Abstract
At presynaptic terminals, intermixing during cycles of exocytosis and endocytosis challenges the molecular identity of the plasma and synaptic vesicle membranes. Although synaptic vesicle components are retrieved during recycling, the extent to which plasma membrane proteins enter the synaptic vesicle recycling pathway has not been examined. The target-SNARE (N-ethylmaleimide-sensitive factor attachment protein receptor) syntaxin-1 was shown previously to be present on putative synaptic vesicular membranes (Koh et al., 1993; Walch-Solimena et al., 1995; Kretzschmar et al., 1996), suggesting that syntaxin may cycle between the synaptic vesicle pool and the cell surface (Walch-Solimena et al., 1995). This implies that the molecular identity of the two membranes is not maintained during synaptic activity. Because the main role of syntaxin-1 is as a target-SNARE for vesicle fusion, appearance on synaptic vesicles could lead to futile interactions with vesicle-SNARE proteins. We investigated whether the subcellular localization of syntaxin-1A, tagged with the pH-sensitive fluorescent tag pHluorin, is regulated during neurotransmission using laser-scanning microscopy. We report here that syntaxin-1A is predominantly localized to the plasma membrane, with a small proportion present in an intracellular compartment with a lumenal pH consistent with synaptic vesicles. However, the internal fraction of syntaxin-1A is excluded from synaptic vesicles that undergo action potential-dependent recycling. These data indicate that the molecular identity of opposing exocytotic membranes is preserved by the sorting of syntaxin-1A from recycling synaptic vesicles.
Keywords: syntaxin, vesicle, pHluorin, recycling, sorting, exocytosis
Introduction
Neurotransmission results from a cascade of molecular interactions that lead from the influx of calcium through voltage-gated calcium channels to the fusion of neurotransmitter-containing synaptic vesicles with the plasma membrane. Although numerous synaptic proteins mediate and/or regulate synaptic vesicle fusion, the soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) proteins vesicle-associated membrane protein (VAMP), SNAP-25, and syntaxin have been shown to be sufficient to fuse biological membranes (Hu et al., 2003). According to current models, synaptic vesicle exocytosis requires the previous formation of a SNARE complex between VAMP located in synaptic vesicles (a vesicle-SNARE) and the target-SNAREs syntaxin and SNAP-25 on the target plasma membrane (Söllner et al., 1993). In support of this, the vesicle-SNARE VAMP is localized primarily on synaptic vesicles, although it is also present on the plasma membrane (Taubenblatt et al., 1999) and disperses onto the plasmalemma during vesicle recycling (Sankaranarayanan and Ryan, 2000). The target-SNARE syntaxin-1 is located in the target plasma membrane (Bennett et al., 1992; Koh et al., 1993; Garcia et al., 1995; Weimer et al., 2003), yet it has also been shown to be present on putative synaptic vesicular membranes (Koh et al., 1993; Walch-Solimena et al., 1995; Kretzschmar et al., 1996). It is therefore unclear whether the molecular identity of exocytotic membranes, as defined by current models of synaptic transmission, is preserved at synapses because these findings open the possibility that syntaxin recycles between the plasma membrane and synaptic vesicles.
The studies suggesting syntaxin is present on synaptic vesicles have relied on immunolocalization in fixed tissue and biochemical analysis of cell fractions, which are both limited by the identification of functional synaptic vesicles in a static system and antibody specificity (Koh et al., 1993; Walch-Solimena et al., 1995; Kretzschmar et al., 1996). We therefore investigated whether syntaxin is present in and recycles with synaptic vesicles that undergo action potential (AP)-evoked recycling in hippocampal neurons by tracking syntaxin-1A (stx) tagged on the C terminus with the pH-dependent fluorescent probe superecliptic pHluorin (stx-SE) (Sankaranarayanan et al., 2000) in living hippocampal neurons in culture. We find that syntaxin-1A is mostly located on the plasma membrane, with a small fraction present in an internal compartment with a lumenal pH similar to synaptic vesicles. However, our results indicate that syntaxin-1A is excluded from recycling synaptic vesicles, thus preserving the molecular identity of opposing exocytotic membranes.
Materials and Methods
Cell culture and transfection. Hippocampal CA1–CA3 regions were dissected from postnatal 2- to 4-d-old rats, and the neurons were dissociated and plated on coverslips coated with 10 μg/ml poly-l-ornithine. Cells were cultured and maintained as described previously (Ryan, 1999). All experiments were performed on cultures of between 2 and 3 weeks of age. A fusion protein was generated by subcloning rat stx cDNA into a vector encoding stx-SE (Miesenbock et al., 1998). Transfection of stx-SE was performed using a calcium phosphate precipitation technique as described previously (Threadgill et al., 1997). All reagents were obtained from Sigma (St. Louis, MO). All animal experiments and use were approved by the Institutional Animal Care and Use Committee of the Weill Medical College of Cornell University.
Fluorescence microscopy. Coverslips of neuronal cell cultures were mounted in a superfusion chamber equipped with field stimulation electrodes on the stage of a custom-built laser-scanning confocal microscope. Field stimulation was applied by passing 1 msec current pulses across the chamber (10 V/cm) using platinum–iridium electrodes. Unless otherwise noted, cells were superfused at room temperature in a saline solution consisting of 119 mm NaCl, 2.5 mm KCl, 2 mm CaCl2, 2 mm MgCl2, 25 mm HEPES, pH 7.4, 30 mm glucose, 10 μm CNQX (an AMPA receptor antagonist), and 50 μm APV (a NMDA receptor antagonist). As appropriate, 4 μm bafilomycin-A1 was added to block vesicle reacidification 1 min before and throughout data acquisition. The external solution contained 3.5 mm CaCl2 and 0.5 mm MgCl2 for experiments requiring an elevated calcium/magnesium concentration ratio. Stimulation using high KCl was achieved by substituting 40 mm NaCl with KCl. Ammonium chloride solution, pH 7.4, was prepared by substituting 50 mm NaCl in the above saline with NH4Cl. Acidic solution at pH 5.5 was prepared by replacing HEPES in the standard saline with 2-[N-morpholino]ethane sulfonic acid, pK 6.1.
Synaptic vesicle pools were labeled by field stimulating cultures for 30 sec at 20 Hz in the presence of 15 μm FM 4-64 in normal saline. An additional 60 sec of dye exposure was allowed to ensure complete labeling of all recycling vesicles. The cultures were subsequently rinsed in dye-free solution for 10 min before dye destaining. All data were obtained from viable synaptic boutons as determined by their ability to both load and destain FM 4-64 in an activity-dependent manner. Laser-scanning microscope fluorescence images were acquired at a rate of one every 6 sec, as described previously (Sankaranarayanan and Ryan, 2000). Quantitative measurements of fluorescence intensity at or between individual boutons were obtained by averaging a 4 × 4 area of pixel intensities.
Calculation of surface fraction and pH of the intracellular compartment. The surface fraction of stx-SE was calculated using the method of Sankaranarayanan et al. (2000). Briefly, at a given pH, pHluorin will be at equilibrium with protons, so the baseline fraction of deprotonated pHluorin molecules [X] can be predicted by the Henderson-Hasselbach equation:
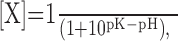 |
(1) |
where pK is the logarithm of the equilibrium constant for protonation. The relative change in stx-SE fluorescence as a function of internal pH, α(pHi), during application of membrane-permeant NH4Cl solution at pH 7.4 can be calculated according to the following:
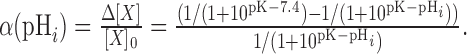 |
(2) |
The total pHluorin fluorescence (Ftotal) is given by the following:
 |
(3) |
where Fsurface is the surface fluorescence and Fintracellular is the fluorescence contributed by any intracellular fraction of pHluorin. Thus, the baseline fluorescence (F0) is equal to the following:
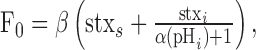 |
(4) |
where stxs and stxi is the amount of surface and intracellular stx-SE, respectively, and β is the fluorescence of stx-SE on the surface. Using a similar notation, the measured fluorescence during application of NH4Cl (FNH4Cl) is as follows:
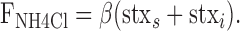 |
(5) |
The surface fraction of stx-SE as a function of intracellular pH f(pHi) can be calculated by first measuring the mean value for the fractional increase in fluorescence during application of NH4Cl solution at pH 7.4 (γ = [FNH4Cl – F0]/F0). Substituting for F0 and FNH4Cl, as defined in Equations 4 and 5, and solving for stxi, we get the following:
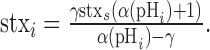 |
(6) |
The surface fraction, f(pHi), is defined by the following expression:
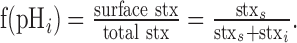 |
(7) |
Substituting for stxi we get the following:
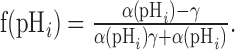 |
(8) |
During application of a pH 5.5 solution, the ratio of surface to internal stx-SE fluorescence as a function of internal pH ϕ(pHi) can be calculated according to the following:
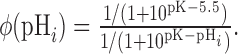 |
(9) |
The expression for measured fluorescence during application of pH 5.5 solution (FpH5.5) is as follows:
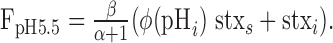 |
(10) |
A value for f(pHi) can be calculated using a mean value for the fractional increase in fluorescence during application of a nonpermeating pH 5.5 solution [ϵ = (F0 – FpH 5.5)/F0]. Substituting for F0 and FpH 5.5, as defined in Equations 4 and 10, respectively, and solving for stxi we get the following:
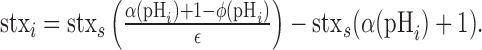 |
(11) |
To calculate f(pHi), we substitute for stxi into Equation 7 to give the following:
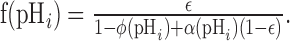 |
(12) |
To derive the pH of the intracellular compartment, we plotted the functions for f(pHi) derived from data using NH4Cl and pH 5.5 solution as detailed above (plotted in Fig. 2C). The point of intersection gave us a readout of intracellular pH. This mean pH value was used to calculate the surface fraction of stx-SE using a measured value for γ for each cell. The level of stx-SE expression was quantified as the fluorescence level during NH4Cl solution application, corrected for laser power and photomultiplier tube gain, and then normalized to the largest expression value.
Figure 2.
Syntaxin-1A is present in an acidic internal compartment. A, Effect of application of external solution at pH 5.5 on fluorescence of stx-SE in arbitrary units (au) at synaptic boutons (filled circles) and nonsynaptic axonal regions (axon; open triangles). B, Effect of application of NH4Cl solution at pH 7.4 on fluorescence of stx-SE in arbitrary units (au) at synaptic boutons (filled circles) and nonsynaptic axonal regions (axon; open triangles). It should be noted that the ΔF/F for the bouton in this example is larger than average. C, Surface fraction of stx-SE as a function of pH of internal compartment calculated from acid quenching data (pH 5.5; thin line; Eq. 12) or NH4Cl data (thick line; Eq. 8), at synaptic boutons. Arrow indicates pH value at which the curves intersect. D, Internal fraction of stx-SE calculated using NH4Cl data at synaptic boutons (filled circles) or nonsynaptic axonal regions (open triangles) as a function of expression level. Linear fits for both synaptic boutons (solid line) or nonsynaptic axonal regions (dashed line) were not significant (p > 0.7; Pearson linear correlation analysis).
Immunocytochemistry. Neurons were processed for immunofluorescence for syntaxin-1 after fixation in 4% paraformaldehyde (Electron Microscopy Science, Fort Washington, PA) and 4% sucrose in PBS for 20 min. Cells were then permeabilized in the same fixative plus 0.25% Triton X-100 for 10 min, blocked in 10% bovine serum albumin (BSA) for 2 hr at 37°C, and incubated overnight with affinity-purified monoclonal anti-syntaxin-1 antibody (1:1000 dilution; Synaptic Systems, Göttingen, Germany), diluted in 1% BSA in PBS. Cells were then incubated with an anti-mouse Alexa 546-conjugated goat IgG secondary antibody (1:1000 dilution; Molecular Probes, Eugene, OR) for 3 hr and mounted for laser-scanning microscopic analysis. Using this analysis, the level of expression of syntaxin-1 was 2.1-fold higher (n = 2) in cells expressing exogenous syntaxin-1A. Calibration of the normalized level of stx-SE expression data, as defined in the previous paragraph, with the syntaxin-1 expression data allowed us to define a range of values between 1.2- and 6.2-fold overexpression of syntaxin-1, assuming linear summation of our fusion protein with an unaltered expression of endogenous syntaxin-1. These values should be considered an underestimate of the overexpression of syntaxin-1A, because the syntaxin-1 antibody used is not specific for the syntaxin-1A subtype.
Results
Vesicle recycling is functional in neurons expressing a syntaxin-1A-pHluorin fusion protein
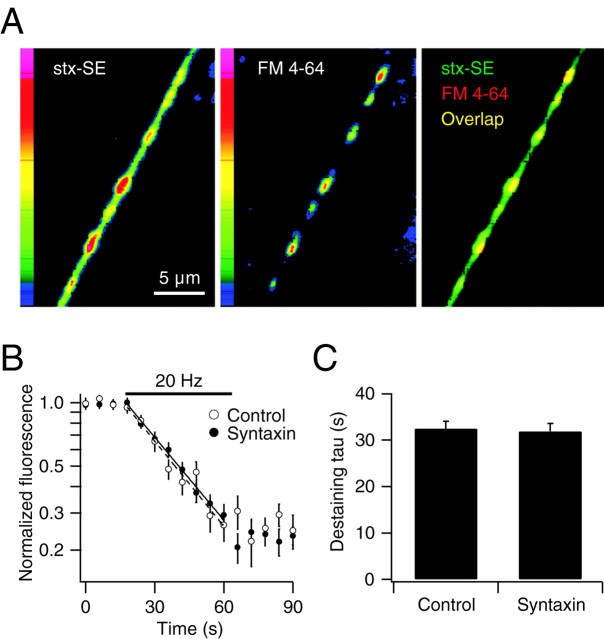
The subcellular localization of syntaxin-1A-pHluorin (stx-SE) expressed in hippocampal neurons in culture was examined by laser-scanning fluorescence microscopy. To distinguish synaptic boutons from nonsynaptic axonal regions and to test the efficiency of vesicle recycling in stx-SE-expressing neurons, we labeled recycling synaptic vesicles with the red-shifted styryl dye FM 4-64 using AP stimulation (Fig. 1A). The time constant for FM 4-64 destaining in stx-SE-expressing synapses measured during a subsequent round of stimulation was not significantly different than in control synapses (n = 4; p > 0.05; paired two-tailed t test) (Fig. 1B,C). These data indicate that synaptic vesicle recycling in the stx-SE-expressing cells was functional under these conditions.
Figure 1.
Vesicle recycling in stx-SE-expressing neurons. A, Laser-scanning fluorescence images of an axon of a neuron expressing stx-SE (left), which was loaded with the syryl dye FM 4-64 with a 30 sec 20 Hz stimulation, followed by 60 sec of dye exposure, defining locations of recycling synaptic vesicles (middle). Fluorescence pseudocolor scale shown for left and middle panels. Right shows overlap (yellow) between images for stx-SE (green) and FM 4-64 (red). B, Semi-log plot of simultaneous destaining of FM 4-64 from synaptic vesicles of control cells and stx-SE-expressing cells, during electrical stimulation at 20 Hz. Solid and dashed lines show single-exponential fits of destaining rates for control and stx-SE-expressing cells, respectively. C, Mean destaining time constant (tau) for control cells and stx-SE-expressing cells (n = 5).
A small fraction of syntaxin-1A is present in an intracellular compartment at nerve terminals
We quantified the localization of stx-SE at functional synaptic boutons for each cell by monitoring changes in stx-SE fluorescence during application of extracellular solution at pH 5.5, to quench surface fluorescence (Fig. 2A), or one containing NH4Cl to clamp internal compartments to pH 7.4 (Fig. 2B). In all cases, functional synaptic boutons were defined as those that were labeled and subsequently destained FM 4-64 in an activity-dependent manner. The change in stx-SE fluorescence normalized to the initial fluorescence (ΔF/F) during application of acidic solution was –96.5 ± 0.5% (mean ± SE; n = 12), indicating quenching of a large surface component of stx-SE. The increase in fluorescence during NH4Cl solution application (ΔF/F = 15.1 ± 3.4%; n = 12) (Fig. 2B) showed that some stx-SE was present in an acidic intracellular compartment. The magnitude of fluorescence changes in response to acidic or NH4Cl-containing solution application depends on the size of the surface fraction, as well as the pH of the internal compartment of stx-SE. Combining these data with the known pK of SE (7.18) (Sankaranarayanan et al., 2000), enables one to calculate values of both parameters (see Materials and Methods). The acid quench and NH4Cl data indicated that the internal stx-SE was in a compartment with a lumenal pH of 5.3 (Fig. 2C), similar to synaptic vesicles (pH 5.5) (Miesenbock et al., 1998; Sankaranarayanan et al., 2000). Intracellular stx-SE represented 12.7 ± 2.5% (n = 12) of the total stx-SE at synaptic boutons. Similar analysis revealed that the fraction of stx-SE on nonsynaptic axonal plasma membrane was 97.9 ± 1.6% (n = 12). The size of the internal fraction of stx-SE was not correlated with the expression levels of stx-SE over a 30-fold range of expression for both synaptic and nonsynaptic axonal regions (Fig. 2D). This corresponds to a 1.2- to 6.2-fold overexpression of total syntaxin-1 (see Materials and Methods). These data show that syntaxin-1A is mainly located on the plasma membrane but that a small fraction is located in a compartment with an acidic lumenal pH at synaptic boutons.
Syntaxin-1A is absent from recycling synaptic vesicles
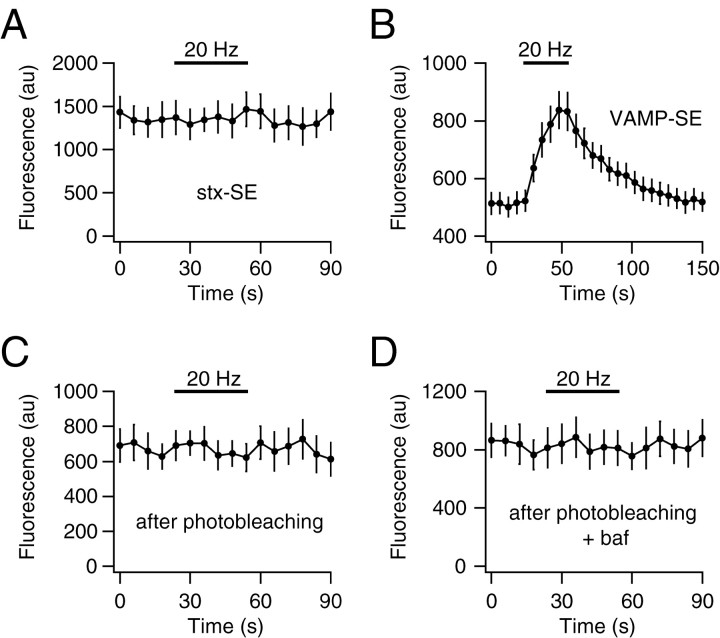
To investigate whether the internal fraction of syntaxin-1A is located in recycling synaptic vesicles, we monitored the fluorescence of stx-SE at synaptic boutons during neurotransmission evoked by electrical stimulation. Because stx-SE is quenched if present in acidic synaptic vesicles and fluorescent when residing on the cell surface, the fluorescence level should change during synaptic activity if syntaxin recycles between the two compartments. A 600 AP stimulus at 20 Hz induced no significant accumulation or loss of stx-SE on the plasma membrane at synaptic boutons (ΔF/F = 2.3 ± 0.9%; n = 17; p > 0.05) (Fig. 3A). By comparison, the same stimulus results in significant accumulation of the vesicle-SNARE VAMP as measured using synapto-pHluorin (Fig. 3B). The ratio of stx-SE fluorescence on the plasma membrane at boutons versus nonsynaptic axonal regions (3.0 ± 0.3; n = 12) was unchanged by stimulation, indicating that the distribution of syntaxin on the cell surface remains unaltered during exocytosis. Similar results were found using stimulation at 20 Hz for 60 sec, 10 Hz for 90 sec, 40 Hz for 30 sec, and 100 Hz for 30 sec (n = 12, 12, 3, and 2, respectively) and during application of high-KCl (40 mm) stimulation for 30 sec (n = 4). Furthermore, stimulation by 600 APs at 20 Hz in the presence of a sevenfold higher calcium/magnesium ion concentration ratio to maximize release probability produced no change in stx-SE fluorescence (ΔF/F = 0.0 ± 1.4%; n = 5).
Figure 3.
Syntaxin-1A is excluded from recycling synaptic vesicles. A, Stx-SE fluorescence during electrical stimulation at 20 Hz for 30 sec under control conditions. B, VAMP-SE fluorescence at synaptic boutons during electrical stimulation at 20 Hz for 30 sec under control conditions. C, Stx-SE fluorescence during electrical stimulation at 20 Hz for 30 sec after photobleaching. D, Stx-SE fluorescence during electrical stimulation at 20 Hz for 30 sec in the presence of 4 μm bafilomycin-A1 (baf) after photobleaching.
It is possible that the recycling fraction of stx-SE was too small to detect above the noise generated by a background signal from the nonrecycling surface fraction. We enhanced the signal-to-noise ratio by photobleaching stx-SE fluorescence by 65 ± 13% (n = 5), increasing the NH4Cl ΔF/F from 15 ± 5to50 ± 9% (n = 4), thereby selectively bleaching the surface fraction of stx-SE. This corresponds to an approximate increase of the internal fraction of stx-SE from 13.0 ± 3.7 to 30.0 ± 9.7% (n = 4). Even under these conditions, 20 Hz stimulation failed to reveal any change in stx-SE fluorescence (ΔF/F = 4.3 ± 2.7%; n = 5) (Fig. 3C). Because it is possible that stx-SE might recycle via a process that is too rapid to detect, in addition to prebleaching of the surface, we made use of the vesicular proton pump blocker bafilomycin-A1 (4 μm) to inhibit reacidification of recycling vesicles. Any stx-SE would thereby be trapped in a fluorescent form after one cycle of exocytosis–endocytosis. This treatment also failed to reveal any accumulation of stx-SE fluorescence during AP firing (ΔF/F = –3.4 ± 1.5%; n = 5) (Fig. 3D). These data indicate that syntaxin-1A is excluded from synaptic vesicles that undergo AP-dependent recycling.
Discussion
In this study, we used a genetically encoded, pH-sensitive tag to enable us to show that syntaxin-1A is present predominantly on the plasma membrane of axons. A small intracellular fraction is located at synaptic boutons in a compartment with a lumenal pH consistent with synaptic vesicles (pH 5.5) (Sankaranarayanan et al., 2000). However, syntaxin did not translocate between plasma membrane and the internal compartment during AP- or KCl-induced exocytosis, suggesting that it is excluded from recycling synaptic vesicles.
Subcellular localization of syntaxin-1A at nerve terminals
Early immunofluorescence studies suggested that syntaxin-1 is a plasma membrane protein (Bennett et al., 1992; Söllner et al., 1993). This finding contributed to a model of membrane fusion that requires syntaxin-1/SNAP-25 on the plasma membrane and VAMP in the vesicle to form the highly stable SNARE complex (Söllner et al., 1993). However, there is conflicting higher-resolution evidence showing that syntaxin-1 is present on both plasma membrane and in vesicles from synaptosomes (Koh et al., 1993; Walch-Solimena et al., 1995; Kretzschmar et al., 1996) or primarily on the plasma membrane of neuron axonal membrane (Weimer et al., 2003). One possible difference may lie in the fact that our measurements, as with those of Weimer et al. (2003), were performed on intact neurons, whereas the previous studies indicating a large internal syntaxin-1 fraction were obtained from synaptosomes. Our data partially consolidates some of these apparently contradictory findings, however, by showing that a small fraction of syntaxin-1A does exist in an intracellular compartment, only at synaptic axonal regions, but that this fraction is not mobilized during evoked exocytosis. Our estimate that only ∼12% of synataxin-1A resides on internal membranes at synapses is in good quantitative agreement with the ultrastructural studies of Weimer et al. who find only ∼10% of synaptic synataxin-1A is found on internal membranes (Erik Jorgensen, personal communication). Although we cannot rule out that our syntaxin-1A fusion protein behaves differently from endogenous syntaxin, the results were invariant with expression level of the tagged protein. If the internal fraction that we observe is present on synaptic vesicles, then our findings show that these synaptic vesicles are not release competent. Alternatively, the vesicles could correspond to active zone precursor vesicles that fuse with the plasma membrane only during synaptogenesis (Zhai et al., 2001; Shapira et al., 2003), or they may be intracellular trafficking vesicles that never fuse with the plasma membrane. It is unclear whether these types of vesicle have a lumenal pH similar to synaptic vesicles in neurons.
Sorting of syntaxin-1A
The data presented here indicate that either the sorting machinery for the endocytic step of vesicle recycling operates at sufficiently high fidelity to exclude internalization of an abundant axonal membrane protein such as syntaxin or that syntaxin itself is restrained from entering sites of synaptic vesicle endocytosis. Either mechanism may rely on local variations in the lipid composition of the plasma membrane, because syntaxin is excluded from sphingomyelin and cholesterol-enriched membranes in liposomes (Saslowsky at al., 2003) and is clustered at exocytotic sites in PC12 cells in a cholesterol-dependent manner (Lang et al., 2001). Exocytosis is thought to occur at or near calcium microdomains produced by clusters of voltage-gated calcium channels (Pumplin et al., 1981; Sugimori et al., 1994; DiGregorio et al., 1999). Retention of syntaxin-1A may also be mediated by voltage-gated calcium channels, because they bind via the synaptic protein interaction (synprint) site (Rettig et al., 1996). No such clustering of syntaxin-1A was observed in hippocampal nerve terminals at the level of resolution used in our experiments, consistent with previous immunocytochemical data (Garcia et al., 1995).
Conclusion
Our data show that an abundant plasma membrane protein, syntaxin-1A, whose correct functioning almost certainly relies on its precise membrane targeting, remains excluded from recycling synaptic vesicles, even during robust vesicle membrane fluxes to and from the plasma membrane during the exo-endocytic cycle at nerve terminals. In this way, the functional identity of exocytotic membranes in nerve terminals is preserved.
Footnotes
This work was supported by National Institutes of Health Grants NS24692 and GM61925 and the Hirschl Trust (T.A.R.). S.J.M. is the recipient of an International Research Fellowship from the Wellcome Trust. We thank W. Yan for technical assistance, K. Nicholson Tomishima for making the stx-SE construct, and members of the Ryan laboratory for discussions.
Correspondence should be addressed to Timothy A. Ryan, Department of Biochemistry, Weill Medical College of Cornell University, 1300 York Avenue, New York, NY 10021. E-mail: taryan@med.cornell.edu.
DOI:10.1523/JNEUROSCI.0174-04.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/244884-05$15.00/0
References
- Bennett MK, Calakos N, Scheller RH (1992) Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257: 255–259. [DOI] [PubMed] [Google Scholar]
- DiGregorio DA, Peskoff A, Vergara JL (1999) Measurement of action potential-induced presynaptic calcium domains at a cultured neuromuscular junction. J Neurosci 19: 7846–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia EP, McPherson PS, Chilcote TJ, Takei K, De Camilli P (1995) rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J Cell Biol 129: 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Ahmed M, Melia TJ, Söllner TH, Mayer T, Rothman JE (2003) Fusion of cells by flipped SNAREs. Science 300: 1745–1749. [DOI] [PubMed] [Google Scholar]
- Koh S, Yamamoto A, Inoue A, Inoue Y, Akagawa K, Kawamura Y, Kawamoto K, Tashiro Y (1993) Immunoelectron microscopic localization of the HPC-1 antigen in rat cerebellum. J Neurocytol 22: 995–1005. [DOI] [PubMed] [Google Scholar]
- Kretzschmar S, Volknandt W, Zimmermann H (1996) Colocalization on the same synaptic vesicles of syntaxin and SNAP-25 with synaptic vesicle proteins: a re-evaluation of functional models required? Neurosci Res 26: 141–148. [DOI] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R (2001) SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 20: 2202–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192–195. [DOI] [PubMed] [Google Scholar]
- Pumplin DW, Reese TS, Llinas R (1981) Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci USA 78: 7210–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA (1996) Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci USA 93: 7363–7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA (1999) Inhibitors of myosin light chain kinase block synaptic vesicle pool mobilization during action potential firing. J Neurosci 19: 1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA (2000) Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol 2: 197–204. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA (2000) The use of pHluorins for optical measurements of presynaptic activity. Biophys J 79: 2199–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslowsky DE, Lawrence JC, Henderson RM, Edwardson JM (2003) Syntaxin is efficiently excluded from sphingomyelin-enriched domains in supported lipid bilayers containing cholesterol. J Membr Biol 194: 153–164. [DOI] [PubMed] [Google Scholar]
- Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC (2003) Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron 38: 237–252. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE (1993) A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418. [DOI] [PubMed] [Google Scholar]
- Sugimori M, Lang EJ, Silver RB, Llinas R (1994) High-resolution measurement of the time course of calcium-concentration microdomains at squid presynaptic terminals. Biol Bull 187: 300–303. [DOI] [PubMed] [Google Scholar]
- Taubenblatt P, Dedieu JC, Gulik-Krzywicki T, Morel NJ (1999) VAMP (synaptobrevin) is present in the plasma membrane of nerve terminals. J Cell Sci 112: 3559–3567. [DOI] [PubMed] [Google Scholar]
- Threadgill R, Bobb K, Ghosh A (1997) Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron 19: 625–634. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R (1995) The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol 128: 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM (2003) Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci 6: 1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC (2001) Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron 29: 131–143. [DOI] [PubMed] [Google Scholar]