Abstract
Autism is a neurodevelopmental disorder characterized by impairments in reciprocal social interaction, deficits in verbal and nonverbal communication, and a restricted repertoire of activities or interests. We performed a magnetic resonance imaging study to better define the neuropathology of autistic spectrum disorders. Here we report findings on the amygdala and the hippocampal formation. Borders of the amygdala, hippocampus, and cerebrum were defined, and their volumes were measured in male children (7.5-18.5 years of age) in four diagnostic groups: autism with mental retardation, autism without mental retardation, Asperger syndrome, and age-matched typically developing controls. Although there were no differences between groups in terms of total cerebral volume, children with autism (7.5-12.5 years of age) had larger right and left amygdala volumes than control children. There were no differences in amygdala volume between the adolescent groups (12.75-18.5 years of age). Interestingly, the amygdala in typically developing children increases substantially in volume from 7.5 to 18.5 years of age. Thus, the amygdala in children with autism is initially larger, but does not undergo the age-related increase observed in typically developing children. Children with autism, with and without mental retardation, also had a larger right hippocampal volume than typically developing controls, even after controlling for total cerebral volume. Children with autism but without mental retardation also had a larger left hippocampal volume relative to controls. These cross-sectional findings indicate an abnormal program of early amygdala development in autism and an abnormal pattern of hippocampal development that persists through adolescence. The cause of amygdala and hippocampal abnormalities in autism is currently unknown.
Keywords: Asperger, amygdaloid complex, development, mental retardation, MRI, neuroanatomy
Introduction
Autism is a lifelong neurodevelopmental disorder that is diagnosed in early childhood and characterized by a core deficit in social interaction (American Psychiatric Association, 1994). Other components of the disorder may include language impairments, stereotypical behaviors, and unusual fear or anxiety. Since Kanner (1943) initially described the disorder over 60 years ago, the definition of the autistic spectrum has evolved and now encompasses a wide range of severity of social and emotional abnormalities with varying levels of cognitive and linguistic functioning. The disorder ranges from low functioning with mental retardation [low-functioning autism (LFA)] to high functioning with normal intelligence quotient (IQ) [high-functioning autism (HFA)]. Almost simultaneously with Kanner, Asperger (1944) described a group of children with a narrow range of interests and impaired social interaction similar to high-functioning autism but whose development of verbal ability was not delayed. The distinction of Asperger syndrome (ASP) from high-functioning autism is controversial (Klin et al., 1995; Ozonoff et al., 2000; Howlin, 2003).
The neuropathology of autism has not yet been clearly established. Among the brain regions that have been implicated are the cerebellum, brainstem nuclei, amygdala, hippocampal formation, and various cortical areas. Bauman and Kemper (1985) were the first to observe neuropathology of the amygdala and hippocampus in postmortem cases. They reported abnormally small and densely packed cells, particularly in the medial portion of the amygdala and CA1 and subiculum of the hippocampal formation. However, their findings have not yet been replicated.
Structural magnetic resonance imaging (MRI) studies of the amygdala have provided inconsistent results (for review, see Cody et al., 2002). Some studies have reported decreased volumes (Aylward et al., 1999; Pierce et al., 2001), whereas others have reported increased volumes (Howard et al., 2000; Sparks et al., 2002); still others have found no difference (Haznedar et al., 2000). Abell et al. (1999) used voxel-based morphometry and demonstrated decreased gray matter at anterior levels of the amygdala but increased gray matter through posterior levels. Structural MRI studies of the hippocampus have also provided inconsistent results (for review, see Cody et al., 2002). Some studies have reported decreased volumes of the hippocampus (Aylward et al., 1999), whereas others have reported increased volumes (Sparks et al., 2002), and still others have found no significant differences (Piven et al., 1998; Haznedar et al., 2000; Howard et al., 2000). A number of factors may contribute to the inconsistent findings, including subject diagnostic criteria (e.g., whether study participants with autism or Asperger syndrome were included), exclusionary criteria (e.g., whether study participants with a seizure disorder were included), the age group measured, and the neuroanatomical definition of the amygdala and hippocampus.
Although individuals diagnosed with mental retardation make up ∼70% of the population of children on the autistic spectrum (Fombonne, 2003), no MRI study to date has evaluated children with autism and mental retardation separately from those without mental retardation. The two major objectives for this study were: (1) to compare volume measurements of the amygdala and hippocampus in children across the autistic spectrum and (2) to attempt to reconcile contradictory results in previously published MRI studies on the autistic amygdala and hippocampus.
Materials and Methods
A parent or guardian for each study participant gave informed consent and children with typical cognitive development gave their assent to participate in these studies as approved by the Institutional Review Boards of the University of California Davis and Stanford University. Study participants were recruited through local advocacy groups, the Stanford Neuropsychiatry-Pervasive Developmental Disorders Clinic, and the M.I.N.D. (Medical Investigation of Neurodevelopmental Disorders) Institute Clinic. Ninety-eight male volunteers between the ages of 7.5 and 18.5 participated in this study. All participants were healthy volunteers who met criteria in one of four diagnostic groups: LFA (n = 19), HFA (n = 27), ASP (n = 25), and typically developing controls (CON) (n = 27). Autism is diagnosed in four times as many males as females (Fombonne, 2003). To eliminate variability in brain measurements attributable to gender, only males were included in this study.
Diagnostic assessments were conducted either at the M.I.N.D. Institute Clinic of the University of California Davis Medical Center or at Stanford University in the Division of Child and Adolescent Psychiatry and Child Development. Clinicians (B.L.G.-J. and L.J.L.) experienced in the diagnosis of autism spectrum disorders were formally trained to administer the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994) and Autism Diagnostic Observation Schedule-Generic (ADOS-G) (DiLavore et al., 1995; Lord et al., 2000) and obtained reliability with an author of these measures (C. Lord) before beginning this study. The ADI-R is a comprehensive parent interview administered by a trained clinician using a semistructured interview format that probes for symptoms of autism. It is closely linked to the diagnostic criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (American Psychiatric Association, 1994) and relies on cutoff scores for the diagnosis of autism. The ADOS-G is a semistructured interactive assessment conducted with the child during an evaluation for autism spectrum disorders.
An IQ exam was also administered to all participants; the particular test used was based on their verbal ability. Higher-functioning children and typically developing controls were given either the Wechsler Intelligence Scale for Children (Wechsler, 1991) or the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Participants who were nonverbal, including all those in the low-functioning autism group, were given the Leiter International Performance Scale-Revised (Roid and Miller, 1997).
Participants who met criteria for autism with a full-scale IQ of >70 were included in the high-functioning autism group. Participants with a diagnosis of autism and a full-scale IQ of <70 were included in the low-functioning autism group. A diagnosis of Asperger syndrome was given to study participants who met the criteria for high-functioning autism, as determined by the ADOS-G, met DSM-IV criteria for Asperger syndrome, and had developed phrase language before 36 months of age. A more detailed description of the clinical protocol used in this study has been published previously (Lotspeich et al., 2004).
Typically developing control participants had a full-scale IQ of >70 and did not have a family member with an autism spectrum disorder. Participants were excluded from the study if they had a diagnosis of fragile X, seizure disorder, tuberous sclerosis, obsessive-compulsive disorder, bipolar disorder, or any other major neurological illness. All volunteers with an IQ of <70 who had not been tested previously for fragile X were tested before MRI (Kimball Genetics, Denver, CO); none of the participants in the study tested positively for fragile X.
Neuroimaging
After completion of the diagnostic process, MRI scans were performed at one of three possible sites: University of California Davis Hospital (using a 1.5 T GE Signa Horizon system; GE Healthcare, Waukesha, WI), the University of California Davis Imaging Research Center (using a 1.5 T GE Signa NV/I system; GE Healthcare), or the Richard M. Lucas Center for Magnetic Resonance Spectroscopy and Imaging at Stanford University (using a 3 T GE Signa VH/I system; GE Healthcare).
Because volume measurement accuracy is related to the linearity of magnetic field gradients in each of the MRI systems, an intersite comparison and calibration were conducted before imaging study participants. Images were collected from three healthy adult volunteers (one male and two females) at each of the three MRI sites for in vivo validation of volume measurement accuracy. Images were acquired with the same pulse sequence and analyzed at the Stanford Psychiatry Neuroimaging Laboratory using BrainImage 5.x software (developed by A. L. Reiss in 2002). Total brain and segmented tissue volumes were compared between the three systems. The percentage difference between MRI systems for each participant was averaged across participants to arrive at a mean percentage difference for each volumetric measure. The a priori requirement for combining volume measurements across sites was a difference of no more than 5%. A 1.2% difference for total cerebral tissue and a 1.8% difference for cerebral gray matter were found. Lotspeich et al. (2004) have presented a more detailed description of site comparisons.
The protocol for scanning each participant included a three-dimensional coronal spoiled gradient recalled echo (SPGR) series (repetition time, 35 msec; echo time, 6 msec; field of view, 24 cm; matrix, 256 × 256; section thickness, 1.5 mm; number of slices, 124; total scan time, 14 min and 24 sec) that was used for the volumetric assessment of the amygdala. In addition, a two-dimensional (2D) sagittal T1 spin echo, a 2D proton density/T2 interleaved double echo, and a diffusion tensor sequence were collected on all participants for other analyses.
A parent or guardian for each participant signed consent before the child entered the MRI scanner and was present throughout the duration of the scan in an adjacent waiting room. Those study participants requiring anesthesia (isoflurane) to undergo MRI (19 LFA, 13 HFA, and 7 ASP) were imaged at University of California Davis Hospital. All remaining participants were scanned at either the University of California Davis Research Imaging Center (6 HFA, 9 ASP, and 18 CON) or at Stanford University (8 HFA, 9 ASP, and 9 CON). After reviewing the images, 13 participants were excluded from the study (1 LFA, 6 HFA, 1 ASP, and 5 CON) because of excessive movement, distorted images resulting from orthodontics, or additional diagnostic information that precluded the series from being used. Within each diagnostic group, excluded participants did not differ from included participants with respect to age, IQ, or symptom severity.
Volumetric analysis of the amygdala
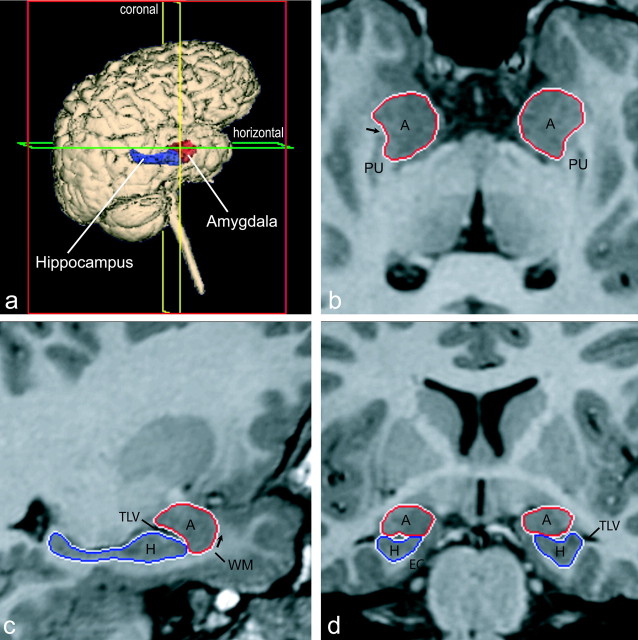
After completion of the MRI acquisition, all images were transferred to the University of California Davis for volumetric analysis of the amygdala and cerebrum. Each coronal SPGR series was imported into Analyze 5.0 (Biomedical Imaging Resource, Rochester, MN) (Robb et al., 1989) and converted to cubic voxel dimensions of 0.469 mm using a cubic spline interpolation algorithm. Images were reoriented so that the horizontal axis was parallel to a line from the rostral to the caudal pole of the hippocampus (Fig. 1b). Coronal sections were viewed perpendicular to the horizontal axis (Fig. 1d).
Figure 1.
Orthogonal views for segmenting the amygdala and hippocampus on MRI sections. A three-dimensional reconstruction of images (a) in which lines indicate the position of the horizontal plane (b), sagittal plane (c), and coronal plane (d) is shown. The arrow in b indicates the best-fit line along the white matter separating the amygdala from the putamen; the arrow in c represents the white matter that forms the ventral border of the rostral amygdala. A, Amygdala; EC, entorhinal cortex; H, hippocampus; PU, putamen; TLV, temporal horn of the lateral ventricle; WM, subamygdaloid white matter.
On each coronal image that contained the amygdala, the amygdala was manually outlined based on a detailed set of tracing guidelines. These guidelines were developed by first studying the anatomy of the human amygdala and surrounding structures in histological sections cut perpendicular to the horizontal axis of the hippocampus. Two raters (C.M.S. and J.H.) who were blind to subject identity manually traced the amygdala after establishing reliability on MRI scans from 30 subjects with an inter-rater reliability correlation of 0.92 for the left amygdala and 0.93 for the right amygdala. Each rater obtained an intrarater reliability of >0.95.
The initial tracing process involved defining the borders in coronal sections starting with the most caudal level in which the amygdala was visible. Outlines were also checked in horizontal (axial) and sagittal views (Fig. 1) that were simultaneously available to the rater while tracing the amygdala. The following guidelines detail the procedures used for systematic outlining of the amygdala.
Caudal third of the amygdala. At its caudal extent (Fig. 2a), the amygdala is bordered dorsally by the substantia innominata, laterally by the putamen, and ventrally by the temporal horn of the lateral ventricle. The medial surface of the amygdala abuts the optic tract. The outline (Fig. 2a) at this level started at the dorsolateral extent of the optic tract and extended laterally to the junction of the amygdala and putamen. The outline continued ventrally along the lateral border of the amygdala until the lateral ventricle was reached. The outline was then extended medially along the dorsal surface of the ventricle to the ventrolateral extent of the optic tract and completed along the optic tract to the starting point. The lateral border of the amygdala with the putamen is not always clear in the coronal views. Therefore, the border was first drawn in the coronal views and further edited in the horizontal view during the revision process (see below) (Fig. 1b).
Figure 2.
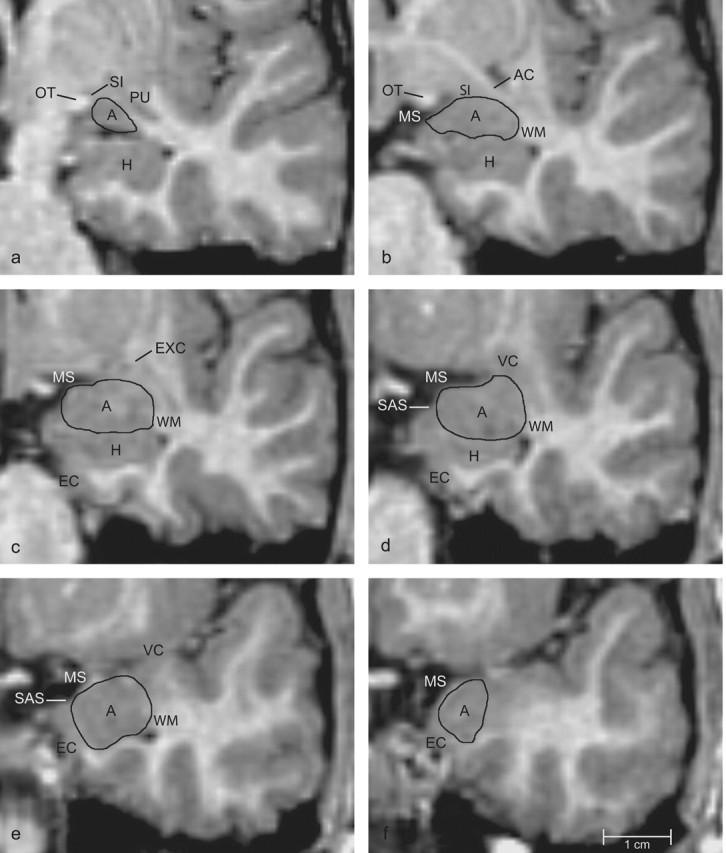
Series of coronal images arranged from caudal (a) to rostral (f), indicating boundaries of the amygdala. A, Amygdala; AC, anterior commissure; H, hippocampus; EC, entorhinal cortex; EXC, external capsule; MS, medial surface of the brain; OT, optic tract; PU, putamen; SAS, semiannular sulcus; SI, substantia innominata; VC, ventral claustrum; WM, subamygdaloid white matter.
Proceeding rostrally, the amygdala increases in size (Fig. 2b). It is bordered dorsally by the substantia innominata and fibers of the anterior commissure. The lateral border is formed by white matter of the temporal lobe. The ventral surface is formed by the temporal horn of the lateral ventricle. However, because the hippocampus often appears to be fused with the ventral surface of the amygdala, a more reliable boundary is the alveus, the white matter that forms the dorsal surface of the hippocampus. The amygdala forms part of the medial surface of the brain.
The starting point of the outline at this level is the dorsomedial extent of the amygdala. If the medial extent of the optic tract extends more laterally than the medial surface of the brain formed by the temporal lobe, then this remains the starting point as described for the caudal section. However, if the surface of the brain extends farther laterally than the optic tract, then the starting point becomes the dorsolateral extent of the medial surface. The outline of the amygdala then extends from this point laterally until it meets the fibers of the anterior commissure. The outline follows the white matter along the lateral surface of the amygdala to the ventricle (or dorsal surface of the hippocampus) and is then drawn medially along the alveus to the medial surface of the brain. The outline was completed along the medial surface of the amygdala.
Midrostrocaudal third of the amygdala. At midrostrocaudal levels (Fig. 2c), the amygdala is outlined in much the same way as just described. In more rostral sections (Fig. 2d), the hippocampus decreases in size and the entorhinal cortex begins to form part of the medial surface of the amygdala. At this point, a thin band of white matter separates the amygdala from the entorhinal cortex.
Outlining at this level was initiated at the dorsolateral extent of the medial surface of the brain and continued laterally to the white matter of the temporal lobe. The outline then follows the white matter along the lateral surface of the amygdala until it reaches the ventricle (or dorsal surface of the hippocampus). The outline continues medially along the alveus of the hippocampus to the white matter tract that separates the amygdala from the entorhinal cortex. The outline then follows the white matter tract to a point on the medial surface of the brain that coincides with the semiannular sulcus. The outline is then completed along the medial surface of the amygdala.
Rostral third of the amygdala. In rostral sections (Fig. 2e), the dorsomedial surface of the amygdala forms a portion of the medial surface of the brain. The amygdala is bordered laterally by white matter of the temporal lobe, ventrally by the temporal horn of the lateral ventricle and by subamygdaloid white matter, and ventromedially by the entorhinal cortex. The outline begins at the semiannular sulcus on the medial surface of the brain and continues laterally along the dorsal surface of the amygdala. It is then extended ventrally along the white matter that lines the lateral surface of the amygdala to the ventricle. The outline is then drawn medially along the white matter that forms the ventral surface of the amygdala and dorsomedially along the white matter that separates the amygdala from the entorhinal cortex until the semiannular sulcus is reached. However, the white matter that separates the amygdala from the entorhinal cortex is not always clear. In this case, a diagonal line is drawn from the most medial point of the subamygdaloid white matter that is visible to the semiannular sulcus.
At the rostral pole of the amygdala (Fig. 2f), the outlining rules are very similar to what has just been described above. However, the gray-matter-white-matter boundaries are more difficult to delineate. Therefore, it was necessary to confirm the rostral boundary of the amygdala by reviewing the outlines in sagittal images (see below) (Fig. 1c).
Editing the amygdala in horizontal and sagittal views. The outline was then reviewed systematically in the horizontal and sagittal planes (Fig. 1b,c, arrows). Two areas consistently needed revision. These included (1) the dorsolateral border of the amygdala with the putamen and (2) the rostral extent of the amygdala. The horizontal plane provided a more reliable view of the border between the amygdala and putamen. In cases in which the putamen had been included in the original outline of the amygdala, the boundary between the structures was straightened by providing a best-fit line along the white matter separating the amygdala from the putamen (Fig. 1b, arrows). This line was determined by connecting the white-matter tracts on the medial and lateral portions of the rostral pole of the putamen.
As noted above, the rostral pole of the amygdala was also difficult to define in coronal sections. Thus, the rostral border was reviewed in sagittally oriented sections. The white matter that forms the ventral border of the rostral amygdala (Fig. 1c, arrows) can be followed dorsally around the rostral limit of the amygdala and was used to correct the rostral border of the amygdaloid complex.
Finally, we again reviewed the amygdala in coronal sections to ensure that the outlines had not been erroneously altered. Once the outlines were completed, we used Analyze software to calculate the volume of the left and right amygdala.
Volumetric analysis of the hippocampus
After measurement of the amygdala, the hippocampus was outlined manually on each coronal image in which it was present based on a detailed set of tracing guidelines (see below). Before outlining the hippocampus, the z-axis for each set of images was converted to 0.938 mm slice thickness to reduce the number of images in which the hippocampus was defined.
In previous experimental studies from our laboratory, the hippocampal formation has included the dentate gyrus, the CA fields of the hippocampus, the subiculum, presubiculum, and parasubiculum, and the entorhinal cortex (Amaral, 1994). For the current study, the entorhinal cortex was not included in volume measurements, and the remaining regions (i.e., dentate gyrus, CA fields, subiculum, presubiculum, and parasubiculum) of the hippocampal formation will be referred to as the hippocampus. The fornix, fimbria, and alveus were not included in the volumetric measurements.
Two raters (C.M.S. and J.H.) who were blind to subject diagnosis manually traced the hippocampus after establishing reliability of tracing methods on MRI scans from 30 subjects with an inter-rater correlation of 0.96 for the left hippocampus and 0.97 for the right hippocampus. Each rater obtained an intrarater reliability of >0.96.
The hippocampus was initially defined in coronal sections starting with the most caudal level in which it was visible (Fig. 3). Outlines were reviewed in the horizontal and sagittal views (Fig. 1) that were simultaneously available while tracing the hippocampus. The following guidelines detail the procedures used for systematically outlining the hippocampus.
Figure 3.
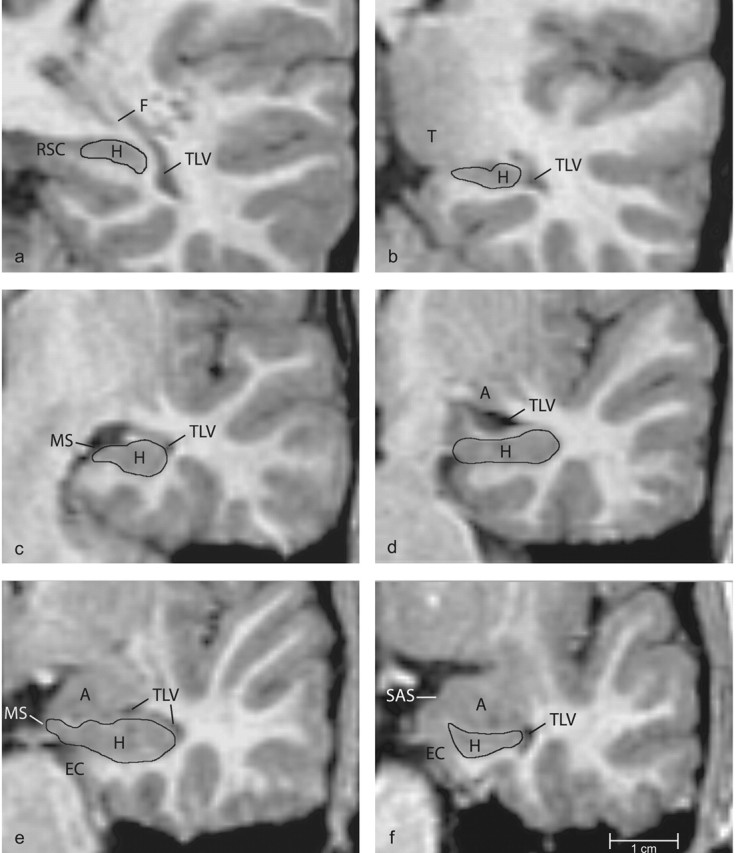
Series of coronal images arranged from caudal (a) to rostral (f), indicating boundaries of the hippocampus. A, amygdala; EC, entorhinal cortex; F, fornix; H, hippocampus; LV, Lateral ventricle; MS, medial surface of the brain; RSC, retrosplenial cortex; SAS, semiannular sulcus; T, pulvinar of the thalamus; TLV, temporal horn of the lateral ventricle.
Caudal third of the hippocampus. At its caudal extent (Fig. 3a), the hippocampus has a dorsoventral orientation and is primarily encapsulated by white matter. The retrosplenial cortex appears along the medial surface of the hippocampus and is separated from it by a thin band of white matter. The dorsal border is also formed by white matter. The hippocampus is bordered laterally by the fornix. White matter of the temporal lobe forms the ventral surface of the hippocampus. The outline at this level (Fig. 3a) started at the dorsomedial extent of the hippocampus and extended laterally to the white matter of the fornix. It extended ventrally from the fornix along the lateral surface of the hippocampus to subhippocampal white matter. The outline continued along the subhippocampal white matter to the starting point.
Proceeding rostrally (Fig. 3b), the dorsomedial extent of the hippocampus forms a portion of the medial surface of the brain. The pulvinar of the thalamus also appears along the dorsomedial surface. The ventral surface of the thalamus is separated from the dorsal surface of the hippocampus by CSF on the medial surface of the brain. The dorsolateral surface of the hippocampus is formed by the fornix. The lateral border is formed by the temporal horn of the lateral ventricle. The ventral surface of the hippocampus is formed by white matter of the temporal lobe. The outline at this level (Fig. 3b) began at the medial extent of the hippocampus, which is ventral to the thalamus. It was extended laterally along the dorsal surface of the hippocampus until it reached the fornix. The outline continued ventrally along the temporal horn of the lateral ventricle to the subhippocampal white matter and continued medially along the white matter to the starting point.
Midrostrocaudal third of the hippocampus. In the body of the hippocampus (Fig. 3c), the medial surface of the hippocampus forms the medial surface of the brain. At this level, white matter (the alveus) makes up the dorsal surface of the hippocampus. A small section of the temporal horn of the lateral ventricle may be visible along the dorsolateral surface of the hippocampus. The ventral border is formed by subhippocampal white matter. The starting point of the outline at this level (Fig. 3c) is the medial extent of the hippocampus on the medial surface of the brain. The outline was drawn laterally along the dorsal surface of the hippocampus, ventral to the alveus, to the temporal horn of the lateral ventricle. The outline continued ventrally and then medially along the white matter of the temporal lobe to the starting point.
Proceeding rostrally (Fig. 3d), the hippocampus is outlined in much the same way as just described. The starting point for the outline at this level (Fig. 3d) was the medial surface of the hippocampus. The outline was drawn dorsally along the medial surface of the hippocampus to the alveus. The outline continued laterally along the dorsal surface of the hippocampus until it reached either the temporal horn of the lateral ventricle or the white matter of the temporal lobe. The outline was then drawn ventrally along the lateral ventricle or temporal lobe white matter to the subhippocampal white matter. It was completed medially along the white matter to the medial surface of the brain.
Rostral third of the hippocampus. In rostral sections (Fig. 3e), the hippocampus forms part of the medial surface of the brain and is limited dorsally by the alveus, laterally by the temporal horn of the lateral ventricle, and ventrally by white matter of the temporal lobe. The outline (Fig. 3e) was started at the medial surface of the brain and extended laterally along the alveus to the temporal horn of the lateral ventricle. The outline was continued ventrally along the lateral ventricle to the subhippocampal white matter. The outline was completed by tracing medially along the subhippocampal white matter to the medial surface of the brain.
At its rostral extent (Fig. 3f), the hippocampus decreases in size and is mainly subiculum. The entorhinal cortex begins to form part of the medial surface of the hippocampus. The two structures are separated by a band of fibers extending from the subhippocampal white matter to the semiannular sulcus on the medial surface of the brain. The dorsal surface of the hippocampus often appears to be fused with the ventral surface of the amygdala at this level, but the two structures are separated by the alveus or by a thin portion of the lateral ventricle. The hippocampus continues to be bordered laterally by the temporal horn of the lateral ventricle and ventrally by white matter of the temporal lobe. The outline (Fig. 3f) began at the dorsomedial extent of the hippocampus. This point is found at the junction of the alveus and the band of fibers extending from the subhippocampal white matter toward the semiannular sulcus. From this point, the outline continued laterally along the alveus, then ventrally along the temporal horn of the lateral ventricle, until the subhippocampal white matter was reached. The outline was completed medially along the white matter, which separates the hippocampus from the entorhinal cortex, to the starting point. Once the outlines were completed, we used the Analyze software package to calculate the volumes of the left and right hippocampus.
Total cerebral volume measurement
To obtain a measure of total cerebral volume, each series of images was edited manually to remove nonbrain structures, the brainstem, and the cerebellum. Using a Gaussian cluster multispectral thresholding tool, the ventricles were defined and excluded. The remaining brain tissue was considered to be a measure of total cerebral volume. Two raters established reliability with an inter-rater reliability correlation of 0.96. Total cerebral volume was then measured with Analyze software.
Statistical analyses
Differences between study participant diagnostic groups for age, IQ scores, and unadjusted amygdala, hippocampal, and total cerebral volume were tested by ANOVA using Statistical Program for the Social Sciences Edition 12.0 statistical software (SPSS, Chicago, IL). Post hoc comparisons were performed using a Tukey test to identify differences between groups. Using an ANCOVA, a comparison was performed to explore effects of amygdala and hippocampal volume after adjusting for covariates such as MRI site (University of California Davis vs Stanford University), total cerebral volume, and age. In a separate analysis, performance IQ was used as a covariate for analyzing the HFA, ASP, and CON groups. The LFA group was not included in this analysis, because performance IQ was not acquired. Full-scale IQ was not used as a covariate because (1) a score of <70 is inherently part of the diagnosis for LFA and (2) it includes a verbal IQ score and verbal ability is taken into account when distinguishing HFA from ASP. A Pearson correlation was performed to detect a potential relationship of amygdala and hippocampal volume to the age of the participant at the time of MRI.
Results
Age and IQ measures: all subjects
Participant demographic information was analyzed for the four clinically defined diagnostic groups (Table 1). There was no difference in the age of the groups at the time of MRI acquisition. However, there was a significant group effect for full-scale IQ (F = 58.7; p < 0.01). As would be expected, post hoc tests found that the full-scale IQ for LFA was lower than all other groups (p < 0.01). The full-scale IQ for HFA was lower than for ASP (p < 0.05) and for CON (p < 0.01). There was no difference in full-scale IQ between the ASP and CON groups.
Table 1.
Mean diagnostic data for all male participants 7.5-18.5 years of age
|
|
LFA (n = 18) |
HFA (n = 21) |
ASP (n = 24) |
CON (n = 22) |
|---|---|---|---|---|
| Age (years) at time of MRI | 13.1 ± 3.0 | 12.7 ± 3.5 | 13.0 ± 2.9 | 13.1 ± 3.1 |
| Full-scale IQa | 56 ± 10b | 91 ± 15b | 106 ± 20 | 115 ± 11 |
| Verbal IQ | NA | 86 ± 18b | 111 ± 21 | 112 ± 14 |
| Performance IQ |
NA |
99 ± 17 |
106 ± 28 |
114 ± 12 |
NA, Not applicable.
LFA subjects were given a nonverbal Leiter IQ exam; all other subjects were given the Wechsler Abbreviated Scale of Intelligence or Wechsler Intelligence Scale for Children exam.
Significantly different from controls at p < 0.01.
We found a significant group effect (F = 14.1; p < 0.01) when verbal IQ was evaluated in the HFA, ASP, and CON groups. Verbal IQ was lower in the HFA group than in the ASP and CON groups (p < 0.01). There was no difference in verbal IQ between ASP and CON. There was a trend for a group effect for performance IQ (F = 2.8; p = 0.06).
Volumetric measures: all subjects
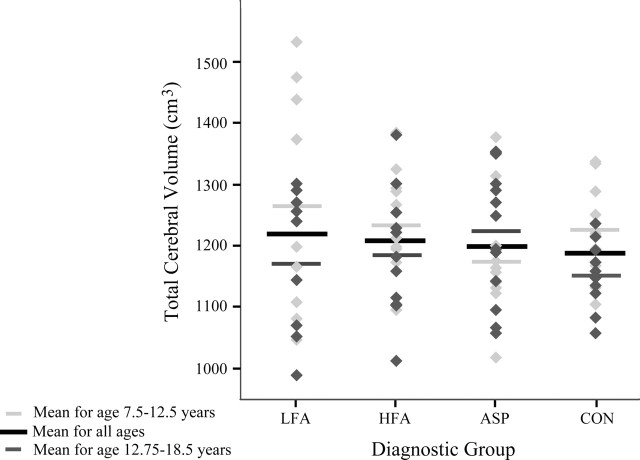
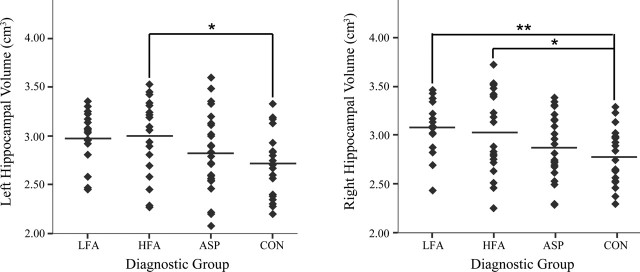
Mean volumetric data for all participants are given in Table 2. There was no difference across diagnostic groups in terms of total cerebral volume (Fig. 4). However, there was a significant main group effect for absolute right (F = 3.8; p < 0.05) amygdala volume. Post hoc tests indicated that the LFA group had a larger right amygdala volume than the CON group (p < 0.05). There was also a significant main group effect for absolute right (F = 5.4; p < 0.01) and left (F = 3.5; p < 0.05) hippocampal volume (Fig. 5). Post hoc tests indicated that the LFA group had a larger right hippocampal volume than CON subjects (p < 0.01). There was a trend for the left hippocampus to be larger in the LFA group than in the CON group (p = 0.06). HFA subjects had larger right and left hippocampal volumes than the CON groups (p < 0.05). When MRI site (i.e., University of California Davis vs Stanford University) was considered as a covariate for analyzing amygdala or hippocampal volumes, no diagnostic group-by-site interactions were found. Therefore, the MRI site was not included as a covariate in additional analyses. When total cerebral volume was included as a covariate, there was no diagnostic group-by-total cerebral volume interaction, and the volume of the right amygdala remained significantly larger in the LFA group versus the CON group (p < 0.05). Right hippocampal volume also remained significantly larger in the HFA and LFA groups than in the CON group (p < 0.05). When performance IQ was considered as a covariate, the right hippocampus remained significantly larger in the HFA group compared with the CON group (p < 0.05).
Table 2.
Mean volumetric (cm3 ± SD) data for all male participants 7.5-18.5 years of age
|
|
LFA (n = 18) |
HFA (n = 21) |
ASP (n = 24) |
CON (n = 22) |
|---|---|---|---|---|
| Right amygdala volume | 2.20 ± 0.22* | 2.13 ± 0.18 | 2.08 ± 0.28 | 1.96 ± 0.30 |
| Left amygdala volume | 2.09 ± 0.27 | 2.06 ± 0.20 | 2.01 ± 0.20 | 1.94 ± 0.30 |
| Right hippocampal volume | 3.11 ± 0.29** | 3.06 ± 0.43* | 2.87 ± 0.32 | 2.75 ± 0.30 |
| Left hippocampal volume | 2.99 ± 0.27 | 3.00 ± 0.38* | 2.83 ± 0.41 | 2.71 ± 0.32 |
| Total cerebral volume |
1224 ± 158 |
1214 ± 97 |
1204 ± 103 |
1190 ± 77 |
Significantly different from controls at p < 0.05.
Significantly different from controls at p < 0.01.
Figure 4.
Total cerebral volume (in cubic centimeters) for subjects 7.5-18.5 years of age by diagnostic group.
Figure 5.
Absolute left (a) and right (b) hippocampal volume (in cubic centimeters) by diagnostic group for subjects 7.5-18.5 years of age (*p < 0.05; **p < 0.01).
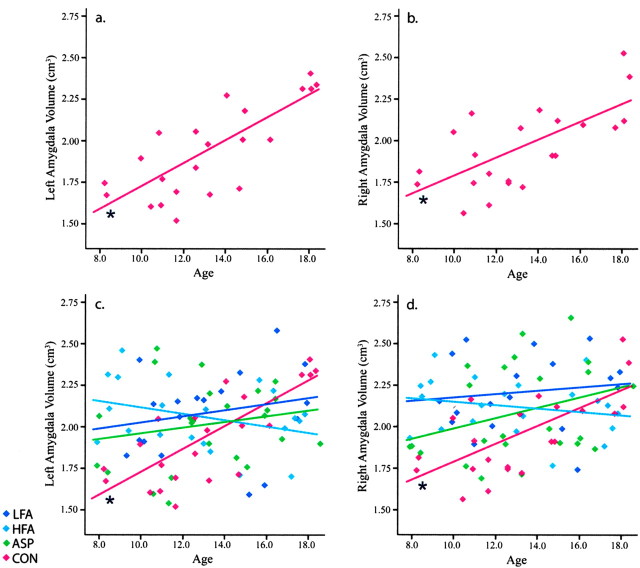
Age was considered as a covariate to determine whether the volume of the amygdala or hippocampus was associated with the age of the participant. There was a significant diagnosis-by-age interaction for right (p < 0.05) and left (p < 0.01) amygdala volume (LFA: right, r = -0.00, left, r = 0.40; HFA: right, r = 0.23, left, r = 0.26; ASP: right, r = 0.16, left, r = 0.24; CON: right, r = -0.09, left, r = -0.07) but not for the hippocampus. Correlation analyses for age and amygdala volume were also performed for each diagnostic group (Fig. 6). Age did not correlate with right or left amygdala volume in the LFA (right, r = 0.14; left, r = 0.21), HFA (right, r = -0.21; left, r = -0.32), or ASP (right, r = 0.32; left, r = 0.20) groups. However, in the CON group, age significantly correlated with right (r = 0.67; p < 0.05) and left (r = 0.77; p < 0.05) amygdala volume.
Figure 6.
Linear regression scatterplot for absolute amygdala volume (in cubic centimeters) by age. Typically developing subjects show a positive correlation (*p < 0.05) of age with amygdala volume for both the left amygdala [a; CON, r2 = 0.59] and right amygdala [b; CON, r2 = 0.45]. Amygdala volume in participants with autism was not correlated with age. c, Left amygdala: LFA, r2 = 0.05; HFA, r2 = 0.10; ASP, r2 = 0.04. d, Right amygdala: LFA, r2 = 0.02; HFA, r2 = 0.05; ASP, r2 = 0.10.
Given the observation that the volume of the amygdala appears to be increasing with age from 7.5 to 18.5 years in typically developing children, we performed additional analyses of the amygdala by dividing the study groups into two age ranges: 7.5-12.5 years of age and 12.75-18.5 years of age (see below). When the control group is broken up into participants in these two age ranges, the volume of the right and left amygdala in the older group is significantly greater than in the younger group (right: younger mean ± SD, 1.81 ± 0.18 cm3, older mean ± SD, 2.10 ± 0.22 cm3, F = 11.8, p < 0.01; left: younger mean ± SD, 1.77 ± 0.18 cm3, older mean ± SD, 2.11 ± 0.25 cm3, F = 13.6, p < 0.01). In no other group was this difference significant. For total cerebral volume, the older control group is significantly smaller than the younger control group (younger mean ± SD, 1231 ± 78 cm3; older mean ± SD, 1150 ± 99 cm3; F = 7.9; p < 0.05). There was no difference in total cerebral volume between the younger and older children with autism or Asperger syndrome.
Analyses of 7.5- to 12.5-year-old subjects
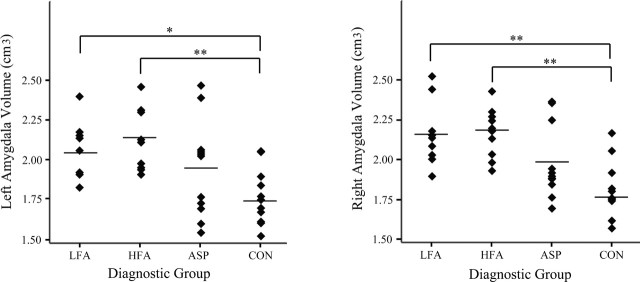
Amygdala and cerebral mean volumetric data for male participants in the 7.5- to 12.5-year-old age range are given in Table 3. When only subjects in this age range were considered, there was a main group effect for the volume of the right amygdala (F = 10.8; p < 0.01) and for the volume of the left amygdala (F = 6.4; p < 0.01). Post hoc tests indicated that the volumes of the right amygdala in the LFA and HFA groups were significantly larger than in the CON group (p < 0.01) (Fig. 7). There was also a trend for the volume of the right amygdala to be larger in the ASP group relative to the CON group (p = 0.06). The volume of the left amygdala in the LFA and HFA groups was larger than in the CON group (p < 0.05 and p < 0.01, respectively).
Table 3.
Amygdala and cerebral mean volumetric (cm3 ± SD) data for male participants 7.5-12.5 years of age
|
|
LFA (n = 9) |
HFA (n = 10) |
ASP (n = 11) |
CON (n = 11) |
|---|---|---|---|---|
| Right amygdala volume | 2.16 ± 0.20** | 2.17 ± 0.15** | 1.98 ± 0.23 | 1.81 ± 0.18 |
| Left amygdala volume | 2.04 ± 0.19* | 2.14 ± 0.19** | 1.94 ± 0.31 | 1.77 ± 0.18 |
| Total cerebral volume |
1269 ± 186 |
1243 ± 85 |
1181 ± 96 |
1231 ± 78 |
Significantly different from controls at p < 0.05.
Significantly different from controls at p < 0.01.
Figure 7.
Absolute left (a) and right (b) amygdala volume (in cubic centimeters) by diagnostic group for subjects 7.5-12.5 years of age (*p < 0.05; **p < 0.01).
There was no significant main effect for total cerebral volume in the 7.5-12.5 age range (Fig. 4). It is interesting to note, however, that for LFA, the estimates of total cerebral volume seemed to partition the group into those whose brains were approximately similar in volume to the controls and a group with brain volumes that were larger and outside of the range of typically developing children. When total cerebral volume was included as a covariate, LFA and HFA right amygdala volume remained significantly different from CON (p < 0.01). For the left amygdala volume, both the LFA group and the HFA group also remained significantly different from the CON group (p < 0.05). Covarying the performance IQ with amygdala volume produced the same pattern of results.
Analysis of 12.75- to 18.5-year-old subjects
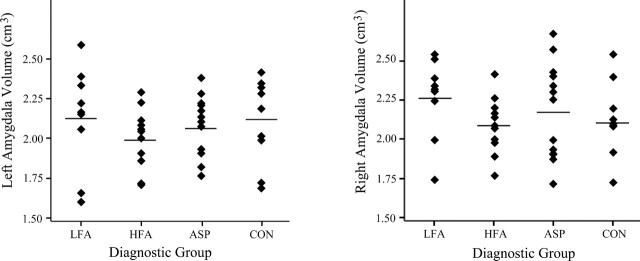
Amygdala and cerebral mean volumetric data for male participants in the 12.75- to 18.5-year-old age range are given in Table 4. When this older subject group was analyzed, there was no main group effect for right or left amygdala volume (Fig. 8) or total cerebral volume (Fig. 4). There were no significant group differences even when total cerebral volume or performance IQ was included as a covariate.
Table 4.
Amygdala and cerebral mean volumetric cm3 ± SD) data for male participants 12.75-18.5 years of age
|
|
LFA (n = 9) |
HFA (n = 11) |
ASP (n = 13) |
CON (n = 11) |
|---|---|---|---|---|
| Right amygdala volume | 2.26 ± 0.25 | 2.08 ± 0.18 | 2.17 ± 0.30 | 2.10 ± 0.22 |
| Left amygdala volume | 2.12 ± 0.32 | 1.99 ± 0.19 | 2.06 ± 0.19 | 2.11 ± 0.25 |
| Total cerebral volume |
1180 ± 118 |
1188 ± 104 |
1224 ± 108 |
1150 ± 99 |
Figure 8.
Absolute left (a) and right (b) amygdala volume (in cubic centimeters) by diagnostic group for subjects 12.75-18.5 years of age.
Discussion
We used structural MRI to analyze the volume of the amygdala, hippocampus, and total cerebrum in male children 7.5-18.5 years of age. There were four subgroups: children with autism and with mental retardation, children with autism without mental retardation, children with Asperger syndrome, and typically developing controls.
The amygdala in autism
One striking finding was that the amygdala in typically developing children increased in size from ∼1.7 cm3 at age 8 to ∼2.3 cm3 at age 18, representing a nearly 40% increase over this age range. This growth occurred in the context of a slight decrease in overall cerebral volume. Our cross-sectional findings closely replicate structural MRI results from Giedd (1997) and Giedd et al. (1996), who reported that the amygdala increases in volume by 50% in typically developing males (but not females) from 4 to 18 years of age with little change in overall cerebral volume.
In our clinical populations, we found that younger children with autism plus mental retardation had a 16% larger right amygdala and a 13% larger left amygdala than typically developing controls. Younger children with autism but without mental retardation also had a 17% larger right and left amygdala than controls. These findings indicate not only that the amygdala in autism is initially larger than typically developing controls but also that the increase is related to autism rather than mental retardation. An enlarged amygdala in autism is not paralleled by an overall enlarged brain, because there were no group differences in cerebral volume.
Our results extend previous findings by Sparks et al. (2002) pertaining to children 3-4 years of age. The amygdala in male children with an autism spectrum disorder was 14% larger on the left and 22% larger on the right than in typically developing children of the same age, and the right amygdala remained significantly different when corrected for total cerebral volume.
In our older group of children with autism, there was no difference in either amygdala or cerebral volume. Thus, it appears that the amygdala in children with autism is initially larger than normal but does not undergo the age-related increase in volume that takes place in typically developing children. These findings help explain variability in previous structural MRI studies of autism. Studies that focus on young children (Sparks et al., 2002) have found that the amygdala is larger in autism. However, studies on adults or on a wide age range find that the autistic amygdala is no different (Haznedar et al., 2000) or even possibly smaller (Aylward et al., 1999; Pierce et al., 2001) than controls. These data are entirely consistent with the results of the present study.
Our data also indicate that the magnitude of amygdala enlargement relates to the clinical diagnosis. We found that young children with autism on average have a 16% larger amygdala than controls, whereas young children with Asperger syndrome have a 9% larger amygdala than controls. The hypothesis that abnormal amygdala volume may be more pronounced in autism relative to other diagnoses on the autistic spectrum is again consistent with the findings of Sparks et al. (2002). They found that when the autism spectrum group was differentiated into autism and pervasive developmental disorder-not otherwise specified (PDD-NOS), the children with autism had an even larger amygdala than those with PDD-NOS. In children with an autism diagnosis, the amygdala was 14% larger on the right and 12% larger on the left compared with children with a PDD-NOS diagnosis.
We found that the amygdala of older children with autism is approximately the same size as older typically developing children. However, the amygdala of children with autism reaches adult size before adolescence, whereas typically developing children undergo a progressive growth of the amygdala through adolescence. We would predict that although the amygdala is of equal size, fundamental aspects of its neuroanatomical or functional organization are different in children with autism compared with typically developing controls. Many factors contribute to brain volume, including number and size of neurons and glial cells, number and collateralization of afferent and efferent fibers, myelination, and even the density of vasculature. These factors are affected by various influences, including genetics, growth factors, hormones, nutrients, and environmental stimulation of the developing nervous system (for review, see McAllister, 2000). Although Bauman and Kemper (1985) observed increased packing density of neurons in the amygdala, there have been no quantitative studies published to date that provide accurate estimates of the number of neurons in the normal and autistic amygdala. Thus, the cause of the enlarged amygdala in autism is currently unknown.
If the amygdala does develop abnormally in autism, what behavioral symptoms might be expected? The amygdala has been implicated in the mediation of social behavior (Brothers et al., 1990) and many other cognitive processes in humans. These include face processing (Grelotti et al., 2002; Haxby et al., 2002), recognition of emotions (Adolphs, 2002; Adolphs et al., 2003), enhancement of memory for emotionally significant events (Cahill et al., 1995; Canli et al., 2000), and predicting reward values (Gottfried et al., 2003). This has lead some to suggest that the amygdala might be the primary structure responsible for the social impairments in autism (Baron-Cohen et al., 2000). However, studies of human and nonhuman primates with amygdala lesions argue against this conclusion (Amaral et al., 2003). Human patients with Urbach-Wiethe, a disease that results in destruction of the amygdala, do not display core autistic symptomotology. In addition, nonhuman primates that sustained amygdala damage early in development are able to produce species-typical social behaviors (Prather et al., 2001). The view from our animal studies, which is consistent with human lesion studies, is that dysfunction of the amygdala is not responsible for the core social deficits of autism.
There is an abundance of evidence from animal (LeDoux, 2000; Davis et al., 2003) and human (Adolphs et al., 1994, 1995; Buchel and Dolan, 2000) studies to implicate the amygdala in the detection of danger and the production of fear and anxiety. In fact, children with generalized anxiety disorder have a 16% larger right amygdala than typically developing controls (De Bellis et al., 2000). The presence of anxiety has been noted in descriptions of autism (Wing and Gould, 1979; American Psychiatric Association, 1994), and recent studies suggest that anxiety is a common feature of the autism spectrum disorders (Muris et al., 1998). Abnormal processing of fear during development may contribute to behavioral symptoms seen in autism. The role of the amygdala in processing stimuli related to potential threat may extend to complex judgments on whether to approach or trust other people, a function in which both human patients with amygdala lesions and individuals with autism are impaired (Adolphs et al., 1998, 2001).
The hippocampus in autism
We found that male children with low-functioning autism have a 12% larger right hippocampus and a 9% larger left hippocampus relative to age- and sex-matched typically developing children. Male children with high-functioning autism have a 10% larger right and left hippocampus than typically developing controls. Consistent with Giedd (1997) and Giedd et al. (1996), we also found that the hippocampus does not increase in size through adolescence in typically developing controls or in children on the autistic spectrum.
There have been relatively few studies of autism to date that have published volumetric analyses of the hippocampal formation. In general, studies focusing primarily on adults have found no difference in hippocampal volume between autism and control subjects (Piven et al., 1998; Haznedar et al., 2000; Howard et al., 2000). One exception is Aylward et al. (1999), who reported a decrease in hippocampal volume in adolescents and adults with autism after controlling for total cerebral volume. Saitoh et al. (2001) measured the cross-sectional area of the dentate gyrus and CA4 in three contiguous 5 mm sections and found that it was smaller than normal in children and adults with autism, with the most significant difference in subjects 2-4 years of age.
Sparks et al. (2002) have published the only study of hippocampal volume that focused on young children. They found that the right and left hippocampus of their cohort of male children 3 and 4 years of age with an autism spectrum disorder was 9% larger than typically developing controls. Our results extend these findings through late childhood and adolescence.
An enlarged hippocampal formation in autism may be a precursor to, or a consequence of, autistic symptomotology. The increased size could result from pathological development or experience-dependent increase of function. The smaller size and increased packing density of neurons reported by Bauman and Kemper (1994) suggest that development of the autistic hippocampal formation has been disrupted, perhaps because of reduced programmed cell death. This could be confirmed by using stereological techniques to count the total number of neurons in the postmortem autistic hippocampus. Another possibility is that the increased size of the hippocampus indicates a use-dependent expansion of hippocampal connections. There is substantial evidence in the animal literature that the volume of the hippocampus is correlated with spatial memory function. Species that develop complex spatial maps, such as food-caching birds, have larger hippocampi than members of the same species who do not engage in food caching (Clayton and Krebs, 1994). Similar data have been demonstrated for London taxi-cab drivers who must memorize the complex roadway system of the city (Maguire et al., 2000, 2003). If the larger size of the autistic hippocampal formation was evidence of use-dependent enlargement, one might expect enhanced spatial or episodic memory function in autism. This possibility has not yet been fully explored, but there is some evidence that this might be the case.
Several studies have shown that declarative memory function is fairly normal in higher-functioning autistic subjects (Ameli et al., 1988; Rumsey and Hamburger, 1988; Bennetto et al., 1996; Minshew and Goldstein, 2001). Dawson et al. (1998) have studied visual object recognition in children with autism using “delayed nonmatching to sample” (DNMS) and found that the children with autism were impaired on DNMS. Recently, however, Dawson et al. (2001) performed a “paired comparison task” study to selectively test object recognition without requiring the child to form a stimulus-reward association (Diamond, 1995) and found that children with autism were not impaired.
In contrast to the notion that autism may be associated with impaired hippocampal memory function, Kanner (1943) originally described children with autism as having an extraordinary ability to learn geography and recite long lists of items or facts. Recently, Caron et al. (2004) found that individuals with autism are not only equally capable of learning a route on a map but are superior to typically developing controls on the speed and accuracy at which they memorize and reproduce the map. These studies support the hypothesis that an abnormally large hippocampus in autism may result from or yield enhanced function. This hypothesis is in need of comprehensive assessment.
Conclusions
The present study provides additional evidence that the amygdala and hippocampus are structurally abnormal in autism. It will be of interest to determine whether an enlarged amygdala and hippocampus are characteristic of all children with autism or distinguish a particular phenotype. Understanding the genesis and functional implications of an early enlargement may be amenable to molecular neurobiological approaches using animal models. The consequences of an abnormal amygdala and hippocampus in autism, and how these might be effectively treated, will be elucidated by both animal and human studies on the normal function of these structures.
Footnotes
This work was supported by National Institutes of Health Grants MH41479, MH01832, MH01142, MH50047, NS16980, and HD31715 and by the M.I.N.D. Institute. We thank Meridith Brandt for participant recruitment and scheduling at University of California Davis and John Ryan for assistance in carrying out MRI acquisition. We also thank the study participants and their families for their contribution.
Correspondence should be addressed to Dr. David G. Amaral, University of California Davis, M.I.N.D. Institute, 2825 50th Street, Sacramento, CA 95817. E-mail: dgamaral@ucdavis.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/246392-10$15.00/0
References
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U (1999) The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. NeuroReport 10: 1647-1651. [DOI] [PubMed] [Google Scholar]
- Adolphs R (2002) Neural systems for recognizing emotion. Curr Opin Neurobiol 12: 169-177. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A (1994) Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372: 669-672. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR (1995) Fear and the human amygdala. J Neurosci 15: 5879-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR (1998) The human amygdala in social judgment. Nature 393: 470-474. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Sears L, Piven J (2001) Abnormal processing of social information from faces in autism. J Cogn Neurosci 13: 232-240. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR (2003) Dissociable neural systems for recognizing emotions. Brain Cogn 52: 61-69. [DOI] [PubMed] [Google Scholar]
- Amaral DG (1994) Hippocampal formation. In: Encyclopedia of human behavior, Vol II (Ramachandran V, ed), pp 509-515. Los Angeles: Academic. [Google Scholar]
- Amaral DG, Bauman MD, Schumann CM (2003) The amygdala and autism: implications from non-human primate studies. Genes Brain Behav 2: 295-302. [DOI] [PubMed] [Google Scholar]
- Ameli R, Courchesne E, Lincoln A, Kaufman AS, Grillon C (1988) Visual memory processes in high-functioning individuals with autism. J Autism Dev Disord 18: 601-615. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, Ed 4. Washington, DC: American Psychiatric Association.
- Asperger H (1944) Die “autistischen Psychopathen” im Kindesalter. Arch Psychiatr Nervenkr 117: 76-136. [Google Scholar]
- Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD (1999) MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 53: 2145-2150. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC (2000) The amygdala theory of autism. Neurosci Biobehav Rev 24: 355-364. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35: 866-874. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL (1994) Neuroanatomic observations of the brain in autism. In: The neurobiology of autism (Bauman M, Kemper TL, eds), pp 119-145. Baltimore: Johns Hopkins UP.
- Bennetto L, Pennington BF, Rogers SJ (1996) Intact and impaired memory functions in autism. Child Dev 67: 1816-1835. [PubMed] [Google Scholar]
- Brothers L, Ring B, Kling A (1990) Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res 41: 199-213. [DOI] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ (2000) Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol 10: 219-223. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL (1995) The amygdala and emotional memory. Nature 377: 295-296. [DOI] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L (2000) Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci 20: RC99(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron MJ, Mottron L, Rainville C, Chouinard S (2004) Do high functioning persons with autism present superior spatial abilities? Neuropsychologia 42: 467-481. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Krebs JR (1994) Hippocampal growth and attrition in birds affected by experience. Proc Natl Acad Sci USA 91: 7410-7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody H, Pelphrey K, Piven J (2002) Structural and functional magnetic resonance imaging of autism. Int J Dev Neurosci 20: 421-438. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM (2003) Role of the amygdala in fear extinction measured with potentiated startle. Ann NY Acad Sci 985: 218-232. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J (1998) Neuropsychological correlates of early symptoms of autism. Child Dev 69: 1276-1285. [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Osterling J, Rinaldi J, Carver L, McPartland J (2001) Brief report: recognition memory and stimulus-reward associations: indirect support for the role of ventromedial prefrontal dysfunction in autism. J Autism Dev Disord 31: 337-341. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, Axelson DA, Frustaci K, Boring AM, Hall J, Ryan ND (2000) A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry 48: 51-57. [DOI] [PubMed] [Google Scholar]
- Diamond A (1995) Evidence of robust recognition memory early in life even when assessed by reaching behavior. J Exp Child Psychol 59: 419-456. [DOI] [PubMed] [Google Scholar]
- DiLavore PC, Lord C, Rutter M (1995) The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord 25: 355-379. [DOI] [PubMed] [Google Scholar]
- Fombonne E (2003) Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 33: 365-382. [DOI] [PubMed] [Google Scholar]
- Giedd JN (1997) Normal development. In: Child and adolescent psychiatric clinics of North America neuroimaging (Peterson BS, ed), pp 265-282. Philadelphia: Saunders.
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL (1996) Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. J Comp Neurol 366: 223-230. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ (2003) Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 301: 1104-1107. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT (2002) Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev Psychobiol 40: 213-225. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI (2002) Human neural systems for face recognition and social communication. Biol Psychiatry 51: 59-67. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, Hollander E (2000) Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry 157: 1994-2001. [DOI] [PubMed] [Google Scholar]
- Howard MA, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N (2000) Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. NeuroReport 11: 2931-2935. [DOI] [PubMed] [Google Scholar]
- Howlin P (2003) Outcome in high-functioning adults with autism with and without early language delays: implications for the differentiation between autism and Asperger syndrome. J Autism Dev Disord 33: 3-13. [DOI] [PubMed] [Google Scholar]
- Kanner L (1943) Autistic disturbances of affective contact. Nerv Child 2: 217-250. [PubMed] [Google Scholar]
- Klin A, Volkmar FR, Sparrow SS, Cicchetti DV, Rourke BP (1995) Validity and neuropsychological characterization of Asperger syndrome: convergence with nonverbal learning disabilities syndrome. J Child Psychol Psychiatry 36: 1127-1140. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23: 155-184. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A (1994) Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24: 659-685. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook Jr EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M (2000) The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30: 205-223. [PubMed] [Google Scholar]
- Lotspeich LJ, Kwon H, Schumann CM, Fryer SL, Goodlin-Jones BL, Buonocore MH, Lammers CR, Amaral DG, Reiss AL (2004) Investigation of neuroanatomical differences between autism and Asperger syndrome. Arch Gen Psychiatry 61: 291-298. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000) Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97: 4398-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N (2003) Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus 13: 250-259. [DOI] [PubMed] [Google Scholar]
- McAllister AK (2000) Cellular and molecular mechanisms of dendrite growth. Cereb Cortex 10: 963-973. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G (2001) The pattern of intact and impaired memory functions in autism. J Child Psychol Psychiatry 42: 1095-1101. [DOI] [PubMed] [Google Scholar]
- Muris P, Steerneman P, Merckelbach H, Holdrinet I, Meesters C (1998) Comorbid anxiety symptoms in children with pervasive developmental disorders. J Anxiety Disord 12: 387-393. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, South M, Miller JN (2000) DSM-IV defined Asperger syndrome: cognitive, behavioral, and early history differentiation from high-functioning autism. Autism 4: 29-46. [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E (2001) Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain 124: 2059-2073. [DOI] [PubMed] [Google Scholar]
- Piven J, Bailey J, Ranson BJ, Arndt S (1998) No difference in hippocampus volume detected on magnetic resonance imaging in autistic individuals. J Autism Dev Disord 28: 105-110. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, Amaral DG (2001) Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience 106: 653-658. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC (1989) Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph 13: 433-454. [DOI] [PubMed] [Google Scholar]
- Roid GH, Miller LJ (1997) Leiter International Performance Scale-Revised. Wood Dale, IL: Stoelting.
- Rumsey JM, Hamburger SD (1988) Neuropsychological findings in high-functioning men with infantile autism, residual state. J Clin Exp Neuropsychol 10: 201-221. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Karns CM, Courchesne E (2001) Development of the hippocampal formation from 2 to 42 years: MRI evidence of smaller area dentata in autism. Brain 124: 1317-1324. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR (2002) Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59: 184-192. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1991) Wechsler Intelligence Scale for Children, Ed 3. San Antonio, TX: The Psychological Corporation.
- Wechsler D (1999) Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation.
- Wing L, Gould J (1979) Severe impairments of social interaction and associated abnormalities in children: epidemiology and classification. J Autism Dev Disord 9: 11-29. [DOI] [PubMed] [Google Scholar]