Abstract
Brain-derived neurotrophic factor (BDNF) plays a critical role in nervous system and cardiovascular development and function. Recently, a common single nucleotide polymorphism in the bdnf gene, resulting in a valine to methionine substitution in the prodomain (BDNFMet), has been shown to lead to memory impairment and susceptibility to neuropsychiatric disorders in humans heterozygous for the variant BDNF. When expressed by itself in hippocampal neurons, less BDNFMet is secreted in an activity-dependent manner. The nature of the cellular defect when both BDNFMet and wild-type BDNF (BDNFVal) are present in the same cell is not known. Given that this is the predominant expression profile in humans, we examined the effect of coexpressed BDNFMet on BDNFVal intracellular trafficking and processing. Our data indicate that abnormal trafficking of BDNFMet occurred only in neuronal and neurosecretory cells and that BDNFMet could alter the intracellular distribution and activity-dependent secretion of BDNFVal. We determined that, when coexpressed in the same cell, ∼70% of the variant BDNF forms BDNFVal·BDNFMet heterodimers, which are inefficiently sorted into secretory granules resulting in a quantitative decreased secretion. Finally, we determined the form of BDNF secreted in an activity-dependent manner and observed no differences in the forms of BDNFMet or the BDNFVal·BDNFMet heterodimer compared with BDNFVal. Together, these findings indicate that components of the regulated secretory machinery interacts specifically with a signal in the BDNF prodomain and that perturbations in BDNF trafficking may lead to selective impairment in CNS function.
Keywords: brain derived neurotrophic factor, polymorphism, prodomain, proneurotrophin, intracellular trafficking, regulated secretion
Introduction
Brain-derived neurotrophic factor (BDNF) has been established to play critical roles in vertebrate nervous system and cardiovascular development and function (Tessarollo, 1998; Huang and Reichardt, 2001; Chao, 2003). Recently, a polymorphism in the bdnf gene leading to a valine (Val) to methionine (Met) substitution at position 66 in the prodomain (BDNFMet) has been associated with increased susceptibility in humans heterozygous for the polymorphism to neuropsychiatric disorders, including Alzheimer's disease (Ventriglia et al., 2002), Parkinson's disease (Momose et al., 2002), bipolar disorder (Neves-Pereira et al., 2002; Sklar et al., 2002), depression (Sen et al., 2003), eating disorder (Ribases et al., 2003), and obsessive compulsive disorder (Hall et al., 2003). In addition, humans heterozygous for BDNFMet were shown to have memory impairments (Egan et al., 2003; Hariri et al., 2003). This polymorphism represents the first association of an alteration in BDNF to clinical dysfunction. Less is known about the molecular mechanisms underlying altered BDNF functioning. In one study, when expressed by itself in hippocampal neurons, less BDNFMet is secreted in an activity-dependent manner (Egan et al., 2003).
Trafficking of BDNF from the biosynthetic pathway is a complex, highly regulated process, the mechanisms of which remain unclear. In this context, several questions have yet to be addressed regarding the specificity of this effect of the Met substitution on BDNF trafficking. First, does this substitution mutation in BDNF produce a general or cell type-specific defect in trafficking of this neurotrophin (NT)? Currently, only the trafficking of BDNFMet in hippocampal neurons has been examined (Egan et al., 2003). Decreased expression of BDNF in the certain peripheral cell populations, such as endothelial and vascular smooth muscle cells, have significant developmental consequences, including vascular destabilization and intracardiac hemorrhage (Donovan et al., 2000). Second, does coexpression of the BDNFMet alter the trafficking or processing of the wild-type BDNF (BDNFVal)? Previous studies have shown that coexpressing different neurotrophins in the same cell can alter the trafficking fate of one of the neurotrophins (Farhadi et al., 2000). It remains unclear the nature of the trafficking defect when both BDNFMet and BDNFVal are expressed in the same cell. This is relevant because it has been estimated that 20–30% of the human population is heterozygous for the Met polymorphism (Neves-Pereira et al., 2002; Egan et al., 2003; Hariri et al., 2003; Sen et al., 2003) in which the wild-type and variant BDNF are coexpressed equally. The major phenotype observed in humans, memory impairment, has been shown to be present in these subjects heterozygous for the Met polymorphism (Egan et al., 2003; Hariri et al., 2003).
To address these questions on the role of the Met polymorphism on BDNF trafficking, we conducted an analysis of the intracellular and extracellular fates of BDNFMet and BDNFVal in non-neuronal and neuronal cells that endogenously produce and secrete BDNF. We conclude that the BDNF polymorphism affects only trafficking in neuronal cells and determined that, when expressed together in the same cell, BDNFMet alters the trafficking of BDNFVal through the formation of heterodimers that are less efficiently sorted into the regulated secretory pathway.
Materials and Methods
Reagents and antibodies. Murine NGF was obtained from Harlan Bioproducts (Indianapolis, IN), and human recombinant BDNF was obtained from PeproTech (Rocky Hill, NJ). Anti-FLAG antibodies were obtained from Sigma (St. Louis, MO) (monoclonal, M2, and polyclonal), and anti-HA (hemagglutinin) antibodies were obtained from Covance (Princeton, NJ) (monoclonal, HA.11) and Sigma (polyclonal). Anti-secretogranin II (SecII) antibodies were from Biodesign (Saco, ME). Anti-MAP2 (microtubule-associated protein 2) antibody was from Sigma, and anti-Tau1 antibody was from Chemicon (Temecula, CA). Polyclonal anti-proBDNF and anti-proNGF antibodies were generated as described previously (Beattie et al., 2002). ϵ-Amino-caproic acid (EACA) was purchased from American Diagnostica (Stamford, CT). Fluorescent secondary antibodies were from Molecular Probes (Eugene, OR) and Jackson ImmunoResearch (West Grove, PA). The restriction enzymes were purchased from Fermentas (Hanover, MD), and Pfu Turbo DNA was from Stratagene (La Jolla, CA). All other compounds were from Sigma.
Plasmid constructs. The human BDNF and NGF cDNA were subcloned into pCDNA3.1hygro expression vector (Invitrogen, Carlsbad, CA) using HindIII and XhoI sites. The HA or FLAG epitope tag was added to the 3′ end of the BDNF or NGF cDNA by PCR method. Additionally, the residues RR near the C terminus of NGF were mutated to AA to eliminate a potential proteolytic cleavage site. The Val to Met mutation at position 66 was generated by means of two-step PCR. Using PCR-based mutagenesis, a silent NotI site was generated immediately after the signal peptide of human BDNF or human NGF to facilitate generating dual-epitope-tagged HA-BDNF-FLAG and HA-NGF-FLAG constructs. The HA tag was inserted just after signal peptide of BDNF or NGF, and the FLAG tag was attached to the C terminal of BDNF or NGF. All of the constructs were confirmed by DNA sequence to exclude potential PCR-introduced mutations.
PC12 and COS-7 cell culture and immunoprecipitation and immunoblotting. PC12 (clone 615) cells, stably overexpressing TrkA receptors (Hempstead et al., 1992), were maintained in DMEM (Invitrogen) containing 10% fetal bovine serum (Invitrogen), 5% horse serum (Invitrogen), supplemented with 100 U/ml penicillin–streptomycin (Invitrogen), and 2 mm glutamine plus 200 μg/ml G418 (Invitrogen). COS-7 cells were maintained in DMEM containing 10% fetal bovine serum, supplemented with 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mm glutamine. Lipofectamine 2000 (Invitrogen) was used to transfect DNA in cells. Forty-eight hours after transfection, lysates or condition medium were collected and analyzed by Western blot as described previously (Lee and Chao, 2001). Briefly, for sequential immunoprecipitation experiments, lysates were collected and incubated with polyclonal HA antibody (Sigma), followed by incubation with protein A Sepharose beads (Sigma). The remaining lysates were collected and incubated with polyclonal FLAG antibody (Sigma). Western blots were performed by separating proteins via SDS-PAGE, transferring to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA), and immunoblotting with HA.11 (Covance) or FLAG M2 antibody (Sigma). The immunoreactive protein bands were detected by enhanced chemiluminescence (Pierce, Rockford, IL). For densitometric analysis, immunoreactive bands were scanned and intensity quantitated using NIH Image (Scion, Frederick, MD).
Smooth muscle cell cultures. Temperature-sensitive mouse smooth muscle cells that were initially grown from aortic explants of a transgenic mouse line expressing a temperature-sensitive simian virus 40 (SV40) T antigen (Kraemer et al., 1999) were cultured in DMEM containing 10% fetal calf serum supplemented with 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mm glutamine at 33°C, a temperature at which the T antigen is expressed. One day after transfecting the BDNF plasmids, using Lipofectamine 2000 (Invitrogen), the temperature was raised to 39.5°C, a temperature at which the T antigen is degraded and the cells exhibit a more differentiated phenotype. All experiments were performed 48 hr after transfection.
Endothelial cell culture. Immortalized endothelial cells containing an SV40 T antigen (Schweitzer et al., 1997) were cultured in DMEM containing 10% fetal calf serum supplemented with 100 U/ml penicillin, 100 U/ml streptomycin, and 2 mm glutamine. Lipofectamine 2000 (Invitrogen) was used to transfect DNA in cells. All experiments were performed 48 hr after transfection.
Cortical cell cultures. Dissociated primary cultures of cortical neurons from embryonic day 18 rats were prepared from timed-pregnant Sprague Dawley rats. Fetuses were removed under sterile conditions and kept in PBS on ice for microscopic dissection of the cerebral cortex. The meninges were removed, and the tissue was placed in Neurobasal media (Invitrogen). The tissue was briefly minced with fine forceps and then triturated with a fire-polished Pasteur pipette. Cells were counted and plated on culture wells coated with 0.01 mg/ml poly-d-lysine overnight in a Neurobasal media containing B27 supplement (Invitrogen) and l-glutamine (0.5 mm) (Invitrogen). Experiments were conducted 4 d after plating.
Immunocytochemical staining and fluorescence microscopy. PC12 (615) cells or cortical neurons were grown on glass coverslips (Corning, Corning, NY), coated with poly-l-lysine (Sigma), and transfected with epitope-tagged neurotrophin constructs. Forty eight hours after transfection, cells were fixed with a 4% paraformaldehyde solution in PBS and permeabilized with brief treatment with cold methanol (5 min), followed by washing with PBS three times. Cells were blocked with 5% goat serum in PBS for 30 min. Specimens were incubated with primary antibodies and then incubated with subtype-specific fluorescenated secondary antibodies. Stained PC12 specimens were examined by epifluorescence microscopy using a CoolSNAP CCD camera (PhotoMetrics, Huntington Beach, CA) mounted on a Nikon (Tokyo, Japan) TE2000 microscope equipped with a motorized Z drive and either a 100×, 1.4 numerical aperture objective or 60× 1.4 numerical aperture objective and standard FITC–Texas Red dichroic filter sets. Confocal fluorescence microscopy was performed on cortical neuron specimens using a Zeiss (Oberkochen, Germany) LSM510 microscope fitted with a Zeiss 63×, 1.4 numerical aperture objective with standard filter sets and standard (one Airy disc) pinhole.
Quantitative analysis of fluorescent images. To quantitate the amount of staining of BDNF in different regions of PC12 cells or cortical neurons, ∼20 cells were examined at random that were expressing the epitope-tagged BDNF. For PC12 cells, a three-dimensional (3D) stack of images was obtained by the use of the Z drive mounted on a Nikon TE2000 microscope. For cortical neurons, the entire 3D stack of the cell was imaged by confocal microscopy. For each 3D stack of images, the background was subtracted from each individual slice, and the stack of the images was reconstructed using the using the 3D reconstruction module in the MetaMorph software (Universal Imaging Corporation, West Chester, PA). Two regions were delineated within each cell to denote cell body and processes (see Fig. 4 D). The integrated intensities for each region in all of the slices were summed. In the case of experiments in which two species of BDNF were cotransfected in the same cell, staining intensities of the BDNFVal species was quantitated in the same manner as for the single staining conditions. The results of four independent experiments were compiled, and SEM between these experiments is indicated by the error bars.
Figure 4.
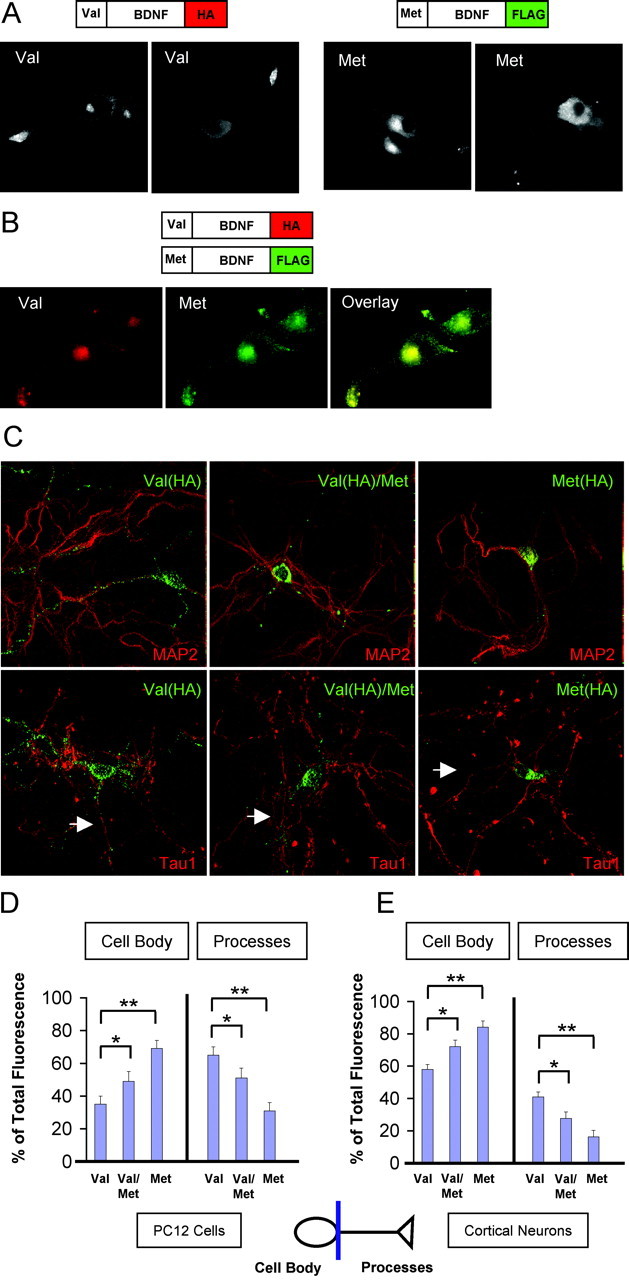
Processing and localization of variant and wild-type BDNF. A, Differentiated PC12 cells were transfected with BDNFVal (Val) or BDNFMet (Met) containing a C-terminal HA epitope or C-terminal FLAG epitope tag, respectively. Cells were fixed after 48 hr and permeabilized, and subcellular distribution of neurotrophins was visualized by indirect immunofluorescence microscopy, as described in Materials and Methods, using ant-HA or anti-FLAG antibodies. Representative epifluorescence images are shown. B, Differentiated PC12 cells were transfected with both BDNFVal containing a C-terminal HA epitope and BDNFMet containing a C-terminal FLAG epitope. Cells were fixed after 48 hr and permeabilized, and subcellular distribution of neurotrophins was visualized by indirect immunofluorescence microscopy using epitope antibodies. Representative epifluorescence images are shown. C, Cortical neurons were transfected with BDNFVal (Val) containing a C-terminal HA epitope, BDNFMet (Met) containing a C-terminal FLAG epitope, or both BDNFVal containing a C-terminal HA epitope and BDNFMet containing a C-terminal FLAG epitope (Val/Met). Cells were fixed after 48 hr and permeabilized, and subcellular distribution of neurotrophins was visualized by confocal microscopy, as described in Materials and Methods, using epitope antibodies. In the case of the coexpressed BDNFVal and BDNFMet, staining for the HA epitope was performed. In addition, all neurons were costained with either MAP2 or Tau1 to identify BDNF localization in dendrites and axons (white arrow), respectively. Representative reconstructed images are shown. D, Quantitative analysis of PC12 cell results illustrated in B, as described in Materials and Methods. The proportion of total fluorescence localized to the cell body or processes is presented as a mean ± SEM determined from analysis of four independent experiments. (*p < 0.01; **p < 0.001; Student's t test). E, Quantitative analysis of cortical neuron results illustrated in C, as described in Materials and Methods. The proportion of total fluorescence localized to the cell body or processes is presented as a mean ± SEM determined from analysis of four independent experiments. (*p < 0.01; **p < 0.001; Student's t test).
To quantitate the proportion of colocalization between BDNF and the secretory granule marker SecII, PC12 cells were examined by epifluorescence microscopy using appropriate filter sets to selectively detect Alexa488 (FLAG) and Cy3 (SecII). Optical slices were obtained by the use of the Z drive mounted on a Nikon TE2000 microscope, and the slice containing the maximal fluorescent intensity was chosen. For cortical neurons, multiple optical confocal images (0.6 μm) were obtained across the z-axis, and the slice containing the maximal fluorescent intensity was chosen. Staining intensity for each fluor and the proportion of colocalization was quantitated using the colocalization module in the MetaMorph software (Universal Imaging Corporation). Approximately 20 cells were examined at random. The results of four independent experiments were compiled, and SEM between these experiments is indicated by the error bars.
ELISA. PC12 (615) cells (4.5 × 105) were seeded to six-well plates. One day before transfection, cells were placed in differentiation media (1.5% horse serum, 1% fetal bovine serum, and 50 ng/ml NGF). Then, 4 μgof BDNFVal, 4 μg of BDNFMet, or 2 μg of BDNFVal plus 2 μg of BDNFMet construct were added per well. Forty eight hours after transfection, cells were washed three time with Krebs'-Ringer's-Henseleit (KRH) buffer with the following composition (in mm): 125 NaCl, 4.8 KCl, 2.6 CaCl2, 25 HEPES, 1.2 MgSO4, 5.6 glucose, 1 sodium ascorbate, and 1.2 KH2PO4, adjusted to pH 7.4 with NaOH. The conditioned media were collected after a 2 hr incubation at 37°C and used as a measure of constitutive secretion. To determine regulated secretion, cells were washed three times with KRH buffer, followed by a 10 min incubation at 37°C in stimulated media [KRH buffer with an increased KCl concentration (56 mm) and decreased NaCl concentration (75 mm)]. The BDNF protein concentrations in the respective media samples were determined using the BDNF Emax immunoassay system (Promega, Madison, WI) with recombinant BDNF as a standard. Standards and samples were performed in duplicates, and each group contained 12 independent samples. After collecting condition media, cells were fixed and immunostained to determine the transfection efficiency. Each sample had a consistent transfection efficiency of ∼30%.
Analysis of secreted forms of BDNF. Differentiated PC12 (615) cells were transfected with 4 μg of BDNFVal, 4 μg of BDNFMet, or 2 μg of BDNFVal plus 2 μg of BDNFMet constructs. Forty-eight hours after transfection, cells were washed three times with KRH buffer and preincubated for 20 min at 37°C in the absence or presence of EACA (10 mm), aprotinin (10 μg/ml), and α2-plasmin inhibitor (100 nm). Cells were then incubated for 10 min at 37°C in stimulated KRH media containing an increased KCl concentration (56 mm) and decreased NaCl concentration (75 mm). The immunoreactive protein bands were detected by enhanced chemiluminescence (Pierce).
Results
Identification and imaging of pro forms of BDNF
To visualize intracellular localization and trafficking of BDNF, we generated a series of epitope-tagged neurotrophin expression constructs bearing combinations of C- and/or N-terminal epitope tag sequences. Immunodetection of C- and N-terminal epitopes on the same BDNF molecule would allow for BDNF pro and mature forms to be imaged by immunofluorescent microscopy. This system allows us to assess both processing and trafficking alterations produced by the Met substitution in the same cell.
Because it has already been observed that BDNF and NGF have different intracellular processing patterns (Mowla et al., 1999, 2001), we also made dual-epitope-tagged NGF constructs to compare with BDNF. By Western blot analysis of transfected COS-7 cell lysates, we initially confirmed that immunoblotting for the C-terminal epitope (FLAG) recognizes both species of neurotrophins (pro and mature) for BDNF and NGF, whereas the N-terminal epitope (HA) recognizes only the pro form (Fig. 1A). Of note, no smaller bands (<32 kDa) were detected with the N-terminal-directed antibody, suggesting that N-terminal antibody only recognized the pro form of neurotrophin and that the cleaved prodomain was not detected. Western blot analysis of transfected PC12 cell lysates confirmed that immunoblotting for a C-terminal epitope (HA) on BDNF and NGF recognize both pro and mature forms (Fig. 1B). Antibodies against the mature domain of BDNF and NGF (Fig. 1B) produced similar results in recognizing both pro and mature forms (Fig. 1B).
Figure 1.
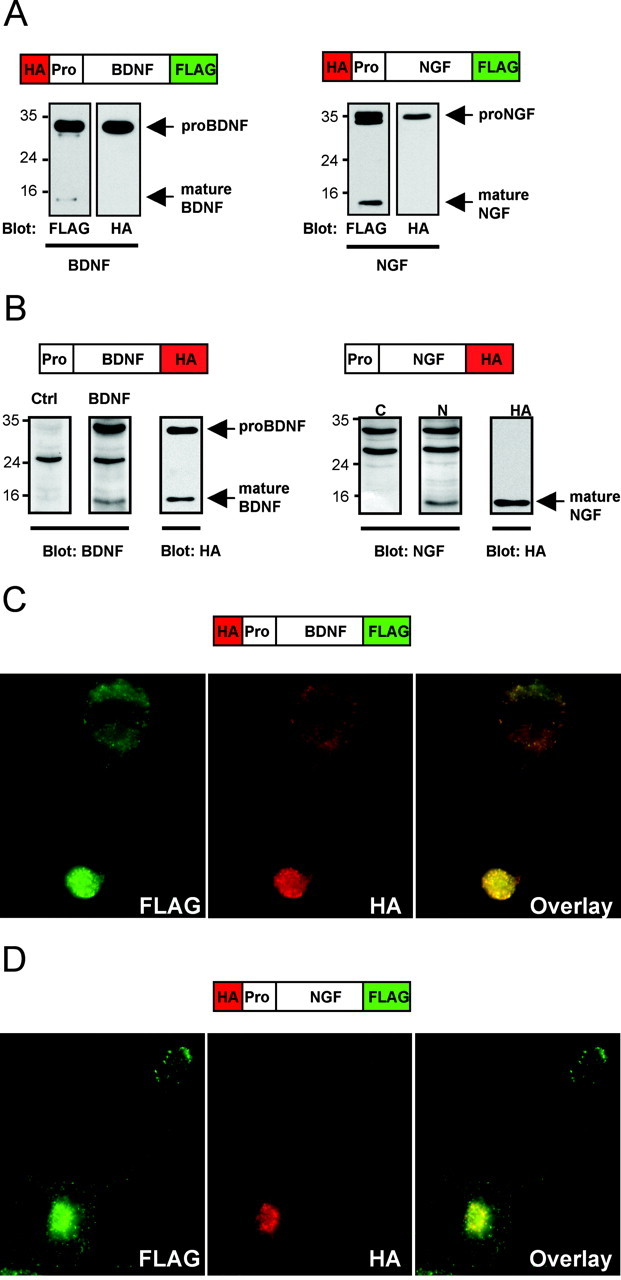
Processing and localization of epitope-tagged neurotrophins. A, Dual N-terminal HA-tagged and C-terminal FLAG-tagged BDNF or NGF was transfected into COS-7 cells, and, after 48 hr, lysates were prepared as described in Materials and Methods and analyzed by immunoblotting with anti-HA and anti-FLAG antibodies. B, Single C-terminal HA-tagged BDNF or NGF was transfected into differentiated PC12 cells. After 48 hr, lysates were prepared as described in Materials and Methods and analyzed by immunoblotting with C-terminal antibodies for BDNF or NGF or with anti-HA antibodies. Dual-epitope-tagged BDNF (C) and NGF (D) were transfected in differentiated PC12 cells. After 48 hr, cells were fixed and permeabilized, and subcellular distribution of neurotrophins was visualized by indirect immunofluorescence microscopy using epitope antibodies. Representative epifluorescence images are shown.
To image the intracellular locations of pro and mature neurotrophins, we transfected PC12 cells with the dual-epitope-tagged neurotrophin constructs and stained for the C-terminal FLAG and N-terminal HA epitopes. We observed almost complete overlap of the N- and C-terminal epitope tag staining patterns for wild-type BDNF (Fig. 1C). By Western blot analysis of COS-7 and PC-12 cell lysates (Fig. 1A,B), the antibodies directed against the C-terminal epitope detect both pro and mature forms, whereas the N-terminal epitope antibodies detect only the pro form. The predominant overlapping immunostaining of both C- and N-terminal epitopes suggests that the pro form of BDNF is the major intracellular form. To confirm that this staining pattern was for proBDNF, PC12 cells were stained with pro-specific antibodies for BDNF. Similar to the dual-epitope staining pattern, the majority of immunoreactivity for prodomain staining was found in punctate structures in the distal processes of the PC12 cells and overlapped with the C-terminal antibody staining (data not shown). In contrast, N- and C-terminal epitope immunostaining for NGF in PC12 cells displayed minimal overlap, except in a perinuclear compartment (Fig. 1D). These results are consistent with past biochemical studies demonstrating that the majority of NGF appears to be cleaved in the Golgi apparatus, whereas BDNF is more predominantly in the pro form in neuronal cells (Mowla et al., 1999). Thus, our dual-epitope-tagged neurotrophin staining system allowed for subcellular visualization of both pro and mature forms for BDNF and NGF.
Processing and trafficking of BDNFMet in non-neuronal cells
The single nucleotide polymorphism in the BDNF that leads to a valine to methionine substitution at position 66 has been shown to lead to impaired BDNF secretion in cultured primary hippocampal neurons (Egan et al., 2003). We wanted to use our fluorescent imaging system to examine the specificity underlying defective BDNFMet trafficking. Our first question focused on whether this defect in secretion was specific to neuronal cells. We chose to study non-neuronal cells that endogenously produce and secrete BDNF. Endothelial cells and vascular smooth muscle cells in the cardiovascular system secrete and use BDNF as an autocrine or local paracrine mediator of cardiovascular development, maintenance of intracardiac vessel stability, as well as response to vascular lesioning (Donovan et al., 1995, 2000; Wang et al., 2000). The amount of BDNF in the vasculature and heart are at levels comparable with the CNS (Scarisbrick et al., 1993; Donovan et al., 1995; Hiltunen et al., 1996). These peripheral cells differ from neurons in two important aspects. They lack the specialized depolarization-dependent secretion pathway, and they do not have a highly polarized morphology of neuronal cells.
In an endothelial cell line (Schweitzer et al., 1997), we observed a uniform distribution of wild-type and variant BDNF staining (Fig. 2A). In addition, for both wild-type and variant BDNF, there was almost complete overlap of the N- and C-terminal epitope tag staining patterns, suggesting that the predominant species was the pro form (Fig. 2A). Similarly, by immunostaining in an immortalized vascular smooth muscle cell line (Kraemer et al., 1999; Donovan et al., 2000), there was no difference in the distribution or the almost complete overlap of the N- and C-terminal epitope tag staining of transfected BDNF variant compared with wild-type BDNF (Fig. 2B). We also confirmed that staining for the N-terminal epitope antibody overlapped with staining with the proBDNF-specific antibody(data not shown). Furthermore, we measured by ELISA no functional difference between variant and wild-type BDNF secretion in these cells (data not shown). This is in marked contrast to BDNFMet trafficking in hippocampal neurons (Egan et al., 2003) in which there has been shown to be defects in intracellular localization and regulated secretion. Together, these results suggest that, in cells that endogenously produce and secrete BDNF but lack the depolarization-dependent regulated secretory machinery, there is no apparent defect in processing, localization, or secretion of BDNFMet. Thus, BDNFMet does not have a generalized defect in secretion but rather a defect in a specific cell population, neuronal cells.
Figure 2.
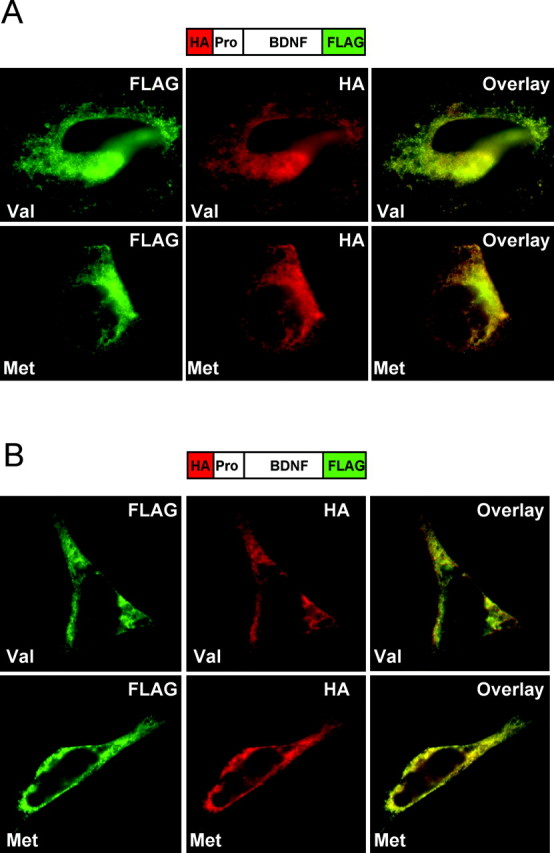
Variant BDNF has no defect in localization and secretion in endothelial and vascular smooth muscle cells. A, Localization of dual-epitope-tagged BDNF species in endothelial cells. After transfection, fixation, and permeabilization, subcellular distribution of wild-type (Val) and variant (Met) BDNF was visualized by indirect immunofluorescence microscopy using anti-HA and anti-FLAG antibodies. B, Localization of dual-epitope-tagged BDNFVal in vascular smooth muscle cells. After transfection, fixation, and permeabilization, subcellular distribution of BDNFVal (Val) and variant (Met) BDNF was visualized by indirect immunofluorescence microscopy using anti-HA and anti-FLAG antibodies.
Dimerization and trafficking of coexpressed BDNFVal and BDNFMet in neuronal cells
Because the biosynthetic trafficking pathway in neuronal cells appears to be the primary pathway affected by this polymorphism, the second question we focused on was the effect of expression of BDNFMet on BDNFVal trafficking and processing. Coexpression of the wild-type and variant BDNF would closely reflect the condition in human populations that are heterozygous for this polymorphism and for which a clinical phenotype in memory, as well susceptibility to neuropsychiatric disorders, has been observed (Egan et al., 2003; Hariri et al., 2003; Lu, 2003b). First, we hypothesized that, when coexpressed in the same cell, pro forms of BDNFMet and BDNFVal may heterodimerize, because it has been demonstrated that heterodimers of BDNF with other neurotrophins can be formed (Heymach and Shooter, 1995; Farhadi et al., 2000). To determine quantitatively, the relative proportion of BDNFMet and BDNFVal heterodimerization when both forms are expressed in the same cell, we performed a series of sequential coimmunoprecipitation experiments. We initially determined whether we could quantitatively detect different degrees of heterodimerizatiom of two epitope-tagged forms of wild-type BDNF. We coexpressed in COS-7 cells wild-type C-terminal FLAG-BDNFVal and HA-BDNFVal with increasing proportion of FLAG-BDNFVal compared with HA-BDNFVal and subjected the lysates to an initial HA antibody immunoprecipitation (IP#1) and then a FLAG antibody immunoprecipitation (IP#2). As a control, we determined that the first immunoprecipitation with HA antibodies (IP#1) pulled down >95% of all of the detectable HA-tagged BDNF in the cell lysate, by performing a second immunoprecipitation with HA antibodies and quantitating the resultant immunocomplex by Western blot analysis (data not shown). The relative degree of FLAG-BDNFVal·HA-BDNFVal complex formation was determined by quantitation of the proportion of FLAG-BDNFVal coimmunoprecipitated by HA antibodies (Fig. 3A, IP#1, Blot: FLAG) compared with the total amount of FLAG-BDNFVal immunoprecipitated by both immunoprecipitation steps (Fig. 3A, IP#1, IP#2, Blot: FLAG). We confirmed that we could detect different proportions of epitope-tagged BDNF heterodimers with this sequential coimmunoprecipitation procedure (Fig. 3A,C). Increasing the ratio of FLAG-tagged BDNF compared with HA-tagged BDNF (FLAG/HA) from 1:3 to 3:1 led to a decrease in proportion of FLAG-BDNF·HA-BDNF heterodimer formed from 96.5% to 36.1%, respectively, with an concomitant increase in FLAG-BDNF homodimers (Fig. 3A,C).
Figure 3.
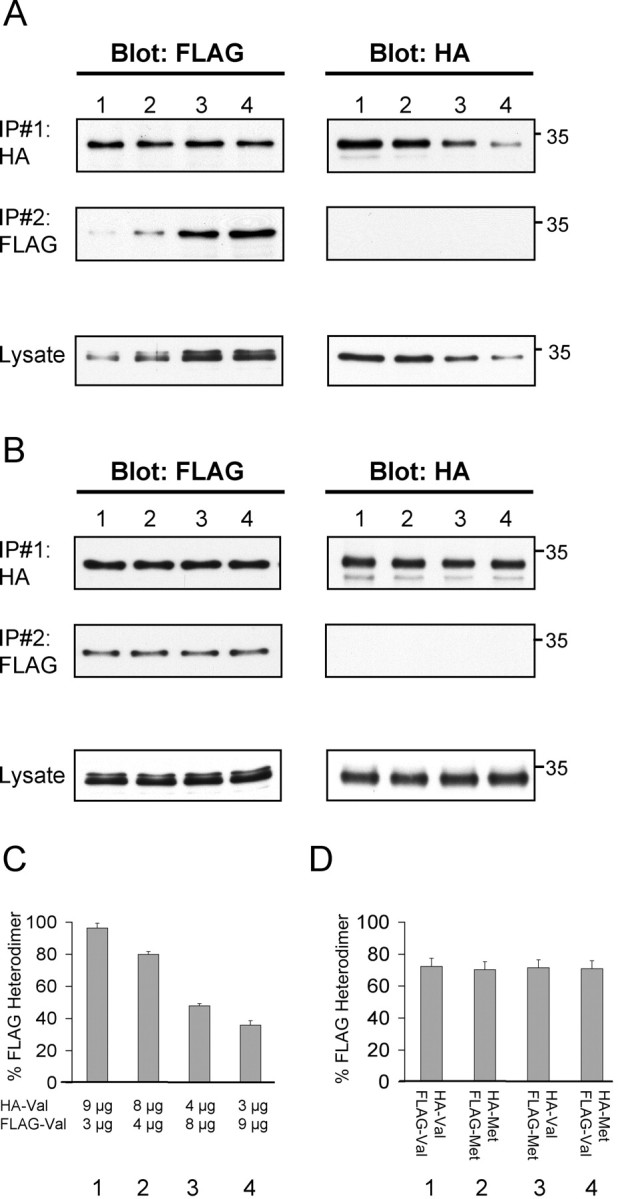
Coimmunoprecipitation of BDNFVal and BDNFMet heterodimers. A, To detect different degrees of heterodimerizatiom of two epitope-tagged forms of wild-type BDNF, COS-7 cells were transfected with two wild-type BDNF constructs bearing different C-terminal epitopes (HA or FLAG). The amount of cotransfected FLAG-BDNF and HA-BDNF was varied while maintaining a constant amount of total DNA (12 μg) transfected. Cell lysates were prepared as described in Materials and Methods, and the first immunoprecipitations were performed with HA antibodies (IP#1 indicates HA). A second immunoprecipitation was subsequently performed on the remaining lysates with FLAG antibodies (IP#2 indicates FLAG), and immunoprecipitated samples were analyzed by Western blot. In addition, initial lysates (20 μg of total protein) were analyzed by Western blot for levels of transfected FLAG-BDNF and HA-BDNF. B, To detect different degrees of heterodimerizatiom of epitope-tagged forms of wild-type and variant BDNF, COS-7 cells were transfected with combinations of BDNFVal and BDNFMet constructs bearing different C-terminal epitopes (HA-BDNFVal plus FLAG-BDNFVal, HA-BDNFMet plus FLAG-BDNFMet, HA-BDNFVal plus FLAG-BDNFMet, or FLAG-BDNFVal plus HA-BDNFMet). A constant amount (6 μg) of each epitope-tagged BDNF species was transfected to maintain a constant amount of total DNA (12 μg) transfected. Cell lysates were prepared as described in Materials and Methods, and sequential immunoprecipitations were performed (IP#1 indicates HA; IP#2 indicates FLAG), as described in A, and analyzed by Western blot. In addition, initial lysates (20 μg of total protein) were analyzed by Western blot for levels of transfected BDNFVal and BDNFMet. C, Quantitation of Western blots in A as a proportion of FLAG-BDNF immunoreactivity found in IP#1 over the total FLAG immunoreactivity obtained from both immunoprecipitation steps (IP#1 and IP#2, Blot: FLAG). Mean ± SEM proportions were determined from analysis of three independent experiments. D, Quantitation of Western blots in B as a proportion of FLAG-BDNF immunoreactivity found in IP#1 over the total FLAG immunoreactivity obtained from both immunoprecipitation steps (IP#1 and IP#2, Blot: FLAG). Mean ± SEM proportions were determined from analysis of three independent experiments.
We then coexpressed equal amounts of C-terminal FLAG-BDNFMet and HA-BDNFVal in COS-7 cells and confirmed that, like wild-type BDNF, the predominant form in the cell lysates was the 32 kDa pro form. Sequential coimmunoprecipitations were performed with HA antibodies (IP#1) and then FLAG antibodies (IP#2) (Fig. 3B, lane 3). We confirmed that the initial immunoprecipitation with HA antibodies was able to pull down all detectable FLAG-BDNFMet·HA-BDNFVal complexes (Fig. 3B). The relative degree of FLAG-BDNFMet·HA-BDNFVal complex formation was determined by quantitation of the proportion of FLAG-BDNFMet coimmunoprecipitated by HA antibodies compared with the total amount of FLAG-BDNFMet immunoprecipitated by both immunoprecipitation steps. We determined that 71.5% of the BDNFMet was complexed with BDNFVal. This proportion was equivalent to the proportion of heterodimerization of coexpressed wild-type BDNF forms with different epitope tags (Fig. 3B, lane 1) or coexpressed variant BDNF forms with different epitope tags (Fig. 3B, lane 2). In addition, switching epitope tags on BDNFMet and BDNFVal did not alter this level of complex formation (Fig. 3B, lane 4). These experiments provide quantitative evidence that, when both BDNFMet and BDNFVal are coexpressed in the same cell, the majority of BDNFMet (>70%) is heterodimerized with BDNFVal (Fig. 3D).
Second, we determined whether there was a quantitative difference in intracellular distribution of coexpressed variant and wild-type BDNF in PC12 cells, an established neuronal cell line with a polarized morphology, and cortical neurons by use of the C-terminal epitope tag staining. We initially observed that, when expressed individually, BDNFMet was localized more in the cell body compared with BDNFVal in both PC12 cells and cortical neurons (Fig. 4A,C). We then observed in PC12 cells (Fig. 4B) and cortical neurons (data not shown) that, when coexpressed, the majority of FLAG-BDNFMet staining overlapped with HA-BDNFVal staining, with little FLAG- or HA-alone staining observed. Quantitative analysis of colocalization of BDNFMet and BDNFVal showed that >70% of wild-type and variant BDNF colocalized in the same cell (data not shown). This high degree of colocalization was equivalent to that observed when we cotransfected two wild-type BDNF constructs with different epitope tags (HA and FLAG) (data not shown). This high degree of colocalization is also consistent with the high degree of heterodimerization of pro forms of BDNFMet and BDNFVal (Fig. 3D).
To determine whether coexpression of BDNFMet with BDNFVal altered BDNFVal trafficking, we transfected equal amounts of the respective DNA constructs, which produced equal levels of BDNF protein expression, by Western blot analysis (data not shown). With the use of two different C-terminal epitope tags on the two species of neurotrophin (FLAG-BDNFMet and HA-BDNFVal), we were able to follow the intracellular distributions of both wild-type and variant BDNF. In PC12 cells, we determined that, compared with wild-type BDNF transfection alone, cotransfection of both variant and wild-type BDNF leads to a significantly greater distribution of wild-type BDNF in the cell body (p < 0.01) (Fig. 4B,D). In addition, in these cells coexpressing both wild-type and variant BDNF, BDNFMet was also significantly localized in the cell body (p < 0.01) (data not shown). In cortical neurons, we observed a similar shift in distribution for BDNFVal when coexpressed with BDNFMet (Fig. 4C). In these neurons, we quantitated the relative distribution of BDNFVal in the cell body and processes by 3D reconstruction of the entire neuron in x, y, and z planes and determined that there was a significantly greater distribution of wild-type BDNF in the cell body compared with individually expressed BDNFVal (p < 0.01) (Fig. 4E). In addition, in these cells coexpressing both wild-type and variant BDNF, BDNFMet was also significantly distributed in the cell body (p < 0.01) (data not shown). For the cortical neuronal cultures, we also costained for neuronal markers of dendrites (MAP2) or axons (Tau1) to confirm their identity as neurons (Fig. 4C) and also noted no quantitative difference in selective distribution of the variant BDNF in dendrites or axons under any of the expression conditions (data not shown). These series of experiments suggested that BDNFMet can alter the trafficking of BDNFVal from the biosynthetic pathway leading to more concentrated distribution in the cell body of polarized neurosecretory cells.
Identification of aberrant BDNF trafficking step involved in coexpressed BDNFVal and BDNFMet
It has been shown previously that, when expressed alone, BDNFMet has decreased distribution to distal neuronal processes, as well as decreased colocalization with secretory granule markers (Egan et al., 2003). In this context, the altered intracellular distribution of BDNFVal when coexpressed with BDNFMet could be attributable to two potential trafficking abnormalities: (1) a defect in sorting to the proper secretory organelle in transport to distal processes and/or (2) defect in transport to distal processes. To determine which step in the biosynthetic trafficking pathway led to the altered intracellular distribution of coexpressed BDNFVal, we first assessed colocalization with SecII, an established marker of secretory granules in the regulated secretory pathway (Huttner et al., 1991; Halban and Irminger, 1994; Ozawa and Takata, 1995). When BDNFMet and BDNFVal were cotransfected into differentiated PC12 cells or cortical neurons, less BDNFVal colocalized with the secretory granule marker (SecII) compared with BDNFVal control condition (Fig. 5A,B). Becuase the majority of endogenous SecII staining was in the distal processes, it remained a possibility that the quantitative lack of colocalization was attributable to the decreased transport of BDNF to the distal processes.
Figure 5.
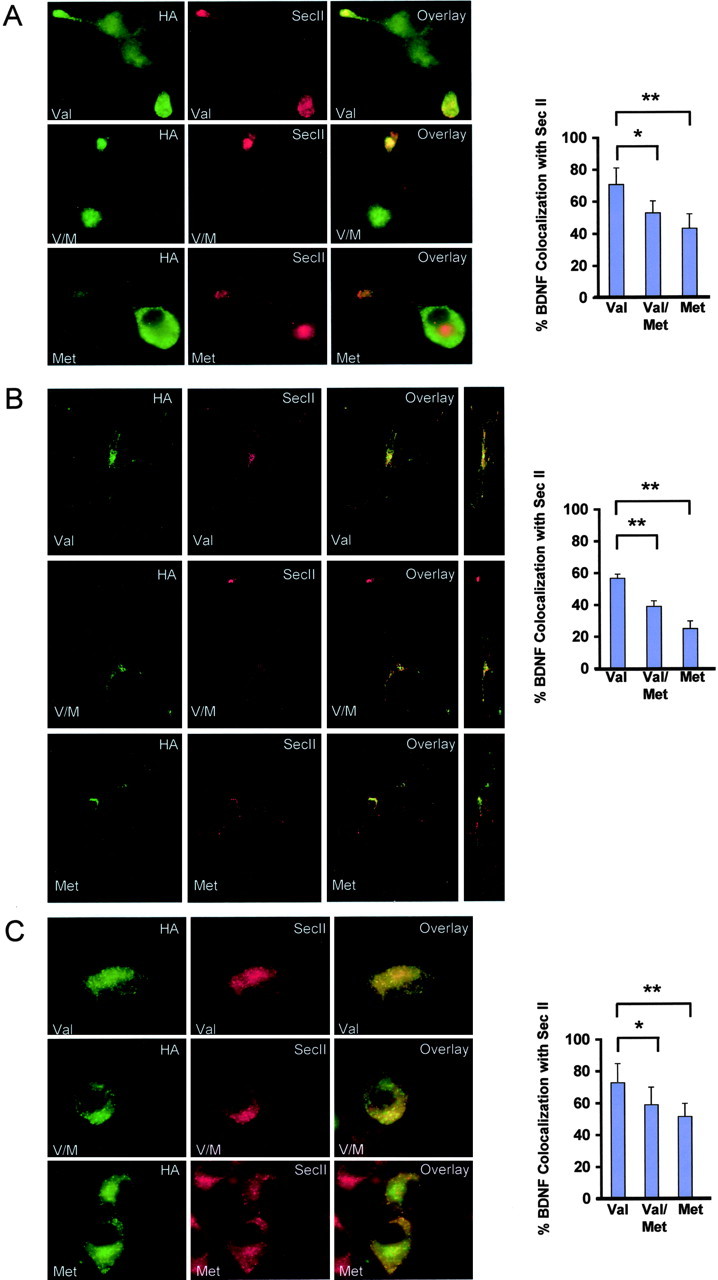
Subcellular colocalization of variant and wild-type BDNF with organelle markers. Differentiated PC12 cells (A), cortical neurons (B), and undifferentiated PC12 cells (C) were transfected with C-terminal HA-tagged variant (Met), C-terminal HA-tagged wild-type BDNF (Val), or both C-terminal FLAG-tagged variant and HA-tagged wild-type BDNF (V/M). Cells were fixed after 48 hr and permeabilized. Colocalization of BDNF and the secretory granule marker SecII was visualized by costaining with anti-HA and anti-SecII antibodies. Confocal three-dimensional reconstruction of serial z planes were obtained, and alternate rotation angle is also shown for each representative cell. Quantitative analysis of results illustrated in A–C was conducted by scoring ∼25 cells for each BDNF condition. The proportion of colocalization between BDNF and SecII is presented as a mean ± SEM determined from analysis of four independent experiments (*p < 0.01; **p < 0.001; Student's t test).
To control for the possibility that there was a defect in transport from the cell body to distal processes, we assessed BDNF localization in nonpolarized undifferentiated PC12 cells, which contain similar regulated secretion machinery as in differentiated PC12 cells (Schubert and Klier, 1977), in the presence and absence of BDNFMet. In undifferentiated PC12 cells, BDNFVal and BDNFMet were in punctate structures and were evenly distributed throughout the cell (Fig. 5C). We first observed that, when expressed alone, there was significantly less colocalization of BDNFMet with SecII staining (p < 0.001) (Fig. 5C). When coexpressed, BDNFMet (data not shown) and BDNFVal also were observed to have a significantly decreased colocalization with SecII staining (p < 0.01) (Fig. 5C). These results suggest that the decreased BDNFVal colocalization with SecII was not attributable to decreased transport from the cell body to the distal processes. Together, these results suggest that, when coexpressed, the resulting BDNFMet and BDNFVal complex is less efficiently sorted from the Golgi apparatus to secretory granules destined for the regulated secretory pathway.
Functional consequences of aberrant intracellular trafficking on BDNFMet secretion
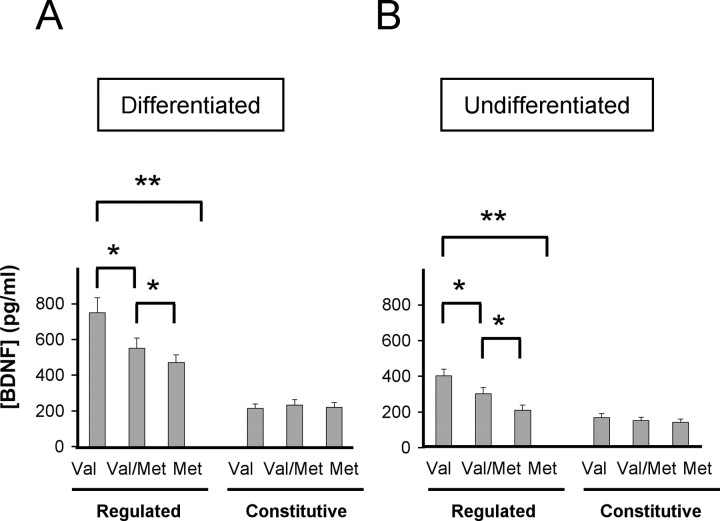
To determine whether the altered colocalization of BDNFVal, when coexpressed with BDNFMet, with secretory granule markers has functional consequences, we assessed in PC12 cells whether BDNF constitutive or regulated secretion was affected. Using a BDNF ELISA assay, we observed first that BDNFMet (Met), transfected alone in differentiated or undifferentiated PC12 cells, has decreased regulated secretion compared with BDNFVal (p < 0.001) (Fig. 6A,B). This finding from the undifferentiated PC12 cells confirms our immunofluorescent results (Fig. 5C), suggesting that trafficking defect associated with the Met polymorphism is one attributable to inefficient sorting to secretory granules rather than a transport defect. In addition, when both BDNFVal and BDNFMet were transfected together (Val/Met), the amount of total BDNF secreted in the regulated pathway was decreased compared with wild-type BDNF (p < 0.01) but higher than BDNFMet alone (Fig. 6A,B). There was no significant difference in constitutive secretion in either differentiated or undifferentiated PC12 cells (Fig. 6A,B). Together, these results suggest that the presence of a single phenocopy of BDNFMet is sufficient to impair wild-type BDNF regulated or activity-dependent secretion.
Figure 6.
Regulated and constitutive secretion of BDNFVal and BDNFMet. Differentiated (A) and undifferentiated (B) PC12 cells were transfected with BDNFVal (Val), BDNFMet (Met), or both BDNF species (Val/Met). After 48 hr, media was collected under depolarization and constitutive secretion conditions, as described in Materials and Methods, and analyzed by ELISA. Results are presented as a mean ± SEM determined from analysis of three independent experiments (*p < 0.01; **p < 0.001; Student's t test).
Identification of secreted forms of BDNFVal and BDNFMet
Our studies suggest that BDNFVal· BDNFMet heterodimers are inefficiently sorted into the regulated secretory pathway and may be responsible for the observed clinical phenotypes. Because pro and mature forms of neurotrophins can induce opposing biological functions, apoptosis and survival, respectively (Lee et al., 2001; Beattie et al., 2002), it remains a possibility that an aberrant form of secreted BDNF heterodimer could also contribute to the observed biological effects. Thus, it was important to determine the actual forms of BDNF that are secreted in an activity-dependent manner. Although the forms of BDNF released constitutively from neuronal and neurosecretory cells has been observed previously (Mowla et al., 1999), it is not known what forms of BDNF are released from the regulated secretory pathway. We initially determined the specific forms of wild-type or variant BDNF that were secreted from PC12 cells when expressed alone after KCl-induced depolarization. After a 10 min depolarization with KCl, BDNF was harvested from the media of PC12 cells transfected with either a C- or N-terminal HA-tagged BDNF construct and immunoprecipitated with HA antibodies. First, we observed that there was a decrease in the amount of total BDNF released in the BDNFMet and BDNFMet plus BDNFVal conditions (Fig. 7A), consistent with the secretion results from the ELSA (Fig. 6). Then, we observed that, by immunoprecipitating a C-terminal HA-tagged BDNF, two forms of secreted BDNF were observed. These resolved as a major 19 kDa and a minor 14 kDa form (Fig. 7A). No BDNF was observed after immunoprecipitating an N-terminal HA-tagged BDNF (Fig. 7B), suggesting that the 19 and 14 kDa forms of BDNFVal and BDNFMet lacked the N-terminal epitope as a result of cleavage of the prodomain. During cotransfection of HA epitope-tagged BDNFVal and BDNFMet constructs in PC12 cells, the secreted forms of BDNF were identical to those of individually expressed BDNFVal or BDNFMet. Specifically, only a predominant 19 kDa and minor 14 kDa form were detected by Western blot analysis during immunoprecipitation of the C-terminal HA-BDNF, and no BDNF forms were detected during immunoprecipitation of the N-terminal HA-BDNF (Fig. 7A,B).
Figure 7.
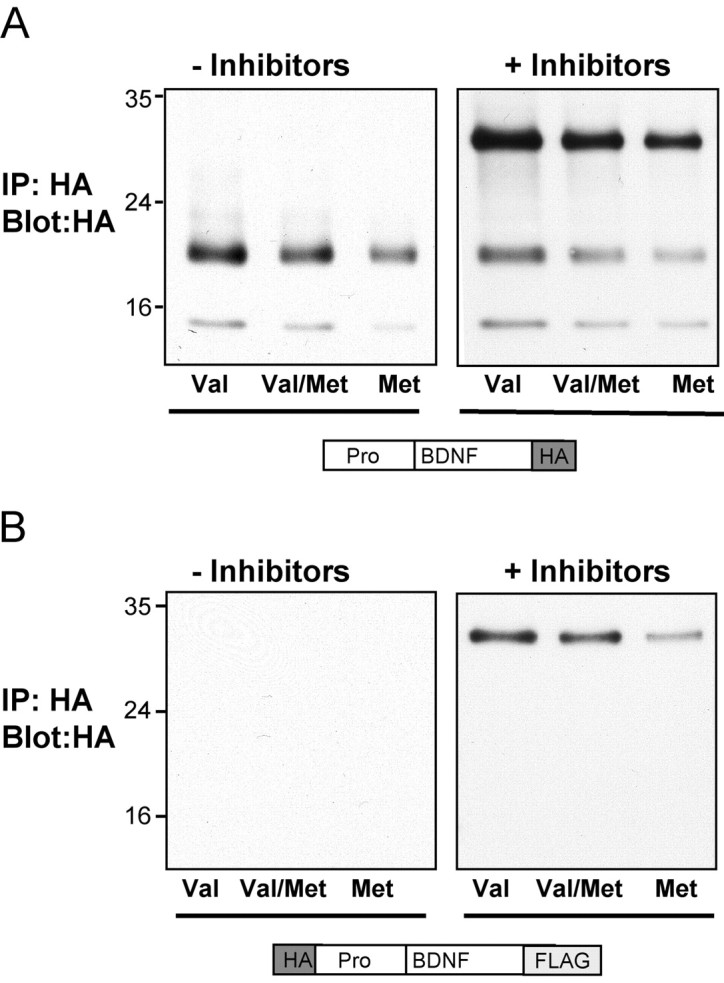
Identification of secreted forms of BDNFMet. Differentiated PC12 cells were transfected with BDNFVal (Val), BDNFMet (Met), or both BDNFVal and BDNFMet (Val/Met). The BDNF constructs contained either a single C-terminal HA epitope tag (A) or dual N-terminal HA and FLAG C-terminal tags (B). After 48 hr, media was collected under depolarization conditions, as described in Materials and Methods, in the absence or presence of protease inhibitors. BDNF in the media was immunoprecipitated with polyclonal anti-HA antibodies and analyzed by immunoblotting with monoclonal anti-HA antibodies. Representative blots from three independent experiments are shown.
These results were unexpected because, by both Western bot analysis of PC12 lysates (Fig. 1B) and by immunofluorescent microscopy (Fig. 1C), the predominant intracellular form of BDNF was the uncleaved pro form containing intact N-terminal and C-terminal epitope tags. To determine whether a rapid extracellular cleavage event was responsible for the generation of the lower molecular mass forms, similar secretion studies were performed in the presence of protease inhibitors. BDNFVal, BDNFMet, or BDNFVal and BDNFMet were harvested, after a 10 min depolarization with KCl in the presence of plasmin and metalloprotease inhibitors, from the media of PC12 cells, transfected with either a C-terminal or N-terminal HA-tagged BDNF construct. Western blot analysis of immunoprecipitates with HA antibodies revealed that the secreted forms of BDNF were predominantly the 32 kDa form, with minor 19 and 14 kDa forms (Fig. 7A). Parallel studies using a BDNFVal or BDNFMet construct, with an N-terminal HA tag and C-terminal FLAG tag revealed only the higher 32 kDa form (Fig. 7B), confirming that the full-length pro form of BDNFVal or BDNFMet is secreted during depolarization. The minimally higher migration pattern of the dual-epitope BDNF species is likely attributable to the presence of two epitope tags instead of one. In the presence of protease inhibitors, the secreted forms of coexpressed BDNFVal and BDNFMet were also identical to that of BDNFVal, with the major form migrating at 32 kDa (Fig. 7A,B). Together, these results suggest that BDNF is secreted from the regulated secretory pathway as an intact proBDNF form, which then undergoes rapid cleavage during release. This result is consistent with previous studies demonstrating that constitutively secreted proBDNF is capable of being cleaved extracellularly by plasmin and metalloproteases (Lee et al., 2001). Importantly, the polymorphism at position 66 in the BDNF prodomain does not alter the relative proportion of pro and mature forms of BDNF secreted in an activity-dependent manner.
Discussion
Our present studies on the intracellular and extracellular fate of BDNF and a BDNF polymorphism, BDNFMet, provide several new insights into the trafficking defect associated with this BDNF polymorphism. First, through immunofluorescent microscopy and secretion studies, we observed that this prodomain alteration impairs intracellular trafficking and regulated secretion of BDNF in primary cortical neurons and neurosecretory cells but not in endothelial and vascular smooth muscle cells. This finding is significant, because it suggests that the BDNF-producing neuronal cell populations in the CNS, but not those in the cardiovascular system, are specifically affected by this Met substitution in the BDNF prodomain. This finding is consistent with the functional deficits associated with the BDNF polymorphism in human subjects. The BDNFMet polymorphism is relatively common in the human population with a prevalence for heterozygotes between 20 and 30% and the prevalence for the homozygotes at ∼4% (Neves-Pereira et al., 2002; Egan et al., 2003; Hariri et al., 2003; Sen et al., 2003). To date, several genetic linkage and behavioral studies have shown that this polymorphism has been associated with only neuropsychiatric disorders and memory impairments (Momose et al., 2002; Neves-Pereira et al., 2002; Sklar et al., 2002; Ventriglia et al., 2002; Egan et al., 2003; Hall et al., 2003; Hariri et al., 2003; Ribases et al., 2003; Sen et al., 2003). It is notable that no cardiovascular system impairments have been reported. BDNF has been established to play a significant role in heart development and postnatal cardiovascular system function. Deficient levels of BDNF in the cardiovascular system lead to vascular destabilization and intracardiac hemorrhage (Donovan et al., 1995, 2000; Kraemer et al., 1999; Wang et al., 2000). Our studies provide a plausible explanation for a mutation in BDNF causing a CNS phenotype but no obvious phenotypic defect in the cardiovascular system. The Met substitution in the prodomain leads to a selective impairment in regulated BDNF secretion, which is the primary means of BDNF release from neuronal cells but not from vascular cells.
A second finding from these studies is that the intracellular trafficking fate, as well as regulated secretion, of wild-type BDNF is altered by coexpression with the variant BDNF. From sequential coimmunoprecipitation studies, we demonstrate that, when coexpressed, >70% of BDNFMet is in BDNFVal·BDNFMet heterodimers (Fig. 3D). This finding is consistent with previous studies that showed that coexpressing different neurotrophins can lead to the formation of heterodimers and that their trafficking fates can be altered (Heymach and Shooter, 1995; Farhadi et al., 2000; Hibbert et al., 2003). Although it has been assumed that BDNF does not form heterodimers in vivo with the other neurotrophins (NGF, NT-3, and NT-4), it is known that humans heterozygous for the BDNFMet polymorphism do coexpress both BDNFVal and BDNFMet. Our coexpression studies are consistent with the functional studies from human subjects heterozygous for this polymorphism, in which having one copy of the bdnfMet gene led to deficits in cognitive tasks (Egan et al., 2003; Hariri et al., 2003). Thus, our studies provide insight into how the presence of one copy of the BDNFMet gene can lead to a decrease in the amount of total BDNF released from cells in an activity-dependent manner. Through the predominant formation of BDNFVal·BDNFMet heterodimers, there is less efficient BDNF trafficking into the regulated secretory pathway in neurons. This decreased regulated secretion of BDNF may explain the behavioral deficits observed in humans heterozygous for the relatively common Met polymorphism.
Third, we determined the form of BDNF secreted in an activity-dependent manner to be the 32 kDa pro form that is rapidly cleaved to mature form after secretion (Fig. 7). It has been shown previously that proBDNF can be constitutively secreted from cultured neurons (Heymach et al., 1996; Mowla et al., 1999, 2001; Farhadi et al., 2000) and endothelial cells (Lee et al., 2001). However, it has also been established that the majority of BDNF released from neurons is attributable to activity-dependent secretion from the regulated secretory pathway rather than the constitutive secretory pathway (Lu, 2003b). Our findings provide, for the first time, evidence that the 32 kDa pro form of BDNF is the major form released from the regulated secretory pathway. This is significant because many biological functions mediated by BDNF in the nervous system are from BDNF released in this activity-dependent manner. In particular, there is accumulating evidence that activity-dependent release of BDNF rapidly modulates synaptic transmission and neural plasticity in a variety of different experimental paradigms (Poo, 2001; Lu, 2003a). BDNF causes increases in EPSCs in hippocampal neurons (Kang and Schuman, 1995; Levine et al., 1995, 1996). The maintenance of long-term potentiation, the hallmark cellular basis for learning and memory, in the CA1 region of the hippocampus is also impaired in slices from mice deficient in BDNF (Korte et al., 1995). In addition, proneurotrophins such as proNGF has been shown to have biological activity through their selective activation of the p75 neurotrophin receptors (Lee et al., 2001; Beattie et al., 2002), whereas the mature forms interact selectively with Trk neurotrophin receptors. Our studies suggest that a significant proportion of the processing of proBDNF to mature BDNF may occur extracellularly. Thus, proteases on the cell surface or extracellular space will play a critical role in determining the proportion of BDNF forms (pro or mature) produced and thus the biological activity of BDNF that is released after depolarization.
In this context, we observed that the forms of BDNFMet released after depolarization, whether expressed alone or with BDNFVal, did not differ from wild-type BDNF. We determined that there was no difference in proportions of pro and mature forms of BDNFMet released compared with BDNFVal (Fig. 7). We assume that the 19 kDa form of BDNF, produced after cleavage of the 32 kDa form, is attributable to cleavage occurring ∼80 amino acids from the N terminus, which would remove the portion containing the Met polymorphism. Thus, the molecular mechanism underlying defective BDNFMet function appears to be attributable not to the form of BDNF secreted but more simply the amount of BDNF released in an activity-dependent manner.
These findings demonstrating altered intracellular trafficking of wild-type BDNF trafficking, with coexpression of the variant BDNF, as well as the lack of proteolytic processing defects for the secreted BDNFVal·BDNFMet heterodimers, provide a more specific mechanistic framework to understand the secretion defect caused by the Met substitution. Our findings point to the presence of a specific trafficking signal in the BDNF prodomain region containing the Met substitution that is required for efficient BDNF sorting to the regulated secretory pathway. Importantly, through the formation of heterodimers with the wild-type BDNF, BDNFMet is capable of altering the trafficking fate when both wild-type and variant BDNF are present in the same cell. These findings emphasize the importance of intracellular sorting of pro forms of BDNF into the cell-specific secretory pathways as a general mechanism of regulating appropriate biological activity. Thus, defects in the fidelity of these sorting events may lead to vulnerability to a wide range of CNS dysfunctions. An important direction for future studies will be to link the cell biological defect in BDNFMet trafficking to in vivo functional deficits found in human heterozygous for this polymorphism.
Footnotes
This work was supported by the DeWitt-Wallace Fund of the New York Community Trust (F.S.L.), the National Alliance for Research on Schizophrenia and Depression (F.S.L.), the Nancy Pritzker Depression Network (F.S.L., P.D.P.), Foundation for the Author of National Excellent Doctoral Dissertation of China Grant 200229 (Z.-Y.C.), Shanghai Youth Science and Technology Phosphor Grant 01QB14001 (Z.-Y.C.), National Natural Science Foundation of China Grant 30000048 (Z.-Y.C.), and National Institute of Neurological Disorders and Stroke Grant NS30687 (B.L.H., K.K.T.). We thank Alessandro Ieraci for cortical neuronal cultures, Rosemary Kraemer for vascular smooth muscle cell cultures, and Margit Burmeister, Moses Chao, and Pilar Perez for helpful discussions.
Correspondence should be addressed to Francis S. Lee, Weill Medical College of Cornell University, 1300 York Avenue, Box 244, New York, NY 10021. E-mail: fslee@med.cornell.edu.
DOI:10.1523/JNEUROSCI.0348-04.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/244401-11$15.00/0
References
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO (2002) ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 36: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309. [DOI] [PubMed] [Google Scholar]
- Donovan M, Miranda R, Kraemer R, McCaffrey T, Tessarollo L, Mahadeo D, Sharif S, Kaplan D, Tsoulfas P, Parada L, Hempstead B (1995) Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol 147: 309–324. [PMC free article] [PubMed] [Google Scholar]
- Donovan M, Lin M, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez C, Rafii S, Hempstead B (2000) Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 127: 4531–4540. [DOI] [PubMed] [Google Scholar]
- Egan M, Kojima M, Callicott J, Goldberg T, Kolachana B, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger D (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257–269. [DOI] [PubMed] [Google Scholar]
- Farhadi H, Mowla S, Petrecca K, Morris S, Seidah N, Murphy R (2000) Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. J Neurosci 20: 4059–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Irminger JC (1994) Sorting and processing of secretory proteins. Biochem J 299: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D, Dhilla A, Charalambous A, Gogos JA, Karayiorgou M (2003) Sequence variants of the brain-derived neurotrophic factor (BDNF) gene are strongly associated with obsessive-compulsive disorder. Am J Hum Genet 73: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23: 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL, Rabin SJ, Kaplan L, Reid S, Parada LF, Kaplan DR (1992) Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron 9: 883–896. [DOI] [PubMed] [Google Scholar]
- Heymach Jr JV, Shooter EM (1995) The biosynthesis of neurotrophin heterodimers by transfected mammalian cells. J Biol Chem 270: 12297–12304. [DOI] [PubMed] [Google Scholar]
- Heymach Jr JV, Kruttgen A, Suter U, Shooter EM (1996) The regulated secretion and vectorial targeting of neurotrophins in neuroendocrine and epithelial cells. J Biol Chem 271: 25430–25437. [DOI] [PubMed] [Google Scholar]
- Hibbert AP, Morris SJ, Seidah NG, Murphy RA (2003) Neurotrophin-4, alone or heterodimerized with brain-derived neurotrophic factor, is sorted to the constitutive secretory pathway. J Biol Chem 278: 48129–48136. [DOI] [PubMed] [Google Scholar]
- Hiltunen JO, Arumae U, Moshnyakov M, Saarma M (1996) Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ Res 79: 930–939. [DOI] [PubMed] [Google Scholar]
- Huang E, Reichardt L (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Gerdes HH, Rosa P (1991) The granin (chromogranin/secretogranin) family. Trends Biochem Sci 16: 27–30. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267: 1658–1662. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA 92: 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer R, Nguyen H, March KL, Hempstead B (1999) NGF activates similar intracellular signaling pathways in vascular smooth muscle cells as PDGF-BB but elicits different biological responses. Arterioscler Thromb Vasc Biol 19: 1041–1050. [DOI] [PubMed] [Google Scholar]
- Lee F, Chao M (2001) Activation of Trk neurotrophin receptors in the absence of neurotrophins. Proc Natl Acad Sci USA 92: 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng K, Hempstead B (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945–1948. [DOI] [PubMed] [Google Scholar]
- Levine E, Dreyfus C, Black I, Plummer M (1996) Selective role for trkB neurotrophin receptors in rapid modulation of hippocampal synaptic transmission. Mol Brain Res 38: 300–303. [DOI] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR (1995) Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA 92: 8074–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B (2003a) BDNF and activity-dependent synaptic modulation. Learn Mem 100: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B (2003b) Pro-region of neurotrophins. Role in synaptic modulation. Neuron 39: 735–738. [DOI] [PubMed] [Google Scholar]
- Momose Y, Murata M, Kobayashi K, Tachikawa M, Nakabayashi Y, Kanazawa I, Toda T (2002) Association studies of multiple candidate genes for Parkinson's disease using single nucleotide polymorphisms. Ann Neurol 51: 133–136. [DOI] [PubMed] [Google Scholar]
- Mowla S, Pareek S, Farhadi H, Petrecca K, Fawcett J, Seidah N, Morris S, Sossin W, Murphy R (1999) Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J Neurosci 19: 2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla S, Farhadi H, Pareek S, Atwal J, Morris S, Seidah N, Murphy R (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276: 12660–12666. [DOI] [PubMed] [Google Scholar]
- Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy J (2002) The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet 71: 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa H, Takata K (1995) The granin family—its role in sorting and secretory granule formation. Cell Struct Funct 20: 415–420. [DOI] [PubMed] [Google Scholar]
- Poo M (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–31. [DOI] [PubMed] [Google Scholar]
- Ribases M, Gratacos M, Armengol L, De Cid R, Badia A, Jimenez L, Solano R, Vallejo J, Fernandez F, Estivill X (2003) Met66 in the brain-derived neurotrophic factor (BDNF) precursor is associated with anorexia nervosa restrictive type. Mol Psychiatry 8: 745–751. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Jones EG, Isackson PJ (1993) Coexpression of mRNAs for NGF, BDNF, and NT-3 in the cardiovascular system of the pre- and postnatal rat. J Neurosci 13: 875–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Klier FG (1977) Storage and release of acetylcholine by a clonal cell line. Proc Natl Acad Sci USA 74: 5184–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer K, Vicart P, Delouis C, Paulin D, Drager A, Langenhuijsen M, Weksler B (1997) Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest 76: 25–36. [PubMed] [Google Scholar]
- Sen S, Nesse R, Stoltenberg S, Li S, Gleiberman L, Chakravarti A, Weder A, Burmeister M (2003) A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology 28: 397–401. [DOI] [PubMed] [Google Scholar]
- Sklar P, Gabriel S, McInnis M, Bennett P, Lim Y, Tsan G, Schaffner S, Kirov G, Jones I, Owen M, Craddock N, JR D, Lander E (2002) Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry 7: 579–593. [DOI] [PubMed] [Google Scholar]
- Tessarollo L (1998) Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev 9: 125–137. [DOI] [PubMed] [Google Scholar]
- Ventriglia M, Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M (2002) Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer's disease. Mol Psychiatry 7: 136–137. [DOI] [PubMed] [Google Scholar]
- Wang S, Bray P, McCaffrey T, March K, Hempstead BL, Kraemer R (2000) p75(NTR) mediates neurotrophin-induced apoptosis of vascular smooth muscle cells. Am J Pathol 157: 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]