Abstract
Many mammalian species form dominance hierarchies, but it remains unknown whether differences in social status correspond to structural differences in the brain. Stressful experiences may arise naturally during the establishment of dominance, and stress has been linked to adult neurogenesis in the hippocampus. To determine whether position in a dominance hierarchy leads to changes in adult neurogenesis in the hippocampus, we examined the brains of rats housed in a visible burrow system (VBS), a seminaturalistic environment with opportunities for social interaction. Dominance hierarchies emerged among the males in all colonies within 3 d of living in the VBS. Although cell proliferation in the dentate gyrus did not differ between the groups, more new neurons were observed in the dentate gyrus of the dominant males compared with both subordinates and controls. Dominant and subordinate animals showed similar basal, stress, and recovery from stress levels of corticosterone, as well as similar thymus, adrenal gland, and body weights, suggesting that variables other than stress are responsible for the observed changes in adult neurogenesis. The differences in brain structure persisted among the animals that had no access to the burrow system after the dominance hierarchy stabilized, suggesting that social status rather than living in a complex environment accounts for the effect of dominance on adult neurogenesis.
Keywords: neurogenesis, dentate gyrus, hippocampus, dominance, visible burrow system, aggression
Introduction
Individual differences in behavioral, endocrine, and neural responses to potentially stressful experiences have been reported in humans (Lawler et al., 2001; Halpern et al., 2002; Koehl et al., 2002; Pujol et al., 2002; Stein et al., 2002). However, the origins of these differences and their implications for the development of psychopathology remain elusive.
Despite the fact that individual differences are a hallmark of the human brain and behavior, most animal studies are designed to minimize intragroup variability. Strains are bred to decrease the variability that could obscure differences across experimental groups. Housing conditions are standardized to minimize the potential for unique experiences, which could confound the measures of interest. Although there are advantages to these controlled approaches, the artificial standardization of the animals may render such studies inapplicable to certain human conditions.
Some laboratory studies have examined animals under conditions that elicit individual differences in behavior. One such paradigm is the visible burrow system (VBS), a seminaturalistic laboratory environment that presents opportunities for exploration and social interaction (Blanchard et al., 1991, 2001). When housed in this environment, rats form a dominance hierarchy, characterized by individual differences in offensive and defensive behavior. Numerous studies have examined the effects of social stress on the brains of subordinate animals living in dominance hierarchies, but less attention has been paid to the brains of animals displaying dominant behavioral characteristics. Some previous experiments report few, if any, differences between the dominant animals and controls (Holmes et al., 1995), but evidence also supports the possibility that dominant animals are stressed relative to controls, although to a lesser degree than subordinates. For example, dominant as well as subordinate Long-Evans rats exhibit stress-related reductions in dendritic length and branching in the CA3 pyramidal neurons of the hippocampus, compared with control animals living in standard laboratory conditions (McKittrick et al., 2000).
The hippocampus is a brain region known for its functional and structural plasticity. Throughout adulthood, the hippocampus generates a large number of new neurons. Production and survival of these new neurons are modulated by stress (Gould and Tanapat, 1999) and environmental complexity (Kempermann et al., 1998; Nilsson et al., 1999), among other factors. No previous studies have examined the possibility that individual changes in adult neurogenesis may arise when animals are exposed to seminaturalistic conditions that elicit behavioral differences. To determine whether position within a dominance hierarchy affects the production of new neurons in the hippocampus, we examined the brains of adult male rats living in the VBS. Here we show that social dominance does not alter cell proliferation but is associated with enhanced survival of new neurons in the dentate gyrus. Neither stress nor environmental complexity can account for the relationship between dominance status and adult neurogenesis.
Materials and Methods
Animal care and treatment. Adult male Sprague Dawley rats, weighing 250-320 gm at the start of the study (Taconic, Germantown, NY), were used. Animal procedures were conducted in accordance with The National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animals were marked with black spray paint for identification on videotapes and housed in groups of four in the VBS or in standard laboratory cages. The VBS was adapted from Blanchard et al. (1991), who designed it to study dominance hierarchies in Long-Evans rats. Relative to standard laboratory cages, the VBS presents greater opportunities for spatial exploration, interaction with conspecifics, competition for nourishment, and physical activity (Fig. 1 A). In this experiment, the VBS was made from wood and Plexiglas, with opaque side panels and transparent tunnels and chambers. It consisted of an open field (91 cm2), covered by bedding material; the field was lit by an incandescent light bulb during the light phase and an infrared light bulb during the dark phase of the 12 hr light/dark cycle. The open field was surrounded by clear tunnels leading to several interconnected chambers. These cylindrical chambers varied in height and diameter and were generally used for sleeping or hiding. Minor adaptations were made to facilitate competition among Sprague Dawley rats, a strain known to be more docile than Long-Evans. Specifically, our VBS included narrow ramps that lead to food and water, accessible to only one animal at a time. Two sexually mature females were housed with every group of four males to stimulate competition among the animals.
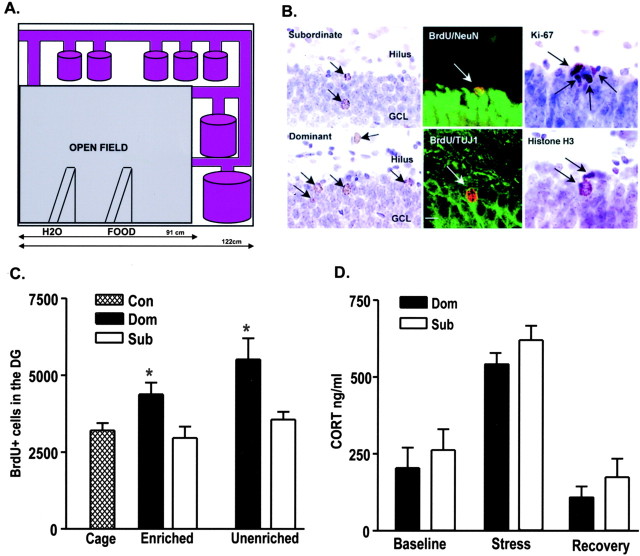
Figure 1.
A, Diagram of the visible burrow system, a seminaturalistic environment for the rat: a large enclosure (in gray), surrounded by tunnels and chambers of different size (in purple). B, New cells in the dentate gyrus of subordinate (top left) and dominant (bottom left) animals. More BrdU-labeled cells were observed in the dentate gyrus of dominant relative to subordinate and control animals (controls not shown). Confocal laser-scanning images of cells in the GCL of animals living in the VBS. Double-labeling of BrdU (red) with a neuronal marker NeuN (green). Another BrdU-labeled cell is positive for TUJ1 (green), a marker of both immature and mature neurons. The majority of BrdU-labeled cells colabeled with one of the neuronal markers, and the rate of colabeling did not vary across groups. Proliferating cells in the GCL of animals living in the VBS. No differences were detected in the number of cells expressing endogenous markers of cell proliferation, Ki-67, or phosphorylated-histone-H3. Scale bar, 10 μm. C, BrdU counts in the dentate gyrus of dominant, subordinate, and cage control animals. Two weeks after a single BrdU injection, dominant animals had more BrdU-labeled cells in the dentate gyrus compared with subordinate animals and cage controls. This difference was maintained in animals whether or not they had access to a more enriched environment during the survival time after BrdU injection. Bars represent mean ± SEM. Asterisks represent significant difference from subordinates and controls; p < 0.05. D, Plasma corticosterone (CORT) response to restraint stress. Samples were collected before restraint, after 0.5 hr long restraint, and after 3.5 hr of recovery. Corticosterone values did not differ between dominant and subordinate animals. Bars represent mean ± SEM.
To determine whether male Sprague Dawley rats establish dominance hierarchies, groups were videotaped for 10 hr during the dark phase for three nights in the VBS, a time sufficient for establishing dominance hierarchies in Long-Evans rats (Blanchard et al., 1995).
Behavioral analysis. In each cohort, a dominant animal was selected based on the quantity of offensive and defensive behavior. Behavioral measurements were sampled hourly from videotapes, in 10-min-long intervals, adding up to the total of 300 min per colony. Male to male dyadic interactions involving lateral attacks, boxing, mounting, and fighting on ramps were scored as instances of offensive behavior; flight and underside exposure were the typically observed defensive actions (Blanchard et al., 1995). For every male, the number of defensive acts was subtracted from the number of offensive acts, yielding one score. The animal with the highest score was designated as the dominant, and the other three males in the colony were considered subordinate. One subordinate animal was chosen randomly from each cohort for all comparisons with the dominant animals to balance the group sizes.
Dominance, environmental complexity, and neurogenesis. To examine whether dominance status affects the number of new neurons, animals living in a dominance hierarchy for three nights were injected with the thymidine analog bromodeoxyuridine (BrdU; 200 mg/kg) on day 4 and perfused 2 weeks later. This survival time after BrdU injection is sufficient for the majority of new cells to express markers of mature neurons (Gould et al., 1999).
To determine whether differences in neurogenesis between dominants and subordinates stem from an interaction between dominance status and environmental enrichment, cohorts of rats were housed under enriched (n = 5 dominants; n = 5 subordinates) or unenriched (n = 4 dominants; n = 4 subordinates) conditions after BrdU injection. Some cohorts were allowed access to the entire VBS (including the tunnels and chambers) during the 2 week survival period, whereas others were returned to the same apparatus but were not permitted access to the burrows (the purple area in Fig. 1 A was blocked off). Five control animals were housed in standard laboratory cages, injected with BrdU, and returned to their cages for 14 d until perfusion.
Dominance and cell proliferation. To determine whether dominance influences cell proliferation in the adult dentate gyrus, additional rats were housed in the VBS for 3 d and were perfused on day 4, together with age-matched controls living in standard cages (n = 4 dominants; n = 4 subordinates; n = 5 controls). Their brains were processed for two endogenous markers of cell proliferation: phospho-histone-H3, expressed by cells in late G2 and M phases of the cell cycle (Zindy et al., 1999; Polioudaki et al., 2004), and Ki-67, expressed by cells during all phases of the cell cycle (Kee et al., 2002).
Histological procedures and microscopy. The animals were anesthetized with Nembutal and perfused with 4.0% paraformaldehyde in 0.1 m phosphate buffer. After post-fixation, 40-μm-thick coronal sections were cut through one half of each brain using an oscillating tissue slicer.
For BrdU peroxidase labeling, the tissue was heated in 0.1 m citric acid, denatured in 2 N HCl, and incubated overnight at 4°C with primary mouse anti-BrdU antibody (Vector Laboratories, Burlingame, CA). Next, sections were reacted with a mouse ABC kit (Vector Laboratories).
For double labeling of BrdU and neuronal markers, the tissue was pretreated in 2N HCl and incubated in primary rat anti-BrdU (Accurate Chemical, Westbury, NY) and either mouse anti-NeuN or mouse anti-TUJ1 (Chemicon, Temecula, CA). Next, the sections were incubated in biotinylated anti-rat (Chemicon), followed by streptavidin Alexa 568 and goat anti-mouse Alexa 488 (Molecular Probes, Eugene, OR) for NeuN or TUJ1.
For labeling with endogenous markers of cell proliferation, the tissue was incubated with rabbit polyclonal anti-Ki-67 (Vector Laboratories) or anti-phospho-histone-H3 (Upstate Groug, Waltham, MA). Then, the tissue was reacted for peroxidase staining using a standard rabbit ABC kit (Vector Laboratories).
For microscopic data analysis, the slides were coded and BrdU-labeled cells in the granule cell layer (GCL), subgranular zone (SGZ), the hilus, and the subventricular zone (SVZ) were counted on every twelfth half-section through the dentate gyrus with 100× oil objective on an Olympus (Tokyo, Japan) BX-50 microscope. Ki-67 and histone-H3-labeled cells were counted in the GCL and the SGZ. Labeled cells in the outermost focal plane were excluded from the analysis. The numbers were tallied for the dentate gyrus, GCL-SGZ, or the SVZ and multiplied by 24 to obtain a stereological estimate for the different brain regions. For double labeling, the percentage of BrdU-labeled GCL cells expressing NeuN or TUJ1 was determined by analyzing 25 BrdU-labeled cells using a Zeiss Axiovert confocal laser-scanning microscope. These data were analyzed using a one-way or a two-way ANOVA followed by Newman-Keuls post hoc comparisons.
Corticosterone measures. Four additional cohorts of rats (n = 4 dominants; n = 4 subordinates) were used to examine whether dominant and subordinate animals have different basal corticosterone blood levels and whether they respond differently to stress. Because stress downregulates cell proliferation in the dentate gyrus (Gould and Tanapat, 1999), blood samples using the tail-bleed method could not be obtained from animals included in the neurogenesis study. On day 4 of living in the VBS (at the same time of day the animals in the adult neurogenesis experiment were injected with BrdU), blood samples were taken from the tails of rats at three different time points: (1) immediately after being placed into the restrainer (baseline), (2) after being in the restrainer for 0.5 hr (stress), and (3) 3.5 hr after being released from the restrainer and returned to the home cage (recovery). These animals were weighed and decapitated; adrenal and thymus glands were removed and weighed. After the blood was centrifuged for 20 min at 3000 rpm, plasma was collected and circulating corticosterone (in nanograms per milliliter) was measured using the solid-phase radioimmunoassay system (Coat-A-Count; Diagnostic Products Corporation, Los Angeles, CA). Assay sensitivity was 99.1%, with an interassay variability of <5%. The results were analyzed using a two-way ANOVA followed by Newman-Keuls post hoc comparisons.
Results
Sprague Dawley rats housed in the VBS establish dominance hierarchies
During the first three nights of living in the VBS, one male engaged in substantially more offensive behavior relative to the other males within the same cohort (t = 2.391; p = 0.028). Whereas the overall level of aggression varied across cohorts, on average the dominant animals engaged in twice as many offensive actions per hour of observation, compared with nondominant animals. In some cohorts, the dominant animals engaged in substantially more aggressive behavior than all of the subordinates; in others, there was evidence of more extended fighting for dominance between two most aggressive males. By the end of the third day of living in the VBS, the level of aggression dropped off in all cohorts, suggesting that dominance hierarchies had been established.
Dominants have more new neurons in the dentate gyrus than subordinates, regardless of environmental enrichment
Dominant animals living in the VBS had more BrdU-labeled cells in the dentate gyrus compared with subordinate animals (F(1,17) = 14.48; p = 0.0019). Access to the tunnels was not necessary for the maintenance of the dominance-related difference in BrdU cell number. Dominants with access to a more complex environment during the survival period exhibited more BrdU-labeled cells in the dentate gyrus relative to subordinates and controls (Fig. 1B,C) (F(2,12) = 4.992; p = 0.0265). Similarly, dominant animals living in an unenriched environment had more BrdU-labeled cells relative to subordinates living in the same conditions as well as controls (F(2,12) = 8.618; p = 0.0067). Most BrdU-labeled cells in the GCL of all animals expressed the markers of mature or immature neurons: ∼75% colabeled with NeuN whereas ∼88% expressed TUJ1 (Fig. 1B). There were no differences in the percentage of BrdU-labeled cells that expressed either neuronal marker among any of the groups examined, suggesting that the increase in BrdU-labeled cell number associated with dominance represents enhanced neurogenesis.
The range in the number of BrdU-labeled cells among animals living in the VBS (both dominants and subordinates) was greater than in control animals (the difference between maximum and minimum numbers of BrdU-labeled cells in the enriched groups was 4128, in the unenriched groups it was 3648, and in the control group it was 1296). No differences in the number of BrdU-labeled cells in the SVZ of VBS-housed dominants, subordinates, and controls were observed (F(2,12) = 0.002; p = 0.9867).
Dominance hierarchy does not influence cell proliferation in the dentate gyrus
No differences were observed in the number of Ki-67 or histone-H3-labeled cells (Fig. 1B) in the GCL and SGZ of dominant, subordinate, and control animals (for Ki-67: F(2,11) = 0.6891; p = 0.5225; for histone-H3: F(2,11) = 0.5458; p = 0.5943), indicating that dominance status does not affect cell proliferation in the dentate gyrus.
Stress measures do not differ between dominant and subordinate animals
The levels of circulating corticosterone were not statistically different between dominant and subordinate animals at baseline, stress, or recovery time points (Fig. 1D) (F(1,18) = 2.387; p = 0.1398). No differences between dominant and subordinate animals in the ratio of adrenal gland weight to body weight were detected (mean + SEMDom/100 gm of body weight = 13.08 ± 0.96 mg; mean ± SEMSub = 13.98 ± 0.62 mg; p > 0.05). Likewise, no differences were found in the ratio of thymus gland weight to body weight (mean ± SEMDom/100 gm of body weight = 76.91 ± 9.43 mg; mean ± SEMSub = 82.01 ± 4.66 mg; p > 0.05), or in total body weight (mean ± SEMDom = 437.5 ± 14.2 gm; mean ± SEMSub = 415.5 ± 12.6 gm; p > 0.05).
Discussion
We have shown that living in a seminaturalistic environment elicits differences in behavior among adult male rats that correspond to differences in the rate of neurogenesis in the dentate gyrus. Dominant rats produced more new neurons in the dentate gyrus compared with subordinate rats and controls, whereas the number of new SVZ cells did not differ among the groups. Our results suggest that dominance increases the number of new neurons by enhancing cell survival rather than cell proliferation. Despite differences in the number of BrdU-labeled cells that express neuronal markers, we detected no differences in the number of cells expressing endogenous markers of cell proliferation between dominants and subordinates.
It is unlikely that previous individual differences in adult neurogenesis account for different behavioral tendencies in the VBS. First, Sprague Dawley rats housed in laboratory cages are not characterized by particularly varied behavior or high levels of aggression. Bred to be docile, animals of this strain engage in uniform, predominantly nonaggressive social behavior under control settings (Becker et al., 1999). Second, the range of the numbers of BrdU-labeled cells in the dentate gyrus was more narrow in the control animals than in those living in the VBS. The lack of substantial individual differences in behavior and adult neurogenesis among control rats suggests that placing animals in the VBS actually elicits the changes in behavior and, hence, in adult neurogenesis. Future studies designed to manipulate position in the dominance hierarchy, either by placing preselected dominants together in a new cohort or by removing a dominant from an established hierarchy, will assess whether changes in social status over time mirror changes in adult neurogenesis.
Dominant and subordinate animals showed similar corticosterone baseline, stress and recovery levels, and similar body weights and the weights of organs known to be sensitive to stress, raising the likelihood that mechanisms other than stress underlie the observed changes in brain structure. These data stand in contrast to previous studies reporting increased basal levels of corticosterone, increased adjusted adrenal weights, and decreased thymus and body weights in subordinates, relative to controls and sometimes relative to dominants as well (Blanchard et al., 1993, 1995). The differences between these studies and our results are likely caused by the fact that Sprague Dawley rats are less aggressive than Long-Evans rats. Additional support for the view that Sprague Dawley rats living in a dominance hierarchy are not stressed relative to controls is the lack of a difference in cell proliferation. Studies have demonstrated significant decreases in cell proliferation in the dentate gyrus after stress (Gould et al., 1997; Tanapat et al., 2001; Malberg and Duman, 2004); neither dominants nor subordinates showed such decreases in our study.
Other aspects of living in the VBS, not directly related to stressful experiences, may explain increased neurogenesis in the dominants. Living in an “enriched” environment is known to enhance adult neurogenesis in the dentate gyrus of rats and mice, although all of these studies used females to prevent the formation of a dominance hierarchy (Kempermann et al., 1998, 2002; Nilsson et al., 1999). The VBS, with its tunnels and chambers, is a complex environment relative to a standard laboratory cage. Thus, one possible interpretation of our findings is that dominant animals exhibit an increase in neurogenesis because they are living in an enriched environment. The difference between dominant and subordinate animals, then, may be that subordination prevents the positive effects of living in an enriched environment. The results of our experiments, however, have dissociated the effect of dominance from environmental enrichment. We found that living in the VBS for 2 weeks failed to increase adult neurogenesis beyond the enhancing effect of dominance. The absence of an enrichment effect in our study is not surprising, because earlier studies have found increases in neurogenesis in animals living in enriched environments for longer periods of time (2-10 months) (Kempermann et al., 1998, 2002; Nilsson et al., 1999). In addition, most of these studies have included running wheels in the enriched environment, and running by itself has been shown to increase adult neurogenesis (van Praag et al., 1999). It remains to be determined whether dominants and subordinates would respond similarly to living for a prolonged period of time in an environment that is complex enough to enhance adult neurogenesis.
The difference in adult neurogenesis between dominants and subordinates is likely to be related to their varied experiences. By definition, dominant animals display higher levels of aggression. In addition, dominant animals spend more time in physical contact with females, engaging in anogenital sniffing and mating (Blanchard et al., 2001). Both aggression and mating are linked to enhanced levels of testosterone (Taylor et al., 1985; Albert et al., 1992). Thus, dominant animals may have more new neurons as a result of experience-dependent increases in testosterone. Indeed, a positive relationship between testosterone and adult neurogenesis has been reported in several forebrain areas of voles and birds (Louissant et al., 2002; Fowler et al., 2003; Absil et al., 2003).
Future studies will be necessary to fully understand the effects of living in a dominance hierarchy on the relationship between behavior and brain structure in adult animals. However, it is clear that, in comparison to laboratory controls, animals living in naturalistic environments that allow the formation of dominance hierarchies show more individual differences in brain structure, which may present a more accurate reflection of the normal brain.
Footnotes
This work was supported by National Institutes of Health Grant MH59740 and the Conte Center for the Neuroscience of Mental Disorders (MH58922). We thank Deanna Samburg, Gwendolyn Wood, and Alexander Raines for technical assistance.
Correspondence should be addressed to Elizabeth Gould at the above address. E-mail: goulde@princeton.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/246755-05$15.00/0
References
- Absil P, Pinxten R, Balthazart J, Eens M (2003) Effect of age and testosterone on autumnal neurogenesis in male European starlings (Sturnus vulgaris). Behav Brain Res 143: 15-30. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Jonik RH, Walsh ML (1992) Hormone-dependent aggression in male and female rats: experiential, hormonal, and neural foundations. Neurosci Biobehav Rev 16: 177-192. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B (1999) Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology 144: 333-338. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Cholvanich P, Blanchard RJ, Clow DW, Hammer Jr RP, Rowlett JK, Bardo MT (1991) Serotonin, but not dopamine, metabolites are increased in selected brain regions of subordinate male rats in a colony environment. Brain Res 568: 61-66. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ (1993) Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res 58: 113-121. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR (1995) Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology 20: 117-134. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Dulloog L, Markham C, Nishimura O, Nikulina Compton J, Jun A, Han C, Blanchard DC (2001) Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol Behav 72: 245-254. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Freeman ME, Wang Z (2003) Newly proliferated cells in the adult male amygdala are affected by gonadal steroid hormones. J Neurobiol 57: 257-269. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P (1999) Stress and hippocampal neurogenesis. Biol Psychiatry 46: 1472-1479. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E (1997) Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci 17: 2492-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ (1999) Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci 2: 260-265. [DOI] [PubMed] [Google Scholar]
- Halpern CT, Campbell B, Agnew CR, Thompson V, Udry JR (2002) Associations between stress reactivity and sexual and nonsexual risk taking in young adult human males. Horm Behav 42: 387-398. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Blanchard DC, Blanchard RJ, Brady LS, Crawley JN (1995) Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacol Biochem Behav 50: 655-660. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002) The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115: 97-105. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH (1998) Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci 18: 3206-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH (2002) Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 52: 135-143. [DOI] [PubMed] [Google Scholar]
- Koehl M, Lemaire V, Mayo W, Abrous DN, Maccari S, Piazza PV, Le Moal M, Vallee M (2002) Individual vulnerability to substance abuse and affective disorders: role of early environmental influences. Neurotox Res 4: 281-296. [DOI] [PubMed] [Google Scholar]
- Lawler KA, Kline KA, Adlin RF, Wilcox ZC, Craig FW, Krishnamoorthy JS, Piferi RL (2001) Psychophysiological correlates of individual differences in patterns of hemodynamic reactivity. Int J Psychophysiol 40: 93-107. [DOI] [PubMed] [Google Scholar]
- Louissaint Jr A, Rao S, Leventhal C, Goldman SA (2002) Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34: 945-960. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS (2003) Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28: 1562-1571. [DOI] [PubMed] [Google Scholar]
- McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR (2000) Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse 36: 85-94. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS (1999) Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 39: 569-578. [DOI] [PubMed] [Google Scholar]
- Polioudaki H, Markaki Y, Kourmouli N, Dialynas G, Theodoropoulos PA, Singh PB, Georgatos SD (2004) Mitotic phosphorylation of histone H3 at threonine 3. FEBS Lett 560: 39-44. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T (2002) Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage 15: 847-855. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ (2002) Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry 159: 1675-1681. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E (2001) Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol 437: 496-504. [DOI] [PubMed] [Google Scholar]
- Taylor GT, Weiss J, Rupich R (1985) Suprathreshold manipulations of testosterone and reproductive functioning in gonadally intact sexually experienced and inexperienced male rats. Physiol Behav 35: 735-739. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH (1999) Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2: 266-270. [DOI] [PubMed] [Google Scholar]
- Zindy F, Cunningham JJ, Sherr CJ, Jogal S, Smeyne RJ, Roussel MF (1999) Postnatal neuronal proliferation in mice lacking Ink4d and Kip1 inhibitors of cyclin-dependent kinases. Proc Natl Acad Sci USA 96: 13462-13467. [DOI] [PMC free article] [PubMed] [Google Scholar]