Abstract
Norepinephrine (NE) plays a complex role in the behavioral state-dependent regulation of sensory processing. However, the role of forebrain NE action in modulating high-order sensory activity has not been directly addressed. In this study, we take advantage of the discrete, feedforward organization of the avian song system to identify a site and mechanism of NE action underlying state-dependent modulation of sensory processing. We have developed an experimental paradigm in which brief arousal repeatedly suppresses song system auditory responsiveness. Using pharmacological manipulations in vivo, we show that infusion of α-adrenergic antagonists into the NIf (nucleus interfacialis of the nidopallium), an auditory forebrain area, blocks this state-dependent modulation. We also demonstrate dose-dependent enhancement and suppression of song system auditory response properties by NE and adrenergic agonists. Our results demonstrate that noradrenergic release in a single forebrain area is a mechanism underlying behavioral state-dependent regulation of auditory processing in a neural system specialized for vocal learning.
Keywords: song system, arousal, norepinephrine, auditory, behavioral state, forebrain
Introduction
Ascending modulatory input, such as that from the cholinergic, noradrenergic, and histaminergic systems, robustly alters mammalian brain responses to sensory inputs in a behavioral state-dependent manner (Steriade and McCarley, 1990; McCormick, 1992). Extensive evidence suggests that changes in locus ceruleus (LC) activity influence sensory processing in the thalamus and cortex (Berridge and Foote, 1991; Steriade et al., 1993; Waterhouse et al., 1998). LC firing rates are higher during wakefulness than sleep and increase before and during periods of arousal and vigilance (Hobson et al., 1975; Foote et al., 1980; Aston-Jones and Bloom, 1981; Rajkowski et al., 1994). In addition, local application of norepinephrine (NE) alters the responsiveness of cortical neurons (Foote et al., 1975; Waterhouse et al., 1980, 1988; Kasamatsu and Heggelund, 1982; Armstrong-James and Fox, 1983; Videen et al., 1984; Devilbiss and Waterhouse, 2000). However, no studies have directly isolated the impact of NE on the regulation of high-order sensory processing by controlled changes in behavioral state.
The avian song system offers a uniquely well suited model in which to study neuromodulatory influences on behavioral state-dependent sensory processing. The ascending auditory pathway of the song system primarily consists of feedforward projections between discrete brain nuclei, allowing isolated pharmacological manipulations of specific areas (Fig. 1A). We have previously observed that auditory responses in the higher-order song system nuclei NIf (nucleus interfacialis of the nidopallium) and HVC (used as proper name), but not the primary thalamorecipient area Field L, are completely suppressed by arousal (Cardin and Schmidt, 2003, 2004). This state-dependent modulation can be evoked consistently and appears to be predominantly restricted to forebrain auditory areas.
Figure 1.
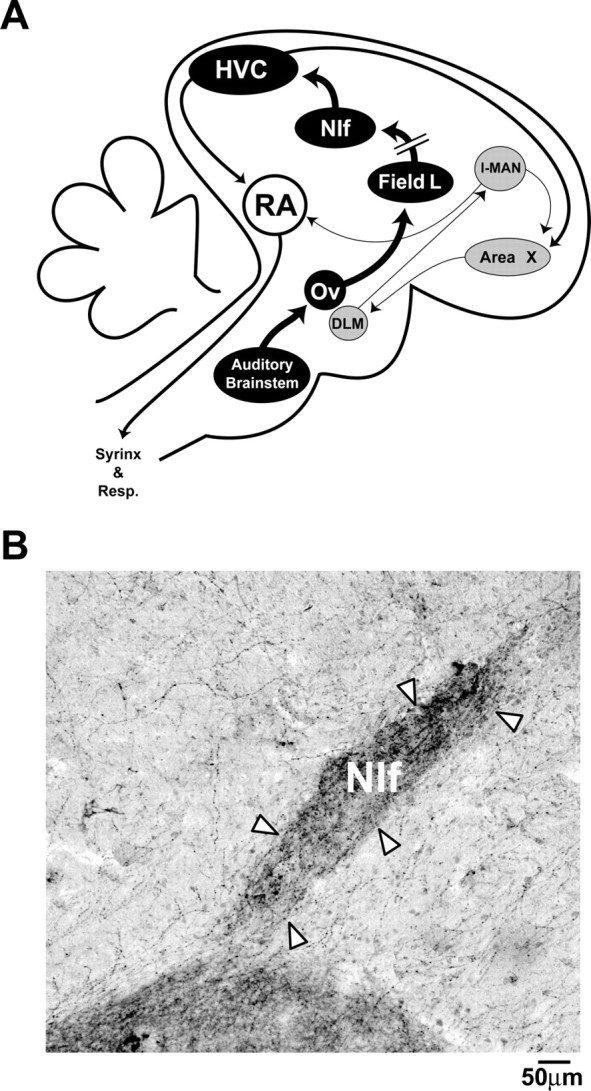
Song system schematic and tyrosine hydroxylase staining in NIf. A, Schematic diagram of the three major song system pathways: premotor, anterior forebrain, and ascending auditory. HVC, which is part of all three pathways, receives auditory input indirectly from Field L via NIf. Field L is the primary forebrain structure that receives auditory input from the thalamic nucleus Ovoidalis. NIf provides the major auditory input to HVC. The anterior forebrain pathway includes HVC, Area X, DLM, LMAN, and RA. The premotor pathway includes HVC and RA. Area X, Song-related region of the basal ganglia; DLM, medial portion of the dorsolateral nucleus of the anterior thalamus; Field L, primary thalamorecipient auditory area of the telencephalon; LMAN, lateral portion of the magnocellular nucleus of the anterior nidopallium; Ov, nucleus Ovoidalis of the thalamus; Resp., respiratory areas of the brainstem; Syrinx, avian vocal organ. B, Parasaggital brain section labeled with an antibody to tyrosine hydroxylase (TH). NIf is densely innervated with TH-positive fibers. Arrowheads indicate borders of NIf.
Previous studies have found that song system auditory responses are affected by neuromodulatory influences (Dave et al., 1998; Shea and Margoliash, 2003). Immunohistochemical evidence indicates that NIf, in particular, contains fibers immunoreactive for tyrosine hydroxylase (Fig. 1B) and dopamine-β-hydroxylase (Soha et al., 1996; Harding et al., 1998; Mello et al., 1998) and is thus a potential site of noradrenergic action. Auditory responsiveness in NIf is robustly altered by changes in behavioral state (Cardin and Schmidt, 2004), suggesting that NIf may be a target of state-dependent modulatory action. Furthermore, because auditory responses in HVC depend on NIf inputs (Cardin and Schmidt, 2004), modulation of NIf activity will also indirectly regulate auditory input to the rest of the song system.
In the present study, we show dose-dependent noradrenergic effects on NIf and HVC auditory responses. Whereas low levels of NE in NIf enhance auditory responses, higher levels completely suppress responses. These effects appear to be mediated byα-adrenergic receptors. Most strikingly, α-adrenergic antagonists in NIf prevent the arousal-mediated suppression of HVC auditory responses. These results strongly suggest a role for NE in the behavioral state-dependence of song system auditory responsiveness. Some of these results have been presented previously in abstract form.
Materials and Methods
Animals and auditory stimuli. Adult male zebra finches (Taeniopygia guttata) ranging from 120 to 500 d of age were obtained from our breeding colony and from a local supplier. The song of each bird was recorded in a sound attenuation chamber and digitized at 40 kHz with GoldWave (GoldWave, St. John's, NFLD, Canada). All auditory stimuli were two motifs of the bird's own song (BOS).
Acute recordings. Surgery procedures were as described in detail in previous work (Cardin and Schmidt, 2003, 2004). The bird was given intramuscular injections of 7.5 mg/kg diazepam and 0.1 cc of 5% dextrose (Abbott Labs, Irving, TX) and a small subcutaneous injection of 2% lidocaine hydrochloride (Phoenix Pharmaceuticals, Mountain View, CA) and secured in a stereotaxic apparatus by a previously cemented head post. Sharp and patch electrodes (5-20 MΩ) were pulled on a Micropipette Puller P-97 (Sutter Instruments, Novato, CA) and lowered into HVC and NIf through small incisions in the dura. All single units were recorded using a loose-patch technique as described in previous work (Cardin and Schmidt, 2003). Multiunit recordings represent the activity of 5-20 neurons. Once the electrodes were in place, HVC and NIf auditory responses were confirmed. The NIf electrode was then replaced by a drug-filled infusion pipette connected to a Neurophore BH-2 Pneumatic Pump Module (Harvard Apparatus, Holliston, MA). After a series of baseline auditory trials, a small amount (50-100 nl) of drug was infused by pressure injection into NIf over the course of 1-2 min. Additional auditory trials were presented after drug administration to assess changes in auditory responsiveness and recovery.
We used norepinephrine alone at four doses (low doses, 0.5, 1 mm; high doses, 5, 8 mm; Sigma-Aldrich, St. Louis, MO). α1-Adrenergic drugs were the α1 agonists cirazoline (2, 8 mm; Sigma-Aldrich) and phenylephrine (1, 6 mm; Sigma-Aldrich) and the α1 antagonists benoxathian (5 mm; Sigma-Aldrich) and HEAT (2-β-4-hydroxy-3-iodophenyethylaminomethyltetralone) (1 mm; Tocris Cookson, Ellisville, MO). α2-adrenergic drugs were the α2 agonists clonidine (1, 5 mm; Sigma-Aldrich) and guanabenz acetate (0.25, 1 mm; Tocris Cookson) and the α2 antagonists idazoxan (5 mm; Sigma-Aldrich) and rauwolscine (1 mm; Tocris Cookson). β-Adrenergic drugs were the β2 agonist isoproterenol (2, 5 mm; Tocris Cookson) and the β2 antagonist ICI 118,551 ((+)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol) (2 mm; Tocris Cookson). All drugs were dissolved in 0.9% saline and pH was adjusted to between 7.3 and 7.7.
During most experiments and all control injections, the drug solution contained 10% biotinylated dextran amine (BDA) (Molecular Probes, Eugene, OR). Staining for BDA allowed confirmation of the location and approximate spread of the microinjection. Data from experiments in which the staining spread beyond the borders of NIf were discarded.
Arousal experiments. To assess the effects of drug application on behavioral state-dependent changes in auditory responsiveness, we used a previously characterized protocol in which an air puff to the chest arouses the bird (Cardin and Schmidt, 2003, 2004). Birds were lightly sedated by an intramuscular dose of 0.08 cc of 1.5 mg/ml diazepam. Arousal experiments were composed of 120 identical auditory stimulus trials. The time interval between trials was varied during each experiment and ranged from 5 sec to 3 min. On randomly interleaved trials, the bird was aroused by a puff of air to the chest 1 sec before the onset of the BOS stimulus. Neural data from the acute experiments was amplified and filtered between 500 Hz and 10 kHz and digitized at 20 kHz. Acquisition software was written in LabVIEW (National Instruments, Austin, TX) by A. Leonardo (Caltech, Pasadena, CA).
In these experiments, a baseline series of 40 auditory trials was performed to confirm suppression of HVC auditory responses during arousal. On 20 randomly interleaved trials, the bird was aroused by the air puff. Drugs were then infused into NIf. A second series of 40 trials was performed to assess the effects of the drugs on the arousal-mediated suppression. The bird was then allowed to rest for 15-20 min to allow the effects of the drugs to dissipate and a final series of 40 trials was performed to confirm recovery.
Histology. For identification of microinjection sites, tissue was fixed with 4% paraformaldehyde, cryoprotected in 30% sucrose, sectioned on a freezing microtome, and processed with an avidin-biotin-horseradish peroxidase complex kit followed by a reaction with a peroxidase substrate kit (Vector Laboratories, Burlingame, CA).
Data analysis. Data were analyzed using Matlab (MathWorks, Natick, MA) routines written by J.A.C. and M.F.S. Spike events in the single- and multiunit data were measured using a peak-detection algorithm. For each data set, the threshold was visually positioned at a point clearly above background noise but low enough to detect all observed spike events. Peristimulus time histograms (PSTH) were calculated by binning spike events (10 msec bin size) during each trial and summing the resulting raster plots over 15-30 auditory trials.
Auditory ratio. The auditory ratio (AR) was defined as the ratio of stimulus-evoked activity to spontaneous activity. For each auditory response, we used the neural activity recorded during 30 presentations of the auditory stimulus. The AR was calculated as follows:
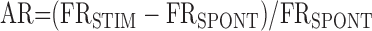 |
FRSTIM is the mean firing rate during presentation of the auditory stimulus, and FRSPONT is the mean firing rate over the baseline period of equal duration preceding each stimulus presentation. FRSPONT and FRSTIM were also used separately as measures of spontaneous and evoked firing rates, respectively. An AR of 0 indicates no change in firing in response to the auditory stimulus, a positive AR indicates an increase in firing in response to the stimulus, and a negative AR indicates a decrease in firing in response to the stimulus. All AR and firing rate values are shown as mean ± SEM.
Results
Dose-dependent effects of NE on HVC auditory responsiveness
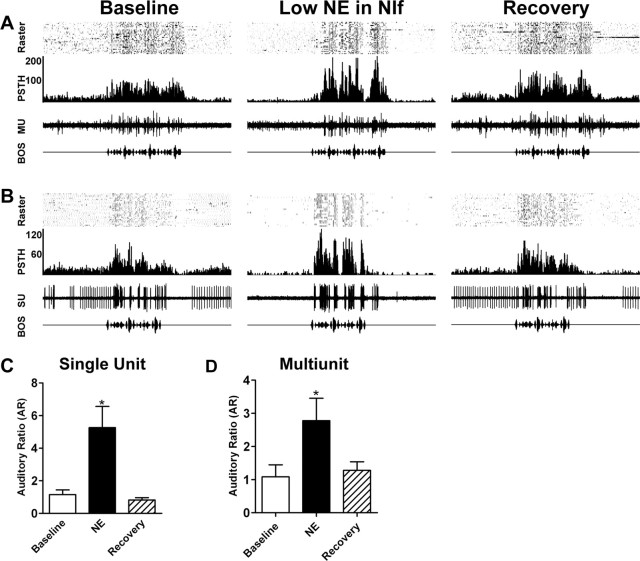
We recorded single- or multiunit HVC activity in lightly sedated birds while infusing small volumes (50-100 nl) of NE into the ipsilateral NIf. Injections of 0.5 and 1 mm NE were defined as low doses and injections of 5 and 8 mm NE as high doses. In all cases, the auditory stimulus was the BOS. Auditory responses were quantified by an AR measure (see Materials and Methods). An AR of 0 indicates no change in firing during the auditory stimulus, a positive AR indicates an increase in firing in response to the stimulus, and a negative AR indicates a decrease in firing in response to the stimulus. Figure 2, A and B, shows a multiunit and a single-unit recording, respectively, in HVC during administration of a low dose of NE to NIf. In each case, auditory responses were recorded under baseline conditions (left), after a low dose of NE was delivered to NIf (center), and after recovery (right). In the example shown in Figure 2A, 0.5 mm NE was infused into NIf during a multiunit recording of the ipsilateral HVC. NE administration caused a decrease in spontaneous HVC activity and an increase in the HVC auditory response relative to spontaneous firing (center). Recovery was observed after 10 min (right). Similarly, the unit shown in Figure 2B also demonstrated an enhancement of auditory responsiveness after low-dose NE administration to NIf. After infusion of 1 mm NE into NIf, spontaneous firing was reduced and the auditory response relative to spontaneous firing was increased. Recovery was observed after 6 min.
Figure 2.
Low doses of NE in NIf increase HVC auditory responsiveness relative to spontaneous activity. In each of these examples, recordings were made in HVC during baseline auditory trials (left), after low-dose NE (0.5 or 1 mm) administration in the ipsilateral NIf (center), and after recovery (right). The top portion of each panel shows the raster plot of spike events during 30 auditory trials and the middle portion is the cumulative PSTH. The neural record is an example of one auditory trial. The bottom trace is the amplitude waveform of the auditory stimulus. A, After administration of a low dose of NE to NIf, spontaneous multiunit (MU) HVC activity was decreased and auditory responsiveness, as measured by the AR, was increased. Recovery was observed 6 min later. B, Single HVC unit (SU) recorded during administration of a low dose of NE to NIf. This unit demonstrated decreased spontaneous firing and an increased AR after low-dose NE in NIf. Recovery was observed 6 min later. C, Mean single-unit AR for HVC was significantly increased by application of a low dose of NE to the ipsilateral NIf (n = 5). D, Likewise, mean multiunit HVC AR was significantly increased by low NE in NIf (n = 5). Baseline AR values are shown as open bars, AR values after NE as black bars, and AR values after recovery as hatched bars. *p < 0.05. Error bars represent SEM.
We tested the effects of low-dose NE in NIf on five single units and five multiunit recording sites in HVC in 10 birds. Low doses of NE (0.5 or 1 mm) significantly decreased spontaneous HVC firing rates. The mean single-unit spontaneous firing rate decreased from 8.8 ± 2.5 to 1.5 ± 0.9 spikes/sec after administration of low-dose NE to NIf (p < 0.05; one-way ANOVA with Dunnett's multiple comparison test). Similarly, the mean multiunit spontaneous firing rate was reduced from 16.1 ± 5.4 to 4.3 ± 1.3 spike events/sec after low-dose NE (p < 0.05). In two individual cases, including that shown in Figure 2B, the mean stimulus-evoked firing rate of HVC units was significantly increased by low-dose NE in NIf (p < 0.05; unpaired t test). However, this was not consistent across all cells because overall mean single-unit evoked activity was not significantly altered between baseline (10.2 ± 4.0 spikes/sec) and low-dose NE (6.0 ± 4.5 spikes/sec) conditions (p > 0.05). Mean multiunit evoked activity was similarly unchanged between baseline (19.9 ± 5.6 spike events/sec) and low-dose NE (25.3 ± 4.0 spike events/sec) conditions (p > 0.05). In each case, however, there was an increase in the HVC auditory response to the auditory stimulus. The mean single-unit AR increased from 1.2 ± 1.3 to 5.3 ± 1.3 (p < 0.01) (Fig. 2C) and the mean multiunit AR increased from 1.0 ± 0.4 to 2.8 ± 0.7 (p < 0.05) (Fig. 2D). Recovery of baseline auditory responses and spontaneous activity was observed 6-15 min later. Low levels of NE in NIf thus increase the magnitude of HVC auditory responses relative to spontaneous activity. Because the mean evoked firing rates were not significantly altered by NE application, the observed increase in AR values appears to be driven predominantly by a decrease in spontaneous activity. However, as apparent in Figure 2A and B, NE-induced changes in stimulus-evoked firing patterns led to increased peak height and deeper troughs in the PSTHs.
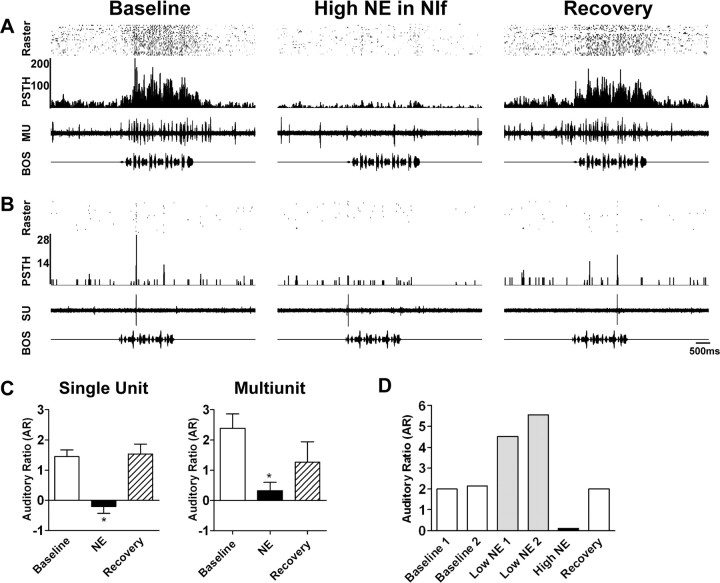
In contrast to the effects of low-dose NE, application of higher doses (5 or 8 mm) of NE to NIf caused a robust suppression of both spontaneous and evoked activity in HVC. Figure 3,A and B, shows a multiunit and a single-unit recording, respectively, in HVC during application of a higher dose of NE to NIf. In the example shown in Figure 3A, 5mm NE was infused into NIf during a multiunit recording of the ipsilateral HVC. NE administration caused a decrease in both the spontaneous and evoked activity of the HVC recording site (center). Recovery was observed after 12 min (right). Similarly, the unit shown in Figure 3B showed a complete loss of auditory responsiveness after administration of 8 mm NE to the ipsilateral NIf (center) and recovery after 10 min (right).
Figure 3.
High doses of NE in NIf eliminate HVC auditory responsiveness. In contrast to the effects of low doses of NE in NIf, higher doses (5 or 8 mm) led to a suppression of HVC auditory responsiveness. A, Multiunit (MU) recording in HVC during baseline recordings (left), after application of a high dose of NE to the ipsilateral NIf (center), and after 12 min of recovery (right). High-dose NE decreased both spontaneous and auditory activity in HVC. B, Single-unit (SU) recording in HVC during a similar experiment. Application of high-dose NE to NIf resulted in a complete loss of auditory responsiveness by this unit (center). Recovery was observed 10 min later (right). C, Mean single-unit AR for HVC was significantly decreased by application of a high dose of NE to the ipsilateral NIf (n = 7) (left). Likewise, mean multiunit AR for HVC was significantly decreased by high-dose NE in NIf (n = 5) (right). Baseline AR values are shown as open bars, AR values after NE as black bars, and AR values after recovery as hatched bars. *p < 0.05. Error bars represent SEM. D, To determine whether individual HVC units demonstrate both enhancement and suppression of auditory responsiveness, single HVC units were recorded throughout a series of doses of NE to the ipsilateral NIf. Whereas the baseline AR values of this unit were stable (open bars), sequential application of two low doses of NE to the ipsilateral NIf each caused an increase in the auditory response (gray bars). A subsequent high dose of NE to NIf caused a complete loss of auditory responsiveness (black bar). The auditory response returned to baseline levels after 15 min. The total time between the first baseline trial and the last recovery trial was 123 min.
We tested the effects of higher doses of NE in NIf on seven single units and five multiunit recording sites in HVC in 12 birds. Mean HVC spontaneous firing rates were significantly decreased by NE application in all cases. High-dose NE in NIf caused the mean single-unit spontaneous firing rate to decrease from 2.7 ± 1.0 to 0.7 ± 0.3 spikes/sec (p < 0.05) and the mean multiunit spontaneous firing rate to decrease from 22.6 ± 4.3 to 4.6 ± 1.6 spike events/sec (p < 0.01). Mean single-unit stimulus-evoked activity decreased from 4.4 ± 1.3 to 1.0 ± 0.4 spikes/sec after high-dose NE (p < 0.05). Similarly, mean multiunit evoked activity decreased from 33.7 ± 5.5 to 6.0 ± 2.6 spike events/sec (p < 0.05). These changes in spontaneous and evoked activity completely eliminated HVC auditory responsiveness. The mean single-unit AR was reduced from 1.5 ± 0.2 to -0.2 ± 0.2 (p < 0.01) (Fig. 3C, left) and the mean multiunit AR decreased from 2.4 ± 0.5to0.3 ± 0.3 (p < 0.05) (Fig. 3C, right) after high-dose NE to NIf. Recovery of both spontaneous and auditory activity was observed after 10-15 min.
To determine whether individual HVC neurons could show both enhancement and suppression of auditory responses, we recorded from single HVC units during multiple administrations of NE to NIf (n = 3 units in three birds). Figure 3D shows data from one experiment in which a single HVC unit was recorded during successive applications of NE to the ipsilateral NIf. The AR values of the unit were stable during baseline recordings but increased in response to a first low dose of NE (0.5 mm) to NIf. A second low dose of NE to NIf resulted in a small additional increase. Infusion of a high dose of NE (5 mm) to NIf caused the unit to lose all auditory responsiveness. Recovery was observed 15 min after the last dose of NE. We observed similar effects on two additional units. These results suggest that individual HVC neurons are capable of exhibiting both enhancement and suppression of auditory responsiveness after NE application in NIf. Interestingly, whereas the spontaneous activity of all three units was significantly decreased by low-dose NE (baseline, 6.4 ± 1.5 spikes/sec; low NE, 1.1 ± 0.9 spikes/sec; one-way ANOVA with Tukey's post hoc test; p < 0.01), subsequent high-dose NE led to a decrease in AR values without an additional reduction in spontaneous firing (high NE, 0.8 ± 0.4 spikes/sec; p > 0.05).
In control experiments, we tested the effects of saline infusion into NIf on multiunit HVC spontaneous firing rates and AR values (n = 7 sites in seven birds). Multiunit HVC spontaneous firing rates were unchanged between baseline (17.3 ± 3.8 spike events/sec) and saline injection (17.0 ± 4.0 spike events/sec) conditions (p > 0.05). Similarly, mean AR values for HVC were not altered between baseline (2.1 ± 0.3) and saline injection (2.3 ± 0.4) conditions (p > 0.05). We also tested the effects of infusing low (n = 3 sites in three birds) or high (n = 4 sites in four birds) doses of NE 100-125 μm dorsal or posterior to NIf. Multiunit HVC spontaneous firing rates were unchanged between baseline recordings (19.4 ± 3.8 spike events/sec) and low NE injection at distant sites (21.0 ± 4.1 spike events/sec) (p > 0.05). Likewise, mean AR values for HVC were not altered between baseline recordings (1.6 ± 0.4) and low NE injection at distant sites (1.5 ± 0.6) (p > 0.05). Multiunit HVC spontaneous firing rates were similar between baseline recordings (16.7 ± 3.2 spike events/sec) and high NE injection at distant sites (16.4 ± 4.4 spike events/sec) (p > 0.05). Mean AR values for HVC were unchanged between baseline (1.2 ± 0.3) and distant high-dose NE (1.4 ± 0.4) conditions (p > 0.05). In summary, there were no significant changes in HVC spontaneous or evoked activity after saline infusion into NIf or low- or high-dose NE infusion outside of NIf.
Dose-dependent effects of NE on NIf auditory responsiveness
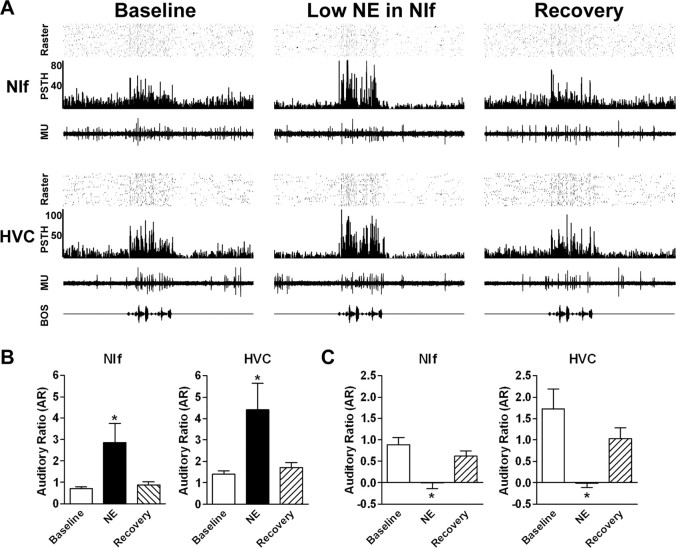
Because NIf is a major source of input to HVC (Cardin and Schmidt, 2004), the observed effects on HVC activity after infusion of NE into NIf likely result from altered NIf output. To test this hypothesis, we recorded simultaneously from NIf and HVC during administration of either low (n = 3 sites in three birds) or high (n = 4 sites in four birds) doses of NE to NIf (Fig. 4). An example of the comodulation of activity in NIf and HVC by administration of low-dose NE in NIf is shown in Figure 4A. In this experiment, 0.5 mm NE was infused into NIf during simultaneous recordings in NIf and HVC. After NE application, spontaneous activity in both NIf and HVC was reduced and the auditory responses in both areas were increased relative to spontaneous firing (center). After 14 min, both NIf and HVC showed recovery (right). These effects were observed in all similar experiments. Mean NIf spontaneous activity decreased from 13.1 ± 1.0 to 1.8 ± 0.3 spike events/sec (p < 0.01) and mean HVC spontaneous activity decreased from 16.1 ± 4.0 to 7.5 ± 2.2 spike events/sec (p < 0.05) after low-dose NE infusion into NIf. Mean NIf evoked firing rates were not significantly changed between baseline (28.5 ± 2.2 spike events/sec) and low-dose NE (36.5 ± 3.1 spike events/sec) conditions (p > 0.05). Mean HVC evoked activity was similarly unchanged between baseline (22.1 ± 4.0 spike events/sec) and low-dose NE (23.7 ± 2.0 spike events/sec) conditions (p > 0.05). However, the mean NIf AR increased from 0.7 ± 0.1 to 2.9 ± 0.9 (p < 0.05) (Fig. 4B, left) and the mean HVC AR increased from 1.4 ± 0.2 to 4.4 ± 1.2 (p < 0.05) (Fig. 4B, right).
Figure 4.
NE in NIf modulates NIf and HVC auditory responsiveness. A, In this experiment, multiunit (MU) activity in an ipsilateral NIf-HVC pair was recorded simultaneously during application of a low dose of NE to NIf. Both NIf (top) and HVC (bottom) demonstrated baseline auditory responses (left). After administration of low-dose NE to NIf, auditory responsiveness was increased relative to spontaneous activity at both recording sites (middle). Both NIf and HVC recovered auditory responses similar to the original control responses after 14 min (right). B, In all similar experiments (n = 3), low doses of NE to NIf caused a significant increase in both NIf (left) and HVC (right) AR values. C, In contrast, administration of high-dose NE to NIf (n = 4) significantly decreased both NIf (left) and HVC (right) AR values. Baseline AR values are shown as open bars, AR values after NE as black bars,and AR values after recovery as hatched bars. *p < 0.05. Error bars represent SEM.
In contrast, high-dose NE reduced auditory responsiveness in both NIf and HVC (Fig. 4C). Mean NIf spontaneous activity decreased from 15.8 ± 4.9 to 3.8 ± 1.8 spike events/sec after high-dose NE application (p < 0.01). Similarly, mean HVC spontaneous activity was reduced from 13.9 ± 3.2 to 6.1 ± 2.5 spike events/sec after high-dose NE in NIf (p < 0.05). Mean NIf evoked activity decreased from 32.3 ± 8.1 to 9.4 ± 3.7 spike events/sec (p < 0.05) and mean HVC evoked activity decreased from 19.0 ± 4.9 to 4.0 ± 2.6 spike events/sec (p < 0.05) after high-dose NE in NIf. In addition, the mean NIf AR decreased from 0.9 ± 0.2 to -0.01 ± 0.1 (p < 0.05) (Fig. 4C, left) and the mean HVC AR decreased from 1.7 ± 0.5 to -0.01 ± 0.1 after a high dose of NE to the ipsilateral NIf (p < 0.05) (Fig. 4C, right). In summary, these results indicate that NE can act in NIf to modulate spontaneous and evoked activity in NIf and HVC.
Effects of NE in NIf are replicated by α-adrenergic agonists
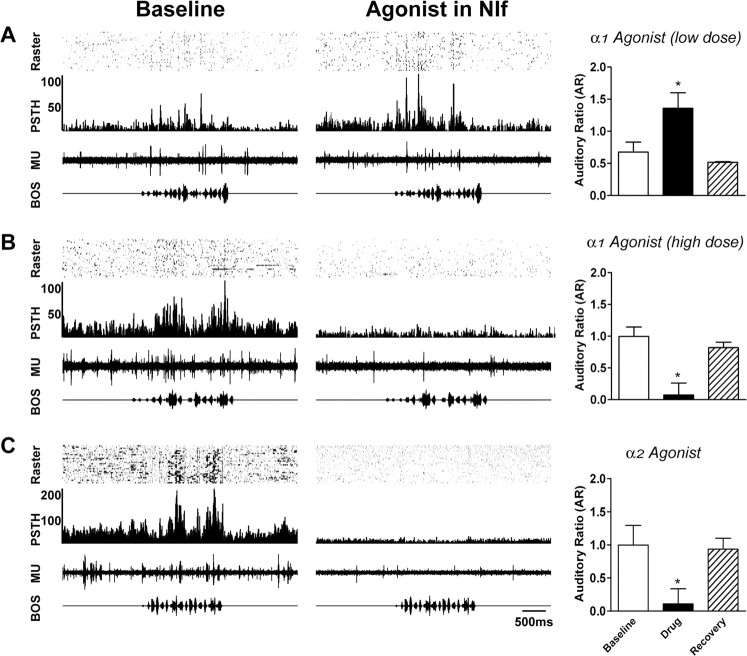
We used pharmacological agents to identify the subtypes of adrenergic receptors responsible for the effects of NE in NIf. The major subtypes expressed in the avian telencephalon are the α1, α2, and β2 adrenergic receptors (Balthazart and Ball, 1989; Balthazart et al., 1989; Fernandez-Lopez et al., 1997). We first tested the effects of infusing α1 and α2 agonists into NIf. As in the NE experiments, we used a low and a high dose of each type of agonist (see Materials and Methods). Infusion of low doses of the α1-adrenergic agonists phenylephrine (n = 3 sites in three birds) or cirazoline (n = 1 site in one bird) into NIf caused an increase in HVC auditory responsiveness similar to that observed with low NE administration. Because similar results were obtained with the two drugs, the data for all low-dose α1 agonist experiments were combined. In the example shown in Figure 5A, infusion of 1 mm phenylephrine into NIf caused the HVC auditory response to increase relative to spontaneous activity (center). After 15 min of recovery, the HVC auditory response returned to baseline levels. Mean spontaneous HVC firing rates were not significantly different between baseline (10.9 ± 1.4 spike events/sec) and low-dose α1 agonist (7.2 ± 2.0 spike events/sec) conditions (p > 0.05). However, mean evoked HVC firing rates increased from 21.1 ± 3.0 to 39.9 ± 3.2 spike events/sec after low-dose α1 agonist application (p < 0.05). Mean AR values for HVC were significantly increased from 0.7 ± 0.2 to 1.4 ± 0.2 after infusion of a low dose of α1 agonist into NIf (p < 0.05) (Fig. 5A, right).
Figure 5.
α-Adrenergic agonists in NIf replicate the effects of NE. In these experiments, we tested the specificity of the noradrenergic effects on auditory responsiveness by injecting receptor-specific agonists into NIf while recording from HVC. Bar graphs on the right represent cumulative data from all experiments targeting a given receptor subtype. The mean AR values during baseline recordings are shown as open bars, mean AR values after agonist administration as blackbars, and mean AR values after recovery as hatched bars. A, Auditory responses at a multiunit (MU) recording site in HVC (left) were increased after administration of a low dose of 1 mm phenylephrine, an α1-receptor specific agonist (center). As shown in the graph on the right, mean AR values were significantly increased by low doses of α1 agonists (n = 4). B, Administration of a high dose of 8 mm cirazoline, another α1-receptor-specific agonist, resulted in a significant decrease in HVC spontaneous activity and auditory response. High doses of α1 agonists significantly decreased HVC AR values in all similar experiments (n = 4) (right). C, Both auditory responsiveness and spontaneous activity at this multiunit HVC recording site were decreased by administration of 5 mm clonidine, an α2-receptor specific agonist, to the ipsilateral NIf. The mean AR value for HVC was significantly reduced by α2 agonists in NIf (n = 6) (right). *p < 0.05. Error bars represent SEM.
Higher doses of the α1 agonists phenylephrine (n = 3 sites in three birds) or cirazoline (n = 2 sites in two birds) resulted in suppression of HVC auditory responsiveness. Because similar results were obtained for both phenylephrine and cirazoline, the data were combined. Figure 5B shows an example of a multiunit recording in HVC before and after administration of 8 mm cirazoline to the ipsilateral NIf. Immediately after drug application, HVC spontaneous and auditory activity was reduced (middle). Recovery was observed after 15 min. Mean spontaneous HVC firing rates decreased from 19.5 ± 4.6to1.6 ± 1.0 spike events/sec after administration of a high dose of α1 agonist to NIf (p < 0.05). Mean evoked HVC firing rates decreased from 28.9 ± 2.7 to 4.6 ± 1.6 spike events/sec after high-dose α1 agonist application (p < 0.01). Similarly, the mean AR for HVC decreased from 1.0 ± 0.2 to 0.1 ± 0.2 (p < 0.01) (Fig. 5B, right).
We next tested the effects of α2 adrenergic agonists in NIf. All doses of the α2 agonists clonidine (n = 3 sites in three birds) and guanabenz acetate (n = 3 sites in three birds) (see Materials and Methods) resulted in similar suppression of HVC auditory responsiveness, and the data from these experiments were combined. In the example shown in Figure 5C, 5 mm clonidine applied to NIf completely eliminated HVC auditory responsiveness (middle). Recovery was observed 15 min later. Although α2 agonists sometimes caused a significant reduction in spontaneous activity, as shown in Figure 5C, over all experiments mean spontaneous HVC firing rates were not significantly decreased between baseline recordings (40.6 ± 14.0 spike events/sec) and α2 agonist application to NIf (26.1 ± 7.5 spike events/sec) (p > 0.05). However, mean evoked HVC firing rates decreased from 44.4 ± 4.3 to 14.8 ± 2.1 spike events/sec after α2 agonist administration (p < 0.01). Similarly, the mean AR value for HVC decreased from 1.0 ± 0.3 to 0.1 ± 0.2 after α2 agonist administration to NIf (p < 0.05) (Fig. 5C, right).
Although there is very little β1 adrenergic receptor expression, β2 receptors are prevalent throughout the avian telencephalon (Dermon and Kouvelas, 1988; Fernandez-Lopez et al., 1997; Revilla et al., 1998). We therefore tested the effects of the β2 agonist isoproterenol in NIf (n = 3 sites in three birds). Mean spontaneous HVC firing rates were not significantly altered between baseline recordings (18.2 ± 5.5 spike events/sec) and infusion of β2 agonist into NIf (16.7 ± 6.0 spike events/sec) (p > 0.05). Mean evoked HVC firing rates were also unchanged between baseline recordings (29.6 ± 3.8 spike events/sec) and β2 agonist application (25.1 ± 5.2 spike events/sec) (p > 0.05). Similarly, mean AR values for HVC were not significantly different between baseline recordings (2.6 ± 0.9) and infusion of β2 agonist into NIf (2.7 ± 1.2) (p > 0.05). Together with the above results, these data suggest that the dose-dependent effects of NE in NIf may be mediated by α1 and α2, but not β2, adrenergic receptors.
Adrenergic antagonists in NIf do not alter HVC activity
Activity in HVC was not significantly altered by application of the α1 antagonists benoxathian (n = 4 sites in four birds) or HEAT (n = 1 site in one bird) in NIf. Mean HVC spontaneous firing rates were unchanged between baseline recordings (17.8 ± 3.9 spike events/sec) and α1 antagonist application (15.5 ± 4.2 spike events/sec) (p > 0.05). Similarly, mean AR values for HVC were not altered between baseline recordings (1.8 ± 0.6) and α1 antagonist application (1.9 ± 0.5) (p > 0.05). There were no significant effects of the α2 antagonists idazoxan (n = 3 sites in three birds) or rauwolscine (n = 3 sites in three birds) on either spontaneous or auditory activity in HVC. Mean HVC spontaneous firing rates were unchanged between baseline (21.2 ± 4.0 spike events/sec) and α2 antagonist (23.4 ± 5.7 spike events/sec) conditions (p > 0.05). Similarly, mean AR values for HVC were not altered between baseline recordings (1.6 ± 0.5) and α2 antagonist application (1.5 ± 0.6) (p > 0.05). Likewise, there was no significant effect of the β2 antagonist ICI 118,551 (n = 3 sites in three birds) on either spontaneous or stimulus-evoked HVC activity. Mean HVC spontaneous firing rates were unchanged between baseline recordings (16.5 ± 4.2 spike events/sec) and β2 antagonist application (17.4 ± 3.3 spike events/sec) (p > 0.05). Similarly, mean AR values for HVC were not altered between baseline (1.19 ± 0.4) and β2 antagonist (1.24 ± 0.6) conditions (p > 0.05). In summary, we observed no significant impact of noradrenergic antagonists in NIf on spontaneous or stimulus-evoked HVC activity under conditions of resting sedation.
Adrenergic antagonists block behavioral state-dependent modulation of auditory processing
In previous studies, we demonstrated that auditory responsiveness in NIf and HVC, but not Field L, is completely suppressed by arousal from a resting state (Cardin and Schmidt, 2003, 2004). The suppression of auditory responses by NE and α-adrenergic agonists in NIf suggests that noradrenergic inputs to NIf may play a role in this state-dependent modulation. To test this hypothesis, we assessed the ability of α-adrenergic antagonists in NIf to block the arousal-mediated suppression of song system auditory responses.
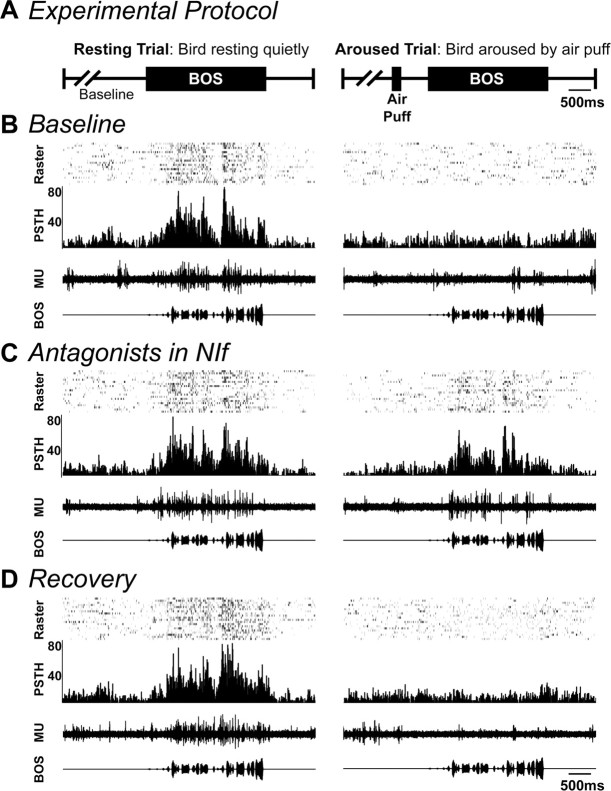
In these experiments, we recorded from HVC during randomly interleaved resting and arousal trials (Fig. 6A). During the first portion of each experiment, we assessed the effects of arousal on HVC auditory responsiveness in the absence of pharmacological manipulations in NIf. We then administered a combination of α1- and α2-adrenergic antagonists to the ipsilateral NIf and repeated the paradigm of resting and arousal trials. After 15 min, additional resting and arousal trials were performed to assess recovery. A typical example of these experiments is shown in Figure 6B-D. Under baseline conditions (Fig. 6B), multiunit recordings in HVC demonstrated a robust auditory response to the BOS stimulus during resting trials (left) and complete suppression of auditory responsiveness by arousal (right). Administration of the α1 antagonist benoxathian (5 mm) and the α2 antagonist idazoxan (5 mm) to the ipsilateral NIf eliminated this suppression (Fig. 6C). Under these conditions, HVC showed strong auditory responses even when the bird was aroused. After 18 min of recovery, arousal again resulted in elimination of HVC auditory responses (Fig. 6D).
Figure 6.
Injection of α-adrenergic antagonists into NIf blocks arousal-mediated suppression of HVC responses. A, Schematic of the two types of randomly intermixed auditory trials used in arousal experiments. During resting trials, a lightly sedated bird was presented with the BOS stimulus. During aroused trials, the bird was aroused by an air puff to the chest 1 sec before the beginning of the BOS stimulus. The two types of trials were then separated for analysis. B, Multiunit (MU) HVC auditory responses were recorded during resting (left) and aroused (right) auditory stimulus trials in the absence of any manipulation of NIf. Arousal resulted in complete suppression of HVC auditory responses. C, After infusion of α1 and α2 receptor-specific antagonists (5 mm benoxathian and 5 mm idazoxan) to the ipsilateral NIf, a second series of intermixed resting and aroused trials was performed. α-Adrenergic antagonists prevented the arousal-mediated suppression of HVC auditory responses. D, After 15 min of recovery, arousal again elicited a suppression of HVC auditory responses.
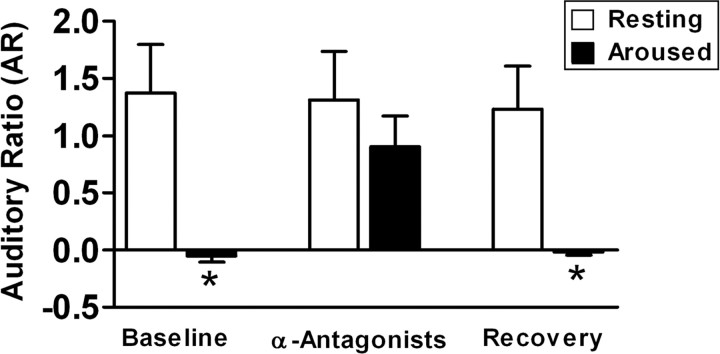
In all cases (n = 7 sites in seven birds), α-adrenergic antagonists in NIf blocked the suppressive effect of arousal on HVC auditory responsiveness. Under baseline conditions, the mean AR value for HVC was 1.4 ± 0.4 during resting trials and -0.05 ± 0.05 during arousal (p < 0.05; paired t test) (Fig. 7, left bars). However, after infusion of α-adrenergic antagonists into the ipsilateral NIf, mean AR for HVC was 1.3 ± 0.4 during resting trials and 0.9 ± 0.3 during arousal (p > 0.05) (Fig. 7, center bars). After recovery, mean AR for HVC was 1.2 ± 0.4 during resting and -0.02 ± 0.02 during arousal (p < 0.05) (Fig. 7, right bars). α-Adrenergic antagonists in NIf thus prevented the behavioral state-dependent suppression of HVC auditory responses.
Figure 7.
α-Adrenergic antagonists in NIf block state-dependent changes in HVC auditory activity. Cumulative data from arousal experiments (n = 7). Under baseline conditions (left bars), arousal caused a complete suppression of HVC auditory responses. After administration of α1- and α2-adrenergic receptor antagonists into the ipsilateral NIf, HVC demonstrated auditory responses even in the aroused state (center bars). There was no significant difference between resting and aroused auditory responsiveness, as measured by AR, after drug application to NIf. Similarly, the antagonists in NIf did not significantly affect resting auditory responses in HVC. After 15 min, arousal again suppressed auditory responses (right bars). In each pair of bars, the resting AR values are shown as open bars and aroused AR values are shown as black bars. * p < 0.05. Error bars represent SEM.
Discussion
In this study, we investigated the role of noradrenergic modulation in regulating auditory responsiveness in NIf and its target structure HVC. Our results represent the first evidence that neuromodulatory transmitter action is capable of both enhancing and suppressing song system auditory responsiveness in a dose-dependent manner. Strikingly, α-adrenergic antagonists infused into NIf blocked the behavioral state-dependent suppression of HVC auditory responses. These results strongly implicate noradrenergic input in the behavioral state-dependent regulation of song system auditory responses.
Noradrenergic effects on sensory responsiveness
Noradrenergic inputs from the LC participate in the processes of arousal and alerting in mammals and profoundly influence sensory processing throughout the brain. LC neurons are more active immediately before and during periods of arousal and vigilance (Hobson et al., 1975; Foote et al., 1980; Aston-Jones and Bloom, 1981; Rajkowski et al., 1994), and LC stimulation in anesthetized animals results in excitation of thalamocortical neurons (Steriade et al., 1993), activation of cortical EEGs (Berridge and Foote, 1991), and enhancement of responses in primary sensory neurons (Waterhouse et al., 1998). In addition, NE alters cortical sensory receptive field properties (Waterhouse et al., 1990; George, 1992; McLean and Waterhouse, 1994; Manunta and Edeline, 1997). Local application of NE decreases spontaneous cortical activity (Foote et al., 1975; Armstrong-James and Fox, 1983) and increases and decreases the responsiveness of cortical neurons in vitro in a dose-dependent manner (Foote et al., 1975; Waterhouse et al., 1980, 1988; Kasamatsu and Heggelund, 1982; Videen et al., 1984; Devilbiss and Waterhouse, 2000). However, despite the abundance of data implicating NE in state-dependent changes in sensory processing, no study to date has used clearly defined, replicable behavioral paradigms to directly study the influence of NE on state-dependent sensory responsiveness in high-order brain areas. Here, we used repeated arousal during an acute recording paradigm to study the role of NE in modulating a well characterized, state-dependent change in sensory processing. The predominantly linear, feedforward anatomy of this portion of the ascending auditory system allowed us to pharmacologically isolate a discrete site of NE action in NIf.
We found that noradrenergic manipulations in NIf alter song system auditory responsiveness in a dose-dependent manner. Low doses of NE applied locally to NIf caused a significant increase in NIf and HVC auditory responses relative to spontaneous firing. Rather than an absolute increase in stimulus-evoked firing, the enhanced auditory responsiveness was predominantly caused by a specific decrease in spontaneous firing rates that resulted in an increased “signal-to-noise” ratio (Foote et al., 1975; Waterhouse et al., 1980; Kasamatsu and Heggelund, 1982; Videen et al., 1984). In contrast, higher doses of NE suppressed both spontaneous and evoked activity in NIf and HVC. These results agree well with previous data from mammalian studies suggesting a dose-dependent enhancement and suppression of cortical responsiveness by local NE application (Bevan et al., 1977; Rogawski and Aghajanian, 1980; Waterhouse et al., 1981, 1982; Armstrong-James and Fox, 1983; Mouradian et al., 1991; Pralong and Magistretti, 1995). The dose-dependent effects of NE in NIf suggest that neuromodulatory input to NIf may dynamically regulate the flow of auditory information to the rest of the song system.
Application of high-dose α1 agonists replicated the effects of high-dose NE, including elimination of evoked activity, decreased spontaneous firing, and decreased AR values. A range of doses of α2 agonists likewise reduced evoked activity and AR values but did not consistently lead to decreased spontaneous firing. Like low-dose NE, low-doseα1 agonists led to increased AR values, although these were attributable to increased evoked activity rather than to decreased spontaneous firing. Interestingly, we did observe some cases in which low-dose NE led to an increase in single-unit evoked firing rates in HVC, suggesting that the effects of low-dose α1 agonists on AR values in HVC might represent one aspect of NE action in NIf. Together, these results suggest that α1 and α2 agonists individually replicate some, but not all, noradrenergic effects on HVC activity. One possible explanation for this disparity is that NE may activate an additional receptor type(s), not pharmacologically activated in these experiments, that leads to decreased spontaneous activity. An alternative possibility is that simultaneous activation of both α1 and α2 receptors by NE may result in changes not observed when either type is activated alone.
The results of the present study are somewhat similar to those observed in mammalian systems, in which noradrenergic enhancement of responses is primarily the result of α1 receptor activation and suppressive effects have variously been shown to result from α2 receptor activation, higher levels of α1 receptor activation, and β receptor-dependent enhancement of inhibitory synaptic transmission (Bevan et al., 1977; Rogawski and Aghajanian, 1980; Waterhouse et al., 1981, 1982; Armstrong-James and Fox, 1983; Mouradian et al., 1991; Pralong and Magistretti, 1995; Devilbiss and Waterhouse, 2000). Although the data presented here suggest thatα1 and α2 adrenergic receptors in NIf are involved in the modulation of activity in NIf and HVC, it is still unknown whether NE is acting directly on projection neurons, indirectly via local inhibitory inter-neurons, or both. In addition, as in mammalian cortex, NE in NIf may play a role in altering synaptic efficacy (McCormick et al., 1993) or in synaptic plasticity (Kirkwood et al., 1999). Future experiments using intracellular recordings in vitro may elucidate the specific cellular mechanisms by which adrenergic action in NIf modulates spontaneous and evoked activity.
Noradrenergic regulation of state-dependent auditory responsiveness
Previous studies have demonstrated state-dependent modulation of auditory responses in NIf, HVC, and the robust nucleus of the arcopallium (RA) (Dave et al., 1998; Schmidt and Konishi, 1998; Nick and Konishi, 2001; Cardin and Schmidt, 2003, 2004). Arousal of a lightly sedated bird results in a complete suppression of HVC (Cardin and Schmidt, 2003) and NIf (Cardin and Schmidt, 2004) auditory responses. Similarly, arousal from sleep results in a transient suppression of HVC auditory responsiveness (Nick and Konishi, 2001; Cardin and Schmidt, 2003). In the present study, we found that infusion of α1- and α2-adrenergic receptor antagonists into NIf prevented the arousal-mediated suppression of HVC auditory responses. This is the first direct demonstration of a neuromodulatory mechanism underlying the behavioral state-dependence of song system auditory responses.
Noradrenergic innervation of NIf is unlikely to be the sole modulatory mechanism involved in regulating song system auditory responsiveness because activation of cholinergic inputs to HVC and RA (Shea and Margoliash, 2003) and administration of high doses (20 mm) of NE to HVC (Dave et al., 1998) both suppress song system auditory responses. Together with our results, these observations indicate that ascending neuromodulatory systems exert a complex influence over auditory processing within several sensorimotor areas of the song system. Noradrenergic modulation in the forebrain is one mechanism by which the flow of ascending input to high-order sensory areas may be regulated in a behavioral state-dependent manner.
In previous experiments, we observed that auditory responsiveness in NIf and HVC during wakefulness covaries over the course of the day, showing distinct increases and decreases over short periods (Cardin and Schmidt, 2004). The dose-dependent enhancement and suppression of NIf and HVC responses observed here, as well as the block of arousal-mediated suppression by adrenergic antagonists in NIf, suggest that small changes in NE levels in NIf are a potential mechanism mediating the continual response modulation observed during wakefulness. In mammals, changes in locus ceruleus activity and NE release are associated with changes in attentional state (Foote et al., 1980; Rajkowski et al., 1994; Aston-Jones et al., 2000). Likewise, the observed variability in HVC responses may be associated with NE-dependent shifts in attentional states. This idea is consistent with recent findings that suggest a role for selective attention in modulating auditory responses in HVC (A. P. Alderete, J. Cynx, and M. F. Schmidt, unpublished observations). Such attentional modulation of auditory responsiveness might play a critical role in regulating auditory input to the song system during song learning (ten Cate 1984; Tchernichovski et al., 2001), song perception (Scharff et al. 1998), and auditory feedback of the bird's own song. A challenge for future experiments will be to identify the role of norepinephrine in shaping auditory response properties during these different natural behavioral conditions.
Footnotes
This research was supported by a predoctoral National Research Service Award to J.A.C., a March of Dimes Basil O'Connor Award to M.F.S., and a Sloan Foundation Award to M.F.S. We thank G. Aston-Jones, M. J. Higley, D. J. Perkel, and the members of the Schmidt laboratory for helpful discussions and comments during the preparation of this manuscript.
Correspondence should be addressed to Dr. Marc F. Schmidt, University of Pennsylvania, 312 Leidy Laboratories, 415 South University Avenue, Philadelphia, PA 19104. E-mail: marcschm@sas.upenn.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247745-09$15.00/0
References
- Armstrong-James M, Fox K (1983) Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J Physiol (Lond) 335: 427-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1: 876-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J (2000) Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res 126: 165-182. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF (1989) Effects of the noradrenergic neurotoxin DSP-4 on luteinizing hormone levels, catecholamine concentrations, alpha 2-adrenergic receptor binding, and aromatase activity in the brain of the Japanese quail. Brain Res 492: 163-175. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF, McEwen BS (1989) An autoradiographic study of alpha 1-adrenergic receptors in the brain of the Japanese quail (Coturnix coturnix japonica). Cell Tissue Res 258: 563-568. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Foote SL (1991) Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci 11: 3135-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan P, Bradshaw CM, Szabadi E (1977) The pharmacology of adrenergic neuronal responses in the cerebral cortex: evidence for excitatory alpha- and inhibitory beta-receptors. Br J Pharmacol 59: 635-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF (2003) Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness, and suppressed by arousal. J Neurophysiol 90: 2884-2899. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF (2004) Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J Neurophysiol 91: 2148-2163. [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D (1998) Behavioral state modulation of auditory activity in a vocal motor system. Science 282: 2250-2254. [DOI] [PubMed] [Google Scholar]
- Dermon CR, Kouvelas ED (1988) Binding properties, regional ontogeny and localization of adrenergic receptors in chick brain. Int J Dev Neurosci 6: 471-482. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD (2000) Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 37: 273-282. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lopez A, Revilla V, Candelas MA, Gonzalez-Gil J, Diaz A, Pazos A (1997) A comparative study of alpha2- and beta-adrenoceptor distribution in pigeon and chick brain. Eur J Neurosci 9: 871-883. [DOI] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP (1975) Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res 86: 229-242. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE (1980) Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci USA 77: 3033-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MJ (1992) Modification of receptive fields of posteriomedial barrel subfield neocortical single units by known concentrations of iontophoresed noradrenaline in the rat. Int J Neurosci 65: 69-81. [DOI] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA (1998) Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol 34: 329-346. [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW (1975) Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science 189: 55-58. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Heggelund P (1982) Single cell responses in cat visual cortex to visual stimulation during iontophoresis of noradrenaline. Exp Brain Res 45: 317-327. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rozas C, Kirkwood J, Perez F, Bear MF (1999) Modulation of long-term synaptic depression in visual cortex by acetylcholine and norepinephrine. J Neurosci 19: 1599-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM (1997) Effects of noradrenaline on frequency tuning of rat auditory cortex neurons. Eur J Neurosci 9: 833-847. [DOI] [PubMed] [Google Scholar]
- McCormick DA (1992) Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol 9: 212-223. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Wang Z, Huguenard J (1993) Neurotransmitter control of neocortical neuronal activity and excitability. Cereb Cortex 3: 387-398. [DOI] [PubMed] [Google Scholar]
- McLean J, Waterhouse BD (1994) Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res 667: 83-97. [DOI] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S (1998) Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol 400: 207-228. [PubMed] [Google Scholar]
- Mouradian RD, Sessler FM, Waterhouse BD (1991) Noradrenergic potentiation of excitatory transmitter action in cerebrocortical slices: evidence for mediation by an alpha 1 receptor-linked second messenger pathway. Brain Res 546: 83-95. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M (2001) Dynamic control of auditory activity during sleep: correlation between song response and EEG. Proc Natl Acad Sci USA 98: 14012-14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralong E, Magistretti PJ (1995) Noradrenaline increases K-conductance and reduces glutamatergic transmission in the mouse entorhinal cortex by activation of alpha 2-adrenoreceptors. Eur J Neurosci 7: 2370-2378. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G (1994) Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res Bull 35: 607-616. [DOI] [PubMed] [Google Scholar]
- Revilla R, Fernandez-Lopez C, Revilla V, Fernandez-Lopez A (1998) Preand post-hatching developmental changes in beta-adrenoceptor subtypes in chick brain. Brain Res Dev Brain Res 111: 159-167. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK (1980) Activation of lateral geniculate neurons by norepinephrine: mediation by an alpha-adrenergic receptor. Brain Res 182: 345-359. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J (1998) Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol 36: 81-90. [PubMed] [Google Scholar]
- Schmidt MF, Konishi M (1998) Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci 1: 513-518. [DOI] [PubMed] [Google Scholar]
- Shea SD, Margoliash D (2003) Basal forebrain cholinergic modulation of auditory activity in the zebra finch song system. Neuron 40: 1213-1226. [DOI] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ (1996) Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol 29: 473-489. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW (1990) Brainstem control of wakefulness and sleep. New York: Plenum.
- Steriade M, Amzica F, Nunez A (1993) Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol 70: 1385-1400. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F (2001) Dynamics of the vocal imitation process: how a zebra finch learns its song. Science 291: 2564-2569. [DOI] [PubMed] [Google Scholar]
- ten Cate C (1984) The influence of social relations on the development of species recognition in zebra finch males. Behaviour 91: 263-285. [Google Scholar]
- Videen TO, Daw NW, Rader RK (1984) The effect of norepinephrine on visual cortical neurons in kittens and adult cats. J Neurosci 4: 1607-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ (1980) Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative neurotransmitters. Exp Neurol 69: 30-49. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ (1981) Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology 20: 907-920. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Yeh HH, Woodward DJ (1982) Norepinephrine enhancement of inhibitory synaptic mechanisms in cerebellum and cerebral cortex: mediation by beta adrenergic receptors. J Pharmacol Exp Ther 221: 495-506. [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC (1988) New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull 21: 425-432. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Azizi SA, Burne RA, Woodward DJ (1990) Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res 514: 276-292. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ (1998) Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res 790: 33-44. [DOI] [PubMed] [Google Scholar]