Abstract
We combined the use of knock-out mice and subtype-selective antagonists [2-methyl-6-(phenylethynyl)pyridine (MPEP) and (E)-2-methyl-6-(2-phenylethenyl)-pyridine (SIB1893)] to examine whether endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the pathophysiology of nigro-striatal damage in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of parkinsonism. High doses of MPTP (four injections of 20 mg/kg, i.p., every 2 hr) induced a high mortality rate and a nearly total degeneration of the nigro-striatal pathway in wild-type mice. mGlu5 knock-out mice were less sensitive to MPTP toxicity, as shown by a higher survival and a milder nigro-striatal damage. Protection against MPTP (80 mg/kg) toxicity was also observed after MPEP injections (four injections of 5 mg/kg, i.p., 30 min before each MPTP injection). MPEP treatment did not further increase neuroprotection against 80 mg/kg of MPTP in mGlu5 knock-out mice, indicating that the drug acted by inhibiting mGlu5 receptors. In wild-type mice, MPEP was also neuroprotective when challenged against lower doses of MPTP (either 30 mg/kg, single injection, or four of 10 mg/kg injections). The action of MPEP was mimicked by SIB1893 but not by the mGlu1 receptor antagonist 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester. MPEP did not change the kinetics of 1-methyl-4-phenylpyridinium ion formation in the striatum of mice injected with MPTP. We conclude that mGlu5 receptors act as amplifiers of MPTP toxicity and that mGlu5 receptor antagonists may limit the extent of nigro-striatal damage in experimental models of parkinsonism.
Keywords: basal ganglia, dopamine, MPTP toxicity, mGlu 5 receptors, neuroprotection, experimental parkinsonism
Introduction
Although l-3,4-dihydroxyphenylalanine is still the mainstay in the treatment of Parkinson's disease (PD), this drug does not reduce the progression of the disease, and its chronic use is complicated by the occurrence of motor fluctuations (such as wearing-off and on–off phenomena) and dyskinesias, which are related to the ongoing degeneration of nigro-striatal dopaminergic neurons (Bedard et al., 1999). Thus, the identification of drugs that slow the progression of nigro-striatal damage will be a major breakthrough in the treatment of PD, particularly if these drugs can also relieve motor symptoms. The use of NMDA receptor antagonists has shown that endogenous excitotoxic mechanisms contribute to the development of nigro-striatal damage in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of parkinsonism (Turski et al., 1991; Brouillet and Beal, 1993; Lange et al., 1993; Srivastava et al., 1993; Lange and Riederer, 1994; Loschmann et al., 1994; Ossowska, 1994; Vaglini et al., 1994; Kanthasamy et al., 1997; Sonsalla et al., 1998; Araki et al., 2001; but see Kupsch et al., 1992; Michel and Agid, 1992; Sonsalla et al., 1992). However, a long-term treatment with NMDA receptor antagonists is limited by the occurrence of sedation, ataxia, deficits in learning and memory, and psychotomimetic effects, although the use of NR2B-selective antagonists or fast NMDA channel blockers may partially overcome these limitations (Parsons et al., 1999; Loftis and Janowsky, 2003). Metabotropic glutamate (mGlu) receptors are alternative targets for neuroprotective drugs, because they modulate excitatory synaptic transmission and are implicated in the processes of neurodegeneration–neuroprotection (for review, see Bruno et al., 2001). These receptors form a family of eight subtypes, of which mGlu1 and mGlu5 receptors are coupled to inositol phospholipid hydrolysis, whereas all other subtypes are negatively coupled to adenylyl cyclase in heterologous expression systems. We focused on mGlu5 receptors, because endogenous activation of these receptors contributes to the development of excitotoxic neuronal death both in vitro and in vivo (Bruno et al., 2000; Battaglia et al., 2001). mGlu5 receptors are physically coupled to the NR2 subunit of NMDA receptors through a chain of anchoring proteins, which includes PSD-95 (postsynaptic density-95) or other “MAGUK” (membrane-associated guanylate kinase) proteins, SHANK, and the long isoforms of Homer proteins (Tu et al., 1999). Activation of mGlu5 receptors positively modulates NMDA receptors by relieving the Mg2+ blockade of the NMDA channel or through other mechanisms (for review, see Bruno et al., 2001), whereas activation of NMDA receptors amplifies mGlu5 responses by preventing receptor desensitization (Alagarsamy et al., 1999). This reciprocal interaction between mGlu5 and NMDA receptors led us to predict that pharmacological blockade of mGlu5 receptors isolates NMDA receptors from their natural partner, thus reducing the extent of an excitotoxic insult (Bruno et al., 2001). Here, we combined the use of mGlu5 knock-out mice and systemic injections of mGlu5 receptor antagonists to show that endogenous activation of mGlu5 receptors plays a permissive role on the development of nigro-striatal damage induced by MPTP in mice.
Materials and Methods
Materials. 2-Methyl-6-(phenylethynyl)pyridine (MPEP), (E)-2-methyl-6-(2-phenylethenyl)-pyridine (SIB1893), and 7-hydroxyiminocyclopropan[b]chromen-1a-carboxylic acid ethyl ester (CPCCOEt) were purchased from Tocris Cookson (Bristol, UK). All other chemicals were purchased from Sigma (Milano, Italy).
Animals. Heterozygous mGlu5 receptor knock-out mice were obtained from Jackson Laboratories (Bar Harbor, ME). Mice heterozygous for the targeted mutation were intercrossed to homozygosity. Homozygous females and males were fertile but poor breeders. Thus, all mice were generated by heterozygous breeding. Mice were kept under environmentally controlled conditions (ambient temperature, 22°C; humidity, 40%) on a 12 hr light/dark cycle with food and water ad libitum. Mice were identified by PCR analysis on tail samples after birth. The absence of mGlu5 receptors in knock-out mice was confirmed by Western blot analysis performed in the cerebral cortex (see below) when they were killed for the assessment of nigro-striatal damage. Experiments were performed following the National Institutes of Health Guidelines for Animal Care and Use.
Treatments. Groups of wild-type and mGlu5 knock-out mice were injected intraperitoneally with 24 mg/kg of MPTP hydrochloride (corresponding to 20 mg/kg of free MPTP), repeated four times with 2 hr of interval (cumulative dose, 80 mg/kg of free MPTP). Control mice were treated with saline. Additional groups of wild-type and knock-out mice were treated with MPEP (5 mg/kg, four injections) administered 30 min before each injection of MPTP or saline. The actions of MPEP (10 mg/kg, i.p., if injected once; 5 mg/kg, i.p., if injected four times) and other mGlu receptor antagonists (SIB1893, 10 mg/kg; CPCCOEt, 10 mg/kg; both injected once) were also tested against lower doses of MPTP (either a single injection of 36 mg/kg, i.p., of MPTP hydrochloride or four consecutive injections of 12 mg/kg, i.p., of MPTP hydrochloride corresponding to 30 and 10 × 4 mg/kg of free MPTP, respectively). All surviving mice were killed by decapitation 7 d after the treatments.
TH, dopamine transporter, and GFAP immunostaining. Animals were perfused with 4% paraformaldehyde, and brains were dissected out and maintained in 4% paraformaldehyde overnight. Brains were cryopreserved in 30% sucrose in PBS and then stored at –80°C until used. Eight micrometer sections were incubated overnight with monoclonal mouse antibody (1:2000; Sigma), monoclonal rat antibody (1:4000; Chemicon, Temecula, CA), or monoclonal mouse antibody (1:400; Sigma) for the detection of TH, dopamine transporter (DAT), and GFAP, respectively, and then for 1 hr with secondary antibodies. 3,3′-diaminobenzidine tetrachloride immunostaining was used for the detection of TH (ABC elite kit; Vector Laboratories, Burlingame, CA), and Cy3 goat anti-rat immunoglobulin IgG (1:400; Chemicon) and fluorescein isothiocyanate-conjugated horse anti-mouse IgG (1:100; Vector Laboratories) were used for the detection of DAT and GFAP, respectively. Control staining was performed without the primary antibodies. TH and DAT immunoreactivity was quantified by measuring the relative optical densities of the dorsal striatum in the stained sections using a computer-based microdensitometer (NIH Image Software, Bethesda, MD). TH-positive neurons were counted in the substantia nigra pars compacta.
Monoamine assay. The corpus striatum was homogenized by sonication in 0.6 ml of ice-cold 0.1 m perchloric acid (PCA). Fifty microliters of the homogenate was used for protein determination (Lowry et al., 1951). The remaining aliquot was centrifuged at 8000 × g for 10 min, and 20 μl of the supernatant was injected into an HPLC equipped with an autosampler 507 (Beckman Instruments, Fullerton, CA), a programmable solvent module 126 (Beckman Instruments), an analytical C-18 reverse-phase column kept at 30°C (Ultrasphere ODS; 5 μm; 80 Å pore; 250 × 4.6 mm; Beckman Instruments), and a Coulochem II electrochemical detector (ESA, Chelmsford, MA). The holding potentials were set at 350 and –350 mV for the detection of DA, 3,5-dihydroxyphenylactic acid (DOPAC), homovanillic acid (HVA), 5-hydroxytriptamine (5-HT), 5-hydroxyindolacetic acid (5-HIAA), and norepinephrine (NE). The mobile phase consisted of 80 mm sodium phosphate, 40 mm citric acid, 0.4 mm EDTA, 3 mm 1-heptansulphonic acid, and 8.5% methanol, brought to pH 2.75, with phosphoric acid (run under isocratic conditions at 1 ml/min).
Western blot analysis of mGlu5 receptor mutant mice. Cortex was homogenized at 4°C in ice-cold lysis buffer with a motor-driven Teflon glass homogenizer (1700 rpm). Five microliters from tissues and cellular extracts was used for protein determinations; 30 μg of proteins was resuspended in SDS-bromophenol blue reducing buffer with 40 mm DTT and used for protein identification. Western blot analyses were performed using 8% SDS-PAGE run on a minigel apparatus (Mini Protean II Cell; Bio-Rad, Milano, Italy); gels were electroblotted on ImmunBlot polyvinylidene difluoride membrane (Bio-Rad) for 1 hr using a semi-dry electroblotting system (Bio-Rad Trans-blot system SD), and filters were blocked overnight in TTBS buffer (100 mm Tris-HCl, 0.9% NaCl, 0.1% Tween 20, pH 7.4) containing 5% nonfat dry milk. Blots were then incubated for 1 hr at room temperature with primary polyclonal antibodies (1 μg/ml), which recognize a specific carboxy-terminal epitope of mGlu receptors (Upstate Biotechnology, Milano, Italy). Blots were washed three times with TTBS buffer and then incubated for 1 hr with secondary antibodies (peroxidase-coupled anti mouse; Amersham Biosciences, Arlington Heights, IL) diluted 1:10.000 with TTBS. Immunostaining was revealed by ECL (Amersham Biosciences).
Assay of MPTP and 1-methyl-4-phenylpyridinium ion levels. Groups of five mice treated with saline or MPEP (10 mg/kg, i.p.) and injected intraperitoneally with 36 mg/kg of MPTP hydrochloride in a single injection, and additional groups of five mice treated with saline or MPEP (5 mg/kg, i.p., four injections with 2 hr of interval) and injected with 12 mg/kg of MPTP hydrochloride (corresponding to 10 mg/kg of free MPTP) repeated four times with 2 hr of interval, were used for measurements of MPTP and 1-methyl-4-phenylpyridinium ion (MPP+) levels in the striatum at different time points after the last MPTP injection. The corpus striatum was homogenized by sonication in 0.6 ml of ice-cold 0.1 m PCA. Fifty microliters of the homogenate was used for protein determination (Lowry et al., 1951). The remaining aliquot was centrifuged at 8000 × g for 10 min, and 20 μl of the supernatant was injected into an HPLC equipped with an autosampler 507 (Beckman Instruments), a programmable solvent module 125 (Beckman Instruments), an analytical C-18 reverse-phase column kept at 30°C (Ultrasphere ODS; 5 μm; 80 Å pore; 150 × 2 mm; Beckman Instruments), and a 166 UV detector (Beckman Instruments). The mobile phase consisted of 0.1 m sulfuric acid, 10% acetonitrile, and 75 mm triethylamine at pH 2.3 (run under isocratic conditions at 0.3 ml/min). The UV detector was set to 295 nm for MPP+ detection and was automatically switched to 245 nm for MPTP as described previously (Fornai et al., 1997).
In vitro assay of MPTP transformation into MPP+. Mouse brains were homogenized in 250 mm sucrose and 10 mm phosphate buffer, pH 7.4 (1:10 w/v). Homogenates were centrifuged for 10 min at 600 × g. The supernatant was centrifuged at 6500 × g for 20 min. The pellets were resuspended in 5 ml of sucrose buffer and centrifuged at 6500 × g for 20 min. After removing the supernatant, the crude mitochondrial preparation was lysed in 2 ml of ice-cold distilled water using a Teflon homogenizer. The protein concentration was determined in the lysed crude mitochondrial homogenate (Lowry et al., 1951). Incubation mixtures (500 μl of final volume in sodium phosphate buffer, pH 7.4) contained MPTP (100 μm), the crude mitochondrial homogenate (protein 0.5 mg/ml), and the appropriate concentrations of MPEP (1 nm to 1 mm). Samples were incubated at 37°C for 45 min, during which the rate of oxidation of MPTP remained constant. Samples were protected from light by covering the incubation tubes with aluminum foil. The reaction was stopped by the addition of 20 μl of 70% PCA, and samples were centrifuged at maximal speed for 30 min in a refrigerate microfuge. The supernatant was analyzed for MPP+ content as described above.
Measurement of [3H]-MPP+ uptake in striatal synaptosomes. Synaptosomes were prepared as described previously (Bonnet and Costentin, 1989) with minor modifications. Briefly, striata from wild-type and mGlu5 receptor knock-out mice were homogenized in 20 vol of 0.32 m sucrose using a Teflon glass homogenizer. The homogenates were then centrifuged at 1000 × g for 10 min at 4°C to give a nuclear pellet. Supernatants were stored at 4°C, and the pellet was resuspended in 20 vol of 0.32 m sucrose and centrifuged for 10 min at 1000 × g. The two supernatants were then pooled and centrifuged at 17,500 × g for 30 min at 4°C, after which the supernatant was discarded and the final pellet resuspended in ice-cold Krebs–Ringer buffer (120 mm NaCl, 4.8 mm KCl, 1.3 mm CaCl2, 1.2 mm MgSO4, 1.2 mm KH2PO4, 25 mm NaHCO3, 6mm glucose, pH 7.6). Synaptosomes (25 μg of protein) were incubated in 1 ml of Krebs–Ringer buffer with [3H]-MPP+ (5 nm; specific activity, 83.5 mCi/mmol; PerkinElmer Life Sciences, Boston, MA) for 2 min at 37°C. Nonspecific MPP+ uptake was measured in the presence of 500 μm MPP+. When present, MPEP (1 μm) was preincubated for 5 min. MPP+ uptake was stopped by the addition of 3 ml of ice-cold Krebs–Ringer buffer. The suspension was immediately filtered under vacuum through Whatman glass fiber/C filters. Radioactivity was determined by liquid scintillation spectrometry. Specific [3H]-MPP+ uptake, defined as the difference between [3H]-MPP+ accumulated at 37°C in the presence and absence of MPP+, was expressed as femtomoles per milligram of protein. Protein content was measured by the method of Lowry et al. (1951) using bovine serum albumin as standard.
Results
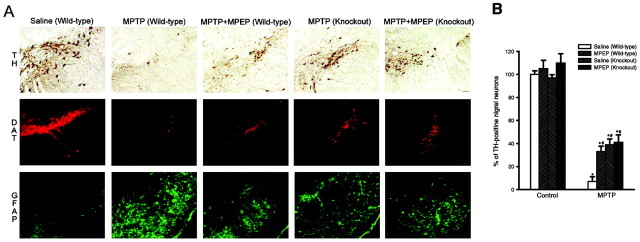
Wild-type and mGlu5 knock-out mice were challenged with 80 mg/kg of MPTP (20 mg/kg, i.p., injected four times with a 2 hr interval), which we reported to induce a high degree of mortality (from 65 to >90% in different experiments) and >90% loss of nigro-striatal dopaminergic fibers in surviving mice (Battaglia et al., 2003). MPTP induced a lower mortality rate in mGlu5 knock-out mice than in wild-type mice (Table 1). Seven days after MPTP injection, we assessed nigro-striatal degeneration in all surviving mice by immunohistochemical analysis of TH, DAT, and GFAP in groups of perfused mice or by biochemical determinations of DA, DOPAC, and HVA levels in the striatum from independent groups of mice. NE, 5-HT, and 5-HIAA levels were also measured for comparative purposes. Immunohistochemical analysis of the caudate nucleus (Fig. 1) and substantia nigra (Fig. 2) showed that mGlu5 knock-out mice were partially protected against MPTP toxicity. Protection was more remarkable by assessing DAT immunostaining as an indication of surviving striatal fibers (Fig. 1). Biochemical analysis of DA, DOPAC, HVA, NE, 5-HT, and 5-HIAA levels in the striatum did not show any difference between wild-type and knock-out mice under basal conditions. The drop in striatal DA levels induced by MPTP was attenuated in mGlu5 knock-out mice, although levels were still substantially reduced as compared with saline-injected mice. In contrast, injection of MPTP in mGlu5 knock-out mice almost failed to lower DOPAC and HVA levels in the striatum (Fig. 3). No changes in NE, 5-HT, or 5-HIAA levels were induced by MPTP in wild-type or knock-out mice (data not shown). Western blot analysis of mGlu5 receptors in the cerebral cortex confirmed the absence of the receptor protein in all surviving mGlu5 knock-out mice tested for nigro-striatal damage (see Fig. 3 for an example). In this assay, the mGlu5 receptor protein corresponded to the top of the two bands detected between 130 and 145 kDa in the cortex of wild-type mice (Fig. 3).
Table 1.
Mortality in mice treated with high doses of MPTP ± MPEP
|
|
Number of dead mice/total mice (percentage of mortality) |
||
|---|---|---|---|
| Treatments |
Wild-type mice |
mGlu5 knock-out mice |
|
| MPTP (20 mg/kg, i.p., four times) | 54/72 (75%) | 20/39 (51%) | |
| MPTP plus MPEP (5 mg/kg, i.p., four times) |
18/38 (47%) |
10/20 (50%) |
|
Figure 1.
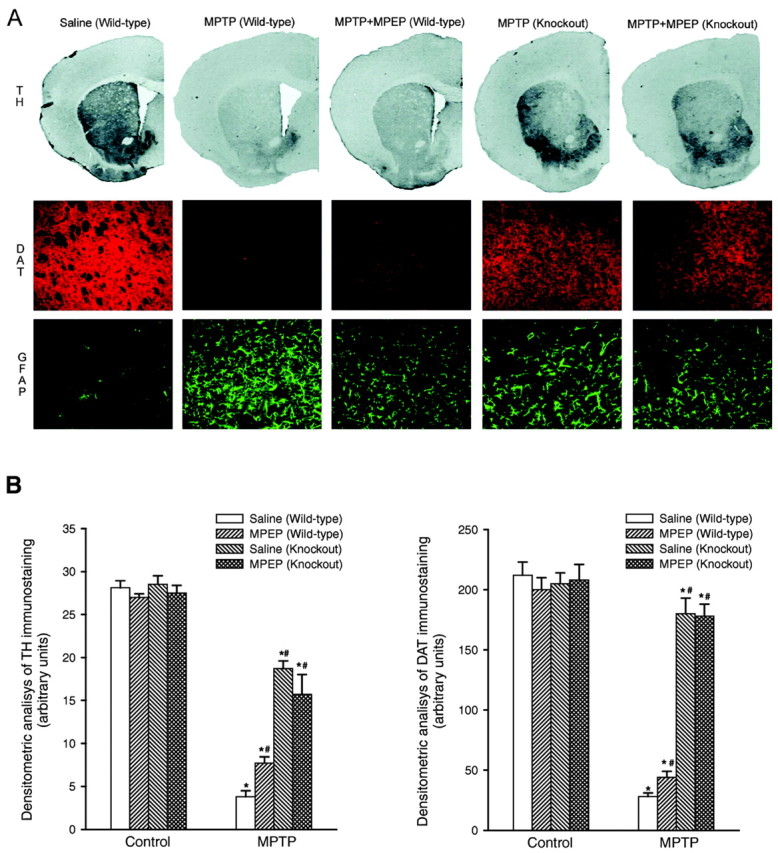
A, Immunohistochemical analysis of TH, DAT, and GFAP in the corpus striatum of wild-type and mGlu5 knock-out mice injected with saline or MPTP (20 mg/kg, i.p., injected 4 times with a 2 hr interval) alone or combined with MPEP (5 mg/kg, i.p., injected 4 times, 30 min before each saline or MPTP injection). Scale bar, 50 μm. B, Densitometric analysis of striatal TH and DAT immunostaining was performed on comparable sections from five to six mice. *p < 0.05 (one-way ANOVA plus Fisher's PLSD) versus the respective control mice treated with saline. #p < 0.05 (one-way ANOVA plus Fisher's PLSD) versus wild-type mice treated with MPTP and saline. Note that the lateral ventricle is not visible in the two representative sections of mGlu5 knock-out mice. This particular feature was observed in most of the sections from knock-out mice and was apparently attributable to a hypertrophy of the choroid plexus.
Figure 2.
A, Immunohistochemical analysis of TH, DAT, and GFAP in the substantia nigra pars compacta of wild-type and of mGlu5 knock-out mice treated as in Figure 1. Scale bar, 50 μm. B, Values in the graph (means ± SEM) are expressed as a percentage of controls (wild-type mice treated with saline) and were calculated by counting the number of TH+ neurons of the pars compacta of the substantia nigra from four comparable sections from five to six mice per group. *p < 0.05 (one-way ANOVA plus Fisher's PLSD) versus the respective control mice treated with saline. #p < 0.05 (one-way ANOVA plus Fisher's PLSD) versus wild-type mice treated with MPTP and saline.
Figure 3.
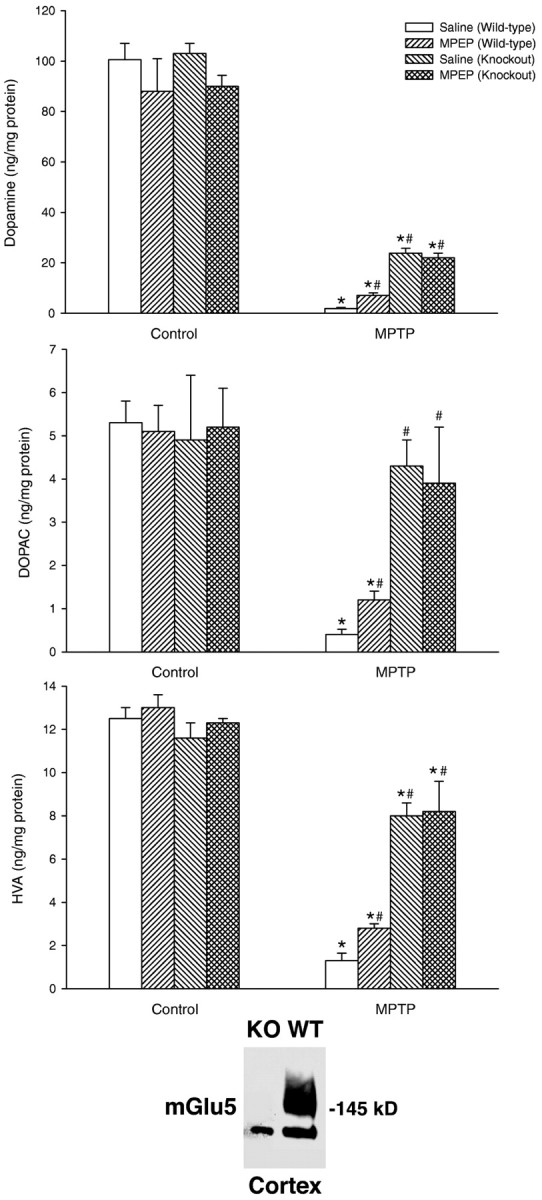
Striatal DA, DOPAC, and HVA levels in wild-type or mGlu5 receptor knock-out mice treated with high doses of MPTP ± MPEP, as described in Figures 1 and 2. Values are mean ± SEM of determinations from six mice. *p<0.05 (one-way ANOVA plus Fisher's PLSD) versus the respective control mice treated with saline. #p < 0.05 (one-way ANOVA plus Fisher's PLSD) versus wild-type mice treated with MPTP and saline. A representative immunoblot of mGlu5 receptors in the cerebral cortex of wild-type and knock-out mice is also shown. Thirty micrograms of proteins was loaded per lane. The top band at ∼145 kDa corresponding to the mGlu5 receptor monomer is visible only in the cortex of the wild-type mouse.
In additional groups of wild-type or knock-out mice, we examined the effect of the mGlu5 receptor antagonist MPEP on nigro-striatal degeneration induced by 80 mg/kg of MPTP. Wild-type mice receiving MPTP plus MPEP (5 mg/kg, i.p., administered 30 min before each MPTP injection for a cumulative dose of 20 mg/kg of MPEP) showed a lower rate of mortality as compared with mice injected with MPTP alone. However, MPEP did not further reduce the mortality rate in mGlu5 knock-out mice treated with 80 mg/kg of MPTP (Table 1). Immunohistochemical and biochemical analysis of surviving mice showed a limited protection by MPEP against nigro-striatal damage. MPEP did not further enhance the protection observed in mGlu5 knock-out mice treated with 80 mg/kg of MPTP (Figs. 1, 2, 3). We also examined the effect of MPEP on mice injected with a lower dose of MPTP (either 30 mg/kg, i.p., injected once or 10 mg/kg, i.p., injected four times with a 2 hr interval). These animals were used exclusively for the biochemical determination of monoamine levels in the striatum. “Low doses” of MPTP induced ∼50% reduction in the levels of DA and its metabolites in the striatum. This reduction was attenuated in mice treated with MPEP (either 10 mg/kg, i.p., injected 30 min before 30 mg/kg of MPTP or 5 mg/kg, i.p., injected 30 min before each injection of 10 mg/kg of MPTP four times) (Fig. 4A,B). The action of MPEP was mimicked by the mGlu5 receptor antagonist SIB1893 (10 mg/kg, i.p.) but not by the mGlu1 receptor antagonist CPCCOEt (10 mg/kg, i.p.; tested exclusively in mice treated with 30 mg/kg of MPTP) (Fig. 4A).
Figure 4.
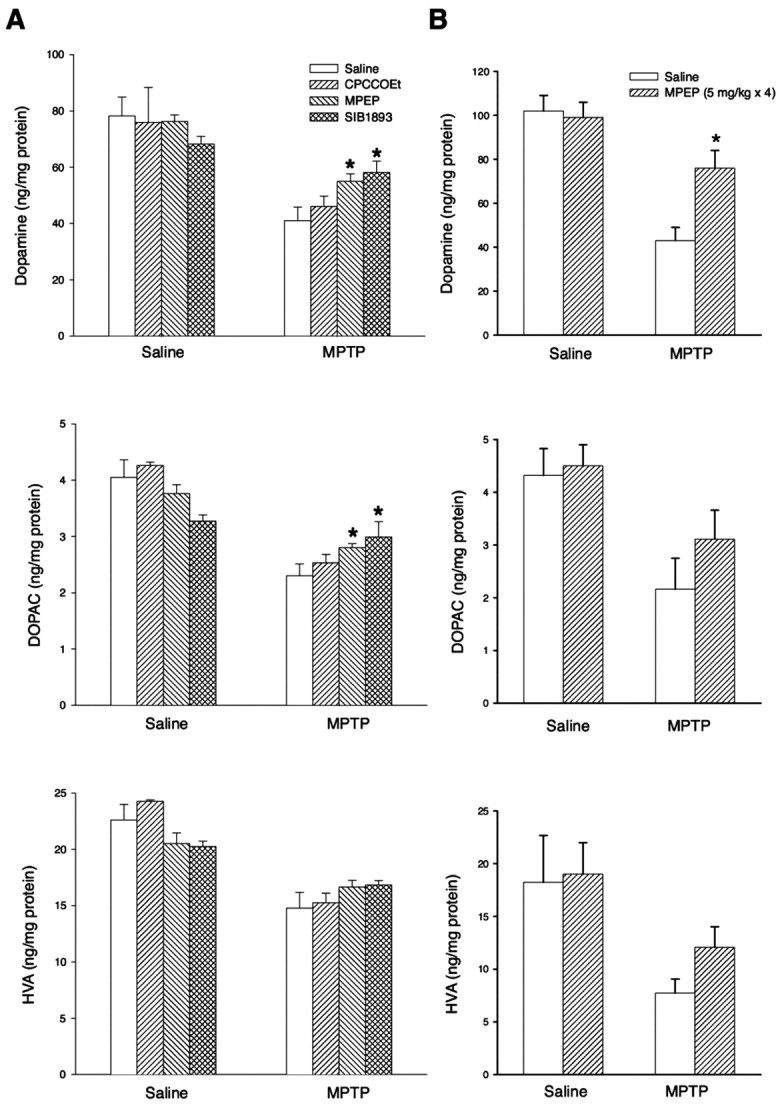
A, B, Striatal DA, DOPAC, and HVA levels in wild-type mice injected with MPTP (30 mg/kg, i.p., single injection) and MPEP, SIB1893, or CPCCOEt (all at 10 mg/kg, i.p., administered 30 min before MPTP) (A), or MPTP (10 mg/kg, i.p., injected 4 times with a 2 hr interval) and MPEP (5 mg/kg, i.p., injected 4 times, 30 min before each MPTP injection) (B). Values are mean ± SEM of 6–10 determinations. *p < 0.05 (one-way ANOVA plus Fisher's PLSD) versus mice treated with MPTP and saline.
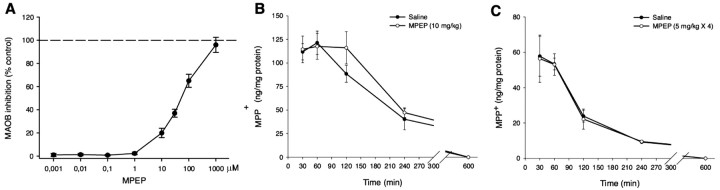
Because of the structural similarity between MPEP and MPTP, we examined whether MPEP could affect the conversion of MPTP into the active metabolite MPP+, a reaction catalized by monoamine oxidase B. When we performed an in vitro assay using lysates of a crude mitochondrial preparation from the mouse brain, we found that MPEP did not affect the conversion of MPTP into MPP+ up to 1 μm. However, higher concentrations of MPEP inhibited the conversion of MPTP into MPP+ (20% inhibition at 10 μm and 100% inhibition at 1 mm) (Fig. 5A). For this reason, we decided to perform in vivo experiments measuring the striatal levels of MPP+ in mice at different times after injection of 30 mg/kg of MPTP plus or minus 10 mg/kg of MPEP, or after four injections of 10 mg/kg of MPTP plus 5 mg/kg of MPEP. MPEP failed to affect the kinetics of MPP+ formation in the mouse striatum (Fig. 5B,C). Striatal MPTP levels were only detected 30 min after MPTP injection and did not differ between control mice and mice pretreated with MPEP (MPTP, 30 mg/kg, 0.012 + 0.001; MPTP, 30 mg/kg, plus MPEP, 10 mg/kg, 0.011 + 0.001 ng/mg of protein).
Figure 5.
A, In vitro transformation of MPTP into MPP+ in the presence of the mGlu5 receptor antagonist MPEP (1 nm to 1 mm). Values are mean ± SEM of five determinations. B, In vivo measurements of MPP+ levels in mouse striatal homogenates at different times after injection of 30 mg/kg of MPTP plus 10 mg/kg of MPEP. Values are mean ± SEM of five determinations. C, Same as in B but in striatal homogenates from mice treated with four consecutive doses of MPTP (10 mg/kg, i.p., 4 times) and MPEP (5 mg/kg, i.p., injected 4 times, 30 min before each MPTP injection. Values are means ± SEM of four determinations.
Because mGlu5 receptors are found in striatal dopaminergic terminals where they negatively modulate DAT activity (Page et al., 2001), we measured the specific uptake of [3H]MPP+ in striatal synaptosomes prepared from wild-type and mGlu5 receptor knock-out mice in the absence or presence of MPEP. The specific uptake of [3H]MPP+ was increased by >90% in striatal synaptosomes from mGlu5 knock-out mice as compared with wild-type mice, whereas a 10 min preincubation with MPEP (1 μm) induced a smaller increase in [3H]MPP+ uptake in wild-type mouse striatal synaptosomes (∼43%). MPEP did not affect [3H]MPP+ uptake in synaptosomes from mGlu5 knock-out mice (Table 2).
Table 2.
[3H]MPP+ uptake in striatal synaptosomes from wild-type or mGlu5 knock-out mice
|
|
fmol/mg of protein |
|---|---|
| Wild-type mice | 176 ± 19 |
| mGlu5 knock-out mice | 339 ± 16* |
| Wild-type mice plus in vitro MPEP, 1 μm | 252 ± 16* |
| mGlu5 knock-out mice plus in vitro MPEP, 1 μm
|
318 ± 27* |
Values are means ± SEM from three independent experiments performed in triplicate. *p<0.05 (one-way ANOVA plus Fisher's PLSD), versus values obtained in synaptosomes from wild-type mice without in vitro addition of MPEP.
Discussion
The combination of results obtained with mGlu5 knock-out mice and noncompetitive mGlu5 receptor antagonists indicates that endogenous activation of mGlu5 receptors amplifies nigrostriatal damage in mice treated with MPTP. This action might be exterted at different levels. Both mGlu1 and mGlu5 receptors are expressed by nigral dopaminergic neurons of the pars compacta of substantia nigra, where they are commonly found at the edges of asymmetric postsynaptic densities (Hubert et al., 2001; Smith et al., 2001). In addition, mGlu5 receptors are found in striatal dopaminergic terminals, where they negatively modulate DAT activity (Page et al., 2001). This is supported by our finding that both MPEP and the lack of mGlu5 receptors enhanced the uptake of [3H]MPP+, a substrate of DAT (Kopin, 1987; Trevor et al., 1987), in striatal synaptosomes. Although this finding warrants additional investigation, it suggests that the lower MPTP toxicity observed in mGlu5 knock-out mice or in mice treated with MPEP does not result from a limited access of MPP+ to the site of action. We speculate that the combined activation of NMDA and mGlu5 receptors in nigro-striatal dopaminergic neurons is translated into a death signal under conditions of energy loss (i.e., when the mitochondrial respiratory chain is inhibited by MPP+) (for review, see Przedborski and Jackson-Lewis, 1998). However, we cannot exclude that mGlu5 receptor blockade or deletion in the subthalamic nucleus could have palliative effects by reducing the excitatory drive to the pars compacta of the substantia nigra, thus limiting excitotoxicity (Awad et al., 2000; Blandini et al., 2001). Although mGlu5 knock-out mice were resistant to MPTP toxicity, the degree of protection varied depending on how we assessed nigro-striatal degeneration, with DAT immunostaining and striatal DA levels at the two extremes. All methods used for the assessment of nigro-striatal damage incorporate some limitations. For example, DA–DOPAC–HVA levels and TH–DAT immunostaining may reflect functional changes occurring in surviving fibers, whereas the extent of gliosis in knock-out mice may be affected by the absence of mGlu5 receptors in reactive astrocytes (Aronica et al., 2000, 2001; Ulas et al., 2000; Ferraguti et al., 2001). It is likely that more nigro-striatal dopaminergic terminals are spared in mGlu5 knock-out mice treated with MPTP; however, these terminals are overactive in terms of DA synthesis, release, re-uptake, and metabolism. This may explain why the loss of striatal DA levels was only partially reduced in knock-out mice treated with MPTP in spite of the nearly complete rescue of DOPAC levels and DAT immunostaining. An increased activity of DAT, resulting from the absence of mGlu5 receptors (Page et al., 2001; our data), might have facilitated the conversion of DA into DOPAC in the surviving terminals of knock-out mice treated with MPTP.
In pharmacological experiments, we used MPEP and SIB1893, because these drugs are subtype selective, can cross the blood-brain barrier, and act as noncompetitive antagonists (i.e., their action is independent of the amount of extracellular glutamate) (Gasparini et al., 1999; Varney et al., 1999a,b). One potential problem was related to the chemical structure of both drugs, which are phenylpyridine derivatives and are therefore similar to MPTP. For this reason, we examined whether MPEP could interfere with the transformation of MPTP into its active metabolite, MPP+, a reaction catalized by monoamine oxidase B (for review, see Nicotra and Parvez, 2000). In vitro experiments showed that MPEP was in fact able to inhibit the transformation of MPTP into MPP+ but only at high concentrations (>1–10 μm). However, injection of MPEP to mice at the same doses used for toxicity experiments did not change the kinetics of striatal MPP+ formation, indicating that the protective effect of MPEP was not caused by a reduced formation MPP+. This was also an indirect demonstration that striatal levels of MPEP were <1–10 μm, which is relevant because the drug is highly specific for mGlu5 receptors at concentrations <1 μm, although it can inhibit NMDA channels (O'Leary et al., 2000; Movsesyan et al., 2001) and positively modulate mGlu4 receptors (Mosolff Mathiesen et al., 2003) at concentrations >5–10 μm. The specificity of MPEP for mGlu5 receptors in our experiments was confirmed by the evidence that neuroprotection by the drug was not observed in mGlu5 knockout mice, thus excluding any contribution by nonspecific mechanisms. The limited amounts of MPEP reaching the nigro-striatal system may help explain why mice treated with the drug were less resistant to MPTP than mGlu5 knock-out mice.
In conclusion, our data support a role for mGlu5 receptors in the pathophysiology of experimental parkinsonism and suggest that mGlu5 receptor antagonists may delay the progression of nigro-striatal damage (Battaglia et al., 2002). These drugs may also relieve motor symptoms caused by a reduced activity of the nigro-striatal dopaminergic pathway, as shown by the efficacy of MPEP in improving bradykinesia in 6-hydroxydopamine-treated rats (Breysse et al., 2002).
Based on the lack of effect of systemically injected CPCCOEt, we cannot conclude that mGlu1 receptors do not contribute to the development of nigro-striatal damage induced by MPTP, because the ability of CPCCOEt to cross the blood-brain barrier is uncertain. Intracerebroventricular infusion of (R,S)-1-aminoindan-1,5-dicarboxylic acid (a competitive mGlu1 receptor antagonist) is shown to protect nigral neurons against MPTP toxicity (Aguirre et al., 2001). Studies with mGlu1 knock-out mice or with potent and brain-permeant subtype-selective antagonists are needed to clarify the role of mGlu1 receptors in the development of nigro-striatal damage. Finally, we have shown a protective role for mGlu2/3 receptors against MPTP toxicity using the potent and selective agonist 1R,4R,5S,6R-2-oxa-4-aminobicyclo[3.1.0.]hexane-4,6-dicarboxylic acid (Battaglia et al., 2003). Local infusion of (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (a mixed agonist of mGlu2/3 and NMDA receptors) is also reported to protect striatal dopaminergic terminals against MPP+ toxicity via the production of brain-derived neurotrophic factor by the reactive microglia (Matarredona et al., 2001; Venero et al., 2002). Thus, both mGlu5 receptor antagonists and mGlu2/3 receptor agonists are potential candidates as neuroprotective drugs in the treatment of parkinsonism. The strategy of blocking mGlu5 receptors may be promising for the dual role of these receptors in the pathophysiology of nigro-striatal damage and in the trans-synaptic mechanisms that control the development of motor symptoms (Pisani et al., 1997, 2001; Liu et al., 2001; Marino et al., 2001, 2002a,b, 2003; Popoli et al., 2001; Sung et al., 2001; Bell et al., 2002; Diaz-Cabiale et al., 2002; Marino and Conn, 2002). However, little is known about the adverse effects induced by long-term use of mGlu5 receptor antagonists. The permissive role of mGlu5 receptors in synaptic plasticity, learning, and memory (Lu et al., 1997; Jia et al., 1998, 2001; Bortolotto et al., 1999; Doherty et al., 2000; Balschun and Wetzel, 2002; Petersen et al., 2002; Rodrigues et al., 2002; Gubellini et al., 2003) raises a major concern for the frequent association between parkinsonism and dementia (Korczyn, 2001; Mark, 2001). The clinical profile of mGlu2/3 receptor agonists is more established, and these drugs appear to be safe and suitable for long-term use in humans (Grillon et al., 2003; Holden, 2003). However, an intrinsic limitation to the use of receptor agonists is that their efficacy critically depends on the levels of saturation of mGlu2/3 receptors by the endogenous glutamate (Battaglia et al., 2003).
Footnotes
This work was supported by Telethon-Italy Grant 1238.
Correspondence should be addressed to Dr. Ferdinando Nicoletti, Department of Human Physiology and Pharmacology, Piazzale Aldo Moro, 5,00185 Roma, Italy. E-mail: nicoletti@neuromed.it.
DOI:10.1523/JNEUROSCI.3831-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/240828-08$15.00/0
References
- Aguirre JA, Andbjer B, Gonzalez-Baron S, Hansson A, Stromberg I, Agnati LF, Fuxe K (2001) Group I mGluR antagonist AIDA protects nigral DA cells from MPTP-induced injury. NeuroReport 12: 2615–2617. [DOI] [PubMed] [Google Scholar]
- Alagarsamy S, Marino MJ, Rouse ST, Gereau IVRW, Heinemann SF, Conn PJ (1999) Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci 2: 234–240. [DOI] [PubMed] [Google Scholar]
- Araki T, Kumagai T, Tanaka K, Matsubara M, Kato H, Itoyama Y, Imai Y (2001) Neuroprotective effect of riluzole in MPTP-treated mice. Brain Res 918: 176–181. [DOI] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA (2000) Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci 12: 2333–2344. [DOI] [PubMed] [Google Scholar]
- Aronica E, Catania MV, Geurts J, Yankaya B, Troost D (2001) Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience 105: 509–520. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ (2000) Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci 20: 7871–7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wetzel W (2002) Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav 73: 375–380. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Bruno V, Pisani A, Centonze D, Catania MV, Calabresi P, Nicoletti F (2001) Selective blockade of type-I metabotropic glutamate receptors induces neuroprotection by enhancing GABAergic transmission. Mol Cell Neurosci 17: 1071–1083. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F (2002) Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci 22: 2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Busceti LC, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F (2003) Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in mice. Neuropharmacology 45: 155–166. [DOI] [PubMed] [Google Scholar]
- Bedard PJ, Blanchet PJ, Levesque D, Soghomonian JJ, Grondin R, Morissette M, Goulet M, Calon F, Falardeau P, Gomez-Mancilla B, Doucet JP, Robertson GS, DiPaolo T (1999) Pathophysiology of L-dopa-induced dyskinesias. Mov Disord 14: 4–8. [PubMed] [Google Scholar]
- Bell MI, Richardson PJ, Lee K (2002) Functional and molecular characterization of metabotropic glutamate receptors expressed in rat striatal cholinergic interneurones. J Neurochem 81: 142–149. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Greenamyre JT (2001) Subthalamic infusion of an NMDA antagonist prevents basal ganglia metabolic changes and nigral degeneration in a rodent model of Parkinson's disease. Ann Neurol 49: 525–529. [PubMed] [Google Scholar]
- Bonnet JJ, Costentin J (1989) Correlation between [3H]-dopamine specific uptake and [3H]-GBR 12783 specific binding during the maturation of rat striatum. Life Sci 44: 1759–1765. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Fitzjohn SM, Collingridge GL (1999) Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol 299–304. [DOI] [PubMed]
- Breysse N, Baunez C, Spooren W, Gasparini F, Amalric M (2002) Chronic but not acute treatment with a metabotropic glutamate 5 receptor antagonist reverses the akinetic deficits in a rat model of parkinsonism. J Neurosci 22: 5669–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillet E, Beal MF (1993) NMDA antagonists partially protect against MPTP induced neurotoxicity in mice. NeuroReport 4: 387–390. [DOI] [PubMed] [Google Scholar]
- Bruno V, Ksiazek I, Battaglia G, Lukic S, Leonhardt T, Sauer D, Gasparini F, Kuhn R, Nicoletti F, Flor PJ (2000) Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology 39: 2223–2230. [DOI] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Copani A, D'Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F (2001) Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab 21: 1013–1033. [DOI] [PubMed] [Google Scholar]
- Diaz-Cabiale Z, Vivo M, Del Arco A, O'Connor WT, Harte MK, Muller CE, Martinez E, Popoli P, Fuxe K, Ferre S (2002) Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett 324: 154–158. [DOI] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Bortolotto ZA, Hargreaves A, Kingston AE, Ornstein PL, Schoepp DD, Lodge D, Collingridge GL (2000) A novel, competitive mGlu(5) receptor antagonist (LY344545) blocks DHPG-induced potentiation of NMDA responses but not the induction of LTP in rat hippocampal slices. Br J Pharmacol 131: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Corti C, Valerio E, Mion S, Xuereb J (2001) Activated astrocytes in areas of kainite-induced neuronal injury upregulate the expression of the metabotropic glutamate receptors 2/3 and 5. Exp Brain Res 137: 1–11. [DOI] [PubMed] [Google Scholar]
- Fornai F, Alessandrí MG, Torracca MT, Bassi L, Corsini GU (1997) Effects of noradrenergic lesions on MPTP/MPP+ kinetics and MPTP-induced nigrostriatal dopamine depletions. J Pharmacol Exp Ther 283: 100–107. [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeirer H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçelebi G, Kuhn R (1999) 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 38: 1493–1503. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Levine LR, Morgan III CA (2003) Anxyolitic effects of a novel group II metabotropic glutamate receptor agonist (LY354740) in the fear-potentiated startle paradigm in humans. Psychopharmacology 168: 446–454. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Costa C, Tropepi D, Bernardi G, Conquet F, Calabresi P (2003) Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology 44: 8–16. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J (1998) Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem 5: 331–343. [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Lu YM, Agopyan N, Roder J (2001) Gene targeting reveals a role for the glutamate receptors mGluR5 and GluR2 in learning and memory. Physiol Behav 73: 793–802. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kanthasamy A, Matsumoto RR, Vu TQ, Truong DD (1997) Neuroprotective effects of the strychnine-insensitive glycine site NMDA antagonist (R)-HA-966 in an experimental model of Parkinson's disease. Brain Res 759: 1–8. [DOI] [PubMed] [Google Scholar]
- Kopin IJ (1987) MPTP: an industrial chemical and contaminant of illicit narcotics stimulates a new era in research of Parkinson's disease. Environ Health Perspect 75: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczyn AD (2001) Dementia in Parkinson's disease. J Neurol 248[Suppl 3]: 1–4. [DOI] [PubMed] [Google Scholar]
- Kupsch A, Loschmann PA, Sauer H, Arnold G, Renner P, Pufal D, Burg M, Wachtel H, ten Bruggencate G, Oertel WH (1992) Do NMDA receptor antagonists protect against MPTP-toxicity? Biochemical and immunocytochemical analyses in black mice. Brain Res 592: 74–83. [DOI] [PubMed] [Google Scholar]
- Holden C (2003) Excited by glutamate. Science 300: 1866–1868. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Parquet M, Smith Y (2001) Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey substantia nigra. J Neurosci 15: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Riederer P (1994) Glutamatergic drugs in Parkinson's disease. Life Sci 55: 2067–2075. [DOI] [PubMed] [Google Scholar]
- Lange KW, Loschmann PA, Sofic E, Burg M, Horowski R, Kalveram KT, Wachtel H, Riederer P (1993) The competitive NMDA antagonist CPP protects substantia nigra neurons from MPTP-induced degeneration in primates. Naunyn Schmiedebergs Arch Pharmacol 348: 586–592. [DOI] [PubMed] [Google Scholar]
- Liu F, Ma XH, Ule J, Bibb JA, Nishi A, DeMaggio AJ, Yan Z, Nairn AC, Greengard P (2001) Regulation of cyclin-dependent kinase 5 and casein kinase 1 by metabotropic glutamate receptors. Proc Natl Acad Sci USA 98: 11062–11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A (2003) The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation and clinical implications. Pharmacol Ther 97: 55–85. [DOI] [PubMed] [Google Scholar]
- Loschmann PA, Lange KW, Wachtel H, Turski L (1994) MPTP-induced degeneration: interference with glutamatergic toxicity. J Neural Transm Suppl 43: 133–143. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275. [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC (1997) Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 17: 5196–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark MH (2001) Lumping and splitting the Parkinson Plus syndromes: dementia with Lewy bodies, multiple system atrophy, progressive supranuclear palsy, and cortico-basal ganglionic degeneration. Neurol Clin 19: 607–627. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ (2002) Modulation of the basal ganglia by metabotropic glutamate receptors: potential for novel therapeutics. Curr Drug Target CNS Neurol Disord 1: 239–250. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ (2001) Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci 21: 7001–7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Awad H, Poisik O, Wittmann M, Conn PJ (2002a) Localization and physiological roles of metabotropic glutamate receptors in the direct and indirect pathways of the basal ganglia. Amino Acids 23: 185–191. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Awad-Granko H, Ciombor KJ, Conn PJ (2002b) Haloperidol-induced alteration in the physiological actions of group I mGlus in the subthalamic nucleus and the substantia nigra pars reticulata. Neuropharmacology 43: 147–159. [DOI] [PubMed] [Google Scholar]
- Marino M, Valenti O, Conn PJ (2003) Glutamate receptors and Parkinson's disease: opportunities for intervention. Drugs Aging 20: 377–397. [DOI] [PubMed] [Google Scholar]
- Matarredona ER, Santiago M, Venero JL, Cano J, Machado A (2001) Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+-induced neurotoxicity along with brain-derived neurotrophic factor induction. J Neurochem 76: 351–360. [DOI] [PubMed] [Google Scholar]
- Michel PP, Agid Y (1992) The glutamate antagonist, MK-801, does not prevent dopaminergic cell death induced by the 1-methyl-4-phenylpyridinium ion (MPP+) in rat dissociated mesencephalic cultures. Brain Res 597: 233–240. [DOI] [PubMed] [Google Scholar]
- Mosolff Mathiesen J, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT (2003) Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol 138: 1026–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan VA, O'Leary DM, Fan L, Bao W, Mullins PG, Knoblach SM, Faden AI (2001) mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther 296: 41–47. [PubMed] [Google Scholar]
- Nicotra A, Parvez SH (2000) Cell death induced by MPTP, a substrate for monoamino oxidase B. Toxicology 153: 157–166. [DOI] [PubMed] [Google Scholar]
- O'Leary DM, Movsesyan V, Vicini S, Faden AI (2000) Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol 131: 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K (1994) The role of excitatory amino acids in experimental models of Parkinson's disease. J Neural Transm Park Dis Dement Sect 8: 39–71. [DOI] [PubMed] [Google Scholar]
- Page G, Peters M, Najimi GM, Maloteaux JM, Hermans E (2001) Modulation of the neuronal dopamine transporter activity metabotropic glutamate receptors mGuR5 in rat striatal synaptosomes through phosphorylation mediated processes. J Neurochem 76: 1282–1290. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G (1999) Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist-a review of preclinical data. Neuropharmacology 38: 735–767. [DOI] [PubMed] [Google Scholar]
- Petersen S, Bomme C, Baastrup C, Kemp A, Christoffersen GR (2002) Differential effects of mGluR1 and mGluR5 antagonism on spatial learning in rats. Pharmacol Biochem Behav 73: 381–389. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G (1997) Enhancement of NMDA responses by group-I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol 120: 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Bernardi G, Calabresi P (2001) Functional coexpression of excitatory mGluR1 and mGluR5 on striatal cholinergic interneurons. Neuropharmacology 40: 460–463. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pezzola A, Torvinen M, Reggio R, Pintor A, Scarchilli L, Fuxe K, Ferre S (2001) The selective mGlu(5) receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: interactions with adenosine A(2a) receptors. Neuropsychopharmacology 25: 505–513. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V (1998) Mechanisms of MPTP toxicity. Mov Disord 13[Suppl 1]: 35–38. [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE (2002) The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci 22: 5219–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Charara A, Parquet M, Kieval JZ, Pare JF, Hanson JE, Hubert GW, Kuwajima M, Levey AI (2001) Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. J Chem Neuroanat 22: 13–42. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Zeevalk GD, Manzino L, Giovanni A, Nicklas WJ (1992) MK-801 fails to protect against the dopaminergic neuropathology produced by systemic 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in mice or intranigral 1-methyl-4-phenylpyridinium in rats. J Neurochem 58: 1979–1982. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Albers DS, Zeevalk GD (1998) Role of glutamate in neurodegeneration of dopamine neurons in several animal models of parkinsonism. Amino Acids 14: 69–74. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Brouillet E, Beal MF, Storey E, Hyman BT (1993) Blockade of 1-methyl-4-phenylpyridinium ion (MPP+) nigral toxicity in the rat by prior decortication or MK-801 treatment: a stereological estimate of neuronal loss. Neurobiol Aging 14: 295–301. [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM (2001) Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol 86: 2405–2412. [DOI] [PubMed] [Google Scholar]
- Trevor AJ, Singer TP, Ramsay RR, Castagnoli Jr N (1987) Processing of MPTP by monoamine oxidases: implications for molecular toxicology. J Neural Transm Suppl 23: 73–89. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF (1999) Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23: 583–592. [DOI] [PubMed] [Google Scholar]
- Turski L, Bressler K, Rettig KJ, Loschmann PA, Wachtel H (1991) Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature 349: 414–418. [DOI] [PubMed] [Google Scholar]
- Ulas J, Satou T, Ivins KJ, Kesslak JP, Cotman CW, Balazs R (2000) Expression of metabotropic glutamate receptor 5 is increased in astrocytes after kainite-induced epileptic seizures. Glia 30: 352–361. [PubMed] [Google Scholar]
- Vaglini F, Fascetti F, Fornai F, Maggio R, Corsini GU (1994) (+)MK-801 prevents the DDC induced enhancement of MPTP toxicity in mice. Brain Res 668: 194–203. [DOI] [PubMed] [Google Scholar]
- Varney MA, Cosford N, Jachec C, Rao SP, Sacaan A, Lin FF, Bleicher L, Santori EM, Flor PJ, Allgeier H, Gasparini F, Kuhn R, Hess SD, Veliçelebi G, Jonhson C (1999a) SIB-1757 and SIB-1893: selective, non-competitive antagonists of metabotropic glutamate receptor type 5. J Pharmacol Exp Ther 290: 170–181. [PubMed] [Google Scholar]
- Varney MA, Cosford N, Jachec C, Rao SP, Sacaan A, Santori EM, Allgeier H, Gasparini F, Flor PJ, Kuhn R, Hess SD, Veliçelebi G, Jonhson C (1999b) Characterization of SIB-1757 and SIB-1893: highly selective non-competitive antagonists at metabotropic glutamate receptor subtype 5 (mGluR5). Soc Neurosci Abstr 25: 976. [PubMed] [Google Scholar]
- Venero JL, Santiago M, Tomas-Camardiel M, Matarredona ER, Cano J, Machado A (2002) DCG-IV but not other group-II metabotropic receptor agonists induces microglial BDNF mRNA expression in the rat striatum. Correlation with neuronal injury. Neuroscience 113: 857–869. [DOI] [PubMed] [Google Scholar]