Abstract
Although l-dopa remains the most effective treatment of Parkinson disease, its long-term administration is hampered by the appearance of dyskinesia. Hypersensitivity of dopamine D1 receptors in the striatum has been suggested to contribute to the genesis of these delayed adverse effects. However, D1 receptor amounts are unchanged in Parkinson disease, suggesting alterations of downstream effectors. In rodents, striatal D1 receptors activate adenylyl cyclase through olfactory type G-protein α subunit (Gαolf) and G-protein γ 7 subunit (Gγ7). We found that Gαolf was enriched in human basal ganglia and was markedly diminished in the putamen of patients with Huntington disease, in relation with the degeneration of medium spiny neurons. In contrast, in the putamen of patients with Parkinson disease, Gαolf and Gγ7 levels were both significantly increased. In the rat, the degeneration of dopamine neurons augmented Gαolf levels in the striatal neurons, specifically at the plasma membrane, an effect accounting for the increase of D1 response on cAMP production in dopamine-depleted striatum. In lesioned rats, Gαolf levels were normalized by a 3 week treatment with l-dopa or a D1 agonist but not with aD2-D3 agonist, supporting a Gαolf regulation by D1 receptor usage. In contrast, the increases of Gαolf levels in patients were not affected by the duration of l-dopa treatment but correlated with duration of disease. In conclusion, our results revealed in the parkinsonian putamen a prolonged elevation of Gαolf levels that may lead to a persistent D1 receptor hypersensitivity and contribute to the genesis of long-term complications of l-dopa.
Keywords: Parkinson disease, dopamine, G-protein, striatum, l-dopa, 6-hydroxydopamine
Introduction
The main feature of Parkinson disease (PD) is the loss of dopaminergic transmission in the striatum that underlies a large part of the symptoms of disease. Although the dopamine precursor l-dopa remains the most effective treatment of parkinsonian symptoms, its efficacy decreases and fluctuates during chronic administration and is complicated by the development of dyskinesia (Obeso et al., 2000). Hypersensitivity of dopamine receptors after dopamine denervation may play a crucial role in the genesis of long-term l-dopa adverse effects (Bezard et al., 2001). Activation of dopamine receptors has important consequences on neuronal plasticity (Greengard et al., 1999; Berke and Hyman, 2000; Gerfen, 2000a), and modifications of dopamine receptor signaling in PD may lead to the development of abnormal responses to l-dopa. In support of this hypothesis, D1 receptor (D1R) hypersensitivity is associated with alterations of synaptic plasticity in a rat model of l-dopa-induced dyskinesia (Picconi et al., 2003), and dopamine-stimulated adenylyl cyclase is increased in PD (Pifl et al., 1992; Tong et al., 2004). The mechanisms of D1R hypersensitivity are unclear, because in contrast to D2 receptor (D2R) (Creese et al., 1977; Lee et al., 1978), D1R number appears unchanged (Shinotoh et al., 1993; Turjanski et al., 1997; Hurley et al., 2001). Although functional D1 hypersensitivity could be mediated by an increase in the activity of downstream effectors, several key proteins mediating D1R signaling, including protein kinase A and dopamine- and cAMP-regulated phosphoprotein Mr-32,000 (DARPP-32), are unaltered in PD (Girault et al., 1989; Nishino et al., 1993). However, the possibility of alterations at the level of G-proteins that couple D1R to adenylyl cyclase has not yet been explored. In rodents, this coupling is mediated through the olfactory type G-protein α subunit (Gαolf), and not through the ubiquitous isoform of stimulatory G-protein α subunit, Gαs (Zhuang et al., 2000; Corvol et al., 2001). Gαolf, first identified in the olfactory epithelium (Jones et al., 1990; Belluscio et al., 1998), is enriched in striatal medium-size neurons (Drinnan et al., 1991; Herve et al., 1993), and its absence in a mutant mouse strain abolishes the coupling of D1R to adenylyl cyclase (Corvol et al., 2001). In human, Gαolf protein was detected in the whole brain (Zigman et al., 1993), but its regional distribution has not been examined. Gαolf is a good candidate to explain potential D1R signaling changes in PD, because its levels are limiting for D1R responses and are regulated by dopamine in rodents (Herve et al., 2001). We examined Gαolf distribution in human brain and quantified its levels in postmortem putamen of patients with PD or other basal ganglia diseases. We explored the functional relevance of Gαolf alterations and its regulation by l-dopa and dopamine agonists in 6-hydroxydopamine (6-OHDA) lesioned rats. In PD patients, Gαolf levels were found increased in the striatum as in 6-OHDA lesioned rats. In lesioned rats, Gαolf upregulation was concomitant of an increased D1R signaling. Alteration of D1R signaling by persistent increase in Gαolf in PD may contribute in the genesis of long-term adverse effects of l-dopa.
Materials and Methods
Antibodies. Selective antibodies against Gαolf and Gαs were obtained by immunization of rabbit with full-length recombinant Gαolf and Gαs proteins, followed by adsorption on Gαs and Gαolf columns as described previously (Corvol et al., 2001). For immunoblotting, antibodies were used with the following dilutions: anti-Gαolf (1:2000), anti-Gαs (1:1000), anti-tyrosine hydroxylase (TH) (1:4000, monoclonal; Chemicon, Temecula, CA), anti-Gq protein α subunit (Gαq) (1:1000, monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA), anti-β-actin (1:5000, monoclonal; Chemicon), and anti-G-protein γ 7 (Gγ7) (1:2000, polyclonal; gift from J. D. Robishaw, Geisinger Clinic, Danville, PA).
Human brain samples and Western blots. Brain samples were provided by the Brain Bank of the Institut National de la Santé et de la Recherche Médicale Unité 289 laboratory (Pitié-Salpétrière Hospital, Paris, France). Ten patients with clinical PD were compared with 14 control subjects who were free of any neurological disorders. The PD patients were all treated with l-dopa. However, because the patients as well as the control subjects died between 1978 and 1980, most of the PD patients developed symptoms before the generalization of dopatherapy in the 1970s and received l-dopa during a relatively short period of time compared with the disease duration. l-Dopa-induced dyskinesia was reported for five patients and was very disabling for three of them. In addition, five patients with clinical progressive supranuclear palsy and four with Huntington disease (HD) confirmed by neuropathological examination were analyzed. Retrospective analysis of clinical data was used to calculate a premortem severity index (PMSI) that is easily, objectively, and reproducibly assessed (Monfort et al., 1985). Briefly, the PMSI score (from 0 to 6) is the sum of a hypoxia and a hypovolaemia index scaled from 0 to 3 as follows: 0, absence of hypoxia or hypovolaemia; 1, duration <1 hr; 2, duration between 1 and 24 hr; 3, duration exceeding 24 hr.
Samples of regions of interest (∼20 mg of tissue) were stored at -80°C. They were homogenized by sonication in 1% SDS and boiled for 5 min. Protein concentration was measured with bicinchoninic acid-based (BCA) method (Smith et al., 1985), and equal amounts of protein (80-100 μg) were separated by SDS-PAGE (10%) before electrophoretic transfer onto nitrocellulose membrane (Hybond Pure; Amersham Biosciences, Orsay, France). For Gγ7 Western blots, putamen homogenates were separated in 15% polyacrylamide gel with 10% glycerol. Nitrocellulose membranes were blocked for 1 hr at room temperature in Tris-buffered saline (TBS; 100 mm NaCl, 10 mm Tris, pH 7.5) with 0.05% Tween 20 and 5% nonfat dry milk and incubated overnight at 4°C with the primary antibodies. Bound antibodies were detected with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibodies (Amersham Biosciences; 1:4000) and visualized by enhanced chemiluminescent detection (Amersham Biosciences). When necessary, membranes were stripped in buffer containing 100 mm glycine, pH 2.5, 200 mm NaCl, 0.1% Tween 20, and 0.1% β-mercaptoethanol for 1 hr at room temperature, followed by extensive washing in TBS before reblocking and reprobing with other antibodies.
Rat lesioning and treatments. Male Sprague Dawley rats weighing 200-250 gm (Iffa-Credo, L'Arbresle, France) were lesioned as described previously (Herve et al., 1993). Briefly, stereotaxic injection of 1 μl of saline (shams) or 6-OHDA (6 μg; Sigma-Aldrich, St. Louis, MO) was performed unilaterally in the medial forebrain bundle (2.2 mm caudal, 1.9 mm lateral to bregma, 8.4 mm under surface of skull), according to the atlas by Paxinos and Watson (1997), under ketamine anesthesia (150 mg/kg; Imalgene; Virbac, Carros, France), 30 min after 25 mg/kg desipramine to protect noradrenergic neurons. Lesion efficacy was controlled by postmortem TH quantification in immunoblots, and only rats with >90% decrease were retained for subsequent analysis. Three weeks after lesioning, groups of rats were treated with a single acute intraperitoneal injection of saline or 50 mg/kg l-dopa-methyl-ester (LDME) (gift from Chiesi Farmaceutici, Parma, Italy) plus 10 mg/kg benserazide (gift from Roche, Basel, Switzerland) plus 0.02% ascorbic acid. For 3 weeks, other groups of rats received two daily intraperitoneal injections (12 hr interval) of either saline or 10 mg/kg per day LDME plus 10 mg/kg benserazide, or 100 mg/kg per day LDME plus 20 mg/kg benserazide, in the presence of 0.02% (w/v) ascorbic acid. Additional groups received saline or 1 mg/kg per day (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1M-3-benzazepine (SKF81297) (Sigma-Aldrich) or 1 mg/kg per day ropinirole (gift from GlaxoSmithKline, Marly-le-Roi, France). Animals were killed 2 or 6 hr after acute injection and 12 hr after the last injection of intermittent chronic treatment. Brains were rapidly dissected out, frozen in dry ice, and sectioned into 500 μm coronal slices at -10°C with a freezing microtome. Tissue microdisks were punched out from the caudate-putamen using a stainless steel cylinder (2.2 mm diameter) and stored at -80°C before homogenization and Western blotting that were performed as described for human brain samples.
Adenylyl cyclase assay. Six weeks after lesioning, rats were killed and brains were sectioned in 300 μm slices in ice-cold Ca2+-free artificial CSF (125 mm NaCl, 2.4 mm KCl, 1.93 mm MgCl2, 0.5 mm KH2PO4, 0.5 mm Na2SO4) using a refrigerated Vibroslice apparatus. Microdisks were punched out from the caudate-putamen and homogenized in 2 mm Tris maleate, pH 7.2, containing 2 mm EGTA and 300 mm sucrose, in a Potter Elvehjem apparatus (Wheaton Scientific, Millville, NJ). Adenylyl cyclase activity was measured at 30°C by the conversion of α-32P-ATP into cyclic 32P-cAMP for 7 min as described previously (Corvol et al., 2001) and reported to the amount of protein concentration measured by BCA method. Adenylyl cyclase activity was expressed as percentage of control basal activity (baseline).
Electron microscopy. Rat brains were rapidly dissected and left for 48 hr in 4% paraformaldehyde; 40 μm sections were prepared with a vibratome and stored in PBS (100 mm NaHPO4, 0.9% NaCl, pH 7.4). Immunohistochemistry was performed as described previously (Muriel et al., 1999). Briefly, sections were washed in PBS and incubated in PBS-0.2% BSA for 1 hr, followed by incubation with Gαolf antibody (1:1000; 48 hr at 4°C). Sections were rinsed in PBS-0.2% BSA and incubated in PBS-0.5% BSA (1 hr at room temperature) supplemented with 0.1% fish gelatin (Aurion, Biovalley, Conches, France) before incubation in the presence of goat anti-rabbit IgG secondary antibody (1:50; 24 hr at room temperature) conjugated to ultrasmall gold particles (0.8 nm; Aurion). Sections were rinsed three times in 2% sodium acetate (w/v), and argentic amplification was performed in darkness (30 min at room temperature) and stopped by two washes in 2% sodium acetate. The signal was intensified and stabilized by immersion of the sections in 0.05% gold chloride (w/v) for 10 min at 4°C and in 0.3% sodium thiosulfate twice for 10 min at 4°C. Sections were postfixed (15 min) in 1% (w/v) osmium tetroxide and embedded in Epon. Ultrathin sections (50 nm) were cut using an ultramicrotome (Ultracut E; Leica, Rueil-Malmaison, France) contrasted with uranyl acetate and lead citrate and observed with a JEOL (Akishima, Japan) 1200EX II electron microscope at 80 kV. For some of the sections processed for immunohistochemical analysis, the primary antibody was omitted from the reaction to test for nonspecific staining. No significant staining was observed under these conditions.
For quantitative analysis, 40 neurons (20 randomly selected neurons in two independent tissue blocks) with surrounding dendrites were analyzed for each rat. Morphometric analysis was performed using Explora Nova Mercator software (La Rochelle, France). For each neuron analyzed, gold particles localized at the plasma membrane in the cell bodies or dendrites as well as those in the cytoplasm of cell bodies were counted; the length of the cell body or dendrite membrane and the surface of the cytoplasm (surface of the nucleus subtracted from the whole surface) were measured. Results were expressed as: (1) density of gold particles per micrometer for those localized at the membrane of cell bodies or dendrites, and (2) density of gold particles per square micrometer for those localized in the cytoplasm. The mean density of gold particles overlying the cytoplasm or the membranes (linear density) was calculated for each rat.
Statistical analysis. After Western blotting, the relevant immunoreactive bands on films were quantified with laser scanning densitometry using Scion (Frederick, MD) Image software (β 4.0.2, 2000). To allow comparison between different immunoblots, the density of various bands was normalized to that obtained for the β-actin band in the same sample and was expressed as the percentage of controls. Results are expressed as means ± SEM. Statistical analysis was done with Student's t or Mann-Whitney U test or with one-way or two-way ANOVA followed by appropriate post hoc tests, depending on the nature of the data, as indicated in Results and figures. In correlation analyses between protein levels and PD duration (continuous variable) or PMSI (discontinuous variable), we used the Pearson's coefficient and the nonparametric Spearman's coefficient, respectively. Statistical analyses were performed with PRISM 3.0 software (San Diego, CA).
Results
Brain samples and patient characteristics
We compared the levels of various G-protein subunits in postmortem samples of putamen from PD and control patients. Studies of protein levels in human postmortem samples could be affected by several confounding variables, including postmortem delay (PMD), age, and gender (Girault et al., 1989). To evaluate the effects of such variables, we first examined the levels of Gαolf, Gαs, Gαq, and Gγ7 proteins as well as those of TH, DARPP-32, and β-actin in putamen samples of 23 controls (13 men and 10 women) from 56 to 90 years of age, with a PMD ranging from 1.3 to 24 hr. In agreement with previous studies on Gαs and Gαq (Dwivedi et al., 2002), PMD, age, and gender had no significant effect on the levels of the various G-protein subunits or on TH or β-actin (Table 1). In contrast, DARPP-32 levels were inversely correlated with PMD (Table 1), as described previously (Girault et al., 1989). To eliminate bias between groups, patients and controls were matched for PMD, age, and sex. Patient characteristics are summarized in Tables 2 and 3. Ten PD patients (eight men and two women, from 57 to 82 years of age) with mean disease duration of 12 ± 2 years and a mean l-dopa treatment duration of 6 ± 2 years were compared with 14 matched control subjects (eight men and six women, from 45 to 90 years of age) free of any brain pathology (Table 3). The PMSI could be assessed in 10 controls and 9 patients with PD and was not significantly different (2.8 ± 1.6 and 3.0 ± 1.4; Mann-Whitney U test; p = 0.84). There was no correlation between PMSI and Gαolf protein levels (Spearman's coefficient, r = 0.27; p = 0.25).
Table 1.
Influence of PMD, age, and gender on protein levels in control patients
|
|
PMD |
Age |
|
||||
|---|---|---|---|---|---|---|---|
| Protein |
r2
|
p
|
r2
|
p
|
Gender p (t test) |
||
| TH | 0.01 | 0.66 | 0.00 | 0.95 | 0.94 | ||
| Gαolf | 0.00 | 0.99 | 0.00 | 0.76 | 0.52 | ||
| Gαs | 0.01 | 0.76 | 0.18 | 0.20 | 0.87 | ||
| Gαq | 0.00 | 0.95 | 0.00 | 0.84 | 0.20 | ||
| Gγ7 | 0.07 | 0.48 | 0.07 | 0.48 | 0.71 | ||
| DARPP32 | 0.44 | 0.03 | 0.21 | 0.25 | 0.90 | ||
| β-Actin |
0.09 |
0.20 |
0.00 |
0.91 |
0.73 |
||
Correlation analyses were performed using a two-tailed linear regression analysis to calculate the Pearson's coefficient (r2) and the p value for slope difference from zero. The effect of gender was evaluated by Student's t test.
Table 2.
Patient characteristics
|
Diagnostic |
Sex |
Age (years) |
PMD (hours) |
Cause of death |
Disease duration (years) |
l-Dopa duration (years) |
|---|---|---|---|---|---|---|
| PD | Male | 66 | Nd | Collapse | 13 | 6 |
| PD | Male | 48 | 7 | Nd | 9 | 2 |
| PD | Female | 62 | 17 | Sepsis | 7 | 7 |
| PD | Male | 82 | 15 | Bronchopneumonia | 6 | 6 |
| PD | Male | 75 | 24 | Bronchopneumonia | 9 | 6 |
| PD | Female | 57 | 15.5 | Sudden death | 16 | 10 |
| PD | Male | 67 | 15.5 | Sepsis | 11 | 10 |
| PD | Male | 72 | 6 | Bronchopneumonia | 10 | 2 |
| PD | Male | 79 | 24 | Collapse | 14 | 8 |
| PD | Male | 59 | 9 | Bronchopneumonia | 22 | 6 |
| Control | Female | 74 | Nd | Pulmonary embolism | ||
| Control | Male | 65 | 5 | Bronchopneumonia | ||
| Control | Male | 75 | 1.25 | Bronchopneumonia | ||
| Control | Male | 45 | 10 | Sepsis | ||
| Control | Male | 56 | 4 | Bronchopneumonia | ||
| Control | Male | 83 | 16 | Sudden death | ||
| Control | Female | 85 | 4 | Myocardial infarction | ||
| Control | Male | 76 | 14 | Nd | ||
| Control | Male | 67 | 10 | Acute leukemia | ||
| Control | Female | 77 | 11.7 | Cardiac insufficiency | ||
| Control | Female | 90 | 16.75 | Nd | ||
| Control | Female | 85 | 13 | Nd | ||
| Control | Female | 84 | 14 | Sudden death | ||
| Control | Male | 83 | 18 | Bronchopneumonia | ||
| PSP | Male | 65 | 8.5 | Nd | ||
| PSP | Female | 75 | 5 | Nd | ||
| PSP | Female | 79 | 11.5 | Nd | ||
| PSP | Male | 65 | Nd | Nd | ||
| PSP | Male | 63 | Nd | Nd | ||
| HD | Male | 66 | 30 | Nd | ||
| HD | Female | 59 | 3 | Nd | ||
| HD | Female | 79 | 22 | Nd | ||
| HD |
Male |
65 |
22 |
Nd |
|
|
Nd, Not documented.
Table 3.
Comparison between patients with Parkinson disease and controls
|
|
Controls (n = 14) |
Parkinson disease (n = 10) |
p |
|---|---|---|---|
| Age | 75 ± 13 | 67 ± 11 | 0.14 |
| Sex ratio | 1.3 | 4.0 | 0.10 |
| PMD (hr) | 10.6 ± 5.5 | 14.8 ± 6.6 | 0.12 |
| PMSI |
2.8 ± 1.6 |
3.0 ± 1.4 |
0.84 |
For statistical analysis, we used Student's t test for age and PMD, χ2 test for sex ratio, and Mann-Whitney U test for PMSI.
Gαolf is prominently expressed in human striatum
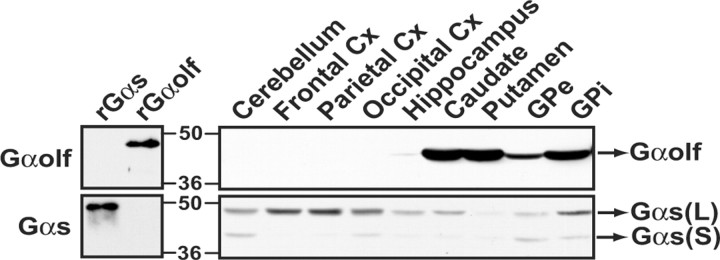
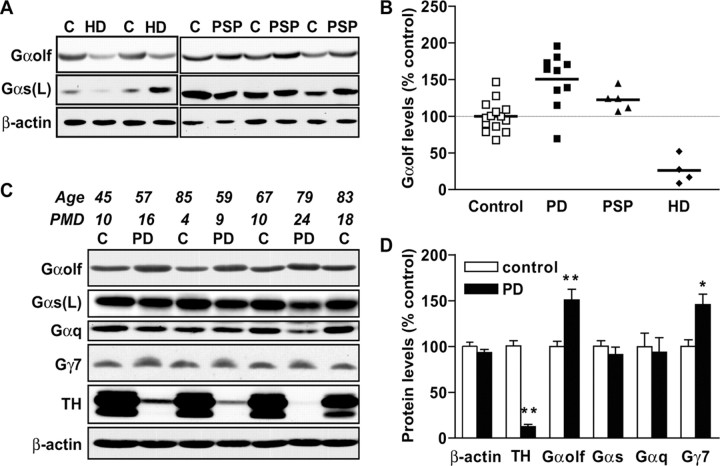
Regional distribution of Gαolf and Gαs was investigated in control human brain using affinity-purified antibodies that showed no cross-reactivity between Gαolf and Gαs proteins (Fig. 1). Gαolf antibodies recognized a single band (∼46 kDa), whereas Gαs antibody identified two bands (∼52 and 46 kDa) corresponding to the long and short splice isoforms of the protein (Robishaw et al., 1986). In human brain, Gαolf was abundant in the striatum, including putamen and caudate nucleus as well as internal and external globus pallidus, whereas very low levels were found in the hippocampus, and no signal was detected in the neocortex and the cerebellum (Fig. 1). Conversely, Gαs, primarily the long isoform, was expressed in all tested brain regions, although it was much less abundant in the striatum than in the neocortex (Fig. 1). The selective expression of Gαolf in human striatum is concordant with data in rodents (Drinnan et al., 1991; Herve et al., 1993), in which Gαolf is abundantly expressed in striatal efferent neurons. For testing a similar localization in human striatum, Gαolf levels were evaluated in patients with HD, an inherited neurodegenerative disorder in which striatal efferent GABAergic medium-size neurons are selectively affected. Gαolf levels were dramatically reduced in the putamen of the four HD patients tested (26 ± 9% of levels in controls; p < 0.001) (Fig. 2A,B), confirming that Gαolf is expressed in efferent striatal neurons. It is noteworthy that no significant alteration in Gαs levels was observed in the putamen of HD patients (Fig. 2B).
Figure 1.
Gαolf localization in human brain. Immunoblotting using purified recombinant proteins showed that affinity-purified anti-Gαolf (top left panel) and anti-Gαs (bottom left panel) antibodies recognized specifically full-length Gαolf (rGαolf) and Gαs (rGαs), respectively. These antibodies revealed the presence of Gαolf in human brain regions corresponding to the basal ganglia, where Gαs levels were lower (right panels). The positions of the long (L) and short (S) isoforms of Gαs are indicated. GPe and GPi correspond to external and internal segments of globus pallidus, respectively. Cx, Cortex.
Figure 2.
G-protein levels in postmortem putamen of patients with neurodegenerative disease. A, Representative immunoblots of postmortem putamen samples from HD, PSP, and control (C) subjects. B, Scatter diagram of Gαolf levels for PD (filled square), PSP (filled triangle), HD (filled diamond), and control (open squares) subjects. C, Immunoblots of postmortem putamen samples from PD or control subjects (C); age and PMD are indicated. D, Quantification of the relevant immunoreactive bands detected on immunoblots normalized to β-actin levels and expressed as percentage of controls; *p < 0.05, **p < 0.001 compared with controls (two-way ANOVA followed by Bonferroni multiple comparison test; n = 10 and n = 14 for PD and controls, respectively). Gαs(L), Long isoform of Gαs.
Gαolf and Gγ7 protein levels are increased in the putamen of PD patients
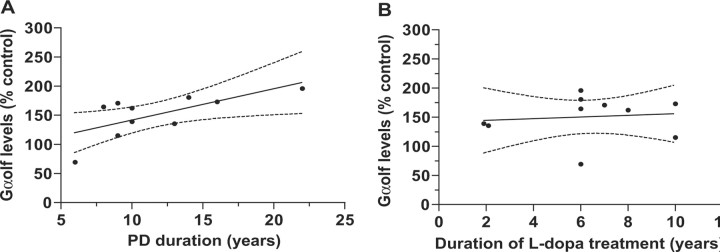
Nigrostriatal dopaminergic denervation was severe in the putamen of patients with PD, as demonstrated by a dramatic reduction in TH levels compared with controls (Fig. 2C,D). In contrast, Gαolf levels were increased by 50% in PD, whereas β-actin, Gαs, and Gαq levels were not significantly altered (Fig. 2C,D). We examined Gαolf levels in patients with another disease of basal ganglia, progressive supranuclear palsy (PSP), which shares with PD a severe dopaminergic denervation but includes degeneration of additional neuronal populations (Levy et al., 1995). An increase in Gαolf levels was also observed in the putamen of five patients with PSP, although it was less pronounced than in PD and did not reach statistical significance (Fig. 2A,B). The G-protein gamma subunit Gγ7 displays a restricted striatal expression in rodents and associates with Gαolf for coupling D1R to adenylyl cyclase (Wang et al., 2001). We found that Gγ7 levels were increased by 46% in the putamen of patients with PD (Fig. 2A,B). Upregulation of Gαolf and Gγ7 may result from similar mechanisms, because the levels of these two proteins were significantly correlated in the putamen of patients with PD (Pearson's coefficient, r2 = 0.44; p < 0.05). The increase in Gαolf levels showed a significant correlation with disease duration (Fig. 3A). In our study, all of the patients received an oral chronic intermittent treatment with l-dopa for 2-10 years until their death. However, no significant correlation was observed between Gαolf levels and duration of l-dopa treatment (Fig. 3B). The magnitude of l-dopa-induced dyskinesia was not available for each patient, preventing a systematic study of the correlation between elevation of Gαolf and l-dopa dyskinesia. However, we noticed that the three patients with the highest Gαolf levels (196, 181, and 173% of controls, respectively) were those with the most disabling dyskinesia reported in the medical record.
Figure 3.
Correlation between Gαolf protein level and Parkinson disease duration. A, Plot of Gαolf protein levels as a function of the duration of PD. Pearson's coefficient, r2 = 0.47; p < 0.05. B, Plot of Gαolf protein level as a function of the duration of l-dopa therapy. Pearson's coefficient, r2 = 0.01; p > 0.05. In both cases, the regression (solid) lines and the 95% confidence interval (dotted) lines are plotted. Each dot corresponds to one patient.
In 6-OHDA lesioned rats, D1R hypersensitivity is associated with an increase in Gαolf at the neuronal plasma membrane
To further examine the causes and functional consequences of the alterations in G-proteins in the striatum of PD patients, we used a rat model of dopaminergic denervation in which a similar upregulation of Gαolf has been observed (Herve et al., 1993; Marcotte et al., 1994). Because only membrane-associated G-proteins can functionally couple receptors to adenylyl cyclase (Milligan, 1993), we investigated adenylyl cyclase activity and the membrane localization of Gαolf in unilaterally 6-OHDA lesioned rats. Six weeks after the lesion, Gαolf levels were increased in the denervated caudate-putamen compared with sham-operated rats (154 ± 12%; p < 0.01) (Fig. 4A), in agreement with previous studies (Herve et al., 1993; Marcotte et al., 1994). No significant variation in Gαolf levels was detected in the caudateputamen contralateral to 6-OHDA microinjection (data not shown). In lesioned rats, an increase in Gαolf levels was associated with an increase in dopamine-dependent adenylyl cyclase activity without change in its apparent affinity for dopamine, supporting the hypothesis of postdenervation D1R hypersensitivity (Fig. 4B). We performed an electron microscopy immunogold-labeling study of Gαolf to examine the precise location of this protein in the caudate-putamen of control and 6-OHDA lesioned rats (Fig. 4C). Gαolf immunoreactivity in control rats was found in virtually all medium-sized spiny neurons, and gold particles were predominantly associated with the plasma membrane of dendrites and cell bodies (Fig. 4C) (78 ± 2% of the total gold particles in cell bodies were at the plasma membrane). In the striatum of 6-OHDA lesioned rats, Gαolf remained preferentially associated with the plasma membranes (75 ± 2%). Quantification of immunogold particles in 6-OHDA lesioned and sham-operated rats revealed a significant increase of particle numbers at the plasma membrane in cell bodies and dendrites (132 ± 18 and 167 ± 21% of particle numbers in shams, respectively) (Fig. 4D). In contrast, the number of cytoplasmic gold particles was not significantly altered (Fig. 4E). The increase in Gαolf levels at the plasma membrane strongly suggests that it is functional and that D1R hypersensitivity is directly related to Gαolf upregulation.
Figure 4.
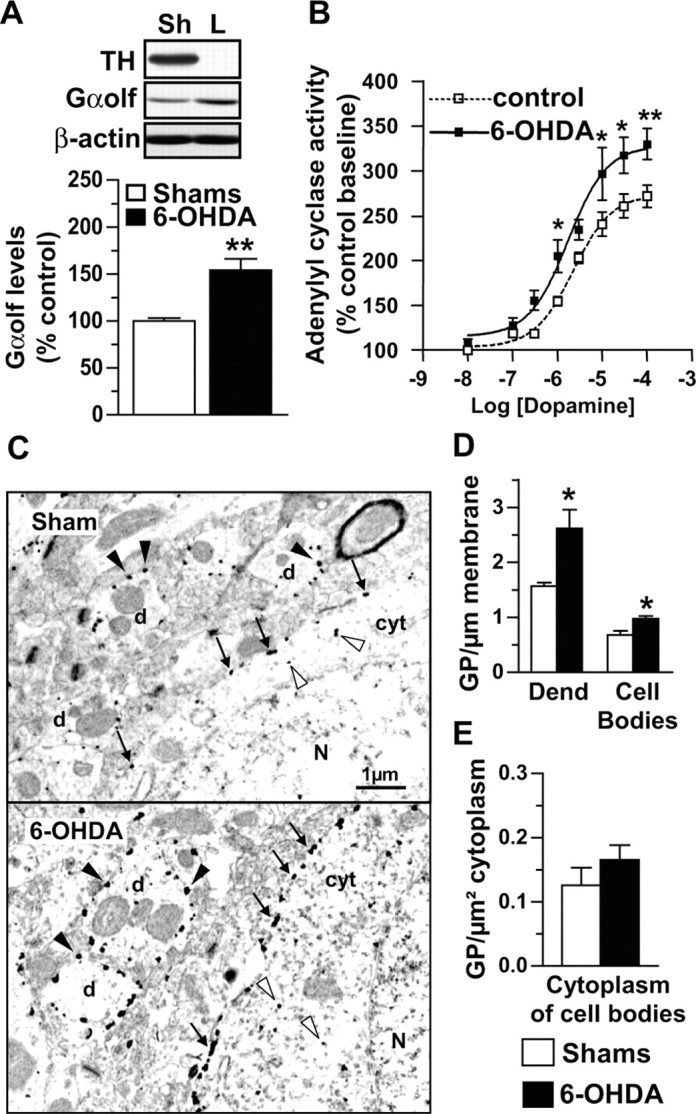
Functional role of Gαolf increase in the striatum of 6-OHDA lesioned rats. A, Immunoblot of Gαolf in caudate-putamen extracts and means ± SEM of Gαolf levels on the lesioned side of unilaterally 6-OHDA lesioned (L; n = 6) or sham-operated (Sh; n = 6) rats. Student's t test: **p < 0.01. B, Dopamine effects on adenylyl cyclase activity in striatal membranes from lesioned (filled square) and control (open square) sides of unilaterally 6-OHDA lesioned rats; values are expressed as percentage of control basal activity (baseline) and are means ± SEM of three independent experiments. Two-way ANOVA followed by Bonferroni multiple comparison test: *p < 0.05 and **p < 0.01 compared with controls. C, Electron microscopy photographs of medium spiny neurons and dendrites in the caudate-putamen of the lesioned side of unilaterally 6-OHDA lesioned or sham-operated rats; gold particles were observed at the plasma membrane of the cell body (arrows) and dendrites (black arrowheads) or within the cytoplasm (white arrowheads). N, Nucleus; cyt, cytoplasm; d, dendrite. D, E, Quantification of number of gold particles (GP) expressed per micrometer of plasma membrane in the cell body or dendrites (Dend) and per square micrometer of cytoplasm in cell body; n = 3 rats per group. Student's t test: *p < 0.05.
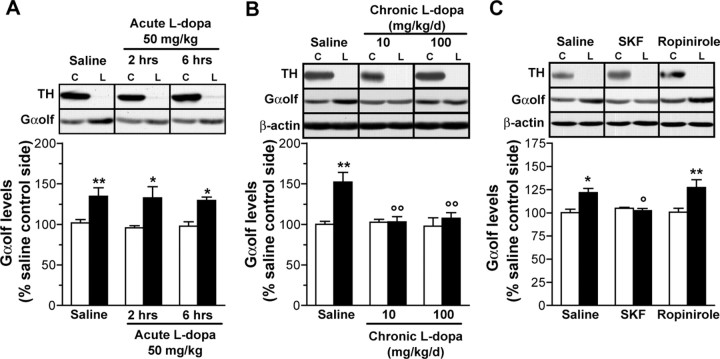
Gαolf levels are normalized by l-dopa therapy in 6-OHDA lesioned rats through dopamine D1 receptor stimulation
Because loss of dopamine transmission leads to increased Gαolf levels in the striatum, we examined the effects on Gαolf levels of l-dopa treatment, which, in part, restores dopamine transmission. We explored striatal Gαolf levels in rats with unilateral 6-OHDA lesions after acute and chronic l-dopa therapy. Acute injection of LDME (50 mg/kg, i.p.) did not modify Gαolf levels in denervated striatum, which remained similarly increased for 2 or 6 hr after injection (Fig. 5A). In contrast, treatments with LDME (5 or 50 mg/kg twice daily, i.p.) for 3 weeks reversed Gαolf increase, which returned to normal levels (Fig. 5B). Gαolf levels were not significantly changed by chronic LDME therapy (50 mg/kg for 12 hr) in the striatum contralateral to the lesion (98 ± 10%; p > 0.05) or in sham-operated animals (107 ± 13%; p > 0.05; data not shown).
Figure 5.
Regulation of Gαolf levels by l-dopa therapy and dopamine receptor agonists. Immunoblots and quantification of Gαolf levels in the lesioned (L) or control (C) striatum of unilaterally 6-OHDA lesioned rats. A, Three weeks after operation, rats were killed 2 or 6 hr after an acute injection of saline or l-dopa-methyl-ester 50 mg/kg (l-dopa). B, Three weeks after operation, rats were treated for 3 weeks with saline or l-dopa-methyl-ester intermittently (5 or 50 mg/kg twice daily, 12 hr interval; n = 6 per group). C, Similarly, lesioned rats were treated for 3 weeks with saline (n = 6), agonist SKF81297 (0.5 mg/kg twice daily; n = 4), D2 agonist ropinirole (0.5 mg/kg twice daily; n = 6). Two-way ANOVA followed by Bonferroni's multiple comparison test: *p < 0.05, **p < 0.01 lesioned compared with control side with the same treatment;  p < 0.05 and
p < 0.05 and  p < 0.01 treated compared with saline. Open columns, Control; filled column, lesioned.
p < 0.01 treated compared with saline. Open columns, Control; filled column, lesioned.
To investigate whether Gαolf down-regulation by l-dopa therapy in the dopamine-depleted striatum was a D1R- or D2R-dependent mechanism, different groups of lesioned rats were treated with either the D1R full agonist SKF81297 (1 mg/kg per day) or the D2-D3 receptor agonist ropinirole (1 mg/kg per day) for 3 weeks (Fig. 5C). Gαolf levels in the denervated striatum were normalized in animals treated with SKF81297, whereas the D2-D3 agonist did not modify the lesion-induced Gαolf increase (Fig. 5C). Altogether, these results demonstrated that Gαolf levels were downregulated by sub-chronic stimulation of D1R in the denervated striatum.
Discussion
We report here the first direct evidence for Gαolf expression in human striatum and for increased levels of Gαolf and Gγ7 in the putamen of patients with Parkinson disease. Upregulation of Gαolf may result in an enhanced D1R signaling as shown by studies in the model of hemiparkinsonian rats. Our results identify increased Gαolf and enhanced D1R signaling as a factor possibly contributing to the genesis of l-dopa-induced dyskinesia.
Expression of Gαolf in human basal ganglia
The present study demonstrates that Gαolf is the predominant adenylyl cyclase-stimulatory G-protein α subunit expressed in the human striatum. The pattern of Gαolf distribution appeared similar with that described in rat, in which Gαolf expression was observed in striatal medium spiny neurons (Herve et al., 1993, 2001). In human brain, high Gαolf expression in medium spiny neurons was indicated by the drastic decrease in Gαolf levels in the putamen of HD patients, in which medium spiny neurons specifically degenerate. This strongly suggests that Gαolf, but not Gαs, couples D1R to adenylyl cyclase in human striatum as in rodents (Zhuang et al., 2000; Corvol et al., 2001). The physiological significance of selective Gαolf expression in striatal neurons remains unclear, because Gαolf and Gαs share 80% amino acid sequence identity and similarly activate adenylyl cyclase and couple β-adrenergic receptors in cell culture (Jones et al., 1990). However, because of lower affinity for GDP and more rapid GTP dissociation, Gαolf has a higher constitutive activity than Gαs in vitro, which may be critical for basal adenylyl cyclase activity (Liu et al., 2001), as supported by the decreased basal adenylyl cyclase activity in the striatum of Gαolf knock-out mice (Corvol et al., 2001).
Upregulation of Gαolf in the putamen of patients with Parkinson disease
In rodents, Gαolf and Gγ7, which are very likely associated in the same heterotrimeric G-protein, are required for the coupling of D1R to the adenylyl cyclase in the striatum (Zhuang et al., 2000; Corvol et al., 2001; Schwindinger et al., 2003). We observed in the putamen of PD patients a clear increase in the levels of both proteins. The present results suggest a coregulation of Gαolf and Gγ7, in agreement with the findings in Gγ7 knock-out mice, in which Gαolf levels were reduced in the striatum (Schwindinger et al., 2003). Coregulation of G-protein subunits may be linked to their early association during intracellular maturation and their coordinated trafficking to the plasma membrane (Michaelson et al., 2002).
The increase in Gαolf levels after dopamine denervation in PD may result from the lack of D1R stimulation in the denervated striatum. Previous studies in transgenic mice have suggested a dependence of Gαolf levels on receptor usage (Herve et al., 2001). Indeed, Gαolf amounts were increased in the striatum of D1R knock-out mice and conversely decreased in dopamine transporter knock-out mice, in which D1R is chronically stimulated by increased extracellular dopamine concentration (Giros et al., 1996; Herve et al., 2001). In PD, Gαolf amounts probably increase by a post-transcriptional mechanism, because Gαolf mRNA levels were unchanged after dopamine denervation in rats (Herve et al., 1993). Supporting this hypothesis, studies in cell cultures showed that receptor stimulation results in Gαs degradation, possibly related to its agonist-dependent depalmitoylation and cellular redistribution (Levis and Bourne, 1992; Milligan, 1993; Adie and Milligan, 1994).
In agreement with the regulatory role of D1R activation on Gαolf levels, restoration of D1R activation by a 3 week treatment with either l-dopa or a D1 agonist reversed Gαolf upregulation in the striatum of lesioned rats, whereas a 3 week D2R stimulation by ropinirole was ineffective. In contrast, Gαolf levels remained elevated in parkinsonian patients, although they had received l-dopa during a long period. Persistent increase in Gαolf, despite chronic l-dopa therapy, may be related to differences between human and rat either in the pharmacokinetics of l-dopa or in Gαolf regulation. However, differences in the modalities of degeneration of dopamine neurons or l-dopa treatments appear to be more likely. First, the lesion of dopamine neurons was unilateral in rat, whereas it was bilateral in PD patient. In rat, bilateral and unilateral lesions produce distinct physiological effects, as indicated by different changes in striatal expression of enkephalin (Salin et al., 1996). The long durations of disease (6-22 years) and l-dopa treatment (2-10 years) in human, compared with those in rat (6 week lesion and 3 week treatment) are also parameters to consider for explaining the differences in l-dopa-induced effects on Gαolf. Whatever the cause of difference between human and rat, the correlation between the duration of disease and the increase of Gαolf levels observed in the PD patients suggests that Gαolf levels increase with disease progression.
Significance of Gαolf increase for D1 receptor signaling
Several lines of evidence indicate that increased Gαolf levels result in increased D1R-activated signaling in PD patients. As in the 6-OHDA lesioned rat model (Herve et al., 1993), dopamine-stimulated adenylyl cyclase activity is increased in the putamen of parkinsonian patients (Pifl et al., 1992; Tong et al., 2004). In both lesioned rats and parkinsonian patients, the increased D1-stimulated cAMP production occurs in the absence of significant increase in the D1R levels (Shinotoh et al., 1993; Turjanski et al., 1997; Hurley et al., 2001; Cai et al., 2002). This discrepancy suggests the existence of an alteration in the protein(s) responsible for coupling D1R with adenylyl cyclase. The upregulation of Gαolf and Gγ7 reported here is in good agreement with such a hypothesis and is consistent with evidence showing that Gαolf levels are limiting for the D1 response in the striatum (Herve et al., 2001). It is also consistent with a recent study in 6-OHDA-lesioned rats that provided evidence for enhanced coupling of D1R with G-proteins in dopamine-denervated striatum indicated by a larger proportion of D1 binding sites in a high affinity state for agonist, an increased association of D1R with G-proteins detected by coimmunoprecipitation, and an enhancement of dopamine-induced G-protein activation and palmitoylation (Cai et al., 2002). Our results provide an explanation for these results, suggesting that increased Gαolf levels are responsible, at least in part, for increased coupling of D1R to adenylyl cyclase by mass action law. Our electron microscopy results in 6-OHDA-treated rats support this hypothesis, because Gαolf upregulation took place at the plasma membrane of medium-size spiny neurons, where it is expected to be functionally associated with D1R. Interestingly, after dopamine neuron lesion, D1R supersensitivity was also apparent in behavioral responses and in striatal gene induction (Trugman and Wooten, 1987; Berke et al., 1998). Thus, G-protein regulation appears to be a critical step in functional supersensitized responses, because the other components of D1R-related signaling were unaffected by the dopamine neuron lesion (Girault et al., 1989; Nishino et al., 1993).
Hypersensitivity of D1 receptor and genesis of dyskinesia
Pulsatile stimulation of supersensitive D1R has been implicated in the appearance of dyskinesia in l-dopa-treated PD patients (Gerfen, 2000b). l-Dopa that stimulates both D1R and D2R produced significantly more dyskinetic disorders in PD patients than the D2R-selective agonist ropinirole (Rascol et al., 2000). However, D1R hypersensitivity cannot be directly responsible for triggering dyskinesia episodes. If this were the case, dyskinesia would appear at the first l-Dopa administration, because D1R hypersensitivity resulting from dopamine depletion is present before any treatment. In fact, it takes repetitive administrations, sometimes for a long period of time, for the development of dyskinesia to be complete.
The Gαolf-linked increase in D1R signaling could constitute a crucial parameter for the generation of aberrant circuits responsible for dyskinetic movements. Activation of D1R modulates neuronal synaptic plasticity in the striatum through a cascade of intracellular events controlled by cAMP-dependent protein kinase (Greengard, 2001). In lesioned animals, D1R hypersensitivity is responsible for the induction by l-dopa of a complex gene program selectively in the medium spiny neurons of the direct pathway in the basal ganglia (Berke et al., 1998), leading in particular to a long-lasting accumulation of dynorphin and transcription factor ΔFosB in these neurons (Doucet et al., 1996; Steiner and Gerfen, 1998; Kelz and Nestler, 2000; Tekumalla et al., 2001). Such alterations could promote a progressive unbalance between direct and indirect pathways and facilitate development of dyskinesia (Gerfen, 2000b). In addition, D1R stimulation by l-dopa contributes to the progressive overexpression of D3 receptor (Bordet et al., 1997; Guillin et al., 2001), a critical mechanism of dyskinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys (Bezard et al., 2003). We therefore suggest that Gαolf upregulation may facilitate the genesis of dyskinesia in PD by increasing D1R signaling. Interestingly, the patients displaying the most severe l-dopa-induced dyskinesia were those with the highest Gαolf levels. Although the number of patients was too small for drawing final conclusions, our results support an important role of Gαolf upregulation in the functional hypersensitivity of D1R in PD. Treatments capable of normalizing Gαolf levels in PD might have beneficial therapeutic implications, especially for delaying the adverse consequences of long-term l-dopa therapy.
Footnotes
This work was supported in part by grants from Fondation pour la Recherche Médicale, Fondation Schlumberger pour l'Enseignement et la Recherche, and Fondation Liliane Bettencourt (J.-A.G.). J.-C.C. was supported by a fellowship (Poste d'accueil) from Institut National de la Santé et de la Recherche Médicale. l-Dopa-methyl-ester, benserazide, and ropinirole were generous gifts from Dr. Cudenec (Chiesi Farmaceutici Group, Parma, Italy), Roche Laboratories (Basel, Switzerland), and GlaxoSmithKline Laboratories (Marly-le-Roi, France), respectively. Anti-Gγ7 polyclonal antibody was kindly provided by J. D. Robishaw (Geisinger Clinic, Danville, PA).
Correspondence should be addressed to Dr. Jean-Christophe Corvol, Institut National de la Santé et de la Recherche Médicale U536, Institut du Fer à Moulin, 75005 Paris, France. E-mail: jean-christophe.corvol@psl.ap-hop-paris.fr.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247007-08$15.00/0
References
- Adie EJ, Milligan G (1994) Regulation of basal adenylate cyclase activity in neuroblastoma x glioma hybrid, NG108-15, cells transfected to express the human beta 2 adrenoceptor: evidence for empty receptor stimulation of the adenylate cyclase cascade. Biochem J 303: 803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R (1998) Mice deficient in G(olf) are anosmic. Neuron 20: 69-81. [DOI] [PubMed] [Google Scholar]
- Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515-532. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR (1998) A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci 18: 5301-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Brotchie JM, Gross CE (2001) Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci 2: 577-588. [DOI] [PubMed] [Google Scholar]
- Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P (2003) Attenuation of levodopa-induced dyskinesia by normalizing dopamine D(3) receptor function. Nat Med 9: 762-767. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC (1997) Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci USA 94: 3363-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Wang HY, Friedman E (2002) Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J Pharmacol Exp Ther 302: 1105-1112. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Studler JM, Schonn JS, Girault JA, Herve D (2001) Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem 76: 1585-1588. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH (1977) Dopamine receptor binding enhancement accompanies lesion-induced behavioral supersensitivity. Science 197: 596-598. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St. Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS (1996) Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci 8: 365-381. [DOI] [PubMed] [Google Scholar]
- Drinnan SL, Hope BT, Snutch TP, Vincent SR (1991) Golf in the basal ganglia. Mol Cell Neurosci 2: 66-70. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2002) mRNA and protein expression of selective alpha subunits of G proteins are abnormal in prefrontal cortex of suicide victims. Neuropsychopharmacology 27: 499-517. [DOI] [PubMed] [Google Scholar]
- Gerfen CR (2000a) Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci 23: S64-S70. [DOI] [PubMed] [Google Scholar]
- Gerfen CR (2000b) Dopamine-mediated gene regulation in models of Parkinson's disease. Ann Neurol 47: S42-S50. [PubMed] [Google Scholar]
- Girault JA, Raisman-Vozari R, Agid Y, Greengard P (1989) Striatal phosphoproteins in Parkinson disease and progressive supranuclear palsy. Proc Natl Acad Sci USA 86: 2493-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606-612. [DOI] [PubMed] [Google Scholar]
- Greengard P (2001) The neurobiology of slow synaptic transmission. Science 294: 1024-1030. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23: 435-447. [DOI] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411: 86-89. [DOI] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, Girault JA (1993) G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci 13: 2237-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Le Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, Jaber M, Studler JM, Girault JA (2001) Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J Neurosci 21: 4390-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MJ, Mash DC, Jenner P (2001) Dopamine D(1) receptor expression in human basal ganglia and changes in Parkinson's disease. Brain Res Mol Brain Res 87: 271-279. [DOI] [PubMed] [Google Scholar]
- Jones DT, Masters SB, Bourne HR, Reed RR (1990) Biochemical characterization of three stimulatory GTP-binding proteins. The large and small forms of Gs and the olfactory-specific G-protein, Golf. J Biol Chem 265: 2671-2676. [PubMed] [Google Scholar]
- Kelz MB, Nestler EJ (2000) deltaFosB: a molecular switch underlying long-term neural plasticity. Curr Opin Neurol 13: 715-720. [DOI] [PubMed] [Google Scholar]
- Lee T, Seeman P, Rajput A, Farley IJ, Hornykiewicz O (1978) Receptor basis for dopaminergic supersensitivity in Parkinson's disease. Nature 273: 59-61. [DOI] [PubMed] [Google Scholar]
- Levis MJ, Bourne HR (1992) Activation of the alpha subunit of Gs in intact cells alters its abundance, rate of degradation, and membrane avidity. J Cell Biol 119: 1297-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Ruberg M, Herrero MT, Villares J, Javoy-Agid F, Agid Y, Hirsch EC (1995) Alterations of GABAergic neurons in the basal ganglia of patients with progressive supranuclear palsy: an in situ hybridization study of GAD67 messenger RNA. Neurology 45: 127-134. [DOI] [PubMed] [Google Scholar]
- Liu HY, Wenzel-Seifert K, Seifert R (2001) The olfactory G protein G(alphaolf) possesses a lower GDP-affinity and deactivates more rapidly than G(salphashort): consequences for receptor-coupling and adenylyl cyclase activation. J Neurochem 78: 325-338. [DOI] [PubMed] [Google Scholar]
- Marcotte ER, Sullivan RM, Mishra RK (1994) Striatal G-proteins: effects of unilateral 6-hydroxydopamine lesions. Neurosci Lett 169: 195-198. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Ahearn I, Bergo M, Young S, Philips M (2002) Membrane trafficking of heterotrimeric G proteins via the endoplasmic reticulum and Golgi. Mol Biol Cell 13: 3294-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G (1993) Agonist regulation of cellular G protein levels and distribution: mechanisms and functional implications. Trends Pharmacol Sci 14: 413-418. [DOI] [PubMed] [Google Scholar]
- Monfort JC, Javoy-Agid F, Hauw JJ, Dubois B, Agid Y (1985) Brain glutamate decarboxylase in Parkinson's disease with particular reference to a premortem severity index. Brain 108: 301-313. [DOI] [PubMed] [Google Scholar]
- Muriel MP, Bernard V, Levey AI, Laribi O, Abrous DN, Agid Y, Bloch B, Hirsch EC (1999) Levodopa induces a cytoplasmic localization of D1 dopamine receptors in striatal neurons in Parkinson's disease. Ann Neurol 46: 103-111. [DOI] [PubMed] [Google Scholar]
- Nishino N, Kitamura N, Hashimoto T, Tanaka C (1993) Transmembrane signalling systems in the brain of patients with Parkinson's disease. Rev Neurosci 4: 213-222. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Olanow CW, Nutt JG (2000) Levodopa motor complications in Parkinson's disease. Trends Neurosci 23: S2-S7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, Ed 3. San Diego: Academic. [DOI] [PubMed]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-dopa-induced dyskinesia. Nat Neurosci 6: 501-506. [DOI] [PubMed] [Google Scholar]
- Pifl C, Nanoff C, Schingnitz G, Schutz W, Hornykiewicz O (1992) Sensitization of dopamine-stimulated adenylyl cyclase in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys and patients with idiopathic Parkinson's disease. J Neurochem 58: 1997-2004. [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. 056 Study group. N Engl J Med 342: 1484-1491. [DOI] [PubMed] [Google Scholar]
- Robishaw JD, Smigel MD, Gilman AG (1986) Molecular basis for two forms of the G protein that stimulates adenylate cyclase. J Biol Chem 261: 9587-9590. [PubMed] [Google Scholar]
- Salin P, Hajji MD, Kerkerian-le Goff L (1996) Bilateral 6-hydroxydopamine-induced lesion of the nigrostriatal dopamine pathway reproduces the effects of unilateral lesion on substance P but not on enkephalin expression in rat basal ganglia. Eur J Neurosci 8: 1746-1757. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Betz KS, Giger KE, Sabol A, Bronson SK, Robishaw JD (2003) Loss of G protein gamma 7 alters behavior and reduces striatal alpha olf level and cAMP production. J Biol Chem 278: 6575-6579. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Inoue O, Hirayama K, Aotsuka A, Asahina M, Suhara T, Yamazaki T, Tateno Y (1993) Dopamine D1 receptors in Parkinson's disease and striatonigral degeneration: a positron emission tomography study. J Neurol Neurosurg Psychiatry 56: 467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76-85. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR (1998) Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res 123: 60-76. [DOI] [PubMed] [Google Scholar]
- Tekumalla PK, Calon F, Rahman Z, Birdi S, Rajput AH, Hornykiewicz O, Di Paolo T, Bedard PJ, Nestler EJ (2001) Elevated levels of DeltaFosB and RGS9 in striatum in Parkinson's disease. Biol Psychiatry 50: 813-816. [DOI] [PubMed] [Google Scholar]
- Tong J, Fitzmaurice PS, Ang LC, Furukawa Y, Guttman M, Kish SJ (2004) Brain dopamine-stimulated adenylyl cyclase activity in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol 55: 125-129. [DOI] [PubMed] [Google Scholar]
- Trugman JM, Wooten GF (1987) Selective D1 and D2 dopamine agonists differentially alter basal ganglia glucose utilization in rats with unilateral 6-hydroxydopamine substantia nigra lesions. J Neurosci 7: 2927-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turjanski N, Lees AJ, Brooks DJ (1997) In vivo studies on striatal dopamine D1 and D2 site binding in L-dopa-treated Parkinson's disease patients with and without dyskinesias. Neurology 49: 717-723. [DOI] [PubMed] [Google Scholar]
- Wang Q, Jolly JP, Surmeier JD, Mullah BM, Lidow MS, Bergson CM, Robishaw JD (2001) Differential dependence of the D1 and D5 dopamine receptors on the G protein gamma 7 subunit for activation of adenylylcyclase. J Biol Chem 276: 39386-39393. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Belluscio L, Hen R (2000) G(olf)alpha mediates dopamine D1 receptor signaling. J Neurosci 20: RC91(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Westermark GT, LaMendola J, Boel E, Steiner DF (1993) Human G(olf) alpha: complementary deoxyribonucleic acid structure and expression in pancreatic islets and other tissues outside the olfactory neuroepithelium and central nervous system. Endocrinology 133: 2508-2514. [DOI] [PubMed] [Google Scholar]