Abstract
The establishment of topographic projections in the developing visual system depends on the spatially and temporally controlled expression of axon guidance molecules. In the developing chick tectum, the graded expression of the repulsive guidance molecule (RGM) has been proposed to be involved in controlling the topography of the retinal ganglion cell (RGC) axon termination zones along the anteroposterior axis of the tectum. We now show that there are three mouse proteins homologous to chick RGM displaying similar proteolytic processing but exhibiting differential cell-surface targeting by glycosyl phosphatidylinositol anchor addition. Two members of this gene family (mRGMa and mRGMb) are expressed in complementary patterns in the nervous system, and mRGMa is expressed prominently in the superior colliculus at the time of anteroposterior targeting of RGC axons. The third member of the family (mRGMc) is expressed almost exclusively in skeletal muscles. Functional studies in the mouse reveal a role for mRGMa in controlling cephalic neural tube closure, thus defining an unexpected role for mRGMa in early embryonic development. In contrast, mRGMa mutant mice did not exhibit defects in anteroposterior targeting of RGC axons to their stereotypic termination zones in the superior colliculus.
Keywords: RGM, retinal topography, exencephaly, neural tube closure, GPI, axon guidance
Introduction
The precise temporal and spatial interplay of different extracellular proteins is essential for the establishment of correct morphology and patterning of the nervous system at early developmental stages as well as for the assembly of neuronal circuits later in development. Many extracellular proteins have been studied for their role in controlling axon outgrowth to target regions in an attempt to address the question of how axonal projections of different neuronal populations achieve precise targeting to the region they innervate in the mature nervous system. These studies have led to the concept that extracellular guidance molecules can act in both attractive and repulsive manners (Tessier-Lavigne and Goodman, 1996) and much is known about the mechanisms by which these molecules direct axons toward their targets (Yu and Bargmann, 2001; Dickson, 2002).
One system in which the underlying molecular mechanisms controlling the development of precise axonal projections has been studied extensively is the projection of retinal ganglion cells (RGCs) to either the chick tectum or the rodent superior colliculus (Sperry, 1963; McLaughlin et al., 2003). The anatomical arrangement of retinocollicular projections during rodent development and in the mature system has been well defined (Simon and O'Leary, 1992). In the mature retinocollicular system there is a precise topography of projections from the retina to the superior colliculus: Temporal RGC axons consistently terminate in the anterior superior colliculus, whereas nasal RGC axons project to the posterior superior colliculus (Sperry, 1963; McLaughlin et al., 2003). This observation has led to the proposal that RGC axons terminating in the anterior superior colliculus may be repelled by molecular cues found at a higher concentration in the posterior relative to the anterior superior colliculus (Sperry, 1963). Consistent with this model, temporal RGCs are repelled by membranes isolated from the posterior chick tectum and grow preferentially on anterior tectal membranes (Walter et al., 1987). The isolation of molecules expressed in a low anterior to high posterior gradient in the chick tectum led to the discovery that members of the Ephrin family (in particular EphrinA2 and EphrinA5) possess such a repulsive activity (Drescher et al., 1995; Nakamoto et al., 1996; Monschau et al., 1997). Both EphrinA2 and EphrinA5 are expressed in a gradient in the chick tectum and the mouse superior colliculus (Drescher et al., 1995; Cheng et al., 1995; Feldheim et al., 2000). Functional evidence supports the idea that EphrinA2 and EphrinA5 act as repulsive guidance cues, confining the termination zones of temporal RGCs to more anterior positions (Nakamoto et al., 1996; Feldheim et al., 2000; McLaughlin et al., 2003).
Chick RGM (cRGM) has been reported to possess an in vitro activity similar to the Ephrins, causing growth cone collapse and preferential guidance of temporal RGC axons (Monnier et al., 2002). In contrast to Ephrins, however, the in vivo role of RGM remains unclear. To determine whether RGM does indeed play a role in the establishment of retinocollicular projections in vivo we decided to isolate the corresponding mouse gene and examine the neural phenotype of mice lacking RGM gene function.
We discovered three genes with homology to cRGM within the mouse genome. All three murine members of this protein family (mRGMa, mRGMb, and mRGMc) show a C-terminal GPI-anchor consensus sequence, but the efficiency of cell-surface transport of the different family members is highly variable. The expression of mRGMa and mRGMb is confined primarily to the nervous system, in which they are expressed in complementary patterns. In contrast, the most prominent expression of mRGMc is found in skeletal muscles. Analysis of mRGMa mutant mice demonstrated that the development of RGC projections from the retina to the colliculus and topographic mapping of these projections to defined anteroposterior positions within the superior colliculus was normal. In contrast, ∼50% of mRGMa mutant mice showed defects in cephalic neural tube closure. Together, these findings identify a novel family of extracellular GPI anchored proteins in the mouse, with homology to cRGM, and reveal an unexpected role for mRGMa in the process of neural tube closure.
Materials and Methods
Characterization of mRGM gene family and histology. The three members of the mRGM gene family were isolated by database searches [GenBank accession numbers AI118914 (mRGMa), BG519283 (mRGMb), and AA656608 (mRGMc)]. Signal peptide and GPI anchor cleavage sites were determined using the following programs [http://www.cbs.dtu.dk/services/SignalP-2.0/#submission (Nielsen et al., 1999) and http://mendel.imp.univie.ac.at/gpi/gpi_server.html (Eisenhaber et al., 1999)]. Percentage identity of mRGM family members to cRGMa was determined using National Center for Biotechnology Information (Bethesda, MD) BlastP version 2.2.6. To determine N-terminal proteolytic cleavage sites, cRGMa (Monnier et al., 2002) and mRGMs were cloned into pSecTag2A (Invitrogen, San Diego, CA) in frame with the His-tag (cRGMa, 93–1217 bp; mRGMa, 91–1232 bp; mRGMb, 144–1243 bp; mRGMc, 94–1178 bp). COS-7 cell (American Type Culture Collection, Manassas, VA) conditioned medium was collected 2–5 d after transfection, and proteins were purified over an Ni-nitrilotriacetic acid-column (Qiagen, Basel, Switzerland). N-terminal sequencing was performed by Edman degradation (Analytical Research and Services, University of Bern, Bern, Switzerland).
For in situ hybridization analysis, sections were hybridized with digoxigenin-labeled probes (Schaeren-Wiemers and Gerfin-Moser, 1993) directed against mouse mRGMa, mRGMb, mRGMc, cRGMa (Monnier et al., 2002), mEphrinA5 (BG921710), mEphrinA2 (AA170896), and mPea3 (Livet et al., 2002). Antibodies used in this study were: rabbit anti-GAP-43 (Arber et al., 1999), rabbit anti-mRGMa (peptide antibody to amino acids 316–331), rabbit anti-mRGMb (peptide antibody to amino acids 256–270), rabbit anti-mRGMc (peptide antibody to amino acids 314–328), rabbit anti-cRGMa (peptide antibody to amino acids 319–332), mouse anti-bromodeoxyuridine (BrdU; Becton Dickinson, San Jose, CA), mouse anti-Myc (9E10; American Type Culture Collection), mouse anti-skeletal α-actinin (Sigma, Buchs, Switzerland), goat anti-β-galactosidase (Arnel, New York, NY) and sheep anti-enhanced green fluorescent protein (eGFP; Biogenesis, Kingston, NH). Chick electroporations were performed as described previously (Briscoe et al., 2000). Cryostat sections were processed for immunohistochemistry as described previously (Arber et al., 1999), using fluorophore-conjugated secondary antibodies (1:1000; Molecular Probes, Eugene, OR).
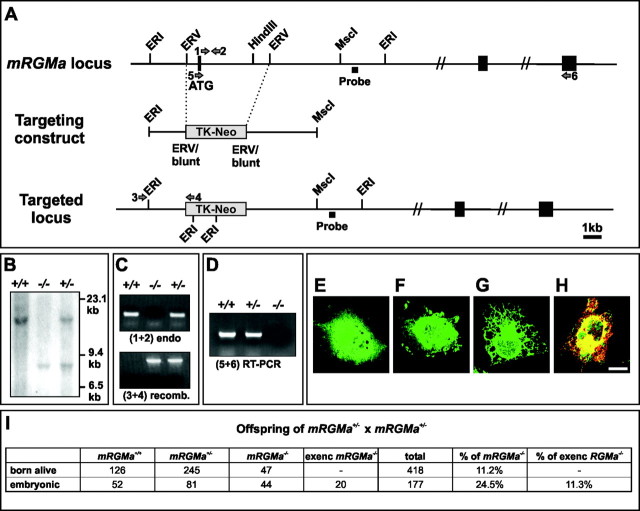
Generation and analysis of mRGMa mutant mice. A mouse genomic library was screened using an mRGMa-specific probe (Incyte Genomics, Palo Alto, CA). An EcoRV genomic fragment containing the exon coding for the N-terminal methionine and signal peptide was replaced by a thymidine kinase (TK)-neomycin cassette using homologous recombination in embryonic stem (ES) cells (targeting frequency, ∼1:2000). ES cell recombinants were screened by genomic Southern blot (EcoRI digest; 3′ probe (∼200 bp): oligonucleotides (A) 5′-TTGACCTGCCGCTGAGCACA-3′ and (B) 5′-CTGGGCACTGAGTGGTAAGG-3′) and verified by PCR (see Fig. 5 for position of oligonucleotides: [3], 5′-CATCCAACAAGGCTCCACTGGAAGG-3′; [4], 5′-TGCGAAGTGGACCTGGGACCGCG-3′). The identification of mRGMa–/– mice was performed by genomic Southern blotting and PCR (see Fig. 5 for position of oligonucleotides: [1], 5′-CAGGTAGGCACAACTCCTTGGTGG-3′; [2], 5′-TTAGCACGTCTGAGCCTGTGTCCG-3′). Reverse transcription (RT)-PCR was performed according to the instructions of the manufacturer (Promega, Madison, WI) using postnatal day 0 (P0) mouse brain total RNA and the following oligonucleotides: [5], 5′-CTTCCTTCTCTGCAGCTTCCCCGC-3′; [6], 5′-CTGGCGCGCCAGCTTGGTAGACTTTCTGGTCC-3′. The lack of antibodies recognizing endogenous mRGMa prohibited us from confirming the absence of mRGMa protein in mRGMa–/– mice.
Figure 5.
Generation of mRGMa mutant mice. A–D, Targeting strategy for homologous recombination in ES cells to eliminate mRGMa gene function. An EcoRV fragment including the exon containing the methionine of the signal peptide for targeting of mRGMa to the endoplasmic reticulum was replaced by a TK-neomycin resistant cassette (light gray). Coding exons for mRGMa and the probe used for genomic Southern analysis (B) are indicated by black boxes. Oligonucleotides to determine the absence of the methionine-containing exon are indicated by arrows 1 and 2 (C), oligonucleotides to verify 5′ homologous recombination by arrows 3 and 4 (C), and oligonucleotides used for RT-PCR by arrows 5 and 6 (D). RT-PCR analysis (D) was used to verify the absence of the exon containing the signal peptide on mRGMa mRNA in mRGMa mutant mice (arrows 5 and 6 in A). E–H, Immunocytochemistry of COS-7 cells transfected with a cDNA construct containing mRGMa exons 3′ of the targeted exon and eGFP on a bicistronic plasmid (E, F) or a cDNA construct containing an additional artificial in-frame N-terminal methionine and an eGFP containing a signal peptide for targeting to the endoplasmic reticulum (G, H). Cells were stained for eGFP (green) and mRGMa (red). In E and G, the incubation of cells with antibody to mRGMa was performed before the fixation and permeabilization of cells to label cell-surface-associated mRGMa. In F and H, the fixation and permeabilization of cells was performed before incubation with antibodies. Note that even the addition of an artificial N-terminal methionine in frame with the C-terminal mRGMa exons did not result in cell-surface-exposed mRGMa after transfection (G, H). Scale bar, 15 μm. I, Statistical analysis of the offspring recovered from matings of mRGMa+/– breeder pairs. exenc, Exencephalic mRGMa–/– embryos.
BrdU experiments were performed by the intraperitoneal injection of BrdU (50 μg/gm body weight; Sigma, Buchs, Switzerland) 2 hr before killing, and BrdU was detected as described previously (Arber et al., 1999). Terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) to detect apoptotic cells in whole-mount embryos was performed as described by the manufacturer (Roche, Rotkreuz, Switzerland). Anterograde labeling of retinocollicular projections was done essentially as described previously (Simon and O'Leary, 1992). Briefly, 5% DiI (Molecular Probes) in dimethyl formamide solution was injected into the retina at P0 or the nasal or temporal extreme of the retina at P9–P12 with a fine glass micropipette using a Picospritzer III (Parker, Fairfield, NJ). One day later for fills at P0 or 2 d later for focal injections, colliculi were analyzed blind to genotype with confocal microscopy (Olympus, Hamburg, Germany). The retinas were examined to verify a single injection point for focal injections.
Results
Isolation of three genes in the mouse genome homologous to cRGM
To isolate genes homologous to cRGM (Monnier et al., 2002) in the mouse genome, we searched the database for mouse expressed sequence tags and genomic sequences with a high degree of identity to cRGM. We found that the mouse genome contains three genes with homology to cRGM (in this paper, now referred to as cRGMa). Mouse RGMa (mRGMa) is most closely related to cRGMa and shows an identity of 80% to cRGMa at the amino acid level (Fig. 1A). The two more distantly related members of the RGM family of proteins, which we called mRGMb and mRGMc, show identities to cRGMa of 50 and 42%, respectively (Fig. 1A). Additional evidence that mRGMb may be more closely related to mRGMa than mRGMc comes from an analysis of the organization of the respective genomic loci. The positions of intron–exon junctions as well as the sizes of introns are highly conserved between mRGMa (chromosome 7) and mRGMb (chromosome 17; data not shown). In addition, two homologous genes [chromodomain helicase DNA binding protein 1 (CHD1) and chromodomain helicase DNA binding protein 2 (CHD2)] are located in close proximity to mRGMb (mRGMb/CHD1) and mRGMa (mRGMa/CHD2), respectively, suggesting that mRGMa and mRGMb may have evolved by gene duplication. In contrast, the genomic organization of mRGMc is highly divergent to mRGMa or mRGMb (data not shown).
Figure 1.
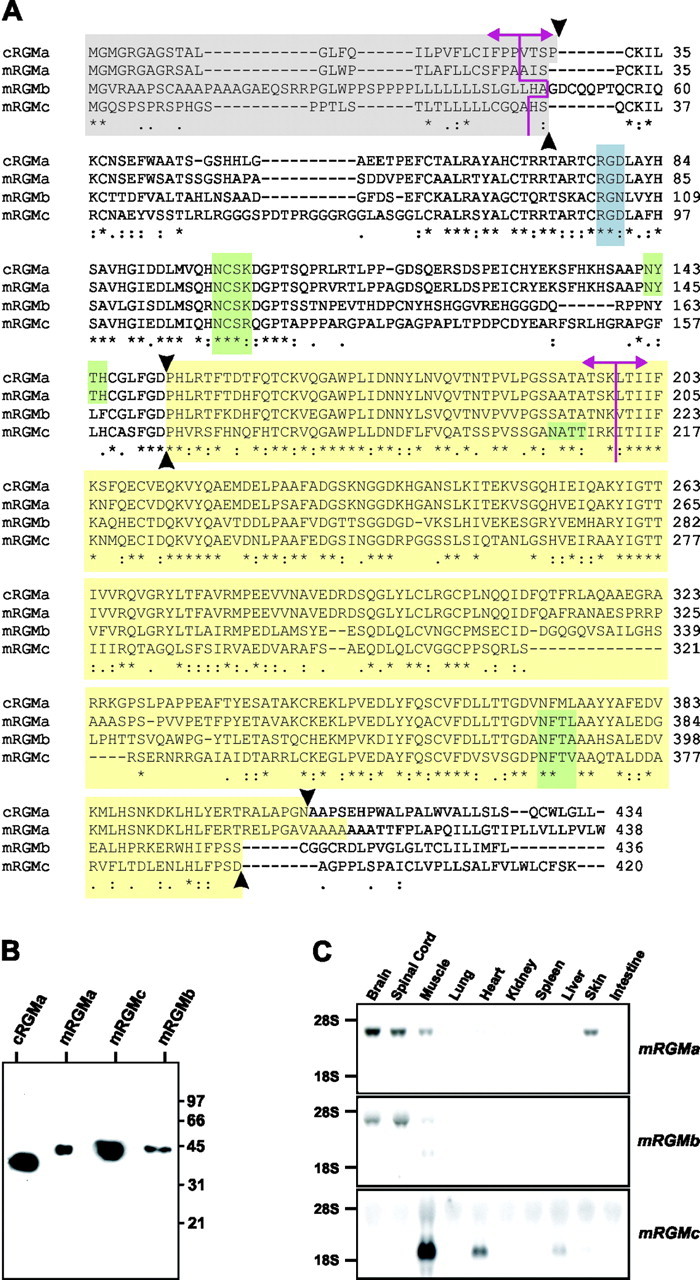
Characterization of the murine RGM protein family. A, Protein sequence alignment of cRGMa, mRGMa, mRGMb, and mRGMc. Asterisks indicate identical amino acids; pink lines, intron–exon junctions; the gray box, predicted signal peptides; the blue box, potential integrin binding sites (RGD); green boxes, predicted N-glycosylation sites; the yellow box, mature C-terminal RGM fragments after full proteolytic cleavage and C-terminal GPI anchor addition (proteolytic cleavage sites indicated by arrowheads). B, Western blot analysis of supernatant collected from COS-7 cells transfected with C-terminally truncated histidine–Myc-labeled cRGMa, mRGMa, mRGMc, and mRGMb detected with an anti-Myc antibody. Molecular weight standards in kilodaltons are indicated on the right. C, Northern blot analysis on total RNA from a variety of P3 mouse tissues as indicated, using mRGMa, mRGMb, and mRGMc as probes.
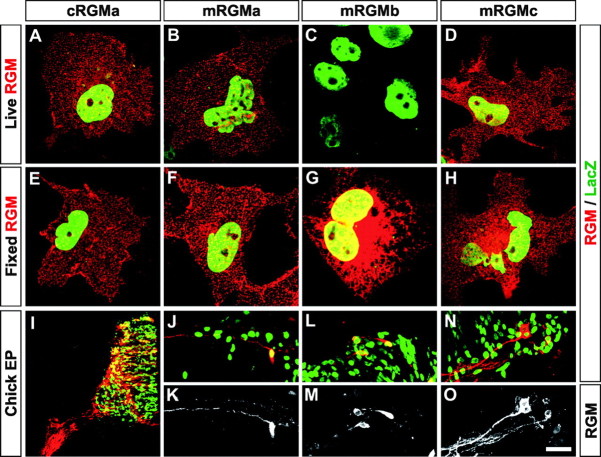
We found that like cRGMa, all three members of the mouse family of RGM proteins contain an N-terminal consensus signal peptide for targeting to the endoplasmic reticulum (Fig. 1A, gray box) and a C-terminal GPI anchor consensus sequence (Fig. 1A). However, the quality and score of the best site predicted for the addition of a GPI anchor varied significantly among the three mouse homologues of RGM and cRGMa (cRGMa, 7.93; mRGMa, 1.14; mRGMb, 2.72; mRGMc, 6.63), raising the possibility that not all members of the RGM family are processed with the same efficiency by the addition of a C-terminal GPI anchor. Because differential processing could affect the efficiency of protein targeting to the plasma membrane, in which GPI anchored proteins are usually localized to lipid rafts (Sharom and Lehto, 2002), we assessed the subcellular localization of cRGMa, mRGMa, mRGMb, and mRGMc by transfecting full-length RGMs and a cDNA encoding nuclear β-galactosidase from a bicistronic mRNA into COS-7 cells. To label cell-surface accumulated proteins, we incubated live transfected cells with primary antibodies specific to individual RGM family members before fixation and permeabilization of cells. For the identification of transfected cells, we stained them with an antibody to β-galactosidase after fixation and permeabilization. Although strong cell-surface labeling was detected on cells transfected with cRGMa and mRGMc, mRGMa-expressing cells appeared to be labeled somewhat less intensely, and very low if any staining was detected on the plasma membrane of cells transfected with mRGMb (Fig. 2A–D). When transfected cells were fixed and permeabilized before incubation with the primary antibody, all four RGMs displayed strong labeling (Fig. 2E–H). However, mRGMb protein was highly concentrated in the perinuclear endoplasmic reticulum/Golgi compartment, consistent with the observation that mRGMb protein is not efficiently targeted to the cell surface (Fig. 2G). Consistent with these data, supernatant collected from COS-7 cells transfected with individual RGM cDNA constructs contained high levels of mRGMc or cRGMa protein but only very low levels of mRGMb protein (data not shown), indicating that cell-surface-exposed RGMs may also be processed and released into the extracellular medium. To determine whether the differential subcellular distribution was also found in neurons in vivo, we electroporated embryonic day 3 (E3) chick spinal cords with vectors expressing mRGMa, mRGMb, or mRGMc. Whereas mRGMa and mRGMc proteins were expressed and efficiently transported into neuronal processes by E5, mRGMb appeared to be concentrated predominantly in neuronal cell bodies and proximal axonal processes, consistent with our findings in transfected COS-7 cells (Fig. 2I–O).
Figure 2.
Differential cell-surface targeting of mRGMs. A–H, Expression of full-length cDNAs coding for cRGMa (A, E), mRGMa (B, F), mRGMb (C, G), and mRGMc (D, H) andβ-galactosidase on the same plasmid using an internal ribosome entry site in COS-7 cells. Transfected COS-7 cells are identified by staining forβ-galactosidase after the fixation and permeabilization of cells (green). RGMs (red) are detected either before fixation and permeabilization of cells (A–D) to detect cell-surface-accumulated RGM or after fixation and permeabilization of cells (E–H) to detect all RGM in transfected cells. I–O, Chick spinal cords electroporated with cDNAs coding for cRGMa (I), mRGMa (J, K), mRGMb (L, M), and mRGMc (N, O) and β-galactosidase on the same plasmid using an IRES. Sections were stained for RGM (red:I,J,L,N;white:K,M,O) and β-galactosidase (green:I,J,L,N). Note the extensive labeling of axonal processes in I,J,K,N, and O and the predominant cell-body and proximal axonal labeling in L and M. Scale bar: (in O) A–H, 15 μm; I, 150 μm; J–O, 60 μm.
It has been shown previously that in addition to the proteolytic processing of the N-terminal signal peptide, cRGMa is cleaved once more to yield two proteolytic fragments, an N-terminal fragment containing an integrin-binding RGD motif and a C-terminal GPI-anchored fragment (Monnier et al., 2002). To determine whether this is also the case for mRGM proteins, we expressed mRGMa, mRGMb, and mRGMc in COS-7 cells, replacing the GPI anchor consensus sequence by a Myc-labeled hexahistidine tag. The molecular weights of these mRGM proteins collected from COS-7 cell supernatants were ∼42 kDa, whereas the molecular weight of cRGMa expressed using the same strategy was ∼35 kDa (Fig. 1B). Nevertheless, N-terminal end sequencing using Edman degradation showed identical cleavage sites within cRGMa, mRGMa, mRGMb, and mRGMc (Fig. 1A). The difference in the detected molecular weight is most likely caused by glycosylation (Fig. 1A, green boxes) or other post-translational modifications. In summary, the three murine members of the RGM family are proteolytically processed in a manner analogous to cRGMa, but whereas mRGMa and mRGMc are transported to the plasma membrane, mRGMb appears to be predominantly accumulated in intracellular compartments.
Differential expression of mRGM family members during development
Using a Northern blot analysis we found that the most abundant expression of mRGMa and mRGMb was detected in the nervous system, whereas mRGMc was expressed predominantly in striated muscle tissues, with the highest level of expression detected in skeletal muscles (Fig. 1C). To determine the specific sites of expression of mRGMa and mRGMb during embryonic development, we performed in situ hybridization experiments. Both mRGMa and mRGMb are expressed specifically at the tips of the neural folds of mouse embryos from E8 to E9, coincident with the expression of mEphrinA5 (Fig. 3A–C) (Holmberg et al., 2000). Later, both mRGMa and mRGMb are expressed at discrete sites in the developing CNS, but in nonoverlapping and highly complementary patterns. mRGMa expression in the brain is found surrounding the ventricles, whereas mRGMb expression is often found laterally apposed to mRGMa in early postmitotic neurons (Fig. 3E–G, E12.5 spinal cord; H–K, E14.5 thalamus). In addition, a high level of mRGMb expression was also detected in developing dorsal root ganglia (DRG) (Fig. 3F,G) and at later developmental stages, mRGMa and mRGMb are also expressed in distinct nuclei of the brain (E17.5; data not shown). Consistent with the data from our Northern blot analysis, the expression of mRGMc is confined to striated muscles, where it is found in both the muscles of the extremities (Fig. 3L–O) and of the face (Fig. 3J,K). No expression of mRGMc was detected in embryonic brain or spinal cord (Fig. 3J; data not shown).
Figure 3.
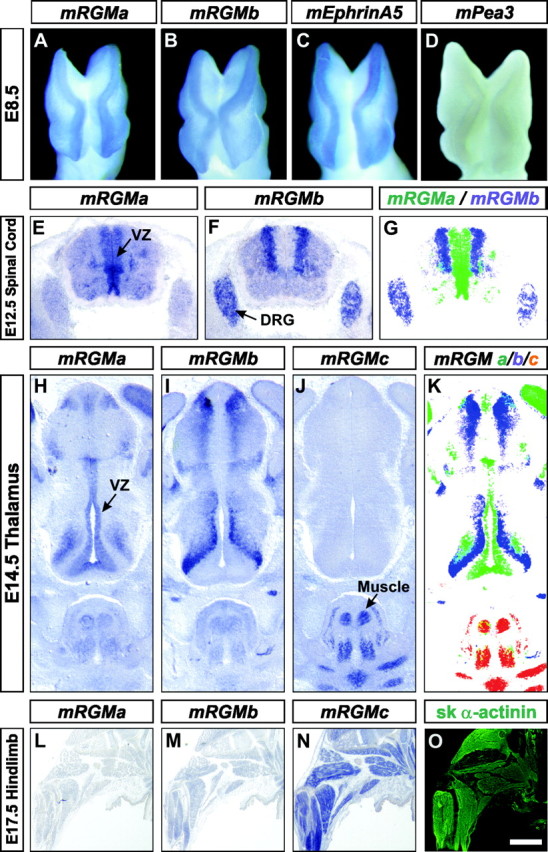
Embryonic expression of mRGMs in complementary patterns. A–D, Whole-mount in situ hybridization of E8.5 mouse embryos using mRGMa (A), mRGMb (B), mEphrinA5 (C), and mPea3 (D) as probes. Note the absence of mPea3 expression from the tips of neural folds. E–G, Expression of mRGMa (E) and mRGMb (F) in E12.5 mouse spinal cord. Arrows point to the ventricular zone (VZ) in E and the DRG in F, detected by in situ hybridization on transverse sections. G, Artificial overlay (mRGMa in green, mRGMb in blue) to demonstrate complementarity of expression patterns. H–K, Expression of mRGMa (H, VZ), mRGMb (I), and mRGMc (J) in E14.5 mouse thalamus by in situ hybridization on coronal brain sections. The arrow in J points to the signal of mRGMc in skeletal muscles. K shows an artificial overlay (mRGMa in green, mRGMb in blue, and mRGMc in red) to demonstrate the complementarity of expression patterns. L–O, Expression of mRGMa (L), mRGMb (M), and mRGMc (N) by in situ hybridization and skeletal α-actinin (O) by immunohistochemistry on E17.5 mouse hindlimbs. Scale bar: (in O) A–D, 0.15 mm; E–G, 0.35 mm; H–K, 0.32 mm; L–O, 1.8 mm.
In summary, the most striking feature revealed by this in situ hybridization analysis was an essentially complete lack of overlap and a strong complementarity in the expression of mRGMa and mRGMb in the developing nervous system. Whereas mRGMa expression is consistently found in subventricular zones surrounding the ventricles, mRGMb expression is often found laterally apposed to mRGMa and mRGMc is expressed predominantly in skeletal muscles.
Expression analysis of mouse RGMs in the visual system
To address which of the mouse RGM family members might play a dominant role in the development of the retinocollicular system, we performed in situ hybridization experiments in the mouse superior colliculus at P0, a stage just before targeting of RGC axons to defined anteroposterior positions occurs (Simon and O'Leary, 1992). We found that mRGMa, the closest homolog of cRGMa, was prominently expressed in the superior colliculus at this stage (Fig. 4A,D). However, in contrast to chick (Monnier et al., 2002) (Fig. 4F), we failed to detect a gradient in the level of mRGMa expression along the anteroposterior axis of the superior colliculus (Fig. 4A,D). In addition, and as described previously, mEphrinA5 was expressed in a clear anteroposterior gradient in the mouse superior colliculus (Fig. 4B,E) (Frisen et al., 1998; Feldheim et al., 2000). At earlier developmental stages (E15.5), mRGMa is also expressed prominently in the superior colliculus, whereas only faint expression of mRGMb was detected (Fig. 4G–I). In the retina, mRGMb but not mRGMa or mRGMc is expressed in RGCs (Fig. 4 J–L). Thus, the mRGM family member expressed most prominently in the superior colliculus, at a time just before targeting of RGC axons to defined anteroposterior positions occurs, is mRGMa.
Figure 4.
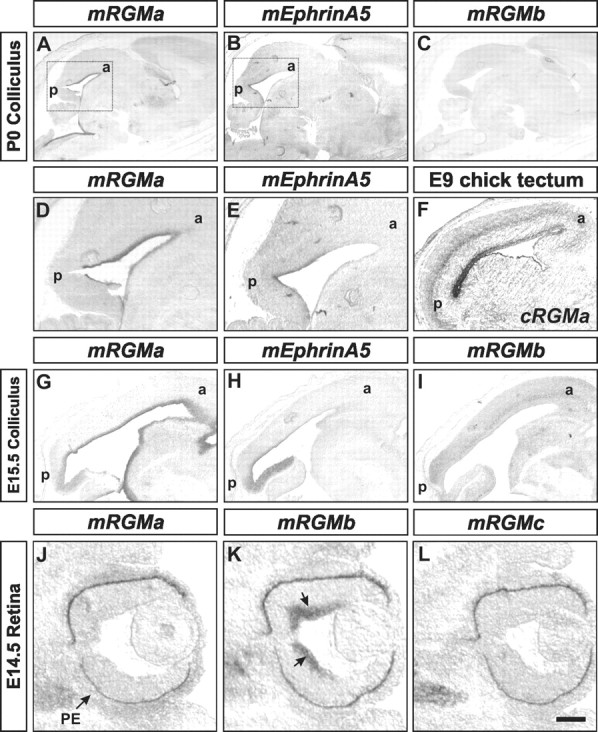
Expression of mRGMs in the developing retinocollicular system. A–E, Expression of mRGMa (A, D), mEphrinA5 (B, E), and mRGMb (C) in the superior colliculus of P0 sagittal brain sections detected by in situ hybridization. Boxes in A and B indicate regions shown in higher magnification in D and E. a, Anterior; p, posterior. F, Expression of cRGMa in E9 chick tectum detected by in situ hybridization. G–I, Expression of mRGMa (G), mEphrinA5 (H), and mRGMb (long exposure to detect faint expression; I) in the superior colliculus of E15.5 sagittal brain sections detected by in situ hybridization. J–L, Expression of mRGMa (J), mRGMb (K), and mRGMc (L) in E14.5 retina detected by in situ hybridization. Note the expression of mRGMb in RGCs (K, arrows). Pigment epithelium is marked by an arrow in J. Scale bar: (in L) A–C, 1 mm; D, E, 0.4 mm; F, 0.85 mm; G–I, 0.25 mm; J–L, 0.13 mm.
Generation of mRGMa mutant mice
To investigate the function of mRGMa in retinocollicular topographic mapping, we performed homologous recombination in embryonic stem cells to eliminate mRGMa gene function. We replaced the exon encoding the N-terminal signal peptide responsible for targeting mRGMa protein to the endoplasmic reticulum with a TK-neomycin cassette (Fig. 5A). The transfection of a cDNA construct coding for the remaining C-terminal exons of mRGMa into COS-7 cells showed that none of the transfected (eGFP+) COS-7 cells expressed the C-terminal fragment of mRGMa, thus demonstrating that all cell-surface-targeted mRGMa protein is eliminated with this targeting strategy (Fig. 5E–H).
Successful homologous recombination in embryonic stem cells using this targeting construct was detected at a very low frequency (∼1:2000). Heterozygous mRGMa+/– mice were phenotypically normal, and interbreeding resulted in the generation of homozygous viable mRGMa–/– mice (Fig. 5I; data not shown). Both PCR from genomic DNA as well as RT-PCR from total RNA of homozygous mRGMa–/– mice showed successful elimination of the exon encoding the signal peptide of mRGMa (Fig. 5C, D). Although mRGMa–/– mice were detected at postnatal stages, the frequency of recovered viable mutants was non-Mendelian (Fig. 5I).
Mutation in mRGMa results in an exencephalic phenotype in utero
To assess whether a fraction of mRGMa–/– embryos die in utero, we examined the frequency and appearance of mRGMa–/– embryos at various prenatal stages. At E16.5, we recovered two phenotypically different types of mRGMa–/– embryos: ∼50% of mRGMa–/– embryos had an appearance indistinguishable from wild-type embryos (Fig. 5I; data not shown), whereas in the remaining ∼50% of mRGMa–/– embryos, the brain was exposed to the exterior environment and cranial skull tissue was absent (Figs. 5I, 6D). Dissection of the brains from these affected mRGMa–/– embryos revealed that the ventral side of the brains as well as the brainstem and spinal cord were anatomically normal compared to wild-type brains and spinal cords (Fig. 6E,F). In contrast, dorsal and lateral views of the brain from affected mRGMa–/– embryos revealed major defects in the morphogenesis of dorsal brain structures (Fig. 6B,E; data not shown).
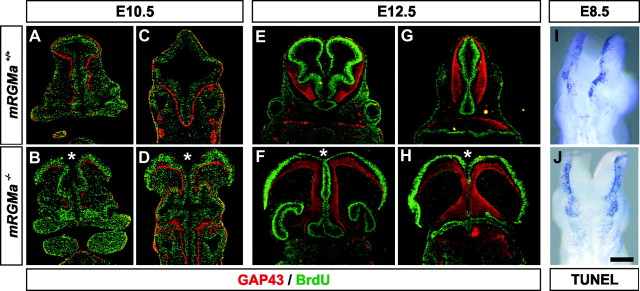
Figure 6.
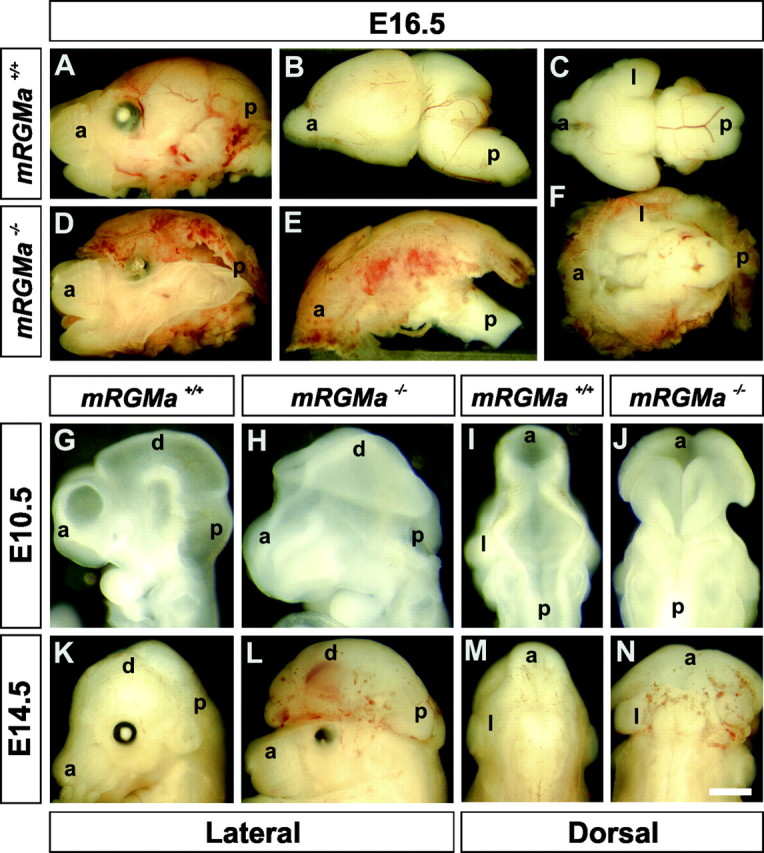
mRGMa mutant mice show an exencephalic phenotype. A–F, Lateral (A, B, D, E) or ventral (C, F) view of E16.5 heads (A, D) or dissected brains (B, C, E, F) from mRGMa+/+ (A–C) and exencephalic mRGMa–/– (D–F) embryos. G–J, Lateral (G, H) or dorsal (I, J) head view of E10.5 mRGMa+/+ (G, I) and mRGMa–/– (H, J) embryos. K–N, Lateral (K, L) or dorsal (M, N) head view of E14.5 mRGMa+/+ (K, M) and mRGMa–/– (L, N) embryos. a, Anterior; p, posterior; d, dorsal; l, lateral. Scale bar: (in N) A, D, 1.5 mm; B, E, 1 mm; C, F, 1.1 mm; G–J, 0.6 mm; K–N, 1.2 mm.
To define the nature of the observed defects more precisely, we performed a time course analysis at different embryonic stages. At E8, all mRGMa–/– embryos were indistinguishable from wild-type embryos (data not shown). By E8.5–E9, when the closure of neural folds in wild-type embryos has been initiated at both cephalic and spinal cord levels (Theiler, 1989), ∼50% of mRGMa–/– embryos did not show signs of efficient cephalic closure, leading to a lack of closure at the cephalic level by E10.5 (Fig. 6G–J; data not shown). In contrast, the closure of the neural folds at the level of the spinal cord was never affected in mRGMa–/– embryos (Fig. 6I,J; data not shown). This defect has been described previously as exencephaly (Harris and Juriloff, 1997). Exencephaly is caused by a failure of the cephalic neural folds to fuse, resulting in an exvagination of the developing cephalic tissue at later developmental stages, when many neurons are born and brain size increases (Harris and Juriloff, 1997). By E14.5, exencephalic mRGMa–/– embryos show a pronounced exposure of the developing brain structures and third ventricle to the external environment, as well as a lack of development of cranial skull tissue (Fig. 6K–N). In addition, the exencephalic tissue of mRGMa–/– embryos was highly vascularized (Fig. 6E,L), another feature frequently observed in exencephalic brains (Harris and Juriloff, 1997). Exencephalic mRGMa–/– embryos removed surgically immediately before birth (E19) displayed reflexive motion and breathing behavior but died shortly thereafter (data not shown). In contrast, exencephalic mRGMa–/– embryos delivered by natural birth were stillborn and anencephalic (lacking brain tissue entirely), presumably because of a lack of protection of the brain by the overlying skull during the process of birth (data not shown).
These findings suggest that an early function of mRGMa at the site of neural tube closure where both mRGMa and mRGMb are expressed at this time may be responsible for the exencephalic phenotype observed in ∼50% of mRGMa–/– embryos.
Exencephalic mRGMa mutants show no defects in early brain patterning
Does the absence of mRGMa lead to defects in neuronal patterning, cell proliferation, or apoptosis? We first performed BrdU pulse labeling experiments to assess proliferation and neuronal differentiation in mRGMa–/– embryos. We found that the amount of cellular proliferation was not obviously changed in either exencephalic or anatomically normal mRGMa–/– embryos compared with wild-type brains or spinal cords (Fig. 7A–H; data not shown). However, because of the lack of brain closure in exencephalic mRGMa–/– embryos, proliferating cells in dorsal brain structures now appeared on the outside of the brain, whereas GAP43+ differentiating neurons were facing the lumen (Fig. 7B,D,F,H). BrdU+ and GAP43+ cells were not intermingled in exencephalic mRGMa–/– embryos, indicating that mRGMa is not required for the initial segregation of proliferating cells and differentiating neurons in the brain. We also did not find defects in cellular proliferation and pan-neuronal differentiation in the spinal cords of mRGMa–/– embryos (data not shown). Similarly, many genes expressed in defined cell types of the brain and spinal cord were expressed normally in mRGMa–/– embryos (data not shown). In addition, no aberrant increase or decrease in apoptotic cell death could be observed in cephalic neural folds of exencephalic mRGMa–/– embryos at E8.5 (Fig. 7I,J). Together, these findings suggest that the exencephalic phenotype observed in a subpopulation of mRGMa–/– embryos is most likely not caused by defects in neuronal patterning or cell proliferation, nor does the exencephalic phenotype caused by absence of mRGMa lead to such defects.
(Figure 7.
Exencephalic mRGMa mutant embryos do not show defects in proliferation in the brain. A–H, Immunohistochemical analysis (GAP-43: red; BrdU: green) of coronal brain sections from E10.5 (A–D) and E12.5 (E–H) BrdU pulse-labeled mRGMa+/+ (A, C, E, G) and exencephalic mRGMa–/– (B, D, F, H) embryos. *Open ventricle. I, J, Whole-mount TUNEL of E8.5 mRGMa+/+ (I) and exencephalic mRGMa–/– (J) embryos in dorsal view. Scale bar: (in J) A, B, E, F, 0.38 mm; C, D, G, H, 0.5 mm; I, J, 0.13 mm.
Viable mRGMa mutants show no defects in anteroposterior retinocollicular projections
To determine whether the surviving mRGMa–/– mice show defects in the anteroposterior mapping of retinocollicular projections, as suggested from in vitro studies in the developing chick embryo (Monnier et al., 2002), we first injected the lipophilic tracer DiI into the eyes of P0 mice, attempting to label many RGC axons projecting from the retina to the brain. In viable mRGMa–/–, as in wild-type mice, the entire superior colliculus was filled with axons and, as expected at this developmental stage (Simon and O'Leary, 1992), a significant number of axons also projected into the inferior colliculus (n = 5) (Fig. 8A,B).
Figure 8.
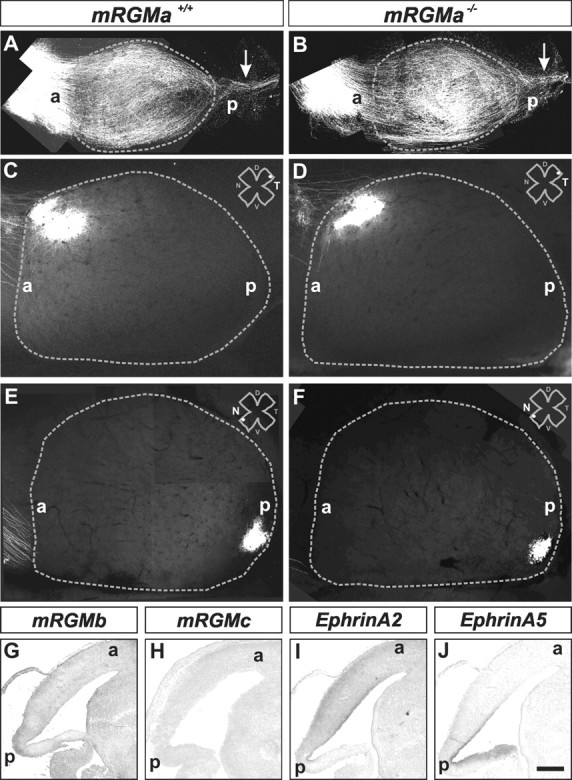
Lack of retinocollicular projection phenotype in mRGMa mutant mice. A, B, Dorsal views of superior and inferior colliculi from mRGMa+/+ (A) and mRGMa–/– (B) mice after anterograde Dil labeling from the retina at P0 to label many RGC axons. The gray dashed line outlines the superior colliculus; arrows point to RGC axons projecting to the inferior colliculus. C–F, Anterograde Dil labeling from the temporal (C, D) or nasal (E, F) retina to the superior colliculus (outlined by gray dashed lines) after focal Dil injection into the retina of mRGMa+/+ (C, E) and mRGMa–/– (D, F) mice at P10 (injection point in the retina indicated by the white dot the top right corner: T, Temporal; N, nasal; D, dorsal; V, ventral). G–J, Expression of mRGMb (G), mRGMc (H), mEphrinA2(I), and mEphrinA5 (J) in superior colliculi of E16.5 sagittal brain sections from mRGMa–/– mice detected by in situ hybridization. a, Anterior; p, posterior. Scale bar: (in J) A, B, 0.5 mm; C–F, 0.33 mm; G–J, 0.3 mm.
By P8, RGC axons within the superior colliculus have segregated into defined anteroposterior positions, reaching topographic positions found in the mature superior colliculus (Simon and O'Leary, 1992; Frisen et al., 1998). To determine whether in viable mRGMa–/– mice, retinocollicular axonal projections terminate in the appropriate anteroposterior position within the superior colliculus, we focally injected DiI into specific regions of the retina of P9–P12 animals. In this analysis, we first focused our attention on injections into the temporal RGC population known to project normally to the anterior and to be repelled by the posterior superior colliculus (Walter et al., 1987; Godement and Bonhoeffer, 1989; Roskies and O'Leary, 1994). Analysis of the projections of these DiI+ temporal RGC axons showed a single focal termination zone, in the predicted anteroposterior position of both wild-type and viable mRGMa–/– mice (n ≥ 15) (Fig. 8C,D). In addition, focal injections of DiI into the nasal retina of both wild-type and viable mRGMa–/– mice revealed that these axons also project and terminate normally in the posterior superior colliculus (n = 10) (Fig. 8E,F). To assess whether the lack of phenotype observed in retinocollicular projections of viable mRGMa–/– mice could be caused by compensatory upregulation of either other mRGM family members or members of the Ephrin family of genes, we performed in situ hybridization experiments. We found that expression of mRGMb, mRGMc, EphrinA2, and EphrinA5 in the superior colliculus of mRGMa–/– mice was not altered when compared with wild-type littermates (Fig. 8G–J). Together, these findings suggest that the lack of mRGMa gene function does not seem to impair anteroposterior mapping of RGC axons to the superior colliculus.
Discussion
In this study we found that cRGMa is a member of a novel family of GPI-anchored proteins, of which we identified and characterized three murine members: mRGMa, mRGMb, and mRGMc. Two members of this gene family are expressed predominantly in the developing nervous system in nonoverlapping and distinct expression patterns (mRGMa and mRGMb), whereas the third member is expressed most abundantly in skeletal muscles (mRGMc). Previous in vitro studies in the chick have suggested that cRGMa may play a role in the establishment of appropriate anteroposterior RGC termination zones within the tectum. Our functional studies in the mouse now showed that mRGMa mutant mice did not exhibit defects in the development of topographically appropriate projections in the anteroposterior dimension of the superior colliculus. Instead, a significant proportion of mRGMa mutant mice showed an exencephalic defect caused by failure of the cephalic neural tube to close during development. We will discuss these findings in the context of (1) the distinct sites of expression and subcellular localization of different RGM family members and (2) the in vivo function of mRGMa in the developing mouse nervous system.
Identification of a novel family of GPI-anchored proteins homologous to cRGMa
The identification of three genes homologous to cRGMa in the mouse genome has allowed us to study the precise developmental time course of expression, subcellular targeting, and proteolytic processing of these proteins. Two members of this gene family (mRGMa and mRGMb) show abundant expression in the developing mouse nervous system. It is interesting to note that the two genes expressed in the nervous system appear to have evolutionarily arisen by gene duplication. Preliminary evidence suggests that there is a homolog of mRGMb in the chick genome with an expression pattern confined to the nervous system (R. Salie, V. Niederkofler, and S. Arber, unpublished observations), indicating that this gene duplication must have occurred evolutionarily earlier than in the chick. A striking feature we observed in the expression of mRGMa and mRGMb within the nervous system is that their sites of expression are highly distinct and nonoverlapping. Although mRGMa is consistently expressed in ventricular zones in which proliferating cells are found, the expression of mRGMb is almost exclusive to domains in which early postmitotic neurons are found and not in ventricular zones. In addition to these sites of expression, distinct groups of postmitotic neurons also express mRGMa or mRGMb in nonoverlapping patterns. In contrast, the third member of the family (mRGMc) is expressed in skeletal muscles and is evolutionarily more distantly related to mRGMa and mRGMb. Given the function of mRGMa in controlling the process of alignment of dorsal neural folds ultimately leading to neural tube closure, it is tempting to speculate that mRGMc might be involved in the process of myogenesis, during which mononucleated myoblasts fuse to form multinucleated myotubes. Consistent with this idea, mRGMc expression is upregulated during the process of myogenic differentiation in vitro (Niederkofler, Salie, and Arber, unpublished observations).
All three mouse homologues of cRGMa have predicted GPI anchor consensus sequences and undergo proteolytic cleavage at several sites within the protein, raising the question of the subcellular localization of the mature mRGM proteins. Our analysis revealed that there is an internal cleavage site within all three mRGM proteins that is highly conserved in sequence between the three mRGM members and cRGMa. This internal protein cleavage produces two fragments, one N-terminal fragment containing an integrin-binding RGD site and a C-terminal fragment with a GPI anchor consensus site. To our knowledge, the sequence of this cleavage site has not been described previously in other proteins, and it will be interesting to determine which protease(s) recognize(s) this cleavage site and whether similar proteolytic cleavage sites are present in other proteins. In addition, such a protease may be expressed differentially in different cell types, leading to variable efficiencies in proteolytic processing depending on the amount and activity of protease present in a given cell type.
In contrast to the proteolytic cleavage reactions in the N-terminal region detected in all mRGM proteins, subcellular targeting of different mRGM members to the cell surface does not occur with equal efficiency for all RGM family members. Although mRGMc is as efficiently targeted to the cell surface as cRGMa, less mRGMa and almost no mRGMb appears to reach the cell surface. This differential subcellular localization of the RGM family of proteins could be caused by differential efficiency in the addition of GPI anchors and may have important implications for the function of individual RGM family members in the extracellular space. Differential subcellular targeting has been suggested recently as a mechanism by which several proteins with proposed extracellular functions regulate their activities. For example, only small amounts of NogoA protein reach the cell surface of oligodendrocytes and the most prominent localization of NogoA is found in the endoplasmic reticulum (Chen et al., 2000; GrandPre et al., 2000). It has been suggested that NogoA protein may be released from oligodendrocytes only after lesion of the nervous system, thus preventing NogoA from acting under normal circumstances (Brittis and Flanagan, 2001). Moreover, Commissureless in Drosophila has recently been described to sort Roundabout, the receptor for the repulsive guidance molecule Slit, to the endosomal compartment to prevent commissural neurons from responding to Slit before midline crossing (Keleman et al., 2002). Similarly, differential in vivo processing and subcellular localization of different members of the RGM family of proteins could regulate the degree of activity in a particular cell.
In vivo function of mRGMa in the developing nervous system
Two major candidate protein families have been implicated in anteroposterior topographic mapping in the retinocollicular system (McLaughlin et al., 2003). Ephrins and cRGMa have both been shown to exhibit comparable in vitro activities guiding RGC axons (Drescher et al., 1995; Cheng et al., 1995; Monschau et al., 1997; Monnier et al., 2002). However, whereas genetic evidence supports a role for Ephrins in anteroposterior topographic mapping in the superior colliculus (Feldheim et al., 2000; McLaughlin et al., 2003), our data on mRGMa–/– mice suggest that mRGMa is not essential for this process. However, our findings reveal that although there does not appear to be a similarity in the phenotype of EphrinA5–/– and mRGMa–/– mice in the retinocollicular system, both mRGMa and EphrinA5 are involved in the process of cephalic neural tube closure, albeit at a different level of genetic penetrance (Holmberg et al., 2000). Consistent with this phenotype, both EphrinA5 (Holmberg et al., 2000) and mRGMa are expressed in the dorsal edges of the cranial neural folds.
Why do mRGMa–/– mice not show a defect in the establishment of anteroposterior retinocollicular topographic mapping? It is unlikely that a compensatory upregulation of gene expression of other mRGM or Ephrin family member(s) can account for the lack of phenotype in retinocollicular mapping of mRGMa–/– mice because the expression of both mRGMb and mRGMc as well as EphrinA2 and EphrinA5 is unchanged in mRGMa–/– mice. Moreover, mRGMa appears to be the member of the mRGM gene family expressed at the highest level in the mouse superior colliculus at the time RGC axons segregate into their final anteroposterior position within the target region, suggesting that mRGMa should play the dominant role among mRGM family members in controlling RGC targeting. In contrast, developing RGCs express high levels of mRGMb and no or only low levels of mRGMa. Because previous evidence suggests that the expression of Ephrins does not only contribute to the development of retinocollicular projections through expression in the target region itself but also through expression in RGCs (Hornberger et al., 1999), it will be interesting to determine in future experiments how mRGMb gene function contributes to the development of retinocollicular projections. The generation of mRGMa/mRGMb double mutant mice should be able to address this question definitively in the future. Another possible explanation for a lack of phenotype in mRGMa–/– mice could come from the observation that although the expression of EphrinA2 and EphrinA5 in the mouse superior colliculus is graded (Feldheim et al., 2000), mRGMa exhibits no such anteroposterior gradient. Thus, the function of mRGMa may have changed during evolution, maybe because the chick tectum is significantly bigger than the mouse superior colliculus (McLaughlin et al., 2003) potentially requiring additional cues to acquire precision in the development of projections from the retina to the tectum. Alternatively, mRGMa may function only in combination with EphrinA2 and EphrinA5 present in the superior colliculus of the mouse and to reveal a retinocollicular phenotype may require the generation of mRGMa–/–/EphrinA2–/–/EphrinA5–/– mice. It has indeed been suggested that in addition to EphrinA2 and EphrinA5 other guidance cues ought to be present in the mouse to explain the anteroposterior topographic mapping in the superior colliculus because disruption of both EphrinA2 and EphrinA5 does not lead to a complete loss in anteroposterior retinocollicular topography (Feldheim et al., 2000). Future work will determine whether mRGMa does indeed act as a cofactor in combination with other guidance molecules to restrict RGC axons to their correct anteroposterior position in the superior colliculus.
Footnotes
This work was supported by a grant from the Swiss National Science Foundation, by the Kanton of Basel-Stadt, and MigraGen. We thank Monika Mielich, Jean-Francois Spetz, Patrick Kopp, and Bernard Kuchemann for excellent technical assistance, Bernhard Mueller for support and valuable insights, Robert Hindges for expert advice on retinocollicular tracing experiments, and Thomas Jessell for discussions and helpful comments on this manuscript.
Correspondence should be addressed to Dr. Silvia Arber, Biozentrum, Department of Cell Biology, University of Basel, Klingelbergstrasse 70, 4056 Basel, Switzerland. E-mail: silvia.arber@unibas.ch.
DOI:10.1523/JNEUROSCI.4610-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/240808-11$15.00/0
V.N. and R.S. contributed equally to this work.
References
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S (1999) Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23: 659–674. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J (2000) A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101: 435–445. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Flanagan JG (2001) Nogo domains and a Nogo receptor: implications for axon regeneration. Neuron 30: 11–14. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME (2000) Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403: 434–439. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG (1995) Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell 82: 371–381. [DOI] [PubMed] [Google Scholar]
- Dickson BJ (2002) Molecular mechanisms of axon guidance. Science 298: 1959–1964. [DOI] [PubMed] [Google Scholar]
- Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F (1995) In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell 82: 359–370. [DOI] [PubMed] [Google Scholar]
- Eisenhaber B, Bork P, Eisenhaber F (1999) Prediction of potential GPI-modification sites in proprotein sequences. J Mol Biol 292: 741–758. [DOI] [PubMed] [Google Scholar]
- Feldheim DA, Kim YI, Bergemann AD, Frisen J, Barbacid M, Flanagan JG (2000) Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron 25: 563–574. [DOI] [PubMed] [Google Scholar]
- Frisen J, Yates PA, McLaughlin T, Friedman GC, O'Leary DD, Barbacid M (1998) Ephrin-A5 (AL-1/RAGS) is essential for proper retinal axon guidance and topographic mapping in the mammalian visual system. Neuron 20: 235–243. [DOI] [PubMed] [Google Scholar]
- Godement P, Bonhoeffer F (1989) Cross-species recognition of tectal cues by retinal fibers in vitro. Development 106: 313–320. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM (2000) Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature 403: 439–444. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM (1997) Genetic landmarks for defects in mouse neural tube closure. Teratology 56: 177–187. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Clarke DL, Frisen J (2000) Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature 408: 203–206. [DOI] [PubMed] [Google Scholar]
- Hornberger MR, Dutting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U (1999) Modulation of EphA function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron 22: 731–742. [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ (2002) Comm sorts robo to control axon guidance at the Drosophila midline. Cell 110: 415–427. [DOI] [PubMed] [Google Scholar]
- Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S (2002) ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron 35: 877–892. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, O'Leary DD (2003) Regulation of axial patterning of the retina and its topographic mapping in the brain. Curr Opin Neurobiol 13: 57–69. [DOI] [PubMed] [Google Scholar]
- Monnier PP, Sierra A, Macchi P, Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B, Bonhoeffer F, Mueller BK (2002) RGM is a repulsive guidance molecule for retinal axons. Nature 419: 392–395. [DOI] [PubMed] [Google Scholar]
- Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger MR, Loschinger J, Pasquale EB, Siever DA, Verderame MF, Muller BK, Bonhoeffer F, Drescher U (1997) Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. EMBO J 16: 1258–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto M, Cheng HJ, Friedman GC, McLaughlin T, Hansen MJ, Yoon CH, O'Leary DD, Flanagan JG (1996) Topographically specific effects of ELF-1 on retinal axon guidance in vitro and retinal axon mapping in vivo. Cell 86: 755–766. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Brunak S, von Heijne G (1999) Machine learning approaches for the prediction of signal peptides and other protein sorting signals. Protein Eng 12: 3–9. [DOI] [PubMed] [Google Scholar]
- Roskies AL, O'Leary DD (1994) Control of topographic retinal axon branching by inhibitory membrane-bound molecules. Science 265: 799–803. [DOI] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A (1993) A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100: 431–440. [DOI] [PubMed] [Google Scholar]
- Sharom FJ, Lehto MT (2002) Glycosylphosphatidylinositol-anchored proteins: structure, function, and cleavage by phosphatidylinositol-specific phospholipase C. Biochem Cell Biol 80: 535–549. [DOI] [PubMed] [Google Scholar]
- Simon DK, O'Leary DD (1992) Development of topographic order in the mammalian retinocollicular projection. J Neurosci 12: 1212–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry R (1963) Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA 50: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274: 1123–1133. [DOI] [PubMed] [Google Scholar]
- Theiler K (1989) The house mouse: atlas of embryonic development. New York: Springer.
- Walter J, Henke-Fahle S, Bonhoeffer F (1987) Avoidance of posterior tectal membranes by temporal retinal axons. Development 101: 909–913. [DOI] [PubMed] [Google Scholar]
- Yu TW, Bargmann CI (2001) Dynamic regulation of axon guidance. Nat Neurosci 4 [Suppl]: 1169–1176. [DOI] [PubMed] [Google Scholar]