Abstract
Chronic opiate use produces persistent changes in brain neurons that are expressed as adverse effects, including physical dependence and compulsive drug-seeking behavior. Dysregulation of the hypothalamic-pituitary-adrenal response to stress also occurs with chronic opiate administration and has been implicated as a contributing factor to continued substance abuse. This study provides the first evidence for dysregulation of the central noradrenergic response to stress by chronic opiates. Chronic morphine selectively sensitized locus ceruleus (LC)-norepinephrine (NE) neurons to corticotropin-releasing factor (CRF), an integral mediator of the stress response. CRF doses that were inactive in vehicle-treated rats produced a near-maximal activation of LC neurons of rats chronically administered morphine. LC sensitization to CRF was not solely a pharmacological phenomenon but was expressed as hyperresponsivity to physiological stress. Finally, opiate-induced LC sensitization translated to a change in the behavioral repertoire in response to environmental stress (swim stress) such that NE-mediated hyperactive behaviors predominated. The opiate-induced sensitization of the central NE response to stress predicts that chronic opiate administration increases vulnerability to certain stress-related symptoms (e.g., hyperarousal, attentional dysfunction), and this may contribute to the maintenance of opiate-seeking behavior.
Keywords: corticotropin-releasing factor, locus ceruleus, morphine, μ-opiate receptor, swim stress, 8-bromo-cAMP
Introduction
Chronic opiate use has long-term adverse consequences including tolerance, physical dependence, and compulsive drug-seeking behavior, that result from persistent alterations in brain neurons (Nestler, 2001). One consequence that may contribute to drug-seeking behavior is dysregulation of the stress response (Kreek and Koob, 1998; Houshyar et al., 2001; Schluger et al., 2003). Dysregulation of the stress response may additionally pre-dispose chronic opiate users to stress-related diseases.
One site at which opiates regulate central responses to stress is the locus ceruleus (LC)-norepinephrine (NE) system. This system is implicated in arousal and setting the attentional mode (Aston-Jones et al., 2000) and is engaged as part of the stress response to facilitate arousal (Page et al., 1993; Valentino and Van Bockstaele, 2002). Stress-elicited activation of the LC-NE system and subsequent forebrain arousal is mediated in part by the stress-related neuropeptide corticotropin-releasing factor (CRF) (Valentino et al., 1991; Page et al., 1993; Kawahara et al., 2000).
Convergent findings suggest that the LC-NE system is reciprocally regulated by CRF and endogenous opioids during stress. For example, LC dendrites receive convergent input from CRF- and enkephalin-containing axon terminals (Tjoumakaris et al., 2003). CRF receptor (CRF-R) activation and μ-opiate receptor (μ-OR) activation have opposing effects on LC neurons, with CRF increasing discharge rate (Curtis et al., 1997) and μ-OR activation decreasing discharge rate (Korf et al., 1974). The functional impact of this reciprocal regulation is apparent in the response of the LC-NE system to hypotensive stress elicited by nitroprusside infusion. During this challenge, CRF afferents are engaged to increase LC neuronal activity, and this is temporally correlated with, and necessary for, cortical EEG activation (Valentino et al., 1991; Page et al., 1993). After stress termination, opioid release inhibits LC neurons, restoring basal activity (Curtis et al., 2001) and presumably protecting against adverse consequences of continued activation (e.g., hyperarousal).
Previous studies provided evidence for dysregulation of the hypothalamic-pituitary-adrenal axis by chronic opiates (Kreek and Koob, 1998; Houshyar et al., 2001; Schluger et al., 2003). Given the role of the LC-NE system in stress and its regulation by opioids, this system presents an additional potential target through which chronic opiates could act to dysregulate the stress response. In the present study, single-unit LC activity was recorded and neuronal responses to CRF and to hypotensive stress (elicited by nitroprusside infusion) were compared in morphine- and vehicle-treated rats. These experiments tested the hypothesis that chronic opiate use alters the balance of CRF-opioid regulation of the LC and thereby the sensitivity of this central arousal system to stress.
Materials and Methods
Animals. The subjects were adult male Sprague Dawley rats (300 gm; Taconic Farms, Germantown, NY). Care and use of animals was approved by the Children's Hospital of Philadelphia Institutional Animal Care and Use Committee and was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic morphine administration. Morphine was chronically administered as previously described (Valentino and Wehby, 1989). Rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and an osmotic minipump (no. 2001; Alzet, Cupertino, CA) that contained 0.2 ml of sterile saline or morphine sulfate (50 mg/ml in sterile saline) was implanted subcutaneously. Tubing connected the outflow of the pump to a cannula implanted in the lateral ventricle so that solution from the pump was delivered intracerebroventricularly (1 μl/hr). Rats were individually housed after surgery.
Electrophysiological recording. Rats were anesthetized with halothane (1-2%) and surgically prepared for recording extracellular single-unit LC activity 1 (1 d morphine) or 7 (7 d morphine or vehicle) days after pump implantation (Curtis et al., 1997). The femoral vein and artery were cannulated for nitroprusside infusion (to produces hypotensive stress) and blood pressure recording, respectively. Double-barrel glass micropipettes were used to simultaneously record LC discharge and microinfuse substances (Curtis et al., 1997). These consisted of a recording pipette, filled with 2% Pontamine Sky Blue dye (PSB) in 0.5 m sodium acetate, and a calibrated microinfusion pipette that was filled with agents to be administered intra-LC.
Experimental protocol. Discharge rate was recorded for at least 9 min before microinfusion of agents or nitroprusside administration. Agents were microinfused into the LC by applying small pulses of pressure (over 20-60 sec) to the microinfusion pipette using a picospritzer (Parker Hannifin, Fairfield, NJ) to deliver a total volume of 30 nl. CRF, vasoactive intestinal peptide (VIP), and hypotensive challenge were tested on only one cell in an individual rat. For experiments in which the effects of 8-bromo-cAMP (8-Br-cAMP) were examined, 0.1 mm was first tested on one LC neuron, and 1.0 mm was tested on a cell in the contralateral LC of the same rat.
Histology. The recording site was marked by PSB iontophoresis (-15 μA; 10 min), and neutral red dye was injected to verify the patency of the intracerebroventricular cannula. Rats were killed by halothane overdose, and the brains were removed and sectioned for localization of the PSB spot. Data are from neurons that were histologically identified as being within the LC (Valentino et al., 1983).
Electron microscopy. Animals were anesthetized with sodium pentobarbital (60 mg/kg) and perfused transcardially with 50 ml of 3.8% acrolein (Electron Microscopy Sciences, Fort Washington, PA) and 200 ml of 2% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4. The brains were removed, cut into 1-3 mm coronal slices, and postfixed for an additional 30 min. Sections (40 μm) were cut through the LC using a Vibratome and collected into 0.1 m PB. These were placed in 1% sodium borohydride to remove reactive aldehydes and rinsed before the primary antibody incubation. The sections were incubated in goat anti-CRF-R antiserum (C-20; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-μ-OR antiserum (1:2500; Chemicon, Temecula, CA) for 15-18 hr at room temperature. The CRF-R antiserum recognizes both CRF-R1 and CRF-R2. The μ-OR antiserum recognizes the C-terminal domain of the μ-OR. Control sections were run in parallel in which one of the primary antisera was omitted, but the rest of the processing procedure was identical. Sections were rinsed in 0.1 m PBS and incubated in the secondary antiserum at room temperature. For CRF-R immunocytochemistry, sections were incubated in biotinylated donkey anti-goat IgG (1:400; Vector Laboratories, Burlingame, CA) for 30 min followed by incubation in avidin-biotin complex (Vector Laboratories). This was visualized by reaction with 3-3′ diaminobenzidine (Sigma, St. Louis, MO) and H2O2 in 0.1 m PBS. For μ-OR immunocytochemistry, sections were incubated in 0.01 m PBS containing 0.1% gelatin and 0.8% bovine serum albumin (BSA) for 30 min. Sections were then incubated in donkey anti-rabbit IgG conjugated to 1 nm gold particles (Amersham Biosciences, Piscataway, NJ) for 2 hr at room temperature. These were rinsed and incubated in 1.25% glutaraldehyde (Electron Microscopy Sciences, Fort Washington, PA) followed by a wash in 0.01 m PBS and then in 0.2 m sodium citrate buffer, pH 7.4. Silver intensification of the gold particles was achieved using a silver enhancement kit (Amersham Biosciences). Sections were rinsed and incubated in 2% osmium tetroxide (Electron Microscopy Sciences) for 1 hr, rinsed, dehydrated, and flat embedded in Epon 812 (Electron Microscopy Sciences). Thin sections (80-100 nm) were cut from the outer surface of the tissue, collected on grids, and counterstained with uranyl acetate and Reynolds lead citrate.
Western blot analysis. Rats were killed by decapitation, and brains were placed in a brain block from which a slice (2 mm) containing the LC was cut. The cerebellum was removed, and tissue containing the LC was microdissected using a trephine. Samples were homogenized and centrifuged. Protein content was determined using the BCA method. Protein extracts (15 μg) were subjected to SDS-PAGE gel electrophoresis, and proteins were transferred to polyvinylidene difluoride membrane. Membranes were incubated in the goat anti-CRF-R antiserum (1:500) for 1 hr at room temperature, rinsed, and incubated with peroxidase-conjugated donkey anti-goat antiserum (1:10,000; Jackson ImmunoResearch, West Grove, PA). Nonspecific binding was blocked by incubation of the membranes in BSA (0.2%). The membranes were developed using an ECL kit (Amersham Biosciences) and exposed to X-OMAT AR film (Eastman Kodak, Rochester, NY). After probing for CRF-R, the membranes were stripped using Re-Blot Plus (Chemicon), blocked in the BSA solution, and incubated in mouse anti-β-actin antiserum (1:5000; Sigma). Membranes were processed as above using donkey anti-mouse antiserum. Films were scanned and band density was quantified using UN-SCAN-IT (Silk Scientific, Orem, UT). CRF-R-to-actin ratios were calculated for each sample. Duplicate or triplicate samples were averaged for an individual rat.
Behavioral testing. Seven days after implanting osmotic pumps, rats were placed into a glass cylinder (46 cm high; 20 cm diameter) filled to a 30 cm depth with water at room temperature for 15 min. Behavior was videotaped for subsequent treatment-blind analysis by a trained observer. Behaviors were scored using a time sampling procedure (Detke et al., 1995). At the end of each 5 sec epoch, behavior was scored as (1) immobility [floating without struggling (i.e., the rat makes only those movements necessary to keep its head above the water)], (2) swimming (swimming movements that result in a change in position of at least one-fourth of the cylinder circumference), or (3) climbing (active movements with forepaws in and out of the water, usually directed against the cylinder walls). Diving was added to swimming scores in the final analysis. The incidence of each behavior was summed and compared between groups at the end of each 5 min period and at the end of the 15 min test.
Drugs. Ovine CRF (Dr. J. Rivier, The Salk Institute, San Diego, CA) and VIP (Sigma) were dissolved in water, aliquoted, concentrated, and stored at -70°C. They were dissolved in the appropriate volume of artificial cerebrospinal fluid (ACSF) on the day of the experiment. Sodium nitroprusside (Sigma) was dissolved in saline (0.33 mg/ml) and infused intravenously at a rate of 30 μl/min for 15 min. 8-Br-cAMP (Sigma) was dissolved in ACSF on the day of the experiment. Morphine sulfate was obtained from the National Institute of Drug Abuse.
Data analysis. Mean baseline LC discharge rate was determined over three 1 min intervals before microinfusion of substances into the LC or intravenous nitroprusside administration. Discharge rates after microinfusion or nitroprusside administration were expressed as a percentage of this mean. A repeated-measures ANOVA was used to determine the effect of an individual manipulation as a function of time. Two- or three-way ANOVAs were used to compare groups. The CRF dose-response curve was generated by determining the maximum percentage increase produced by a dose of CRF during the first 15 min after injection in individual cases. The mean maximum effect was then determined and compared between groups. Data are expressed as mean ± SEM.
Results
Chronic morphine sensitizes LC neurons to CRF
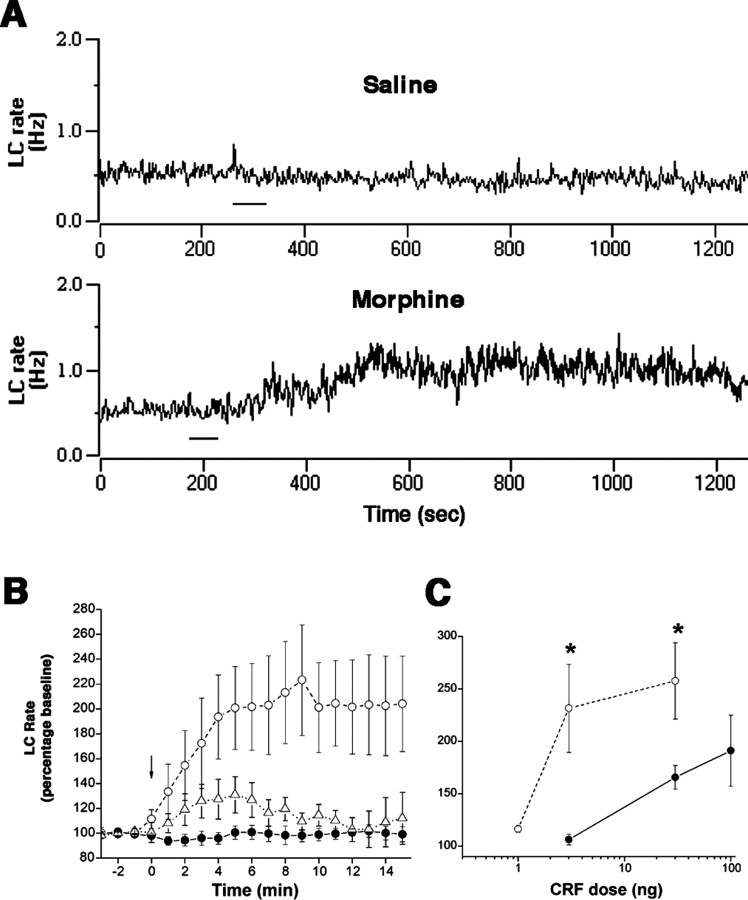
Mean baseline LC discharge rates in 1 d morphine rats (0.6 ± 0.1 Hz; n = 6) and 7 d morphine rats (0.8 ± 0.1 Hz; n = 31) were not different and were less (p < 0.001) than that recorded in vehicle-treated rats (1.4 ± 0.1; n = 31). CRF microinfusion (1-100 ng in 30 nl) produced a dose-related increase in LC discharge rates of all rats (Fig. 1C). However, LC neurons of 7 d morphine rats were sensitized to CRF (Fig. 1). A dose of CRF (3 ng) that was ineffective in vehicle-treated rats or 1 d morphine rats produced a near-maximal activation of LC neurons of 7 d morphine rats (Fig. 1). In the 7 d morphine group, 3 ng of CRF was equieffective as 100 ng in vehicle-treated rats (Fig. 1C). LC sensitization to CRF in 7 d morphine rats was unrelated to the lower basal LC discharge rate because LC neurons of 1 d morphine rats, which had similarly low basal rates, were not sensitized to CRF (Fig. 1B).
Figure 1.
Chronic morphine sensitizes LC neurons to CRF. A, Traces representing LC discharge rate in saline-treated and 7 d morphine rats before and after 3 ng of CRF (bar). Baseline rates were comparable. B, Mean effect of CRF (3 ng) as a function of time in vehicle (solid circles; n = 5), 1 d morphine (open triangles; n = 6), and 7 d morphine (open circles; n = 7) rats. CRF was administered at arrow (Time = 0). There was an effect of treatment (F(2,15) = 4.75; p < 0.05), time (F(18,270) =4.3;p<0.0001), and a time-treatment interaction (F(36,270) =3.22;p < 0.0001). C, CRF dose-response curves in vehicle (solid circles) or 7 d morphine (open circles) rats. Points represent the mean maximum effect produced by CRF at any time after injection (n = 5-7 determinations). *p < 0.05, Student's t test for independent samples. Bars in B and C indicate SEM.
Because CRF receptor activation has been linked to activation of adenyl cyclase (Chalmers et al., 1996), the effects of 8-Br-cAMP and VIP, which activates adenyl cyclase in LC neurons (Wang and Aghajanian, 1990), were examined. Microinfusion of 8-Br-cAMP (0.1 and 1.0 mm) increased LC discharge rates of morphine- and vehicle-treated rats by the same magnitude. In vehicle-treated rats, 0.1 mm and 1.0 mm 8-Br-cAMP increased LC discharge rate by 16 ± 6% (n = 6) and 45 ± 12% (n = 6), respectively. In 7 d morphine rats, the percentage increase in LC discharge rate produced by 0.1 mm and 1.0 mm 8-Br-cAMP was 17 ± 8% (n = 5; p = 0.9) and 63 ± 34% (n = 5; p = 0.6), respectively. LC responses to VIP were also comparable in vehicle-treated rats versus chronic morphine rats. Thus, in vehicle-treated rats, 0.3 ng and 1.0 ng VIP produced a 38 ± 10% (n = 7) and 203 ± 36% (n = 7) increase in LC discharge rate, respectively. In chronic morphine rats, the same doses produced a 34 ± 7% (n = 9; p = 0.4) and 338 ± 137% (n = 6; p = 0.3) increase in LC discharge rate.
Chronic morphine sensitizes LC neurons to stress
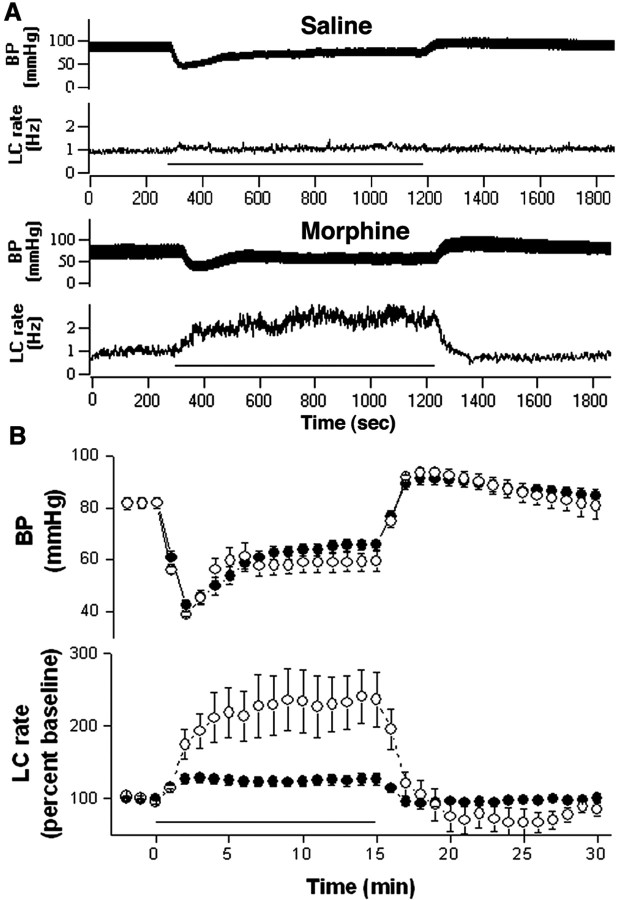
Consistent with postsynaptic sensitization to CRF, LC activation by the same magnitude of hypotensive challenge was substantially greater in rats chronically administered morphine (Fig. 2). A two-way repeated-measures ANOVA on LC discharge rates during the 15 min from the start of the infusion revealed a statistically significant effect of treatment (F(1,11) = 8; p < 0.02), time (F(5,55) = 16; p < 0.0001), and a time-treatment interaction (F(5,55) = 7.7; p < 0.0001).
Figure 2.
Chronic morphine sensitizes LC neurons to stress. A, Blood pressure (top trace) and LC discharge rate (bottom trace) in response to nitroprusside infusion (bar) in vehicle- and 7 d morphine-treated rats. Baseline rates are comparable. B, Mean effect of nitroprusside infusion on blood pressure (top) and LC discharge rate (bottom) of vehicle-treated (solid circles; n = 7) and 7 d morphine (open circles; n = 6) rats. Bars indicate SEM.
CRF-R and μ-OR are colocalized in dendrites in the rat LC
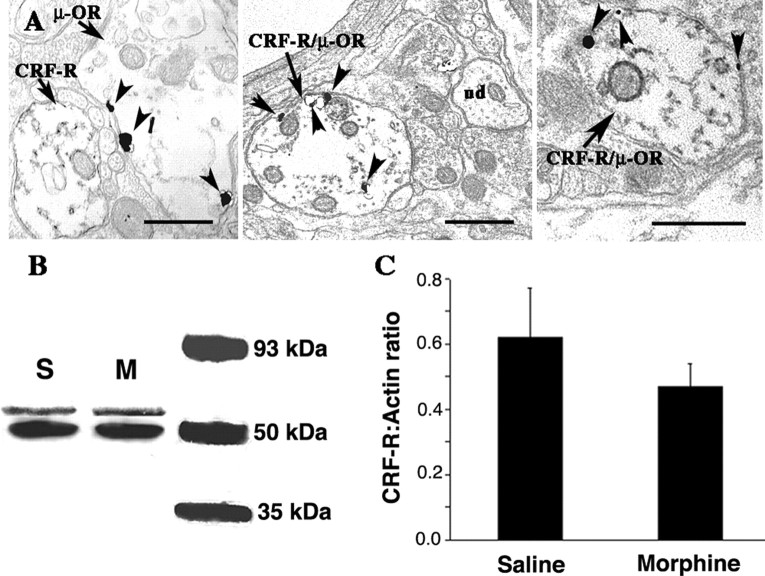
At the ultrastructural level, gold-silver labeling for μ-OR could be identified along the plasma membrane of dendritic processes, as previously described (Van Bockstaele et al., 1996). Peroxidase labeling for CRF-R was also associated with the plasmalemma, as well as within cytoplasmic portions of dendritic profiles. Immunolabeling for CRF-R and μ-OR was detected in individual dendrites in the LC (Fig. 3A, left). However, CRF-R and μ-OR were often colocalized within the same dendritic process (Fig. 3A, middle and right).
Figure 3.
Colocalization of μ-OR and CRF-R in LC processes. A, Electron photomicrographs showingμ-OR (immunogold) and CRF-R (immunoperoxidase) labeling in LC dendrites. In the left panel, CRF-R and μ-OR labeling are in separate dendrites (arrows). Arrowheads indicate gold-silver particles for μ-OR, whereas peroxidase labeling for CRF-R is identified as electron-dense reaction product that is distributed along the plasma membrane, as well as diffusely distributed within the cytoplasm of the dendrite. The middle and right panels show dendrites that colocalize CRF-R and μ-OR (CRF-R/μ-OR). In the middle panel, an unlabeled dendrite (ud) can be seen in the same field as the dual-labeled dendrite. Arrowheads point to immunogold particles. Scale bars, 500 nm. B, Western blot showing CRF receptor immunoreactivity in LC tissue from vehicle (S) versus 7 d morphine rat (M). Top and bottom bands are CRF-R and actin, respectively. An image of film showing the actin bands was superimposed with an image of the film showing the CRF-R bands for the same samples. C, The bar graph indicates the mean CRF-R-to-actin ratio in LC tissue punches from seven vehicle-treated rats and 10 morphine-treated rats. Lines indicate SEM.
There was no difference in CRF-R protein levels from micropunches of LC tissue from morphine-versus vehicle-treated rats. Figure 3, B and C, shows representative immunoblots of CRF-R and actin from rats administered morphine or vehicle for 7 d and the mean CRF-R-to-actin ratios.
Behavioral manifestation of LC sensitization to CRF
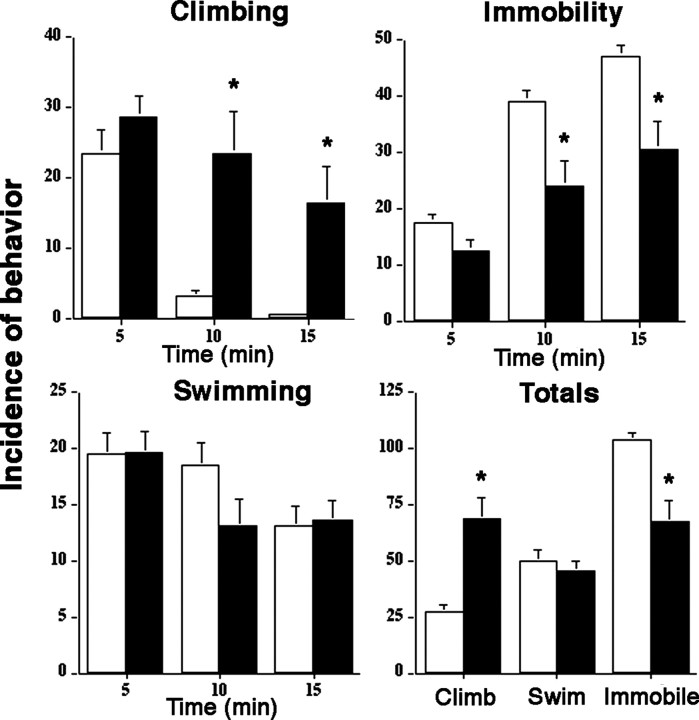
LC sensitization translated to a change in the behavioral repertoire of the animal in response to swim stress. As previously described (Porsolt et al., 1977; Detke et al., 1995), swim stress elicited active (e.g., climbing, swimming) and passive (e.g., immobility) behaviors. Vehicle-treated rats showed a typical behavioral pattern with active responses being prominent at the onset of stress and declining during the later stages of the stress when immobility predominates (Fig. 4). In marked contrast, in chronic morphine rats, climbing behavior was prominent and was often maintained throughout the entire 15 min of the swim stress. The increase in climbing occurred with no change in the incidence of swimming and a decrease in immobility.
Figure 4.
Chronic morphine alters the pattern of behavioral responses to swim stress. Bars indicate the mean incidence of different behaviors in 5 min epochs of a 15 min swim stress. The “Totals” graph indicates the mean incidence of climbing, swimming, or immobility over the entire 15 min stress. Lines above bars indicate SEM. Vehicle (n = 8; open bars) and 7 d morphine rats (n = 8; filled bars) are compared. ANOVA indicated an effect of treatment (F(1,14) = 13.8; p < 0.005) and time (F(2,28) = 16.3; p < 0.0001) on climbing, but no time-treatment interaction (F(2,28) = 3). There was a statistically significant effect of treatment (F(1,14) = 11.3; p < 0.005) and time (F(2,28) = 54.2; p < 0.0001) on immobility and a time-treatment interaction (F(2,28) = 3.6; p < 0.05). *p < 0.01, Student's t test for independent samples.
Discussion
LC-NE activation is an important component of the stress response that facilitates arousal and adaptive alterations in attention (Valentino and Van Bockstaele, 2002). Recent physiological evidence suggests that this is maintained in part through reciprocal regulation of the LC-NE system by CRF and opioids (Curtis et al., 2001). Convergence of CRF- and enkephalin-immunoreactive axon terminals onto LC dendrites (Tjoumakaris et al., 2003), taken with the present evidence for coexistence of CRF-R and μ-OR in dendrites in the LC, provides anatomical support for this. Given this reciprocal regulation, it was predicted that manipulation of either the CRF or opioid influence would have a long-term impact on the sensitivity of the LC-NE system to stress. Consistent with this, in the present study, chronic but not acute morphine sensitized LC neurons selectively to CRF, and this was translated to enhanced activation by a physiological stressor. Importantly, LC sensitization had behavioral consequences that were expressed as hyperactivity in response to an environmental stress. These findings underscore the importance of integrity in the CRF-opioid balance in the LC and have particular clinical relevance for individuals that are chronically taking opiates.
Potential mechanisms of opiate-induced sensitization
The cellular mechanisms underlying opiate-induced sensitization to CRF remain unknown. Western blot analysis of protein levels suggested that this was not attributable to simple upregulation of CRF-R, although a potential increase in the expression of a receptor that is not detected by the Western blot antiserum cannot be ruled out. Chronic morphine upregulates components of the cAMP pathway in LC neurons, specifically, adenyl cyclase (type I and VIII) and cAMP-dependent protein kinase (Lane-Ladd et al., 1997; Nestler, 2001). Because CRF receptors are Gs-coupled and linked to adenyl cyclase activation (Dautzenberg and Hauger, 2002), upregulation of these components of the cascade are potential mechanisms for sensitization. The finding that 8-Br-cAMP was similarly effective in morphine- and vehicle-treated rats argues against mechanisms distal to the formation of cAMP (i.e., upregulation of protein kinase A). Consistent with this, LC responses to VIP were comparable in morphine- and vehicle-treated rats. Like CRF, VIP increases LC discharge rate, and this is thought to occur via Gs-coupled receptor-induced activation of adenyl cyclase and formation of cAMP (Wang and Aghajanian, 1990). Thus, these peptides may converge on common intracellular signaling mechanisms. The finding that VIP and 8-Br-cAMP were similarly effective in vehicle- and morphine-treated rats underscores the selectivity of the sensitization and suggests that mechanisms underlying CRF sensitization occur at a level proximal to the formation of cAMP.
Opiate-induced sensitization to CRF is reminiscent of the in vitro phenomenon of heterologous sensitization to agonists of Gs-coupled receptors that occurs with persistent activation of Gi/o-coupled receptors (Watts, 2002). Several mechanisms have been proposed for this phenomenon that could account for the present observations, including altered coupling of the Gs protein (Watts, 2002).
Functional expression of LC sensitization
LC sensitization to CRF was not merely a pharmacological phenomenon, but was functionally evident as enhanced neuronal activation by hypotensive challenge. Similarly, neuronal activation produced by other stimuli that release CRF in the LC would be predicted to be enhanced. Most importantly, a behavioral manifestation of opiate-induced LC sensitization to CRF was readily apparent in the response to swim stress. The active behaviors of climbing and swimming have been attributed to activation of brain noradrenergic and serotonergic systems, respectively (Detke et al., 1995). The unusual prevalence of climbing behavior throughout the 15 min swim stress is completely consistent with a heightened behavioral response of the central NE system.
Clinical relevance
The present results have implications for individuals that are chronically exposed to opiates as a result of abuse or for therapeutic reasons. The findings predict that these individuals will be especially vulnerable to stress-related pathology involving the LC-NE system. For example, hyperresponsivity of the central norepinephrine system could be expressed as hyperarousal, attentional dysfunctions, and/or anxiety. Consistent with this, opiate addicts exhibit sleep disturbances, characterized by difficulty in initiating or maintaining sleep and inadequate sleep quantity and quality (Kay et al., 1981; Oyefeso et al., 1997). The results are also consistent with the higher incidence of symptoms of post-traumatic stress disorder (PTSD) in opiate users (Cottler et al., 1992). It is noteworthy that in that study, the onset of PTSD was preceded by substance abuse, suggesting that drug use increased vulnerability to PTSD symptoms.
Finally, in addition to increasing vulnerability to stress-related pathology, opiate-induced sensitization of the LC-NE system may favor opiate-seeking behavior in an effort to counteract the hypersensitivity of the LC-NE system to stress. The end result would be the cyclical perpetuation of the sensitization as a result of drug use and continued drug-seeking behavior to attenuate the consequences of sensitization.
Footnotes
This work was supported by Public Health Service Grants DA09082, MH40008, and MH02006 (a Research Scientist Award to R.J.V.) and a National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award (R.J.V.). We thank Andrew P. Danziger for expert technical assistance and Dr. Kathryn Commons for comments on this manuscript.
Correspondence should be addressed to Rita J. Valentino, The Children's Hospital of Philadelphia, 402 C Abramson Pediatric Research Center, Philadelphia, PA 19104. E-mail: valentino@email.chop.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248193-05$15.00/0
References
- Aston-Jones G, Rajkowski J, Cohen J (2000) Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res 126: 165-182. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB (1996) Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci 17: 166-172. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Compton III WM, Mager D, Spitznagel EL, Janca A (1992) Post-traumatic stress disorder among substance users from the general population. Am J Psychiatry 149: 664-670. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ (1997) Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther 281: 163-172. [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ (2001) Evidence for functional release of endogenous opioids in the locus ceruleus during stress termination. J Neurosci 21: RC152(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL (2002) The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci 23: 71-77. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 121: 66-72. [DOI] [PubMed] [Google Scholar]
- Houshyar H, Galigniana MD, Pratt WB, Woods JH (2001) Differential responsivity of the hypothalamic-pituitary-adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: possible mechanisms involved in facilitated and attenuated stress responses. J Neuroendocrinol 13: 875-886. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH (2000) The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol 387: 279-286. [DOI] [PubMed] [Google Scholar]
- Kay DC, Pickworth WB, Neider GL (1981) Morphine-like insomnia from heroin in nondependent human addicts. Br J Clin Pharmacol 11: 159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf J, Bunney BS, Aghajanian GK (1974) Noradrenergic neurons: morphine inhibition of spontaneous activity. Eur J Pharmacol 25: 165-169. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF (1998) Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51: 23-47. [DOI] [PubMed] [Google Scholar]
- Lane-Ladd SB, Pineda J, Boundy VA, Pfeuffer T, Krupinski J, Aghajanian GK, Nestler EJ (1997) CREB (cAMP response element-binding protein) in the locus coeruleus: biochemical, physiological and behavioral evidence for a role in opiate dependence. J Neurosci 17: 7890-7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119-128. [DOI] [PubMed] [Google Scholar]
- Oyefeso A, Sedgwick P, Ghodse H (1997) Subjective sleep-wake parameters in treatment-seeking opiate addicts. Drug Alcohol Depend 48: 9-16. [DOI] [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ (1993) Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci Lett 164: 81-84. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pinchon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266: 730-732. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Bart G, Green M, Ho A, Kreek MJ (2003) Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subject. Neuropsychopharmacology 28: 985-994. [DOI] [PubMed] [Google Scholar]
- Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ, Van Bockstaele EJ (2003) Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus. J Comp Neurol 466: 445-456. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele EJ (2002) Corticotropin-releasing factor: putative neurotransmitter actions of a neurohormone. In: Hormones, brain and behavior (Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, eds), pp 81-102. San Diego: Academic.
- Valentino RJ, Wehby RG (1989) Locus coeruleus discharge characteristics of morphine-dependent rats: effects of naltrexone. Brain Res 488: 126-134. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G (1983) Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res 270: 363-367. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL (1991) Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res 555: 25-34. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Cheng P, Moriwaki A, Uhl GR, Pickel VM (1996) Ultrastructural evidence for prominent distribution of the i `-opioid receptor at extrasynaptic sites on noradrenergic dendrites receiving excitatory-type afferents in the rat nucleus locus coeruleus. J Neurosci 16: 5037-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Aghajanian GK (1990) Excitation of locus coeruleus neurons by vasoactive intestinal peptide: role of cAMP and protein kinase A. J Neurosci 10: 3335-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts VJ (2002) Molecular mechanisms for heterologous sensitization of adenylate cyclase. J Pharmacol Exp Ther 302: 1-7. [DOI] [PubMed] [Google Scholar]