Abstract
It is generally assumed that the inhibitory neurotransmitter GABA and the stimulatory neurotransmitter glutamate are released from different neurons in adults. However, this tenet has made it difficult to explain how the same afferent signals can cause opposite changes in GABA and glutamate release. Such reciprocal release is a central mechanism in the neural control of many physiological processes including activation of gonadotropin-releasing hormone (GnRH) neurons, the neural signal for ovulation. Activation of GnRH neurons requires simultaneous suppression of GABA and stimulation of glutamate release, each of which occurs in response to a daily photoperiodic signal, but only in the presence of estradiol (E2). In rodents, E2 and photoperiodic signals converge in the anteroventral periventricular nucleus (AVPV), but it is unclear how these signals differentially regulate GABA and glutamate secretion. We now report that nearly all neurons in the AVPV of female rats express both vesicular glutamate transporter 2 (VGLUT2), a marker of hypothalamic glutamatergic neurons, as well as glutamic acid decarboxylase and vesicular GABA transporter (VGAT), markers of GABAergic neurons. These dual-phenotype neurons are the main targets of E2 in the region and are more than twice as numerous in females as in males. Moreover, dual-phenotype synaptic terminals contact GnRH neurons, and at the time of the surge, VGAT-containing vesicles decrease and VGLUT2-containing vesicles increase in these terminals. Thus, we propose a new model for ovulation that includes dual-phenotype GABA/glutamate neurons as central transducers of hormonal and neural signals to GnRH neurons.
Keywords: VGAT, VGLUT, LHRH, GnRH, estradiol, ovulation
Introduction
Ovulation is triggered through estradiol (E2)-dependent activation of gonadotropin-releasing hormone (GnRH) neurons in the preoptic area (POA) (Levine et al., 1991). Evidence from rodent models indicates that this activation is indirect and mediated primarily by the anteroventral periventricular nucleus (AVPV; a region of the POA). The AVPV is a sexually dimorphic region (Simerly, 1998) with abundant estrogen receptors (ERs) (Simerly et al., 1990; Shughrue et al., 1997). Lesions of the AVPV (Wiegand et al., 1980; Wiegand and Terasawa, 1982; Ronnekleiv and Kelly, 1986; Petersen et al., 1989) or microimplants of anti-estrogen (Petersen and Barraclough, 1989) placed into the region block luteinizing hormone (LH) surge release. Furthermore, the AVPV provides the majority of ERα-containing cells that innervate the rostral POA (rPOA), where most GnRH neurons participating in the LH surge reside (Simonian et al., 1999). Finally, the AVPV receives inputs from numerous brain regions that convey sensory and autonomic signals relevant to reproduction (Simerly, 1998). Thus, this nucleus is a critical region for integrating hormonal and environmental signals and communicating them to GnRH neurons.
The AVPV cells responsible for communicating signals to GnRH neurons have not been identified; however, it seems likely for several reasons that they are GABAergic and glutamatergic neurons. First, glutamatergic (Eyigor et al., 2004) and GABAergic (Flugge et al., 1985) neurons in the AVPV contain ER. Furthermore, GABAergic neurons in the AVPV, but not those surrounding GnRH neurons in the rPOA, exhibit changes in GAD67 gene expression that parallel GABA release on the day of LH surge release (Curran-Rauhut and Petersen, 2002). Consistent with these findings, GABA and glutamate terminals provide most of the synaptic input to GnRH neurons (Herbison, 1998; Kiss et al., 2003; Lin et al., 2003; Petersen et al., 2003; Eyigor et al., 2004; Han et al., 2004), and receptors for GABA and glutamate are among the few types found on GnRH neurons (Petersen et al., 2003). Moreover, agonists or antagonists to these receptors disrupt E2-dependent LH surge release (Herbison and Dyer, 1991; Donoso et al., 1994; Brann and Mahesh, 1995). Other neurotransmitters also regulate the LH surge, but many of these act by regulating GABA and glutamate release (Hartman et al., 1990; Brann and Mahesh, 1995; Bhat et al., 1998; Herbison, 1998; Gore, 2001). Taken together, these data suggest that GABAergic and glutamatergic neurons of the AVPV are likely targets of E2 and central to the generation of GnRH and LH surge release.
Although under some conditions GABA may be stimulatory to GnRH neurons (Ondo, 1974; DeFazio et al., 2002), the preponderance of evidence indicates that in postpubertal animals, GABA inhibits them (Herbison, 1998; Han et al., 2002, 2004). In contrast, activation of glutamate receptors consistently stimulates GnRH neurons and LH surge release (Brann and Mahesh, 1995; Gore, 2001; Kuehl-Kovarik et al., 2002). Consistent with these findings, induction of LH surge release requires suppression of GABA and stimulation of glutamate release into the POA (Petersen et al., 2003), changes that occur at the onset of the afternoon surge (Jarry et al., 1995; Mitsushima et al., 2002). These changes are triggered, at least in part, by neurons of the suprachiasmatic nucleus (SCN) that communicate photoperiodic signals to ER-containing cells in the AVPV (de la Iglesia et al., 1995; Watson et al., 1995).
It is not clear how a photoperiodic signal in the presence of E2 simultaneously activates glutamate release and inhibits GABA release from AVPV neurons, but two possibilities seem most likely. First, it is possible that GABAergic and glutamatergic neurons are separate cells, and the same afferent signal has opposite effects on the two populations. This could be accomplished if only one population contained ER and E2 changed the responsiveness of that cell type to the photoperiodic signal. An alternative model is that GABA and glutamate are released from the same neurons and that these dual-phenotype neurons have ER. In this model, reciprocal release might be accomplished by photoperiodic signals and autofeedback mechanisms that are regulated by E2.
A previous obstacle to differentiating between these models was that no reliable method of detecting glutamatergic neurons existed. This obstacle was recently overcome with the characterization of vesicular glutamate transporters (VGLUTs) as specific markers of glutamatergic neurons (Hisano, 2003). Using these markers and markers of GABA neurons, we now report that most of the neurons in the AVPV of females are both GABAergic and glutamatergic under physiological conditions. In addition, we show that these dual-phenotype neurons are the major targets of E2 in the AVPV and that the incidence of these neurons differs between sexes. Finally, we demonstrate that during the time period in which GnRH and LH surge release begins, E2 decreases markers of GABAergic vesicles and increases markers of glutamatergic vesicles in terminals contacting GnRH neurons.
Materials and Methods
Animals. Adult Sprague Dawley rats (Zivic Miller, Zelienople, PA) were maintained according to NIH Guidelines for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of the University of Massachusetts approved all treatment protocols. Animals were housed in a temperature- and light-controlled room (14/10 hr light/dark cycle; lights on at 5:00 A.M.) with food and water provided ad libitum.
Dual-label in situ hybridization histochemical studies. All dual-label in situ hybridization histochemical (ISHH) studies used orchidectomized (ORX) male and/or ovariectomized (OVX) female rats. Gonadectomies were performed under isofluorane anesthesia, and animals were killed 1 week later with CO2. Brains were frozen in powdered dry ice at the time of sacrifice, then wrapped in Parafilm and stored at -80°C in sealed tubes. We obtained serial 12 μm coronal cryosections through the POA region that contains the AVPV, the medial preoptic nucleus (MPO), and the periventricular POA (PePO) (-0.00 to -0.26 from bregma) (Swanson, 1998), as well as through regions containing the hippocampal formation (-2.45 to -4.60 from bregma) and cortex (-2.6 from bregma).
Three dual-label ISHH studies were performed. First, we colocalized mRNAs for glutamic acid decarboxylase (GAD; marker of GABA neurons) and VGLUT2 (marker of hypothalamic glutamatergic neurons) (Ziegler et al., 2002) in the AVPV and in the medial preoptic area (MPO) of OVX rats (n = 5). In separate studies, we verified that vesicular GABA transporter (VGAT) mRNA and GAD mRNA were colocalized in all neurons of the AVPV (E. Ottem and S. Petersen, unpublished observations). As a control study, we also colocalized GAD and VGLUT1 mRNAs in the hippocampal formation and cortex. In these regions, VGLUT1 predominates and GABAergic neurons are separate from glutamatergic neurons.
Second, we examined sex differences in the incidence of cells containing both VGLUT2 and GAD mRNAs in the AVPV (n = 5 OVX and 5 ORX rats). The rationale for this study was that if the dual-phenotype GABA/glutamate neurons in AVPV are important for LH surge release, there might be sex differences in the incidence of such neurons because only females exhibit the surge in response to E2.
Third, anti-estrogen microimplants placed into the AVPV block LH surge release (Petersen and Barraclough, 1989), but the affected neurons are unknown; therefore, we next used dual-label ISHH to examine whether the dual-phenotype GABA/glutamate neurons in the AVPV of females were targets of E2. ERα mRNA is the most abundant isoform in the POA (Shughrue et al., 1997), and in this region, ERβ expression is generally in the same cells as ERα expression (Shughrue et al., 1998). Similarly, preliminary studies showed that GAD-containing neurons were more abundant than VGLUT2-containing neurons, and all VGLUT2 mRNA was found in GAD-containing cells in the AVPV. Therefore, to maximize detection, we used cRNA probes for ERα and GAD mRNAs. We performed these studies in OVX rats (n = 5) because endogenous ovarian steroids suppress ER expression (Zhou et al., 1995; Greco et al., 2001).
The cDNA transcription template for probes to VGLUT2 mRNA was a 705 bp fragment corresponding to bases 3197-3901 of the rat VGLUT2 cDNA (GenBank accession number AF271235). It was prepared as described previously (Ottem et al., 2002) using reverse transcription-PCR with a forward primer sequence of 5′-GGAACTCACACCACAAAGC-3′ and a reverse primer sequence of 5′- CAGCAAGGGTTATGGTCACA-3′. The cDNA template for VGLUT1 (predominant form found in the hippocampus and cortex) was a 555 bp fragment corresponding to bases 24-768 of the rat VGLUT1 cDNA (GenBank accession number U07609). It was prepared using a forward primer sequence of 5′-ATAGGAACCGCAAAAGGCTG-3′ and reverse primer sequence of 5′-GGGGGATTGGCAGGGGAC-3′. Fragments were cloned into a TOPO-TA vector (Invitrogen, Carlsbad, CA) and sequenced to verify identity. The cDNA template for ERα was an 880 bp fragment subclone of the full-length clone (Koike et al., 1987) (provided by M. Muramatsu, University of Tokyo, Tokyo, Japan). We used standard in vitro transcription to prepare 35S-labeled cRNA probes using each of these cDNA templates (Ottem et al., 2002).
To maximize detection of GABAergic cells, we used a mixture of three digoxigenin-labeled cRNA probes for GAD mRNAs as described previously (Hays et al., 2002). The cDNA templates for GAD65 probes corresponded to bases 315-944 and 944-1769 of the full-length clone (Erlander et al., 1991), and the template for the GAD67 probe to bases 232-767 of the full-length clone (Erlander et al., 1991) (both provided by A. Tobin, University of California-Los Angeles, Los Angeles, CA). Digoxigenin-labeled probes for GAD65 and GAD67 mRNA were prepared and used in dual-label ISHH studies as described previously (Hays et al., 2002). Sections were thawed, fixed, and prehybridized before applying a mixture of 35S-labeled cRNA probes for VGLUT1, VGLUT2, or ERα (1 × 106 cpm), and 0.5 μl each of the digoxigenin-labeled GAD65 (0.5:l each of two probes) and GAD67 (0.5 μl) probes in 0.5 μl of hybridization buffer. To verify specificity, we hybridized representative sections to 35S-labeled sense strand probes in buffer with or without digoxigenin-labeled cRNA probes for GAD mRNAs.
Sections were hybridized at 52°C overnight under glass coverslips, washed, and processed for immunocytochemical (ICC) detection of digoxigenin-labeled probes for GAD mRNAs. We then used standard emulsion autoradiographical procedures (NTB3 emulsion; Eastman Kodak, Rochester, NY) to visualize radiolabeled probes for VGLUT1 or VGLUT2 mRNAs (5 d exposure for each) or ERα (7 d exposure).
To analyze dual-label ISHH studies, we used BioQuant Windows (R & M Biometrics, Nashville, TN) interfaced to a Leitz Laborlux microscope through a 3CCD color video camera (Hitachi Denshi America, Woodbury, NY). Four to six sections from the region of interest in each animal were analyzed to determine the mean number of neurons containing GAD mRNA, as well as the mean number containing VGLUT2 or ERα mRNA. We also determined the mean percentage of GAD mRNA-positive neurons that contained VGLUT2 or ERα mRNAs and the mean percentage of VGLUT2 or ERα mRNA-containing neurons that also contained GAD mRNA. Grand means for each group were obtained from means of individual animals. In studies comparing males and females, data were analyzed using unpaired two-tailed t tests.
ICC studies. We performed three ICC studies with laser confocal analysis. First, we examined whether GABAergic and glutamatergic vesicles were colocalized in the rPOA, a major projection field of ER-containing neurons in the AVPV (Simonian et al., 1999) and a region that contains abundant GnRH neurons (OVX rats; n = 5). Second, we tested whether rPOA terminals that contained both GABAergic and glutamatergic vesicles had synaptic specializations as indicated by the presence of synaptophysin (SYN) (Wiedenmann and Franke, 1985) (OVX rats; n = 5). Finally, we asked whether levels of GABAergic and glutamatergic vesicles in the dual-phenotype terminals contacting GnRH neurons changed during the day of LH surge release in response to E2. We examined OVX animals with and without E2 at 9:00 A.M. when LH release is suppressed, at 12:00 P.M. (around the so-called “critical period” just before LH surge begins), and at 3:30 P.M. during the LH surge in our animal model (Petersen and Barraclough, 1989).
For each study, animals were killed with an overdose of pentobarbital and perfused transcardially with 50 ml of 0.9% saline, followed by 200 ml of 4% (wt/vol) paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.2. Brains were postfixed in the same solution at 4°C for 1 hr and placed in a solution of 30% sucrose in 0.1 m PB at 4°C. Forty-micrometer serial cryosections were obtained through the rPOA region (+0.45 to -3.25 from bregma) (Swanson, 1998). Sections were stored in cryoprotectant (30% sucrose, 0.1% polyvinyl pyrolidone-40, in ethylene glycol, and 0.1 m PB) (Watson et al., 1986).
Free-floating sections were washed in phosphate-buffered saline (PBS), pH 7.4, then incubated in blocking buffer (10% normal donkey serum, 0.2% Triton X-100, and 0.02% sodium azide in PBS) at room temperature for 1 hr. To detect GABAergic vesicles, we used antibody to VGAT (McIntire et al., 1997), rabbit polyclonal anti-VGAT (1:1000; Chemicon, Temecula, CA). We incubated sections overnight in blocking buffer containing anti-VGAT, as well as guinea pig polyclonal anti-VGLUT2 (final dilution, 1:5000; Chemicon). To verify that these vesicles were synaptic structures, we also included mouse anti-SYN (1:5000; Chemicon) in the buffer. In studies to examine VGAT and VGLUT2 contacts on GnRH neurons, mouse monoclonal anti-GnRH (1:100; Acris GmbH, Hiddenhausen, Germany) was substituted for anti-SYN.
After incubation in primary antisera, sections were washed in PBS and incubated at room temperature for 1 hr in a mixture of secondary antibodies (diluted 1:150 in PBS containing 0.2% Triton X-100 and 0.02% sodium azide) obtained from Jackson ImmunoResearch (West Grove, PA). We detected immunoreactive (IR) VGAT with Cy5-conjugated donkey anti-rabbit IgG, IRVGLUT2 with Texas Red-conjugated donkey anti-guinea pig IgG, IRSYN with fluorescein (FITC)-conjugated donkey anti-rabbit IgG, and GnRH with FITC-conjugated donkey anti-mouse IgG. After incubation with secondary antibodies, sections were washed in PBS, mounted on glass slides, and allowed to air dry before applying coverslips with Vectashield Mounting Medium (Vector Laboratories, Burlingame, CA).
To verify specificity of primary antibodies, we used standard preabsorption protocols, as well as procedures to rule out cross-reactivity between primary and secondary antibodies (Smith et al., 2000). Our findings verified results of previous studies that have extensively characterized the specificity and reactivity of anti-VGLUT2 (Lin et al., 2003; Todd et al., 2003), anti-VGAT (McIntire et al., 1997; Holderith et al., 2003), and anti-SYN (Wiedenmann and Franke, 1985; Masliah et al., 2001).
ICC results were analyzed using a Zeiss LSM 510 META confocal microscope. For image scans, the pinhole diameter was optimized to 1.11 Airy disk, and image size was set at 512 × 512 pixels. All VGAT and VGLUT2 were found in punctate structures; therefore, confocal images were obtained using a high-power objective (63× oil immersion objective; 1.4 numerical aperture) and 2.5× digital zoom. To determine whether IRVGLUT2, IRVGAT, and IRSYN were colocalized, we performed scans at each wavelength independently to eliminate “bleed-through” between individual channels.
To determine whether IRVGAT- and IRVGLUT2-containing terminals contacted GnRH neurons, individual IRGnRH neurons were visualized at 40× and 63× magnification under epifluorescent (mercury vapor) illumination. Next, the illumination was switched to confocal mode (laser), and optical sectioning of a selected GnRH neuron was performed using a 63× oil immersion objective and 3× digital zoom. For each GnRH neuron, we obtained stacks of 20-35 optical sections (Z-thickness, 0.45 μm), depending on the orientation of the individual neuron. Each Z-series was composed of scans through three separate channels (488, 543, and 643 nm excitation). Images from single scans and compiled Z-series were captured and saved. Composite images, as well as individual images from each channel, were converted to TIFF format, saved, and subsequently analyzed using BioQuant Windows Image Analysis software described below. We also used Zeiss LSM 5 Image Browser software that allows virtual rotation of cells to verify that terminals actually contact neurons in multiple fields of view.
Based on previous work showing preferential localization of NMDA-type glutamate receptors in medial subppopulations of GnRH neurons, in each animal we examined 50 neurons in both the medial and lateral subdivisions as defined previously (Ottem et al., 2002). We determined the mean number of terminals that were in contact or closely apposed to each GnRH cell body and that contained IRVGAT only, IRVGLUT2 only, or both IRVGAT and IRVGLUT2. Grand means for each group were obtained and analyzed using two-way ANOVA to determine whether the number of terminals in contact with GnRH neurons changed because of E2 or time of day. Medial and lateral populations were analyzed separately, and interactions between main effects were probed with Bonferroni's t tests.
To determine whether the levels of VGLUT2- and VGAT-containing vesicles changed in contacts with both proteins, we measured the area of each reaction product in terminals contacting perikarya of medial GnRH neurons (n = 50/animal) in animals treated with E2 or oil and killed at 12:00 P.M. or 3:30 P.M. Using Bioquant Windows software, we set a threshold that digitally highlighted pixels corresponding to red signal from IRVGLUT2 or blue signal from IRVGAT and then determined the mean number of highlighted pixels corresponding to each color. Grand means of individual animals in a treatment group were analyzed using two-sample t tests to determine whether the mean level of IRVGAT or IRVGLUT2 in dual-phenotype terminals contacting medial GnRH changed between 12:00 P.M. and 3:30 P.M.
Results
AVPV GABA neurons in females have VGLUT2 mRNA
Results of dual-label ISHH studies showed that in the AVPV of females, most neurons that contained GAD mRNA (as well as VGAT mRNA; data not shown) also contained VGLUT2 mRNA (Fig. 1A). Likewise, virtually all VGLUT2 mRNA-containing neurons also contained GAD mRNA, indicating that few cells are solely GABAergic or solely glutamatergic in the AVPV of females. The absence of either signal in sense-strand control assays verified the specificity of the probes (data not shown). In contrast to the AVPV, the MPO (Fig. 2C) and PePO (data not shown) had only scattered neurons that expressed both GAD and VGLUT2 mRNAs (Fig. 2C). As expected in control studies, we found that VGLUT1 mRNA was found in cells separate from those containing GAD mRNA in the cortex (Fig. 1C) and hippocampus (Fig. 1D).
Figure 1.
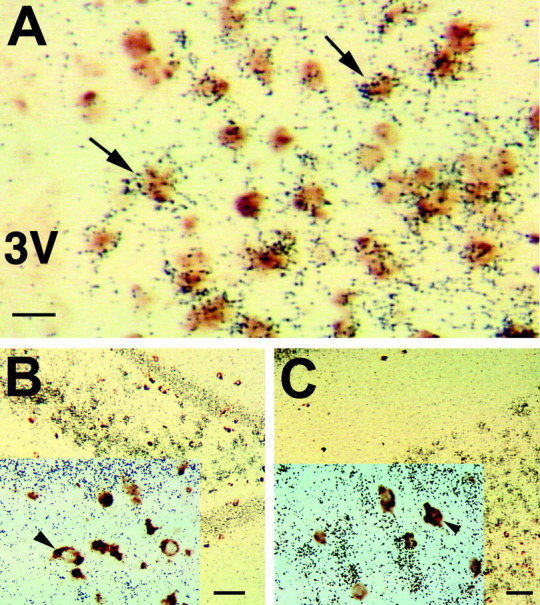
Most GABAergic neurons are also glutamatergic in the AVPV of female rats. A, VGLUT2 mRNA (marker of hypothalamic glutamate neurons detected with 35S-labeled cRNA probes; black grains) and GAD mRNA (marker of GABA neurons detected with digoxigenin-labeled cRNA probes; brown stain) are extensively colocalized in the AVPV nucleus (arrows indicate examples of colocalization). VGLUT1 (marker of extrahypothalamic glutamatergic neurons; black grains) and GAD (brown stain) mRNAs are not found in the same cells in either the CA3 region of the hippocampus (B) or in the sommatosensory cortex (C). Scale bars, 5 μm. 3V, Third ventricle.
Figure 2.
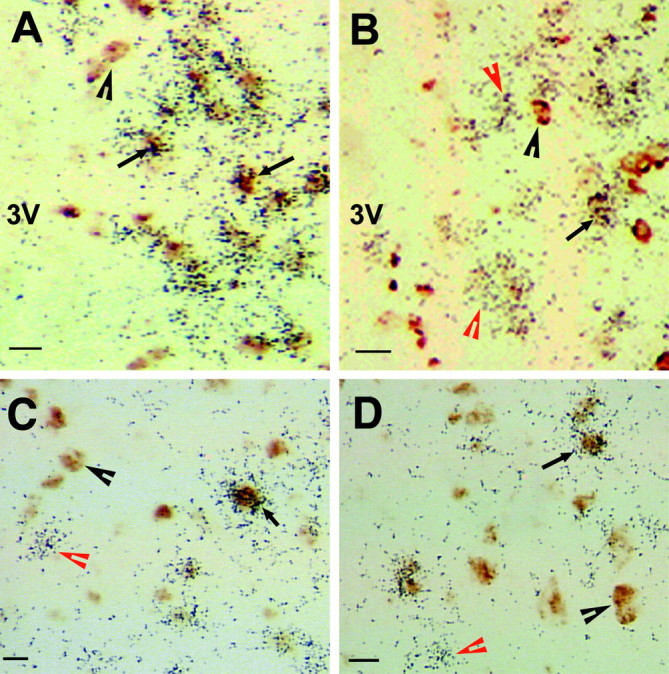
Sex differences in VGLUT2 and GAD mRNA colocalization. In females (A), but not males (B), most VGLUT2 mRNA (marker of glutamate neurons; black grains) is in GABAergic neurons (brown stain) in the AVPV. However, in the MPO of both females (C) and males (D), most neurons contain either VGLUT2 or GAD mRNA, not both. The black arrows indicate neurons positive for VGLUT2 and GAD mRNAs. The black arrowheads denote cells positive only for GAD mRNA, and the red arrowheads indicate cells positive only for VGLUT2 mRNA. Scale bars, 5 μm. 3V, Third ventricle.
Sex differences in dual-phenotype AVPV neurons
Photomicrographs of studies examining colocalization of GAD and VGLUT2 mRNAs in gonadectomized males and females are shown in Figure 2, A and B, and Table 1 summarizes the data. Most cells in the AVPV of females contained both GAD and VGLUT2 mRNAs, and females had 2.5 times as many of these dual-phenotype neurons as males. In addition, females had nearly twice as many GAD mRNA-positive cells and also more VGLUT2 mRNA-positive cells than males, consistent with previous evidence that the AVPV of females is significantly larger than that of males (Simerly, 1998). Although the AVPV of males had dual-phenotype neurons, there were more cells that contained only VGLUT2 mRNA or only GAD mRNA than cells that contained both. No such sex differences were seen in the MPO (Fig. 2C,D).
Table 1.
Sex differences in the number of GABA, glutamate, and dual-phenotype neurons in the AVPV (mean ± SEM)
|
|
GAD mRNA |
VGLUT2 mRNA |
Percentage of GAD-positive cells with VGLUT2 mRNA |
Percentage of VGLUT2-positive cells with GAD mRNA |
|---|---|---|---|---|
| Female | 676 ± 10.4 | 632 ± 13.9 | 95.5 ± 0.8 | 98.5 ± 0.3 |
| Male |
307 ± 8.0a
|
459 ± 24.5a
|
76.8 ± 1.0a
|
48.6 ± 1.8a
|
Significantly different from corresponding values in females (p<0.001).
Most ERα mRNA in the AVPV is in GAD mRNA-positive cells
ERα mRNA was present in 94.5 ± 0.8% of GAD mRNA-containing neurons of the AVPV, and 95.1 ± 0.7% of ERα-positive cells contained GAD mRNA (Fig. 3). Thus, in this brain region, nearly all GABAergic neurons contained ERα and nearly all ERα expression was in GABAergic neurons. This is a significantly higher percentage of ER-containing GABA cells than detected when the POA is considered as a whole (Flugge et al., 1985). This is consistent with our observation that relatively few GAD-containing neurons in the MPO or other regions of the POA contained ER (data not shown). These findings show that dual-phenotype GABA/glutamate neurons are the major targets of E2 in the AVPV of females and therefore are probably crucial for the signal that activates GnRH neurons and LH surge release.
Figure 3.
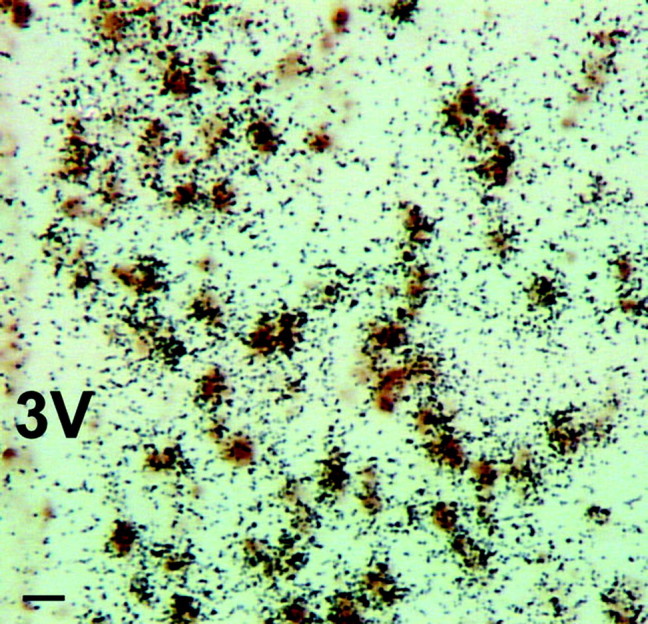
Virtually all ERα mRNA is found in GABAergic cells of female AVPV. A photomicrograph shows results of dual-label ISHH studies using 35S-labeled cRNA probes for ERα mRNA (black silver grains) and digoxigenin-labeled cRNA probes for GAD mRNAs (brown stain). Scale bar, 10 μm. 3V, Third ventricle.
VGLUT2 and VGAT in synaptic contacts on GnRH cells
The strongest ER-containing projections to GnRH neurons comes from the AVPV region (Simonian et al., 1999). In view of our finding that dual-phenotype GABA/glutamate neurons contain most, if not all, ERα expression in the AVPV, it seemed likely that dual-phenotype terminals from this nucleus comprise the projections. Consistent with this idea, we observed numerous examples of structures containing IRVGLUT2 and IRVGAT in the rPOA, where GnRH neurons reside (Fig. 4). All vesicular transporter immunoreactivity in these structures was colocalized with SYN and, as in a previous report, confined to typical punctate structures characteristic of synaptic vesicles (Lin et al., 2003) (Fig. 5).
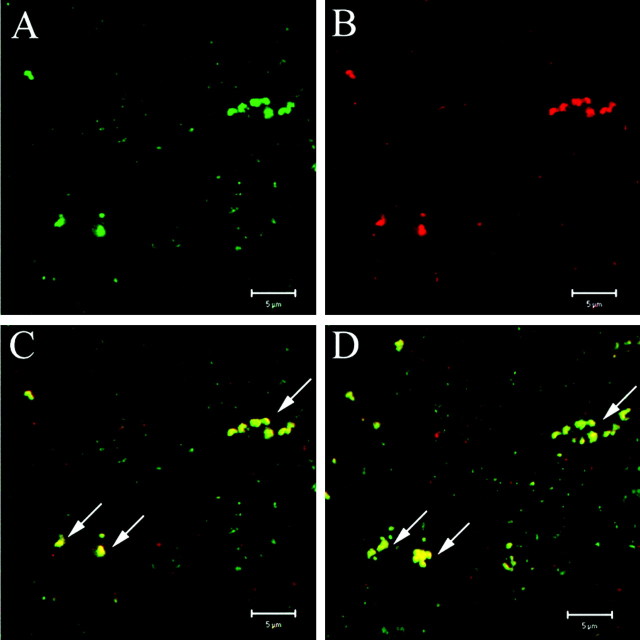
Figure 4.
Immunoreactivity for VGAT and VGLUT2 is colocalized in the rPOA projection site of AVPV neurons. A single laser confocal optical section shows clustering of IRVGAT (A) and IRVGLUT2 (B). Merged images show colocalization of VGAT and VGLUT2 signals (C). The projected image was obtained from 10 stacked 1 μm optical sections in the same field as images in A-C at 63× with 4× zoom. The arrows indicate colocalization of IRVGAT and IRVGLUT2.
Figure 5.
Structures positive for VGLUT2 and VGAT also contain the synaptic marker SYN. Single laser confocal optical sections of triple-label fluorescent ICC for VGLUT2 (A), SYN (B), and VGAT (C) in the rPOA are shown. In the overlay (D), virtually all IRVGAT (green) and IRVGLUT2 (red) overlaps with IRSYN (blue) in punctate, terminal-like structures (63× with 2× zoom). The arrows indicate IRVGLUT2, IRrSYN, and IRVGAT contained in triple-labeled structures.
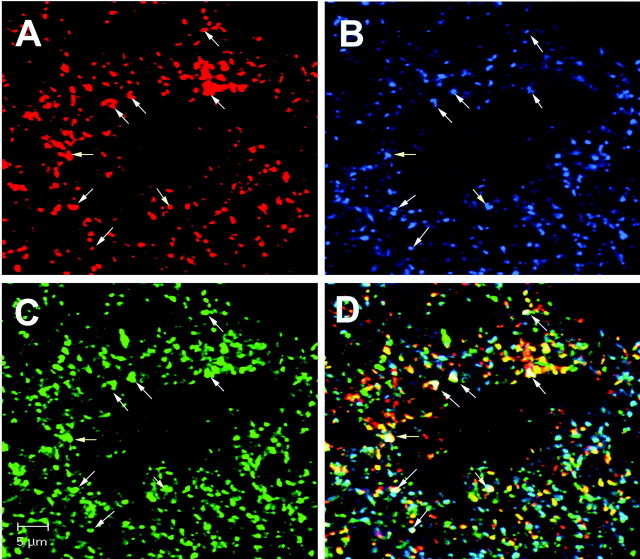
We observed contacts with both IRVGLUT2 and IRVGAT on all GnRH neurons in the medial subpopulation of neurons in tissues collected at 12:00 P.M. and 3:30 P.M., but only in E2-treated animals (Fig. 6). Only 18.4 ± 1.0% of GnRH neurons in lateral subgroups had dual-phenotype contacts. This is an important finding because previous work demonstrates that most medial, but not lateral, subpopulations of GnRH neurons contain NMDA-type glutamate receptors (Ottem et al., 2002), the receptor subtype primarily involved in LH surge release (Brann and Mahesh, 1991). Moreover, we previously showed that a majority of GnRH neurons express the β-3 GABAA receptor subunit (Petersen et al., 1993), with all medial neurons positive for the subunit (S. McCrone and S. Petersen, unpublished observations).
Figure 6.
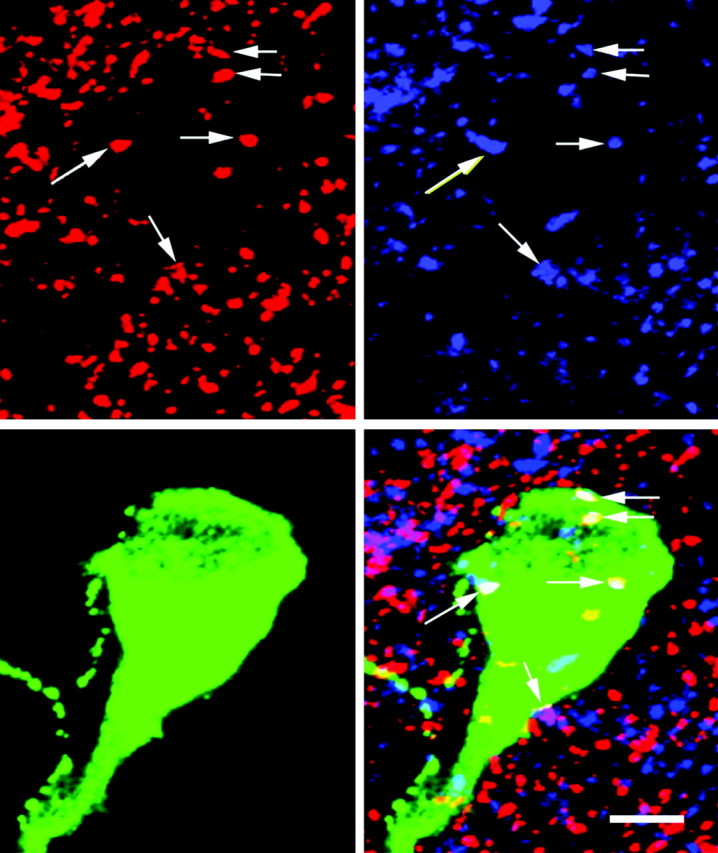
Dual-phenotype GABAergic/glutamatergic terminals contact medial GnRH neurons. A compilation of 30 optical sections from a Z-series (Z-thickness, 0.45 μm) from an OVX, E2-treated rat killed at 12:00 P.M. shows triple-label ICC for VGLUT2 (A), VGAT (B), and GnRH (C). The overlay (D) shows terminal-like structures containing both IRVGAT and IRVGLUT2 in contact with a GnRH neuron. The white arrows indicate terminals with both IRVGAT and IRVGLUT2. Scale bar, 5 μm.
E2-induced changes in IRVGAT and IRVGLUT2 levels
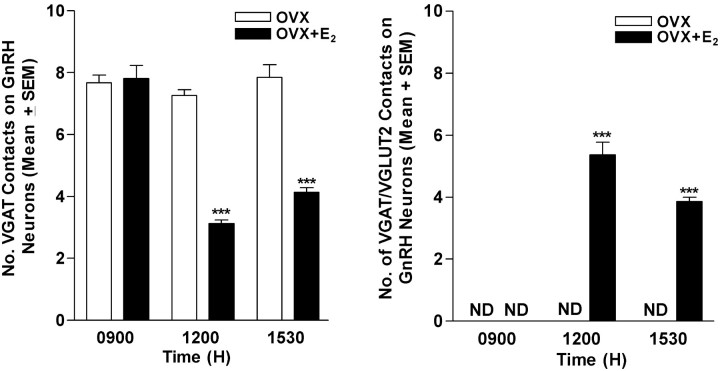
In medial, but not lateral, subpopulations of GnRH neurons, the mean number of dual-phenotype terminals increased between morning and afternoon in OVX E2-treated animals (Fig. 7A). There were no dual-phenotype contacts at any time in OVX animals, and the number of IRVGAT only or IRVGLUT2 only did not change during the day in these animals.
Figure 7.
Effects of E2 on temporal changes in the number of dual-phenotype IRVGAT/IRVGLUT2 or IRVGAT contacts on medial subpopulations of GnRH neurons. Right, Dual-phenotype terminals were observed in OVX rats that received E2 (n = 5/group), but not in those that received vehicle (n = 5/group). Dual-phenotype terminals were seen only in animals examined in the afternoon. Left, The number of terminals containing only IRVGAT declined during the period in which LH surge begins in our animal model. ND, Nondetectable. ***Significantly greater than observed at 9:00 A.M. or in OVX animals (p < 0.001).
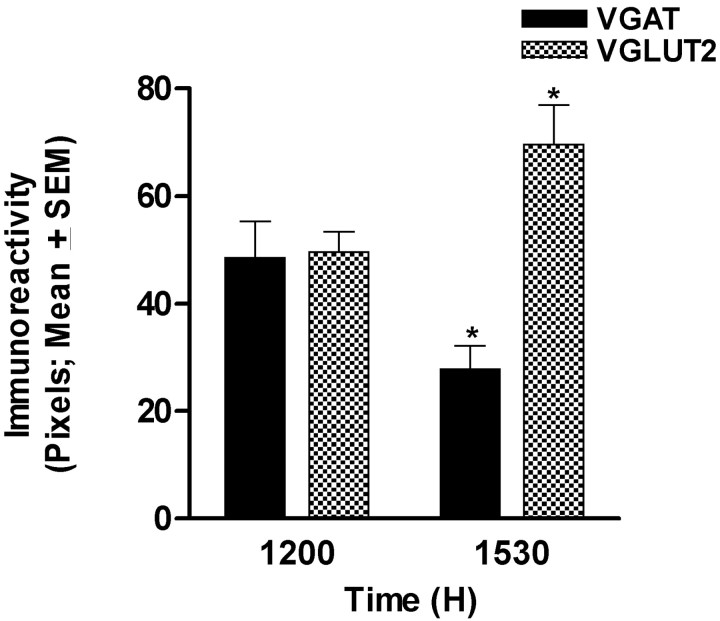
Overall, neither the number of IRVGLUT2-containing contacts nor the total number of contacts changed during the day (data not shown). However, the number of contacts with IRVGAT alone was lower at 12:00 P.M. and 3:30 P.M. than during the morning (Fig. 7B). We reasoned that these data could be explained if IRVGAT declined in terminals and IRVGLUT2 reached detectable levels, thus causing the appearance of more dual-labeled structures. To test this idea, we examined levels of IRVGAT and IRVGLUT2 reaction product in dual-labeled terminals. Consistent with our hypothesis, IRVGLUT2 increased significantly, whereas levels of IRVGAT decreased between 12:00 P.M. and 3:30 P.M. in E2-treated, but not oil-treated, animals (Fig. 8). These findings support the idea that dual-phenotype terminals on medial GnRH neurons are responsible for the reciprocal changes in GABA and glutamate that are necessary for LH surge release.
Figure 8.
Reciprocal changes in levels of IRVGAT and IRVGLUT2 in dual-phenotype terminals contacting medial GnRH neurons in OVX, E2-treated animals (no such terminals are seen in OVX animals treated with oil or in animals examined during the morning hours; see Fig. 7). At the time of the LH surge (3:30 P.M. in our animal model), IRVGAT decreases and IRVGLUT2 increases in dual-phenotype terminals. *Significantly different from corresponding values at 12:00 P.M.; p < 0.05.
Discussion
Our results provide compelling evidence that in the AVPV of female rats, GABA and glutamate are released from the same neurons. We also made the surprising discovery that this unique population of neurons is sexually dimorphic and the major target of E2 in the AVPV. Finally, we showed that during the afternoon, IRVGAT declines and IRVGLUT2 rises in dual-phenotype terminals contacting GnRH neurons, but only in animals treated with E2. The timing of these changes is similar to previously described daily photoperiodic signals necessary for the LH surge (Chappell et al., 2000). Moreover, the direction of the changes is consistent with demonstrated roles of these neurotransmitters in the induction of the surge (Petersen et al., 2003). Together, our findings indicate that dual-phenotype GABA/glutamate neurons of the AVPV integrate hormonal and photoperiodic signals necessary for LH surge release and ovulation.
GABA/glutamate neurons in the female AVPV were densely packed and appeared to be the major neural phenotype of the AVPV. In contrast, few such dual-phenotype neurons were found in the adjacent MPO region, and none was detected in either the hippocampus or cortex. Therefore, it seems likely that these neurons are important for a function unique to the AVPV, namely the induction of LH surge release. In support of this idea, we found that most ERα gene expression in the AVPV was in GABA/glutamate neurons. Thus, these neurons are likely the targets through which AVPV anti-estrogen microimplants block both E2-dependent LH surge release and changes in GnRH gene expression (Petersen and Barraclough, 1989; Petersen et al., 1989).
We found that the ER-containing GABA/glutamate neurons were 2.5 times more prevalent in the AVPV of females than of males. This is significant because E2 elicits LH surge release only in females (Gorski, 1979). It is notable that a previous study did not detect any dual-function neurons in the hypothalamus or POA of males (Ziegler et al., 2002), but the method they used was different than the one used in the present studies. Regardless, it is clear from the present data that the dual-phenotype GABA/glutamate neurons are sexually dimorphic populations and likely to be important for female-specific LH surge release.
Other evidence that dual-phenotype neurons play a role in the LH surge comes from our findings that in the projection fields of these neurons, terminals containing both IRVGAT and IRVGLUT2 contacted GnRH neurons. These contacts were observed only in E2-treated animals and only during the afternoon at the onset of the LH surge in this model. Moreover, dual-phenotype contacts were found on all medial GnRH neurons examined, but on few lateral GnRH neurons. This is a key finding because in rats the majority of medial, but not lateral, GnRH neurons contains functional NMDA receptors (Ottem et al., 2002), a glutamate receptor subtype that regulates GnRH synthesis (Petersen et al., 1991; Liaw and Barraclough, 1993; Gore and Roberts, 1994), and surge release (Brann and Mahesh, 1995). All midline GnRH neurons also contain GABAA receptors (Petersen et al., 1993; Petersen, unpublished) and GABAB receptors (Petersen et al., 2003). Consistent with these findings, midline green fluorescent protein-expressing GnRH neurons exhibit altered firing patterns when glutamate and GABA receptors are blocked, whereas lateral GnRH neurons do not (Nunemaker et al., 2002). Interestingly, it is also the medial population of GnRH neurons that is initially activated on the day of LH surge release (Hiatt et al., 1992; Rubin et al., 1995). These findings indicate that dual-phenotype GABA/glutamate neurons are important for GnRH regulation.
In addition to this neuroanatomical evidence for a role of GABA/glutamate terminals in LH surge release, we found physiologically relevant changes in dual-phenotype terminals. During the period preceding LH surge release in E2-treated animals, IRVGAT decreased and IRVGLUT2 increased in dual-phenotype terminals on medial GnRH neurons. These fluctuations occurred around the time GABA release decreases and glutamate release into the POA increases in this animal model (Jarry et al., 1995). Such changes appear to be necessary for the LH surge because GABA generally inhibits (Herbison, 1998; Han et al., 2004) and glutamate stimulates (Brann and Mahesh, 1995; Spergel et al., 1999; Kuehl-Kovarik et al., 2002) GnRH activity in adult animals. This interpretation is in line with evidence that GABA signaling must be inhibited in order for stimulatory signals to trigger LH surge release (Hartman et al., 1990).
Although we did not show directly that the changes we observed in IRVGAT and IRVGLUT2 are in terminals of AVPV GABA/glutamate neurons, there is reason to presume that this is the case. First, our present work identifies AVPV GABA/glutamate neurons as the E2-sensitive cells that send strong projections to GnRH neurons (Simonian et al., 1999). Similarly, AVPV GABA/glutamate neurons are most likely the ER-containing targets to which the SCN afferents project (de la Iglesia et al., 1995; Watson et al., 1995) and therefore the neurons that receive the daily signal necessary for the LH surge (Legan and Karsch, 1975). This interpretation is supported by recent work demonstrating that a daily rise in cAMP levels occurs in AVPV neurons (Chappell et al., 2000). Importantly, this rise is necessary for the LH surge, and it coincides with a rise in GAD67 gene expression in neurons found specifically in the AVPV (Curran-Rauhut and Petersen, 2002). These changes also coincide with the timing of E2-dependent changes in IRVGAT and IRVGLUT2 we observed, as well as in GABA and glutamate release into the POA observed by others using this animal model (Jarry et al., 1995). This body of work provides convincing evidence that AVPV GABA/glutamate neurons communicate hormonal and photoperiodic information to GnRH neurons and that this information is encoded as a change in the balance between GABA and glutamate release from dual-phenotype terminals.
Without electrophysiological evidence, which would be difficult to obtain in this system, we cannot unambiguously show that VGAT- and VGLUT2-containing vesicles in dual-phenotype terminals are released. However, there is strong indirect evidence to support such a notion. First, the number of synaptic contacts between GnRH neurons and IRVGAT and IRVGLUT2 terminals is similar to the total number of synapses found on GnRH neurons (Witkin et al., 1995). Considering that GABA and glutamate provide most of the input to GnRH neurons (Herbison, 2003), it seems likely that they are released from the VGAT- and VGLUT2-containing vesicles we observed in contact with GnRH neurons. Second, the decline in IRVGAT before the onset of LH surge parallels the E2-dependent suppression of GABA release into the POA (Jarry et al., 1992, 1995), as well as the decline in GAD67 gene expression in AVPV neurons (Curran-Rauhut and Petersen, 2002). Notably, work examining other brain regions shows that VGAT and GAD67 mRNA levels are correlated with GABA release (Litwak et al., 1990; Drengler and Oltmans, 1993; Falkenberg et al., 1997; Lamas et al., 2001; Ramirez and Gutierrez, 2001; Gutierrez, 2002; Kang et al., 2003). No similar correlates of glutamate release are available; however, it has been suggested that in other brain regions, VGLUT2-containing vesicles are found in terminals that have a high probability of release (Fremeau et al., 2004). Together, these data support the idea that reciprocal changes in IRVGAT and IRVGLUT2 in terminals contacting GnRH neurons are responsible for corresponding changes in GABA and glutamate release required for LH surge release.
The present work identified a population of dual-phenotype GABA/glutamate neurons in the AVPV. Previously, the main evidence for such neurons came from work showing that glutamatergic granule cells of the dentate gyrus contain low levels of GABA (Sandler and Smith, 1991; Bergersen et al., 2003), as well as GAD67 (Sloviter et al., 1996). Seizure activity upregulates these levels (Schwarzer and Sperk, 1995; Lehmann et al., 1996) and triggers simultaneous release of GABA and glutamate (Gutierrez, 2000; Gutierrez and Heinemann, 2001). Importantly, GAD67, but not GAD65, gene expression in these neurons is also upregulated in an activity-dependent manner in the absence of seizures (for review, see Gutierrez, 2003). This finding is similar to our previous observation that changes in GAD67 mRNA levels in AVPV GABA/gluamate neurons also reflect changes in GABA release, whereas levels of GAD65 mRNA do not (Curran-Rauhut and Petersen, 2002). Likewise, changes in levels of VGAT mRNA are linked to GABA release in granule cells (Lamas et al., 2001), and we found that IRVGAT in AVPV projection fields decreased at the time GABA release into the region declines (Jarry et al., 1995). However, one difference is that we found relatively high expression of IRVGAT in terminals, as well as high VGAT mRNA (data not shown) in cell bodies of dual-phenotype GABA/glutamate neurons in AVPV neurons. In contrast, IRVGAT is undetectable (Chaudhry et al., 1998; Sperk et al., 2003) in granule cells, and VGAT mRNA is present only at low levels in adults (Lamas et al., 2001). Regardless of these differences, work from granule cells and our present findings from AVPV neurons provide compelling evidence that dual-phenotype GABA/glutamate neurons are not artifacts, but rather serve important physiological functions.
Based on our findings, we propose that the physiological function of AVPV GABA/glutamate neurons is to integrate hormonal and environmental signals and communicate them to GnRH neurons. Specifically, we propose that in the presence of E2, a photoperiodic cue to dual-phenotype neurons inhibits GABA release while stimulating glutamate release. These changes allow other neurotransmitters and neuropeptides to elicit GnRH hypersecretion and the preovulatory surge of LH release. The intracellular mechanisms regulating the switch from GABA to glutamate release remain to be determined; however, the fact that glutamate is converted by GAD to GABA suggests one possibility. In prepubertal monkeys, central administration of GAD67 anti-sense oligonucleotides suppresses GABA and then stimulates glutamate release (Terasawa et al., 1999). In our E2-treated OVX rat model, GAD67 gene expression in the AVPV declines (Curran-Rauhut and Petersen, 2002) during the same period VGAT decreased and VGLUT2 increased in the present study. These changes also coincide with a decline in GABA and rise in glutamate release into the region (Jarry et al., 1995). Therefore, it is possible that in the presence of E2, the daily signal to AVPV neurons inhibits GAD67 synthesis, thereby decreasing utilization of glutamate for GABA synthesis and providing more glutamate for release.
Our findings relate directly to the mechanisms through which steroids regulate the neural control of ovulation. However, considering that the balance between GABA and glutamate release regulates myriad functions, it will be important to determine whether dual-phenotype GABA/glutamate neurons are present in other brain regions in which they might regulate sexually dimorphic neural functions and susceptibility to disease.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant HD27305 to S.L.P. and NIH Training Grant MH20051 fellowship to E.N.O. We thank Princy Quadros, Mary Packard, and James Ashley as well as Dr. Clifford D. Carpenter, Vivian Budnik, and Michael Gorczyca for assistance. We also thank Drs. Nancy Forger, Deborah Good, and Tom Zoeller for helpful comments on previous versions of this manuscript.
Correspondence should be addressed to Dr. Sandra L. Petersen, Department of Biology, 611 North Pleasant Street, University of Massachusetts-Amherst, Amherst, MA 01003. E-mail: sandyp@bio.umass.edu.
E. N. Ottem's present address: Department of Biology, Neuroscience Program, Michigan State University, 108 Giltner Hall, East Lansing, MI 48824.
J. G. Godwin's present address: Harvard Gene Therapy Institute, Harvard Medical School, 77 Avenue Louis Pasteur, Boston, MA 02115.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248097-09$15.00/0
References
- Bergersen L, Ruiz A, Bjaalie JG, Kullmann DM, Gundersen V (2003) GABA and GABAA receptors at hippocampal mossy fibre synapses. Eur J Neurosci 18: 931-941. [DOI] [PubMed] [Google Scholar]
- Bhat GK, Mahesh VB, Ping L, Chorich L, Wiedmeier VT, Brann DW (1998) Opioid-glutamate-nitric oxide connection in the regulation of luteinizing hormone secretion in the rat. Endocrinology 139: 955-960. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB (1991) Endogenous excitatory amino acid involvement in the preovulatory and steroid-induced surge of gonadotropins in the female rat. Endocrinology 128: 1541-1547. [DOI] [PubMed] [Google Scholar]
- Brann DW, Mahesh VB (1995) Glutamate: a major neuroendocrine excitatory signal mediating steroid effects on gonadotropin secretion. J Steroid Biochem Mol Biol 53: 325-329. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Lee J, Levine JE (2000) Stimulation of gonadotropin-releasing hormone surges by estrogen. II. Role of cyclic adenosine 3′5′-monophosphate. Endocrinology 141: 1486-1492. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J (1998) The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci 18: 9733-9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran-Rauhut MA, Petersen SL (2002) Regulation of glutamic acid decarboxylase 65 and 67 gene expression by ovarian steroids: identification of two functionally distinct populations of GABA neurons in the preoptic area. J Neuroendocrinol 14: 310-317. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL (1995) The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. NeuroReport 6: 1715-1722. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM (2002) Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16: 2872-2891. [DOI] [PubMed] [Google Scholar]
- Donoso AO, Seltzer AM, Navarro CE, Cabrera RJ, Lopez FJ, Negro-Vilar A (1994) Regulation of luteinizing hormone-releasing hormone and luteinizing hormone secretion by hypothalamic amino acids. Braz J Med Biol Res 27: 921-932. [PubMed] [Google Scholar]
- Drengler SM, Oltmans GA (1993) Rapid increases in cerebellar Purkinje cell glutamic acid decarboxylase (GAD67) mRNA after lesion-induced increases in cell firing. Brain Res 615: 175-179. [DOI] [PubMed] [Google Scholar]
- Erlander MG, Tillakarantne NJK, Feldblum S, Patel N, Tobin A (1991) Two genes encode distinct glutamate decarboxylases. Neuron 7: 91-100. [DOI] [PubMed] [Google Scholar]
- Eyigor O, Lin W, Jennes L (2004) Identification of neurones in the female rat hypothalamus that express oestrogen receptor-alpha and vesicular glutamate transporter-2. J Neuroendocrinol 16: 26-31. [DOI] [PubMed] [Google Scholar]
- Falkenberg T, Lindefors N, O'Connor WT, Zachrisson O, Camilli F, Ungerstedt U (1997) GABA release and GAD67 mRNA expression in rat hippocampus following entorhinal cortex activation. Brain Res Mol Brain Res 48: 413-416. [DOI] [PubMed] [Google Scholar]
- Flugge G, Oertel WH, Wuttke W (1985) Evidence for estrogen-receptive GABAergic neurones in the preoptic/anterior hypothalamic area of the rat brain. Neuroendocrinology 43: 1-5. [DOI] [PubMed] [Google Scholar]
- Fremeau Jr RT, Voglmaier S, Seal RP, Edwards RH (2004) VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci 27: 98-103. [DOI] [PubMed] [Google Scholar]
- Gore AC (2001) Gonadotropin-releasing hormone neurons, NMDA receptors, and their regulation by steroid hormones across the reproductive life cycle. Brain Res Brain Res Rev 37: 235-248. [DOI] [PubMed] [Google Scholar]
- Gore AC, Roberts JL (1994) Regulation of gonadotropin-releasing hormone gene expression by the excitatory amino acids kainic acid and N-methyl-d,l-aspartate in the male rat. Endocrinology 134: 2026-2031. [DOI] [PubMed] [Google Scholar]
- Gorski RA (1979) The neuroendocrinology of reproduction: an overview. Biol Reprod 20: 111-127. [DOI] [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD (2001) Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology 142: 5172-5181. [DOI] [PubMed] [Google Scholar]
- Gutierrez R (2000) Seizures induce simultaneous GABAergic and glutamatergic transmission in the dentate gyrus-CA3 system. J Neurophysiol 84: 3088-3090. [DOI] [PubMed] [Google Scholar]
- Gutierrez R (2002) Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibers in vitro. J Neurophysiol 87: 2562-2570. [DOI] [PubMed] [Google Scholar]
- Gutierrez R (2003) The GABAergic phenotype of the “glutamatergic” granule cells of the dentate gyrus. Prog Neurobiol 71: 337-358. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Heinemann U (2001) Kindling induces transient fast inhibition in the dentate gyrus-CA3 projection. Eur J Neurosci 13: 1371-1379. [DOI] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE (2002) Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143: 1459-1466. [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE (2004) Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145: 495-499. [DOI] [PubMed] [Google Scholar]
- Hartman RD, He J-R, Barraclough CA (1990) γ-Aminobutyric acid-A and -B receptor antagonists increase luteinizing hormone-releasing hormone neuronal responsiveness to intracerebroventricular norepinephrine in ovariectomized estrogen-treated rats. Endocrinology 127: 1336-1345. [DOI] [PubMed] [Google Scholar]
- Hays LE, Carpenter CD, Petersen SL (2002) Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ Health Perspect 110: 369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE (1998) Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19: 302-330. [DOI] [PubMed] [Google Scholar]
- Herbison AE (2003) GnRH neuron. In: Encyclopedia of hormones (Henry HL, Norman AW, eds), pp 171-177. San Diego: Academic.
- Herbison AE, Dyer RG (1991) Effect on luteinizing hormone secretion of GABA receptor modulation in the medial preoptic area at the time of proestrous luteinizing hormone surge. Neuroendocrinology 53: 317-320. [DOI] [PubMed] [Google Scholar]
- Hiatt ES, Brunetta PG, Seiler GR, Barney SA, Selles WD, Wooledge KH, King JC (1992) Subgroups of luteinizing hormone-releasing hormone perikarya defined by computer analyses in the basal forebrain of intact female rats. Endocrinology 130: 1030-1043. [DOI] [PubMed] [Google Scholar]
- Hisano S (2003) Vesicular glutamate transporters in the brain. Anat Sci Int 78: 191-204. [DOI] [PubMed] [Google Scholar]
- Holderith NB, Shigemoto R, Nusser Z (2003) Cell type-dependent expression of HCN1 in the main olfactory bulb. Eur J Neurosci 18: 344-354. [DOI] [PubMed] [Google Scholar]
- Jarry H, Hirsch B, Leonhardt S, Wuttke W (1992) Amino acid neurotransmitter release in the preoptic area of rats during the positive feedback actions of estradiol on LH release. Neuroendocrinology 56: 133-140. [DOI] [PubMed] [Google Scholar]
- Jarry H, Leonhardt S, Schwarze T, Wuttke W (1995) Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology 62: 479-486. [DOI] [PubMed] [Google Scholar]
- Kang TC, An SJ, Park SK, Hwang IK, Bae JC, Won MH (2003) Changed vesicular GABA transporter immunoreactivity in the gerbil hippocampus following spontaneous seizure and vigabatrin administration. Neurosci Lett 335: 207-211. [DOI] [PubMed] [Google Scholar]
- Kiss J, Kocsis K, Csaki A, Halasz B (2003) Evidence for vesicular glutamate transporter synapses onto gonadotropin-releasing hormone and other neurons in the rat medial preoptic area. Eur J Neurosci 18: 3267-3278. [DOI] [PubMed] [Google Scholar]
- Koike S, Sakai M, Muramatsu M (1987) Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res 15: 2499-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM (2002) Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci 22: 2313-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Gomez-Lira G, Gutierrez R (2001) Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fiber synaptosomes. Brain Res Mol Brain Res 93: 209-214. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ (1975) A daily signal for the LH surge in the rat. Endocrinology 96: 57-62. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Ebert U, Loscher W (1996) Immunocytochemical localization of GABA immunoreactivity in dentate granule cells of normal and kindled rats. Neurosci Lett 212: 41-44. [DOI] [PubMed] [Google Scholar]
- Levine JE, Bauer-Dantoin AC, Besecke LM, Conaghan LA, Legan SJ, Meredith JM, Strobl FJ, Urban JH, Vogelsong KM, Wolfe AM (1991) Neuroendocrine regulation of the luteinizing hormone-releasing hormone pulse generator in the rat. Recent Prog Horm Res 47: 97-153. [DOI] [PubMed] [Google Scholar]
- Liaw JJ, Barraclough CA (1993) N-methyl-d,l-aspartic acid differentially affects LH release and LHRH mRNA levels in estrogen-treated ovariectomized control and androgen-sterilized rats. Brain Res Mol Brain Res 17: 112-118. [DOI] [PubMed] [Google Scholar]
- Lin W, McKinney K, Liu L, Lakhlani S, Jennes L (2003) Distribution of vesicular glutamate transporter-2 messenger ribonucleic Acid and protein in the septum-hypothalamus of the rat. Endocrinology 144: 662-670. [DOI] [PubMed] [Google Scholar]
- Litwak J, Mercugliano M, Chesselet MF, Oltmans GA (1990) Increased glutamic acid decarboxylase (GAD) mRNA and GAD activity in cerebellar Purkinje cells following lesion-induced increases in cell firing. Neurosci Lett 116: 179-183. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L (2001) Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci USA 98: 12245-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM (1997) Identification and characterization of the vesicular GABA transporter. Nature 389: 870-876. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Shwe TT, Funabashi T, Shinohara K, Kimura F (2002) GABA release in the medial preoptic area of cyclic female rats. Neuroscience 113: 109-114. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM (2002) Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143: 2284-2292. [DOI] [PubMed] [Google Scholar]
- Ondo JG (1974) Gamma-aminobutyric acid effects on pituitary gonadotropin secretion. Science 186: 738-739. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Petersen SL (2002) Glutamatergic signaling through the N-methyl-d-aspartate receptor directly activates medial subpopulations of luteinizing hormone-releasing hormone (LHRH) neurons, but does not appear to mediate the effects of estradiol on LHRH gene expression. Endocrinology 143: 4837-4845. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA (1989) Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogen into the preoptic brain. Brain Res 484: 279-289. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Cheuk C, Hartman RD, Barraclough CA (1989) Medial preoptic microimplants of the antiestrogen, Keoxifene, affect luteinizing hormone-releasing hormone mRNA levels, median eminence luteinizing hormone-releasing hormone concentrations and luteinizing hormone release in ovariectomized, estrogen-treated rats. J Neuroendocrinol 1: 279-289. [DOI] [PubMed] [Google Scholar]
- Petersen SL, McCrone S, Keller M, Gardner E (1991) Rapid increase in LHRH mRNA levels following NMDA. Endocrinology 129: 1679-1681. [DOI] [PubMed] [Google Scholar]
- Petersen SL, McCrone S, Coy D, Adelman JP, Mahan LC (1993) GABAA receptor subunit mRNAs in cells of the preoptic area: colocalization with LHRH mRNA using dual-label in situ hybridization histochemistry. Endocrine J 1: 29-34. [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD (2003) Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod 69: 1771-1778. [DOI] [PubMed] [Google Scholar]
- Ramirez M, Gutierrez R (2001) Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res 917: 139-146. [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ (1986) Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 43: 564-576. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Mitchell S, Lee CE, King JC (1995) Reconstructions of populations of luteinizing hormone releasing hormone neurons in young and middle-aged rats reveal progressive increases in subgroups expressing Fos protein on proestrus and age-related deficits. Endocrinology 136: 3823-3830. [DOI] [PubMed] [Google Scholar]
- Sandler R, Smith AD (1991) Coexistence of GABA and glutamate in mossy fiber terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol 303: 177-192. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G (1995) Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience 69: 705-709. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I (1997) Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 507-25 388: 507-525. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I (1998) Evidence for the colocalization of estrogen receptor-beta mRNA and estrogen receptor-alpha immunoreactivity in neurons of the rat forebrain. Endocrinology 139: 5267-5270. [DOI] [PubMed] [Google Scholar]
- Simerly RB (1998) Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 92: 195-203. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294: 76-95. [DOI] [PubMed] [Google Scholar]
- Simonian SX, Spratt DP, Herbison AE (1999) Identification and characterization of estrogen receptor alpha-containing neurons projecting to the vicinity of the gonadotropin-releasing hormone perikarya in the rostral preoptic area of the rat. J Comp Neurol 411: 346-358. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL (1996) Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol 373: 593-618. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Jiennes L, Wise PM (2000) Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology 141: 4317-4320. [DOI] [PubMed] [Google Scholar]
- Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH (1999) GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19: 2037-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Heilman J, Furtinger S, Reimer RJ, Edwards RH, Nelson N (2003) Expression of plasma membrane GABA transporters but not of the vesicular GABA transporter in dentate granule cells after kainic acid seizures. Hippocampus 13: 806-815. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1998) Brain maps: structure of the rat brain, Ed 2. Amsterdam: Elsevier.
- Terasawa E, Luchansky LL, Kasuya E, Nyberg CL (1999) An increase in glutamate release follows a decrease in gamma aminobutyric acid and the pubertal increase in luteinizing hormone releasing hormone release in the female rhesus monkeys. J Neuroendocrinol 11: 275-282. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ (2003) The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci 17: 13-27. [DOI] [PubMed] [Google Scholar]
- Watson Jr RE, Wiegand SJ, Clough RW, Hoffman GE (1986) Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155-159. [DOI] [PubMed] [Google Scholar]
- Watson Jr RE, Langub Jr MC, Engle MG, Maley BE (1995) Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Res 689: 254-264. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW (1985) Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell 41: 1017-1028. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E (1982) Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34: 395-404. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW (1980) Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31: 147-157. [DOI] [PubMed] [Google Scholar]
- Witkin JW, O'Sullivan H, Silverman AJ (1995) Novel associations among gonadotropin-releasing hormone neurons. Endocrinology 136: 4323-4330. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shughrue PJ, Dorsa DM (1995) Estrogen receptor protein is differentially regulated in the preoptic area of the brain and in the uterus during the rat estrous cycle. Neuroendocrinology 61: 276-283. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP (2002) Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol 448: 217-229. [DOI] [PubMed] [Google Scholar]