GABAB receptors on GABAergic terminals to reduce GABA release.
A postsynaptic mechanism (i.e., receptor desensitization) is a possible, but less likely, explanation of the response depression found in our study. Although AMPA receptor desensitization has been shown to play a role in synaptic depression in avian auditory neurons (e.g., the nucleus magnocellularis) (Zhang and Trussell, 1994), this type of postsynaptic change has not been shown in mammalian auditory neurons (e.g., mouse medial nucleus of the trapezoid body) (Wang and Kaczmarek, 1998) or most other central synapses that have been studied (Hestrin, 1992; Dittman and Regehr, 1998; Chen et al., 1999; Hjelmstad et al., 1999; Chen et al., 2002). Nevertheless, AMPA receptor desensitization could serve as a mechanism for synaptic depression at specialized synapses that have a high probability of transmitter release and a long time course of transmitter clearance (Chen et al., 2002). Excitatory synapses in the ICC are the nonspecialized bouton type (Oliver, 1985; Shneiderman and Oliver, 1989). Thus, receptor desensitization would not likely account for the depression of AMPA EPSCs in the ICC. Also desensitization of the GABA receptor, which develops slowly (within seconds) (Hosomi et al., 1997; Mellor and Randall, 2001; Behrends et al., 2002), is not a likely candidate for explaining the depression of GABAA IPSCs observed within the millisecond range in the present study.
Role of GABAA receptors
In the ICC, GABAA receptor-mediated IPSPs result in membrane hyperpolarization (Ma et al., 2002b) caused by the influx of Cl- ions (Metherate and Ashe, 1993; Hosomi et al., 1997). Hyperpolarization has different effects on AMPA and NMDA responses. After the GABAergic response is blocked, the initial EPSP mediated by AMPA receptors increases, and a slow and long-lasting EPSP mediated by NMDA receptors appears. These results suggest that hyperpolarization normally counteracts and reduces AMPA receptor-mediated response and suppresses activation of NMDA receptors (Ma et al., 2002a). Other studies have shown that GABAA receptors limit the extent of temporal summation of NMDA receptor-mediated responses in the piriform cortex (Kanter et al., 1996).
The present study also shows an interaction between GABAergic inhibition and NMDA receptor-mediated excitation. Pharmacological block of GABAA receptors revealed a long-lasting NMDA receptor-mediated response. Thus, under normal conditions, the presence of GABAergic inhibition likely controls and regulates the expression of NMDA responses to acoustic stimulation.
Usually, expression of NMDA responses is dependent on depolarization of the cell membrane (Hestrin et al., 1990; Ma et al., 2002a). This raises the question of how NMDA receptor-mediated responses emerged after the GABAA receptors were blocked with bicuculline under the voltage-clamp conditions used in the present study. One possibility is that a membrane depolarization occurred at the distal dendrites, although the voltage was clamped at the recording site. Thus, a depolarization might have activated NMDA receptors even under voltage-clamp conditions.
Functional significance in vivo
Electrophysiological studies in vivo have shown that AMPA, NMDA, and GABAA receptors all contribute to auditory responses in adults, although there is a progressive reduction in the proportion of NMDA receptors during early development (Isaacson and Walmsley, 1995; Ma et al., 2002a). For example, antagonists of both AMPA and NMDA receptors reduce the firing rate of ICC neurons over a wide range of sound intensities (Zhang and Kelly, 2002). Inhibition mediated by GABAA receptors in the ICC has been suggested to play an important role in various aspects of auditory processing, e.g., temporal firing patterns (LeBeau et al., 1996), binaural responses (Klug et al., 1995), responses to frequency modulation (Koch and Grothe, 1998), frequency response areas (LeBeau et al., 2001), responses to sound duration (Casseday et al., 2000), and auditory motion-direction sensitivity (Kautz and Wagner, 1998; McAlpine and Palmer, 2002). All these effects result from interaction between glutamatergic excitation and GABAA receptor-mediated inhibition at specific times in response to specific parameters of acoustic stimulation. The ICC is a pivotal place in the central auditory system for the integration of excitatory and inhibitory signals coming from various auditory brainstem nuclei. AMPA receptor-mediated excitation in the ICC is suitable for conveying precise timing information. Although the AMPA EPSCs undergo depression in response to repetitive excitatory inputs, the depression can be offset by GABAergic inhibition if the inhibitory effect occurs as soon as or earlier than the excitatory event. Intracellular recordings from ICC neurons in the intact cat show that the latencies of inhibitory responses are often shorter than those of excitatory ones (Kuwada et al., 1997). Thus, GABAA receptors can exert a powerful moderating effect on the strength of excitatory responses evoked by acoustic stimulation.
NMDA receptors in the ICC are implicated in development (Wenzel et al., 1996; Caicedo and Eybalin, 1999), auditory learning (Feldman and Knudsen, 1998), long-term plasticity (Zhang and Wu, 2000; Wu et al., 2002), and pathological conditions (e.g., audiogenic seizures) (Pierson et al., 1989; Yasuda et al., 2000). The role of NMDA receptors in auditory processing is still not fully understood. The present study demonstrates that the extent of activation of NMDA receptors is activity dependent and NMDA receptors are suppressed while GABAA receptors are activated. The suppression of NMDA receptors is important for retaining temporal precision of physiological responses in the auditory system. GABAergic activity is an important cellular mechanism for establishing the normal balance of excitation and inhibition in the ICC and serves as a gate to control activation and modulation of ongoing activity mediated by glutamate receptors.
Footnotes
This work was supported by research grants from the Natural Sciences and Engineering Council of Canada (S.H.W., J.B.K.) and the Hearing Foundation of Canada (S.H.W.).
Correspondence should be addressed to Dr. Shu Hui Wu, Institute of Neuroscience, 335 Life Sciences Research Building, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6, Canada. E-mail: shwu@ccs.carleton.ca.
Copyright © 2004 Society for Neuroscience 0270-6474/04/244625-10$15.00/0
References
- Barnes-Davies M, Forsythe ID (1995) Pre- and postsynaptic glutamate receptors at a giant excitatory synapse in rat auditory brainstem slices. J Physiol (Lond) 488: 387-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends JC, Lambert JDC, Jensen K (2002) Repetitive activation of postsynaptic GABAA receptors by rapid, focal agonist application onto intact rat striatal neurones in vitro. Eur J Physiol 443: 707-712. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Eybalin M (1999) Glutamate receptor phenotypes in the auditory brainstem and mid-brain of the developing rat. Eur J Neurosci 11: 51-74. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Covey E (1995) Mechanisms for analysis of auditory temporal patterns in the brainstem of echolocating bats. In: Neural representation of temporal patterns (Covey E, Hawkins HL, Port RF, eds) pp 25-51. New York: Plenum.
- Casseday JH, Ehrlich D, Covey E (2000) Neural measurement of sound duration: control by excitatory-inhibitory interactions in the inferior colliculus. J Neurophysiol 84: 1475-1487. [DOI] [PubMed] [Google Scholar]
- Chen C, Blitz DM, Regehr WG (2002) Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron 33: 779-788. [DOI] [PubMed] [Google Scholar]
- Chen CY, Horowitz JM, Bonham AC (1999) A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am J Physiol 277: H1350-H1360. [DOI] [PubMed] [Google Scholar]
- Covey E, Kauer JA, Casseday JH (1996) Whole-cell patch-clamp recording reveals subthreshold sound-evoked postsynaptic currents in the inferior colliculus of awake bats. J Neurosci 16: 3009-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, De Filippi G, Rossi P, Taglietti V (1995) Synaptic excitation of individual rat cerebellar granule cells in situ: evidence for the role of NMDA receptors. J Physiol (Lond) 484: 397-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman JS, Regehr WG (1998) Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci 18: 6147-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Knudsen EI (1998) Pharmacological specialization of learned auditory responses in the inferior colliculus of the barn owl. J Neurosci 18: 3073-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara BM, Casseday JH, Covey E, Schwartz-Bloom RD (1996) Distribution of GABAA, GABAB, and glycine receptors in the central auditory system of the big brown bat, Eptesicus fuscus J Comp Neurol 369: 83-92. [DOI] [PubMed] [Google Scholar]
- Gaza WC, Ribak CE (1997) Immunocytochemical localization of AMPA receptors in the rat inferior colliculus. Brain Res 774: 175-183. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN (1988) Neuroanatomical distribution of receptors for three potential inhibitory neurotransmitters in the brainstem auditory nuclei of the cat. J Comp Neurol 275: 288-308. [DOI] [PubMed] [Google Scholar]
- Glendenning KK, Baker BN, Hutson KA, Masterton RB (1992) Acoustic chiasm V: Inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol 319: 100-112. [DOI] [PubMed] [Google Scholar]
- González-Hernández T, Mantolán-Sarmiento B, González-González B, Pérez-González H (1996) Sources of GABAergic input to the inferior colliculus of the rat. J Comp Neurol 372: 309-326. [DOI] [PubMed] [Google Scholar]
- Hestrin S (1992) Activation and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron 9: 991-999. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P (1990) Analysis of excitatory action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol (Lond) 422: 203-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, Isaac JT, Nicoll RA, Malenka RC (1999) Lack of AMPA receptor desensitization during basal synaptic transmission in the hippocampal slice. J Neurophysiol 81: 3096-3099. [DOI] [PubMed] [Google Scholar]
- Hosomi H, Mori M, Amatsu M, Okada Y (1997) GABA-activated conductance in cultured rat inferior colliculus neurons. J Neurophysiol 77: 994-1002. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B (1995) Receptors underlying excitatory synaptic transmission in slices of the rat anteroventral cochlear nucleus. J Neurophysiol 73: 964-973. [DOI] [PubMed] [Google Scholar]
- Johnston D, Wu SM-S (1995) Foundation of cellular neurophysiology. Cambridge, MA: MIT.
- Kanter ED, Kapur A, Haberly LB (1996) A dendritic GABAA-mediated IPSP regulates facilitation of NMDA-mediated responses to burst stimulation of afferent fibers in piriform cortex. J Neurosci 16: 307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz D, Wagner H (1998) GABAergic inhibition influences auditory motion-direction sensitivity in barn owls. J Neurophysiol 80: 172-185. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Zhang H (2002) Contribution of AMPA and NMDA receptors to excitatory responses in the inferior colliculus. Hear Res 168: 35-42. [DOI] [PubMed] [Google Scholar]
- Klug A, Park TJ, Pollak GD (1995) Glycine and GABA influence binaural processing in the inferior colliculus of the mustache bat. J Neurophysiol 74: 1701-1713. [DOI] [PubMed] [Google Scholar]
- Koch U, Grothe B (1998) GABAergic and glycinergic inhibition sharpens tuning for frequency modulations in the inferior colliculus of the big brown bat. J Neurophysiol 80: 71-82. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Batra R, Yin TCT, Oliver D, Haberly LB, Stanford TR (1997) Intracellular recordings in response to monaural and binaural stimulation of neurons in the inferior colliculus of the cat. J Neurosci 17: 7565-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau FEN, Rees A, Malmieca MS (1996) Contribution of GABA- and glycine-mediated inhibition to the monaural temporal response properties of neurons in the inferior colliculus. J Neurophysiol 75: 902-919. [DOI] [PubMed] [Google Scholar]
- LeBeau FEN, Malmieca MS, Rees A (2001) Iontophoresis in vivo demonstrates a key role for GABAA and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J Neurosci 21: 7303-7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RAJ, Clements JD, Tong G, Westbrook GL, Jahr CE (1994) The time course of NMDA receptor-mediated synaptic currents. In: The NMDA receptor (Collingridge GL, Watkins JC, eds), pp 206-218. New York: Oxford UP.
- Li Y, Evans MS, Faingold CL (1998) In vitro electrophysiology of neurons in subnuclei of rat inferior colliculus. Hear Res 121: 1-10. [DOI] [PubMed] [Google Scholar]
- Liu G, Tsien RW (1995) Properties of synaptic transmission at single hippocampal synaptic boutons. Nature 375: 404-408. [DOI] [PubMed] [Google Scholar]
- Ma CL, Kelly JB, Wu SH (2002a) AMPA and NMDA receptors mediate synaptic excitation in the rat's inferior colliculus. Hear Res 168: 25-34. [DOI] [PubMed] [Google Scholar]
- Ma CL, Kelly JB, Wu SH (2002b) Presynaptic modulation of GABAergic inhibition by GABAB receptors in the rat's inferior colliculus. Neuroscience 114: 207-215. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Siegelbaum SA (2001) Synaptic plasticity: diverse targets and mechanisms for regulating synaptic efficacy. In: Synapses (Cowan WM, Südhof TC, Stevens CF, eds), pp 393-453. Baltimore: Johns Hopkins UP.
- McAlpine D, Palmer AR (2002) Blocking GABAergic inhibition increases sensitivity to sound motion cues in the inferior colliculus. J Neurosci 22: 1443-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JR, Randall AD (2001) Synaptically released neurotransmitter fails to desensitize postsynaptic GABAA receptors in cerebellar cultures. J Neurophysiol 85: 1847-1857. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH (1993) Ionic flux contributions to neocortical slow waves and nucleus basalis-mediated activation: whole-cell recordings in vivo J Neurosci 13: 5312-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Kotak VC, Sanes DH (1998) Commissural and lemniscal synaptic input to the gerbil inferior colliculus. J Neurophysiol 80: 2229-2236. [DOI] [PubMed] [Google Scholar]
- Oliver DL (1985) Quantitative analyses of axonal endings in the central nucleus of the inferior colliculus and distribution of 3H-labeling after injection in the dorsal cochlear nucleus. J Comp Neurol 237: 343-359. [DOI] [PubMed] [Google Scholar]
- Pedemonte M, Torterolo P, Velluti RA (1997) In vivo intracellular characteristics of inferior colliculus neurons in guinea pig. Brain Res 759: 24-31. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RL (1992) Light and electron microscopic localization of AMPA selective glutamate receptors in the rat brain. J Comp Neurol 318: 329-354. [DOI] [PubMed] [Google Scholar]
- Pierson MG, Smith KL, Swann JW (1989) A slow NMDA-mediated synaptic potential underlies seizures originating midbrain. Brain Res 486: 381-386. [DOI] [PubMed] [Google Scholar]
- Reetz G, Ehret G (1999) Inputs from three brainstem sources to identified neurons of the mouse inferior colliculus slice. Brain Res 816: 527-543. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Ostapoff E-M, Morest DK, Wenthold RL (1989) Glycine-immunoreactive projection of the cat lateral superior olive: possible role in midbrain ear dominance. J Comp Neurol 279: 382-396. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Geary WA, Frederick Wooten G, Rubel EW (1987) Quantitative distribution of the glycine receptor in the auditory brain stem of the gerbil. J Neurosci 7: 3793-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL (1989) EM autoradiography study of the projections from the dorsal nucleus of the lateral lemniscus: a possible source of inhibitory inputs to the inferior colliculus. J Comp Neurol 286: 28-47. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K (1996) Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science 274: 594-597. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Pedemonte M, Velluti RA (1995) Intracellular in vivo recording of inferior colliculus auditory neurons from awake guinea-pigs. Arch Ital Biol 134: 57-64. [PubMed] [Google Scholar]
- Trussell LO (2002) Modulation of transmitter release at giant synapses of the auditory system. Curr Opin Neurobiol 12: 400-404. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Schneggenburger R, Wei S, Neher E (1997) Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. J Neurosci 17: 8137-8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T (1996) Lemniscal input to identified neurons of the central nucleus of mouse inferior colliculus: an intracellular brain slice study. Eur J Neurosci 8: 1231-1239. [DOI] [PubMed] [Google Scholar]
- Wang LY, Kaczmarek LK (1998) High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394: 384-388. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Villa M, Mohler H, Benke D (1996) Developmental and regional expression of NMDA receptor subtypes containing the NR2D subunit in rat brain. J Neurochem 66: 1240-1248. [DOI] [PubMed] [Google Scholar]
- Winer JA, Larue DT, Pollak GD (1995) GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol 355: 317-353. [DOI] [PubMed] [Google Scholar]
- Wu L-G, Saggau P (1997) Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci 20: 204-223. [DOI] [PubMed] [Google Scholar]
- Wu SH, Ma CL, Sivaramakrishnan S, Oliver DL (2002) Synaptic modification in neurons of the central nucleus of the inferior colliculus. Hear Res 168: 43-54. [DOI] [PubMed] [Google Scholar]
- Wu SH, Ma CL, Kelly JB (2004) GABAB receptors modulate synaptic excitation in the rat's central and dorsal nuclei of the inferior colliculus. Assoc Res Otolaryngol Abstr 27: 201. [Google Scholar]
- Yasuda S, Ishida N, Higashiyama A, Morinobu S, Kato N (2000) Characterization of audiogenic-like seizures in naïve rats evoked by activation of AMPA and NMDA receptors in the inferior colliculus. Exp Neurol 164: 396-406. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Li L, Kelly JB, Wu SH (1998) GABAergic projections from the lateral lemniscus to the inferior colliculus of the rat. Hear Res 117: 1-12. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kelly JB (2001) AMPA and NMDA receptors regulate responses of neurons in the rat's inferior colliculus. J Neurophysiol 86: 871-880. [DOI] [PubMed] [Google Scholar]
- Zhang S, Trussell LO (1994) Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol (Lond) 480: 123-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wu SH (2000) Long-term potentiation in the inferior colliculus studied in rat brain slice. Hear Res 147: 92-103. [DOI] [PubMed] [Google Scholar]