Abstract
Neuromodulators are integral parts of a neuronal network, and unraveling how these substances alter neuronal activity is critical for understanding how networks generate patterned activity and, ultimately, behavior. In this study, we examined the cellular mechanisms underlying the excitatory action of substance P (SP) on the respiratory network isolated in spontaneously active transverse slice preparation of mice. SP produced a slow depolarization in all recorded inspiratory pacemaker and non-pacemaker neurons. Ion exchange experiments and blockers for different ion channels suggest that the slow depolarization is caused by the activation of a low-threshold TTX-insensitive cationic current that carries mostly Na+. The SP-induced slow depolarization increased tonic discharge in non-pacemaker neurons and primarily enhanced the frequency of bursting in Cd2+-insensitive pacemaker neurons. In the Cd2+-sensitive pacemaker neuron, the burst frequency was not significantly affected, whereas burst duration and amplitude were more enhanced than in Cd2+-insensitive pacemaker neurons. In a subset of non-pacemaker neurons that produced NMDA-dependent subthreshold oscillations, SP caused the production of bursts of action potentials. We conclude that the degree of pacemaker activity in the respiratory network is not fixed but dynamically regulated by neuromodulators such as SP. This finding may have clinical implications for Rett syndrome in which SP levels along with other neuromodulators are decreased in the brainstem.
Keywords: respiratory network, pacemaker neurons, TTX-resistant Na+ current, substance P, neuromodulation, bursting properties
Introduction
Neuronal networks are under the continuous influence of endogenously released neuromodulators, typically peptides and amines (Marder and Calabrese, 1996; Hilaire and Duron, 1999; Haji et al., 2000). Neuromodulators are not only required for normal functioning of neural networks (Peña and Ramirez, 2002; Nusbaum and Beenhakker, 2002) but they are also instrumental in adapting network activity to changes in metabolic and environmental conditions. Our understanding of modulator action in mammalian neuronal networks has been greatly facilitated by the development of the brain slice technique in which spontaneously active and still functional neuronal networks are isolated from the CNS. The respiratory network located within the so-called pre-Bötzinger complex (PBC) (Smith et al., 1991) is perhaps one of the best studied mammalian networks that generates behaviorally well defined neuronal activity even after isolation in brainstem slices (Lieske et al., 2000). Of particular interest is the specific substance P (SP) receptor neurokinin receptor 1 (NK1), which is expressed on respiratory neurons of the PBC. The presence of the NK1 receptor on PBC neurons is so characteristic that NK1 immunoreactivity has been used to anatomically identify the PBC (Gray et al., 1999). Interestingly, whereas NK1 receptor-deficient mice have normal PBC maturation and function at birth (Ptak et al., 2002), depletion of NK1-containing neurons within the PBC in vivo leads to very irregular breathing and, under some conditions, to death (Gray et al., 2001). Furthermore, in vitro blockade of NK1 receptors produces a slower and significantly more irregular respiratory rhythm (Telgkamp et al., 2002). This finding confirms not only the importance of the PBC in respiratory rhythm generation, but it also suggests that within the PBC, SP plays a critical role in controlling normal breathing. It has been hypothesized that NK1 receptors modulate respiratory activity by depolarizing type 1 and type 2 “rhythmogenic” inspiratory neurons (Gray et al., 1999), and it has been shown that exogenously applied SP depolarizes various types of respiratory neurons (Gray et al., 1999; Shvarev et al., 2002, 2003). However, the cellular mechanisms underlying the SP-induced depolarization remain unknown. In particular, for understanding how SP modulates respiratory rhythm generation, it will be important to specifically unravel the cellular mechanisms that lead to the modulation of pacemaker neurons. These pacemaker neurons are critical for respiratory rhythm generation (Smith et al., 1991; Peña et al., 2004).
In the present study, we provide data indicating that SP modulates a tetrodotoxin (TTX)-insensitive low-threshold sodium current in respiratory neurons. The activation of this low-threshold sodium current leads to the activation of other inward currents that are responsible for bursting activity in two types of pacemaker neurons, the Cd2+-sensitive and Cd2+-insensitive pacemaker neurons.
Materials and Methods
Preparation. Experiments were performed on brainstem transverse slices from male and female mice (CD1; postnatal days 1-13) using a preparation technique described in detail previously (Ramirez et al., 1996). The most important steps are summarized here. The animals were decapitated under anesthesia, and the isolated brainstem was placed in ice-cold artificial CSF (aCSF) bubbled with carbogen (95% O2 and 5% CO2). The aCSF contained (in mm) 118 NaCl, 3 KCl, 1.5 CaCl2, 1 MgCl2, 25 NaHCO3, 1 NaH2PO4, and 30 d-glucose, pH 7.4. The brainstem, glued rostral end up onto an agar block, was mounted into a vibratome (Leica Microsystems, Waukegon, IL) and serially sliced until the rostral boundary of the PBC was identified by anatomical landmarks such as disappearance of the facial nucleus and appearance of the inferior olive, the nucleus ambiguus, and the hypoglossal nucleus (see Fig. 1). A single 630- to 690-μm-thick slice was then taken. Slices were transferred into a recording chamber, superfused continuously with oxygenated aCSF, and maintained at a temperature of 29 ± 0.5°C. To initiate and maintain fictive respiratory rhythmic activity, the potassium concentration of the perfusate was raised from 3 to 8 mm over 30 min.
Figure 1.
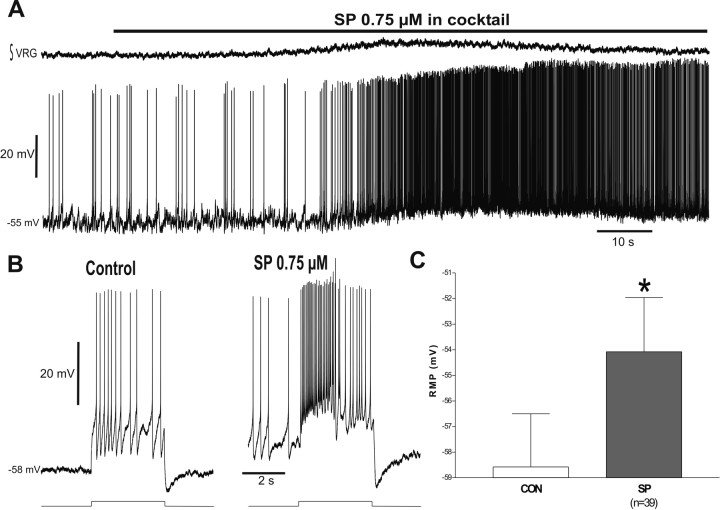
SP excites the respiratory network and depolarizes respiratory neurons. A, Schematic showing anatomical landmarks of a slice from the neonatal mouse medulla and recording sites for both population activity [integrated VRG (∫VRG)] and whole-cell patch-clamp recordings [membrane potential recording (VM)]. Note that application of 0.75 μm SP induces an excitation of integrated population activity (top trace) that is accompanied by a slow depolarization of a rhythmic neuron (bottom trace). The constant hyperpolarizing pulse was applied to test any change in input resistance produced by the application of SP. IO, Inferior olive; NA, nucleus ambiguus; SP5, spinally projecting trigeminal nucleus; XII, hypoglossal nucleus. B, Time course of the effect of SP on integrated population burst frequency. After 3 min of control recording, 0.75 μm SP was added (arrow) and continued for the rest of the experiment (n = 13). C, Time course of the effect of SP on the irregularity score (see Material and Methods) of respiratory activity. Note that after application of SP (arrow), the irregularity score is considerably reduced, indicating that the rhythm became more regular (n = 13). D, Quantification of resting membrane potential before [control (CON)] and after SP application for 15 respiratory neurons showing a significant depolarization. The asterisk denotes a statistically significant difference (p < 0.05). E, Comparison of a constant hyperpolarizing pulse as in A before (Control) and during application of SP, showing that there is no change on input resistance accompanying the depolarization induced by SP.
Drugs and solutions. To block fast synaptic transmission, a mixture of antagonists for NMDA receptors (NMDARs) [10 μm d(-)-3-(2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid (CPP); Tocris Cookson, Ellisville, MO), non-NMDARs (20 μm CNQX; Tocris Cookson), glycine receptors (1 μm strychnine; Sigma-RBI, St. Louis, MO), and GABAA receptors (20 μm bicuculline free base; Sigma-RBI) was added to the bath. SP, tetraethylammonium (TEA), and TTX (Sigma-RBI) were solubilized in distilled water, and the rest of the drugs were initially solubilized in dimethylsulfoxide (Sigma-RBI). For low Ca2+ solution, CaCl2 was substituted for an equimolar amount of MgCl2. For low Na+ solution, NaCl was substituted for an equimolar amount of choline chloride.
Recordings. Population activity recordings were obtained with suction electrodes positioned on the surface of the slice in the ventral respiratory group (VRG) in the area of the nucleus ambiguus (i.e., dorsal to the PBC). The signals were amplified 2000 times, filtered (low pass, 1.5 kHz; high pass, 250 Hz), rectified, and integrated using an electronic filter (time constant of 30-50 msec). Integrated population activity from the VRG was in phase with integrated inspiratory activity of the hypoglossal motor nucleus (Telgkamp and Ramirez, 1999). Therefore, it was used as a marker for inspiratory population activity (see Fig. 1). Intracellular patch-clamp recordings were obtained from PBC neurons (i.e., ventral of the nucleus ambiguus), at variable depths, using the blind patch-clamp technique. Respiratory neurons were identified according to their anatomical location and their discharge characteristics in relation to the population respiratory activity (see Fig. 1). The discharge pattern of each cell type was first identified in the cell-attached mode. Experiments were then performed in the whole-cell patch-clamp mode with the neurons recorded in current clamp at the zero current potential. Establishing the whole-cell patch-clamp configuration did not alter the firing pattern of recorded neurons. The patch electrodes were pulled from filamented borosilicate glass tubes (G150F-4; Warner Instruments, Hamden, CT) and filled with a solution containing (in mm) 140 K-gluconic acid, 1 CaCl2 × 6H2O, 10 EGTA, 2 MgCl2 × 6H2O,4Na2ATP, and 10 HEPES. The K-gluconic acid-containing electrode solution resulted in a significant liquid junction potential (LJP; >12 mV), which affected the measured membrane potentials. All membrane potential values were corrected for this LJP as described by Neher (1992). In some experiments, K-gluconic acid was substituted by an equimolar amount of CsCl.
All recordings were stored on a personal computer using AxoTape (version 2.0; Axon Instruments, Union City, CA) and analyzed off-line using customized analysis software written with IGOR Pro (Wavemetrics, Lake Oswego, OR). We obtained the irregularity score (S) of the respiratory cycle (inspiration plus expiration) using the following formula: Sn = ABS(Pn - Pn - 1)/Pn - 1, where Sn is the score of the nth cycle, Pn is its period, Pn - 1 is the period of the preceding burst, and ABS is the absolute value. Data are expressed as means ± SE. Statistical differences were assessed by Student's t test.
Results
SP and the modulation of network and non-pacemaker activity
Simultaneous recordings from population activity and whole-cell recordings of respiratory neurons in the VRG (Fig. 1A) show that continuous bath application of SP produced an excitation of the respiratory network (Fig. 1A) that is associated with a statistically significant maximal increase in inspiratory burst frequency of 185.5 ± 38.9% of control (p = 0.0002) (Fig. 1B) and a statistically significant reduction of the irregularity score (72.1 ± 9.7% of control; n = 13; p = 0.0008) (Fig. 1C). The excitation produced by SP at the network level correlates with a slow depolarization ranging from 1.1 to 13.7 mV (n = 15) (Fig. 1A,D). As reported previously for respiratory neurons (Gray et al., 1999; Shvarev et al., 2002), the depolarization produced by SP was not associated with a change in input resistance (n = 8) (Fig. 1E).
To test whether an increase in synaptic activity is primarily responsible for the depolarization produced by SP on respiratory neurons, we tested the effect of SP in the presence of a mixture containing specific antagonists for fast synaptic transmission. The mixture contained 10 μm CPP to block NMDARs, 20 μm CNQX to block non-NMDARs, 1 μm strychnine to block glycine receptors, and 20 μm bicuculline to block GABAA receptors. As we reported previously (Peña and Ramirez, 2002; Tryba et al., 2003; Peña et al., 2004), the mixture completely blocked the rhythmic population activity recorded from the VRG (Fig. 2A, top trace), revealing two major groups of inspiratory neurons: non-pacemaker (Fig. 2A) and pacemaker (Figs. 3A, 4A) neurons. As illustrated for a non-pacemaker neuron in Figure 2A, SP also produced the slow depolarization after the blockade of fast synaptic transmission (compare Figs. 2A,C, 1A,D). The depolarization produced by SP in inspiratory non-pacemakers in the presence or absence of the mixture was not significantly different [4.2 ± 0.8 mV (n = 15) and 4.5 ± 0.6 mV (n = 39), respectively; p = 0.39], suggesting that this depolarization was not primarily caused by synaptic inputs. The slow depolarization produced by SP led to an increased generation of spontaneous action potentials (Fig. 2A). The number of action potentials evoked by current injections was increased during application of SP (Fig. 2B).
Figure 2.
The SP-induced slow depolarization does not require synaptic transmission. A, Application of a mixture containing 10 μm CPP, 20 μm CNQX, 1 μm strychnine, and 20 μm bicuculline blocks synaptic transmission and the respiratory rhythm (∫VRG). Under these conditions, SP produces the same slow depolarization compared with the situation in the functional network (compare with Fig. 1A). The neuron presented was previously confirmed as a rhythmic respiratory neuron in the absence of the mixture. B, The SP-induced slow depolarization is accompanied by an increase in excitability. In the presence of SP, the same current injection produced more action potentials and an additional depolarizing drive potential. C, Quantification of resting membrane potential in the presence of the mixture before [control (CON)] and after SP application for 39 respiratory neurons shows a significant depolarization. The asterisk denotes a statistically significant difference (p < 0.05).
Figure 3.
SP potentiates Cd2+-sensitive pacemaker activity. A, Example of a Cd2+-sensitive pacemaker neuron that continues to burst rhythmically (bottom trace) in the absence of rhythmic population activity (top trace). The recording was obtained in the presence of the mixture. The SP-induced slow depolarization is accompanied by a dramatic increase in burst generation leading to action potential inactivation. Note also the amplitude of bursting was affected during the peak depolarization, suggesting also the inactivation of the burst mechanisms themselves. Injection of the constant negative DC current (arrow) restores burst and action potential generation. The neuron presented was previously confirmed as an inspiratory neuron in the absence of the mixture. B, Same pacemaker neuron as A in an expanded timescale showing burst activity before (left), at the beginning of the SP-induced slow depolarization before action potential inactivation (middle), and after applying negative DC current to restore action potential activity (right). C-E, Histograms characterizing bursting properties of Cd2+-sensitive pacemaker neurons before [control (CON)] and during SP application. The asterisk denotes a statistically significant difference (p < 0.05).
Figure 4.
SP excites Cd2+-insensitive pacemaker activity. A, Example of a Cd2+-insensitive pacemaker neuron that continues to burst rhythmically (bottom trace) in the absence of rhythmic population activity (top trace). The SP-induced slow depolarization increases bursting in this type of pacemaker. B, Same pacemaker neuron as A in an expanded time scale showing burst activity before (left) and during SP-induced slow depolarization (right). C-E, Histograms characterizing bursting properties of Cd2+-insensitive pacemaker neurons before [control (CON)] and during SP application. The asterisk denotes statistically significant differences (p < 0.05).
SP and the modulation of pacemaker neurons
As we reported previously (Thoby-Brisson and Ramirez, 2001; Peña and Ramirez, 2002; Peña et al., 2004), there are two major types of pacemaker neurons that can be differentiated by their bursting characteristics and their differential sensitivities to Cd2+: the Cd2+-sensitive pacemakers (Fig. 3) and Cd2+-insensitive pacemakers (Fig. 4). The effect of SP on both types of pacemaker neurons was examined. Pacemaker neurons were initially classified according to criteria described by Toby-Brisson and Ramirez (2001) and Peña et al. (2004); subsequently, in most cases, we added 200 μm Cd2+ at the end of the experiment to pharmacologically confirm the type of the recorded pacemaker. As shown in Figure 3, SP produced a dramatic effect on Cd2+-sensitive pacemaker neurons. The slow depolarization observed in non-pacemaker neurons was also evoked in these pacemakers. The depolarization was of such magnitude that it typically led to the inactivation of action potential generation. The injection of negative DC offset current to hyperpolarize the membrane recovered action potential generation (Fig. 3B). The SP-induced slow depolarization also led to a dramatic change in burst characteristics. This effect was quantified by obtaining for each recorded pacemaker neuron the average burst duration, amplitude, and frequency from five consecutive cycles generated during the peak depolarization. The mean and SE of these averages was obtained for 10 pacemaker neurons. As shown in Figure 3B, SP significantly increased the burst duration and amplitude of the rhythmic pacemaker potential [to 158.2 ± 19.6% of control (p = 0.017) and to 180.7 ± 22.2% of control (p = 0.008), respectively] (Fig. 3D,E) without significantly affecting burst frequency (146.9 ± 28.1% of control; p = 0.27) (Fig. 3C).
SP also produced a slow depolarization in Cd2+-insensitive pacemaker neurons (Fig. 4A), which led to significant changes in burst frequency, duration, and amplitude of the rhythmic pacemaker potential (Fig. 4B). During SP application, the burst frequency increased to 180.4 ± 14.4% of control (p = 0.002) (Fig. 4C), the burst amplitude to 121.3 ± 6.9% of control (p = 0.015) (Fig. 4D), and the burst duration to 128.1 ± 9.1% of control (p = 0.021; n = 27) (Fig. 4E).
SP- and NMDA-evoked activity
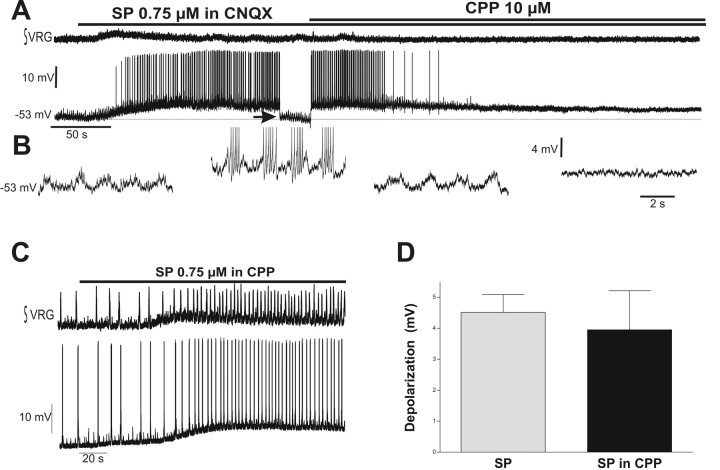
After the elimination of respiratory population activity by blocking just non-NMDARs with 20 μm CNQX, the majority of non-pacemaker neurons would typically lose their bursting activity. Under these conditions, three respiratory neurons (2%) exhibited subthreshold oscillations (Fig. 5A,B, left trace). The SP-induced slow depolarization produced in these neurons bursts of action potentials riding on top of the rhythmic oscillations (Fig. 5B, middle left trace). To examine whether those bursts of action potentials were attributable to an increase in the amplitude of the subthreshold oscillations or attributable to the slow (tonic) depolarization, we applied negative DC current to bring the membrane potential to control values (Fig. 5A). In the presence of the hyperpolarizing offset current, the subthreshold oscillations had the same amplitude as control (Fig. 5B, third trace from left). These subthreshold oscillations were associated with the activation of NMDARs because the bath-applied NMDAR antagonist CCP (10 μm) blocked both the subtreshold oscillations and the SP-evoked bursting (Fig. 5A,B, right trace).
Figure 5.
The SP-induced slow depolarization brings a subset of non-pacemaker neurons to an NMDA-dependent bursting level. A, Blocking just non-NMDARs with 20 μm CNQX is enough to block population respiratory rhythm (top trace). Whereas most of non-pacemaker neurons lose their bursting activity, some respiratory neurons exhibit subthreshold oscillations (bottom trace) that are able to produce CPP-sensitive bursts of action potentials during the SP-induced slow depolarization. B, Same non-pacemaker neuron as A in an expanded time scale showing subthreshold oscillations before (left) and bursts of action potentials (second panel from left) during the SP-induced slow depolarization. Negative DC current (arrow) was applied to bring the membrane potential to the original level (third panel from left). Note that subthreshold oscillations have the same amplitude. After application of 10 μm CPP to block NMDARs, both burst activity and subthreshold oscillations are blocked, whereas depolarization persists (right). C, Application of SP in the presence of 10 μm CPP produces excitation of the respiratory network (top trace) accompanied with depolarization of a respiratory neuron (bottom trace). D, Quantification of the membrane depolarization produced for SP alone and in the presence of 10 μm CPP. Note that there is no difference between the depolarization induced by SP under both conditions.
One of the possibilities for the excitatory effect of SP on the respiratory network is the potentiation of NMDA-mediated processes, as has been described in respiratory-related neurons (Ptak et al., 2000) and other neuronal networks (Liu et al., 1998; Herrero et al., 2000). Therefore, we tested whether SP produced the excitation of the respiratory network after blockade of NMDARs with CPP. As shown in Figure 5C, SP excited the respiratory network similarly as under control conditions (n = 5). At the cellular level, the SP-induced depolarization was not significantly different in the presence or absence of CPP (Fig. 5D) (4.51 ± 0.58 mV in control and 3.95 ± 1.26 mV in CPP; n = 5).
Ionic mechanisms responsible for the SP-evoked depolarization
We demonstrated above that SP induced a slow depolarization in all examined respiratory neurons. This depolarization caused an amplification of bursting properties in Cd2+-sensitive and Cd2+-insensitive pacemaker neurons and an activation of NMDA mechanisms in low-threshold oscillations observed in some non-pacemaker neurons. To examine the ionic basis of the SP-evoked slow depolarization, several sets of experiments were performed in inspiratory neurons consisting of a total of 57 non-pacemaker and 11 pacemaker neurons after the blockade of fast synaptic transmission with the mixture (Fig. 6A). As mentioned above, in the presence of the mixture of antagonists for the GABA, glycine, and glutamate ionotropic receptors (control), SP produced a slow depolarization of the membrane potential of 4.50 ± 0.56 mV (n = 39) (Fig. 6B). To examine the contribution of voltage-sensitive Na+ channels, we tested the effect of SP in the presence of 10 μm TTX. Under these conditions, SP produced a similar depolarization as control (4.79 ± 0.96 mV; n = 6; p = 0.40) (Fig. 6B), suggesting that TTX-sensitive sodium currents are not responsible for the slow SP-induced depolarization.
Figure 6.
SP induces a slow depolarization via a TTX-insensitive Na+ current. A, In the presence of a mixture in aCSF containing 143 mm NaCl and 1.5 mm CaCl2, SP produces a slow depolarization (top trace) that is not affected by the addition of 10 μm TTX (+ TTX) or by substituting CaCl2 for MgCl2 in the aCSF and adding TTX (+ TTX + Low Ca2+) or by adding TTX plus 10 mm TEA (+ TTX + TEA). The depolarization is dramatically reduced by lowering extracellular NaCl in the aCSF to 25 mm (+ Low Na+; bottom left trace). If normal Na+ concentration in the aCSF is reestablished, the SP-induced slow depolarization can be observed in the same neuron (bottom right trace). Note that all recordings were done in the presence of the mixture. B, Quantification of the SP-induced depolarization in a mixture and under the different conditions as described above. Note that where as the SP-induced slow depolarization is not affected by TTX, it is significantly enhanced by low Ca2+ plus TTX and by TEA plus TTX. The SP-induced slow depolarization is dramatically reduced in low Na+. The voltage and time calibration bars apply to all recordings. The asterisk denotes a statistically significant difference (p < 0.05).
To examine whether the activation of Ca2+ channels was responsible for the SP-induced slow depolarization, we tested the effect of SP in a TTX-containing aCSF solution containing low calcium. SP produced a significantly larger depolarization than control (6.64 ± 0.85 mV; n = 5; p = 0.034) (Fig. 6B). The possibility that SP induces the slow depolarization through the activation of the calcium-activated nonspecific cationic current as reported recently (Bell et al., 1998; Oh et al., 2003) was also ruled out. Flufenamic acid (500 μm), a specific blocker of the calcium-activated nonspecific cationic current (Morisset and Nagy, 1999; Partridge and Valenzuela, 2000), did not reduce the SP-induced slow depolarization (4.80 ± 0.66 mV; n = 5). Consistent with our conclusion that the SP-induced slow depolarization does not involve Ca2+ channels or some other calcium-dependent cationic currents, we found that in the presence of Cd2+ (200 μm), the SP-induced depolarization was similar to the control (4.89 ± 1.58 mV; n = 17; p = 0.41).
To examine the potential contribution of K+ channels, we performed the following experiments in the presence of TTX and TEA (10 mm). Under these conditions, SP produced an even larger depolarization than control (8.29 ± 1.84 mV; n = 6; p = 0.048) (Fig. 6B), and in half of the cases, the SP-induced slow depolarization evoked bursts of Ca2+-dependent action potentials (Fig. 6A). Because there are some K+ channels that are not sensitive to TEA, we tested four more cells with an internal electrode solution containing Cs+ (140 mm) to achieve a more complete blockade of K+ channels. The SP-induced depolarization under these conditions was significantly higher compared with the SP-induced slow depolarization evoked during control (6.01 ± 0.53 mV; n = 4; p = 0.037).
Because the SP-induced depolarization was neither blocked by TTX, low calcium, TEA, nor intracellular cesium, we hypothesized that the SP-induced slow depolarization was attributable to the activation of a TTX-insensitive sodium current. To test this hypothesis, we performed the subsequent experiments in aCSF containing low concentrations of extracellular Na+ (25 mm). Under these conditions, the SP-induced slow depolarization was completely abolished (0.56 ± 0.27 mV; n = 4) (Fig. 6B). The SP-induced slow depolarization returned to control levels after reestablishing the normal extracellular Na+ concentration as illustrated in Figure 6A. Another conceivable explanation for this finding is that SP might be acting on the Na+/K+ATPase. However, this possibility is highly unlikely because the SP-induced slow depolarization was not different from control in the presence of ouabain (10 μm; 4.25 ± 0.99 mV; n = 4; p = 0.42).
SP induces respiratory network activity in 3 mm extracellular K+
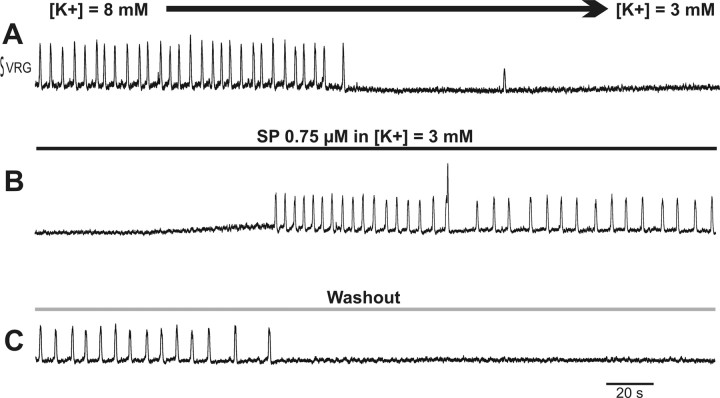
In view of the powerful excitatory effect of SP on the respiratory network, we tested whether SP is able to induce the respiratory rhythm in a silent slice. Previously, we have shown that only 20% of the brainstem slices produce the respiratory rhythm at a normal extracellular K+ concentration (3 mm) (Tryba et al., 2003), and it is necessary to increase the extracellular K+ concentration to 8 mm to observe the respiratory rhythm in 100% of the slices. Figure 7A exemplifies a preparation that fell silent after washing out the 8 mm K+-containing solution with a 3 mm K+-containing solution. SP was able to activate respiratory rhythmic population and cellular activity in all examined slices (n = 6) (Fig. 7B). The brainstem slice fell silent on washout of SP (Fig. 7C).
Figure 7.
SP induces respiratory network activity in 3 mm extracellular K+. A, Recording of the population respiratory activity showing the transition from rhythmic respiratory activity in 8 mm K+ to the absence of rhythmic activity in 3 mm K+. B, Bath application of SP restores the rhythmic activity. C, Washout of SP with a 3 mm K+ containing aCSF brings the network back to a silent state. Time calibration bars apply to all recordings.
Discussion
In this study, we examined the cellular mechanisms underlying the excitatory action of SP on the medullary respiratory network. SP produced a slow depolarization in all recorded neurons, regardless of neuronal type. We propose that the slow depolarization was most likely produced by the activation of a TTX-insensitive cationic current that carries mostly Na+. We hypothesize that this current belongs to the family of TTX-insensitive low-threshold Na+ currents (for review, see Delmas et al., 1997). Although all examined inspiratory neurons were depolarized by SP, the SP-induced slow depolarization led to specific modifications in the firing properties of the different types of respiratory neurons. In non-pacemaker neurons, the SP-induced depolarization led to increased tonic discharge. In Cd2+-insensitive pacemaker neurons, the SP-induced depolarization primarily enhanced the frequency of bursting (by 80%), whereas burst duration and amplitude were enhanced by only 21 and 28%, respectively. In contrast, in the Cd2+-sensitive pacemaker neuron, the SP-induced depolarization did not significantly increase burst frequency, whereas burst duration (58%) and amplitude (81%) were significantly enhanced. The change in burst amplitude induced by SP was significantly larger in Cd2+-sensitive compared with Cd2+-insensitive pacemaker neurons (p = 0.019). The SP-induced slow depolarization also caused the production of bursts of action potentials in a subset of non-pacemaker neurons that produced NMDA-dependent subthreshold oscillations.
The excitatory effect of SP on the respiratory rhythm in vivo and in vitro is well documented (Yamamoto et al., 1992; Gray et al., 1999; Ptak et al., 1999, 2000; Lieske et al., 2000; Telgkamp et al., 2002; Blanchi et al., 2003; Shvarev et al., 2003; Viemari et al., 2003), but we are just beginning to understand the cellular mechanisms underlying this excitatory effect. Previous reports have shown that SP depolarizes respiratory neurons (Gray et al., 1999; Shvarev et al., 2002, 2003) and respiratory-related phrenic and hypoglossal motoneurons (Ptak et al., 1999, 2000; Yasuda et al., 2001). Here, we demonstrate that this depolarization was not specific to a subpopulation of inspiratory PBC neurons. The amplitude of the slow depolarization was not significantly different in non-pacemaker versus pacemaker neurons (p = 0.40) and also did not differ between different types of pacemaker neurons. Previously, both Gray et al. (1999, 2001) and Guyenet et al. (2002) showed that NK1-positive neurons constitute a subset of neurons located in the PBC. More specifically, Guyenet et al. (2002) proposed that these neurons are glutamatergic neurons that might be responsible for the generation of the respiratory rhythm. In the present study, we found a depolarizing response in 100% of the recorded respiratory neurons (n = 150). This finding was unexpected and could suggest that SP depolarizes respiratory neurons not only via NK1 but also other tachykinin receptors like NK2 or NK3 (Severini et al., 2002).
The ionic mechanisms of SP modulation differ considerably among brain areas. SP activates voltage-dependent Ca2+ channels, voltage-dependent K+ conductances, Cl- currents, Ih currents, voltage-dependent Na+ channels, and nonselective cationic channels (Adams et al., 1983; Stanfield et al., 1985; Bley and Tsien, 1990; Shen and North, 1992; Bertrand and Galligan, 1994; Aosaki and Kawaguchi, 1996). In most neurons, SP depolarizes membrane potential by decreasing resting K+ conductances (Akasu et al., 1996; Lepre et al., 1996; Ptak et al., 2000; Yasuda et al., 2001). This seems not to be the case for respiratory neurons because the slow depolarization persisted after blocking K+ channels with TEA externally or cesium internally. We also ruled out that the slow depolarization is attributable to the activation of a calcium-activated nonspecific cationic current (Oh et al., 2003). For example, the calcium-activated nonspecific cationic current that is known to be induced by SP in cholinergic striatal interneurons (Bell et al., 1998) cannot be primarily responsible for depolarizing respiratory neurons because this depolarization was enhanced at low calcium concentrations. Moreover, we found in five neurons that the SP-induced slow depolarization persisted in the presence of flufenamic acid, a specific blocker of calcium-activated nonspecific cationic current. In the peripheral nervous system, SP depolarizes the membrane by increasing nonselective cationic conductances (Spigelman and Puil, 1990; Inoue et al., 1995; Weinreich et al., 1997; Moore et al., 1999; Soejima et al., 1999) that, based on our experiments in low calcium and Cd2+, are also unlikely candidates for mediating the SP effects in respiratory neurons. The absence of a change in input resistance in respiratory neurons could be attributable to a combination of different effects (i.e., the closing of a K+ conductance and opening of a cationic current, as described in the locus coeruleus and brainstem) (Shen and North, 1992; Wang and Robertson, 1998).
Despite the fact that we cannot rule out that SP might induce a slow depolarization by interacting with other neurotransmitter systems or ionic pumps different from Na+/K+ATPase, all our experiments are consistent with the modulation of a low-voltage-activated, TTX-insensitive sodium current (Delmas et al., 1997): (1) this current is activated by various neurotransmitters including SP (Koyano et al., 1993; Aosaki and Kawaguchi, 1996); (2) the current is activated at resting potential (Delmas et al., 1996; Gola et al., 1998a,b); (3) because the slow depolarization persisted in the presence of SP, it does not undergo inactivation; (4) this current is TTX insensitive, yet sodium dependent (Wang and Aghajanian, 1987; Raggenbass and Dreifuss, 1992; Delmas et al., 1996); (5) this current is not blocked by TEA (10-20 mm) (Alberi et al., 1993; Delmas et al., 1996); (6) characterizations of this current have also described the absence of an obvious change in input resistance (for review, see Delmas et al., 1997); and (7) lowering the physiological levels of calcium (2-25 mm) to 0.1 mm reversibly enhances the low-threshold sodium currents (Raggenbass and Dreifuss, 1992; Alberi et al., 1993; Delmas et al., 1996), which explains the observed dramatic depolarization of respiratory neurons caused by SP in low calcium (Fig. 6A).
Over the past decades, increasing evidence indicates that the respiratory network is subject to an extensive neuromodulation mediated by amines, peptides, and acetylcholine (for review, see Hilaire and Duron, 1999; Haji et al., 2000). The cellular mechanisms underlying these modulatory effects are currently investigated. A major objective of our study was to link the cellular effects with those observed at the network level. In contrast to the activation of the 5-HT2A receptor, which affected the persistent sodium current, resulting in a specific modulation of the calcium-insensitive pacemaker neurons (Peña and Ramirez, 2002), modulation of SP was more generalized and led to a more or less uniform depolarization of all examined respiratory neurons in the PBC. In addition to the generalized depolarization, SP also caused specific modulatory effects in the different types of respiratory neurons. These specific effects were presumably secondarily caused by the SP-induced slow depolarization.
The enhancement of bursting properties was the most obvious effect in Cd2+-sensitive pacemaker neurons and may contribute to a stronger contribution of pacemaker activity in the spontaneously active respiratory network. SP caused the most dramatic enhancement in weakly bursting neurons. This finding is consistent with the conclusion that bursting capabilities of pacemaker neurons in the respiratory network are not fixed but are dependent on the presence of neuromodulatory input. This principle has been demonstrated in various invertebrate and vertebrate neuronal networks, in which peptides and amines can induce as well as suppress pacemaker activity in rhythm-generating neurons (Dickinson and Nagy, 1983; Dekin et al., 1985; Harris-Warrick and Flamm, 1987; Straub and Benjamin, 2001; Marder and Thirumalai, 2002; Thoby-Brisson and Simmers, 2002). In the respiratory network, bursting properties are induced in nucleus tractus solitarius neurons by thyrotropin-releasing hormone (Dekin et al., 1985) and in pre-I neurons by SP (Yamamoto et al., 1992). We have previously demonstrated that endogenously released serotonin is required for burst generation in Cd2+-insensitive pacemaker neurons (Peña and Ramirez, 2002), and now we show that SP increases bursting properties in Cd2+-sensitive pacemakers. Thus, different neuromodulators can differentially modulate bursting properties in different types of respiratory neurons and alter the relative contribution of different pacemaker types in the generation of the respiratory rhythm.
Our conclusions may have clinical relevance. It has previously been demonstrated that children with Rett syndrome have significantly reduced SP levels in the CSF (Matsuishi et al., 1997) and a decreased SP immunoreactivity in the brainstem (Deguchi et al., 2000; Dunn and McLeod, 2001; Saito et al., 2001). These children suffer from frequent episodes of irregular breathing and apneas (Lugaresi et al., 1985; Cirignotta et al., 1986; Morton et al., 2000), suggesting that respiratory rhythm generation is severely disturbed in these children. It is possible that this disturbance is the result of a disrupted modulatory milieu, a hypothesis that can be tested in mice that carry the same mutation as these children (Chen et al., 2001; Guy et al., 2001).
Footnotes
This work was supported by fellowships from Pew Charitable Trusts and Consejo Nacional de Cienciay Tecnologia, México to F.P. and by grants from the Rett Syndrome Research Foundation and the National Institutes of Health (HL60120 and HL68860) to J.-M.R.
Correspondence should be addressed to Dr. Fernando Peña, Departamento de Farmacobiología, Centro de Investigación y de Estudios Avanzados, Instituto Politécnico Nacional, Calzada de los Tenorios 235, Colonia Granjas Coapa, 14330 México Distrito Federal, México. E-mail: jfpena@mail.cinvestav.mx.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249549-08$15.00/0
References
- Adams PR, Brown DA, Jones SW (1983) Substance P inhibits the M-current in bullfrog sympathetic neurones. Br J Pharmacol 79: 330-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T, Ishimatsu M, Yamada K (1996) Tachykinins cause inward current through NK1 receptors in bullfrog sensory neurons. Brain Res 713: 160-167. [DOI] [PubMed] [Google Scholar]
- Alberi S, Dubois-Dauphin M, Dreifuss JJ, Raggenbass M (1993) Modulation by divalent cations of the current generated by vasopressin in facial motoneurons. Brain Res 624: 326-330. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kawaguchi Y (1996) Actions of substance P on rat neostriatal neurons in vitro J Neurosci 16: 5141-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MI, Richardson PJ, Lee K (1998) Characterization of the mechanism of action of tachykinins in rat striatal cholinergic interneurons. Neuroscience 87: 649-658. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Galligan JJ (1994) Contribution of chloride conductance increase to slow EPSC and tachykinin current in guinea-pig myenteric neurones. J Physiol (Lond) 481: 47-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, Bevengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, Sieweke MH (2003) MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci 6: 1091-1100. [DOI] [PubMed] [Google Scholar]
- Bley KR, Tsien RW (1990) Inhibition of Ca2+ and K+ channels in sympathetic neurons by neuropeptides and other ganglionic transmitters. Neuron 4: 379-391. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 27: 327-331. [DOI] [PubMed] [Google Scholar]
- Cirignotta F, Lugaresi E, Montagna P (1986) Breathing impairment in Rett syndrome. Am J Med Genet Suppl 1: 167-173. [DOI] [PubMed] [Google Scholar]
- Deguchi K, Antalffy BA, Twohill LJ, Chakraborty S, Glaze DG, Armstrong DD (2000) Substance P immunoreactivity in Rett syndrome. Pediatr Neurol 22: 259-266. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Richerson GB, Getting PA (1985) Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science 229: 67-69. [DOI] [PubMed] [Google Scholar]
- Delmas P, Niel JP, Gola M (1996) Muscarinic activation of a novel voltage-sensitive inward current in rabbit prevertebral sympathetic neurons. Eur J Neurosci 8: 598-610. [DOI] [PubMed] [Google Scholar]
- Delmas P, Raggenbass M, Gola M (1997) Low-threshold Na+ currents: a new family of receptor-operated inward currents in mammalian nerve cells. Brain Res Brain Res Rev 25: 246-254. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Nagy F (1983) Control of a central pattern generator by an identified modulatory interneurone in crustacea. II. Induction and modification of plateau properties in pyloric neurones. J Exp Biol 105: 59-82. [DOI] [PubMed] [Google Scholar]
- Dunn HG, MacLeod PM (2001) Rett syndrome: review of biological abnormalities. Can J Neurol Sci 28: 16-29. [DOI] [PubMed] [Google Scholar]
- Gola M, Delmas P, Chagneux H (1998a) Encoding properties induced by a persistent voltage-gated muscarinic sodium current in rabbit sympathetic neurones. J Physiol (Lond) 510: 387-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M, Delmas P, Chagneux H (1998b) Encoding properties induced by a persistent voltage-gated muscarinic sodium current in rabbit sympathetic neurones. J Physiol (Lond) 510: 387-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL (1999) Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science 286: 1566-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL (2001) Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A (2001) A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet 27: 322-326. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL (2002) Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci 22: 3806-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haji A, Takeda R, Okazaki M (2000) Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol Ther 86: 277-304. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Flamm RE (1987) Multiple mechanisms of bursting in a conditional bursting neuron. J Neurosci 7: 2113-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA (2000) Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol 61: 169-203. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B (1999) Maturation of the mammalian respiratory system. Physiol Rev 79: 325-360. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakazawa K, Inoue K, Fujimori K (1995) Nonselective cation channels coupled with tachykinin receptors in rat sensory neurons. J Neurophysiol 73: 736-742. [DOI] [PubMed] [Google Scholar]
- Koyano K, Velimirovic BM, Grigg JJ, Nakajima S, Nakajima Y (1993) Two signal transduction mechanisms of substance P-induced depolarization in locus coeruleus neurons. Eur J Neurosci 5: 1189-1197. [DOI] [PubMed] [Google Scholar]
- Lepre M, Olpe HR, Brugger F (1996) The effects of neurokinin-1 receptor agonists on spinal motoneurones of the neonatal rat. Neuropharmacology 35: 511-522. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM (2000) Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci 3: 600-607. [DOI] [PubMed] [Google Scholar]
- Liu X, Andre D, Puizillout JJ (1998) Substance P post-synaptically potentiates glutamate-induced currents in dorsal vagal neurons. Brain Res 804: 95-104. [DOI] [PubMed] [Google Scholar]
- Lugaresi E, Cirignotta F, Montagna P (1985) Abnormal breathing in the Rett syndrome. Brain Dev 7: 329-333. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL (1996) Principles of rhythmic motor pattern generation. Physiol Rev 76: 687-717. [DOI] [PubMed] [Google Scholar]
- Marder E, Thirumalai V (2002) Cellular, synaptic and network effects of neuromodulation. Neural Netw 15: 479-493. [DOI] [PubMed] [Google Scholar]
- Matsuishi T, Nagamitsu S, Yamashita Y, Murakami Y, Kimura A, Sakai T, Shoji H, Kato H, Percy AK (1997) Decreased cerebrospinal fluid levels of substance P in patients with Rett syndrome. Ann Neurol 42: 978-981. [DOI] [PubMed] [Google Scholar]
- Moore KA, Taylor GE, Weinreich D (1999) Serotonin unmasks functional NK-2 receptors in vagal sensory neurones of the guinea-pig. J Physiol (Lond) 514: 111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Nagy F (1999) Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci 19: 7309-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RE, Pinnington L, Ellis RE (2000) Air swallowing in Rett syndrome. Dev Med Child Neurol 42: 271-275. [DOI] [PubMed] [Google Scholar]
- Neher E (1992) Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123-131. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP (2002) A small-systems approach to motor pattern generation. Nature 417: 343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EJ, Gover TD, Cordoba-Rodriguez R, Weinreich D (2003) Substance P evokes cation currents through TRP channels in HEK293 cells. J Neurophysiol 90: 2069-2073. [DOI] [PubMed] [Google Scholar]
- Partridge LD, Valenzuela CF (2000) Block of hippocampal CAN channels by flufenamate. Brain Res 867: 143-148. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM (2002) Endogenous activation of serotonin 2A receptors is required for respiratory rhythm generation in vitro J Neurosci 22: 11055-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña F, Parkis MA, Tryba AK, Ramirez JM (2004) Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105-117. [DOI] [PubMed] [Google Scholar]
- Ptak K, Di Pasquale E, Monteau R (1999) Substance P and central respiratory activity: a comparative in vitro study on foetal and newborn rat. Brain Res Dev Brain Res 114: 217-227. [DOI] [PubMed] [Google Scholar]
- Ptak K, Konrad M, Di Pasquale E, Tell F, Hilaire G, Monteau R (2000) Cellular and synaptic effect of substance P on neonatal phrenic motoneurons. Eur J Neurosci 12: 126-138. [DOI] [PubMed] [Google Scholar]
- Ptak K, Burnet H, Blanchi B, Sieweke M, De Felipe C, Hunt SP, Monteau R, Hilaire G (2002) The murine neurokinin NK1 receptor gene contributes to the adult hypoxic facilitation of ventilation. Eur J Neurosci 16: 2245-2252. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Dreifuss JJ (1992) Mechanism of action of oxytocin in rat vagal neurones: induction of a sustained sodium-dependent current. J Physiol (Lond) 457: 131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Richter DW (1996) Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol (Lond) 491: 799-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Ito M, Ozawa Y, Matsuishi T, Hamano K, Takashima S (2001) Reduced expression of neuropeptides can be related to respiratory disturbances in Rett syndrome. Brain Dev 23 [Suppl 1]: S122-S126. [DOI] [PubMed] [Google Scholar]
- Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V (2002) The tachykinin peptide family. Pharmacol Rev 54: 285-322. [DOI] [PubMed] [Google Scholar]
- Shen KZ, North RA (1992) Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience 50: 345-353. [DOI] [PubMed] [Google Scholar]
- Shvarev YN, Lagercrantz H, Yamamoto Y (2002) Biphasic effects of substance P on respiratory activity and respiration-related neurones in ventrolateral medulla in the neonatal rat brainstem in vitro. Acta Physiol Scand 174: 67-84. [DOI] [PubMed] [Google Scholar]
- Shvarev YN, Lagercrantz H, Yamamoto Y (2003) Two types of rhythm in the respiratory network output in the isolated ventrolateral medulla in the neonatal rats. Neurosci Lett 347: 53-56. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima T, Endoh T, Suzuki T (1999) Tachykinin-induced responses via neurokinin-1 and -3 receptors in hamster submandibular ganglion neurones. Arch Oral Biol 44: 455-463. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Puil E (1990) Ionic mechanism of substance P actions on neurons in trigeminal root ganglia. J Neurophysiol 64: 273-281. [DOI] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima Y, Yamaguchi K (1985) Substance P raises neuronal membrane excitability by reducing inward rectification. Nature 315: 498-501. [DOI] [PubMed] [Google Scholar]
- Straub VA, Benjamin PR (2001) Extrinsic modulation and motor pattern generation in a feeding network: a cellular study. J Neurosci 21: 1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Ramirez JM (1999) Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol 82: 2163-2170. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM (2002) Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol 88: 206-213. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Ramirez JM (2001) Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol 86: 104-112. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Simmers J (2002) Long-term neuromodulatory regulation of a motor pattern-generating network: maintenance of synaptic efficacy and oscillatory properties. J Neurophysiol 88: 2942-2953. [DOI] [PubMed] [Google Scholar]
- Tryba A, Pena F, Ramirez JM (2003) Stabilization of bursting in respiratory pacemaker neurons. J Neurosci 23: 3838-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bevengut M, Hilaire G (2003) Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur J Neurosci 17: 1233-1244. [DOI] [PubMed] [Google Scholar]
- Wang X, Robertson D (1998) Substance P-induced inward current in identified auditory efferent neurons in rat brain stem slices. J Neurophysiol 80: 218-229. [DOI] [PubMed] [Google Scholar]
- Wang YY, Aghajanian GK (1987) Excitation of locus coeruleus neurons by an adenosine 3′,5′-cyclic monophosphate-activated inward current: extracellular and intracellular studies in rat brain slices. Synapse 1: 481-487. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Moore KA, Taylor GE (1997) Allergic inflammation in isolated vagal sensory ganglia unmasks silent NK-2 tachykinin receptors. J Neurosci 17: 7683-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Onimaru H, Homma I (1992) Effect of substance P on respiratory rhythm and pre-inspiratory neurons in the ventrolateral structure of rostral medulla oblongata: an in vitro study. Brain Res 599: 272-276. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Robinson DM, Selvaratnam SR, Walsh CW, McMorland AJ, Funk GD (2001) Modulation of hypoglossal motoneuron excitability by NK1 receptor activation in neonatal mice in vitro. J Physiol (Lond) 534: 447-464. [DOI] [PMC free article] [PubMed] [Google Scholar]