Abstract
Presynaptic calcium channels play a central role in chemical synaptic transmission by providing the calcium trigger for evoked neurotransmitter release. These voltage-gated calcium channels are composed of a primary structural subunit, α1, as well as auxiliary β and α2δ subunits. Our previous genetic, molecular, and functional analysis has shown that the cacophony (cac) gene encodes a primary presynaptic calcium channel α1 subunit in Drosophila. Here we report that transgenic expression of a cac-encoded α1 subunit fused with enhanced green fluorescent protein efficiently rescues cac lethal mutations and allows in vivo analysis of calcium channel localization at active zones. The results reported here further characterize the primary role of cac-encoded calcium channels in neurotransmitter release. In addition, these studies provide a unique genetic tool for live imaging of functional presynaptic calcium channels in vivo and define a molecular marker for immunolocalization of other presynaptic proteins relative to active zones. These findings are expected to facilitate additional analysis of synaptic development and function in this important model system.
Keywords: active zone, cacophony, Drosophila, calcium channel, synaptic, GFP
Introduction
Previous studies have described localization of presynaptic calcium channel α1 subunits at active zones, where synaptic vesicles dock and fuse with the plasma membrane, and have considered its important functional implications for neurotransmitter release (for review, see Stanley, 1997; Catterall, 1998). The primary vertebrate presynaptic calcium channels are of the N- and P/Q-type for which the α1 subunits are encoded by the α1B and α1A genes, respectively. Active zone localization of these channels has been shown at native synapses using labeled neurotoxins and calcium channel antibodies (Robitaille et al., 1990; Westenbroek et al., 1995) and in cultured neurons through the use of an epitope tag (Maximov and Bezprozvanny, 2002). Despite this progress, to date, no genetic tools have been available for live imaging of calcium channel localization in vivo. Here we report generation of such a tool and its use in analysis of presynaptic calcium channel distribution and function in Drosophila.
The Drosophila cacophony (cac) locus was originally defined by its role in male courtship behavior (von Schilcher, 1977; Chan et al., 2002) and subsequently found to encode a voltage-gated calcium channel α1 subunit related to those of vertebrate presynaptic calcium channels (Smith et al., 1996). More recently, isolation and analysis of a cac mutant exhibiting rapid temperature-sensitive paralysis has demonstrated that cac encodes a primary presynaptic calcium channel in Drosophila (Dellinger et al., 2000; Kawasaki et al., 2000, 2002). To further examine the role of this calcium channel gene product in neurotransmitter release, we used transgenic methods to express enhanced green fluorescent protein (EGFP)-tagged cac-encoded α1 subunits in vivo.
Materials and Methods
Fly stocks. As described previously (Kawasaki et al., 2002), fly stocks included the cac lethal alleles [l(1)L1320-3 and l(1)L13HC129], the X-linked elav-GAL4 enhancer trap line [P(w+) elavC155], elav-GAL4 l(1)L1320-3, and elav-GAL4 l(1)L13HC129. Wild-type flies were Canton S. Stocks and crosses for this study were maintained at 25°C.
cac1-EGFP fusion construct. Separate PCR products corresponding to the EGFP open reading frame (ORF) and the 3′ end of the cac ORF (excluding the stop codon) were generated using Pfu DNA polymerase (Stratagene, La Jolla, CA) with primers designed to include flanking EcoRI sites. These products were used to generate a fusion construct in which a two amino acid linker (corresponding to the EcoRI site) and the EGFP ORF were appended to the 3′ end of the cac ORF (see Fig. 1 A). The final fusion construct was incorporated into the transformation vector pUAST (Brand and Perrimon, 1993), and transgenic flies were generated as described previously (Kawasaki et al., 2002).
Figure 1.
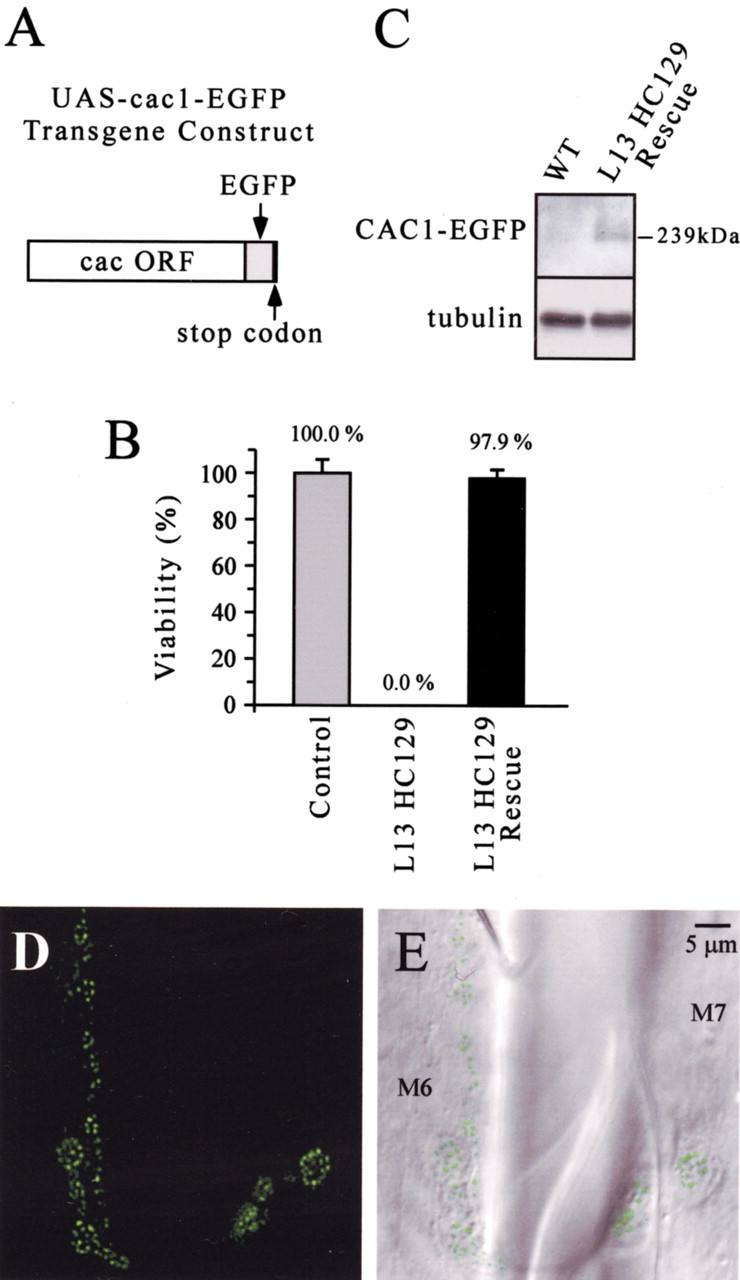
Transformation rescue and live imaging of EGFP-tagged calcium channels. A, The UAS-cac1-EGFP transgene construct for expression of EGFP-tagged cac-encoded α1 subunits. B, Rescue of a cac lethal mutant. Males carrying a cac lethal mutation (L13 HC129) but lacking the transgene exhibited uniform lethality (0% viability); however, cac lethal males carrying the transgene (L13 HC129 Rescue) were clearly rescued and exhibited adult viability similar to that of wild-type controls. Adult viability was determined in five independent rescue experiments for each genotype (n = 5). Viability of L13HC129 rescue males was not significantly different from that of control males (p > 0.05). C, Western analysis. An anti-GFP antibody detected a single band corresponding to the predicted 239 kDa CAC1-EGFP fusion protein in cac lethal flies rescued by transgenic expression of CAC1-EGFP (L13 HC129 Rescue) but not in wild-type (WT) controls. A loading control (tubulin) is also shown. D, E, Live epifluorescence and differential interference contrast imaging reveals CAC1-EGFP puncta and their localization within larval neuromuscular presynaptic terminals. Images were obtained from a cac lethal mutant (L13 HC129) rescued by neural expression of CAC1-EGFP. M6 and M7 mark identified ventral longitudinal muscles 6 and 7.
Transformation rescue. Two transgenic lines carrying insertions on the third chromosome, UAS-cac1-EGFP 786C and UAS-cac1-EGFP 247A, were used to rescue cac lethal mutations as described previously (Kawasaki et al., 2002). Briefly, elav-GAL4 l(1)L13HC129/FM7i and elav- GAL4 l(1)L1320-3/FM7i females were mated to lines homozygous for a UAS-cac1-EGFP insertion. Similar control crosses were performed using +/FM7i females. Percentage viability was calculated as follows: (% non-FM7i male progeny from the rescue cross/% non-FM7i male progeny from the control cross) · 100. Finally, viability of cac lethals in the absence of UAS-cac1 EGFP was determined similarly in crosses of elav-GAL4 l(1)L13HC129/FM7i and elav-GAL4 l(1)L1320-3/FM7i females to wild-type (Canton S) males.
Western blotting. Conventional Western analysis was performed on samples prepared from fly heads. The equivalent of one fly head was loaded per lane. The primary antibody was an anti-GFP monoclonal (Clontech, Palo Alto, CA) used at a dilution of 1:1000. Detection was accomplished using a horseradish peroxidase (HRP)-conjugated secondary antibody (Amersham Biosciences, Arlington Heights, IL) and enhanced chemiluminescence (Amersham Biosciences ECL Plus Western Blotting Detection System). As an internal loading control, tubulin was detected with an anti-tubulin monoclonal antibody (Sigma, St. Louis, MO) at 1:200,000.
Live imaging. CAC1-EGFP fluorescence was observed at larval neuromuscular synapses. Third-instar larvae were dissected in saline solution (in mm: 128 NaCl, 2 KCl, 4 MgCl2, 1.8 CaCl2, 36 sucrose, and 5 HEPES, pH, 7.0), and all nerves projecting from the ventral ganglion were cut to prevent muscle contraction. Epifluorescence images were obtained using a Nikon (Tokyo, Japan) Eclipse E600FN microscope with a Fluor 60× 1.0 numerical aperture water-immersion objective (Nikon) and the following filter set: excitation filter, HQ480/20; dichroic mirror, Q495LP; and emission filter, HQ 525/50 (Chroma, Brattleboro, VT). Images were captured with a CCD camera (ORCA-ER; Hamamatsu Photonics, Hamamatsu, Japan) and acquired and processed using the SimplePCI imaging software package (Compix, Cranberry Township, PA). In this study, all images of larval neuromuscular synapses were obtained from ventral longitudinal muscles 6 and 7 within abdominal segment A2 or A3.
Immunocytochemistry and confocal microscopy. Immunocytochemistry of Drosophila neuromuscular synapses was performed by conventional methods with one exception: typically, and in all experiments shown, detection of CAC1-EGFP signals was performed using Tyramide Signal Amplification (Molecular Probes, Eugene, OR) rather than a fluorescently labeled secondary antibody. A typical double-labeling experiment using tyramide enhancement of the CAC1-EGFP signal was performed as described below.
Dissected third-instar larvae and adults were fixed for 30 min in saline solution (see Live imaging) containing 4% paraformaldehyde (the temperature for all incubations was ambient unless specified). After consecutive 10 min washes with (1) saline solution, (2) PBS, and (3) PBT (PBS containing 0.2% Triton X-100), specimens were incubated in blocking buffer (1% bovine serum albumin in PBT) for 1 hr. Blocked samples were incubated for 2 hr with the primary anti-GFP monoclonal antibody (Clontech) at 1:5000 in blocking buffer (for comparison, this antibody was used at 1:500 for conventional detection with a fluorescently labeled secondary antibody). The primary anti-GFP antibody incubation was followed by three washes with PBT (10 min each) and tyramide enhancement essentially according to the instructions of the manufacturer. Two subsequent washes with PBT and one with PBS (10 min each) were followed by a 30 min incubation with PBS containing 5% normal goat serum (NGS blocking solution).
Incubation in the second primary antibody, either a 1:200 dilution of rabbit anti-HRP antibody (Jackson ImmunoResearch, West Grove, PA) or a 1:2000 dilution of rabbit anti-DPAK (Drosophila p21-activated kinase) antibody (kindly provided by Nicholas Harden), was performed in NGS blocking solution. After five washes with PBS (12 min each), samples were incubated with a fluorescent secondary antibody, Alexa Fluor 546 goat anti-rabbit antibody (Molecular Probes), diluted 1:100 in NGS blocking solution. Samples were finally washed five times with PBS (12 min each) and mounted in a 1:1 mixture of PBS and glycerol. Preparations were imaged using an Olympus Optical (Tokyo, Japan) FV300 confocal microscope with a PlanApo 60× 1.4 numerical aperture oil objective (Olympus Optical) and a z-step size of 0.2 μm. Images shown in Figure 2 I-M were deconvolved and reconstructed using the AutoDeblur and AutoVisualize functions of the Autoquant (Watervliet, NY) software package. The surface projection in Figure 2 M was generated using the Voxel Gradient Shading function of the same package.
Figure 2.
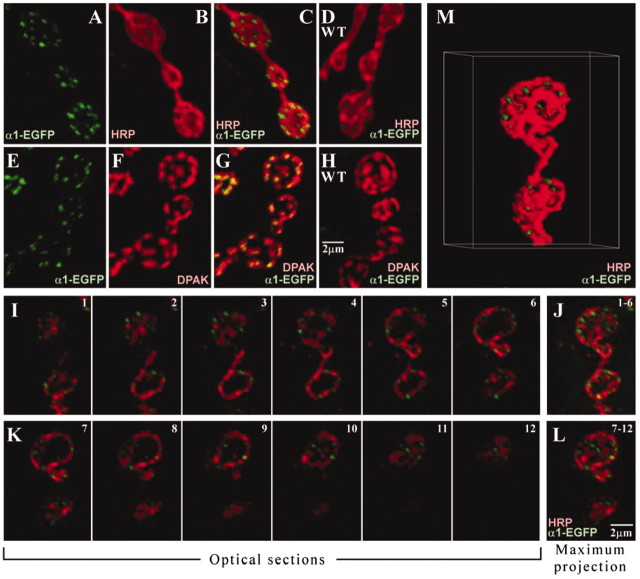
Active zone localization of cac-encoded calcium channels. Images were generated by confocal immunofluorescence microscopy at larval neuromuscular synapses of a cac lethal mutant (L13HC129) rescued by neural expression of CAC1-EGFP. A-C, Double labeling with anti-HRP and anti-GFP showing localization of CAC1-EGFP (α1-EGFP) puncta to presynaptic boutons. D, A wild-type (WT) control lacking the transgene and thus CAC1-EGFP. E-G, Double labeling with anti-DPAK showing active zone localization of CAC1-EGFP puncta. H, A wild-type control lacking the transgene. A-H are each maximum projections of three consecutive optical z-sections. I-M, Analysis of anti-GFP-anti-HRP double labeling showing the three-dimensional distribution of CAC1-EGFP puncta. A series of six optical sections through the z-axis representing the top half of the terminal structure (I) and a maximum projection of these sections showing the distribution of active zones (J). K, L, A series of optical sections and the corresponding maximum projection representing the bottom half of the structure. M, A surface projection for the entire z-series represented in I-L.
Electrophysiology. Recordings of EPSCs from dorsal longitudinal flight muscle (DLM) neuromuscular synapses of the adult were obtained and analyzed as described previously (Kawasaki et al., 1998). Recordings were performed at 20°C using 2- to 4-d-old flies. n refers to the number of recordings; one recording was obtained from each preparation.
Data analysis. Graphing and analysis of numerical data were performed in Microsoft (Seattle, WA) Excel. All data values in the text and bar graphs are given as mean ± SEM. Statistical analysis was performed using the two-tailed Student's t test, and significance was assigned to comparisons for which p ≤ 0.05.
Results
Transformation rescue and live imaging of EGFP-tagged calcium channels
A transgene construct shown previously to rescue cac mutants (Kawasaki et al., 2002) was modified to express a CAC1-EGFP fusion protein by appending EGFP to the α1 subunit C-terminal cytoplasmic tail (Fig. 1A) (see Materials and Methods). As described previously (Kawasaki et al., 2002), the resulting transgene was expressed specifically in the nervous system using the GAL4-UAS system and an elav-GAL4 driver. Rescue of the cac embryonic lethal mutations l(1)L13HC129 and l(1)L1320-3 was examined for two cac1-EGFP transgenic lines, and both lines produced efficient rescue of each lethal to adult viability. Rescue of lethal l(1)L13HC129 by one line, UAS-cac1-EGFP 786c, is shown in Figure 1B. Consistent with our previous findings, these results indicate that neural expression of a CAC-EGFP α1 subunit is sufficient to provide the essential functions of cac-encoded calcium channels. In rescued lethal mutants, the predicted 239 kDa fusion protein was observed by Western analysis using an anti-GFP antibody (Fig. 1C). To examine whether expression of the CAC1-EGFP fusion protein allows live imaging of calcium channel localization in vivo, rescued lethal mutants were imaged by epifluorescence microscopy. These experiments revealed clear punctuate EGFP fluorescence within live neuromuscular presynaptic terminals of third-instar larvae (Fig. 1D,E).
Active zone localization of cac-encoded calcium channels
To further characterize the presynaptic localization of cac-encoded calcium channels, confocal immunofluorescence imaging was used at larval neuromuscular synapses to examine the distribution of EGFP-tagged α1 subunits. Double labeling using a monoclonal anti-GFP antibody and a neuronal plasma membrane-specific antibody, anti-HRP, was performed in a cac lethal mutant rescued by neural expression of CAC1-EGFP. These experiments confirmed the presence of calcium channel puncta within presynaptic boutons of rescued larvae (Fig. 2A-C) but not wild-type controls lacking the transgene (Fig. 2D). The likely possibility that these CAC1-EGFP puncta correspond to active zones was examined by double labeling with an antibody against DPAK, a well characterized marker for the postsynaptic densities closely apposed to presynaptic active zones (Sone et al., 2000). These experiments revealed extensive colocalization of CAC1-EGFP and DPAK, confirming active zone localization of cac-encoded calcium channel α1 subunits (Fig. 2E-H). Consistent with previous studies defining the three-dimensional ultrastructure of larval neuromuscular boutons and the surrounding postsynaptic membrane (Atwood et al., 1993; Jia et al., 1993), active zones exhibited an approximately even distribution over the entire surface of a bouton (Fig. 2I-M). This is revealed in a series of optical sections through the z-axis (Fig. 2I,K), as well as the respective maximum projections representing the top and bottom halves of the image stack (Fig. 2J,L, respectively) and a surface projection for the same structure (Fig. 2M).
cac-encoded calcium channels at adult neuromuscular synapses
Our previous functional analysis of cac-encoded presynaptic calcium channels was performed at adult neuromuscular synapses of the DLM (Kawasaki et al., 2000, 2002). To examine calcium channel distribution in the same preparation, imaging of CAC1-EGFP α1 subunits was performed at DLM neuromuscular synapses of a cac lethal mutant rescued by neural expression of cac1-EGFP. As in the larval preparation, double labeling with anti-GFP and anti-HRP revealed presynaptic CAC1-EGFP puncta (Fig. 3A-C), and active zone localization was confirmed by double-labeling with anti-DPAK (Fig. 3D-F). At DLM synapses, active zones were typically associated with axonal swellings of 1-2 μm in diameter and were distributed along the axon at intervals of ∼1-2 μm. These results extend our previous findings demonstrating that cac-encoded calcium channels function in neurotransmitter release at DLM neuromuscular synapses.
Figure 3.
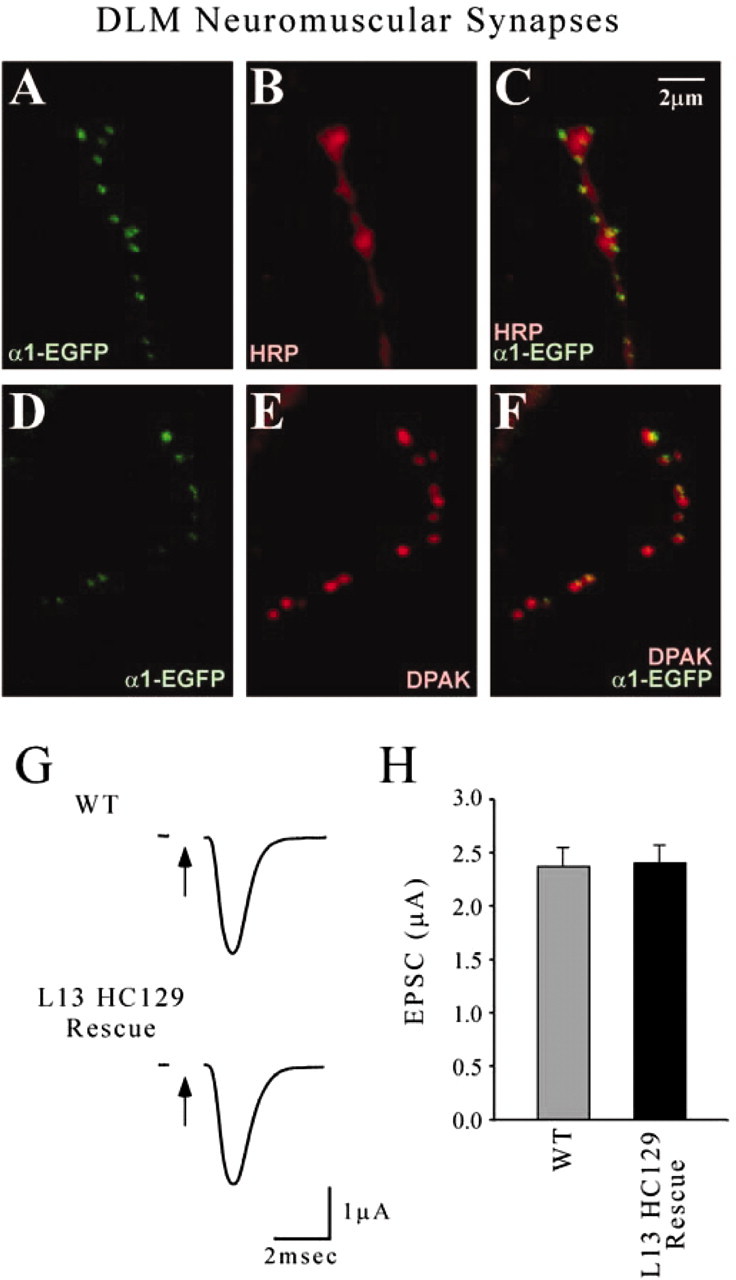
cac-encoded calcium channels at adult neuromuscular synapses. Images and recordings were obtained from a cac lethal mutant (L13HC129) rescued by neural expression of CAC1-EGFP. A-C, Double-labeling experiments with anti-GFP (α1-EGFP) and anti-HRP show localization of CAC1-EGFP within presynaptic terminals of DLM neuromuscular synapses. D-F, Double labeling with anti-DPAK showing active zone localization of CAC1-EGFP puncta. G, H, Voltage-clamp recordings of EPSCs from DLM neuromuscular synapses demonstrate wild-type (WT) synaptic function in the rescued cac lethal mutant. Arrows indicate stimulation of the DLM motor axon. Stimulation artifacts have been removed.
To assess the function of CAC1-EGFP calcium channels in synaptic transmission, analysis of rescued cac lethal mutants included voltage-clamp recordings of DLM EPSCs (Fig. 3G,H). Evoked synaptic currents from wild type and the rescued cac lethal were indistinguishable, exhibiting amplitudes of 2.37 ± 0.18 μA (n = 5) and 2.40 ± 0.17 μA (n = 5), respectively. The activity dependence of synaptic transmission was examined as well. In wild type and the rescued cac lethal mutant, the respective steady-state EPSC amplitudes after 5 sec of 50 Hz stimulation were 39.1 ± 0.84% (n = 4) and 39.9 ± 2.38% (n = 4) of the initial amplitude. Thus, no indication of altered calcium channel function was observed; however, we cannot rule out subtle effects of the EGFP tag on channel properties. The above results provide direct confirmation of cac-encoded calcium channel localization and function at DLM neuromuscular synapses.
Discussion
The results reported here further define the primary role of cac-encoded calcium channels in neurotransmitter release and reveal active zone localization of these channels at two different neuromuscular synapses of Drosophila. A key aspect of this study is transgenic expression of a EGFP-tagged calcium channel α1 subunit that is targeted to active zones and retains its function in neurotransmitter release. In light of the central importance of presynaptic calcium channels and the utility of Drosophila as a model system, the ability to perform live imaging of functional presynaptic calcium channels, as well as immunolocalization of these and other presynaptic proteins relative to active zones, is expected to have general implications for further study of mechanisms operating in the development and function of synapses.
Footnotes
This work was supported by National Institutes of Health Grant R01-NS38064 and National Science Foundation Grant IBN-9986990. We thank Dr. Nicholas Harden (Simon Fraser University, Burnaby, British Columbia, Canada) for generously providing the anti-DPAK antibody. Confocal microscopy was performed in the Pennsylvania State Center for Quantitative Cell Analysis with excellent support from the center staff.
Correspondence should be addressed to Richard W. Ordway, Associate Professor of Biology, Chair, Intercollege Graduate Program in Genetics, Pennsylvania State University, 208 Mueller Laboratory, University Park, PA 16802. E-mail: rwo4@psu.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/240282-04$15.00/0
References
- Atwood HL, Govind CK, Wu C-F (1993) Differential ultrastructure of synpatic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol 24: 1008-1024. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401-415. [DOI] [PubMed] [Google Scholar]
- Catterall WA (1998) Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium 24: 307-323. [DOI] [PubMed] [Google Scholar]
- Chan B, Villella A, Funes P, Hall JC (2002) Courtship and other behaviors affected by a heat-sensitive, molecularly novel mutation in the cacophony calcium-channel gene of Drosophila. Genetics 162: 135-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger BB, Felling R, Ordway RW (2000) Genetic modifiers of the Drosophila NSF mutant, comatose, include a temperature-sensitive paralytic allele of the calcium channel α1 subunit gene, cacophony. Genetics 155: 203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X-X, Gorczyca M, Budnik V (1993) Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol 24: 1025-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Mattiuz AM, Ordway RW (1998) Synaptic physiology and ultrastructure in comatose mutants define an in vivo role for NSF in neurotransmitter release. J Neurosci 18: 10241-10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW (2000) A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci 20: 4885-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Collins SC, Ordway RW (2002) Synaptic calcium channel function in Drosophila: analysis and transformation rescue of temperature-sensitive paralytic and lethal mutations of cacophony. J Neurosci 22: 5856-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Bezprozvanny I (2002) Synaptic targeting of N-type calcium channels in hippocampal neurons. J Neurosci 22: 6939-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R, Adler EM, Charlton MP (1990) Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron 5: 773-779. [DOI] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC (1996) A Drosophila calcium channel α1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci 16: 7868-7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Suzuki E, Hoshino M, Hou D, Kuromi H, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y-I, Hama C (2000) Synaptic development is controlled in the periactive zones of Drosophila synapses. Development 127: 4157-4168. [DOI] [PubMed] [Google Scholar]
- Stanley EF (1997) The calcium channel and the organization of the presynaptic transmitter release face. Trends Neurosci 20: 404-409. [DOI] [PubMed] [Google Scholar]
- von Schilcher F (1977) A mutation which changes courtship song in Drosophila melanogaster. Behav Genet 7: 251-259. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA (1995) Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci 15: 6403-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]