Abstract
The delivery of neurotransmitter receptors into synapses is essential for synaptic function and plasticity. In particular, AMPA-type glutamate receptors (AMPA receptors) reach excitatory synapses according to two distinct routes: a regulated pathway, which operates transiently during synaptic plasticity, and a constitutive pathway, which maintains synaptic function under conditions of basal transmission. However, the specific mechanisms that distinguish these two trafficking pathways are essentially unknown. Here, we evaluate the role of the molecular chaperone hsp90 (heat shock protein 90) in excitatory synaptic transmission in the hippocampus. On one hand, we found that hsp90 is necessary for the efficient neurotransmitter release at the presynaptic terminal. In addition, we identified hsp90 as a critical component of the cellular machinery that delivers AMPA receptors into the postsynaptic membrane. Using the hsp90-specific inhibitors radicicol and geldanamycin, we show that hsp90 is required for the constitutive trafficking of AMPA receptors into synapses during their continuous cycling between synaptic and nonsynaptic sites. In contrast, hsp90 function is not required for either the surface delivery of AMPA receptors into the nonsynaptic plasma membrane or for the acute, regulated delivery of AMPA receptors into synapses during plasticity induction (long-term potentiation). The synaptic cycling of AMPA receptors was also blocked by an hsp90-binding tetratricopeptide repeat (TPR) domain, suggesting that the role of hsp90 in AMPA receptor trafficking is mediated by a TPR domain-containing protein. These results demonstrate new roles for hsp90 in synaptic function by controlling neurotransmitter release and, independently, by mediating the continuous cycling of synaptic AMPA receptors.
Keywords: AMPA receptor trafficking, hippocampus, LTP, radicicol, TPR domain, CA1
Introduction
Most excitatory synaptic transmission in the brain is mediated by AMPA-type glutamate receptors (AMPA receptors), and it is being increasingly appreciated that the targeting and delivery of AMPA receptors into synapses is critical for controlling synaptic function, maturation, and remodeling (Sheng and Lee, 2001; Barry and Ziff, 2002; Malinow and Malenka, 2002; Song and Huganir, 2002). AMPA receptors are hetero-oligomeric molecules composed of different combinations of GluR1 (glutamate receptor subunit 1) to GluR4 subunits (Hollmann and Heinemann, 1994). In hippocampus, most AMPA receptors are composed of a combination of either GluR1/GluR2 subunits or GluR2/GluR3 subunits (Wenthold et al., 1996). Targeting of AMPA receptors into synapses depends mainly on their subunit composition, and two main pathways have been proposed (Malinow et al., 2000). GluR2/GluR3 oligomers are continuously cycling in and out of synapses in a manner essentially independent from synaptic activity (constitutive pathway). This synaptic cycling depends on the direct interaction between GluR2 and the hexameric ATPase N-ethylmaleimide-sensitive fusion protein (NSF) (Nishimune et al., 1998; Song et al., 1998; Lüscher et al., 1999). In contrast, GluR1/GluR2 receptors are added into synapses in an activity-dependent manner after NMDA receptor activation (regulated pathway) (Hayashi et al., 2000; Passafaro et al., 2001; Shi et al., 2001; Esteban et al., 2003). For this regulated delivery, protein–protein interactions mediated by the GluR1 subunit are critical (Hayashi et al., 2000; Shi et al., 2001). According to this scenario, the constitutive pathway would serve to maintain synaptic strength despite protein turnover, and it would act in a relatively fast manner (half-time of minutes). The regulated pathway would act transiently during plasticity induction, leading to long-lasting changes in synaptic strength (Malinow et al., 2000). However, the molecular mechanisms mediating these two trafficking pathways are still unclear.
hsp 90 (heat-shock protein 90) is expressed constitutively in brain from early development into adulthood (D'Souza and Brown, 1998), and it is especially abundant in limbic system-related structures, such as the hippocampus (Izumoto and Herbert, 1993). hsp90 was proposed to be a mediator of protein trafficking over a decade ago (Pratt, 1992, 1993). Since then, ample evidence has accumulated indicating that hsp90 is required for the subcellular targeting of a variety of proteins, including the glucocorticoid receptor (Owens-Grillo et al., 1996; Czar et al., 1997; Silverstein et al., 1999; Galigniana et al., 2001), the dioxin receptor (Kazlauskas et al., 2001), the receptor tyrosine kinase ErbB2 (Xu et al., 2002), the epidermal growth factor receptor (Supino-Rosin et al., 2000), the CFTR (cystic fibrosis transmembrane regulator) chloride channel (Loo et al., 1998) and the G-protein Gα12 (Waheed and Jones, 2002). hsp90 is also required for protein translocation into mitochondria (Young et al., 2003) and peroxisomes (Crookes and Olsen, 1998). The trafficking functions of hsp90 depend on cytoskeletal elements (Galigniana et al., 1998, 2002; Pratt et al., 1999) and involve specific interactions between C-terminal sequences of hsp90 and tetratricopeptide repeat (TPR) domains in several effector molecules (Chen et al., 1996; Young et al., 1998; Russell et al., 1999; Scheufler et al., 2000; Ward et al., 2002). TPR domains are involved in a variety of cellular functions, including protein transport and targeting (for review, see Blatch and Lassle, 1999). Therefore, we considered that hsp90 was an interesting candidate to mediate AMPA receptor trafficking.
Here we examine the potential role of hsp90 in the transport and targeting of AMPA receptors into synapses. Using a combination of biochemical and electrophysiological techniques on organotypic hippocampal slice cultures, we found that hsp90 is a critical component of the cellular machinery that delivers AMPA receptors into synapses during their continuous cycling. In addition, we also describe an independent role for hsp90 in the control of neurotransmitter release at the presynaptic terminal.
Materials and Methods
Expression of recombinant proteins in hippocampal neurons from organotypic slice cultures. The TPR domain (Chen et al., 1996) and the GluR2 (R586Q) (Shi et al., 2001) have been described previously. The TPR domains were coexpressed with GFP (green fluorescent protein) by means of an internal ribosomal entry site construct. All constructs were expressed in hippocampal CA1 pyramidal neurons from organotypic slice cultures using the Sindbis virus expression system (Malinow et al. 1999), except for the coexpression of GluR1 with the constitutively active CaMKII (Ca2+/calmodulin kinase II), in which we used the biolistic delivery method with plasmids bearing the cytomegalovirus promoter (Lo et al., 1994). Briefly, hippocampal slices are prepared from young rats (postnatal days 5–7) and placed in culture on semiporous membranes (Gahwiler et al., 1997). After 4–7 d in culture, the recombinant gene is delivered into the slices with the Sindbis virus expression system (Schlesinger, 1993). This is a replication-deficient, low-toxicity, neurotropic virus that allows us to express recombinant proteins exclusively in neurons by injecting the viral solution extracellularly in the desired area of a hippocampal slice. Expression of the TPR domain was for 15 hr and that of GluR2 (R586Q) and GluR1 was for 36 hr. Neurons remain morphologically and electrophysiologically intact during these expression times. All biosafety procedures and animal care protocols were approved by the University of Michigan.
Electrophysiology. Simultaneous double whole-cell recordings were obtained from nearby pairs of infected and uninfected CA1 pyramidal neurons, under visual guidance using fluorescence and transmitted light illumination. The recording chamber was perfused with 119 mm NaCl, 2.5 mm KCl, 4 mm CaCl2, 4 mm MgCl2, 26 mm NaHCO3, 1 mm NaH2PO4, 11 mm glucose, 0.1 mm picrotoxin, and 2 μm 2-chloroadenosine, pH 7.4 (gassed with 5% CO2–95% O2). Patch recording pipettes (3–6MΩ) were filled with the following (in mm): 115 cesium methanesulfonate, 20 CsCl, 10 HEPES, 2.5 MgCl2, 4 Na2ATP, 0.4 Na3GTP, 10 sodium phosphocreatine, and 0.6 EGTA, pH 7.25. In the rectification experiments, i.e., Figure 5, 0.1 mm spermine was added. Voltage-clamp whole-cell recordings were performed with multiclamp 700A amplifiers (Axon Instruments, Union City, CA). Synaptic responses were evoked with two bipolar electrodes with single voltage pulses (200 μsec, up to 20 V). The stimulating electrodes were placed over Schaffer collateral fibers between 300 and 500 μm from the recorded cells. Synaptic AMPA receptor-mediated responses were measured at –60 mV and NMDA receptor-mediated responses at +40 mV, at a latency when AMPA receptor responses have fully decayed (60 msec). Synaptic responses were averaged over 50–100 trials. In the rectification experiment (see Fig. 5), NMDA receptor-mediated responses were blocked pharmacologically using 0.1 mm dl-APV. Synaptic AMPA receptor-mediated responses were measured at –60 and +40 mV, and their ratio was used as an index of rectification. Electrophysiological experiments in the presence of the pep2m/G10 peptide were performed by including 1 mm peptide in the internal solution, together with the following protease inhibitors (in μm): 100 pepstatin A, 10 leupeptin, and 100 bestatin. Long-term potentiation (LTP) experiments were performed as described previously (Hayashi et al., 2000), by pairing 0 mV postsynaptic depolarization with 3 Hz presynaptic stimulation (300 pulses). All electrophysiological experiments were performed in organotypic hippocampal slices.
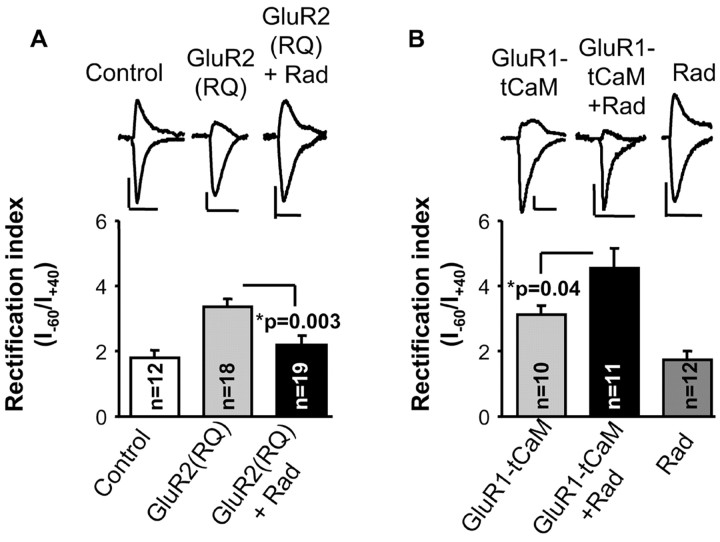
Figure 5.
hsp90 is necessary for the constitutive cycling of GluR2 receptors but not for the stability of GluR1 receptors at synapses. Average rectification values (AMPA-mediated response at –60 mV/AMPA-mediated response at +40 mV) for CA1 neurons in the absence (Control) and presence of radicicol (Rad), as indicated, with or without expression of the rectifying GluR2 (R586Q) (A) or GluR1 plus constitutively active αCaMKII (B). Insets, Sample trace of evoked AMPA receptor-mediated synaptic responses recorded at –60 and +40 mV from control, GluR2 (R586Q)-infected cells or GluR1-tCaM-transfected cells in the absence or presence of radicicol, as indicated. Calibration: 20 pA, 20 msec.
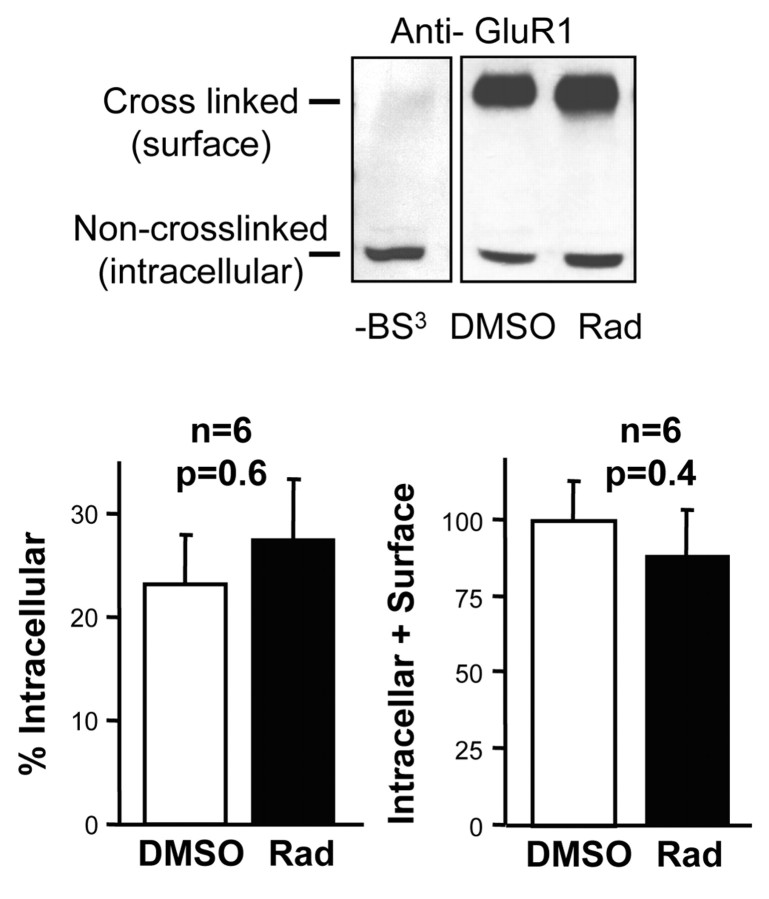
Surface cross-linking assay. Hippocampal slices were treated with either 20 μm radicicol or the vehicle (0.1% DMSO) for 30 min. Slices were then immersed for 5 min in perfusion solution containing 2 mm BS3 (catalog #21580; Pierce, Rockford, IL), a membrane-impermeant cross-linker. This technique has been used previously to determine the fraction of AMPA receptors exposed on the cell surface of hippocampal neurons (Hall and Soderling, 1997) and cerebellar granule cells (Archibald et al., 1998). Extracts from treated hippocampal slices were prepared in homogenization buffer containing protease inhibitors (10 mm HEPES, 500 mm NaCl, 10 mm EDTA, 4 mm EGTA, 0.1 mm PMSF, 2 μg/ml chymostatin, 2 μg/ml leupeptin, 2 μg/ml antipain, 2 μg/ml pepstatin, and 1% Triton X-100). AMPA receptors were then analyzed by Western blot with anti-GluR1 antibody (Chemicon, Temecula, CA). The fraction of intracellular receptors was calculated as the ratio of the intensity of the intracellular (noncross-linked) band over the intensity of the sum of intracellular and surface (cross-linked) bands. All quantifications were performed by densitometric scanning of autoradiographic films under linear exposure conditions.
Results
Distinct presynaptic and postsynaptic roles of hsp90 in synaptic transmission
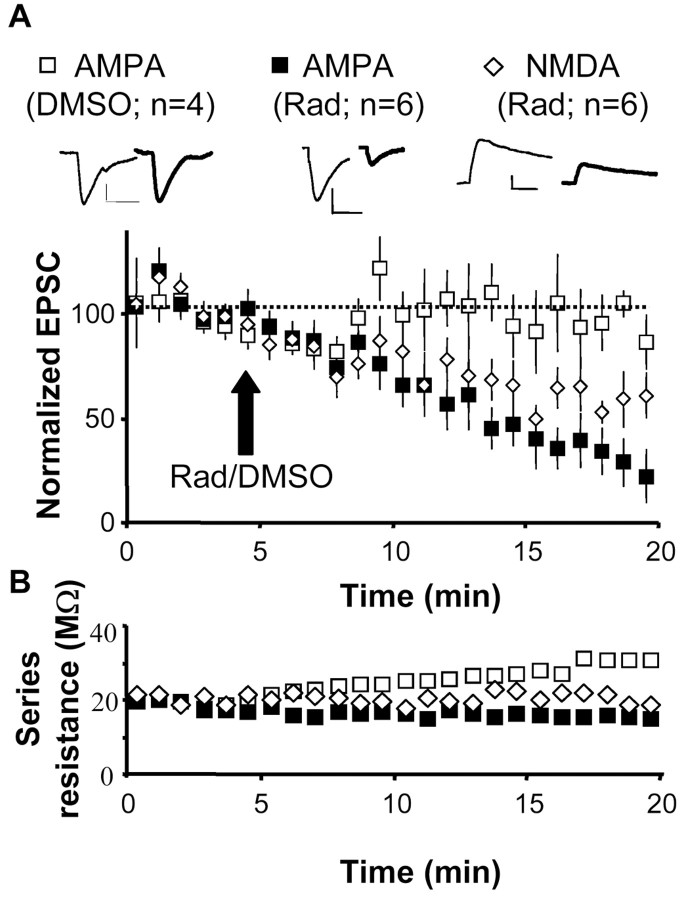
As a first step to evaluate whether hsp90 function is important for synaptic transmission, we monitored evoked AMPA and NMDA receptor-mediated responses in CA1 cells from hippocampal slices after addition of radicicol, a cell-permeable, highly specific inhibitor of hsp90 (Schulte et al., 1999). As shown in Figure 1A, radicicol added to the perfusion system produced a rapid decrease in AMPA receptor-mediated currents. Separate recordings performed at +40 mV also showed a significant, although smaller, depression of NMDA receptor-mediated responses. As control, addition of an equivalent amount of the radicicol vehicle (0.1% DMSO) did not alter AMPA transmission. Figure 1B indicates that the changes in response amplitude shown in Figure 1A are not attributable to systematic variations in access resistance during the recordings.
Figure 1.
Radicicol, an inhibitor of hsp90, diminishes AMPA and NMDA receptor-mediated responses. A, AMPA and NMDA receptor-mediated evoked synaptic responses were recorded in CA1 neurons of the hippocampus during stimulation of the Schaffer collateral pathway. Radicicol (20 μm; Rad) or the equivalent amount of the radicicol vehicle (0.1% DMSO) was added to the perfusion system at the time indicated with an arrow. AMPA and NMDA receptor-mediated currents were recorded in separate experiments, at –60 and +40 mV, respectively. Values are presented as mean ± SEM. Insets, Sample trace of evoked AMPA or NMDA receptor-mediated synaptic responses (as indicated) before (thin line) and 15 min after (thick line) the addition of radicicol or DMSO. Calibration: 20 pA, 20 msec. B, Average series resistance for the recordings shown in A. In addition, we confirmed that radicicol application does not alter membrane input resistance (control, 182 ± 13 MΩ; plus radicicol, 194 ± 15 MΩ).
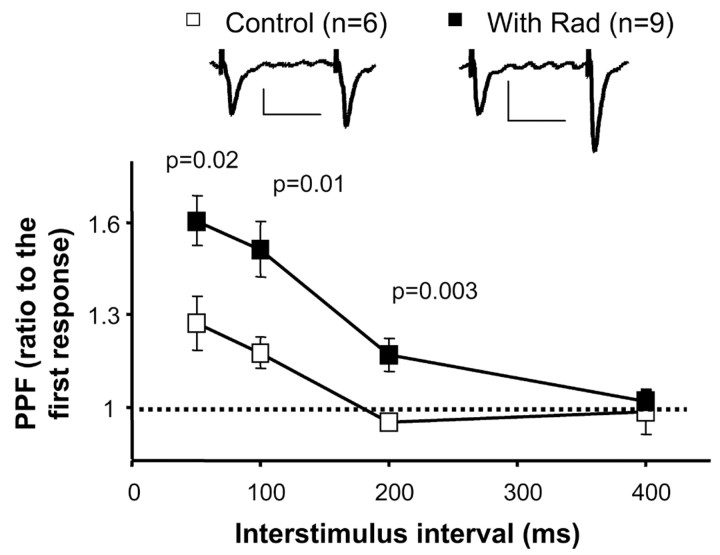
The effect observed on bath application of radicicol may be attributable to the interference with presynaptic and/or postsynaptic functions of hsp90. To evaluate more directly a potential presynaptic function of hsp90, we measured paired-pulse facilitation of synaptically evoked excitatory responses in hippocampal slices. Paired-pulse facilitation is a well established paradigm of short-term plasticity that is very sensitive to changes in the probability of neurotransmitter release (Dobrunz and Stevens, 1997). We compared paired-pulse facilitation between control hippocampal slices and slices that had been under perfusion in the presence of radicicol for at least 30 min before recordings (radicicol was also present during the recordings). As shown in Figure 2, paired-pulse facilitation was significantly enhanced in slices pretreated with radicicol, indicating that the probability of neurotransmitter release was reduced during blockade of hsp90 function. To our knowledge, this is the first evidence of a presynaptic role of hsp90 during synaptic transmission.
Figure 2.
Inhibition of hsp90 enhances paired-pulse facilitation (PPF). Hippocampal slices were perfused with 20 μm radicicol (Rad) for at least 30 min, and then paired-pulse facilitation was monitored at different interstimulus intervals (50, 100, 200, and 400 msec). Paired-pulse facilitation is expressed as the ratio between the amplitude of the second response versus the amplitude of the first response. Note that paired-pulse facilitation was significantly enhanced in slices perfused with radicicol at interstimulus intervals of 50, 100, and 200 msec. At 400 msec, there was no significant facilitation either with or without radicicol. Insets, Sample trace of evoked AMPA receptor-mediated synaptic responses with an interstimulus interval of 100 msec. Calibration: 20 pA, 50 msec.
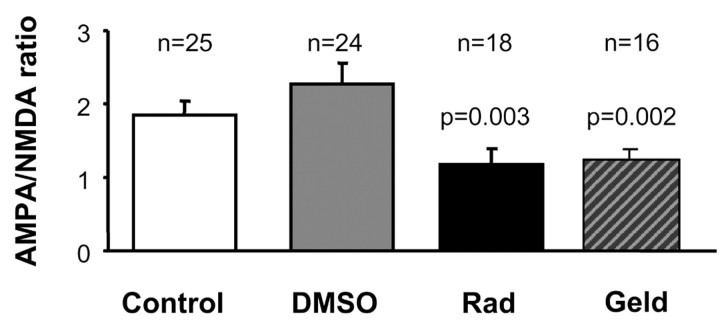
A reduction in neurotransmitter release should affect, to a large extent, similarly AMPA and NMDA receptor-mediated responses. However, our results shown in Figure 1A suggest that the depression induced after blockade of hsp90 function is more pronounced for AMPA receptors than for NMDA receptors. To address this point more rigorously, we kept hippocampal slices under perfusion in the presence of radicicol for at least 30 min and then measured the ratio between AMPA and NMDA receptor-mediated responses from individual cells by recording evoked synaptic currents at –60 mV (AMPA) and +40 mV (NMDA). As shown in Figure 3, slices preincubated in radicicol showed a significant decrease in AMPA/NMDA ratio compared with naive slices or with slices treated with vehicle (DMSO). To further control for the specificity of this effect, we used a chemically different hsp90 inhibitor, geldanamycin. Both radicicol and geldanamycin block hsp90 with high specificity by occupying its atypical nucleotide binding pocket (Roe et al., 1999). Indeed, slices preincubated with geldanamycin also showed significantly reduced AMPA/NMDA ratios (Fig. 3). These results, taken together, strongly suggest that hsp90, in addition to its presynaptic function, plays a direct postsynaptic role in AMPA receptor-mediated transmission. The following experiments are then oriented to test this interpretation and elucidate the postsynaptic function of hsp90.
Figure 3.
Inhibition of hsp90 decreases AMPA/NMDA ratio. Hippocampal slices were perfused with 20 μm radicicol (Rad), 20 μm geldanamycin (Geld), or its vehicle (0.1% DMSO) for at least 30 min, and then AMPA and NMDA responses were recorded from individual cells. Control slices were maintained in regular perfusion solution. Both radicicol and geldanamycin significantly decreased AMPA/NMDA ratio (p is the probability measured by Student's t test comparing AMPA/NMDA ratio in the presence of the vehicle and in the presence of the drug). Values are presented as mean ± SEM.
Inhibition of hsp90 does not alter the fraction of AMPA receptors on the cell surface or the total number of AMPA receptors
The fraction of AMPA receptors present at synapses is a small fraction of the total population present on the neuronal cell surface (synaptic plus extrasynaptic) (Shi et al., 1999). Therefore, to investigate whether hsp90 is required specifically for the synaptic delivery of AMPA receptors or whether it is involved in their global delivery to the cell membrane, we used a surface cross-linking assay (see Materials and Methods). Hippocampal slices were pretreated with radicicol or DMSO for 30 min and then exposed to BS3, a membrane-impermeant bifunctional cross-linker that reacts with primary amine groups in proteins. In the case of AMPA receptors, this reagent cross-links the different subunits of the receptor, leading to a marked increase in its apparent molecular weight in PAGE. As shown in Figure 4, radicicol treatment did not increase the percentage of noncross-linked, intracellular AMPA receptors compared with the slices treated with the vehicle only. Importantly, radicicol did not change the total amount of receptors (intracellular plus surface), suggesting that blocking hsp90 function for 30 min did not cause degradation of AMPA receptors. In conclusion, these data indicate that hsp90 is not needed for the global surface delivery of the receptor. Thus, hsp90 may be important for the local delivery of receptors into synapses, which constitute only a small fraction of the total number of surface AMPA receptors (Shi et al., 1999).
Figure 4.
Surface cross-linking of AMPA receptors in hippocampal slices exposed to radicicol. Top, Western blot analysis of the fraction of AMPA receptor GluR1 subunit cross-linked on the cell surface with the membrane-impermeant cross-linker BS3. Slices were treated with radicicol or DMSO, as indicated, for 30 min. –BS3 indicates control slices not cross-linked. Each lane in the Western blot is the result of pooling together extracts from four slices treated in parallel. Bottom, Quantification by densitometric scanning of six independent experiments as the one shown on top. Quantification of the intracellular fraction was calculated as described in Materials and Methods.
hsp90 is necessary for the continuous synaptic delivery of AMPA receptors
To test directly the role of hsp90 in the delivery of AMPA receptors into synapses, we expressed a recombinant AMPA receptor subunit (GluR2 R586Q) in organotypic hippocampal slices using a viral delivery system (see Materials and Methods). Recombinant GluR2 receptors behave as endogenous GluR2/GluR3 heteroligomers and, therefore, can be used to monitor the constitutive pathway of AMPA receptor synaptic delivery (Shi et al., 2001). In addition, the mutation R586Q, at the channel pore, prevents the receptor from conducting outward currents at positive membrane potentials (inward rectification). Therefore, the synaptic delivery of the recombinant receptor can be detected as an increase in the ratio of the AMPA receptor-mediated response at –60 mV versus the response at +40 mV (rectification index) (Hayashi et al., 2000; Zhu et al., 2000; Shi et al., 2001; Esteban et al., 2003). Importantly, this rectification index is independent from changes in presynaptic function, which would alter similarly responses at –60 and +40 mV. In the absence of radicicol, expression of the recombinant receptor produced inward rectification (increase in the rectification index) [Fig. 5A, compare Control, GluR2(RQ)], as described previously (Shi et al., 2001), indicating the delivery of the homomeric receptor. Thirty minute incubation with radicicol on slices expressing GluR2 (R586Q) blocked this rectification [Fig. 5A, GluR2(RQ)+Rad]. Because GluR2 receptors are constitutively cycling in and out of synapses (Passafaro et al., 2001; Shi et al., 2001), these results suggest that hsp90 is necessary for either the synaptic reinsertion of AMPA receptors during their continuous cycling or their stability once inserted at synapses.
hsp90 inhibition does not affect the noncycling population of AMPA receptors
To test whether hsp90 inhibition affects receptor stability at synapses, we evaluated the effect of blocking hsp90 function on the population of AMPA receptors that is not continuously cycling. To this end, we coexpressed a constitutively active form of αCaMKII (tCaM) (Hayashi et al., 2000; Poncer et al., 2002) with the recombinant GluR1 subunit on organotypic hippocampal slices. Recombinant GluR1 receptors behave as endogenous GluR1/GluR2 heteroligomers and, therefore, can be used to monitor the noncycling receptor population once their synaptic delivery is triggered by active CaMKII (Hayashi et al., 2000). Recombinant GluR1 was detected at synapses when coexpressed with constitutively active CaMKII, as indicated by the increase in rectification index (Fig. 5, compare A, Control with B, GluR1-tCaM) (see also Hayashi et al., 2000). Importantly, 30 min incubation with radicicol on slices expressing GluR1-tCaM did not block this rectification (Fig. 5B, GluR1-tCaM+Rad), indicating that hsp90 is not required for the stability of AMPA receptors at synapses. Furthermore, the rectification index was significantly higher in the presence of radicicol than in its absence. This result is consistent with radicicol preventing reinsertion of the endogenous (nonrectifying) recycling pool of receptors, without affecting the stability of the recombinant (rectifying) receptors. As control, radicicol did not change the rectification index when recombinant GluR2 (R586Q) or GluR1 was not expressed (Fig. 5B, Rad). In conclusion, these results indicate that hsp90 is required for receptor cycling but not for the synaptic stability of the noncycling population of receptors.
hsp90 and NSF act on the same pool of cycling AMPA receptors
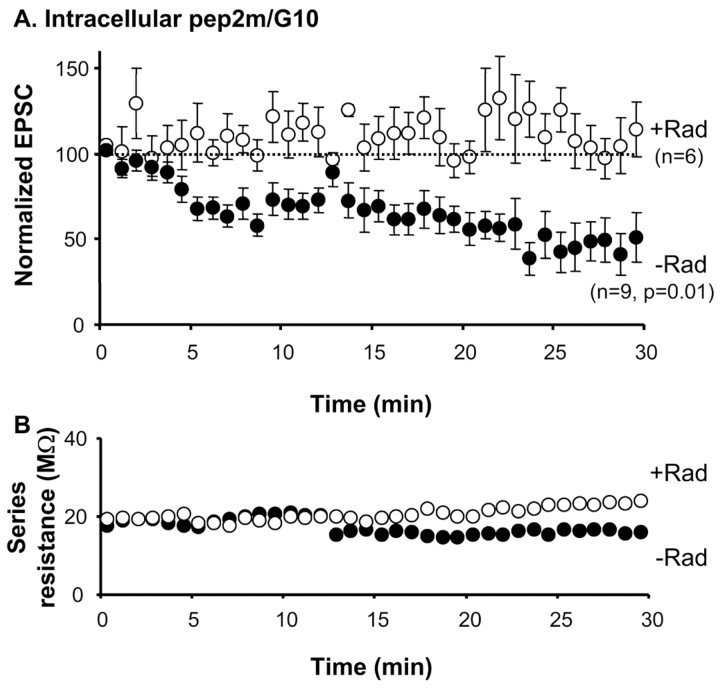
The efficient constitutive cycling of AMPA receptors into synapses requires a direct interaction between GluR2 and NSF. Hence, when this interaction is prevented by intracellular infusion of a peptide containing the NSF-binding sequence of GluR2 (pep2m/G10), AMPA receptor-mediated responses rapidly decline (Nishimune et al., 1998; Song et al., 1998; Lüscher et al., 1999). This is interpreted as the depletion of synapses from the constitutively cycling pool of receptors. To test whether hsp90 and NSF act on the same population of AMPA receptors, we incubated hippocampal slices with radicicol for 30 min and then recorded AMPA receptor-mediated responses while loading the recorded cell with the GluR2/NSF interfering peptide. As shown in Figure 6 A, preincubation with radicicol virtually abolished the depression of AMPA responses induced by the peptide. As control, recordings from nontreated slices showed the expected rundown of AMPA transmission during infusion of the peptide. Figure 6 B indicates that the changes in response amplitude shown in Figure 6 A are not attributable to variations in access resistance during the recordings.
Figure 6.
hsp90 and NSF act on the same pool of cycling AMPA receptors. A, Whole-cell recordings of AMPA receptor-mediated responses in the presence of the pep2M/G10 peptide in the internal solution. Recordings were performed on naive slices or on slices pretreated with radicicol (Rad) for at least 30 min, as indicated. B, Average series resistance from the recordings shown in A.
This result indicates that blocking hsp90 function with radicicol depletes synapses from the same pool of cycling receptors on which NSF acts. This interpretation, based on monitoring endogenous AMPA receptors, is consistent with the results shown in Figure 5A, in which radicicol prevented the constitutive synaptic delivery of recombinant GluR2 receptors.
hsp90–TPR domain interactions are involved in AMPA receptor trafficking
As described in Introduction, all known cases of protein transport mediated by hsp90 are dependent on specific interactions between hsp90 and TPR-containing proteins. To test whether this is the case for AMPA receptors, we overexpressed in CA1 hippocampal neurons the TPR domain of protein phosphatase 5, which binds specifically to hsp90 and acts as a dominant negative on hsp90 function (Chen et al., 1996). As control, we also used a TPR domain with a point mutation that abolishes binding to hsp90 [Arg101 to Ala (Russell et al., 1999)]. It is also important to note that the expression of the TPR constructs is targeted exclusively to the CA1 neurons (by means of local virus injection; see Materials and Methods). Because we monitor synaptic responses from CA3 to CA1 neurons, this experimental configuration implies that the TPR domain is only expressed in the postsynaptic cell. As shown in Figure 7A, overexpression of the TPR domain significantly decreased AMPA receptor-mediated transmission, without altering NMDA receptor-mediated responses (data not shown). Importantly, overexpression of the TPR mutant that does not bind hsp90, TPR (R101A), did not have any effect on AMPA (Fig. 7B) or NMDA (data not shown) transmission. These results support the hypothesis that hsp90 is necessary for the trafficking of AMPA receptor into synapses and that this role may be mediated by a TPR domain-containing protein. Additionally, because the depression of AMPA responses shown in Figure 7A was obtained under conditions of basal transmission, these results suggest that hsp90 is necessary for the constitutive (activity-independent) delivery of AMPA receptors into synapses, supporting the results obtained with radicicol treatment. We can also conclude from these experiments that the depression of NMDA responses during bath application of radicicol (Fig. 1 A) was solely attributable to a reduction in presynaptic function, because postsynaptic expression of the TPR domain did not affect NMDA responses (Fig. 7A).
Figure 7.
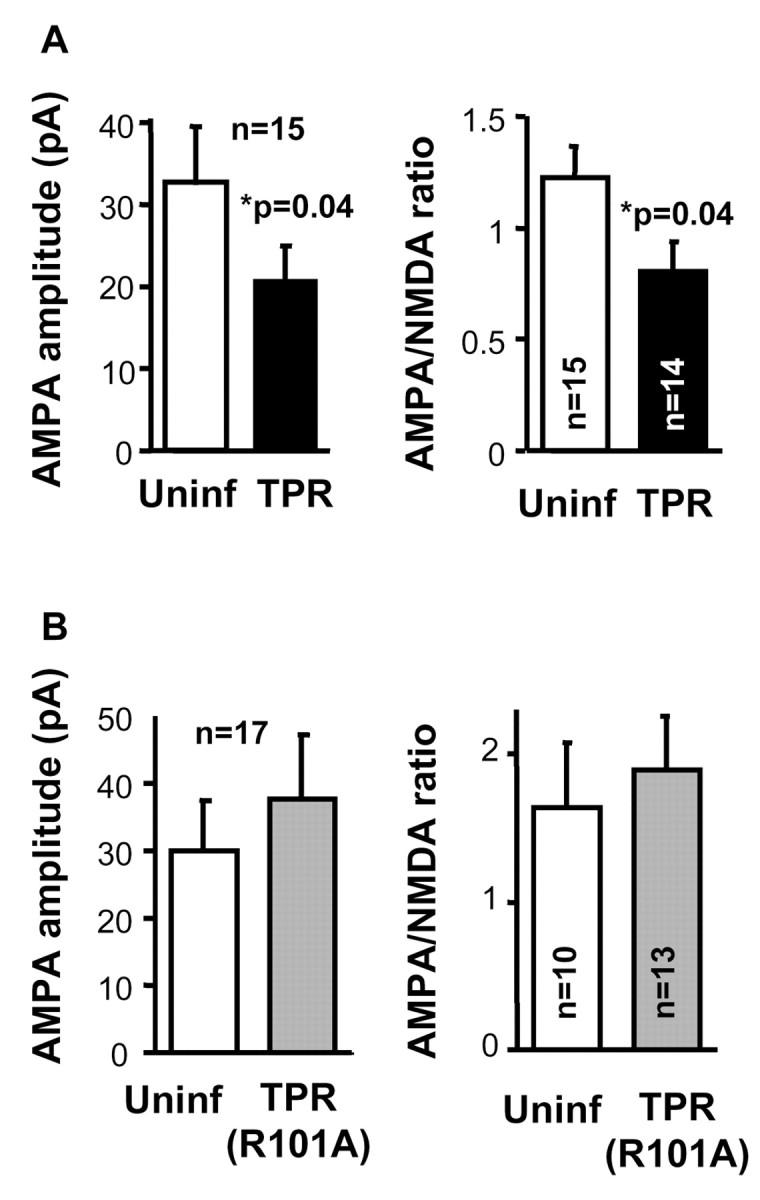
Overexpression of an hsp90-binding TPR domain decreases AMPA receptor-mediated transmission. Left, Average AMPA receptor-mediated current amplitude from infected neurons coexpressing TPR domain and GFP (A) or TPR (R101A) mutant and GFP (B) and control neighboring cells not expressing the recombinant protein (Uninf); n represents the number of pathways from cell pairs. Right, Average AMPA/NMDA ratios for uninfected and infected cells (n represents the number of pathways).
hsp90 is not required for long-term potentiation
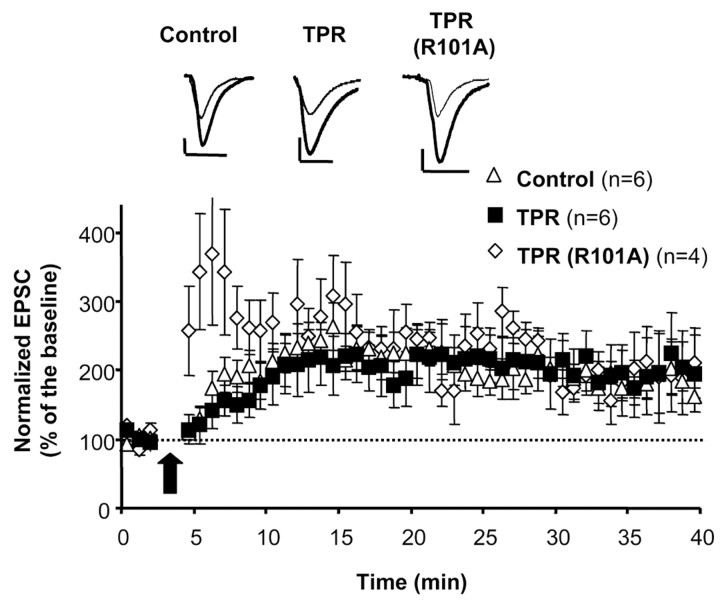
LTP in the CA1 hippocampus is one of the most thoroughly studied forms of synaptic plasticity, and it is accompanied by the synaptic delivery of GluR1-containing AMPA receptors (Hayashi et al., 2000). To test whether hsp90 is necessary for the movement of AMPA receptors during synaptic plasticity, we examined pairing-induced LTP in CA1 neurons expressing the TPR domain or its inactive mutant [TPR (R101A)]. As shown in Figure 8, neurons expressing either TPR or TPR (R101A) exhibit LTP levels similar to those of control, uninfected neurons. Therefore, hsp90 is not required for the acute, activity-dependent delivery of AMPA receptors during synaptic plasticity.
Figure 8.
Blocking hsp90 postsynaptically does not impair LTP. Organotypic slice cultures were infected with virus expressing either TPR or TPR (R101A). Whole-cell recordings were established from neurons expressing the desired proteins or uninfected cells, and LTP was induced by pairing, as described previously (Hayashi et al., 2000). Experiments were done blind with respect to which construct was expressed. Pairing significantly increased AMPA receptor-mediated responses in control, TPR-expressing, and TPR (R101A)-expressing neurons. No significant difference in the amount of potentiation was observed among the three groups at any time point (p = 0.95). Inset, Sample trace of evoked AMPA receptor-mediated synaptic responses before pairing (thin line) and 30 min after pairing (thick line). Calibration: 20 pA, 40 msec.
Discussion
hsp90 is an abundant, constitutively expressed protein in neurons (Gass et al., 1994), and different members of the hsp90 cochaperone machinery have been found in presynaptic and postsynaptic terminals. In particular, a cochaperone complex composed of hsc70 and CSP (cysteine string protein) is present in the presynaptic neurotransmitter vesicles (Tobaben et al., 2001). This complex, together with hsp90, has been proposed to control neurotransmitter release (Sakisaka et al., 2002). Complementary, two members of the hsp90 cochaperone machinery, hsp70 and hsp40, have been found at the postsynaptic density (Suzuki et al., 1999; Walikonis et al., 2000; Moon et al., 2001), although the function of this postsynaptic complex was unknown. Here, using electrophysiological techniques, we show that hsp90 plays important roles in synaptic transmission at both the presynaptic and postsynaptic compartments through independent mechanisms. In particular, we found that hsp90 is necessary for efficient neurotransmitter release at the presynaptic terminal. Although this function had been proposed previously from biochemical studies (Sakisaka et al., 2002), it had never been shown to operate during synaptic transmission. In addition, the most unexpected result of this study is that hsp90 is an essential component of the molecular machinery required for the continuous cycling of AMPA receptors at the postsynaptic membrane.
We have provided three lines of evidence that suggest that hsp90 is required for AMPA receptor synaptic delivery: (1) radicicol decreased AMPA receptor-mediated responses compared with those mediated by NMDA receptors (AMPA/NMDA ratio); (2) radicicol prevented the constitutive synaptic delivery of AMPA receptors, as assayed with recombinant GluR2 receptors and by monitoring endogenous receptor cycling with the GluR2/NSF interfering peptide; and (3) expression of an hsp90-binding TPR domain decreased AMPA receptor-mediated synaptic responses but did not affect NMDA receptor-mediated transmission. In contrast, expression of the TPR domain did not alter LTP induction or expression, which suggests that hsp90 is not involved in the activity-dependent delivery of AMPA receptors. Additionally, surface cross-linking experiments indicated that hsp90 function is not required for the delivery of AMPA receptors into the nonsynaptic cell membrane. Overall, these results strongly suggest that hsp90 has a necessary role in the synaptic delivery of AMPA receptors specifically during their continuous cycling. It is worth mentioning that this specific role in the constitutive pathway is consistent with the small, but significant, reduction of AMPA/NMDA ratio (Figs. 3, 7A) or absolute AMPA receptor-mediated responses (Fig. 7A) observed when blocking hsp90 function. These results suggest that the fraction of cycling AMPA receptors at synapses may range from ∼30 to 50%, in good agreement with previous estimations using GluR2/NSF interfering peptides (Nishimune et al. 1998; Lüscher et al., 1999; Lee et al., 2002), dominant-negative GluR2 C terminus constructs (Shi et al., 2001), and rectifying receptors (Shi et al., 2001).
Although the involvement of hsp90 in the synaptic cycling of AMPA receptors is a novel observation, it is worth mentioning that this is the second chaperone described as an important component of this process. As mentioned above, the hexameric chaperone NSF interacts directly with AMPA receptors, and disruption of this interaction causes a rapid loss of synaptic AMPA receptors (Nishimune et al., 1998; Osten et al., 1998; Song et al., 1998; Lüscher et al., 1999; Luthi et al., 1999; Noel et al., 1999; Kim et al., 2001; Shi et al., 2001). Therefore, our results indicate that both hsp90 and NSF are required for the rapid synaptic cycling of these receptors.
How does hsp90 mediate AMPA receptor synaptic delivery? hsp90 may be acting as a classical molecular chaperone, catalyzing the assembly and disassembly of transient protein complexes required for receptor cycling. This would actually be similar to the role that has been proposed for NSF, which has been shown to catalyze the ATP-dependent dissociation of the complex formed between PICK1 (PKC-interacting protein 1) and GluR2 (Hanley et al., 2002). The formation and dissociation of this complex, together with the one formed between GluR2 and GRIP (glutamate receptor-interacting protein), is thought to regulate receptor delivery into synapses (Chung et al., 2000; Daw et al., 2000; Perez et al., 2001; Braithwaite et al., 2002; Seidenman et al., 2003). We indeed tested whether complex assembly between GluR2, NSF, PICK1, and GRIP was dependent on hsp90 activity, but the results were negative (data not shown).
On the other hand, as mentioned above, hsp90 is known to control intracellular protein transport and targeting. Through its TPR acceptor site, hsp90 interacts with a variety of proteins that bind motor proteins, therefore linking hsp90 with movement along the cytoskeletal tracts (for review, see Pratt et al., 1999; Pratt and Toft, 2003). For instance, the TPR acceptor site of hsp90 interacts with the TPR domain of unc45, and unc45 binds to the actin-dependent motor protein myosin through its C-terminal region (Barral et al., 2002). Interestingly, AMPA receptor presence at synapses is dependent on an intact actin cytoskeleton (Kim and Lisman, 1999, 2001; Shen et al., 2000; Zhou et al., 2001). Our results with the dominant-negative TPR domain are consistent with a model in which hsp90, via a TPR-dependent interaction, mediates the actin-dependent insertion of AMPA receptors into synapses.
In summary, the present findings advance our understanding of the distinct molecular machinery that catalyzes the continuous versus the regulated exocytosis of AMPA receptors. In addition, these results uncover a new role for hsp90 in synaptic function as a mediator of the constitutive delivery of AMPA receptors into synapses.
Footnotes
This work was supported by grants from the Alzheimer's Association and the National Alliance for Research on Schizophrenia and Depression (J.A.E.) and by National Institutes of Health Grants CA28010 (W.B.P.) and DK55877 (M.C.). We thank María S. Soengas for her critical reading of this manuscript.
Correspondence should be addressed to José A. Esteban, 1150 West Medical Center Drive, Ann Arbor, MI 48109-0632. E-mail: estebanj@umich.edu.
DOI:10.1523/JNEUROSCI.0594-04.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/244758-09$15.00/0
References
- Archibald K, Perry MJ, Molnar E, Henley JM (1998) Surface expression and metabolic half-life of AMPA receptors in cultured rat cerebellar granule cells. Neuropharmacology 37: 1345–1353. [DOI] [PubMed] [Google Scholar]
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF (2002) Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295: 669–671. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB (2002) Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12: 279–286. [DOI] [PubMed] [Google Scholar]
- Blatch GL, Lassle M (1999) The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays 21: 932–939. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Xia H, Malenka RC (2002) Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proc Natl Acad Sci USA 99: 7096–7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Silverstein AM, Pratt WB, Chinkers M (1996) The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem 271: 32315–32320. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL (2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 20: 7258–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookes WJ, Olsen LJ (1998) The effects of chaperones and the influence of protein assembly on peroxisomal protein import. J Biol Chem 273: 17236–17242. [DOI] [PubMed] [Google Scholar]
- Czar MJ, Galigniana MD, Silverstein AM, Pratt WB (1997) Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry 36: 7776–7785. [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, Collingridge GL, Isaac JT (2000) PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron 28: 873–886. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF (1997) Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18: 995–1008. [DOI] [PubMed] [Google Scholar]
- D'Souza SM, Brown IR (1998) Constitutive expression of heat shock proteins Hsp90, Hsc70, Hsp70 and Hsp60 in neural and non-neural tissues of the rat during postnatal development. Cell Stress Chaperones 3: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi S-H, Wilson C, Nuriya M, Huganir RL, Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136–143. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM (1997) Organotypic slice cultures: a technique has come of age. Trends Neurosci 20: 471–477. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB (1998) Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol 12: 1903–1913. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB (2001) Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem 276: 14884–14889. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M, Pratt WB (2002) Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry 41: 13602–13610. [DOI] [PubMed] [Google Scholar]
- Gass P, Schroder H, Prior P, Kiessling M (1994) Constitutive expression of heat shock protein 90 (HSP90) in neurons of the rat brain. Neurosci Lett 182: 188–192. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR (1997) Quantitation of AMPA receptor surface expression in cultured hippocampal neurons. Neuroscience 78: 361–371. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Khatri L, Hanson PI, Ziff EB (2002) NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron 34: 53–67. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi S-H, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S (1994) Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108. [DOI] [PubMed] [Google Scholar]
- Izumoto S, Herbert J (1993) Widespread constitutive expression of HSP90 messenger RNA in rat brain. J Neurosci Res 35: 20–28. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Sundstrom S, Poellinger L, Pongratz I (2001) The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol Cell Biol 21: 2594–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lisman JE (1999) A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci 19: 4314–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lisman JE (2001) A labile component of AMPA receptor-mediated synaptic transmission is dependent on microtubule motors, actin, and N-ethylmaleimide-sensitive factor. J Neurosci 21: 4188–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL (2001) Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA 98: 11725–11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M (2002) Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron 36: 661–674. [DOI] [PubMed] [Google Scholar]
- Lo DC, McAllister AK, Katz LC (1994) Neuronal transfection in brain slices using particle-mediated gene transfer. Neuron 13: 1263–1268. [DOI] [PubMed] [Google Scholar]
- Loo MA, Jensen TJ, Cui L, Hou Y, Chang XB, Riordan JR (1998) Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J 17: 6879–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA (1999) Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24: 649–658. [DOI] [PubMed] [Google Scholar]
- Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL (1999) Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron 24: 389–399. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126. [DOI] [PubMed] [Google Scholar]
- Malinow R, Hayashi Y, Maletic-Savatic M, Zaman S, Poncer J-C, Shi S-H, Esteban JA (1999) Introduction of green fluorescent protein into hippocampal neurons through viral infection. In: Imaging living neurons (Yuste R, Lanni F, Konnerth A, eds), pp 58.1-58.9. Cold Spring Harbor, NY: Cold Spring Harbor. [DOI] [PMC free article] [PubMed]
- Malinow R, Mainen ZF, Hayashi Y (2000) LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol 10: 352–357. [DOI] [PubMed] [Google Scholar]
- Moon IS, Park IS, Schenker LT, Kennedy MB, Moon JI, Jin I (2001) Presence of both constitutive and inducible forms of heat shock protein 70 in the cerebral cortex and hippocampal synapses. Cereb Cortex 11: 238–248. [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron 21: 87–97. [DOI] [PubMed] [Google Scholar]
- Noel J, Ralph GS, Pickard L, Williams J, Molnar E, Uney JB, Collingridge GL, Henley JM (1999) Surface expression of AMPA receptors in hippocampal neurons is regulated by an NSF-dependent mechanism. Neuron 23: 365–376. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB (1998) The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron 21: 99–110. [DOI] [PubMed] [Google Scholar]
- Owens-Grillo JK, Czar MJ, Hutchison KA, Hoffmann K, Perdew GH, Pratt WB (1996) A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J Biol Chem 271: 13468–13475. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M (2001) Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci 4: 917–926. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB (2001) PICK1 targets activated protein kinase Cα to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci 21: 5417–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R (2002) Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by α-Ca2+/calmodulin-dependent protein kinase II. J Neurosci 22: 4406–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB (1992) Control of steroid receptor function and cytoplasmicnuclear transport by heat shock proteins. BioEssays 14: 841–848. [DOI] [PubMed] [Google Scholar]
- Pratt WB (1993) The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem 268: 21455–21458. [PubMed] [Google Scholar]
- Pratt WB, Toft DO (2003) Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228: 111–133. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Silverstein AM, Galigniana MD (1999) A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37. Cell Signal 11: 839–851. [DOI] [PubMed] [Google Scholar]
- Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42: 260–266. [DOI] [PubMed] [Google Scholar]
- Russell LC, Whitt SR, Chen MS, Chinkers M (1999) Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem 274: 20060–20063. [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Meerlo T, Matteson J, Plutner H, Balch WE (2002) RabalphaGDI activity is regulated by a Hsp90 chaperone complex. EMBO J 21: 6125–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I (2000) Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101: 199–210. [DOI] [PubMed] [Google Scholar]
- Schlesinger S (1993) Alphaviruses—vectors for the expression of heterologous genes. Trends Biotechnol 11: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Akinaga S, Murakata T, Agatsuma T, Sugimoto S, Nakano H, Lee YS, Simen BB, Argon Y, Felts S, Toft DO, Neckers LM, Sharma SV (1999) Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol Endocrinol 13: 1435–1448. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R (2003) Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci 23: 9220–9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL (2000) Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci 20: 7932–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH (2001) AMPA receptor trafficking and the control of synaptic transmission. Cell 105: 825–828. [DOI] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811–1816. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Kanelakis KC, Radanyi C, Renoir JM, Pratt WB (1999) Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem 274: 36980–36986. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–588. [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL (1998) Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron 21: 393–400. [DOI] [PubMed] [Google Scholar]
- Supino-Rosin L, Yoshimura A, Yarden Y, Elazar Z, Neumann D (2000) Intracellular retention and degradation of the epidermal growth factor receptor, two distinct processes mediated by benzoquinone ansamycins. J Biol Chem 275: 21850–21855. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Usuda N, Murata S, Nakazawa A, Ohtsuka K, Takagi H (1999) Presence of molecular chaperones, heat shock cognate (Hsc) 70 and heat shock proteins (Hsp) 40, in the postsynaptic structures of rat brain. Brain Res 816: 99–110. [DOI] [PubMed] [Google Scholar]
- Tobaben S, Thakur P, Fernandez-Chacon R, Sudhof TC, Rettig J, Stahl B (2001) A trimeric protein complex functions as a synaptic chaperone machine. Neuron 31: 987–999. [DOI] [PubMed] [Google Scholar]
- Waheed AA, Jones TL (2002) Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J Biol Chem 277: 32409–32412. [DOI] [PubMed] [Google Scholar]
- Walikonis RS, Jensen ON, Mann M, Provance Jr DW, Mercer JA, Kennedy MB (2000) Identification of proteins in the postsynaptic density fraction by mass spectrometry. J Neurosci 20: 4069–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BK, Allan RK, Mok D, Temple SE, Taylor P, Dornan J, Mark PJ, Shaw DJ, Kumar P, Walkinshaw MD, Ratajczak T (2002) A structure-based mutational analysis of cyclophilin 40 identifies key residues in the core tetratricopeptide repeat domain that mediate binding to Hsp90. J Biol Chem 277: 40799–40809. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos II J, Niedzielski AS (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci 16: 1982–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Mimnaugh EG, Kim JS, Trepel JB, Neckers LM (2002) Hsp90, not Grp94, regulates the intracellular trafficking and stability of nascent ErbB2. Cell Stress Chaperones 7: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Obermann WM, Hartl FU (1998) Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J Biol Chem 273: 18007–18010. [DOI] [PubMed] [Google Scholar]
- Young JC, Hoogenraad NJ, Hartl FU (2003) Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell 112: 41–50. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Xiao M, Nicoll RA (2001) Contribution of cytoskeleton to the internalization of AMPA receptors. Proc Natl Acad Sci USA 98: 1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R (2000) Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci 3: 1098–1106. [DOI] [PubMed] [Google Scholar]