Abstract
The developing prefrontal cortex receives a dense serotonergic innervation, yet little is known about the actions of serotonin [5-Hydroxytryptamine (5-HT)] in this region during development. Here, we examined the developmental regulation of 5-HT receptors controlling the excitability of pyramidal neurons of this region. Using whole-cell recordings in in vitro brain slices, we identified a dramatic shift in the effects of 5-HT on membrane potential during the postnatal developmental period. In slices derived from young animals [postnatal day (P) 6 to P19], administration of 5-HT elicits a robust depolarization of layer V pyramidal neurons, which gradually shifts to a hyperpolarization commencing during the third postnatal week. This progression is the result of coordinated changes in the function of 5-HT7 and 5-HT2A receptors, which mediate different aspects of the depolarization, and of 5-HT1A receptors, which signal the late developing hyperpolarization. The loss of the 5-HT7 receptor-mediated depolarization and the appearance of the 5-HT1A receptor-mediated hyperpolarization appears to reflect changes in receptor expression. In contrast, the decline in the 5-HT2A receptor depolarization with increasing age was associated with changes in the effectiveness with which these receptors could elicit a membrane depolarization, rather than loss of the receptors per se. Together, these results outline coordinated changes in the serotonergic regulation of cortical excitability at a time of extensive synaptic development and thus suggest a key role for these receptor subtypes in the postnatal development of the prefrontal cortex.
Keywords: 5-HT, serotonin, in situ hybridization, postnatal development, prefrontal cortex, in vitro electrophysiology, single-cell RT-PCR
Introduction
Since the initial report by Harlow (1848), detailing the behavioral sequelae of prefrontal cortex damage, converging work from a variety of disciplines, has identified this region as key to the integration of behavior (Frith and Frith, 1999; Duncan, 2001; Fuster, 2001; Miller and Cohen, 2001) and has implicated it in the pathophysiology of neuropsychiatric disorders (Davidson, 2001; Frith, 2001; Weinberger et al., 2001; Harrison, 2002). In an effort to understand the cellular basis of prefrontal cortical function, numerous studies have focused on the physiology of prefrontal cortical neurons, their function during behavioral tasks (Durstewitz et al., 2000; Fuster, 2001; Miller and Cohen, 2001), and their regulation by neurotransmitters and neuromodulators (Araneda and Andrade, 1991; Tanaka and North, 1993; Otani et al., 1998; Aghajanian and Marek, 1999; Yang et al., 1999; Gao et al., 2001; Seamans et al., 2001). Among the latter, the effects of serotonin [5-Hydroxytryptamine (5-HT)] are of particular interest given its hypothesized role in a variety of neuropsychiatric disorders involving prefrontal cortex.
There is now abundant evidence suggesting a key role for 5-HT in regulating prefrontal cortex function. 5-HT-synthesizing cells of the dorsal and median raphe innervate the prefrontal cortex (Conrad et al., 1974; Azmitia and Segal, 1978; Wilson and Molliver, 1991; Vertes and Kocsis, 1994; Vertes et al., 1999), and neurons of this region express a variety of 5-HT receptors (Pompeiano et al., 1992, 1994; Bruinvels et al., 1994; Gustafson et al., 1996; Waeber et al., 1996; Lopez-Gimenez et al., 1997). In particular, pyramidal cells of the adult prefrontal cortex have been shown to coexpress 5-HT receptors of the 5-HT1A and 5-HT2A subtypes (Martin-Ruiz et al., 2001), and parallel electrophysiological studies have identified that 5-HT1A receptors mediated hyperpolarizing–inhibitory and 5-HT2A receptors mediated depolarizing–excitatory responses in these cells (Davies et al., 1987; Araneda and Andrade, 1991; Tanaka and North, 1993). As such, 5-HT, by acting on multiple 5-HT receptor subtypes, may regulate how pyramidal cells function within prefrontal neuronal circuits.
These studies are beginning to lay the groundwork for an understanding, at the cellular level, of the effects of 5-HT in prefrontal cortex. However, most of these studies have focused on adult animals. This leaves an important gap in our knowledge, because perinatal factors are thought to play an important role in the normal development of cortex and also in the etiological processes leading to mental disorders involving prefrontal cortex, most notably schizophrenia and anxiety (Goldman-Rakic and Selemon, 1997; Raedler et al., 1998). To address this gap, we now investigate the cellular electrophysiological effects of 5-HT in developing prefrontal cortex.
Materials and Methods
Male Sprague Dawley rats (6–19, 25, and 35–45 d of age) were anesthetized with halothane and killed by decapitation. The brain was quickly removed and cooled in ice-cold Ringer solution of the following composition (in mm): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose, bubbled to saturation with 95% O2–5% CO2. The anterior pole of the brain was then isolated and affixed to a stage with cyanoacrylate glue, and coronal slices (300 μm thick) were cut using a vibratome (Lancer series 1000; Ted Pella, Irvine, CA). The slices were then transferred to a holding chamber (Sakmann and Stuart, 1995) where they were allowed to recover for at least 1 hr in Ringer solution with the addition of 30 mm sucrose.
Electrophysiological recordings. Whole-cell patch-clamp recordings were obtained from pyramidal neurons of layer V of the prelimbic or anterior cingulate regions of the medial prefrontal cortex (Krettek and Price, 1977). For recording, slices were transferred one at a time to a recording chamber of standard design (Sakmann and Stuart, 1995) where they were perfused with Ringer (30–33°C) bubbled to saturation with 95% O2–5% CO2. Neurons were targeted using differential interference contrast (DIC) imaging on a fixed-stage upright microscope (Olympus BX50WI; Olympus Optical, Tokyo, Japan).
All current-clamp recordings were performed with the use of an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA), whereas those in voltage-clamp were performed with the use of either an Axoclamp 2A, Axopatch 200, or 200B or with an EPC 10 (Heka Elektronik, Lambrecht/Pfalz, Germany). Electrical signals were recorded on-line with the use of a paper chart recorder (model 3400; Gould Instruments, Valley View, OH) and digitized and stored in an Intel (Santa Clara, CA) processor-based personal computer. The recording pipettes were pulled from borosilicate glass (outer diameter, 1.2 mm) using a Flaming-Brown horizontal puller (model P80; Sutter Instruments, Novato, CA) to give resistance ranging from 4 to 10 MΩ when filled with an internal solution of the following composition (in mm): 115 potassium gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 4 Na2ATP, 0.3 GTP, and 10 Na2 phoshocreatine. The pH was adjusted to 7.3–7.4. The voltages reported in this study were not compensated for liquid junction potentials (∼5–6 mV). The series resistance ranged from 8 to 25 MΩ. Neuron input resistance was determined by applying short-duration (140 msec) hyperpolarizing pulses (40–50 pA). In most voltage-clamp experiments, series resistance was corrected by ∼70%. Experiments in which access resistance increased by more than ∼30% were discarded. Some experiments involved simultaneous recordings from close neighboring cells; the maximal distance between these cells was ∼300 μm.
Agonists [5-HT, (±)-2,5-dimethoxy-4-bromoamphetamine hydrochloride (DOB), α-methyl-5-HT and 5-CT] were applied to the slices either by drop applications (5–20 μl from a 1 mm solution) or by bath administration at known concentrations as indicated. Unless otherwise indicated, agonists were administered only once to each slice to minimize any potential impact of receptor desensitization. All antagonists were applied to the slice by bath administration at known concentration.
Histology. To positively identify pyramidal cells, some electrodes were filled with Alexa Fluor 488-hydrazide (250–500 μm) dissolved in the intracellular solution (Panchuk-Voloshina et al., 1999). Whole-cell recordings were obtained from visually identified putative pyramidal neurons as usual, and recording was maintained for at least 20 min to allow for adequate dye filling. After recording, slices were fixed in 4% paraformaldehyde, rinsed three times in PBS, and whole mounted in aqueous media (Polysciences, Warrington, PA). Filled neurons were imaged using an Olympus Fluoview laser scanning confocal microscope equipped with an argon laser.
Reverse transcriptase-PCR. In the present study, mRNA was amplified either from pooled RNA extracted from medial prefrontal cortex (anterior cingulate and prelimbic subdivisions) or from the cellular content of individual prefrontal cortex pyramidal neurons. Pooled RNA was isolated with the Micro RNA Isolation kit (Stratagene, La Jolla, CA) per the instruction of the manufacturer. For single-cell reverse transcriptase (RT)-PCR, the cytoplasmic content of visually identified pyramidal neurons was harvested essentially as described by Monyer and Jonas (1995). Briefly, recordings were obtained using nominally RNase-free recording solution. Pipette glass was made RNase free by heating at 200°C for at least 1 hr, and the electrode holder and silver chloride wire were treated with RNaseZap (Ambion, Austin, TX). The electrode contents (∼7 μl) were expelled into an Eppendorf tube (Eppendorf Scientific, Westbury, NY) containing 20 U of Super RNasin (20,000 U/ml; Ambion) and sterile water for a final volume of 10 ml. Complete expulsion of the electrode content was obtained by gently breaking the tip on the tube wall while applying positive pressure. All subsequent procedures were the same for mRNA obtained from single cell and whole tissue. The cDNA was synthesized with the use of the SUPERSCRIPT first strand synthesis system (Invitrogen, Grand Island, NY) using 50 ng of random hexamers as per the instruction of the manufacturer. The cDNA generated from the reverse transcription step was subjected to conventional PCR using a programmable thermal cycler (PTC 200; MJ Research, Watertown, MA). Two rounds of PCR amplification were performed, each of 35 cycles. PCR primers were developed using PCGene software (Intelligenetics, Mountain View, CA). Two sets of primers were used to amplify the cDNA obtained from the RT reaction for the 5-HT2A and one set for 5-HT7 receptors. Primer sequences are given in Table 1. The amplification was performed in 10 mm Tris/HCl, 50 mm KCl, 1.5–2.5 mm MgCl, 200 mm dNTP mix, and 200 nmol of each primer, pH 8.3, with 5 U of TaqDNA polymerase (HotStarTaq Master Mix Kit; Qiagen, Chatsworth, CA). Restriction digest used XhoI for the 5-HT2A receptor and TaqI for the 5-HT7 receptor (Promega, Madison, WI). Parallel control experiments in which the cellular template for the RT-PCR reaction was replaced by water were invariably negative. We also conducted additional control experiments involving mock recordings followed by amplification of 5-HT2A and 5-HT7 receptor mRNA from the electrode contents. These control experiments were also generally negative (one of seven experiments resulted in amplification of 5-HT2A receptor message).
Table 1.
Primer sequences for RT-PCR
|
5-HT2A receptor |
Sequence |
Position |
Length of fragment |
|---|---|---|---|
| Primer 1 | |||
| Sense | 5′ AGC CGCTTCAACTCCAGA A | 609-627 | 410 |
| Antisense | 5′ TTTTGCTCATTGCTGATGGA | 1018-999 | |
| Primer 2 | |||
| Sense | 5′ TGTGCGATCTGGATTTAACTGG | 501-522 | 571 |
| Antisense | 5′ GGCACCACATTACAACAAACAGG | 1071-1049 | |
| 5-HT7 receptor |
|
|
|
| Primer 1 | |||
| Sense | 5′ TTACCTCCTCTCTTCGGATG | 767-786 | 660 |
| Antisense |
5′ GTCTTACAGCACAAACTCGG |
1426-1407 |
|
In situ hybridization. Postnatal day (P) 3, P7, P14, P21, and P28 rats were decapitated and the brains postfixed in 4% paraformaldehyde in 0.1 m PBS for 1 week. Coronal brain sections containing tissue from the prefrontal cortex were obtained and processed for free-floating in situ hybridization as described previously (Basura and Walker, 1999; Walker et al., 2000). 5-HT2A receptor cRNA probes were transcribed from a 900 bp PstI fragment subcloned into a bluescript KS+ plasmid (Basura and Walker, 1999). 5-HT7 cRNA probes were generated from BamH1 linearized plasmid BE379 (Heidmann et al., 1998). The resulting 35S-UTP-labeled antisense probes were subjected to alkaline hydrolysis to produce <200 nt probe fragments, which were phenol:chloroform extracted and ethanol precipitated. Before hybridization, tissue slices were partially digested by 10 mg/ml proteinase K and acetylated with 0.25% acetic anhydride. The sections were then incubated in hybridization buffer (50% deionized formamide, 5× saline sodium phosphate EDTA, 10% dextran sulfate, 5× Denhardt's solution, 100 mg/ml denatured ssDNA, 100 mg/ml E. coli tRNA, and 10 mm dithiothreitol) without radio-labeled probe for 60 min at 58°C. Sections were then hybridized in the same buffer overnight with either 2×106 cpm 5-HT7 cRNA probe or 1×106 cpm 5-HT2A receptor cRNA probe at 58°C.
After hybridization, sections were treated with 10 mg/ml RNase A. Slices were mounted onto glass slides and exposed to autoradiographic film (Amersham Biosciences, Arlington Heights, IL) for 3 d (5-HT2A receptors) or 21 d (5-HT7 receptors). Developed films were analyzed as scanned images using a Molecular Dynamics Personal Densitometer and ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Changes in mRNA levels between sections were calculated by measuring the average optical density of the affixed region within the medial aspect of the prefrontal cortex. Background noise was determined by measuring optical density values in the genu of the corpus callosum and subtracted from all data. Graphed data represent arbitrary units on the basis of optical density values obtained from scanned images. Slices from all ages were processed in parallel.
Chemicals. (±) DOB, 5-carboxamidotryptamine (5-CT), 5-HT, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY 100635), and 6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-yl carbamoyl] indoline (SB 242084) were purchased from Sigma (St. Louis, MO). Alexa Fluor 488 hydrazide was purchased from Molecular Probes (Eugene, OR). Tetrodotoxin (TTX) was purchased from Alomone Labs (Jerusalem, Israel). SB 269970 was a generous gift from SmithKline Beecham Pharmaceuticals (Harlow, UK). WAY 100478 was a generous gift from Wyeth-Ayerst Research (Princeton, NJ). R-(+)-α-(2,3-dimethoxyphenyl)-1-[4-fluorophenylethyl]-4-piperidinemethanol (MDL 100907) was a generous gift from Marion Merrel (Dow, Strasbourg, France) and from Dr. Kenner C. Rice (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD).
Results
Electrophysiological characteristics of layer V pyramidal neurons in the developing prefrontal cortex
For these experiments, we focused on pyramidal neurons of layer V of the anterior cingulate and prelimbic subdivisions of the prefrontal cortex starting at P6 and extending to young adults. Pyramidal neurons were selectively targeted for whole-cell recording using DIC imaging and also identified by their electrophysiological characteristics (Connors and Gutnick, 1990). To test the reliability of this targeting procedure, we labeled a subset of these cells (n = 19) with the fluorescent dye Alexa 488 and reconstructed their dendritic arborization using laser scanning confocal microscopy (Fig. 1A). All labeled cells showed the morphological characteristics of cortical pyramidal neurons: an approximately pyramidal shaped soma from which protruded several basal dendrites and a single branching apical dendrite extending toward the brain surface.
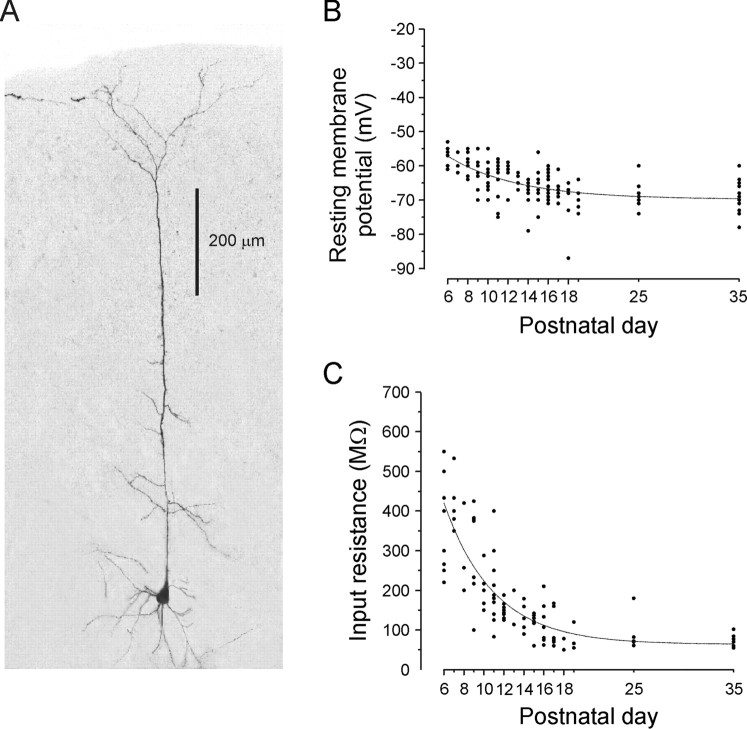
Figure 1.
Electrophysiological properties of layer V pyramidal neurons of developing medial prefrontal cortex. A, Morphological reconstruction of one of the pyramidal neurons included in the present study. This neuron from a P12 animal was filled with Alexa 488 and reconstructed from a Z-stack using laser scanning confocal microscopy. B, Resting membrane potential of layer V pyramidal neurons across postnatal development. Note the tendency of these neurons to display more hyperpolarized membrane potentials with increasing age. C, Input resistance of layer V pyramidal neurons across postnatal development. The input resistance of these cells, determined using short hyperpolarizing current pulses in current clamp, markedly decreased during the first 2 weeks of postnatal life.
The overall mean resting membrane potential of pyramidal neurons in the present study was –72 ± 0.53 mV. As illustrated in Figure 1B, there was a tendency for neurons to express more hyperpolarized potentials with increasing age. This small age-dependent change in resting membrane potential contrasted with the marked reduction in input resistance observed over the same period (Fig. 1C). In principle, such a reduction in input resistance with increasing age might be explained by a concurrent increase in expression of ion channels open at rest, such as the leak potassium channels. Consistent with this idea, we found in voltage-clamp experiments that the inward current induced by 2 mm barium, which blocks most leak potassium channels (Patel and Honore, 2001), became progressively larger with increasing age (P8, –24.1 ± 4.0 pA, n = 5; P11, –68.4 ± 7.9 pA, n = 5; P25, –100.9 ± 11.8 pA, n = 9; data not shown). Similar morphological and physiological changes across early postnatal development have been described previously in prefrontal cortex (Zhang, 2003a) and in cortex (Zhu, 2000; Tyzio et al., 2003) and was not studied further.
The effects of 5-HT on membrane potential of prefrontal cortical neurons exhibit a developmental switch
As illustrated in Figure 2, the ability of 5-HT to regulate membrane potential in layer V pyramidal neurons of the prefrontal cortex was found to exhibit a striking developmental regulation (n > 200 cells). In slices derived from young pups (<P15), applications of 5-HT consistently induced a robust depolarization–inward current (Fig. 2A). In the course of the third postnatal week, however, this depolarization subsided and was gradually replaced by a hyperpolarization–outward current (Fig. 2B). This hyperpolarization–outward current became the dominant response to 5-HT by the fourth postnatal week.
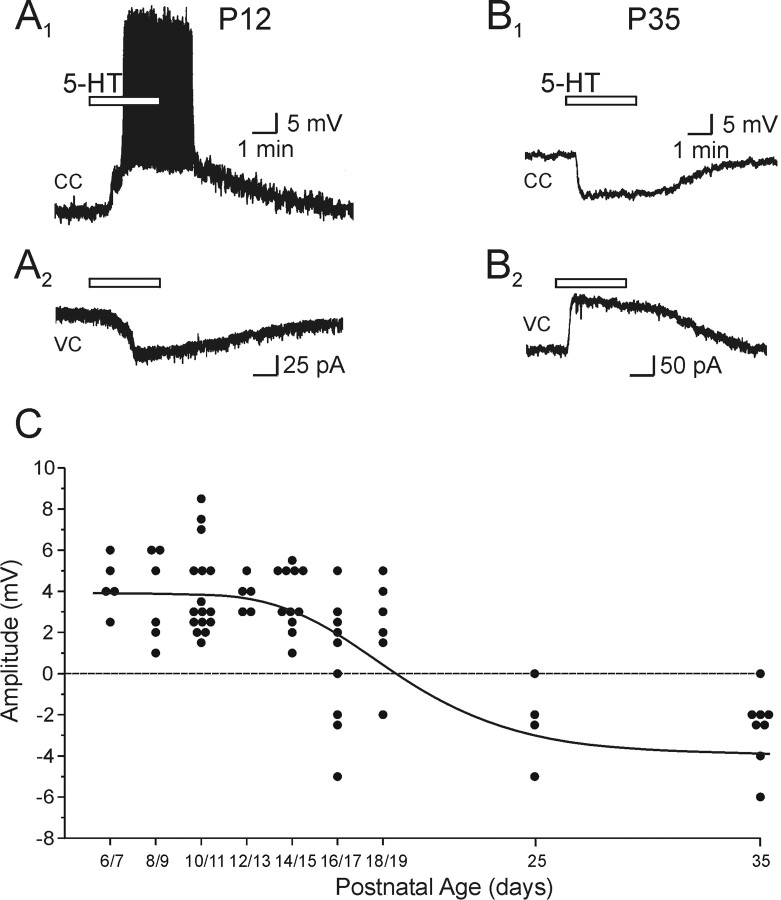
Figure 2.
The effects of 5-HT on membrane excitability of layer V pyramidal neuron are regulated across postnatal development of the rat prefrontal cortex. A1, In a P12 animal, bath administration of 5-HT (30 μm) induced a prominent depolarization of sufficient magnitude to elicit action potential discharge in this layer V pyramidal neuron, as revealed in this current-clamp (CC) recording. The resting membrane potential (Vm) of this neuron was –64 mV. A2, The underlying inward current induced by 5-HT is shown in this current trace obtained in voltage-clamp (VC) mode from the same neuron. B1, In a P35 animal, bath administration of 5-HT (30 μm) induced a membrane hyperpolarization (Vm, –65 mV) in a layer V pyramidal neuron. B2, The underlying outward current is shown in this current trace obtained in voltage-clamp mode from the same neuron. C, Scatter graph showing the peak amplitude of the change in membrane potential of pyramidal neurons induced by drop application of 5-HT in the presence of TTX (1 μm). Data were collected from animals of all ages across the P6–P19 developmental window, binned at 2 d, and of P25 and P35 animals.
Figure 2C illustrates a systematic quantification of this shift. For these experiments, we used brief bolus applications of 5-HT in the presence of TTX (1 μm) to rapidly assess the net effect of 5-HT on membrane potential of layer V pyramidal neurons while eliminating the confound induced by concomitant increases in synaptic activity (see below). Using this method, 5-HT was found to elicit a consistent depolarization between the fifth and fifteenth postnatal day that averaged 3.9 ± 0.26 mV (n = 43 cells). This depolarization was generally accompanied by either a small decrease (n = 12) or no change (n = 8) in input resistance (data not shown). Commencing at P16, an increasing fraction of cells displayed hyperpolarizing rather than depolarizing responses to 5-HT. By P25, most cells examined responded to 5-HT administration with a hyperpolarization that averaged 2.5 ± 0.5 mV (n = 12 cells).
Concomitant with these effects of membrane potential, 5-HT also induced a strong increase in spontaneous synaptic activity at all ages tested (not shown). In the rest of this study, we report on experiments aimed at elucidating the mechanisms underlying the effects of 5-HT on membrane potential during development. A systematic analysis of the 5-HT-induced increase in spontaneous synaptic activity has been published previously (Béïque et al., 2004).
5-HT depolarizes and excites pyramidal cells during early postnatal development
The 5-HT-induced depolarization observed between P6 and P19 ranged from 1 to 8.5 mV and was often sufficient to bring the cell to threshold and initiate firing (Fig. 3A,B). In the current sample, 12 of 29 neurons tested in current clamp in the absence of TTX were depolarized enough to initiate spiking. With the relatively brief applications used for these experiments, no obvious signs of desensitization of the excitatory effects of 5-HT were observed in 10 of 13 neurons tested (Fig. 3A). This ability of 5-HT to induce action potential discharge was observed more frequently, but not exclusively, in neurons from younger animals. Administration of TTX to cells depolarized to spiking by 5-HT revealed robust 5-HT-induced depolarizations (Fig. 3B), suggesting that the direct, postsynaptic, depolarizing effect of 5-HT rather than the increase in spontaneous excitatory synaptic activity was primarily responsible for the spiking activity.
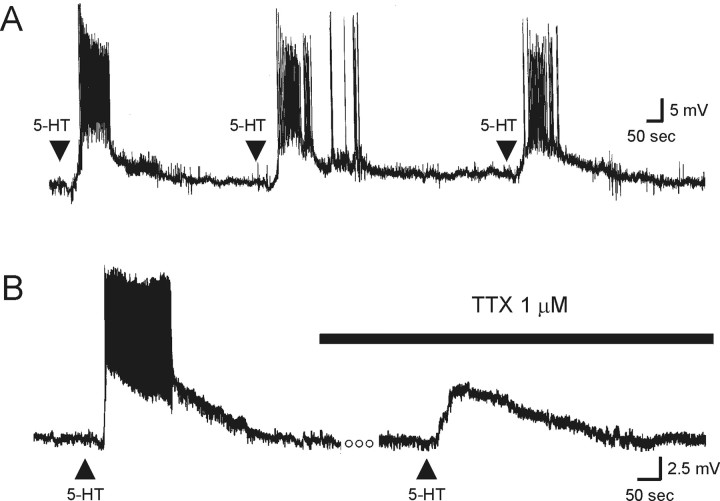
Figure 3.
During the P6–P19 developmental period, 5-HT induces a robust, generally non-desensitizing membrane depolarization of layer V pyramidal neurons. A, Successive drop applications of 5-HT reliably depolarized and induced action potential discharge in this layer V pyramidal neuron from a P6 rat, as shown in this voltage trace (Vm, –66 mV). In this and subsequent figures, the arrow represents the time of agonist application. B, In this neuron from a P9 animal (Vm, –65 mV), application of 5-HT induced a membrane depolarization of sufficient magnitude to induce action potential discharge. TTX (1 μm) completely blocked the 5-HT-induced discharge of action potentials, revealing the underlying membrane depolarization. The open circles represent a period of 4 min.
Activation of 5-HT2A receptors contributes to the 5-HT-induced depolarization
Previous work has shown that activation of different 5-HT receptor subtypes can trigger depolarizing or hyperpolarizing membrane potential responses in various CNS neurons (Andrade and Nicoll, 1987; Araneda and Andrade, 1991; Andrade and Chaput, 1991; Tanaka and North, 1993; Chapin and Andrade, 2001). Therefore, we hypothesized that the shift in the effects of 5-HT on membrane potential observed above could result from a developmentally regulated change in the functional expression of two or more 5-HT receptor subtypes. To test this idea, we conducted a pharmacological analysis of the effects of 5-HT.
5-HT can depolarize pyramidal cells in the adult rat cortex by activating 5-HT2A receptors (Davies et al., 1987; Pierce and Peroutka, 1990; Araneda and Andrade, 1991; Tanaka and North, 1993; Arvanov et al., 1999). We therefore tested whether this receptor subtype could mediate the 5-HT-induced depolarization seen in this region during the first 2–3 postnatal weeks. As illustrated in Figure 4A, brief applications of the broad spectrum 5-HT2 agonist DOB (Titeler et al., 1985; Titeler et al., 1987) depolarized a significant fraction of pyramidal cells (∼50%) and thus partly mimicked the effects of 5-HT (Fig. 4A)(n = 45 of 91 cells tested; P6–P19). However, in contrast to the effect of 5-HT, the DOB-induced depolarization was associated with a small but consistent increase in input resistance (n = 5). This ability of DOB to induce a membrane depolarization was blocked by the 5-HT2 antagonist ketanserin (1 μm; n = 3; data not shown), indicating the involvement of 5-HT2 receptors in this response.
Figure 4.
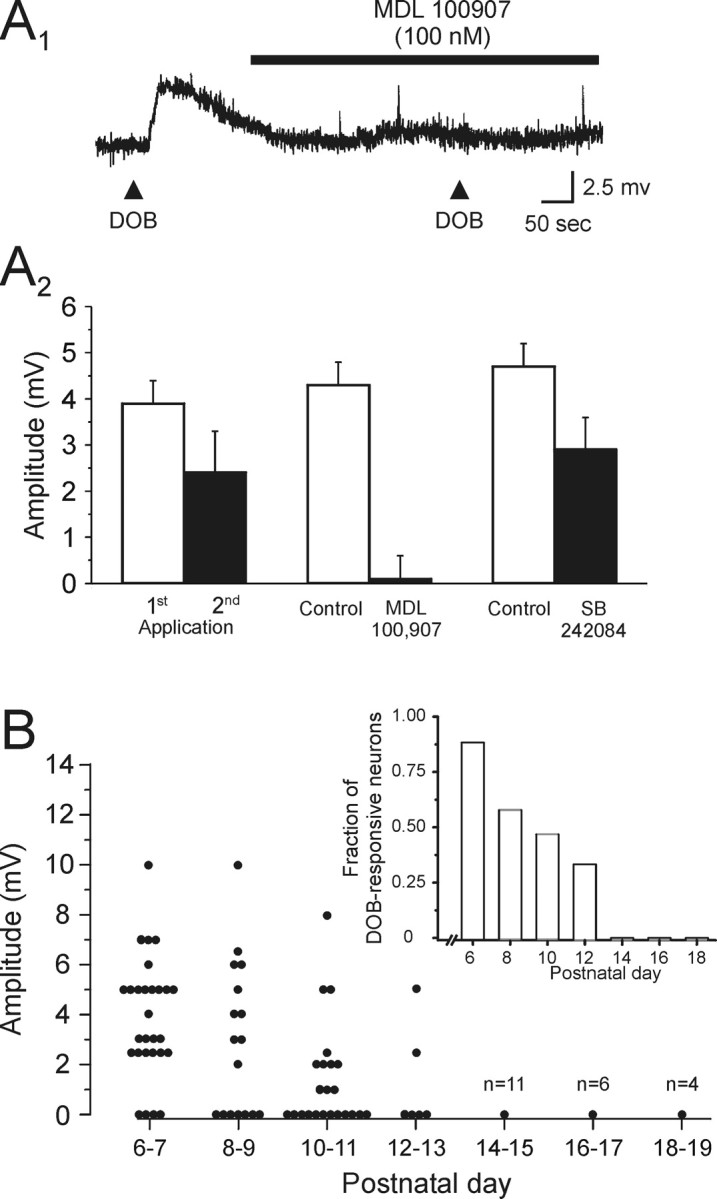
Activation of 5-HT2A receptors depolarizes layer V pyramidal neurons of the rat prefrontal cortex. A1, Application of the selective 5-HT2 receptor agonist DOB induced a depolarization of this layer V pyramidal neuron (P11; Vm, –69 mV). This effect of DOB was blocked by the selective 5-HT2A receptor antagonist MDL 100907 (100 nm). This recording was conducted in the presence of 1 μm TTX. A2, Graph summarizing our pharmacological analysis of the DOB-induced depolarization. The response induced by DOB exhibited a small desensitization determined by two successive applications (8–12 min a part). The depolarizing response to DOB was completely blocked by bath administration of the selective 5-HT2A receptor MDL 100907 (100 nm), whereas the reduction of the DOB-induced depolarization by the selective 5-HT2C antagonist SB 242084 (100 nm) was similar to that expected from desensitization alone. B, Scatter graph showing the peak amplitude of the depolarization induced by application of DOB during the P6–P19 developmental period. Inset, Histogram showing the fraction of neurons that exhibited a depolarizing response to application of DOB across the same developmental window.
The 5-HT2 receptor family is composed of three distinct subtypes (5-HT2A-C). By using subtype-selective antagonists, we next sought to determine which of these accounted for the 5-HT2 receptor depolarization observed here. Because 5-HT2B receptors are not expressed at detectable levels in prefrontal cortex (Pompeiano et al., 1994), the main goal of these experiments was to distinguish between the involvement of 5-HT2A and 5-HT2C receptors. To accomplish this, we compared the abilities of the 5-HT2A selective antagonist MDL 100907 and the 5-HT2C selective antagonist SB 242084 to inhibit the DOB-induced depolarization. This comparison was complicated by the susceptibility of the DOB responses to partially desensitize (Fig. 4A2). Therefore, we tested for an effect of MDL 100907 and SB 242084 beyond the decrement expected from desensitization alone. As illustrated in Figure 4, A1 and A2, when the selective 5-HT2A antagonist MDL 100907 (100 nm; Ki = 0.85 and 88 nm for 5-HT2A and 5-HT2C receptors, respectively) (Kehne et al., 1996; Roth et al., 2000) was administered between the first and second application of DOB, the ability of this agonist to elicit a membrane depolarization was completely suppressed (n = 6 cells; p < 0.01; paired Student's t test). In contrast, when the selective 5-HT2C receptor antagonist SB 242084 (100 nm; Ki = 1 and 158 nm for 5-HT2C and 5-HT2A receptors, respectively) (Kennett et al., 1997; Roth et al., 2000) was administered, the mean depolarization elicited by a second application of DOB was comparable with that seen under control conditions (Fig. 4A2). These results indicate that the depolarization induced by administration of DOB was predominantly, if not exclusively, mediated by activation of 5-HT2A receptors.
Although the vast majority of pyramidal neurons of layer V responded to 5-HT with a depolarization during the P6–P19 developmental period, overall, only approximately half of the cells (45 of 91 cells tested) responded similarly to administration of DOB. During the course of these experiments, we observed that the ability of DOB to elicit a membrane depolarization was age dependent, such that only cells derived from younger animals were responsive to this agonist. In fact, as illustrated in Figure 4B, application of DOB induced a significant depolarization only in neurons derived from rats younger than P14. This decrease was reflected not only in the fraction of cells responding to DOB (Fig. 4B, inset) but also in the amplitude of the DOB response among responsive cells (Fig. 4B). These results indicated a strong developmental regulation of 5-HT2A receptor function.
Activation of 5-HT7 receptors also contributes to the 5-HT-induced depolarization
The results outlined above suggest that activation of 5-HT2A receptors alone cannot account for the depolarizing actions of 5-HT observed during the first 3 weeks of life. Consistent with this idea, the depolarization induced by 5-HT was at least partly resistant to blockade by bath administration of the 5-HT2 receptor antagonist ketanserin (1 μm; five of seven cells tested). These results indicated the presence of a second 5-HT receptor capable of depolarizing pyramidal cells in the developing prefrontal cortex.
Seeking to identify this second receptor, we tested 5-CT, a broad spectrum serotonergic agonist capable of activating a limited subset of 5-HT receptors, most notably those of the 5-HT1 and 5-HT5–7 subtypes. Surprisingly, administration of 5-CT also elicited a membrane depolarization and thus mimicked the effect of 5-HT on pyramidal cells during the first three postnatal weeks (Fig. 5A1,B1) (51 of 59 cells tested). However, this depolarization differed from that elicited by DOB in some important respects. Most notably, it was associated with a small decrease in input resistance (8 of 12 cells tested) and displayed little, if any, desensitization (n = 3 cells). These results suggest that 5-HT acted on an additional, non-5-HT2A receptor to depolarize pyramidal cells of the developing prefrontal cortex.
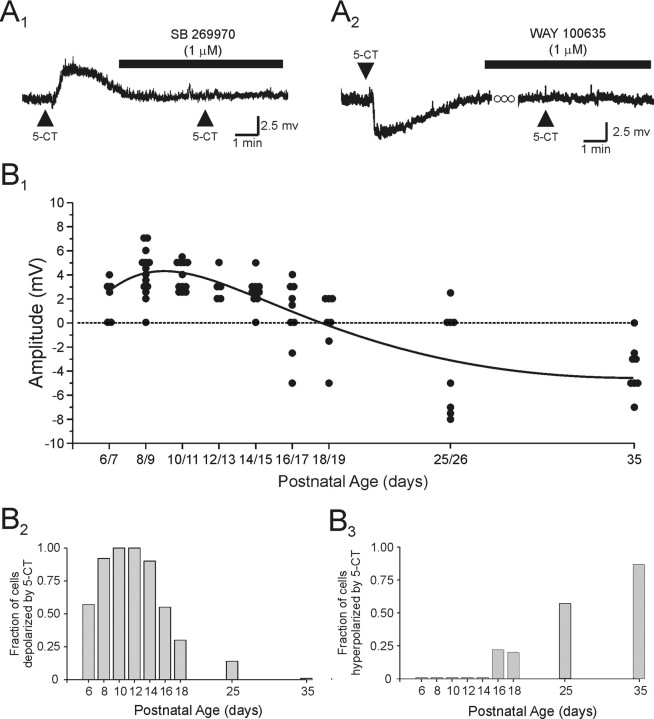
Figure 5.
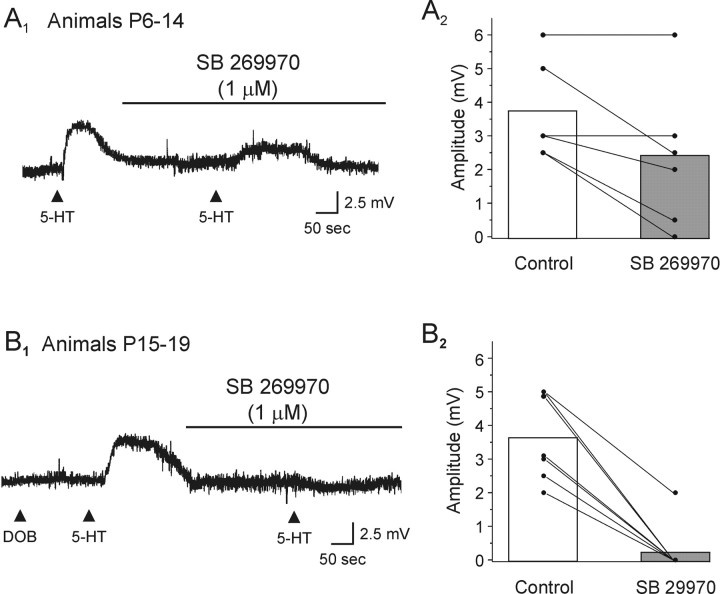
Activation of 5-HT7 receptors induces a membrane depolarization, which is gradually replaced by a 5-HT1A receptor-mediated hyperpolarization with increasing age. A1, In this neuron from a P10 animal, application of the 5-HT1/7 receptor agonist 5-CT induced a depolarization that was completely blocked by bath application of the selective 5-HT7 receptor antagonist SB 269970 (1 μm). This recording was conducted in the presence of 1 μm TTX. Vm, –70 mV. A2, In this neuron from a P25 animal (Vm, –68 mV), application of 5-CT induced a hyperpolarization, which was blocked by bath application of the selective 5-HT1A receptor antagonist WAY 100 635 (1 μm). B1, Scatter graph showing the peak change in membrane potential induced by application of 5-CT across postnatal development. B2, Histogram showing the fraction of neurons tested that exhibited a membrane depolarization in response to application of 5-CT as a function of the age of the animal. B2, Histogram showing the fraction of neurons that exhibited a membrane hyperpolarization in response to application of 5-CT as a function of the age of the animal.
Previous studies in brain have shown that 5-CT, acting on 5-HT7 receptors, can elicit a slow membrane depolarization analogous to that observed here (Cardenas et al., 1999; Chapin and Andrade, 2001). Therefore, we examined the ability of the highly selective 5-HT7 receptor antagonist SB 269970 (Ki = 1 nm for 5-HT7 receptors) (Hagan et al., 2000; Lovell et al., 2000) to inhibit the depolarizing action of 5-CT. As illustrated in Figure 5A, bath application of SB 269970 (1 μm) completely blocked the ability of 5-CT to depolarize pyramidal cells (n = 14), thus identifying the additional receptor involved as belonging to the 5-HT7 subtype.
5-HT2A and 5-HT7 receptors are coexpressed on pyramidal cells of the developing prefrontal cortex
Based on the results outlined above, we hypothesized that, early in postnatal development, the response mediated by 5-HT in an individual neuron would result from the coactivation of 5-HT7 and 5-HT2A receptors, whereas later (P14–P19) it would result predominantly from the activation of 5-HT7 receptors. Consistent with this idea, 5-HT-induced depolarizations were only partially sensitive to blockade by the 5-HT2 receptor antagonist ketanserin and could be mimicked by both DOB and 5-CT in the same neuron (three of four neurons) only during the early postnatal period (<P14). In addition, we found that in animals younger than P14, the depolarization induced by application of 5-HT was only partially inhibited by bath administration of the selective 5-HT7 receptor antagonist SB 269970 (1 μm) (Fig. 6A). In contrast, in animals P14–P19, the 5-HT-induced depolarization was completely blocked by SB 269970 in the vast majority of cells tested (six of seven cells tested) (Fig. 6B). These results support the idea that early in postnatal development (<P14), the depolarization induced by 5-HT generally involves the coactivation of both 5-HT2A and 5-HT7 receptors, whereas later (between P15 and P19) the depolarizing response is mediated primarily by 5-HT7 receptors.
Figure 6.
The 5-HT-induced depolarization is mediated by activation of both 5-HT2A and 5-HT7 receptors. A1, In this neuron from a P10 animal, the depolarization induced by the application of 5-HT was only partially blocked by SB 269970 (1 μm). Vm, –65 mV. A2, Plot illustrating the effects of SB 269970 on the 5-HT-induced depolarization in slices derived from animals P6–P14. SB 269970 only partially antagonized the depolarization elicited by 5-HT in these slices.B1, In this DOB-unresponsive neuron taken from a P17 animal, the depolarization induced by 5-HT was completely blocked by SB 269970 (1 μm). Vm, –71 mV. B2, Plot illustrating the effects of SB 269970 on slices derived from animals P15–P19. SB 269970 (1 μm) essentially abolished the ability of 5-HT to depolarize pyramidal cells in these slices.
The depolarization induced by 5-HT7 receptors is gradually replaced by a 5-HT1A receptor-mediated hyperpolarization during postnatal development
As outlined above, although the overwhelming effect of 5-HT on pyramidal cell membrane potential in the P6–P15 developmental period was a membrane depolarization commencing at approximately P16, a progressively large fraction of cells began to display hyperpolarizing rather than depolarizing responses (Fig. 2B,C). Because adult pyramidal cells express robust 5-HT1A receptor-induced hyperpolarizations (Araneda and Andrade, 1991; Tanaka and North, 1993), we hypothesized that the hyperpolarizations appearing at P16–P19 could represent the beginning of a developmentally regulated increase in 5-HT1A receptor responsiveness. Consistent with this idea, the response to 5-CT, which is also a 5-HT1 receptor agonist, shifted from a membrane depolarization to a hyperpolarization between P16 and P25, thus mimicking the shift seen with 5-HT (Fig. 5B). Most significantly, the hyperpolarizing responses elicited by 5-HT (n = 5) as well as 5-CT (n = 4) were completely blocked by bath administration of the selective 5-HT1A antagonists WAY 100635 (1 μm; Ki = 0.5 nm for 5-HT1A receptors) (Hamon et al., 1990) or WAY 100478 (1 μm; Ki = 10–32 nm for 5-HT1A receptors) (Roth et al., 2000). These results identified the receptor involved in the late developing hyperpolarization as being of the 5-HT1A subtype.
Interestingly, with only few exceptions between P16 and P18, administration of these 5-HT1A antagonists did not unmask depolarizing responses to 5-HT. Likewise, only in one case (P18) was the depolarizing response induced by 5-HT converted to a small hyperpolarization by bath administration of SB 269970 (P18). These observations suggest a relatively rapid, within-cell transition from the expression of a 5-HT7 receptor-mediated depolarization to a 5-HT1A receptor-mediated hyperpolarization. Together, these results outline a developmentally regulated progression in 5-HT responsiveness involving the gradual replacement of the early 5-HT2A–5-HT7 receptor-mediated depolarization by a late developing 5-HT1A receptor-mediated hyperpolarization in the rat prefrontal cortex.
Distinct mechanisms account for the decrease in 5-HT2A and 5-HT7 receptor-mediated depolarizations and the appearance of 5-HT1A receptor-mediated hyperpolarizations during development
In principle, the gradual loss of 5-HT2A and 5-HT7 receptor-mediated depolarizations and the appearance of the 5-HT1A receptor-mediated hyperpolarization could reflect changes in receptor expression, receptor-effector coupling, and the loss–gain of the downstream ion channels responsible for the changes in membrane potential. As a first step to distinguish between these possibilities, we used in situ hybridization and single-cell RT-PCR to directly test for changes in the expression of 5-HT7 and 5-HT2A receptor mRNA during the first postnatal month.
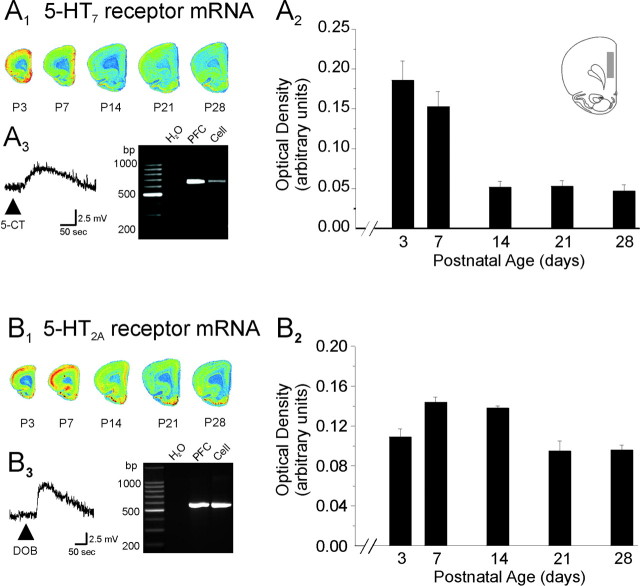
As illustrated in Figure 7A, in situ experiments showed that 5-HT7 receptor mRNA levels in layer V of prefrontal cortex were greatest in the first few days after birth and remained elevated for the first postnatal week. However, 5-HT7 receptor mRNA expression decreased rapidly after that period, attaining low to very low levels by P14–P28. This is consistent with previous observations indicating low levels of 5-HT7 mRNA in adult prefrontal cortex (Ruat et al., 1993; Gustafson et al., 1996; Vizuete et al., 1997; Heidmann et al., 1998). Because pyramidal cells are the predominant neuronal type in layer V, it is likely that a significant fraction of the 5-HT7 mRNA detected by the in situ hybridization reflects expression in these cells. Consistent with this idea, using single-cell RT-PCR, we could amplify 5-HT7 receptor mRNA from layer V pyramidal neurons (19 of 23 cells from P7, P8, P9, and P15 animals). As illustrated in Figure 7A3, 5-HT7 receptor mRNA was detectable in a subset of these cells shown to be responsive to 5-CT before the mRNA harvesting procedure (9 of 11 cells). Together, these observations indicate that a large fraction of layer V pyramidal neurons expresses mRNA coding for 5-HT7 receptors during early postnatal development.
Figure 7.
Expression of 5-HT2A and 5-HT7 receptors mRNA during the postnatal period. A1, B1, Pseudo-color autoradiographic image of cortical slices derived from rats of different ages are shown after hybridization with probes for the 5-HT7 receptor mRNA (A1) and 5-HT2A receptor mRNA (B1). The quantification of 5-HT7 (A2) and 5-HT2A (B2) receptor mRNA expression in the cingulate and prelimbic subdivisions of the medial prefrontal cortex is plotted across postnatal development. The area used to obtain the optical readings is illustrated in the inset of A2. This area corresponds to the area from which the electrophysiological recordings were performed. Graphed data depict optical density measurements expressed in arbitrary units. Data represent the mean ± SEM of four animals per age group. A3, Ethidium bromide-stained agarose gel showing the products of RT-PCR reactions amplifying 5-HT7 receptor mRNA from whole prefrontal cortex and from the pyramidal cell of which the voltage response to 5-CT is shown. Vm, –64 mV; P15 animal. The H2O lane depicts the results of a negative control using water as template. B3, Ethidium bromide-stained agarose gel showing the products of RT-PCR reactions amplifying 5-HT2A receptor mRNA from whole prefrontal cortex and from the pyramidal cell of which the voltage response to DOB is depicted. Vm, –71 mV; P10 animal.
In contrast to the expression pattern seen for 5-HT7 receptors, 5-HT2A receptor mRNA expression in the prefrontal cortex was highest between P7 and P14 and remained elevated throughout the first postnatal month (P3–P28) (Fig. 7B). This persistent expression appears to be at odds with the profound loss of the ability of 5-HT2A receptor activation to elicit a membrane depolarization during the first 2–3 postnatal weeks. Such a discrepancy could be explained if 5-HT2A receptors, as detected by in situ hybridization, were expressed in cells other than those we recorded. However, we could detect 5-HT2A receptor mRNA using single-cell RT-PCR in pyramidal cells during the P6–P15 developing period (17 of 32 cells tested and 6 of 13 cells shown to be responsive to DOB) (Fig. 7B3). These results suggest that the loss of 5-HT2A receptor-induced depolarization seen during the early postnatal period cannot simply be accounted for by a loss of 5-HT2A receptors but rather involves a different mechanism.
The waning of the 5-HT2A receptor-mediated depolarization in the face of persistent 5-HT2A receptor expression could result from a reduction in receptor-effector coupling or from the loss of the ion channels responsible for the depolarization. Our testing protocol may be expected to be very sensitive to changes in receptor coupling, because it relies on brief applications of a partial agonist (DOB). Therefore, we tested whether more prolonged applications of full 5-HT2A agonists could rescue the 5-HT2A receptor-mediated effect on membrane potential at a time when DOB was ineffective (P15–P19). For these experiments, we directly measured the 5-HT2A receptor-induced inward current, because this procedure facilitated the averaging of the response over multiple cells. Bath administration of 5-HT (30 μm; in the presence of 1 μm SB 269970 to block 5-HT7 receptors; n = 7) or α-methyl 5-HT (10 μm; n = 13), a preferential 5-HT2 receptor agonist, elicited small but clearly detectable inward currents (Fig. 8B) (see below). The effect of 5-HT (in SB 269970; n = 4) and α-methyl 5-HT (n = 6) was blocked by MDL 100 907 (300 nm-1 μm; data not shown), indicating that they involved activation of 5-HT2A receptors. As such, these observations support the idea that both the 5-HT2A receptors and the channels responsible for the depolarization–inward current were still expressed in juvenile (P15–P19) pyramidal cells.
Figure 8.
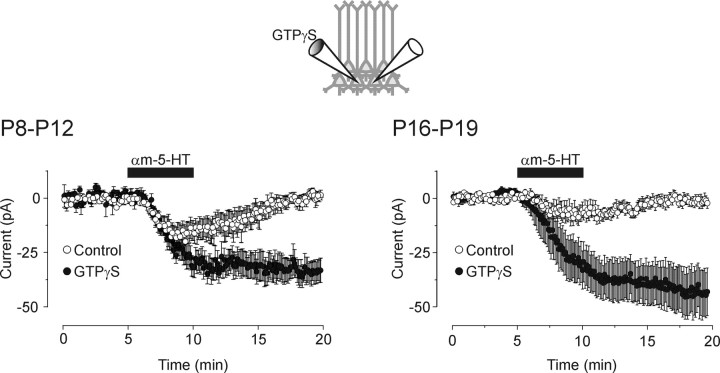
Intracellular GTPγS facilitates the ability of 5-HT2A receptor to elicit an inward current in the developing prefrontal cortex. Left, Ensemble average of voltage-clamp recordings from P8–P12 slices showing that bath administration of αM-5-HT (10 μm) induced a small inward current in control cells (open circles). αM-5-HT (10 μm) induced a larger, nonrecovering, inward current in cells recorded with an intracellular recording solution supplemented with GTPγS (100 μm). Voltage-clamp recordings were simultaneously obtained from two close neighboring cells (as illustrated in the inset) with electrodes containing GTP alone or GTP plus GTPγS. Neurons were held at –70 mV, and the holding currents were sampled every 6 sec and then averaged across recordings. This plot is constructed from data obtained in six paired recordings. Right, Ensemble average of recordings obtained in P16–P19 slices showing that administration of αM-5-HT (10 μm) induced an inward current of much greater amplitude in cells perfused with intracellular GTPγS than in cells recorded with a control intracellular solution (n = 13 pairs). Note that the potentiation induced by GTPγS was itself of greater magnitude in slices derived from the older animals (P16–P19) than in those derived from younger animals (P8–P12).
If this reduction in 5-HT2A responses reflected a weakening in receptor-effector coupling, then it could be expected that manipulations that facilitate G-protein signaling would become more effective with increasing age. To test this prediction, we used the poorly hydrolysable GTP analog GTPγS, a compound that can be expected to amplify G-protein-mediated responses by limiting the rate of GTP hydrolysis by the G-α subunit. If the reduction in 5-HT2A receptor responsiveness during development reflected a weakening of receptor-effector coupling, then it can be expected that GTPγS would facilitate the 5-HT2A receptor-induced inward current more efficiently in slices derived from animals older than P14 than in slices from younger animals. In agreement with this conjecture, infusion of GTPγS only modestly potentiated the 5-HT2A receptor-mediated inward in slices derived from P8–P12 animals (1.75-fold; measured at t = 10 min) (Fig. 8A). However, this same manipulation resulted in a much larger facilitation of the 5-HT2A receptor-induced inward current in slices derived from P16–P19 animals (5.5-fold; p < 0.05) (Fig. 8B). Together, these results suggest that the decrease in 5-HT2A receptor responsiveness observed during development does not result from a reduction in the expression of 5-HT2A receptors or of the ion channels responsible for the depolarization but involve a weakening of the 5-HT2A receptor-effector coupling.
In a final set of experiments, we examined the mechanisms underlying the late appearance of the 5-HT1A receptor-mediated hyperpolarizations. Previous studies have shown that the 5-HT1A receptor-mediated hyperpolarization seen in many regions of the brain is mediated by the activation of G-protein-coupled inwardly rectifying K+ channels (GIRKs; Kir 3.x) (Andrade and Nicoll, 1987; Colino and Halliwell, 1987; Ehrengruber et al., 1997; Luscher et al., 1997). Previous studies have shown that the expression of both 5-HT1A receptors (Daval et al., 1987; Miquel et al., 1994) and GIRK potassium channels (Chen et al., 1997) in cortex increases during the postnatal period. Therefore, the appearance of 5-HT1A receptor-induced depolarizations could have resulted in principle from the expression of either of these proteins, although close to maximal levels of GIRK expression are already seen by P10 (Chen et al., 1997). To distinguish between these possibilities, we took advantage of the fact that GIRK channels can be convergently regulated by 5-HT1A and GABAB receptors (Andrade et al., 1986; Luscher et al., 1997), the latter being, in turn, expressed in cortex during the early postnatal period (Turgeon and Albin, 1994). This allowed us to test for the expression of GIRK channels independently of 5-HT1A receptors. Activation of the GABAB receptors using baclofen (10–30 μm) consistently elicited a membrane hyperpolarization–outward current throughout postnatal development (P12–P25; data not shown; n = 7), indicating the expression of functional GIRK channels during this developmental epoch. These results suggest that the appearance of 5-HT1A receptor-mediated hyperpolarization stems primarily from a developmental regulation of 5-HT1A receptor expression per se rather than from regulation of down-stream effectors.
Discussion
In the present study, we have examined the actions of 5-HT on membrane potential in layer V pyramidal neurons of the rat prefrontal cortex. We find a remarkable shift in the effects of 5-HT across development. During the first 2 postnatal weeks, 5-HT elicits a consistent membrane depolarization that can often reach threshold and initiate spiking activity. However, this effect gradually subsides during the third postnatal week, shifting toward a membrane hyperpolarization. It is this later effect that dominates the membrane response to 5-HT seen in adults. Results from a combined pharmacological, physiological, and molecular biological analysis indicate that this developmental progression is the result of coordinated changes in the expression and function of three distinct 5-HT receptor subtypes.
Early in development, the predominant effect of 5-HT on the membrane potential of pyramidal cells of layer V of prefrontal cortex was a slow membrane depolarization. We found that the main receptor contributing to the 5-HT-induced depolarization in developing prefrontal cortex was of the 5-HT7 subtype. Thus, the 5-HT1/6–7 agonist 5-CT mimicked the depolarizing effect of 5-HT through the first 2 postnatal weeks, and this effect was blocked by bath administration of the selective 5-HT7 receptor antagonist SB 269970. This pharmacological identification is supported by results from in situ hybridization and single-cell RT-PCR experiments that demonstrated the presence of mRNA coding for the 5-HT7 receptor in pyramidal cells derived from animals of this same age group.
However, activation of 5-HT7 receptors cannot completely account for the depolarizing effects of 5-HT. Most notably, during the P6–P15 period, the effect of 5-HT was only partially inhibited by SB 269970, was mimicked by DOB, and was partially inhibited by the 5-HT2 receptor antagonist ketanserin. These results indicate that a 5-HT2 receptor also contributed to the response to 5-HT during this early time period. Consistent with the involvement of 5-HT2A receptors, single-cell RT-PCR demonstrated the expression of 5-HT2A mRNA in pyramidal neurons of layer V, and the DOB-induced depolarization was blocked by the selective 5-HT2A receptor antagonist MDL 100907 but not the selective 5-HT2C receptor antagonist SB 242084. These results identify 5-HT2A receptors as secondary contributors to the depolarization induced by 5-HT during the postnatal period.
The effect of 5-HT on membrane potential exhibited a gradual shift from a depolarization to a hyperpolarization beginning in the third postnatal week. This late developing hyperpolarizing response becomes first detectable at P16–P17 and progresses steadily with age to become the dominant effect of 5-HT on membrane potential in adult animals (P35 and beyond). Pharmacological analysis of this response indicates that it was mimicked by 5-CT and blocked by the selective 5-HT1A receptor antagonists WAY 100635 and WAY 100478, thus identifying the receptor involved as belonging to the 5-HT1A subtype. These results are consistent with previous results identifying 5-HT1A receptor-mediated hyperpolarizations in adult prefrontal cortex (Araneda and Andrade, 1991; Tanaka and North, 1993).
Interestingly, different mechanisms seem to account for the changes in 5-HT7, 5-HT2A, and 5-HT1A receptor function during development in prefrontal cortex. In the case of 5-HT7 and 5-HT1A receptors, these changes appear attributable to changes in receptor expression. Thus, early in development, 5-HT7 receptor mRNA is abundantly expressed in prefrontal cortex, specifically in pyramidal cells, but this expression diminishes dramatically during the second and third postnatal weeks. This reduced mRNA expression is accompanied by a parallel decrease in 5-HT7 receptor function detected by electrophysiological experiments, albeit with a delay of several days. Assuming a half-life for the 5-HT7 receptor protein comparable with that seen for other 5-HT receptors in cortex (Pinto and Battaglia, 1994), the observed decrease in functional expression of 5-HT7 receptors seems likely to be consequential to the decrease in 5-HT7 receptor mRNA. A similar but opposite situation applies to 5-HT1A receptors. Previous studies have shown that 5-HT1A receptors are poorly expressed in the cerebral cortex immediately after birth, but that their expression increases rapidly during the postnatal period (Daval et al., 1987; Miquel et al., 1994). Consistent with these findings, 5-HT1A receptor-mediated hyperpolarization does not become evident until the third postnatal week, despite the expression of GIRK channels during the preceding week (Chen et al., 1997; our results). As such, these results suggest that transcriptional regulation plays a determining role in shaping the pattern of 5-HT7 and 5-HT1A receptor function in developing prefrontal cortex.
In contrast to the changes in 5-HT7 and 5-HT1A receptor function, the reduction in the 5-HT2A receptor-induced depolarization does not appear to result from changes in receptor expression, because 5-HT2A mRNA and protein are abundantly expressed at all ages examined (Mengod et al., 1990; Roth et al., 1991; Morilak and Ciaranello, 1993; Lopez-Gimenez et al., 1997; Jakab and Goldman-Rakic, 1998; Mansour-Robaey et al., 1998; our study). Similarly, this reduction also does not appear to result from a loss of the ion channel responsible for the depolarization, because 5-HT2A receptor activation can induce robust inward currents in the presence of intracellular GTPγS during the P15–P19 period. Rather, the reduction in 5-HT2A receptor responsiveness appears to result, at least in part, from a weakening of the 5-HT2A receptor-effector coupling with increasing age. However, other factors may also contribute to this loss of responsiveness. One additional and likely contributing factor is the decrease in membrane resistance observed during the first 2 weeks of life, which can be expected to result in a reduction in the ability of even a stable 5-HT2A inward to depolarize these cells. Another possible factor could be a developmentally regulated change in subcellular distribution of 5-HT2A receptors. If receptors became localized at sites progressively more electrotonically distal from the soma with increasing age, this could also potentially lead to an apparent loss of 5-HT2A receptor electrophysiological signaling. However, anatomical studies have shown that 5-HT2A receptors do not show a preferential distal distribution over dendrites in adults (Jakab and Goldman-Rakic, 1998), and therefore such a mechanism appears less likely to play a significant role. Future studies will be needed to more precisely evaluate the contribution of these and other potential mechanisms to the decrement in the ability of 5-HT2A receptors to signal a depolarization during development.
Previous studies have reported that activation of 5-HT2A receptors can induce a membrane depolarization of pyramidal neurons in the adult rat prefrontal cortex (Araneda and Andrade, 1991; Tanaka and North, 1993). Although these depolarizations were relatively modest and were seen in only a fraction of cells tested, they nevertheless appeared more readily detectable in these previous studies than in the current recordings. There are a number of methodological differences that could contribute to this apparent discrepancy. First, in the current study, we observed a small but significant 5-HT2A receptor-mediated inward current when using agonists with full intrinsic activity. As such, this difference may be more quantitative than qualitative. Second, the use of different recording methods, “blind” sharp microelectrodes in the previous study, visual targeting of neurons for whole-cell recordings in the current, may have introduced a subtle sampling bias affecting the composition of the cell populations studied. Third, it is possible that the dialysis intrinsic to whole-cell recordings might alter the ability of 5-HT2A receptors to signal a depolarization. Regardless of the exact mechanism accounting for this difference, none of these possibilities is inconsistent with the current results delineating a developmental shift in the ability of 5-HT2A receptors to signal a depolarization nor with our mechanistic interpretation, namely that this developmental shift reflects, at least in part, a reduction in receptor-effector coupling.
Although the effects of 5-HT in adult prefrontal cortex have been extensively studied, only a few studies have examined the effects of 5-HT in regulating membrane excitability in this region during the postnatal period (Zhou and Hablitz, 1999; Lambe and Aghajanian, 2001; Zhang, 2003b). Notably, the large role played by 5-HT7 receptors in developing prefrontal cortex, as well as the remarkable absence of 5-HT1A receptor-mediated hyperpolarizations, has gone either unnoticed or unreported. This may reflect an emphasis on changes in spontaneous synaptic activity by 5-HT and the use, at least in part, of rats older than 3 weeks (Zhou and Hablitz, 1999; Lambe and Aghajanian, 2001). A recent study (Zhang, 2003b), published while this study was being prepared, described a 5-HT-induced depolarization comparable with that reported here but attributed it to the activation of 5-HT2A receptors primarily on the basis of its sensitivity to ketanserin. It is possible that the contribution of 5-HT2A receptors to this depolarization might have been overestimated in that study in light of the non-negligible affinities of ketanserin for 5-HT7 receptors (Shen et al., 1993; Jasper et al., 1997; Adham et al., 1998).
In summary, the present results outline a physiological and mechanistic progression in the ability of 5-HT to regulate the membrane potential of developing layer V pyramidal neurons of the rat prefrontal cortex. Early in the postnatal period, 5-HT depolarizes pyramidal cells by activating receptors of the 5-HT7 and secondarily 5-HT2A subtypes. Beginning in the third postnatal week, however, this depolarizing action begins to subside and is gradually replaced by a hyperpolarizing effect mediated by receptors of the 5-HT1A receptor subtype. Interestingly, different mechanisms appear to underlie these changes in serotonergic function. The decrease in 5-HT7 responsiveness seen during the third postnatal week as well as the concomitant increase in 5-HT1A receptor function are preceded by changes in mRNA expression and most likely reflect changes in receptor expression. In contrast, the gradual reduction in the ability of 5-HT2A receptors to depolarize pyramidal cells during the first 2 postnatal weeks seems to reflect changes in receptor-effector coupling. Future studies will be needed to identify specific actions of each of these receptors on specific developmental processes and to determine whether their disturbance can lead to abnormal behavioral sequela in adulthood.
Footnotes
This study was supported by National Institutes of Health Grant MH43985 (R.A.). M.W.H. is supported by the Veterans Affairs Merit Review Program. J.-C.B. is a recipient of a postdoctoral fellowship from the Canadian Institutes for Health Research. We thank Dr. D. J. Surmeier for his help in the implementation of the single-cell reverse transcriptase-PCR protocol.
Correspondence should be addressed to Jean-Claude Béïque, Department of Psychiatry and Behavioral Neurosciences, 540 East Canfield, Scott Hall, Room 2115, Detroit, MI 48201. E-mail: jbeique@med.wayne.edu.
DOI:10.1523/JNEUROSCI.5113-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/244807-11$15.00/0
References
- Adham N, Zgombick JM, Bard J, Branchek TA (1998) Functional characterization of the recombinant human 5-hydroxytryptamine7(a) receptor isoform coupled to adenylate cyclase stimulation. J Pharmacol Exp Ther 287: 508–514. [PubMed] [Google Scholar]
- Aghajanian GK, Marek GJ (1999) Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res 825: 161–171. [DOI] [PubMed] [Google Scholar]
- Andrade R, Chaput Y (1991) 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J Pharmacol Exp Ther 257: 930–937. [PubMed] [Google Scholar]
- Andrade R, Nicoll RA (1987) Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro J Physiol (Lond) 394: 99–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Malenka RC, Nicoll RA (1986) A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science 234: 1261–1265. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R (1991) 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Magro P, Roberts R, Wang RY (1999) A pre- and postsynaptic modulatory action of 5-HT and the 5-HT2A, 2C receptor agonist DOB on NMDA-evoked responses in the rat medial prefrontal cortex. Eur J Neurosci 11: 2917–2934. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179: 641–667. [DOI] [PubMed] [Google Scholar]
- Basura G, Walker PD (1999) Serotonin 2A receptor mRNA levels in the neonatal dopamine depleted rat striatum remain upregulated following suppression of serotonin hyperinnervation. Brain Res Dev Brain Res 116: 111–117. [DOI] [PubMed] [Google Scholar]
- Béïque JC, Chapin-Pennick EM, Mladenovic L, Andrade R (2004) Serotonergic facilitation of synaptic activity in the developing rat prefrontal cortex. J Physiol (Lond) 556: 739–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM (1994) Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology 33: 367–386. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS (1999) Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol (Lond) 518: 507–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin EM, Andrade R (2001) A 5-HT7receptor-mediated depolarization the anterodorsal thalamus: I. Pharmacological characterization. J Pharmacol Exp Ther 297: 395–402. [PubMed] [Google Scholar]
- Chen SC, Ehrhard P, Goldowitz D, Smeyne RJ (1997) Developmental expression of the GIRK family of inward rectifying potassium channels: implications for abnormalities in the weaver mutant mouse. Brain Res 778: 251–264. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV (1987) Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature 328: 73–77. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ (1990) Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 99–104. [DOI] [PubMed] [Google Scholar]
- Conrad LC, Leonard CM, Pfaff DW (1974) Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol 156: 179–205. [DOI] [PubMed] [Google Scholar]
- Daval G, Verge D, Becerril A, Gozlan H, Spampinato U, Hamon M (1987) Transient expression of 5-HT1A receptor binding sites in some areas of the rat CNS during postnatal development. Int J Dev Neurosci 5: 171–180. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2001) Toward a biology of personality and emotion. Ann NY Acad Sci 935: 191–207. [DOI] [PubMed] [Google Scholar]
- Davies MF, Deisz RA, Prince DA, Peroutka SJ (1987) Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res 423: 347–352. [DOI] [PubMed] [Google Scholar]
- Duncan J (2001) An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci 2: 820–829. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ (2000) Neurocomputational models of working memory. Nat Neurosci 3[Suppl]: 1184–1191. [DOI] [PubMed] [Google Scholar]
- Ehrengruber MU, Doupnik CA, Xu Y, Garvey J, Jasek MC, Lester HA, Davidson N (1997) Activation of heteromeric G protein-gated inward rectifier K+ channels overexpressed by adenovirus gene transfer inhibits the excitability of hippocampal neurons. Proc Natl Acad Sci USA 94: 7070–7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U (2001) Mind blindness and the brain in autism. Neuron 32: 969–979. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U (1999) Interacting minds–a biological basis. Science 286: 1692–1695. [DOI] [PubMed] [Google Scholar]
- Fuster JM (2001) The prefrontal cortex–an update: time is of the essence. Neuron 30: 319–333. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS (2001) Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 98: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD (1997) Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 23: 437–458. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA (1996) A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br J Pharmacol 117: 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR (2000) Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 130: 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Lanfumey L, el Mestikawy S, Boni C, Miquel MC, Bolanos F, Schechter L, Gozlan H (1990) The main features of central 5-HT1 receptors. Neuropsychopharmacology 3: 349–360. [PubMed] [Google Scholar]
- Harlow JM (1848) Passage of an iron bar through the head. Boston Med Surg J 13: 389–393. [Google Scholar]
- Harrison PJ (2002) The neuropathology of primary mood disorder. Brain 125: 1428–1449. [DOI] [PubMed] [Google Scholar]
- Heidmann DE, Szot P, Kohen R, Hamblin MW (1998) Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology 37: 1621–1632. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Goldman-Rakic PS (1998) 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA 95: 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper JR, Kosaka A, To ZP, Chang DJ, Eglen RM (1997) Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b). Br J Pharmacol 122: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C (1996) Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther 277: 968–981. [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP (1997) SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36: 609–620. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL (1977) The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol 171: 157–192. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK (2001) The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci 21: 9955–9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT (1997) Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol 356: 446–454. [DOI] [PubMed] [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, King FD, Middlemiss DN, Rahman SK, Saunders DV, Collin LL, Hagan JJ, Riley GJ, Thomas DR (2000) A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl) phen ol (SB-269970). J Med Chem 43: 342–345. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron [Erratum (1997) 19: 945] 19: 687–695. [DOI] [PubMed] [Google Scholar]
- Mansour-Robaey S, Mechawar N, Radja F, Beaulieu C, Descarries L (1998) Quantified distribution of serotonin transporter and receptors during the postnatal development of the rat barrel field cortex. Brain Res Dev Brain Res 107: 159–163. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F (2001) Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci 21: 9856–9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengod G, Pompeiano M, Martinez-Mir MI, Palacios JM (1990) Localization of the mRNA for the 5-HT2 receptor by in situ hybridization histochemistry. Correlation with the distribution of receptor sites. Brain Res 524: 139–143. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miquel MC, Kia HK, Boni C, Doucet E, Daval G, Matthiessen L, Hamon M, Verge D (1994) Postnatal development and localization of 5-HT1A receptor mRNA in rat forebrain and cerebellum. Brain Res Dev Brain Res 80: 149–157. [DOI] [PubMed] [Google Scholar]
- Monyer H, Jonas P (1995) Polymerase chain reaction analysis of ion channel expression in single neurons of brain slices. In: Single-channel recording (Sakmann B, Neher E, eds), pp 357–373. New York: Plenum.
- Morilak DA, Ciaranello RD 1993. Ontogeny of 5-hydroxytryptamine2 receptor immunoreactivity in the developing rat brain. Neuroscience 55: 869–880. [DOI] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce JM, Crepel F (1998) Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience 85: 669–676. [DOI] [PubMed] [Google Scholar]
- Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY, Haugland RP (1999) Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem 47: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E (2001) Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci 24: 339–346. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Peroutka SJ (1990) d-Lysergic acid diethylamide differentially affects the dual actions of 5-hydroxytryptamine on cortical neurons. Neuropharmacology 29: 705–712. [DOI] [PubMed] [Google Scholar]
- Pinto W, Battaglia G (1994) Comparative recovery kinetics of 5-hydroxytryptamine 1A, 1B, and 2A receptor subtypes in rat cortex after receptor inactivation: evidence for differences in receptor production and degradation. Mol Pharmacol 46: 1111–1119. [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G (1992) Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci 12: 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G (1994) Distribution of the serotonin 5-HT2 receptor family mRNAs: comparison between 5-HT2A and 5-HT2C receptors. Brain Res Mol Brain Res 23: 163–178. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Weinberger DR (1998) Schizophrenia as a developmental disorder of the cerebral cortex. Curr Opin Neurobiol 8: 157–161. [DOI] [PubMed] [Google Scholar]
- Roth BL, Hamblin MW, Ciaranello RD (1991) Developmental regulation of 5-HT2 and 5-HT1c mRNA and receptor levels. Brain Res Dev Brain Res 58: 51–58. [DOI] [PubMed] [Google Scholar]
- Roth BL, Kroeze WK, Lopez E (2000) The multiplicity of serotonin receptors: uselessly diverse molecules or an embarrasment of riches? The Neuroscientist 6: 252–262. [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC (1993) Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90: 8547–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Stuart G (1995) Patch-pipette recordings from the soma, dendrites, and axon of neurons in brain slices. In: Single-channel recording (Sakmann B, Neher E, eds), pp 199–211. New York: Plenum.
- Seamans JK, Gorelova N, Durstewitz D, Yang CR (2001) Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci 21: 3628–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Monsma Jr FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268: 18200–18204. [PubMed] [Google Scholar]
- Tanaka E, North RA (1993) Actions of 5-hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol 69: 1749–1757. [DOI] [PubMed] [Google Scholar]
- Titeler M, Herrick K, Lyon RA, McKenney JD, Glennon RA (1985) [3H]DOB: a specific agonist radioligand for 5-HT2 serotonin receptors. Eur J Pharmacol 117: 145–146. [DOI] [PubMed] [Google Scholar]
- Titeler M, Lyon RA, Davis KH, Glennon RA (1987) Selectivity of serotonergic drugs for multiple brain serotonin receptors. Role of [3H]-4-bromo-2,5-dimethoxyphenylisopropylamine ([3H]DOB), a 5-HT2 agonist radioligand. Biochem Pharmacol 36: 3265–3271. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Albin RL (1994) Postnatal ontogeny of GABAB binding in rat brain. Neuroscience 62: 601–613. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben Ari Y, Khazipov R (2003) Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J Neurophysiol 90: 2964–2972. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B (1994) Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol 340: 11–26. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM (1999) Projections of the median raphe nucleus in the rat. J Comp Neurol 407: 555–582. [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Traiffort E, Vargas C, Machado A, Cano J (1997) Expression of 5-HT7 receptor mRNA in rat brain during postnatal development. Neurosci Lett 227: 53–56. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Bockaert J, Dumuis A (1996) Regional distribution and ontogeny of 5-HT4 binding sites in rat brain. Behav Brain Res 73: 259–262. [DOI] [PubMed] [Google Scholar]
- Walker PD, Andrade R, Quinn JP, Bannon MJ (2000) Real-time analysis of preprotachykinin promoter activity in single cortical neurons. J Neurochem 75: 882–885. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE (2001) Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 50: 825–844. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Molliver ME (1991) The organization of serotonergic projections to cerebral cortex in primates: retrograde transport studies. Neuroscience 44: 555–570. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N (1999) Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology 21: 161–194. [DOI] [PubMed] [Google Scholar]
- Zhang ZW (2003a) Maturation of layer 5 pyramidal neurons in the rat prefrontal cortex: intrinsic properties and synaptic function. J Neurophysiol 91: 1171–1182. [DOI] [PubMed] [Google Scholar]
- Zhang ZW (2003b) Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci 23: 3373–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ (1999) Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol 82: 2989–2999. [DOI] [PubMed] [Google Scholar]
- Zhu JJ (2000) Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol (Lond) 526: 571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]