Abstract
Learning and memory formation in intact animals is generally studied under defined parameters, including the control of feeding. We used associative olfactory conditioning of the proboscis extension response in honeybees to address effects of feeding status on processes of learning and memory formation. Comparing groups of animals with different but defined feeding status at the time of conditioning reveals new and characteristic features in memory formation. In animals fed 18 hr earlier, three-trial conditioning induces a stable memory that consists of different phases: a mid-term memory (MTM), translation-dependent early long-term memory (eLTM; 1–2 d), and a transcription-dependent late LTM (lLTM; ≥3 d). Additional feeding of a small amount of sucrose 4 hr before conditioning leads to a loss of all of these memory phases. Interestingly, the basal activity of the cAMP-dependent protein kinase A (PKA), a key player in LTM formation, differs in animals with different satiation levels. Pharmacological rescue of the low basal PKA activity in animals fed 4 hr before conditioning points to a specific function of cAMP–PKA cascade in mediating satiation-dependent memory formation. An increase in PKA activity during conditioning rescues only transcription-dependent lLTM; acquisition, MTM, and eLTM are still impaired. Thus, during conditioning, the cAMP–PKA cascade mediates the induction of the transcription-dependent lLTM, depending on the satiation level. This result provides the first evidence for a central and distinct function of the cAMP–PKA cascade connecting satiation level with learning.
Keywords: learning, memory, protein kinase, satiety, response, cAMP
Introduction
Formation of memory is a continuous process that has been shown to consist of several phases: a short-term memory (STM) and a mid-term memory (MTM) based on existing proteins and a long-term memory (LTM) that requires translation and transcription (Tully et al., 1994; DeZazzo and Tully, 1995; Huang, 1998). These different phases are induced and maintained by different molecular pathways. For example, the second messenger cAMP, which activates the cAMP-dependent protein kinase A (PKA), plays a key role in the induction of LTM and long-lasting neuronal changes (Davis, 1993, 1996; Frey et al., 1993; Abel et al., 1997; Fiala et al., 1999; Müller, 2000). Although LTM becomes obvious only after days, the action of the cAMP–PKA system is specifically required within a short time window during conditioning (Müller, 2000). Thus, during conditioning the cAMP–PKA cascade is believed to trigger events including the activation of transcription factors such as the cAMP response element-binding protein (CREB) (for review, see Yin and Tully, 1996; Silva et al., 1998). Parameters like the number and temporal succession of conditioning trials or stimulations strongly influence memory formation and induction of neuronal changes (Carew et al., 1972; Byrne, 1987; Tully et al., 1994; Kogan et al., 1997) and thus the underlying molecular mechanisms (Müller and Carew, 1998; Müller, 2000). Although many other factors, such as stress, satiation, etc., influence learning and memory formation, these variables are usually strictly controlled in experiments studying the molecular mechanisms underlying memory formation. In this paper we specifically address the role of the satiation level at the time of conditioning on learning mechanisms, using the associative olfactory conditioning paradigm in honeybees.
Conditioning the proboscis extension response (PER) consists of pairing an odor, the conditioned stimulus (CS), with a subsequent sucrose reward, the unconditioned stimulus (US). A single conditioning trial leads to the induction of a memory that decays over a few days and does not require translation or transcription (for review, see Menzel, 1999; Müller, 2002). If repeated conditioning trials are applied, however, the bees form a stable LTM that consists of a mid-term memory lasting up to several hours, requiring constitutive PKC activity (Grünbaum and Müller, 1998) and an LTM (≥1 d) that requires PKA action during the time window of conditioning (Müller, 1996, 2000; Fiala et al., 1999). Blocking transcription during conditioning, however, impairs only the late LTM (lLTM; ≥3 d) (Grünbaum and Müller, 1998; Wüstenberg et al., 1998), indicating the existence of a dissectible early LTM (eLTM) in the range of 1–2 d. Our results show that memory formation depends strongly on satiation status during conditioning. A slightly different feeding protocol leads to a loss of MTM and LTM induced by multiple trial conditioning and also impairs memory formation after a single conditioning trial. We were able to identify a function of the cAMP–PKA cascade in the induction of the transcription-dependent lLTM, mediated by satiation level. These findings demonstrate the first distinct molecular pathway connecting satiation with distinct aspects of memory formation.
Materials and Methods
(γ-32P)ATP (5000 Ci/mmol) was purchased from PerkinElmer Life Sciences (Brussels, Belgium). Emetine, anisomycin, actinomycin, and all other chemicals were obtained from Sigma (Deisenhofen, Germany).
Animal treatment and olfactory conditioning. Honeybees (Apis mellifera carnica) were caught when leaving the hives for foraging. The experiments were performed from April to October in 2001 with bees from different hives. Bees were immobilized by cooling and harnessed in metal tubes with a strip of tape between the head and the thorax. In the evening, 18 hr before olfactory conditioning, all bees were fed to satiation (1 m sucrose solution) and kept overnight in darkness in a container at 70% relative humidity and 20–25°C. To adjust for different satiation levels, groups of bees were fed with 15 μl sucrose solution (1 m) 4 hr before conditioning (animals are not satiated by this treatment). When pharmacological treatments were given, 1 μl of the drug solution was injected into the hemolymph of the animals 30 min before conditioning using a capillary inserted through a small hole poked into the thorax (Müller and Hildebrandt, 2002).
The responsiveness to sucrose solution was tested 15 min before conditioning. Using an interval of 2 min, two successive sucrose stimulations (0.1 m sucrose followed by 1 m sucrose) were applied to an antenna. The extension of the proboscis caused by sucrose stimulation was monitored and used as a measurement of the sucrose responsiveness of each bee.
The following olfactory conditioning of the PER (Kuwabara, 1957) consisted of a temporal pairing of an odor as CS, with 1 m sucrose (stimulation of an antenna followed by sucrose application to the proboscis) as the US. The animals received either a single conditioning trial or three successive conditioning trials with an intertrial interval (ITI) of 2 min (or 20 min). In the retention test, the CS was presented alone, and the animals responding with a PER were calculated for each group.
All animals were fed to satiation 1 hr after each retrieval test and every evening. Thus, with the exception of supplementary feeding of some animals 4 hr before olfactory conditioning, all bees were treated identically. For statistical analysis, an ANOVA was used for repeated measurements, and if appropriate, a χ2 test was used as a post hoc test.
Determination of basal PKA activity. To determine basal PKA activity in the brain, bees with different satiation levels (fed 4 or 18 hr earlier) or different sucrose responsiveness (0.1 or 1 m) were frozen in liquid nitrogen. The frozen heads were lyophilized at –20°C, and the brains were dissected under liquid nitrogen cooling into optical lobes and central brain. Each sample (either one optic lobe or one-half of the central brain) was transferred into a glass capillary containing 10 μl of extraction buffer containing (in mm): 50 Tris-HCl, pH 7.5, 1 EDTA, 1 EGTA, and 10 2-mercaptoethanol frozen in liquid nitrogen. After the tissue was homogenized on the surface of the frozen buffer, the capillaries were stored in liquid nitrogen. In the subsequent determination of the basal PKA activity, phosphatase inhibitor 1 (I-1) was used as specific PKA substrate (Hildebrandt and Müller, 1995; Müller, 2000). The tissue in the capillaries was thawed and immediately plunged into 10 μl of phosphorylation buffer containing 1 μCi (γ-32P) ATP (5000 Ci/mmol), 30 μm ATP, 20 mm MgCl2, 1 mm EGTA, and 10 mm mercaptoethanol in 50 mm Tris-HCl, pH 7.5, and 1 μg of I-1. Reactions were terminated after 30 sec by adding 5 μl of SDS-PAGE loading buffer (0.5 m Tris-HCl, pH 6.8, with 5% mercaptoethanol, 5% SDS, 20% glycerol, and 0.1% bromophenol blue). After SDS-PAGE, 32P incorporation into inhibitor I-1 was quantified (Hildebrandt and Müller, 1995; Müller, 2000).
Determining the amount of PKA. The relative amount of PKA regulatory subunit II was measured using the ELISA technique as described by Fiala et al. (1999). Each tissue sample, either an optic lobe or half of the central brain, was homogenized in 500 μl of extraction buffer. The microtiter plates (Falcon, Pro Bind) were coated by diluting (1:1) the samples within a row of six wells in PBS, starting with 50 μl of the original sample as the highest concentration. The remaining binding sites of the microtiter were blocked for 1.5 hr with PBS containing 0.5% BSA. Primary antibodies against the regulatory subunit II of PKA (Müller, 1997, 2002) were incubated overnight at 4°C in PBS, 0.5% BSA. After incubation with biotinylated anti-mouse antibody (2 hr, 20°C) and avidin–alkaline phosphatase (1 hr, 20°C), the conversion of o-nitro phenyl phosphate was measured with an ELISA reader at 405 nm (vs 620 nm background).
Results
Acquisition and memory formation depend on the feeding status during associative conditioning
Most studies on learning, and on long-term memory in particular, use a defined protocol regarding animal treatment. In the honeybee, as in other systems, the motivation of the animals is raised by starving them before olfactory conditioning. Thus, our recent studies on learning and memory formation use a protocol in which the bees receive their last food (sucrose solution) ∼15–20 hr before conditioning (Müller, 1996, 2000; Fiala et al., 1999).
To investigate the effect of motivation attributable to satiety on associative learning, we compared animals with a different but defined feeding status at the time of conditioning. Such an additional feeding, however, can change the responsiveness of the bees to sucrose, the US, and thus may interfere with the evaluation of the US in associative conditioning. To minimize this potential effect on US evaluation, we decided to feed animals 4 hr before conditioning, a time during which the transient feeding-induced decrease in responsiveness is hardly detectable (most of the animals show a high responsiveness to sucrose 2–3 hr after feeding) (Scheiner et al., 2003). Nevertheless, we measured the responsiveness of each animal to sucrose. As expected, the feeding protocol that was used caused only minor, but still significant, changes in sucrose responsiveness. The additional feeding 4 hr before testing decreased the number of animals that respond to a stimulation of the antenna with 0.1 m sucrose from 90 to 80% (Table 1) (ANOVA; F = 4.42; p = 0.0006). The remaining animals responded to stimulation with 1 m sucrose, the concentration used in olfactory conditioning. Only 7 of 700 animals failed to respond at all and were excluded from the experiments. It is important to mention that the difference in the feeding status is limited to a time window including conditioning and up to 4 hr after conditioning (4 hr after conditioning animals from both groups were fed to satiation and treated identically).
Table 1.
Sucrose responsiveness of bees at different satiation levels
|
|
% Responding bees (number of bees) |
||
|---|---|---|---|
| Treatment (total number of bees) |
0.1 m Sucrose |
1.0 m Sucrose |
|
| Bees fed 4 hr before conditioning | |||
| Non- and PBS-injected bees (196) | 80 (157) | 20 (39) | |
| Em/An-injected bees (84) | 81 (68) | 19 (16) | |
| BrcAMP-injected bees (38) | 82 (31) | 18 (7) | |
| Bees fed 18 hr before conditioning | |||
| Non- and PBS-injected bees (198) | 91 (182) | 9 (16) | |
| Em/An-injected bees (94) | 93 (87) | 7 (7) | |
| BrcAMP-injected bees (39) |
95 (37) |
5 (2) |
|
Extension of the proboscis caused by sucrose stimulation of the antenna is used as a measure for responsiveness. The indicated values show the percentage of bees that respond to the first 0.1 m sucrose stimulation and the percentage of bees that only respond to the 1 m sucrose stimulation. Bees that fail to respond to 1 m sucrose were excluded from the experiments (∼1%). All bees that received a three-trial conditioning 15 min after testing responsiveness were used for the calculation of the indicated ratios of responding animals. Non-injected and PBS-injected bees were pooled, because responsiveness does not differ between these groups. Injection of emetine/anisomycin (Em/An) or BrcAMP does not affect responsiveness.
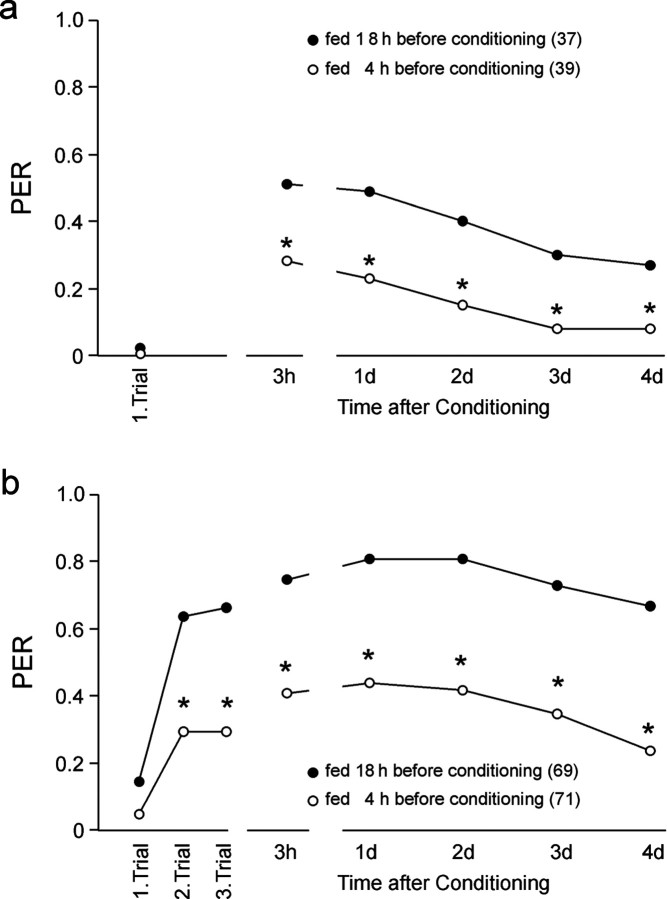
The first experiment clearly shows that a different feeding status at the time of conditioning has a severe impact on learning and memory formation (Fig. 1). Memory formation after a single learning trial (Fig. 1a), independent of translation and transcription (Fig. 2a), is already drastically reduced (ANOVA repeated measurements; F = 8.8; p = 0.0039). Thus, feeding status affects unknown molecular mechanisms implicated in the formation of translation- and transcription-independent memories. In the case of three-trial conditioning (Fig. 1b), acquisition as well as memory formation differ significantly between groups with different feeding status (ANOVA repeated measurements; F = 42.3; p < 0.0001).
Figure 1.
Associative olfactory learning and memory formation induced by single-trial and three-trial conditioning in bees with different satiation levels. All bees were fed 18 hr before conditioning until satiation. To realize different satiation levels during conditioning, half of the bees received an additional feeding 4 hr before conditioning (fed 4 hr previously). After the first retrieval test 3 hr after training, all animals were fed identically. In animals fed 18 hr earlier, single- (a) and three-trial (b) conditioning (ITI, 2 min) led to acquisition and memory formation as reported in previous studies. Only three-trial conditioning (b) induced a long-lasting memory. The additional feeding 4 hr before conditioning affected acquisition and memory formation in both single- and three-trial-conditioned animals. The asterisks indicate significant differences between the groups (χ2 test: *p < 0.02). The number of animals tested in the different groups is indicated in parentheses.
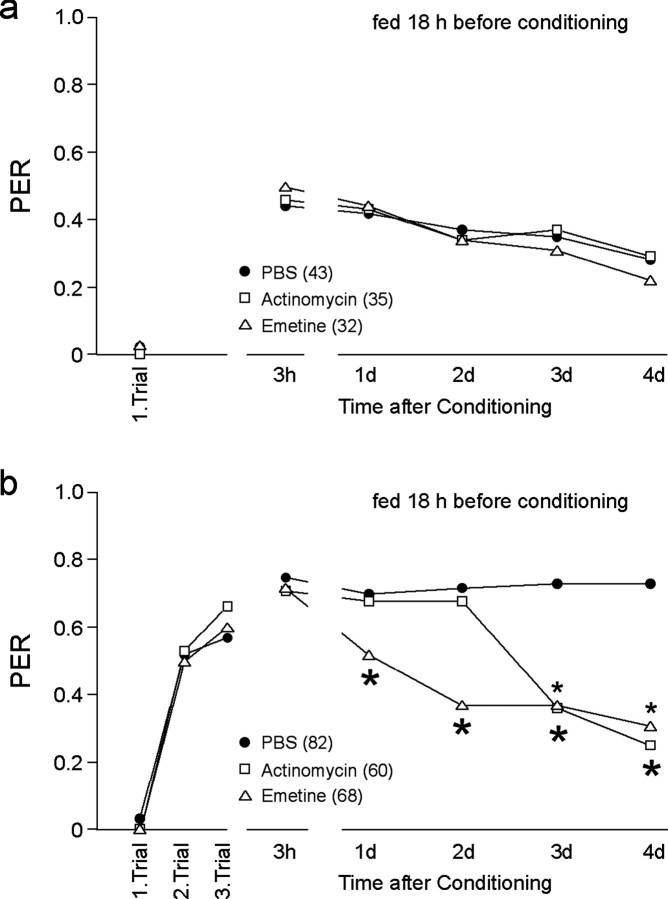
Figure 2.
Inhibition of translation and transcription during conditioning reveals different phases of LTM. Animals were fed 18 hr before conditioning. Thirty minutes before conditioning, animals received an injection (1 μl) of PBS, the transcription blocker actinomycin D (2 mg/ml), or the translation blocker emetine (10 mm). a, Memory induced by single-trial conditioning was affected by neither translation nor transcription blockers. b, Blocking translation or transcription during multiple-trial conditioning specifically impairs distinct phases of LTM. Although blocking transcription during conditioning impairs lLTM (≥ 3 d) (small asterisks), inhibition of translation already affects eLTM (1–2 d) (large asterisks). The number of animals tested in the different groups is indicated in parentheses. Significant differences between the PBS-injected group and the groups injected with drugs are indicated by asterisks (ANOVA repeated measurements and post hoc test; χ2 test: *p < 0.02).
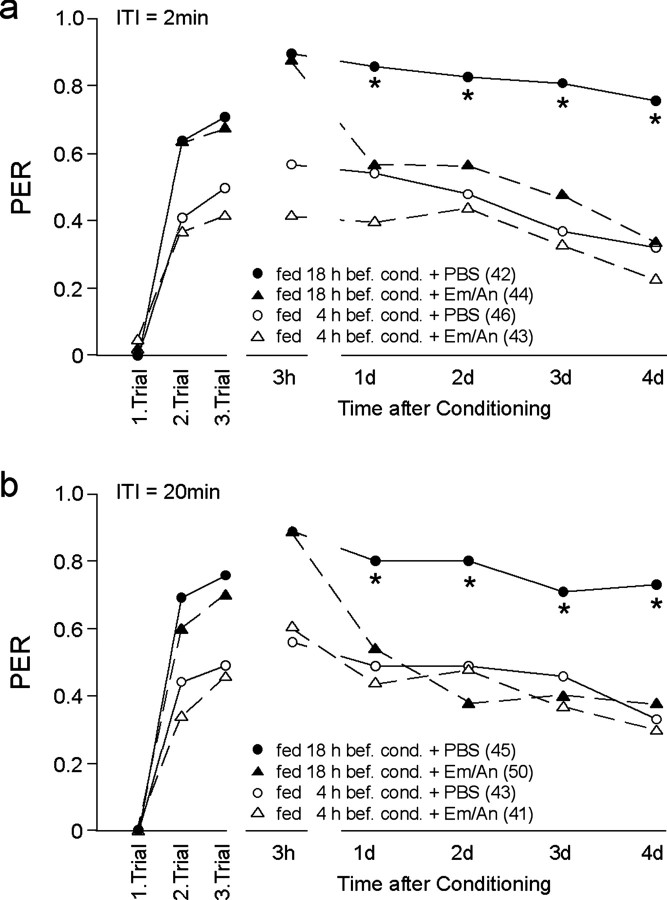
In contrast to single-trial conditioning, animals fed 18 hr before three-trial conditioning induced memory phases that required translation and transcription (Fig. 2). By blocking translation or transcription during conditioning, LTM is dissectible into two different phases (Fig. 2b): an eLTM between 1 and 2 d that requires translation but not transcription, and a lLTM from 3 d that depends on transcription (Fig. 2b). This, together with the results shown in Figure 1b, suggests that feeding status also affects LTM formation. To substantiate this hypothesis, we performed an additional experiment blocking translation in bees with different feeding status during conditioning (Fig. 3). In correlation with the results obtained in Figure 1, bees showed differences in their acquisition and memory formation depending on their feeding status during conditioning (ANOVA repeated measurements; F = 14.4; p < 0.0001). The effect of translation blockers is detectable in bees fed 18 hr before three-trial conditioning (ITI, 2 min) (ANOVA; F = 10.7; p = 0.0015) but not in bees fed 4 hr before conditioning (ANOVA; F = 1.15; p = 0.29). Thus, the feeding status during conditioning interferes with the still-unknown molecular mechanism involved in acquisition and memory consolidation, including formation of translation- and transcription-dependent memories. Sucrose responsiveness is not affected by translation blockers (Table 1).
Figure 3.
A different satiation status during conditioning affects translation-dependent LTM. To establish different satiation levels during conditioning, half of the bees were fed once, 18 hr before conditioning, whereas the others were fed twice, 18 and 4 hr before conditioning. Thirty minutes before conditioning, animals were injected with 1 μl of PBS or Em/An, a mixture of the translation blockers emetine (Em) (10 mm) and anisomycin (An) (10 mm). Bees were trained with three conditioning trials using a 2 min (a) or a 20 min (b) ITI. Regardless of which translation blockers and ITIs were used, the additional feeding (4 hr before conditioning) affected acquisition and memory at 3 hr, eLTM, and lLTM (open symbols). In the group fed 18 hr earlier, translation blockers led to a clear impairment of eLTM and lLTM independent of the ITI (filled symbols). Asterisks indicate significant differences between the PBS-injected and the Em/An-injected groups fed 18 before conditioning (ANOVA, repeated measurements, and post hoc test: χ2 test: *p < 0.01). The number of animals per group is indicated in parentheses.
Because formation of memory depends on the ITIs used for conditioning, we trained in parallel two groups of animals with ITIs of 2 and 20 min. As is true for an ITI of 2 min, application of translation inhibitors during three-trial conditioning (ITI, 20 min) affects memory formation only in animals fed 18 hr earlier (ANOVA; F = 12.4; p = 0.0007). The comparison of the results shown in Figure 3, a and b, reveal no difference between ITIs of 2 and 20 min (ANOVA; F = 0.014; p = 0.9) and excludes the possibility that the observed effect is specific for the ITI used.
Differentiating the effects of sucrose responsiveness and satiation status on learning and memory formation
Categorization of each animal for its sucrose responsiveness allows for differentiation of effects of the feeding status from effects of sucrose responsiveness on associative learning. Because statistical analysis revealed no difference between animals trained with different ITIs or animals that received either a PBS injection or no injection at all, we combined the results of different experiments in this additional analysis. Table 1 summarizes the responsiveness of animals that received three-trial conditioning.
In total, the additional feeding slightly changes the sucrose responsiveness of the animals. Although >90% of the animals fed 18 hr before respond to 0.1 m sucrose, only 80% of the animals that received additional feeding 4 hr before respond to 0.1 m sucrose solution (ANOVA; F = 4.42; p = 0.0006). Injection of translation inhibitors or bromo cAMP (BrcAMP) before conditioning does not affect responsiveness as compared with PBS- and noninjected animals (4 hr: ANOVA; F = 0.29, p = 0.97; 18 hr: ANOVA; F = 0.28, p = 0.75).
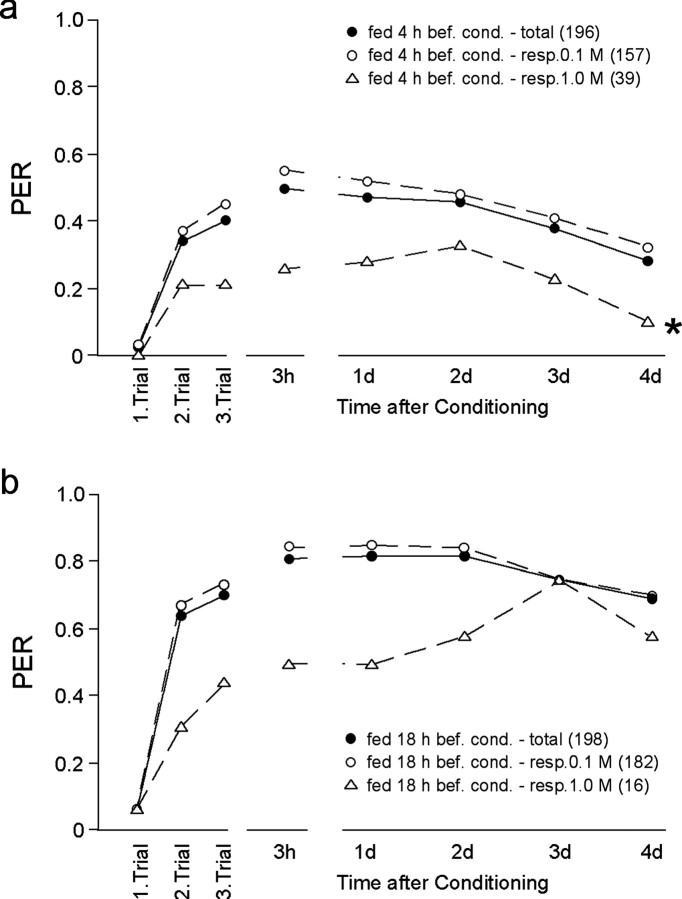
To identify the effects of responsiveness on memory formation, individual animals in each group (fed 4 or 18 hr before training) were sorted with respect to their responsiveness. Within the group of animals fed 4 hr before three-trial conditioning (Fig. 4a), acquisition as well as memory formation differed with respect to sucrose responsiveness (ANOVA; F = 13.29; p = 0.0003). The subgroup of animals (20%) that responded only to 1 m sucrose showed a reduction in acquisition and memory formation as compared with the subgroup (80%) that responded to 0.1 m sucrose and the whole group of animals fed 4 hr before conditioning. This seems to be true also for the group of animals fed 18 hr before conditioning, although the very small number of animals in the subgroup responding to 1 m sucrose (16 of 198) does not allow for a statistical analysis (Fig. 4b).
Figure 4.
Distinguishing effects of satiation status from those of sucrose responsiveness on learning. Sucrose responsiveness of each animal to either 0.1 or 1 m sucrose was tested 15 min before conditioning. The data summarize results of noninjected and PBS-injected animals that received three-trial conditioning (2 and 20 min ITI) (filled circle) and the sorted subgroups (open circle and open triangle) according to their sucrose responsiveness. Although the major subgroups of animals fed 4 hr (a) or 18 hr (b) before conditioning showed the same sucrose responsiveness (0.1 m), they differed in learning and memory formation (a, b, compare open circles). Animals with a reduced sucrose responsiveness (1 m) showed an additional reduction in their performance independent of the satiation status (open triangles). The asterisk in a indicates a significant difference between subgroups with different sucrose responsiveness (ANOVA, repeated measurements). The number of animals per group is indicated in parentheses.
The major subgroups of animals within the groups fed 4 hr (80%) or 18 hr (90%) before conditioning have the same high sucrose responsiveness (0.1 m), however, but they clearly differ in learning and memory formation (Fig. 4, compare a, b). Thus, the effects of satiation levels on learning are clearly different, dissectible from their effects on sucrose responsiveness. Moreover, both effects seem to be additive. A decreased sucrose responsiveness (1 m) leads to a further reduction in acquisition and memory formation on top of the decrease induced by satiation state (Fig. 4a,b, compare open triangles) as compared with the corresponding subgroups with a sucrose responsiveness of 0.1 m.
Feeding status, but not sucrose responsiveness, affects the cAMP-dependent PKA, a central player in LTM formation
In contrast to single-trial conditioning, a lot is known about the molecular mechanisms underlying the formation of LTM. Because feeding status severely affects LTM formation, we tested whether the cAMP–PKA cascade, which is required for LTM formation during conditioning, is involved with this aspect. In a first attempt we tested whether feeding status or sucrose responsiveness influences the cAMP–PKA cascade in the brain of honeybees. Figure 5a shows that the feeding status significantly affects the basal PKA activity in the nervous tissue of bees. In both the optical lobes (t test; p = 0.004) and the central brain (t test; p = 0.017) the basal PKA activity differed with respect to the feeding protocol, indicating a general effect of the feeding status on PKA activity in the nervous system.
Figure 5.
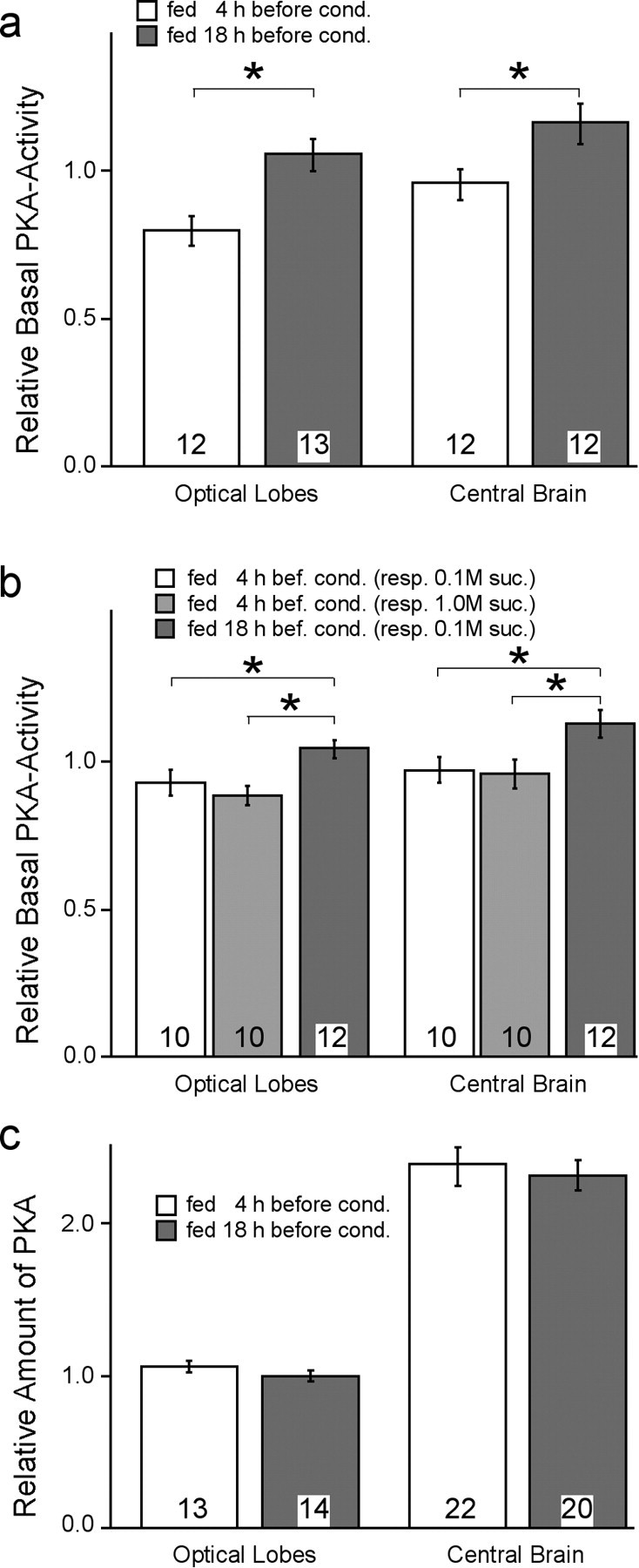
Feeding status but not sucrose responsiveness affects the cAMP–PKA cascade. Bees were fed according to the behavioral experiments described in Figure 1. Bees were shock-frozen in liquid nitrogen either 4 or 18 hr after the last feeding. a, The data show that the relative basal PKA activity (mean ± SEM) as measured in the central brain and the optical lobes is significantly higher in animals fed 18 hr earlier than in animals fed 4 hr before (t test; *p < 0.01). b, Animals with different sucrose responsiveness (0.1 or 1 m) were selected in the group fed 4 hr previously and compared with animals fed 18 hr earlier (all animals responded to 0.1 m sucrose). Only satiation status, not sucrose responsiveness, affects the basal PKA activity (mean ± SEM) in the brain tissue (t test; *p < 0.01). c, The relative total amount of PKA (mean ± SEM) is similar in animals fed 4 or 18 hr previously, as determined by antibodies against PKA. The number of animals tested for each mean is indicated in the columns.
We selected a sufficient number of animals from the group fed 4 hr before testing to determine PKA activity in animals differing in their responsiveness. Interestingly, regardless of their responsiveness, the animals in the group fed 4 hr before testing show a reduction in PKA activity similar to those fed 18 hr before testing (optical lobes: t test, p = 0.01; central brain: t test, p = 0.01) (Fig. 5b). Using antibodies against the PKA type II, which accounts for ∼90% of the total PKA activity in the bee brain (Müller, 1997), we detected no difference in the amount of PKA between animals with different satiation status (optical lobes: t test, p = 0.26; central brain: t test, p = 0.96) (Fig. 5c). This result and the fact that the cAMP–PKA cascade plays an important role in LTM formation prompted us to test whether a pharmacological increase of the basal PKA activity in bees fed 4 hr before training is able to rescue aspects of the impaired learning and memory formation in this group (Fig. 1).
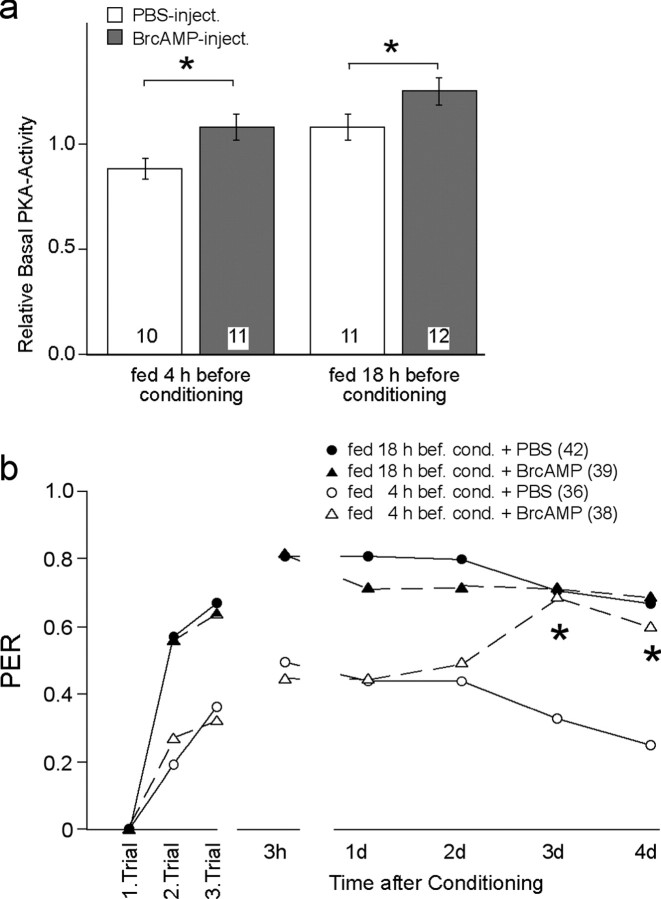
Thirty minutes before conditioning, 1 μl of BrcAMP (1 mm) or PBS was injected into the hemolymph of animals fed 4 or 18 hr earlier. Injection of BrcAMP does not influence responsiveness as compared with PBS-injected bees (Table 1); however, the basal PKA activity in the total brain, as measured at the time point of conditioning, is elevated by ∼15% in the BrcAMP-injected groups (Fig. 6a). In the group of animals fed 18 hr before training, BrcAMP injection before conditioning affected neither acquisition nor memory formation (ANOVA; F = 0.13; p = 0.71) (Fig. 6b). In bees fed 4 hr before conditioning, however, BrcAMP-injected bees showed a specific difference as compared with the PBS-injected controls. Although rescue of basal PKA activity in the brain at the time of conditioning (Fig. 6a) did not improve acquisition, MTM, or translation-dependent eLTM, it did rescue transcription-dependent memory (3 d: χ2; 9.11, p = 0.0025; 4 d: χ2; 8.21, p < 0.0001). This clear dissection provides the first evidence that different molecular pathways underlie the induction of eLTM and lLTM.
Figure 6.
Increase in basal PKA activity during conditioning rescues satiation-dependent loss of late LTM. Animals were fed as described in Figure 1. Thirty minutes after injection (1 μl) of PBS or BrcAMP (1 mm) into the hemolymph, bees were used for determining basal PKA activity in the brain (a) or received a three-trial conditioning with an ITI of 2 min (b). a, Values present the mean (±SEM) of the relative basal PKA activity. The number of bees measured for each value is indicated in the columns (t test; *p < 0.05). b, Injection of BrcAMP before conditioning had no effect on learning and memory formation in animals fed 18 hr earlier (filled symbols). Injection of BrcAMP and thus an increase in basal PKA activity during conditioning specifically rescued lLTM in bees fed 4 hr before conditioning (open symbols). The asterisks at days 3 and 4 mark the significant difference between the PBS- and the BrcAMP-injected subgroups (χ2 test: *p < 0.01). The number of animals tested in the different groups is indicated in parentheses.
Discussion
Conditioning at different satiation levels causes drastic differences in various aspects of learning and memory formation. Only during our regular protocol, when animals are fed 18 hr before multiple-trial conditioning, are the memory phases consisting of MTM, eLTM, and lLTM induced. An additional feeding 4 hr before multiple-trial conditioning is sufficient to impair these memory phases. Our studies provide the first evidence that during conditioning the cAMP–PKA cascade, a central player in LTM formation, is influenced by satiation and mediates transcription-dependent lLTM formation depending on the satiation level.
Distinguishing satiation level and chemosensory responsiveness
Sucrose responsiveness depends on the role of the bees in foraging, their role in the hive, genetic background, age, and probably many other parameters (Pankiw and Page, 1999, 2000; Ben-Sahar and Robinson, 2001; Pankiw et al., 2001; Scheiner et al., 2001b). Bees selected on the basis of their different sucrose responsiveness differ in many respects, including habituation and olfactory and tactile learning (Scheiner et al., 1999, 2001a). The mechanisms that determine how these physiological parameters modulate sucrose responsiveness and how this in turn affects behavior are unclear. At least it is possible to distinguish between processes that differ with respect to kinetic parameters (Scheiner et al., 2003). Feeding to satiation immediately decreases the sucrose responsiveness regardless of the initial responsiveness; however, sucrose responsiveness increases with time after feeding. Although this transient processes has not yet been analyzed thoroughly, the kinetic parameters of the increase in responsiveness after feeding seem to depend on yet unknown physiological and possibly genetic parameters. Animals with initially high responsiveness recover within 20 min. Animals with initially low responsiveness remain at this low level within the first 20 min after feeding but increase their responsiveness drastically above the initially low responsiveness within 90 min (Scheiner et al., 2003). Given this, it is not surprising that 4 hr (or 18 hr) after feeding most of the animals show a high sucrose responsiveness (Table 1).
In this and in all of our previous studies (Grünbaum and Müller, 1998; Fiala et al., 1999; Müller, 2000), we captured forager bees leaving the hives and trained them the next day. Only bees that failed to show a PER after stimulation with a sucrose concentration (1 m) used for feeding or US stimulation were excluded. Measurement of both sucrose responsiveness at the time of conditioning and performance in olfactory learning allows a clear distinction between the effects of sucrose responsiveness and satiation level on learning. First, within the different feeding groups, the major subgroups of animals (>80%) (Table 1) do not differ with respect to sucrose responsiveness, but they do differ in their learning and memory formation (Fig. 4). Second, independent of the feeding protocol (4 or 18 hr before), learning and memory formation differ between animals with low (1 m) and high (0.1 m) sucrose responsiveness (Fig. 4). The latter has also been reported in preselected bees (Scheiner et al., 2001a). This dissection between sucrose responsiveness and satiation level on learning is also confirmed by the function of the cAMP–PKA cascade. Basal PKA activity in the honeybee brain is influenced by feeding status but not by sucrose responsiveness (Fig. 5). This is consistent with a previous study (Scheiner et al., 2003) showing that the measured PKA activity does not correlate with changes in responsiveness over time and that the manipulation of the cAMP–PKA cascade does not affect sucrose responsiveness (Table 1).
Taken together, the effects of chemosensory responsiveness and satiation status are clearly discernible from each other. This is of major importance, because sensory responsiveness is often used as an indicator to evaluate the reward value that directly affects associative learning. Although we and others demonstrated a correlation between sensory responsiveness and memory formation (Fig. 4), the chosen conditions (training 4 or 18 hr after the last feeding) uncovered additional yet unknown processes that impair memory formation independent of the sensory responsiveness. This is a basic prerequisite for unequivocally addressing the impact of satiation-dependent processes on learning and memory formation.
Satiation status during conditioning interferes with different aspects of learning and memory formation
Memory formation is a continuous dynamic process that critically depends on parameters such as the number and temporal succession of conditioning trials. In most species tested to date, a single training trial leads to the formation of short-term and midterm memories, whereas repeated training trials induce long-term memory (Tully et al., 1994; Kogan et al., 1997; Sutton et al., 2002). Single-trial-induced processes characteristically differ from those induced by multiple trials, and both are clearly discernible from each other at the molecular and behavioral levels. In the honeybee, as well as in other systems, pharmacological treatment that leads to a specific loss of LTM affects neither acquisition and mid-term memory nor memory induced by a single-learning trial (Müller, 2002).
This clear separation is disrupted by changing the satiation level during conditioning. Both single- and multiple-trial-induced learning is impaired in animals fed 4 hr before conditioning. Thus, in contrast to pharmacological blockers that impair very distinct memory phases, a change in satiation level affects various features of learning. Three-trial conditioning of animals fed 4 hr earlier induced a memory that resembled features of a single-trial-induced memory in animals fed 18 hr earlier (Fig. 1, compare a, b). Acquisition and memory performance were at a low level, and none of the memories depended on translation or transcription processes.
In animals fed 18 hr earlier, three-trial conditioning triggers different parallel molecular processes responsible for MTM (Grünbaum and Müller, 1998) and LTM formation (Müller, 2000). All of these processes and memory phase are missing in both single-trial and three-trial-conditioned animals fed 4 hr earlier. Thus the simplest explanation would be that the satiation-dependent process interferes with a molecular process of associative learning involved in the computation and integration of repeated learning trials, but because single-trial learning is also reduced to some degree, there may be multiple interaction sites.
Our experiments allow discrimination of at least two basic mechanisms: one that correlates with the sucrose responsiveness and thus may interfere with molecular processes of reward evaluation, and an additional process that is independent of responsiveness and may present yet unknown features of satiation that interfere with memory formation (Fig. 4). These results provide a foundation for future identification of the signaling systems involved in either of these clearly distinguishable mechanisms.
Of special importance is the effect of satiation processes on single-trial conditioning. Until now, no pharmacological treatment has caused an impairment of single-trial-induced processes, and this satiation-dependent decrease in single-trial-induced memory is the first manipulation that allows investigation of the molecular mechanisms underlying this form of memory.
Satiation differentiates between different and parallel functions of the cAMP–PKA cascade during conditioning
In a number of systems, including Aplysia (Schacher et al., 1988; Dash et al., 1990), Drosophila (Drain et al., 1991), honeybees (Müller, 2000), and rodents (Huang et al., 1995; Abel et al., 1997), the cAMP–PKA cascade plays a central function in the induction of long-lasting neuronal changes and LTM. In the honeybee, inhibition of PKA activity (Fiala et al., 1999; Müller, 2000) or blocking of nitric oxide synthase, which mediates PKA action in memory formation (Müller, 1996), leads to an impairment of LTM already detectable 1 d after three-trial conditioning. This time window overlaps with that of eLTM (1–2 d) and lLTM (≥3 d) (Fig. 2b), pointing to a single cAMP–PKA-triggered process responsible for LTM formation. Moreover, we identified a direct connection between training procedure, dynamics of PKA activation, and LTM formation (Müller, 2000). Only multiple-trial conditioning induces a prolonged PKA activation in the range of minutes. Mimicking this prolonged transient PKA activation in combination with a single-conditioning trial leads to LTM formation (≥1 d), again suggesting a single PKA-triggered process required for LTM formation. The same conclusion can be drawn from findings in other systems, in which blocking the cAMP–PKA cascade during conditioning (or stimulation) also leads to an impairment of translation and transcription-dependent LTM (or long-lasting neuronal changes) (Dash et al., 1990; Drain et al., 1991; Abel et al., 1997).
Rescue of basal PKA activity in the brain of animals fed 4 hr earlier, followed by three conditioning trials, leads to a “normal” transcription-dependent lLTM detectable after 3 d (Fig. 6). This normal lLTM, in contrast to a still-impaired acquisition, MTM, and eLTM, makes it a convincing example for molecular cascades acting in parallel in memory formation. One possibility that we can propose is the existence of two parallel and independent (or partially independent) pathways triggered by the cAMP–PKA cascade during conditioning. Whereas one of the cAMP–PKA-dependent processes triggers molecular events leading to translation-dependent eLTM, the other cAMP–PKA-dependent process triggers a cascade responsible for the transcription-dependent lLTM (Fig. 7). It is possible, however, that eLTM and lLTM are triggered initially by a single PKA process that splits into two different and thus dissectible pathways. In this case the pathway that leads to the translation-dependent eLTM must be affected at an additional and different level by satiation-dependent mechanisms.
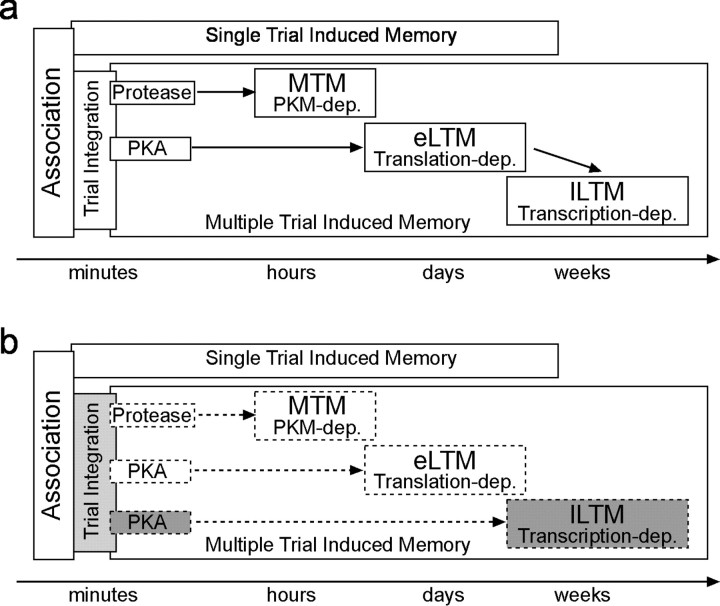
Figure 7.
Memory phases and satiation: dissecting the parallel function of the cAMP–PKA cascade in LTM formation. The diagrams summarize the major molecular mechanisms of memory formation (a) and their modification (b) by the reported interference of satiation on learning. a, All previous experiments used only bees fed 18 hr before conditioning. Single-trial conditioning induces a memory that decays within a few days, whereas multiple-trial conditioning leads to formation of a stable, long-lasting memory. Three-trial conditioning induces different memory phases, including MTM, which requires protein kinase M formed by cleaving protein kinase C during conditioning and an LTM induced in parallel that requires prolonged PKA activation during conditioning. LTM itself can be divided into a translation-dependent eLTM and a transcription-dependent lLTM, both of which are impaired after blocking PKA during conditioning. All of these characteristic memory phases are missing (dotted lines) in bees fed 4 hr before multiple-trial conditioning (b), suggesting an interference of satiation-dependent processes with a site implicated in integrating repeated conditioning trials (dotted area). The specific rescue of lLTM (dark shaded), with still-impaired MTM and eLTM, suggests that two PKA-mediated pathways are required for either eLTM or lLTM induction. Because satiation level also affects single-trial-induced memory, additional molecular interaction sites in addition to the cAMP–PKA pathway must exist between satiation and learning.
Potential interactions between learning and metabolic energy regulation in the brain
Although the brain represents only a miniscule percentage of body weight, it is the major consumer of metabolic energy (Magistretti et al., 1995). Glucose, the major energy substrate, is taken up predominantly by astrocytes that play a key role in supporting the energy flow from blood to neurons. The glycolysis in astrocytes converts glucose into pyruvate and lactate, which are taken up and oxidized by neurons.
Maintenance of brain function even under extreme metabolic situations requires a highly regulated system. This is realized by local regulation of metabolic energy requirements via products of neuronal activity, neurotransmitters, and neuropeptides (Magistretti et al. 1995) and the fact that distinct neuronal circuits of the brain regulate energy homeostasis of the whole organism, including behavioral aspects of food intake (Blevins et al., 2002). Because hormones, neuropeptides, and neurotransmitters mediating aspects of energy metabolism are also involved in neuronal modulation, it is not surprising that they contribute to different aspects of learning (Beinfeld, 2001; Carlini et al., 2002; Figlewicz 2003; Hadjiivanova et al., 2003; Matsushita et al., 2003); however, whether and how factors implicated in regulating energy metabolism interact with the already characterized molecular cascades underlying learning and memory formation have not yet been addressed.
Our finding that elevating the reduced satiation-dependent PKA activity rescues a specific memory phase points to a network of possibly very specific interactions between molecular pathways of satiation and those involved in learning and memory formation. In addition to the cAMP–PKA cascade, other signaling pathways are at least as important for learning and memory formation (Adams and Sweatt, 2002; Wei et al., 2002; Purcell and Carew, 2003) and thus may act as targets of satiation-dependent processes. Identification of additional molecular processes responsible for the satiation-dependent impairment of the other memory phases (Fig. 7b) will not only provide information regarding the interaction between satiation and learning but will also lead to a more complete understanding of the complex network of molecular events underlying the formation of a distinct memory.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (SFB515 and the Graduiertenkolleg 120). We thank M. Wurm for help with this manuscript.
Correspondence should be addressed to Uli Müller, Natural Sciences and Technology III, Zoology (Dep. 8.4), Saarland University, Postfach 151150, 66041 Saarbruecken, Germany. E-mail: uli.mueller@mx.uni-saarlande.de.
A. Friedrich's present address: Department of Biology and Biochemistry, University of Houston, 369 Science and Research Building 2, Houston, TX 77204.
DOI:10.1523/JNEUROSCI.0669-04.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/244460-09$15.00/0
References
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R (1997) Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615–626. [DOI] [PubMed] [Google Scholar]
- Adams JP, Sweatt JD (2002) Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol 42: 135–163. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC (2001) An introduction to neuronal cholecystokinin. Peptides 22: 1197–2200. [DOI] [PubMed] [Google Scholar]
- Ben-Sahar Y, Robinson GE (2001) Satiation differentially affects performance in a learning assay by nurse and forager honey bees. J Comp Physiol [A] 187: 891–899. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG (2002) Peptide signals regulating food intake and energy homeostasis. Can J Physiol Pharmacol 80: 396–406. [DOI] [PubMed] [Google Scholar]
- Byrne JH (1987) Cellular analysis of associative learning. Physiol Rev 67: 329–439. [DOI] [PubMed] [Google Scholar]
- Carew TJ, Pinsker H, Kandel ER (1972) Long-term habituation of a defensive withdrawal reflex in Aplysia. Science 175: 451–454. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Monzon ME, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR (2002) Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun 299: 739–743. [DOI] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER (1990) Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature 345: 718–721. [DOI] [PubMed] [Google Scholar]
- Davis RL (1993) Mushroom bodies and Drosophila learning. Neuron 11: 1–14. [DOI] [PubMed] [Google Scholar]
- Davis RL (1996) Physiology and biochemistry of Drosophila learning mutants. Physiol Rev 76: 299–317. [DOI] [PubMed] [Google Scholar]
- DeZazzo J, Tully T (1995) Dissection of memory formation: from behavioral pharmacology to molecular genetics. Trends Neurosci 18: 212–218. [DOI] [PubMed] [Google Scholar]
- Drain P, Folkers E, Quinn WG (1991) cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron 6: 71–82. [DOI] [PubMed] [Google Scholar]
- Fiala A, Müller U, Menzel R (1999) Reversible downregulation of protein kinase A during olfactory learning using antisense technique impairs long-term memory formation in the honeybee, Apis mellifera. J Neurosci 19: 10125–10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP (2003) Insulin, food intake, and reward. Semin Clin Neuropsychiatry 8: 82–93. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER (1993) Effect of cAMP simulates a late stage of LTP in hippocampal CA1 neurons. Science 260: 1661–1664. [DOI] [PubMed] [Google Scholar]
- Grünbaum L, Müller U (1998) Induction of a specific olfactory memory leads to a long-lasting activation of protein kinase C in the antennal lobe of the honeybee. J Neurosci 18: 4384–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiivanova C, Belcheva S, Belcheva I (2003) Cholecystokinin and learning and memory processes. Acta Physiol Pharmacol Bulg 27: 83–88. [PubMed] [Google Scholar]
- Hildebrandt H, Müller U (1995) Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honeybees. J Neurobiol 27: 44–50. [DOI] [PubMed] [Google Scholar]
- Huang EP (1998) Synaptic plasticity: going through phases with LTP. Curr Biol 8: 350–352. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R (1995) A genetic test of the effects of mutations in PKA of mossy fiber LTP and its relation to spatial and contextual learning. Cell 83: 1211–1222. [DOI] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Blendy JA, Coblentz J, Marowitz Z, Schütz G, Silva AJ (1997) Spaced training induces normal long-term memory in CREB mutant mice. Curr Biol 7: 1–11. [DOI] [PubMed] [Google Scholar]
- Kuwabara M (1957) Bildung des bedingten Reflexes von Pavlov Typus bei der Honigbiene, Apis mellifera. J Fac Sci Hokkaido Univ Ser VI Zool 13: 458–464. [Google Scholar]
- Magistretti PJ, Pellerin L, Martin JL (1995) Brain energy metabolism. In: Psychopharmacology: the fourth generation of progress (Bloom FE, Kupfer DJ, eds), pp 657–670. New York: Raven.
- Matsushita H, Akiyoshi J, Kai K, Ishii N, Kodama K, Tsutsumi T, Isogawa K, Nagayama H (2003) Spatial memory impairment in OLETF rats without cholecystokinin: a receptor. Neuropeptides 37: 271–276. [DOI] [PubMed] [Google Scholar]
- Menzel R (1999) Memory dynamics in the honeybee. J Comp Physiol [A] 185: 323–340. [Google Scholar]
- Müller U (1996) Inhibition of nitric oxide synthase impairs a distinct form of long-term memory in the honeybee, Apis mellifera. Neuron 16: 541–549. [DOI] [PubMed] [Google Scholar]
- Müller U (1997) Neuronal cAMP-dependent protein kinase type II is concentrated in mushroom bodies of Drosophila melanogaster and the honeybee Apis mellifera. J Neurobiol 33: 33–44. [DOI] [PubMed] [Google Scholar]
- Müller U (2000) Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron 27: 159–168. [DOI] [PubMed] [Google Scholar]
- Müller U (2002) Learning in honeybees: from molecules to behaviour. Zoology 105: 313–320. [DOI] [PubMed] [Google Scholar]
- Müller U, Carew TJ (1998) Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron 21: 1423–1434. [DOI] [PubMed] [Google Scholar]
- Müller U, Hildebrandt H (2002) NO/cGMP-mediated protein kinase A activation in the antennal lobes plays an important role in appetitive reflex habituation in the honeybee. J Neurosci 22: 8739–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiw T, Page RE (1999) The effect of genotype, age, sex, and caste on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol [A] 185: 207–213. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Page RE (2000) Response thresholds to sucrose predict foraging behavior in the honey bee (Apis mellifera L.). Behav Ecol Sociobiol 47: 265–267. [Google Scholar]
- Pankiw T, Waddington KD, Page RE (2001) Modulation of sucrose response thresholds in honey bees (Apis mellifera L.): influence of genotype, feeding and foraging experience. J Comp Physiol [A] 187: 293–301. [DOI] [PubMed] [Google Scholar]
- Purcell AL, Carew TJ (2003) Tyrosine kinases, synaptic plasticity and memory: insights from vertebrates and invertebrates. Trends Neurosci 26: 625–630. [DOI] [PubMed] [Google Scholar]
- Schacher S, Castellucci VG, Kandel ER (1988) cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science 240: 1667–1669. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Erber J, Page RE (1999) Tactile learning and the individual evaluation of the reward in honey bees (Apis mellifera L.). J Comp Physiol [A] 185: 1–10. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J (2001a) Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav Brain Res 120: 67–73. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Page RE, Erber J (2001b) The effects of genotype, foraging role and sucrose perception on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol Learn Mem 76: 138–150. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Müller U, Heimburger S, Erber J (2003) Activity of protein kinase A and gustatory responsiveness in the honey bee (Apis mellifera L.). J Comp Physiol [A] 189: 427–434. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S (1998) CREB and memory. Annu Rev Neurosci 21: 127–148. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ (2002) Interaction between amount and pattern of training in the induction of intermediate- and long-term memory for sensitization in Aplysia. Learn Mem 9: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M (1994) Genetic dissection of consolidated memory in Drosophila. Cell 79: 35–47. [DOI] [PubMed] [Google Scholar]
- Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M (2002) Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci 5: 573–579. [DOI] [PubMed] [Google Scholar]
- Wüstenberg D, Gerber BT, Menzel R (1998) Long- but not medium-term retention of olfactory memories in honeybees is impaired by actinomycin D and anisomycin. Eur J Neurosci 10: 2742–2745. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Tully T (1996) CREB and the formation of long-term memory. Curr Opin Neurobiol 6: 264–268. [DOI] [PubMed] [Google Scholar]