Abstract
We recorded hippocampal place cells in two spatial environments: a training environment in which rats underwent fear conditioning and a neutral control environment. Fear conditioning caused many place cells to alter (or remap) their preferred firing locations in the training environment, whereas most cells remained stable in the control environment. This finding indicates that aversive reinforcement can induce place cell remapping even when the environment itself remains unchanged. Furthermore, contextual fear conditioning caused significantly more remapping of place cells than auditory fear conditioning, suggesting that place cell remapping was related to the rat's learned fear of the environment. These results suggest that one possible function of place cell remapping may be to generate new spatial representations of a single environment, which could help the animal to discriminate among different motivational contexts within that environment.
Keywords: activity, aversion, CA1, conditioned (conditioning), extracellular, hippocampus, learning
Introduction
The hippocampus contains place cells that fire preferentially when a rat visits specific locations within a spatial environment (O'Keefe and Dostrovsky, 1971). When a rat first explores a novel environment, hippocampal place cells establish preferred firing locations within that environment (Wilson and McNaughton, 1993), which tend to remain stable during repeated visits to the same environment (Muller et al., 1987; Thompson and Best, 1990). Distributed patterns of stable place fields may thus provide distinct hippocampal representations of each environment visited by the rat (Muller and Kubie, 1987; Bostock et al., 1991; Sharp, 1997; Guzowski et al., 1999; Lever et al., 2002), and this could explain why the hippocampus is often critical for solving tasks that require animals to recognize or discriminate environmental contexts (Kim and Fanselow, 1992; Phillips and LeDoux, 1994; Frankland et al., 1998; Holland and Bouton, 1999; Anagnostaras et al., 2001). Consistent with this view, the stability of place cells is disrupted by genetic, pharmacological, and physiological manipulations that affect contextual learning and hippocampal plasticity (Young et al., 1994; Cho et al., 1998; Kentros et al., 1998; Rotenberg et al., 2000; Dragoi et al., 2003).
However, place cells can change, or remap, their preferred firing locations within a spatial environment, even when the environment itself remains unchanged (Breese et al., 1989; Markus et al., 1995; Sharp et al., 1995; Kobayashi et al., 1997; Frank et al., 2000; Wood et al., 2000; Dragoi et al., 2003) (but see Lenck-Santini et al., 2001). Thus, place cell remapping seems to provide a mechanism by which the hippocampus can generate multiple representations of a single spatial environment, implying that hippocampus not only discriminates among different spatial environments, but also reflects the current temporal and behavioral context within a single environment (Sharp, 1999; Eichenbaum, 2000). However, the causes and functional purpose of such remapping are poorly understood.
In the present study, we addressed this question by investigating whether aversive conditioning induces place cell remapping. We recorded the activity of place cells from the CA1 region of the dorsal hippocampus, because previous studies have reported remapping in these cells (Breese et al., 1989; Markus et al., 1995; Sharp et al., 1995; Kobayashi et al., 1997; Frank et al., 2000; Wood et al., 2000; Dragoi et al., 2003). Rats that were chronically implanted with hippocampal recording electrodes were placed in an experimental chamber in which they foraged for small food pellets dropped from an overhead dispenser (Muller et al., 1987). While foraging, rats underwent fear conditioning in which an auditory conditioned stimulus (CS) was either paired (cued group; n = 8 rats) or explicitly unpaired (context group; n = 8 rats) with an electric shock as unconditioned stimulus (US). Fear learning was assessed by automated scoring of freezing behavior, a standard index of conditioned fear (Blanchard and Blanchard, 1969). Place cells were recorded from the hippocampus in the training box and a neutral control box before and after conditioning (see Fig. 1). The data presented in this study were collected concurrently with data reported in a previous study, from the same group of rats (Moita et al., 2003).
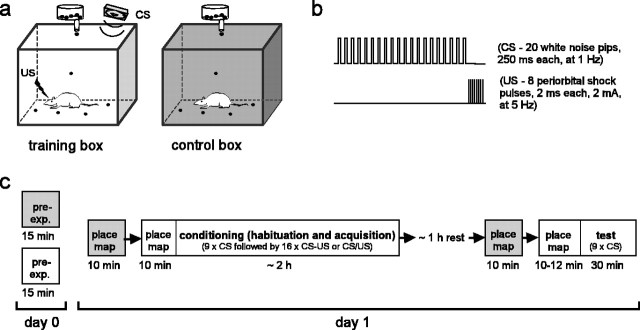
Figure 1.
Experimental design. a, Rats foraged for food pellets in two different chambers (training and control boxes) (see Materials and Methods). b, The CS consisted of a sequence of white-noise pips, and the US, a sequence of electric shocks delivered to the eyelid. c, Rats were preexposed (pre-exp.) for 15 min to each box on the day before conditioning. Three sessions—habituation, acquisition, and test—were given in the training box over a total time period of ∼4 hr. Place cell activity was recorded during place map sessions conducted in the training (open) and control (shaded) boxes at time points indicated in the diagram.
Materials and Methods
Subjects and surgery
Male Sprague Dawley rats, weighing 350-400 gm, were reduced to 85% of their ad libitum weight through limited daily feeding. Under deep Nembutal anesthesia (40 mg/kg), electrode drives consisting of 6-10 independently movable bundles of two (stereotrode) or four (tetrode) formvar-insulated nichrome wires (23 μm diameter; California Fine Wire, Grover Beach, CA) were stereotaxically implanted above the hippocampus (3.3 mm posterior, ±2.0 mm lateral, and 1.5 mm ventral to bregma). Silver wires (75 μm diameter; stripped of insulation 2 mm from the tip) were threaded through the skin of the right eyelid for delivery of the periorbital shock US. Postsurgical analgesics (2 mg/kg ketoprofen) were given daily for 3 d after surgery.
Fear conditioning
One day before fear conditioning, rats were preexposed for 15 min to both the training box (36 × 24 × 44 cm wooden chamber coated with white latex; brown formica floor washed with peppermint soap; enclosed in a bright, sound-attenuating chamber with white foam) and control box (28 × 22 × 37 cm metal chamber coated with dark gray latex; black plastic floor washed with unscented antibacterial soap; enclosed in a dim, sound-attenuating chamber with black foam). Throughout preexposure and subsequent experimental sessions in the boxes, rats constantly foraged for 20 mg food pellets dropped from an overhead dispenser at ∼30 sec intervals. The rat's moment-to-moment position in the chamber was sampled at 60 Hz by an overhead video tracking system that monitored the location of two light-emitting diodes attached to the animal's head stage. Fear conditioning was conducted as illustrated in Figure 1. Habituation and test sessions consisted of 9 presentations of the CS alone. Acquisition consisted of 16 CS-US presentations that were either paired (first US shock pulse occurring 300 msec after the offset of the last CS noise pip) or unpaired (no shock occurring within 30 sec of any pip). Intertrial interval varied pseudorandomly between 95 and 240 sec. Rats continued chasing food pellets in the training box for ∼10 min after the last acquisition trial.
Place cell recording
Beginning 5 d after surgery, daily screening sessions were conducted, in which electrode tips were advanced slowly (∼80 μm/d) until complex spike cells were encountered in the CA1 layer of the hippocampus, which was identified on the basis of EEG signals and single-unit spike patterns (Ranck, 1973; Buzsaki, 1986). Single-unit spikes were identified using on-line and off-line cluster analysis software (Datawave Technologies, Broomfield, CO), which used a multidimensional window discriminator to select units on the basis of waveform parameters such as peak-to-peak amplitude, peak-to-baseline amplitude, spike width, latency-to-peak, and latency-to-valley of the spike. Background noise on the electrode channels was <30 μA during all of the recording sessions. Single units had to meet several criteria for inclusion in the study. First, spike waveforms had to remain stable and well discriminated throughout the experiment. Second, interspike interval histograms had to exhibit a refractory period of at least 2 msec, so that high-frequency multiunit spike waveforms would not be included in the dataset. Third, to insure that remapping was not caused by movement of the recording electrode, cells had to exhibit a stable (non-remapping; defined as r > 0.33) place field during preconditioning and postconditioning sessions in at least one of the boxes. A cell was considered to have a place field during a given session if its peak in-field firing rate was >1.5 Hz, spatial information content was >0.2 bits/spike, and the cell fired at least 50 spikes during the session (see Table 1 for mean firing properties of place cells).
Table 1.
Firing properties of hippocampal place cells
|
|
Context group (n = 26) |
Cued group (n = 27) |
||||
|---|---|---|---|---|---|---|
|
|
Control box |
Training box |
Control box |
Training box |
||
| Peak in-field firing rate (Hz) | 6.9 ± 0.5 | 7.4 ± 0.6 | 7.5 ± 0.8 | 7.4 ± 0.9 | ||
| Mean firing rate (Hz) | 2.0 ± 0.2 | 1.5 ± 0.2 | 1.9 ± 0.3 | 1.7 ± 0.2 | ||
| Spatial information (bits/spike) | 0.7 ± 0.1 | 1.1 ± 0.3 | 1.4 ± 0.4 | 1.5 ± 0.4 | ||
| Field size | 80.7 ± 2.9** | 98.0 ± 4.6 | 82.6 ± 2.8*** | 101.1 ± 3.1 | ||
| Correlation index | 0.59 ± 0.04** | 0.28 ± 0.07# | 0.59 ± 0.06 | 0.49 ± 0.07 | ||
| Peak shift (cm) | 8.4 ± 1.3* | 17.3 ± 3.1 | 9.2 ± 1.4 | 11.3 ± 1.9 | ||
| Gain/loss of fielda
|
8% |
19% |
4% |
12% |
||
*p≤0.05; **p≤0.01; ***p≤0.001; between-box differences within each group.
#p≤0.05; between-group differences within each box.
Field gain/loss percentages are expressed as a proportion of all of the cells recorded from the group.
Data analysis
Freezing. An episode of freezing was defined as a period during which the rat's movement speed was zero (that is, the animal's tracked position did not change) for a period of ≥⅓ sec. To obtain the total amount of time the rat spent freezing during a given time span (e.g., the 20 sec CS period), the durations of all of the freezing episodes that occurred during that time span were summed together. Freezing scores obtained by this method were >90% correlated with scores of experienced human observers.
Place maps. To compensate for different levels of freezing behavior between groups, analyses of place maps included only spikes and position samples from periods when rats were moving at a speed of at least 18 cm/sec (see Results). Pixels were binned at a resolution of 9 cm2, and undersampled pixel bins (those visited for <1 sec during either the preconditioning or postconditioning session) were not included in the place map. Firing rate in each bin was computed by dividing the number of spikes the cell generated in that bin by the number of position samples the rat spent in that bin. Firing-rate maps were smoothed by a single iteration of adjacent pixel averaging. Contour plots were generated using Origin 5.0 (OriginLab, Northampton, MA).
Remapping analysis. The place cell field shift for a given cell was measured by finding the pixel with the highest firing rate in the preconditioning and postconditioning place maps of the cell, and computing the distance (in centimeters) between the centers of these two pixel locations. To compute the Pearson product-moment correlation (r) between firing rates in the pixels of each map, it was necessary to have the same number of pixels in the preconditioning and postconditioning place maps. Therefore, any pixel that was undersampled (see above) during either the preconditioning or postconditioning place map session was excluded from the correlation analysis, so that only those pixels that were well sampled in both sessions were included (note that this is why the white region denoting undersampled pixels is the same for preconditioning and postconditioning maps in the place map diagrams shown in Figs. 4, 5, 6). Similarly, in the analysis of within-session place map stability, the correlation analysis was performed only on those pixels that were adequately sampled during the first and second halves of the session (see Fig. 6). The sampling requirement in each pixel was reduced from 1 to 0.5 sec for the within-session analysis, because the data collection time was halved by splitting the session in two.
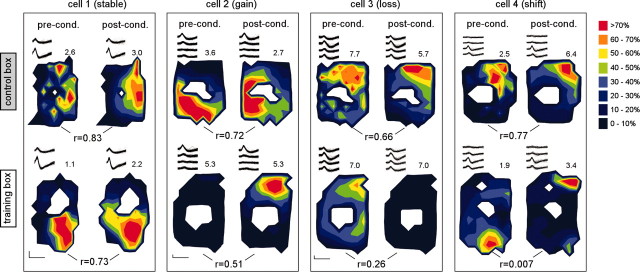
Figure 4.
Example place maps. a, Four place maps [showing preconditioning (pre-cond.) and postconditioning (post-cond.) maps in the control box and training box] are plotted for each of four cells (one from the cued and three from the context group). Color-coded firing rates at each location in the box are plotted as a percentage of a scaling factor, equal to 2 SDs above the mean firing rate (in Hertz) of the cell, shown at top right of each map (white space indicates undersampled pixels) (see Materials and Methods). Pixel-by-pixel correlations (r) between preconditioning and postconditioning maps are shown below each pair of maps. Representative spike traces for each cell in each session are depicted at the top left of each map, and calibration, showing 200 μV (vertical) and 1 msec (horizontal), is plotted in the bottom left corner for each cell.
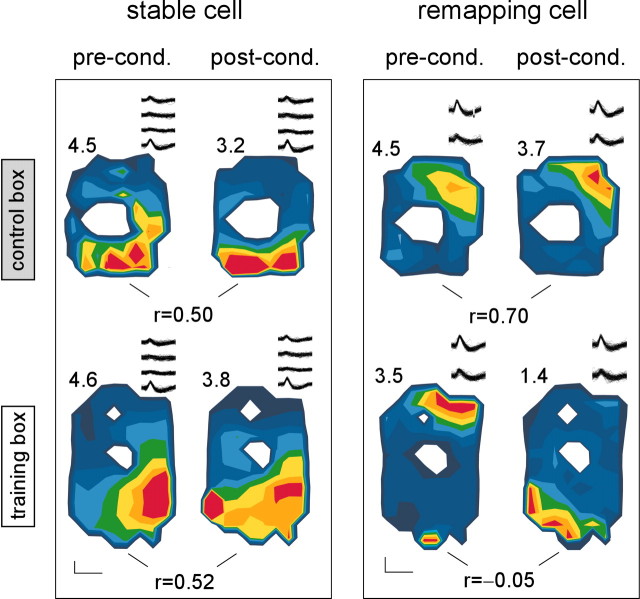
Figure 5.
Partial remapping of hippocampal place cells. Examples of two place cells (one stable and one remapping) recorded simultaneously from the same rat from the context group. Traces, mean firing rates, and correlation indices between maps are shown as in Figure 4. pre-cond., Preconditioning; post-cond., postconditioning.
Figure 6.
Within-session stability of place fields (example cell). On the top row, two maps show the place-specific activity of this cell during the entire place map session before (left) and after (right) conditioning in the training box. On the bottom row, each place map session is divided into two halves (∼5 min/half). Note that, within each session (both before and after conditioning), the place field remained stable, despite the fact that this cell remaps its firing field after conditioning. Mean firing rates and correlation indices are shown as in Figure 4, and traces are shown next to the preconditioning (pre-cond.) and postconditioning (post-cond.) place maps.
Z-score normalized correlation index in the training box (rT). To compare remapping between the context and cued groups, we computed a Z-score for each cell from both groups. The Z-scores were based on the mean correlation index in the control box for each group, which was used as the expected correlation value, because place fields were most stable in this box.
Place cell properties. Mean firing rate was the average rate of all of the pixel bins in a place map. Peak in-field firing rate was the rate of the highest pixel bin in the map. Field size was the summed extent (in square centimeters) of all of the contiguous regions of pixel bins whose firing rate exceeds the mean rate by at least 1 SE. Spatial information content was computed by standard methods (Skaggs et al., 1993).
Spatial uniformity index. The spatial uniformity index (SUI) was computed as ∑i = 1,ρ (pi - 1/ρ)2, where index i sums over the ρ adequately sampled pixel bins that were included in both the preconditioning and postconditioning place maps, and pi is the actual percentage of time the rat spent in the ith pixel bin.
θ Power. To analyze θ rhythm, a dense multiunit spike signal was compiled from one or several hippocampal electrodes in each rat, and a power analysis was performed on the multiunit spike train using Neuro-explorer software (Nex Technologies, Littleton, MA). To analyze θ rhythm specifically during place cell activity, the power analysis was performed only on those multiunit spikes that occurred within 500 msec of a single-unit spike from a given place cell. The t tests on θ power were performed by comparing the summed power from frequency bins in the 6-8 Hz range.
Results
Freezing behavior
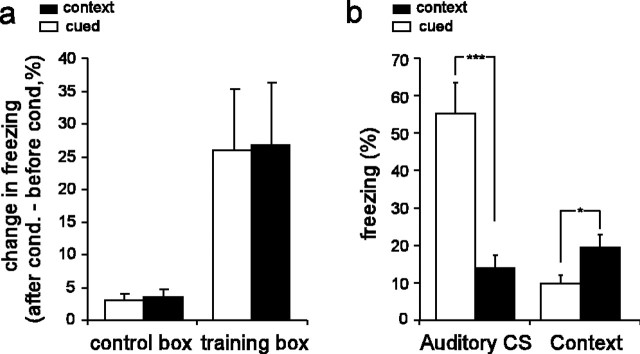
Rats in both groups showed increased freezing in the training box immediately after conditioning, because all of the rats received electrical shocks every 3 min, but no increase in freezing was observed in the control box in which no shocks were delivered (Fig. 2a). A two-way ANOVA, testing for main effect of group and main effect of box, revealed no significant effect of group (F(1) = 0.001; p = 0.97), a significant effect of box (F(1) = 25.36; p < 0.0001), and no significant interaction (F(1) = 0.24; p = 0.88). Because rats were freezing about one-third of the time during the 10 min immediately after conditioning, it was not possible to analyze place cell activity because of poor sampling of the environment by the rats. However, after the 1 hr rest, freezing levels to the context were lower, allowing for good sampling of the environment by the rats, and thus place cell activity was analyzed at this time (postconditioning place map session in Fig. 1c). We next examined whether rats from the cued and context groups displayed differential freezing to the auditory cue and the training context. To do so, we measured the amount of fear of the auditory CS and the training context after conditioning. Freezing scores were taken during the presentation of the auditory CS (in the test session) and during the postconditioning place map session in the training box (Fig. 2b). A two-way ANOVA comparing the amount freezing after conditioning with the auditory CS and context in both groups revealed a significant main effect of group (F(14) = 13.07; p = 0.003), of stimulus type (F(14) = 13.06; p = 0.003), and interaction (F(14) = 20.86; p = 0.0004). Post hoc Newman-Keuls t tests showed that, after conditioning, rats in the cued group froze significantly more to the auditory CS than rats in the context group (p = 0.0006), whereas rats in the context group froze more to the training context than rats in the cued group (p = 0.04). Hence, in accordance with previous studies (Rescorla, 1968; Phillips and LeDoux, 1994), we found that rats in the cued group acquired more fear of the CS than of the context (because the CS is the best predictor of shock when it is paired with the US), whereas rats in the context group acquired more fear of the context than of the CS (because the context is the best predictor of shock when the CS and US are unpaired). Freezing in the control context after conditioning was not significantly different between the cued and context groups (unpaired t(14) = 0.66; p = 0.51), again indicating that contextual fear conditioning was specific to the training context.
Figure 2.
Behavioral analysis of fear conditioning. a, Bar graph shows mean and SE of conditioning-induced increase in freezing behavior, averaged over all rats in the cued (n = 8) and context (n = 8) groups. Training box data compare the 10 min period immediately after the last acquisition trial with the 10 min place map period immediately preceding the first acquisition trial; control box data compare the preconditioning versus postconditioning place map sessions. b, Mean postconditioning freezing behavior to the auditory CS (measured during the test session) and training context (measured during the first 10 min of the postconditioning place map session in the training box) for all rats in the cued (n = 8) and context (n = 8) groups. *p ≤ 0.05; *** p ≤ 0.001.
Place cell remapping
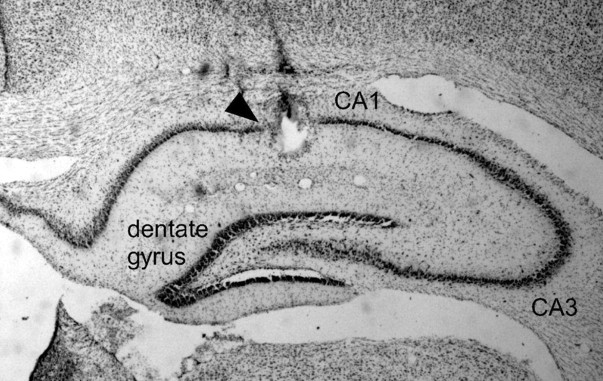
A total of 154 hippocampal θ and complex spike cells was recorded during fear conditioning from the 16 rats in both groups (Fig. 3 shows an example an electrode tip placed in the CA1 region of the dorsal hippocampus). Of these, 53 complex spike cells (cued group, n = 27; context group, n = 26) met criteria for inclusion in the present study (see Materials and Methods). Henceforth, we shall refer to these cells as place cells, because all exhibited spatial firing correlates. Two rats from each group had no cells that met criteria for inclusion in the analysis; consequently, the cells included in this study were recorded from six rats in the cued group and six rats in the context group.
Figure 3.
Electrode placement. Coronal section of dorsal hippocampus. The arrowhead indicates the lesion left by passing current through the tip of the recording electrode at the end of the experiment.
To examine the spatial firing properties of place cells, we plotted place maps to show the firing rate of each cell as a function of the rat's spatial location in the recording chamber (Fig. 4). The general firing properties of place cells in both the control and training boxes are summarized in Table 1. As illustrated in Figure 4, fear conditioning caused some place cells to remap their firing field, either by losing their field, gaining a new field, or shifting their field location. Such remapping responses were more prevalent in the training box than the control box, in which place fields remained mostly stable. For example, place cells were more likely to either lose or gain a field in the training box than in the control box, and also showed a greater tendency to shift their field location in the training box than the control box (Table 1). To quantify these field shifts, we measured the distance (in centimeters) between the location of the peak of the place field during the preconditioning and postconditioning session in each box (Lever et al., 2002). This analysis was performed only on cells that exhibited place fields both before and after conditioning within a given box. Field peak shifts were significantly larger in the training than the control box: straining = 13.95 ± 1.78, and scontrol = 8.76 ± 0.95 (paired t(30) = -2.48; p = 0.02), suggesting that place fields remapped the training box, while remaining stable in the control box.
To further quantify the remapping of place cells caused by fear acquisition, we calculated two correlation indices for each place cell—one for the training (rT) and one for the control box (rC)—by computing the pixel-by-pixel correlation between preconditioning and postconditioning place maps (see Materials and Methods). Low r values indicate that the cell remaps its firing field in a given box after conditioning, whereas high r values indicate that the place map remains stable (only the cells that showed place-specific firing in both boxes were included in this analysis, including those that gained or lost a field). The correlation indices were significantly greater in the control box than the training box: rC = 0.59 ± 0.04 and rT = 0.39 ± 0.05 (paired t(41) = 3.15; p = 0.003). This again indicates that place cell remapping was more likely to occur in the training than in the control box. Despite the increased remapping of place cell firing fields observed in the training box, conditioning did not alter the peak in-field firing rate (paired t(41) = -0.48; p = 0.63), mean firing rate (paired t(41) = -1.08; p = 0.29), mean spatial information content (paired t(41) = -0.78; p = 0.44), or mean size of firing fields (paired t(41) = -0.14; p = 0.89) in the training box.
Although many place cells remapped their firing fields in the training box as a consequence of conditioning, not all of them did. Indeed, even among cells recorded from a single animal, some cells remapped, whereas others did not, a phenomenon that has been referred to as “partial remapping” (Skaggs and McNaughton, 1998) (Fig. 5).
A possible explanation for low rT values observed in the training box could be that place cells became generally unstable in the training box after conditioning, no longer exhibiting coherent place-specific firing. To test this possibility, we analyzed the within-session stability of place cells by dividing each place map session in the training box into two 5 min halves, and then calculating correlation values between the first and second halves of the session (Fig. 6). We found that, in the training box, the within-session correlation after conditioning (rPOST = 0.56 ± 0.04) was not significantly different from within-session correlation before conditioning (rPRE = 0.57 ± 0.03) (paired t(37) = -0.53; p = 0.60), indicating that conditioning did not affect the within-session stability of place fields. Note that this analysis could be performed only on cells that had place fields both before and after conditioning, so gains/losses of field were not included. Furthermore, the within-session correlation value in the training box after conditioning is significantly higher than the between-session correlation value in the training box, that is, rPOST = 0.56 ± 0.04 and rT = 0.39 ± 0.05 (paired t(41) = -2.20; p = 0.03). These results are in accordance with the interpretation that the conditioning-induced remapping reflects the formation of a stable new map of the training environment.
Together, these results show that place cells remained stable in the control environment, which is in accordance with a vast literature showing that hippocampal place cells acquire place fields in a novel environment rapidly, and that these fields are stable over many exposures to that same environment (Muller and Kubie, 1987; Bostock et al., 1991; Sharp, 1997; Guzowski et al., 1999; Lever et al., 2002). Furthermore, they indicate that fear conditioning causes place cells to remap the training box—by losing, gaining, or shifting their firing fields.
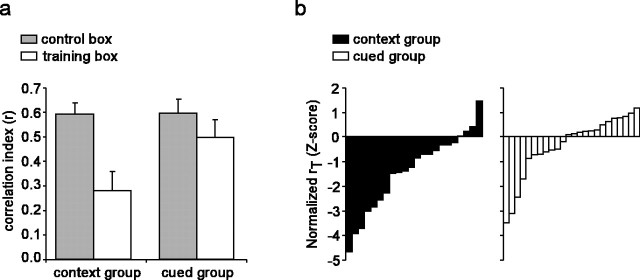
If remapping of the training box is related to the rats' acquired fear of the training environment, it might be expected that remapping should be stronger in rats from the context group than rats from the cued group, because they acquired more fear of the training environment. To compare remapping of the training box (relative to the control box) between the two groups, we normalized the correlation index in the training box (rT) of each cell by computing a Z-score for each cell (see Materials and Methods) on the basis of the mean correlation index in the control box (rC) of each group (no difference was found for rC between the two groups; unpaired t(47) = 0.02; p = 0.98). The rank-ordered distribution of the normalized rT values for cells from the context group is skewed to negative values, indicating that these cells were more likely to remap the training box than the control box (Fig. 7b), whereas this was not the case for cells from the cued group. The normalized rT values were then compared between the cued and context groups. We found that the normalized rT was smaller for cells in the context group than the cued group (Zcontext = -1.36 ± 0.33 and Zcued = -0.34 ± 0.25; unpaired t(44) = 2.47; p = 0.02), suggesting that remapping of the training box relative to the control box was more pronounced in cells recorded from the context group than the cued group. The difference between the cued and context groups in remapping of the training box by place cells was not accompanied by significant between-group differences in peak in-field firing rate (unpaired t(39) = 0.33; p = 0.75), mean firing rate (unpaired t(39) = 0.04; p = 0.97), mean spatial information content (unpaired t(39) = 0.67; p = 0.51), or mean size of firing fields (unpaired t(39) = -1.25; p = 0.21), of place cells in the training box after conditioning. This finding suggests that remapping of the training box by hippocampal place cells was indeed related to acquired fear of the training environment, rather than to differences between the training and control boxes.
Figure 7.
Difference in remapping between the cued and context groups. a, Bars show the mean and SE of the correlation index of the preconditioning versus postconditioning place maps in the training (rT) and the control (rC) box for cells from the context group and cued group. b, Rank-ordered distributions of the normalized rT scores for cells from the context (n = 22) and cued (n = 24) groups that had a place field in the training box. Negative scores indicate more remapping of the training than the control box, whereas positive scores indicate more remapping of the control than the training box.
However, previous studies have shown that place cell remapping can occur in conjunction with changes in the behavior of the rat (McNaughton et al., 1983; Markus et al., 1995). Therefore, it is necessary to consider possible ways in which behavioral differences between the two groups might account for the greater remapping that was observed in the context group.
Behavioral controls
It is not possible to control for every possible behavioral influence on place cell activity, but in this section, we present several analyses of behavioral variables that could possibly account for the remapping observed in the context group.
Although rats in both groups spent most of their time foraging for food pellets throughout the experiment, their behavior after conditioning was not identical. Because of their greater fear of the training box, rats in the context group spent more time freezing (92 ± 21 sec) than rats in the paired group (57 ± 17 sec) during the first 10 min of the postconditioning place map session in the training box. However, because place maps included only data from periods when rats were moving (see Materials and Methods), freezing behavior could not have directly influenced our analysis of place maps. Furthermore, the duration of the postconditioning place map session in the training box was extended to 12 min for rats in the context group (compared with 10 min for the cued group), so that, despite the increased freezing in the context group, there was no significant difference between groups in the mean number of position samples used to create the place map for this session (unpaired t(10) = -0.8; p = 0.44). Still, it remains possible that, after conditioning, rats in one group (but not the other) preferred to spend time in different parts of the training box (compared with before conditioning). To control for this, we performed a correlation analysis on the percentage of time spent in each pixel of the training box before and after conditioning, and found that mean correlation values were similar for rats in the cued and context groups (unpaired t(10) = 0.11; p = 0.91). We also examined whether rats uniformly sampled the training box after conditioning by calculating an SUI for each rat (see Materials and Methods) and found that, while rats were moving, they showed no group differences in their tendency to forage throughout all of the parts of the training box after conditioning (unpaired t(10) = 0.56; p = 0.59).
It is well known that place cells can exhibit different firing fields when rats follow different trajectories through the same environment (McNaughton et al., 1983; Markus et al., 1995; Frank et al., 2000; Wood et al., 2000). Because rats in both groups tended to circle the chamber while searching for food pellets, one possibility is that fear conditioning altered the rats' preferred circling direction, which in turn could account for the remapping effects reported here. To examine this possibility, we measured the ratio of clockwise versus counterclockwise turning movements during the preconditioning and postconditioning sessions, and found that one rat in the cued group and no rats in the context group switched their preferred circling direction after conditioning (no difference between the changes in the ratio of clockwise vs counterclockwise angular velocity was found between the two groups; unpaired t(10) = 1.63; p = 0.13). Thus, changes in circling direction probably did not contribute to place cell remapping.
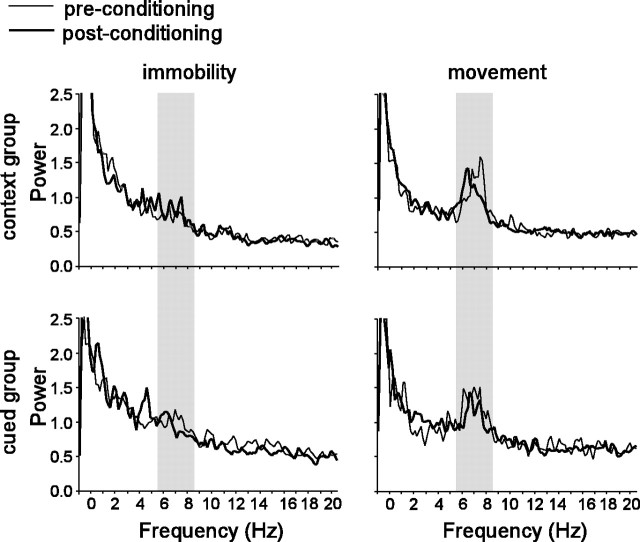
Finally, because the hippocampus exhibits rhythmic activity in the θ frequency band (6-8 Hz) that is known to reflect changes in behavior and arousal state of the rat (Buzsaki, 2002), we investigated whether θ rhythm was differentially affected by fear conditioning in the cued versus context group. We found that, both before and after conditioning, the power spectrum of a multiunit recording signal (see Materials and Methods) showed a characteristic peak in the θ band when the rat was moving, but not when the rat was immobile (Fig. 8). To investigate whether the remapping of the training box by cells in the context group was accompanied by any alteration in θ rhythm during place cell activity, we compared the power of the multiunit signal in the θ band during periods when each cell was active, both before and after conditioning in the training box (this analysis was only performed on place cells that exhibited place fields both before and after conditioning in the training box). Conditioning did not induce any significant change in θ power of the multiunit signal during spike generation by place cells (unpaired t(14) = 1.22; p = 0.24). Thus, the greater remapping that was observed in the context group was not accompanied by any clear changes in the hippocampal θ signal.
Figure 8.
θ Rhythm. Each panel shows the power spectrum (bin width, 0.0256 Hz) of the hippocampal multiunit recording signal during place map sessions in the training box. Shaded region indicates the θ band (6-8 Hz).
Together, these results indicate that the difference in remapping between the cued and context groups is not related in any obvious way to conditioning-induced changes in the rat's behavior. Therefore, our findings suggest that contextual fear conditioning causes place cells to reorganize their spatial representation of the environment in which training occurs.
Discussion
In the present study, we showed that aversive conditioning can cause a subset of hippocampal place cells to change their preferred firing location in the environment in which training occurs. This partial-remapping effect is most pronounced when rats learn to fear the training environment (context conditioning) compared with when rats learn to fear a discrete auditory cue (cue conditioning). What might be the purpose of such place cell remapping during contextual fear conditioning?
Place cell remapping seems to provide a mechanism by which the hippocampus can generate multiple representations of a single spatial environment. This is consistent with the notion that the hippocampus is not only important for discriminating among different spatial environments, but also for encoding specific temporal or behavioral variables within a single environment (Sharp, 1999; Eichenbaum, 2000). If it is true that place cell remapping allows the hippocampus to represent different states of a single environment, then the hippocampus must solve an intriguing stability-plasticity problem (Grossberg, 1988), namely, place cells must somehow decide when the state of a given environment is stable versus when it has changed sufficiently to justify re-representation of that environment by shuffling their preferred firing locations.
In the present experiment, the first experience that rats had in the training environment was the preconditioning session on d 0, during which they chased food pellets and did not receive any aversive stimuli (Fig. 1). The hippocampus probably formed its initial representation of the training environment during this preexposure session (Wilson and McNaughton, 1993). In addition, the rats may also have learned that the training environment was an appetitive location in which food seeking and consumption behaviors should be performed. But the rat's next visit to the environment (d1) was paired with not only appetitive reinforcement (food) but also aversive reinforcement (shock). Thus, an environment that previously predicted food now predicted shock as well. This changed the nature of behaviors that were appropriate in the environment: defensive behaviors became more appropriate than food-seeking behaviors. How might the rat's brain adjust to this new situation? One possibility would be to alter the hippocampal representation of the environment itself, so that the previously learned appetitive associations would no longer be in force. Aversive associations could then quickly be formed with the new hippocampal representation, without competition from previously acquired appetitive associations. Our finding that contextual fear learning alters the hippocampal representation of the training environment suggests that such recoding of the environment may indeed occur when the motivational valence of the environment is changed. This may help to reduce competition between different motivational drives that might otherwise occur if a single environmental representation were permitted to acquire both appetitive and aversive motivational valence. This interpretation is consistent with other studies showing that the hippocampus encodes the same location differently, depending on what behavior the rat intends to perform in the near future (Frank et al., 2000; Wood et al., 2000). Together, the results of the present study and previous studies suggest that the hippocampal code for space may reflect not only the geometry of that space, but also the motivational valence and behavioral responses that are associated with that space.
Remapping of the training environment in the present study was not complete but partial: some place cells retained the same firing fields before and after fear conditioning, whereas other cells remapped (even in the same rat). It is possible that such partial remapping allows the hippocampus to compromise in its attempt to solve the stability-plasticity problem. That is, cells that remap their preferred firing location may allow rapid acquisition of new associations with the context, whereas cells that retain stable firing fields may help to preserve previously learned information about the context. It is tempting to speculate that place cells that remap their firing location in response to a novel reinforcer (such as shock) are more likely to become associated with that reinforcer (and thereby support the contextual prediction of that reinforcer) than place cells that do not remap. It would be difficult to test this idea experimentally. However, it is interesting to note that, in the present study, place cell remapping was more pronounced in rats that primarily learned to fear the training environment (context group) compared with rats that primarily learned to fear the auditory CS (cued group), even though the stimuli (CS, US, and training box) in both tasks were the same. These results suggest that place cell remapping in the hippocampus is not strictly determined by the sensory characteristics of the events an animal experiences, but rather what the animal learns about them. In contextual fear conditioning, the environment acquires a new motivational valence and becomes a predictive cue for making a motivated behavioral choice (to freeze vs forage). In contrast, the motivational valence of the environment does not change as much during cued fear conditioning, because the cue rather than the context provides the best information for choosing the appropriate behavior, and this may be why remapping is less pronounced in this case. This suggests that place cells are most likely to remap an environment in which aversive reinforcement occurs when the environment itself is the best predictor of reinforcement, which provides circumstantial evidence for the hypothesis that cells that remap are more involved in acquiring the new contextual association than non-remapping cells, because the demand for such cells would be greater during context conditioning than cue conditioning.
It is important to note that, even though remapping was less pronounced in the cued group, there were still some cells recorded from this group that remapped the training environment. Because rats that undergo auditory fear conditioning also acquire some fear of the training environment (even though the environment in this case is not the best predictor of shock), it is possible that the lesser remapping exhibited by place cells in the cued group is related to the lesser amount of fear of the training environment that rats in this group acquired with conditioning.
Some previous studies have reported remapping in response to changes in the location of reinforcement (Breese et al., 1989; Markus et al., 1995; Fyhn et al., 2002). In one such study, place cells were recorded in a water maze, and firing fields were observed to migrate to the location of the platform when it was moved to a novel location (Fyhn et al., 2002). However, this remapping effect was temporary, and place cells eventually resumed their original firing fields. This finding raises the question of whether the context conditioning-induced remapping we observed here is long lasting, or whether it might be only temporary. Because of the difficulties associated with recording the same place cells over multiple days, we were unable to address this question in the present study. However, it is worth noting that several studies have shown that the hippocampus can store multiple maps of the same environment across multiple days (Sharp et al., 1995; Frank et al., 2000; Wood et al., 2000), just as it can store stable, long-lasting representations of two spatial environments with different geometric shapes (Lever et al., 2002).
In summary, the results of the present study support the view that hippocampal place cells encode not only the geometry of a spatial environment, but also the motivational and behavioral variables that are associated with the environment. Additional study of this aspect of place cell activity may provide important insights about the role of the hippocampus in motivation, learning, and memory.
Footnotes
M.A.P.M. is supported by Gulbenkian Foundation and Program PRAXIS XXI/Fundação para a Ciência e a Tecnologia under the “Programa Gulbenkian de Doutoramento em Biologia e Medicina.” In addition, this work was supported by National Institute of Mental Health (NIMH) Grant MH12341 to H.T.B. and NIMH Grants MH38774, MH46516, and MH00956 to J.E.L. We thank Valerie Doyere, Jeff Erlich, Karim Nader, and Pat Sharp for helpful comments on previous versions of this manuscript.
Correspondence should be addressed to Dr. Hugh T. Blair, Psychology Department, University of California, Los Angeles, 1285 Franz Hall, Box 951563, Los Angeles, CA 90095-1563. E-mail: blair@psych.ucla.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247015-09$15.00/0
References
- Anagnostaras SG, Gale GD, Fanselow MS (2001) Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus 11: 8-17. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC (1969) Crouching as an index of fear. J Comp Physiol Psychol 67: 370-375. [DOI] [PubMed] [Google Scholar]
- Bostock E, Muller RU, Kubie JL (1991) Experience-dependent modifications of hippocampal place cell firing. Hippocampus 1: 193-205. [DOI] [PubMed] [Google Scholar]
- Breese CR, Hampson RE, Deadwyler SA (1989) Hippocampal place cells: stereotypy and plasticity. J Neurosci 9: 1097-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G (1986) Hippocampal sharp waves: their origin and significance. Brain Res 398: 242-252. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2002) θ oscillations in the hippocampus. Neuron 33: 325-340. [DOI] [PubMed] [Google Scholar]
- Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H (1998) Abnormal hippocampal spatial representations in αCaMKIIT286A and CREBαΔ- mice. Science 279: 867-869. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Harris KD, Buzsaki G (2003) Place representation within hippocampal networks is modified by long-term potentiation. Neuron 39: 843-853. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2000) Hippocampus: mapping or memory? Curr Biol 10: R785-R787. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M (2000) Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27: 169-178. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ (1998) The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci 112: 863-874. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Hollup S, Moser MB, Moser E (2002) Hippocampal neurons responding to first-time dislocation of a target object. Neuron 35: 555-566. [DOI] [PubMed] [Google Scholar]
- Grossberg S (1988) Nonlinear neural networks: principles, mechanisms, and architectures. Neural Networks 1: 17-61. [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF (1999) Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci 2: 1120-1124. [DOI] [PubMed] [Google Scholar]
- Holland PC, Bouton ME (1999) Hippocampus and context in classical conditioning. Curr Opin Neurobiol 9: 195-202. [DOI] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV (1998) Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science 280: 2121-2126. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS (1992) Modality-specific retrograde amnesia of fear. Science 256: 675-677. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Nishijo H, Fukuda M, Bures J, Ono T (1997) Task-dependent representations in rat hippocampal place neurons. J Neurophysiol 78: 597-613. [DOI] [PubMed] [Google Scholar]
- Lenck-Santini P-P, Save E, Poucet B (2001) Place-cell firing does not depend on the direction of turn in a Y-maze alternation task. Eur J Neurosci 13: 1055-1058. [DOI] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O'Keefe J (2002) Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature 416: 90-94. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Qin YL, Leonard B, Skaggs WE, McNaughton BL, Barnes CA (1995) Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci 15: 7079-7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, O'Keefe J (1983) The contributions of position, direction, and velocity to single unit activity in the hippocampus of freely-moving rats. Exp Brain Res 52: 41-49. [DOI] [PubMed] [Google Scholar]
- Moita AP, Rosis S, Zhou Y, LeDoux JE, Blair HT (2003) Hippocampal place cells acquire location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron 37: 485-497. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL (1987) The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci 7: 1951-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck Jr JB (1987) Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci 7: 1935-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34: 171-175. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1994) Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem 1: 34-44. [PubMed] [Google Scholar]
- Ranck Jr JB (1973) Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol 41: 461-531. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1968) Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol 66: 1-5. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Abel T, Hawkins RD, Kandel ER, Muller RU (2000) Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. J Neurosci 20: 8096-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE (1997) Subicular cells generate similar spatial firing patterns in two geometrically and visually distinctive environments: comparison with hippocampal place cells. Behav Brain Res 85: 71-92. [DOI] [PubMed] [Google Scholar]
- Sharp PE (1999) Complimentary roles for hippocampal versus subicular/entorhinal place cells in coding place, context, and events. Hippocampus 9: 432-443. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Blair HT, Etkin D, Tzanetos DB (1995) Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J Neurosci 15: 173-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL (1998) Spatial firing properties of hippocampal CA1 populations in an environment containing two visually identical regions. J Neurosci 18: 8455-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard K, Markus EJ (1993) An information-theoretic approach to deciphering the hippocampal code. In: Advances in neural information processing (Hanson SJ, Cowan JD, Giles CL, eds), pp 1030-1037. San Mateo, CA: Morgan Kaufman.
- Thompson LT, Best PJ (1990) Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res 509: 299-308. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1993) Dynamics of the hippocampal ensemble code for space. Science 261: 1055-1058. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H (2000) Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27: 623-633. [DOI] [PubMed] [Google Scholar]
- Young SL, Bohenek DL, Fanselow MS (1994) NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav Neurosci 108: 19-29. [DOI] [PubMed] [Google Scholar]