Abstract
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) is a member of the tumor necrosis factor (TNF) family of cytokines. It has proangiogenic and proinflammatory properties in vivo and induces cell death in tumor cell lines. TWEAK effects are mediated by the membrane receptor Fn14. In a systematic search for genes regulated in a murine stroke model with the tag-sequencing technique massively parallel signature sequencing, we have identified TWEAK as an induced gene. After 24 hr of focal cerebral ischemia in vivo or oxygen glucose deprivation in primary cortical neurons, both TWEAK and its receptor Fn14 were significantly upregulated. TWEAK induced cell death in primary neurons. Transfection of a nuclear factor (NF)-κB-luciferase fusion gene demonstrated that TWEAK stimulated transcriptional activity of NF-κB through Fn14 and the IκB kinase. Inhibition of NF-κB reduced TWEAK-stimulated neuronal cell death, suggesting that NF-κB mediates TWEAK-induced neurodegeneration at least in part. Intraperitoneal injection of a neutralizing anti-TWEAK antibody significantly reduced the infarct size after 48 hr of permanent cerebral ischemia. In summary, our data show that TWEAK induces neuronal cell death and is involved in neurodegeneration in vivo.
Keywords: stroke, TWEAK, Fn14, NF-κB, transcriptional profiling, neurodegeneration
Introduction
Stroke is a leading cause of death and disability worldwide. Initiation of treatment is often delayed by several hours. The first steps in the pathophysiology of ischemic neurodegeneration such as anoxic depolarization and glutamate receptor activation probably occur before therapy can be initiated (Lee et al., 2000); however, the pathological cascade continues into the potential therapeutic time frame. Inflammation and delayed cell death are events that may be amenable to therapy (Dirnagl et al., 1999). Like other prolonged biological processes, apoptosis and inflammation rely heavily on gene expression. Therefore, transcriptomic technology offers a strategy to characterize the genes that are involved and to find potential drug targets (Trendelenburg et al., 2002; Schneider et al., 2004a,b). Here we describe the application of massively parallel signature sequencing (MPSS) to the study of gene expression in cerebral ischemia. MPSS is a tag-sequencing method that was developed by Sydney Brenner and coworkers in the late 1990s (Brenner et al., 2000). With MPSS, a large number of signatures are obtained in parallel by cloning and ligation-based sequencing of cDNA molecules that are fixed to immobilized microbeads. One of the genes that was found to be upregulated in our study was tumor necrosis factor-like weak inducer of apoptosis (TWEAK), a cytokine of the tumor necrosis factor (TNF)-α superfamily (Aggarwal, 2003; Ware, 2003).
TWEAK is expressed in many tissues, and expression was reported in glial cells in the brain and in peripheral neurons (Chicheportiche et al., 1997; Desplat-Jego et al., 2002; Tanabe et al., 2003). Little is known, however, about its in vivo functions except that TWEAK is an angiogenetic factor in the cornea assay (Lynch et al., 1999; Wiley et al., 2001). In vitro, TWEAK stimulates cell death in some tumor cells (Wiley and Winkles, 2003). Furthermore, it induces the expression of the inflammatory mediators intercellular adhesion molecule-1 (ICAM-1) and interleukin-6 (IL-6), both of which are involved in cerebral ischemia (Saas et al., 2000; Harada et al., 2002; Herrmann et al., 2003). The initial report that TWEAK is a high-affinity ligand for the membrane receptor Apo3 was not confirmed by subsequent studies (Wiley and Winkles, 2003). Recently, an expression cloning technique identified a receptor for TWEAK (Wiley et al., 2001), which was previously cloned as an FGF-inducible gene (Fn14) (Meighan-Mantha et al., 1999). Fn14 is a relatively small transmembrane protein that belongs to the TNF receptor superfamily. In vivo, the expression of Fn14 is upregulated with injury (Tanabe et al., 2003). Although Fn14 does not contain a death domain, it was shown to mediate TWEAK-induced cell death (Nakayama et al., 2003).
Using in vivo and in vitro models of cerebral ischemia, we found that TWEAK and Fn14 are upregulated. TWEAK induced neuronal cell death, partly by activating the transcription factor nuclear factor (NF)-κB through Fn14. Neutralizing TWEAK reduced neurodegeneration in vivo and in vitro, suggesting that TWEAK is a drug target in acute neurodegeneration.
Materials and Methods
Materials. The production of recombinant soluble human TWEAK (rhTWEAK), containing amino-acid residues A106-H249, has been described previously (Jakubowski et al., 2002). For Fc-hTWEAK, a plasmid was generated that contains the sequences corresponding to a human IgG1 Fc fragment (aa 108-338 of GenBank accession number AAC82527 excluding the stop codon), a linker sequence (RSPQPQPKPQPKPEPEGSLQVD), and the receptor-binding domain of TWEAK (aa 106-249). The construct was stably transfected into the 293T cell line. Fc-hTWEAK was purified with protein A. Anti-Fn14 serum was generated by immunizing Fn14 KO mice with purified recombinant murine Fn14 (aa 28-79) containing a myc-His tag at the C-terminal end. Anti-Fn14 monoclonal antibody 1.P1C12.1D8 was generated using the above immunized Fn14 knock-out (KO) mice as described previously (Kennett et al., 1982). SN50 was purchased from Biomol (Hamburg, Germany), murine TNF-α from Sigma (Munich, Germany), and ITEM-4 from eBioscience (San Diego, CA).
MPSS expression profiling. For expression profiling, the filament model of middle cerebral artery occlusion (MCAO) was used. Mice (129X1/SVJ) were anesthetized using 70% N2O, 30% O2, and 1% halothane. A 5-0 nylon filament blunted at the tip was inserted into the common carotid artery. The filament was advanced into the internal carotid artery until the middle cerebral artery was reached. Successful occlusion was monitored using laser Doppler flowmetry (Perimed, Stockholm, Sweden). The 90 min occlusion was followed by a 20 hr reperfusion. Thereafter, animals were killed under deep anesthesia by transcardial perfusion with HBSS. Hemispheric forebrains (cerebellum, olfactory bulb, and brainstem removed) were further processed for RNA using acidic phenol extraction. For expression profiling, RNA from hemispheres (ipsilateral and contralateral) of six animals was pooled to reduce the influence of interindividual variation in infarct severity. Results from two MPSS runs per sample were pooled.
For MPSS, RNA was converted into cDNA and the most 3′ DpnII fragments were recovered. After in vitro cloning, the cDNA templates were immobilized on separate glass beads of 5 μm diameter. Loaded microbeads were placed into a flow cell forming a densely packed monolayer. Short sequences from the free template ends were obtained simultaneously by a fluorescence-based ligation-mediated sequencing method. Obtained signatures (14 bases) were sufficiently long to allow the identification of the vast majority (> 95%) (cf. Velculescu et al., 1995) of the individual cDNAs. Signatures matching more than one gene (taking into account only nonexpressed sequence tags or expressed sequence tag European Molecular Biology Laboratory database entries) could be detected by clustering all matching sequences, excluding putative sequencing errors and sequence polymorphisms.
To prepare cDNA libraries for the MPSS analysis, 5 μg of oligo-dT-cellulose (Peqlab, Erlangen, Germany) -enriched A+ RNA was denatured at 70°C with 50 pmol of BsmBI-oligo-dT18V primer (GGCCAGT GAATTGTAATACGACTCACTATAGGGCTGCATTGAGACGATTCTTTTTTTTTTTTTTTTTTV), cooled on ice, and reverse-transcribed with 200 U of Superscript II at 42°C for 1 hr in 1× reaction buffer, 10 mm dithiothreitol (all reagents from Invitrogen, Karlsruhe, Germany), and 0.5 mm each dNTP (Roche Diagnostics, Mannheim, Germany) in 25 μl. Second-strand cDNA synthesis was performed by adding 40 U of DNA polymerase I, 2 U of RNase H, 10 U of Escherichia coli DNA ligase, and 0.5 mm each dNTP in 1× second-strand buffer (Invitrogen) in a final volume of 100 μl for 2 hr at 16°C. RNA was hydrolyzed in the presence of 100 mm NaOH at 65°C for 20 min. The reaction product was phenol/chloroform-purified and precipitated with ammonium acetate in the presence of PelletPaint (Calbiochem, La Jolla, CA; Novabiochem, Bad Soden, Germany).
Resuspended, double-stranded cDNA was digested with DpnII; 3′DpnII fragments were isolated with streptavidin-coupled paramagnetic beads (Dynal Biotech, Hamburg, Germany) and released from the beads with a BsmBI digest. DpnII-BsmBI double-stranded cDNA fragments were tagged by cloning the fragments into the TAG vector (pLCV), which was digested with BbsI and BamHI. After electroporation of DH10B E. coli (Invitrogen) for each sample, 106 independent clones were harvested, and the plasmid DNA containing the tagged cDNA was extracted. Using PCR, the tagged cDNAs were amplified from the plasmid DNA and mixed with microbeads (LYNX, Hayward, CA) carrying the complementary antitags. The tagged cDNA was loaded onto the microbeads by hybridizing the tags to the antitags. The DNA-loaded beads were loaded into a flow cell and further processed on an MPSS instrument (LYNX) as described (Brenner et al., 2000).
Cell culture and transient transfection. Cortical neurons were prepared from embryonic day 16 (E16) mice. For transfection, cells were plated on 24-well plates precoated with poly-d-lysine (50 μg/ml) at a density of 200,000 cells per well. For RNA preparation 2 million cells per well were plated on six-well plates. Cells were incubated in Neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen), l-glutamine (0.5 mm), penicillin (100 IU/ml), and streptomycin (100 μg/ml). In these cultures >95% of cells were positive for the neuronal marker NeuN. After 10 d in vitro, cells were transfected using Lipofectamine 2000 (Invitrogen) and 1 μg per well of the NF-κB reporter plasmid pNF-κB-Luc, which has five tandem repeats of an NF-κB binding site (Stratagene, Amsterdam, The Netherlands), according to the manufacturer's protocol. After 24 hr, cells were stimulated as indicated and harvested. Luciferase activity was measured as described (Sallmann et al., 2000).
As indicated, cortical neurons were prepared from a transgenic mouse line (NSE-IκBα-SR) that expressed the NF-κB super-repressor, a mutant of IκBα (IκBα-SR), selectively in neurons. In this transgene, two serine residues, which are phosphorylated by the IκB kinase (IKK), are exchanged to alanines. Neuronal expression is driven by a 1.8 kb fragment of the rat neuron-specific enolase (NSE) promoter (Zhang et al., 2004). The cell dissociation was performed individually from the brain of each transgenic or wild-type embryo. To control for transfection efficiency, cortical neurons of NSE-IκBα-SR mice and wild-type littermates were cotransfected with 0.1 μg per well of phRL-TK (Promega, Mannheim, Germany) in addition to pNF-κB-Luc.
Cell death assays. For terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining, the cells were fixed in 4% paraformaldehyde at room temperature for 30 min. Then, cells were washed twice in PBS for 5 min and treated for 2 min with 200 μl of permeabilization solution (0.1% Triton X-100 and 0.1% sodium citrate in PBS) at 4°C. After washing, sections were incubated with 50 μl of TUNEL reaction mix (enzyme solution diluted 1:6 in labeling solution; In Situ Cell Detection Kit, Fluorescein; Roche, Mannheim, Germany) for 1 hr at 37°C in the dark. Then, coverslips were mounted with medium containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Vectashield; Vector Laboratories, Burlingame, CA). TUNEL and DAPI-positive cells were detected under a fluorescent microscope. On each coverslip five randomly selected fields were counted with a 40× objective (corresponding to ∼800 cells per coverslip). Cell Death Detection ELISAPlus (Roche) was performed according to the manufacturer's instructions. Lactate dehydrogenase (LDH) activity in medium was quantified with the Cytotoxicity Detection kit (LDH; Roche). LDH activity in untreated sister cultures was subtracted, and the LDH activity was expressed as percentage of the maximally releasable LDH pool in the presence of 1 mm glutamate.
In situ hybridization and immunocytochemistry. Techniques for in situ analysis have been described in detail previously (Sassoon et al., 1988; Sassoon and Rosenthal, 1993). Fn14 riboprobe templates were generated using specific PCR primers containing a T7 polymerase promoter binding site on either the forward or reverse primer to generate sense or antisense templates, respectively. The 448 bp riboprobe template is specific for nucleotides 9-456 in the murine Fn14 coding domain (GenBank accession number BC025860). High specific-activity probes were synthesized using an Ambion T7 Maxiscript in vitro transcription kit and 33P-radiolabeled UTP (> 3000 Ci/mmol; PerkinElmer Life Sciences, Wellesley, MA), according to the manufacturer's instructions. All probes were used in a hybridization buffer containing 30,000 counts per minute per milliliter final probe concentration. Microscopic analysis of the expression patterns was performed on a Leica DMR system modified for reflective dark-field microscopy. Images were captured using a CoolSnap RGB camera, processed in Open Lab, and polished using Photoshop 7.0.
For immunohistochemistry, cells were fixed for 5 min in acetone at room temperature. Then, cells were dried for 30 min and rehydrated for 5 min in PBS. After 1 hr of blocking with 5% horse serum, polyclonal mouse anti-Fn14 or preimmune sera (1:300 dilution) and culture supernatant containing anti-Fn14 monoclonal antibody (mAb) 1.P1C12.1D8 or DMEM media as control were added and left to stand overnight at 4°C. After washing three times in PBS, biotinylated horse anti-mouse IgG (diluted 1: 200; Vector) and FITC-avidin (1: 200 dilution; Vector) were used for detection. Coverslips were mounted with medium containing DAPI. Cells were analyzed under a fluorescent microscope.
Models of cerebral ischemia. As an in vivo model of permanent focal cerebral ischemia, a distal MCAO was performed (see Figs. 2, 6). At an age of 3-4 months, male C57BL/6 mice were anesthetized by intraperitoneal injection of 150 μl of 2.5% avertin (tribromoethanol) per 10 gm of body weight. A skin incision was made between the ear and the orbit on the left side. The parotid gland and the temporal muscle were removed by electrical coagulation. The stem of the MCA was exposed through a burr hole and occluded by microbipolar coagulation (Erbe, Tübingen, Germany). Surgery was performed under a microscope (Hund, Wetzlar, Germany). Mice were kept at a body temperature of 37°C on a heating pad. The anti-TWEAK antibody AB.G11 (Jakubowski et al., 2002) (200 μg) or the same amount of an unspecific hamster Ig (Jakubowski et al., 2002) was injected intraperitoneally 10 min before MCAO. After 48 hr mice were deeply reanesthetized with Avertin and perfused intracardially with Ringer's solution. The procedure for infarct measurement on cryo-sections and correction for cerebral edema has been described previously (Herrmann et al., 2003). Surgery was performed and infarcts measured without knowledge of the treatment group. In a separate cohort of animals, the femoral artery was cannulated for measurement of arterial blood gases and mean arterial blood pressure. Arterial blood gases, glucose, and hemoglobin were measured immediately before and 15 min into MCAO in a blood sample of 100 μl. For laser Doppler measurements, the probe (P415-205; Perimed) was placed 3 mm lateral and 6 mm posterior to the bregma. Relative perfusion units were determined (Periflux 4001; Perimed).
Figure 2.

TWEAK and Fn14 expression are induced by cerebral ischemia and OGD. A, Twenty-four hours after onset of cerebral ischemia, mRNA accumulation of TWEAK and Fn14 in the ischemic or contralateral cortex was determined by RT-real-time PCR. Values are means ± SE (n = 12) expressed as percentage of the right hemisphere. *p < 0.02; +p < 0.0003 (t test). B, In situ hybridization demonstrated upregulation of Fn14 mRNA 24 hr after onset of MCAO in the cortex adjacent to the infarct. There was no staining in the contralateral cortex. Similar results were obtained after 48 hr of MCAO. Scale bar, 200 μm. C, Immunocytochemistry with an Fn14-specific polyclonal serum or preimmune serum and an anti-Fn14mAb or control (DMEM culture medium) revealed expression of Fn14 in cortical neurons. D, mRNA accumulation of TWEAK (left) and Fn14 (right) in cortical neurons was stimulated by OGD. After OGD for the times indicated, cells were incubated under normal conditions for 24 hr. Values are means ± SE (n = 6) measured in duplicate and expressed as percentage of control cells. +p < 0.006; *p < 0.0001 (t test).
Figure 6.
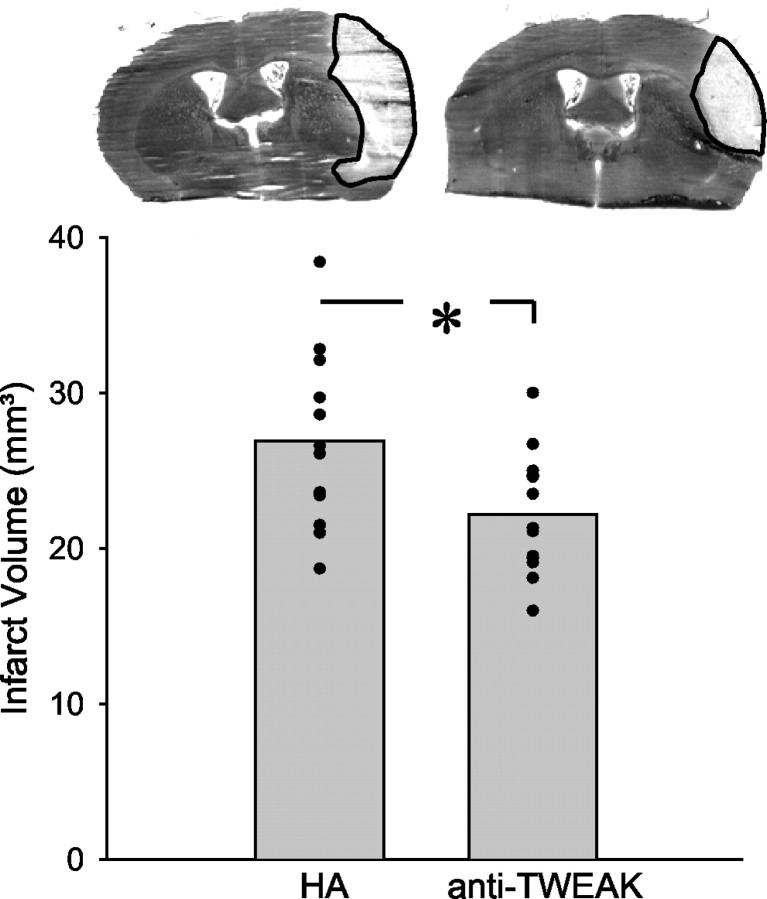
Inhibition of TWEAK reduced the infarct size. Mice were injected with the neutralizing monoclonal anti-TWEAK antibody AB.G11 or an unspecific monoclonal hamster Ig (HA; 200 μg) intraperitoneally immediately before onset of cerebral ischemia and were killed 48 hr later. The infarcts were visualized by silver staining. Typical coronal sections are shown at the top. Below, means and individual values of the corrected infarct volume are shown (n = 12-13). *p < 0.05 (t test).
Oxygen glucose deprivation (OGD) was used as an in vitro model of ischemia. For OGD experiments, primary cortical neurons, which had been in culture for 10 d, were transferred into serum-free medium containing 5 mm 2-deoxy-d-glucose (Merck, Darmstadt, Germany) for 1 hr. Then, the cells were placed in an anaerobic chamber that was flushed for 10 min with a mix of 95% N2 and 5% CO2. After incubation for the indicated times, cells were removed from the anaerobic chamber and incubated under normal conditions for another 24 hr. The control group was cultured in parallel but did not receive 2-deoxy-d-glucose and was not flushed with N2/CO2. Then, RNA was extracted.
Real-time RT-PCR. Mice were reanesthetized and perfused with Ringer's solution 24 hr after MCAO. The ischemic and contralateral cortices were quickly dissected and frozen on dry ice. Tissues were stored at -80°C. RNA from cortex or cultured cells was extracted with peqGOLD RNAPure (PEQLAB, Erlangen, Germany), according to the manufacturer's instructions. RNA (10 μg, cortex; 7.5 μg, cells) was transcribed with Moloney murine leukemia virus reverse transcriptase and random hexamers. The following primers were used for PCR amplification: twk-1, 5′-CGAGCTATTGCAGCCCATTA; twk-2, 5′-CCTGCTTGTGCTCCATCCT (TWEAK, PCR product 62 bp); fn14-1, 5′-GACCTCGACAAGTGCATGGA; fn14-2, 5′-CGCATCCCAGGCAGAAGT (Fn14, PCR product 70 bp); mcycm-1, AGGTCCTGGCATCTTGTCCAT; mcycm-2, GAACCGTTTGTGTTTGGTCCA (cyclophilin, PCR product 51 bp). PCR was performed according to the following protocol: 10 min at 95°C, 15 sec at 95°C, and 1 min at 60°C (40 cycles). Amplification was quantified with the Gene Amp 5700 sequence detector and the SYBR Green kit (PE Diagnostik, Weiterstadt, Germany). A linear concentration-amplification curve was established by diluting pooled samples. Quantified results for individual cDNAs were normalized to cyclophilin. This procedure allows us to quantify results relative to a control group. The purity of the amplified products was checked by the dissociation curve.
Statistical analysis. Data are presented as mean ± SE. Statistical comparisons of three or more groups were made by ANOVA followed post hoc by Fisher's protected least-squares difference (LSD). Two groups were compared by a two-sided t test or by two-sided Mann-Whitney U test in the case of counted data (see Figs. 3A, 5C). Values were considered significant at p < 0.05. In MPSS, the statistical significance of the observed signature frequency distributions was calculated according to Equation 2 of Audic and Claverie (1997). p < 0.001 was considered significant.
Figure 3.

TWEAK induces cell death in primary cortical neurons of the mouse. A, Cortical neurons at 10 d in vitro were exposed to OGD for 4.5 hr and then incubated under standard conditions for 24 hr and stained by the TUNEL reaction. Neuronal cell death was reduced by the monoclonal hamster anti-TWEAK antibody AB.G11 (10 μg/ml). Values are means ± SE of six experiments, each counted in quintuplicate, and are expressed relative to the control group treated with control hamster antibody. *p < 0.0001 (Mann-Whitney U test). B, After exposure of neurons to rhTWEAK (100 ng/ml) or Fc-hTWEAK (100 ng/ml) for 24 hr, cells were stained by DAPI and by the TUNEL reaction. Both forms of TWEAK increased the number of cells with condensed nuclei that were TUNEL positive. C, Quantification of cells with condensed nuclei after DAPI staining (gray columns) or of TUNEL-positive cells (black columns). Values are means ± SE of three experiments, each counted in quintuplicate, and are expressed as percentage of total cell number. CA, Camptothecin (10 μm). *p < 0.005 (ANOVA; LSD post hoc) applying to both methods of apoptosis detection. D, Quantification of histone-associated DNA fragments in cytosolic extracts of cortical neurons after rhTWEAK (100 ng/ml) and Fc-hTWEAK (100 ng/ml) treatment for 24 hr. Values are means ± SE (n = 4) and expressed relative to the untreated control group. *p < 0.03 (ANOVA; LSD post hoc).
Figure 5.
TWEAK-induced neuronal cell death is mediated by NF-κB. A, Cortical neurons were stimulated by drugs for 24 hr and then stained by the TUNEL reaction. The NF-κB inhibitor SN50 (10 μg/ml) increased the basal rate of TUNEL-positive cells but reduced the proapoptotic effect of rhTWEAK (100 ng/ml) and Fc-hTWEAK (100 ng/ml) significantly. Values are means ± SE (n = 3), each performed in duplicate, and are expressed relative to the untreated control. *p < 0.0001 (ANOVA; LSD post hoc test). B, Cortical neurons from mice expressing the NF-κB super-repressor (IκBα-SR) or from wild-type littermates were transfected with the luciferase fusion gene pNF-κB-Luc and the renilla luciferase control plasmid phRL-TK. rhTWEAK (100 ng/ml) stimulated NF-κB less in neurons from IκBα-SR mice than in neurons from wild-type littermates. Values are means ± SE of luciferase activity expressed as percentage of the un-stimulated control of the same genotype (n = 18-22). +p < 0.001 (ANOVA; post hoc test). C, IκBα-SR-expressing neurons were protected from cell death induced by rhTWEAK (100 ng/ml). After 24 hr of treatment, apoptotic cells were determined by the TUNEL reaction. Values are means ± SE of four experiments, each counted in quintuplicate, and are expressed relative to the untreated wild-type group. *p < 0.0001 (Mann-Whitney U test).
Results
Results of two MPSS runs performed with RNA from the ipsilateral forebrain of mice subjected to MCAO versus contralateral forebrain (after 90 min occlusion and 20 hr reperfusion) yielded a total of 174,812 tag sequences for the contralateral and 161,809 tag sequences for the ischemic hemispheres. Identical sequences were clustered to display the expression levels of the corresponding genes, resulting in 58,315 different clusters and including sequences with ambiguities (one or more nucleotides not fully determined). Subsequently, the number of occurrences of individual signatures (pool of two replicate runs) as a direct measure of expression level was compared between the two experimental samples. One of the signatures identified as being induced was GATCCCTGTGGATTTTG, with a tag frequency of 41 in the ipsilateral versus 0 in the contralateral hemisphere (p ∼ 10-12). A database search identified this sequence as mouse TWEAK mRNA (GenBank accession number AF030100). Figure 1 illustrates the distribution of signatures as a scatter plot.
Figure 1.
Result of the MPSS experiment on the comparison of the left (ischemic; y-axis) with the right (nonischemic; x-axis) hemisphere. Each point of the scatter plot represents one particular signature. The absolute counts of the identified signatures are given. The total number of signatures sequenced were 174,812 for the left hemisphere and 161,809 for the right hemisphere. A signature for TWEAK (GATCCCTGTGGATTTTG) was counted 41 times in the ischemic hemisphere and never in the contralateral, nonischemic hemisphere (arrow; p = 1.12 × 10-12).
Induction of TWEAK mRNA by cerebral ischemia was verified by RT-real-time PCR. TWEAK was upregulated more than threefold in the ischemic as compared with the contralateral hemisphere (data not shown). In another stroke model, distal MCAO, there was a 2.0-fold increase in TWEAK mRNA in the ischemic as compared with the contralateral hemisphere 24 hr after occlusion onset (Fig. 2A, left) (p < 0.02). Induction of the TWEAK receptor Fn14, however, was more pronounced. Quantification of Fn14 mRNA by RT-real-time PCR revealed a 22.3-fold upregulation after 24 hr of MCAO compared with the contralateral cortex (Fig. 2A, right). By in situ hybridization, elevated levels of Fn14 transcripts were found in the periphery of the cortical ischemia, the putative penumbra (Fig. 2B).
Fn14 has been shown to be expressed by peripheral neurons (Tanabe et al., 2003), and the in situ hybridization signals were compatible with a predominantly neuronal origin of the Fn14 upregulation in cerebral ischemia. Accordingly, cortical neurons expressed Fn14 in vitro, as demonstrated by immunocytochemistry with anti-Fn14 serum and an Fn14-specific mAb (Fig. 2C). Furthermore, the mRNA could be detected by RT-real-time PCR (Fig. 2D, right). In cortical neurons, OGD, an in vitro model of cerebral ischemia, induced a marked upregulation of Fn14 after 4.5 and 6 hr (Fig. 2D, right). TWEAK mRNA in neurons was also upregulated by OGD, but the induction occurred already at 3 hr and was less pronounced (Fig. 2D, left).
To explore the functional effect of TWEAK upregulation after OGD, we used a neutralizing monoclonal hamster anti-TWEAK antibody, AB.G11 (Jakubowski et al., 2002), and quantified neuronal apoptosis by counting TUNEL-positive cells 24 hr after OGD. Induction of apoptosis by 4.5 hr of OGD was clearly ameliorated by inhibition of TWEAK (Fig. 3A). After 4.5 hr of OGD, the percentage of TUNEL-positive cells of ∼30% represented only a part of neuronal cell death. In the same experiments, LDH release as a measure of necrotic cell death increased to 69.2 ± 6.9% of the maximally releasable LDH pool (n = 18). The anti-TWEAK antibody had only a slight, insignificant effect on LDH release (data not shown). To investigate whether exogenous TWEAK is able to induce neuronal cell death, we used rhTWEAK or the fusion protein Fc-hTWEAK. Human TWEAK has been shown to bind to murine cells (Jakubowski et al., 2002). Fc-hTWEAK or rhTWEAK exposure for 24 hr induced nuclear condensation as shown by a DAPI stain. In addition, the number of cortical neurons that were TUNEL positive increased significantly after exposure to rhTWEAK or Fc-hTWEAK (Fig. 3B,C). An ELISA measuring DNA-histone complexes specific to apoptotic DNA fragmentation supported the notion of programmed cell death being induced by rhTWEAK and Fc-hTWEAK (Fig. 3D). The effect size was comparable with the one with 10 μm camptothecin, a classic inducer of apoptosis by DNA damage (Fig. 3C,D).
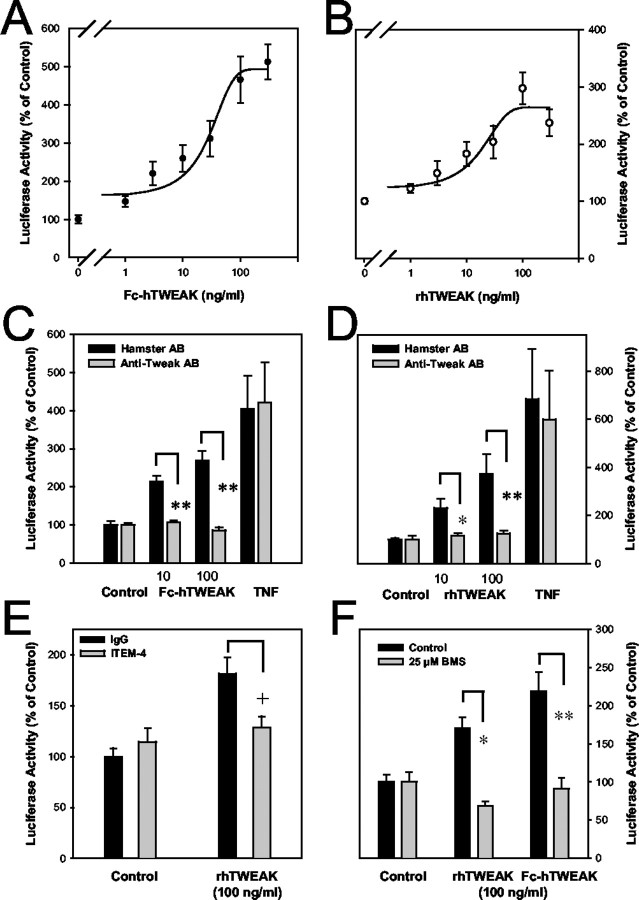
Fn14 does not contain a death domain but binds TNF receptor-associated factors (Wiley et al., 2001; Brown et al., 2003) that are known to link receptors of the TNF receptor superfamily to several signal transduction pathways. Indeed, TWEAK activates the transcription factor NF-κB via Fn14 (Brown et al., 2003). Because NF-κB has important functions in determining neuronal death or survival (Mattson and Camandola, 2001), we wanted to address the question of whether TWEAK also activates NF-κB in cortical neurons. Primary cortical neurons were transfected with a luciferase fusion gene that is under transcriptional control of five NF-κB binding sites. Fc-hTWEAK and rhTWEAK stimulated NF-κB activity in a concentration-dependent manner (Fig. 4A). The effect size varied between experimental series, and there was no consistent difference between Fc-hTWEAK and rhTWEAK (Fig. 4A,B). The neutralizing anti-TWEAK antibody AB.G11 abrogated NF-κB stimulation by Fc-hTWEAK and rhTWEAK in cortical neurons but had no effect on the stimulation by TNF-α, demonstrating that the effect of TWEAK cytokine stimulation was specific (Fig. 4C,D). Fn14 mediates many effects of TWEAK, but there is evidence for a second, still unknown TWEAK receptor (Polek et al., 2003). To test whether Fn14 is responsible for NF-κB stimulation in cortical neurons, we used the anti-Fn14 antibody ITEM-4, which functions as a competitive TWEAK antagonist at the Fn14 receptor and possesses only a low intrinsic agonist activity (Nakayama et al., 2003). When administered alone, ITEM-4 stimulated NF-κB activity slightly, but it inhibited the stimulation of TWEAK in accordance with its function as an antagonist of Fn14 (Fig. 4E). In the classic signaling cascade of NF-κB activation, the IKK plays a pivotal role (Li and Verma, 2002). To test for the involvement of IKK in NF-κB activation by TWEAK, we used BMS-345541, a highly specific inhibitor of the IκB kinase (Burke et al., 2003). BMS-345541 (25 μm) blocked NF-κB activation by rhTWEAK and Fc-hTWEAK, showing that TWEAK stimulates NF-κB via the classic, IKK-dependent pathway in neuronal cells (Fig. 4F).
Figure 4.
TWEAK activates NF-κB through Fn14 and IKK in cortical neurons. Cortical neurons were transfected with pNF-κB-Luc, a luciferase fusion gene that contains five binding sites for NF-κB, and were stimulated by TWEAK for 24 hr. A, B, Fc-hTWEAK (A) and rhTWEAK (B) stimulated NF-κB activity in a concentration-dependent manner. C, D, The stimulation of NF-κB by Fc-hTWEAK (C) or rhTWEAK (D) in a concentration of 10 and 100 ng/ml was abrogated by the neutralizing anti-TWEAK antibody AB.G11 (10 μg/ml added 30 min before stimulation); however, stimulation by TNF-α (10 ng/ml) was not affected by AB.G11. Controls received an unspecific hamster Ig. *p < 0.05; **p < 0.0001 (ANOVA; LSD post hoc test). E, ITEM-4 (1 μg/ml), an anti-Fn14 mAb that blocks TWEAK-Fn14 interaction, was added to the medium 20 min before TWEAK. It partially blocked NF-κB stimulation by rhTWEAK (100 ng/ml). Controls received unspecific mouse Ig (1 μg/ml). +p < 0.005 (ANOVA; LSD post hoc test). F, NF-κB stimulation by rhTWEAK or Fc-hTWEAK was inhibited by the IKK inhibitor BMS-345541 (25 μm). *p < 0.04; **p < 0.0001 (ANOVA; LSD post hoc test). Values are means ± SE (n = 9) of the luciferase activity expressed in percentage of untreated controls.
To examine the role of NF-κB in TWEAK-induced neuronal cell death, its activation was inhibited by SN50. In a concentration of 10 μg/ml, SN50 partially inhibited NF-κB activated by rhTWEAK (data not shown). SN50 itself had toxic effects on neurons; however, it significantly reduced the pro-apoptotic effect of rhTWEAK and Fc-hTWEAK (Fig. 5A). A mouse line that expresses the NF-κB super-repressor selectively in neurons offers the possibility of testing the role of NF-κB with another approach (Zhang et al., 2004). rhTWEAK stimulated NF-κB significantly less in cortical neurons of NSE-IκBα-SR mice than in those of wild-type littermates (Fig. 5B). In parallel to the reduced NF-κB stimulation, TWEAK-induced neuronal cell death was also significantly reduced in neurons of NSE-IκBα-SR mice compared with wild-type littermates (Fig. 5C).
To investigate whether TWEAK-induced neurodegeneration is relevant in vivo, we injected mice intraperitoneally with the neutralizing anti-TWEAK antibody AB.G11 or an unspecific hamster Ig (200 μg per mouse) and subjected them to distal MCAO. Intraperitoneal injection is an effective administration mode for neutralizing antibodies in cerebral ischemia (van Bruggen et al., 1999; Martin-Villalba et al., 2001). The anti-TWEAK antibody had no effect on the various physiological parameters measured (Table 1); however, it significantly reduced the infarct size after 2 d (Fig. 6). This suggests that TWEAK also induces neurodegeneration in vivo.
Table 1.
Physiological parameters immediately before and 15 min after MCAO in mice that were injected intraperitoneally with either 200 μg of unspecific hamster lg or 200 μg of hamster anti-TWEAK AB.G11
|
Parameter |
Control antibody |
Anti-TWEAK AB.G11 |
||||
|---|---|---|---|---|---|---|
|
|
Pre-MCAO |
Post-MCAO |
Pre-MCAO |
Post-MCAO |
||
| MABP (mmHg) | 60.0 ± 2.7 | 51.7 ± 2.1 | 61.3 ± 2.3 | 51.3 ± 3.1 | ||
| Heart rate (per min) | 368.7 ± 7.4 | 391.0 ± 13.3 | 371.5 ± 9.5 | 390.8 ± 12.9 | ||
| Glucose (mg/dl) | 254.6 ± 18.7 | 284.1 ± 33.6 | 231.6 ± 14.2 | 285.3 ± 22.0 | ||
| Arterial PCO2 (mmHg) | 59.6 ± 4.0 | 65.2 ± 3.3 | 59.2 ± 1.8 | 62.0 ± 1.3 | ||
| Arterial PO2 (mmHg) | 92.5 ± 10.3 | 98.0 ± 6.3 | 80.5 ± 6.9 | 85.7 ± 4.9 | ||
| Hb (gm/l) | 14.9 ± 0.2 | 13.6 ± 0.3 | 15.0 ± 0.2 | 13.9 ± 0.2 | ||
| Laser Doppler (relative units) | 84.2 ± 5.1 | 16.4 ± 1.9 | 84.6 ± 4.5 | 13.8 ± 1.4 | ||
| Body weight (gm) |
23.8 ± 0.6 |
|
23.5 ± 0.4 |
|
||
None of the parameters differed significantly among the groups (t test). Values are means ± SE; n = 7-8. MABP, Mean arterial blood pressure; Hb, hemoglobin concentration.
Discussion
Using MPSS, a tag-sequencing technique for digital transcriptional profiling, we have identified TWEAK as one of a number of genes upregulated by cerebral ischemia. TWEAK is a cytokine of the TNF superfamily that has angiogenic, pro-apoptotic, and inflammatory properties. The effects of TWEAK are partly mediated by the membrane receptor Fn14 (Wiley and Winkles, 2003). Given the fact that membrane receptors are the most successful drug targets, we focused on the function of TWEAK and its receptor. Indeed, Fn14 expression was markedly upregulated by cerebral ischemia as judged by RT-real-time PCR, although our MPSS analysis did not detect its induction. Interestingly, induction of Fn14 in cerebral ischemia has been suggested previously using serial analysis of gene expression (Trendelenburg et al., 2002). This illustrates that individual attempts of transcriptional profiling, regardless of the technique that is used, will not detect all induced genes because of technical and statistical limitations. The number of ∼170,000 tags that we have obtained per experimental group in our MPSS study is rather low for the reliable detection of transcripts encoding for rare gene products, such as membrane receptors. If resources were not limiting, one would have to sequence >1 million tags to achieve a comprehensive detection of low-abundance transcripts in a complex transcriptome (Trendelenburg et al., 2002). This may explain why we have missed Fn14 induction in the MPSS experiment. Although Fn14 was discovered as an inducible gene and upregulation has been reported in other experimental paradigms in vivo (Meighan-Mantha et al., 1999; Feng et al., 2000; Tanabe et al., 2003), regulation of TWEAK gene expression has fewer precedents. There is only a single report showing TWEAK mRNAdownregulation in acute and chronic inflammation (Chicheportiche et al., 2000).
Our data are consistent with neurons expressing Fn14 at both the protein and mRNA levels. The neuronal expression is strongly stimulated by oxygen glucose deprivation, an in vitro model of cerebral ischemia. This argues for an upregulation of neuronal Fn14 in cerebral ischemia. We show here that TWEAK triggers cell death in cortical neurons as demonstrated by chromatin condensation and increase of DNA strand breaks. Pro-apoptotic effects of TWEAK have only been observed in tumor cell lines so far (Wiley and Winkles, 2003). Concerning the intracellular mechanism of cell death induction, it is interesting that TWEAK activated the transcription factor NF-κB through Fn14 and the IκBα kinase in neurons. It has been reported recently that in fibroblasts TWEAK induces an early activation of NF-κB p50/p65 dimers by the classic IKK2-NEMO pathway, and a long-lasting NF-κB activation via a noncanonical signaling mechanism, involving IKK1 activation and p100 processing (Saitoh et al., 2003). Because the IKK inhibitor BMS-345541 does not discriminate between the two catalytic subunits IKK1 and IKK2 (Burke et al., 2003), both pathways may be triggered by the TWEAK stimulation of cortical neurons for 24 hr. Because the noncanonical pathway of NF-κB activation is independent from IκBα, this additional mechanism could explain why NF-κB activation by TWEAK was only partly inhibited in neurons from NSE-IκBα-SR mice (Fig. 5B). Our data show that NF-κB mediates TWEAK-triggered neuronal cell death, at least in part. Although well known for its anti-apoptotic action in many cell types, NF-κB actually exerts both proapoptotic and anti-apoptotic effects in neurons, depending on the stimulus (Pizzi et al., 2002; de Erausquin et al., 2003; Fridmacher et al., 2003). Potential target genes that could mediate the proapoptotic effect of NF-κB are death receptors (e.g., DR1, DR2), death receptor ligands such as CD95L or c-myc, and p53 (Aggarwal, 2003). In cell lines there is evidence for multiple pathways of cell death induced by TWEAK (P. Schneider et al., 1999; Nakayama et al., 2002, 2003). This suggests that NF-κB may act in conjunction with other, still unknown signaling pathways to mediate TWEAK-induced neuronal cell death.
Excessive neuronal NF-κB stimulation contributes to cell death in cerebral ischemia (A. Schneider et al., 1999). Indeed, a neutralizing anti-TWEAK antibody reduced the infarct size in a model of permanent cerebral ischemia. Compared with transient cerebral ischemia, models of permanent cerebral ischemia provide a more stringent test of neuroprotection (Chan et al., 1993; Lou et al., 2004). Even at the center of the ischemic region there is residual blood flow (Love, 2003). Thus, the antibody can access the ischemic brain through a permeable blood-brain barrier. In vivo neutralization of TWEAK may protect against ischemic brain damage not only by preventing neuronal cell death, but also by reducing neuroinflammation. In astrocytes, TWEAK stimulates IL-6 and ICAM-1 expression (Saas et al., 2000). Although protective and detrimental effects of IL-6 are apparently balanced in cerebral ischemia (Herrmann et al., 2003), ICAM-1 expression clearly promotes ischemic brain damage (Connolly et al., 1996).
It is intriguing that Tanabe and colleagues (2003) showed that Fn14 stimulates neurite outgrowth in peripheral neurons. A dual function of neuronal membrane receptors in regeneration and neuronal apoptosis is not without precedents. The p75 neurotrophin receptor and the receptor for advanced glycation end products are involved in neurite outgrowth and induce neuronal apoptosis (Huttunen et al., 2000; Gentry et al., 2004). Notably, both receptors also stimulate NF-κB activation. In the case of Fn14, the ligand TWEAK could represent the switch from neurotrophic to neurotoxic effects because the neurotrophic role of Fn14 is independent of TWEAK binding (Tanabe et al., 2003). The differential expression of TWEAK and Fn14 might function as an autocrine or paracrine mechanism regulating neuron survival in cerebral ischemia and other conditions.
Footnotes
This work was supported by Grants Schw 416/4-1 and GK 791from the Deutsche Forschungsgemeinschaft (M.S.) and the Bundesministerium für Bildung und Forschung (M.S., A.S.). We thank A. Pickert, S. Kramps, K. Young, U. Grune, A. Keller, and M. Welschof for performing the MPSS procedures; G. Vogt for bioinformatic and statistical analyses; and T. Zheng, B. Browning, J. Michaelson, S. Cho, Y.-M. Hsu, S. Miklasz, and L. Tarilonte for providing the anti-Fn14 serum and monoclonal antibody. BMS-345541 was kindly provided by J. Burke (Pennington, NJ).
Correspondence should be addressed to either of the following: Dr. Markus Schwaninger, Department of Neurology, Im Neuenheimer Feld 400, D-69120 Heidelberg, Germany, E-mail: markus.schwaninger@med.uni-heidelberg.de; or Dr. Armin Schneider, Axaron Bioscience AG, Im Neuenheimer Feld 515, D-69120 Heidelberg, Germany, E-mail: schneider@axaron.com.
M. Rossner's present address: Max-Planck Institute of Experimental Medicine, Department of Neurogenetics, Hermann-Rein-Strasse 3, D-37075 Göttingen, Germany.
W. Zhang's present address: Department of Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430074, China.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248237-08$15.00/0
References
- Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3: 745-756. [DOI] [PubMed] [Google Scholar]
- Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7: 986-995. [DOI] [PubMed] [Google Scholar]
- Brenner S, Johnson M, Bridgham J, Golda G, Lloyd DH, Johnson D, Luo S, McCurdy S, Foy M, Ewan M, Roth R, George D, Eletr S, Albrecht G, Vermaas E, Williams SR, Moon K, Burcham T, Pallas M, DuBridge RB, et al. (2000) Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat Biotechnol 18: 630-634. [DOI] [PubMed] [Google Scholar]
- Brown SA, Richards CM, Hanscom HN, Feng SL, Winkles JA (2003) The Fn14 cytoplasmic tail binds tumour-necrosis-factor-receptor-associated factors 1, 2, 3 and 5 and mediates nuclear factor-kappaB activation. Biochem J 371: 395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, Yang X, Iotzova VS, Clarke W, Strnad J, Qiu Y, Zusi FC (2003) BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 278: 1450-1456. [DOI] [PubMed] [Google Scholar]
- Chan PH, Kamii H, Yang G, Gafni J, Epstein CJ, Carlson E, Reola L (1993) Brain infarction is not reduced in SOD-1 transgenic mice after a permanent focal cerebral ischemia. NeuroReport 5: 293-296. [DOI] [PubMed] [Google Scholar]
- Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL (1997) TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401-32410. [DOI] [PubMed] [Google Scholar]
- Chicheportiche Y, Fossati-Jimack L, Moll S, Ibnou-Zekri N, Izui S (2000) Down-regulated expression of TWEAK mRNA in acute and chronic inflammatory pathologies. Biochem Biophys Res Commun 279: 162-165. [DOI] [PubMed] [Google Scholar]
- Connolly Jr ES, Winfree CJ, Springer TA, Naka Y, Liao H, Yan SD, Stern DM, Solomon RA, Gutierrez-Ramos JC, Pinsky DJ (1996) Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J Clin Invest 97: 209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Erausquin GA, Hyrc K, Dorsey DA, Mamah D, Dokucu M, Masco DH, Walton T, Dikranian K, Soriano M, Garcia Verdugo JM, Goldberg MP, Dugan LL (2003) Nuclear translocation of nuclear transcription factor-kappa B by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors leads to transcription of p53 and cell death in dopaminergic neurons. Mol Pharmacol 63: 784-790. [DOI] [PubMed] [Google Scholar]
- Desplat-Jego S, Varriale S, Creidy R, Terra R, Bernard D, Khrestchatisky M, Izui S, Chicheportiche Y, Boucraut J (2002) TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol 133: 116-123. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22: 391-397. [DOI] [PubMed] [Google Scholar]
- Feng SL, Guo Y, Factor VM, Thorgeirsson SS, Bell DW, Testa JR, Peifley KA, Winkles JA (2000) The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol 156: 1253-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridmacher V, Kaltschmidt B, Goudeau B, Ndiaye D, Rossi FM, Pfeiffer J, Kaltschmidt C, Israel A, Memet S (2003) Forebrain-specific neuronal inhibition of nuclear factor-κB activity leads to loss of neuroprotection. J Neurosci 23: 9403-9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry JJ, Barker PA, Carter BD (2004) The p75 neurotrophin receptor: multiple interactors and numerous functions. Prog Brain Res 146: 25-39. [DOI] [PubMed] [Google Scholar]
- Harada N, Nakayama M, Nakano H, Fukuchi Y, Yagita H, Okumura K (2002) Pro-inflammatory effect of TWEAK/Fn14 interaction on human umbilical vein endothelial cells. Biochem Biophys Res Commun 299: 488-493. [DOI] [PubMed] [Google Scholar]
- Herrmann O, Tarabin V, Suzuki S, Attigah N, Prinz S, Schneider A, Coserea I, Monyer H, Brombacher F, Schwaninger M (2003) Regulation of body temperature and neuroprotection by endogenous interleukin-6 in focal cerebral ischemia. J Cereb Blood Flow Metab 23: 406-415. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H (2000) Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275: 40096-40105. [DOI] [PubMed] [Google Scholar]
- Jakubowski A, Browning B, Lukashev M, Sizing I, Thompson JS, Benjamin CD, Hsu YM, Ambrose C, Zheng TS, Burkly LC (2002) Dual role for TWEAK in angiogenic regulation. J Cell Sci 115: 267-274. [DOI] [PubMed] [Google Scholar]
- Kennett RH, McKearn TJ, Bechtol KB (1982) Monoclonal antibodies. A new dimension in biological analysis. New York: Plenum.
- Lee JM, Grabb MC, Zipfel GJ, Choi DW (2000) Brain tissue responses to ischemia. J Clin Invest 106: 723-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725-734. [DOI] [PubMed] [Google Scholar]
- Lou M, Eschenfelder CC, Herdegen T, Brecht S, Deuschl G (2004) Therapeutic window for use of hyperbaric oxygenation in focal transient ischemia in rats. Stroke 35: 578-583. [DOI] [PubMed] [Google Scholar]
- Love S (2003) Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry 27: 267-282. [DOI] [PubMed] [Google Scholar]
- Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR (1999) TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 274: 8455-8459. [DOI] [PubMed] [Google Scholar]
- Martin-Villalba A, Hahne M, Kleber S, Vogel J, Falk W, Schenkel J, Krammer PH (2001) Therapeutic neutralization of CD95-ligand and TNF attenuates brain damage in stroke. Cell Death Differ 8: 679-686. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Camandola S (2001) NF-kappaB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107: 247-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, Richards CM, Winkles JA (1999) The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 274: 33166-33176. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Ishidoh K, Kayagaki N, Kojima Y, Yamaguchi N, Nakano H, Kominami E, Okumura K, Yagita H (2002) Multiple pathways of TWEAK-induced cell death. J Immunol 168: 734-743. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Ishidoh K, Kojima Y, Harada N, Kominami E, Okumura K, Yagita H (2003) Fibroblast growth factor-inducible 14 mediates multiple pathways of TWEAK-induced cell death. J Immunol 170: 341-348. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Goffi F, Boroni F, Benarese M, Perkins SE, Liou HC, Spano P (2002) Opposing roles for NF-kappa B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J Biol Chem 277: 20717-20723. [DOI] [PubMed] [Google Scholar]
- Polek TC, Talpaz M, Darnay BG, Spivak-Kroizman T (2003) TWEAK mediates signal transduction and differentiation of RAW264.7 cells in the absence of Fn14/TweakR. Evidence for a second TWEAK receptor. J Biol Chem 278: 32317-32323. [DOI] [PubMed] [Google Scholar]
- Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY (2000) TWEAK stimulation of astrocytes and the proinflammatory consequences. Glia 32: 102-107. [PubMed] [Google Scholar]
- Saitoh T, Nakayama M, Nakano H, Yagita H, Yamamoto N, Yamaoka S (2003) TWEAK induces NF-kappaB2 p100 processing and long lasting NF-kappaB activation. J Biol Chem 278: 36005-36012. [DOI] [PubMed] [Google Scholar]
- Sallmann S, Juttler E, Prinz S, Petersen N, Knopf U, Weiser T, Schwaninger M (2000) Induction of interleukin-6 by depolarization of neurons. J Neurosci 20: 8637-8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D, Rosenthal N (1993) Detection of messenger RNA by in situ hybridization. Methods Enzymol 225: 384-404. [DOI] [PubMed] [Google Scholar]
- Sassoon DA, Garner I, Buckingham M (1988) Transcripts of alpha-cardiac and alpha-skeletal actins are early markers for myogenesis in the mouse embryo. Development 104: 155-164. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M (1999) NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5: 554-559. [DOI] [PubMed] [Google Scholar]
- Schneider A, Fischer A, Spielvogel D, von Ahsen O, Scheeck S, Gümbel C, Rossner M, Hiemisch H, Herrmann O, Bach A, Schwaninger M (2004a) Restriction-mediated differential display (RMDD) identifies pip92 as a pro-apoptotic gene product induced during focal cerebral ischemia. J Cereb Blood Flow Metab 24: 224-236. [DOI] [PubMed] [Google Scholar]
- Schneider A, Laage R, Rossner M, von Ahsen O, Scheek S, Kuner R, Fischer A, Spielvogel D, Gümbel C, Götz B, Hiemisch H, Martin-Villalba A, Bach A, Schwaninger M (2004b) Identification of regulated genes during permanent focal ischemia: characterization of the protein kinase 9b2/MARKL1/MARK4. J Neurochem 88: 1114-1126. [DOI] [PubMed] [Google Scholar]
- Schneider P, Schwenzer R, Haas E, Muhlenbeck F, Schubert G, Scheurich P, Tschopp J, Wajant H (1999) TWEAK can induce cell death via endogenous TNF and TNF receptor 1. Eur J Immunol 29: 1785-1792. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Bonilla I, Winkles JA, Strittmatter SM (2003) Fibroblast growth factor-inducible-14 is induced in axotomized neurons and promotes neurite outgrowth. J Neurosci 23: 9675-9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg G, Prass K, Priller J, Kapinya K, Polley A, Muselmann C, Ruscher K, Kannbley U, Schmitt AO, Castell S, Wiegand F, Meisel A, Rosenthal A, Dirnagl U (2002) Serial analysis of gene expression identifies metallothionein-II as major neuroprotective gene in mouse focal cerebral ischemia. J Neurosci 22: 5879-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bruggen N, Thibodeaux H, Palmer JT, Lee WP, Fu L, Cairns B, Tumas D, Gerlai R, Williams SP, van Lookeren Campagne M, Ferrara N (1999) VEGF antagonism reduces edema formation and tissue damage after ischemia/reperfusion injury in the mouse brain. J Clin Invest 104: 1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velculescu VE, Zhang L, Vogelstein B, Kinzler KW (1995) Serial analysis of gene expression. Science 270: 484-487. [DOI] [PubMed] [Google Scholar]
- Ware CF (2003) The TNF superfamily. Cytokine Growth Factor Rev 14: 181-184. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Winkles JA (2003) TWEAK, a member of the TNF superfamily, is a multifunctional cytokine that binds the TweakR/Fn14 receptor. Cytokine Growth Factor Rev 14: 241-249. [DOI] [PubMed] [Google Scholar]
- Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, Lindner V, Liu H, Daniel TO, Smith CA, Fanslow WC (2001) A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 15: 837-846. [DOI] [PubMed] [Google Scholar]
- Zhang W, Potrovita I, Tarabin V, Herrmann O, Beer V, Weih F, Schneider A, Schwaninger M (2004) Neuronal activation of NF-κβ contributes to cell death in cerebral ischemia. J Cereb Blood Flow Metab, in press. [DOI] [PubMed]