Abstract
Nonlinear microscopy has proven to be essential for neuroscience investigations of thick tissue preparations. However, the optical recording of fast (∼1 msec) cellular electrical activity has never until now been successfully combined with this imaging modality. Through the use of second-harmonic generation microscopy of primary Aplysia neurons in culture labeled with 4-[4-(dihexylamino)phenyl][ethynyl]-1-(4-sulfobutyl)pyridinium (inner salt), we optically recorded action potentials with 0.833 msec temporal and 0.6 μm spatial resolution on soma and neurite membranes. Second-harmonic generation response as a function of change in membrane potential was found to be linear with a signal change of ∼6%/100 mV. The signal-to-noise ratio was ∼1 for single-trace action potential recordings but was readily increased to ∼6–7 with temporal averaging of ∼50 scans. Photodamage was determined to be negligible by observing action potential characteristics, cellular resting potential, and gross cellular morphology during and after laser illumination. High-resolution (micrometer scale) optical recording of membrane potential activity by previous techniques has been limited to imaging depths an order of magnitude less than nonlinear methods. Because second-harmonic generation is capable of imaging up to ∼400 μm deep into intact tissue with submicron resolution and little out-of-focus photodamage or bleaching, its ability to record fast electrical activity should prove valuable to future electrophysiology studies.
Keywords: imaging, action potential, membrane potential, second-harmonic generation, microscopy, nonlinear, optical, voltage-sensitive dye
Introduction
The investigation of the electrical signaling properties of excitable cells such as neurons is predominately accomplished through the use of intracellular microelectrodes. Although these studies are useful for obtaining temporal electrical activity from a few locations on a single neuron (Stuart and Sakmann, 1994), they reveal little about the spatiotemporal modulations of membrane potential (Vm) over the entire cell or the activity of a population of neurons. Optical methods for monitoring Vm enable a greater understanding of the mechanisms underlying single neuron firing properties and cooperative electrical signaling in groups of neurons.
It is currently possible to follow cellular Vm activity optically through the use of linear one-photon methods (i.e., fluorescence, absorption, scattering, and birefringence). Slow transmembrane redistribution of dyes allows for Vm imaging with a high signal-to-noise ratio (S/N) but cannot provide the 1 msec temporal resolution needed to record fast Vm signals (Rink et al., 1980). Elegant green fluorescent protein (GFP) constructs (Knopfel et al., 2003) and fluorescence resonance energy transfer pairs (Gonzalez and Tsien, 1997) have been used to record Vm but can be limited either in their response time or in their ability to stain intact tissue (Zochowski et al., 2000), respectively. Methods using intrinsic changes in linear scattering or birefringence have been used to record action potentials (APs) in thin specimens (Cohen et al., 1968; Stepnoski et al., 1991), but recent attention has focused on fluorescent probes. These fast probes can respond to a Vm change of 100 mV with up to 10–20% changes in fluorescence emission (Grinvald et al., 1982; Loew et al., 1992; Rohr and Salzberg, 1994), although the response is typically limited to ∼1% in practice (Zochowski et al., 2000).
An innovative combination of high dye concentration, large illumination intensities, large collection areas, and/or very sensitive light detectors has allowed researchers to overcome these small signal changes and to image APs with an S/N of ∼10 and subthreshold events by temporal averaging at a spatiotemporal resolution of ∼10 μm and <1 msec (Zochowski et al., 2000). However, in thick preparations, high-resolution (micrometer scale) one-photon techniques are limited to imaging depths of approximately <50 μm by light scattering, making the poor spatial resolution deep in scattering tissues (such as neural tissue) the most severe limitation.
Nonlinear microscopy [such as two-photon fluorescence, second-harmonic (SH) generation (SHG), etc.] has the potential to solve this problem because of its ability to image at depths of up to ∼400 μm in scattering tissues with submicron resolution (Denk et al., 1990; Denk and Svoboda, 1997; Dombeck et al., 2003). Cells labeled with asymmetric styryl dyes generate an SH signal that is sensitive to Vm (Bouevitch et al., 1993; Ben-Oren et al., 1996; Campagnola et al., 1999). These dyes label only the outer leaflet of the lipid bilayer and therefore satisfy the noninversion symmetry requirement for the generation of an SH signal. Like other forms of nonlinear microscopy, SHG microscopy has imaging-resolution advantages over one-photon techniques deep in tissue and therefore has been the focus of considerable research to prove its capability to record fast (∼1 msec) cellular Vm signals such as APs. However, its sensitivity to Vm has only been demonstrated on fast time scales in model membrane systems such as giant unilamellar vesicles (Moreaux et al., 2003; Pons et al., 2003) or on slow time scales (∼1 sec) in sustained cultured cell lines (Milliard et al., 2003). To date, there has been no demonstration of the ability to record fast Vm activity in living cells with any form of nonlinear microscopy, and therefore ∼1 msec, high spatial resolution optical Vm recording has been limited to thin preparations or superficial regions of thick specimens. Here, we report the response of SHG from labeled Aplysia cell membranes during step changes in Vm and demonstrate 0.833 msec and 0.6 μm spatiotemporal resolution of APs with minimal background and photodamage during recording sessions.
Materials and Methods
Primary cell culture. Abdominal and cerebral ganglia from up to 15 different 5–10 gm Aplysia californica were dissociated and cultured with like ganglia (i.e., abdominal with abdominal, cerebral with cerebral) according to Goldberg and Schacher (1998). The cultures were grown in glass-bottom dishes (MatTek, Ashland, MA) at 17–18°C. For recordings, 4- to 14-d-old cultures were used.
Staining and imaging. We used 4-[4-(dihexylamino)phenyl][ethynyl]-1-(4-sulfobutyl)pyridinium (DHPESBP) (inner salt) [molecule B from Moreaux et al. (2003)] because of its proven fast (<150 μsec) response to Vm. SHG from giant unilamellar vesicles stained with this molecule was sensitive to Vm on fast time scales through an electrochromic (rather than reorientational) mechanism (Moreaux et al., 2003). Here, staining was accomplished by extracellular perfusion of 8 μm dye in SL-15 extracellular buffer (Goldberg and Schacher, 1998). The dye remained in the bath during the experiments, and the flip-flop time of a similar dye to the inner leaflet of a stained membrane was found previously to be ∼2 hr (Moreaux et al., 2001). The SHG imaging system has been described previously (Dombeck et al., 2003), except here, the transmitted light-condensing objective was replaced by a 0.9 numerical aperture (NA) condenser, modified to switch easily between differential interference contrast wide-field imaging and SHG collection. The focusing objective was also changed to a 20×, 0.75 NA Zeiss (Thornwood, NY) Fluar, making a diffraction-limited ∼0.6 μm radial diameter focal spot. The Ti:Sapphire laser was operated at 940 nm with ∼12 mW of average power at the sample, and a 460/30 bandpass filter was used for SHG detection in front of the bialkali photomultiplier tube. The excitation polarization was linear.
Optical and electrode voltage recording. Electrophysiological recordings of Aplysia neuron cultures were made at room temperature with an Axoclamp 2B amplifier and pClamp 8.1 software (Axon Instruments, Union City, CA). Two-electrode voltage clamp was used to clamp Vm at defined values. For stimulation of APs, the neuron was impaled with two microelectrodes (8–15 MΩ, filled with 3 m KCl), one for voltage recording and the other for current injection. AP stimulation was possible with <5 nA, ∼50 msec duration current pulses as used previously (Bedi et al., 1998; Rubakhin et al., 1999); however, shorter more intense pulses (30–100 nA, 1.0–3.5 msec duration) were used here to quickly reach threshold. This shorter stimulation protocol afforded an AP temporal stability of <0.5 msec peak voltage drift over the minutes of signal averaging that was not possible with the longer protocol (>3 msec drift), obviating the need for postprocessing. The necessary temporal resolution to optically detect APs with SHG was obtained using the line-scanning mode of the scanning system (600–1200 lines/sec). X–Y images were taken by raster scanning the focal spot over the two-dimensional field of view while one line was repeatedly scanned 256 times during line scanning. Each 8-bit image is built of 256 pixels in each dimension. The line scanning and stimulation or voltage-clamping steps were synchronized through trigger pulses coupling the amplifier and image acquisition software. Exact timing of the amplifier signals and line scanning was accomplished by recording the monitor output of the amplifier in one of the line-scan imaging channels. This enabled an increase in the S/N through line-scan averaging. Each recording session consisted of a number (n) of stimulation–line scans in series with pauses, 4 sec for two-electrode voltage clamp and 3 sec for AP stimulation experiments, to save the images and electrode recordings and reset the system for the next line scan. A typical n = 50 session totaled 2–3 min in duration.
Dye synthesis. The electroNLOchromic dye DHPESBP was obtained via a multistep synthesis scheme involving a Sonogashira key coupling reaction followed by alkylation with 1,4 butane sultone (T. Mallegol, O. Mongin, M. Blanchard-Desce, unpublished procedure).
Results
Spatiotemporal AP recordings with SHG microscopy
Figure 1, A and B, shows SHG images of a cultured Aplysia neuron stained with DHPESBP. Because of the noninversion symmetry requirement of SHG, a signal is only produced at the labeled membranes; no background from randomly oriented dye is seen. During line scanning of the green line in Figure 1B, voltage steps were applied to the cell in voltage clamp. The S/N was ∼1 for single traces, necessitating temporal averaging. This was accomplished by synchronizing the start of the line scan with the amplifier voltage steps. After repeated line-scan averaging (n = 50), the modulations in the SHG emission followed the voltage steps accurately with an S/N of ∼7. This line-scan average is scaled to the dynamic range of the SHG response to visualize the small change in emission (Fig. 1C). Negative voltage steps resulted in increased SHG emission, whereas positive voltage steps resulted in decreased emission (Fig. 1D). A linear response of the SHG with respect to ΔVm (linear best fit: ΔSHG/SHG = –0.06ΔVm; slope error = 0.004) was observed by applying a range of voltage steps (Fig. 1E).
Figure 1.
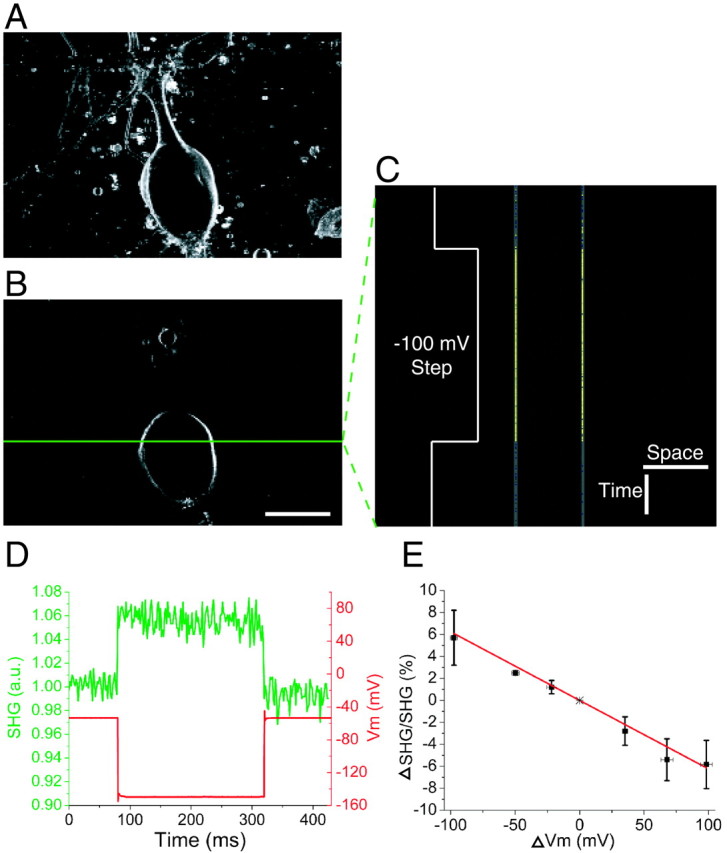
Line-scan recording of Vm with SHG during voltage steps in cultured Aplysia neurons. A, Projection image superimposing 21 ∼1-μm-thick z-sections 2 μm apart. B, A single z-section through the neuron in A at the plane of line scanning. The green line represents the scanned line where membrane potentials are recorded. C, SHG signal changes recorded by line scanning the line denoted in B at 600 lines/sec. The voltage-clamped neuron was given a 240 msec duration –100 mV step after 80 msec of scanning during each line scan. n = 50 line scans were averaged. The line scan image is scaled to visualize the small change in SHG emission. Calibration: 50 μm, 50 msec. D, The green trace, obtained from the left membrane line in C, is a normalized intensity plot of SHG emission versus time. The red line represents the measured Vm during the voltage-clamp step. a.u., Arbitrary units. E, Plot of ΔSHG/SHG over physiologically relevant ΔVm. The functional fit in red shows a linear relationship. Error bars represent SDs of three to five different measurements at each ΔVm ±∼4 mV. Note the inverse relationship between ΔSHG/SHG and ΔVm.
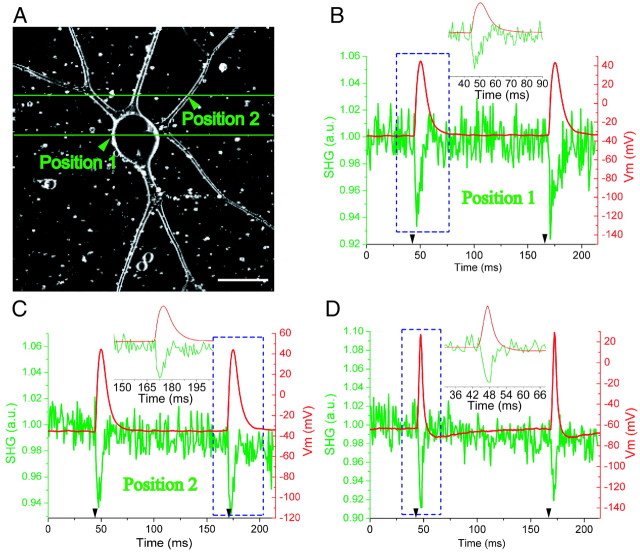
To prove the ability of SHG to record fast neuronal signals, APs were elicited and optically recorded. Line-scanning positions of two different recording sessions on an Aplysia neuron are represented by the green lines in Figure 2A, one through the soma and the other through several neurites. During each line scan, two ∼10- to 15-msec-duration APs, consistent with mixed Ca2+– Na+ APs (Gardner and Kerkut, 1968), were elicited through intracellular electrodes (Fig. 2B,C, red traces). Line-scan averaging (n = 50) showed that the optical intensity modulations in the SHG emission follow the fast Vm events with 0.6 μm spatial and 0.833 msec temporal resolution and an S/N of ∼6 (Fig. 2B,C, green traces). Note that the somatic recording electrode and SHG optical recording positions are different, so the AP shape and duration differ in some instances, as seen previously (Zecevic, 1996). The temporal response of SHG is easily capable of recording faster events such as the ∼4 msec duration APs, consistent with Na+ APs (Gardner and Kerkut, 1968), seen in Figure 2D. After line-scan averaging (n = 70), the S/N was ∼7 for this recording session.
Figure 2.
Fast SHG line-scan recording of APs at multiple sites in cultured Aplysia neurons. A, An SHG projection image of six ∼1-μm-thick z-sections 3 μm apart. Green lines represent the scanned lines. Scale bar, 50 μm. B, C, The green traces, obtained from the averaged line scans, are normalized intensity plots of SHG emission versus time at membrane position 1 and position 2, respectively, in A at 1200 lines/sec (0.833 msec/line). Two APs were elicited by current injection (timing of current pulses given by black arrowheads), one at 45 msec and the other at 170 msec, during each line scan. n = 50 line scans were averaged. The red trace is the Vm from the recording electrode at the soma. The inset is an expanded time base around the AP in the blue dashed box. C reveals the usefulness of high-resolution optical Vm recording by showing the clear difference in AP duration that can occur between the soma and neurites of individual neurons. D, Example of shorter APs from a different neuron than in A. n = 70 line scans were averaged.
The S/N in SHG recording can be understood by analyzing the noise in the baseline of the optical traces. For example, noise of ∼1% is seen in the baseline of Figure 2, B and C. Assuming a shot noise-limited system, this equates to a total of ∼10,000 photons collected per membrane during the 50 traces, yielding ∼200 photons during the laser dwell time across the membrane. Therefore, the noise is ∼7% and the S/N is ∼1 for n = 1.
Minimal phototoxicity during recordings
Little cellular photodamage was detected during the recording sessions with an average power of ∼12 mW, as in those examples presented here. To evaluate this, we first noted that the variation in resting potential was <4 mV during individual sessions, equivalent to nonillumination controls. Second, the elicited APs remained constant in amplitude and duration throughout recording sessions compared with nonillumination controls. Finally, no gross morphological changes were detectable in the soma or processes after individual sessions. Repeated optical recording from single neurons was possible over the course of ∼0.5 hr. When the power was increased to ∼22 mW, damage was observable. Recording from the soma was still possible with little damage, but recording from distal neurites resulted in blebbing or transection of the processes at the sites of line scanning. More studies are needed to investigate long-term damage effects and to determine the cause of the phototoxicity and possible avoidance procedures. Although little loss of the SHG signal was observed at ∼12 mW, it was more apparent at ∼22 mW.
Discussion
An eventual goal of optical Vm recording is to image subthreshold events, without averaging, deep in intact neural tissue with <1 μm and ∼1 msec spatiotemporal resolution. To reach this goal, the SHG S/N for Vm recording and imaging frame rate must be improved (i.e., full two-dimensional images must be obtained at the same temporal resolution demonstrated here for line scanning). Commonly used commercial scanning systems have a frame rate for full images of ∼1 Hz; thus, to obtain the necessary 1 kHz imaging frame rate, new scanning technologies must be implemented. Resonant galvanometer (Tsien and Bacskai, 1995)- and microlens (Kobayashi et al., 2002)-based scanners have been used for this purpose, but current demonstrations of these devices only achieve a frame rate of ∼33 Hz. Newer versions of this technology may show promise for faster scan rates (So, 2003). Another possibility is the use of random access scanning (Bullen et al., 1997; Bullen and Saggau, 1999) to scan over only the membrane region of interest.
Improvements in S/N can come from: (1) increased laser power, (2) higher dye concentrations in the membrane, (3) higher quantum yield detectors, or (4) better SHG molecules, with increases in both hyperpolarizibility and response to Vm. The number of emitted photons is proportional to the square of both the dye concentration and the fundamental laser intensity (Moreaux et al., 2000). Therefore, increases in either should lead to large decreases in the photon shot noise; methods for decreasing photo-damage must be investigated concurrently. Rapid progress is being made on option 3. New GaAsP photomultiplier tubes, for example, have greater quantum efficiency than the bialkali model used here. Option 4 will involve the design and screening of many dyes. It is likely that molecules with greater Vm response and increased hyperpolarizibility will be found.
To achieve the goal of Vm imaging with SHG under the guidelines above, many possible variables may be altered. For example, an ∼20% ΔSHG/100 mV dye with twice the hyperpolarizibility of DHPESBP, stained and illuminated at twice the concentration and incident power used here, should be capable of recording a 10 mV subthreshold event with n = 1 and an S/N of >1.
Recording Vm activity deep in tissue may be possible in the near future, but sample thickness, staining procedures, and tissue type must first be carefully evaluated. Intrinsic SHG imaging at depths of up to 300–400 μm with submicron resolution has been demonstrated previously in hippocampal brain slices (Dombeck et al., 2003). Because SHG is collected in the transmitted light direction, sample thickness is limited to approximately <500 μm. Staining thick turbid media, such as intact ganglia or mammalian neural tissue, has been accomplished by bath-applying dye (Delaney et al., 1994), but other techniques such as pressure injection of dye (Stosiek et al., 2003), intracellular labeling (Antic et al., 1999), novel GFP constructs (Knopfel et al., 2003), or the addition of dye crystals into tissue (Gan et al., 2000; Moreaux et al., 2001) have been needed previously to stain at these depths. Additionally, varying preparations have led previously to different sensitivities of the same Vm dyes (Zochowski et al., 2000), but a greater issue for SHG dyes may be their effects on more delicate mammalian cells.
With these issues in mind, numerous applications should be possible with SHG Vm recording. The current capabilities demonstrated here are most immediately applicable to reproducibly stimulated systems, as are often used for current optical Vm recording techniques (Zecevic, 1996). It should be feasible to investigate spike initiation zones (Zecevic, 1996) and AP propagation properties (Fromherz and Muller, 1994; Meyer et al., 1997) deep in intact ganglia, where one-photon methods are not appropriate. Repeated line scans at varying spatial positions over processes will allow for the generation of high-resolution time series movies of AP propagation, leading to the experimental analysis of electrical propagation properties at complex structures, such as axon bifurcations and varicosities. In addition, SHG Vm recording simultaneously with powerful Ca2+ imaging techniques (Szmacinski et al., 1993; Yuste and Denk, 1995) should prove to be a useful combination.
Any increase in spatial resolution is valuable for the investigation of Vm properties of small structures (i.e., dendritic spines) or for providing higher-resolution data of AP propagation. The spatial resolution of ∼0.6 μm demonstrated here represents the highest-resolution optical recording of fast Vm activity to date. The potential usefulness of this resolution for AP propagation studies in which shape and duration vary depending on position is seen in Figure 2C, where these differences are clear. Theoretically, similar spatial resolution is possible for the fast one-photon techniques while maintaining the same S/N; however, a higher illumination intensity would be needed, likely resulting in greater photodamage. The highest demonstrated resolution of ∼2 μm was demonstrated by one-photon random-access scanning, but like other linear methods, it was limited to thin specimens (Bullen et al., 1997; Bullen and Saggau, 1999).
Here, we demonstrated that SHG microscopy is capable of recording APs from excitable neurons with 0.833 msec temporal resolution at the highest spatial resolution yet reported for fast optical Vm recordings. This technique for optically recording Vm holds the following important advantages over current techniques: submicron resolution at depths up to ∼400 μm in scattering tissue, little out-of-focus photodamage or bleaching, and no background. The above work represents a key step toward ∼1 msec time-scale, high-resolution Vm imaging deep in intact neural tissue. We expect that rapid progress will continue to be made toward this goal in the near future.
Footnotes
This work was supported by Centre National de la Recherche Scientifique (Action Coordonnée Optique and Physique et Chimie du Vivant grants) to M.B.D. and National Institutes of Health Grants 9 P41 EB001976-16 and GM08267 to D.A.D. and W.W.W. We thank T. Mallegol for the synthesis of the DHPESBP dye and J. Mertz, B. Johnson, P. Kloppenburg, and many Webb group members for helpful discussions and careful reading of this manuscript. We appreciate the R. Harris-Warrick laboratory for the use of its microelectrode puller and for electrophysiology advice and guidance.
Correspondence should be addressed to Watt W. Webb, Applied Physics, Cornell University, 223 Clark Hall, Ithaca, NY 14853. E-mail: www2@cornell.edu.
DOI:10.1523/JNEUROSCI.4840-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/240999-05$15.00/0
References
- Antic S, Major G, Zecevic D (1999) Fast optical recordings of membrane potential changes from dendrites of pyramidal neurons. J Neurophysiol 82: 1615–1621. [DOI] [PubMed] [Google Scholar]
- Bedi SS, Salim A, Chen S, Glanzman DL (1998) Long-term effects of axotomy on excitability and growth of isolated Aplysia sensory neurons in cell culture: potential role of cAMP. J Neurophysiol 79: 1371–1383. [DOI] [PubMed] [Google Scholar]
- Ben-Oren I, Peleg G, Lewis A, Minke B, Loew L (1996) Infrared nonlinear optical measurements of membrane potential in photoreceptor cells. Biophys J 71: 1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouevitch O, Lewis A, Pinevsky I, Wuskell JP, Loew LM (1993) Probing membrane potential with nonlinear optics. Biophys J 65: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen A, Saggau P (1999) High-speed, random-access fluorescence microscopy. II. Fast quantitative measurements with voltage-sensitive dyes. Biophys J 76: 2272–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen A, Patel SS, Saggau P (1997) High-speed, random-access fluorescence microscopy. I. High-resolution optical recording with voltage-sensitive dyes and ion indicators. Biophys J 73: 477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnola PJ, Wei MD, Lewis A, Loew LM (1999) High-resolution nonlinear optical imaging of live cells by second harmonic generation. Biophys J 77: 3341–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LB, Keynes RD, Hille B (1968) Light scattering and birefringence changes during nerve activity. Nature 218: 438–441. [DOI] [PubMed] [Google Scholar]
- Delaney KR, Gelperin A, Fee MS, Flores JA, Gervais R, Tank DW, Kleinfeld D (1994) Waves and stimulus-modulated dynamics in an oscillating olfactory network. Proc Natl Acad Sci USA 91: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Svoboda K (1997) Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18: 351–357. [DOI] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248: 73–76. [DOI] [PubMed] [Google Scholar]
- Dombeck DA, Kasischke KA, Vishwasrao HD, Ingelsson M, Hyman BT, Webb WW (2003) Uniform polarity microtubule assemblies imaged in native brain tissue by second-harmonic generation microscopy. Proc Natl Acad Sci USA 100: 7081–7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromherz P, Muller CO (1994) Cable properties of a straight neurite of a leech neuron probed by a voltage-sensitive dye. Proc Natl Acad Sci USA 91: 4604–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW (2000) Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron 27: 219–225. [DOI] [PubMed] [Google Scholar]
- Gardner DR, Kerkut GA (1968) A comparison of the effects of sodium and lithium ions on action potentials from Helix aspersa neurones. Comp Biochem Physiol 25: 33–48. [DOI] [PubMed] [Google Scholar]
- Goldberg D, Schacher S (1998) Culturing large neurons of Aplysia In: Culturing nerve cells, Ed 2 (Banker G, Goslin K, eds), pp 213–236. Cambridge, MA: MIT.
- Gonzalez JE, Tsien RY (1997) Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem Biol 4: 269–277. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Hildesheim R, Farber IC, Anglister L (1982) Improved fluorescent probes for the measurement of rapid changes in membrane potential. Biophys J 39: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopfel T, Tomita K, Shimazaki R, Sakai R (2003) Optical recordings of membrane potential using genetically targeted voltage-sensitive fluorescent proteins. Methods 30: 42–48. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Fujita K, Kaneko T, Takamatsu T, Nakamura O, Kawata S (2002) Second-harmonic generation microscope with a microlens array scanner. Opt Lett 27: 1324–1326. [DOI] [PubMed] [Google Scholar]
- Loew LM, Cohen LB, Dix J, Fluhler EN, Montana V, Salama G, Wu JY (1992) A naphthyl analog of the aminostyryl pyridinium class of potentiometric membrane dyes shows consistent sensitivity in a variety of tissue, cell, and model membrane preparations. J Membr Biol 130: 1–10. [DOI] [PubMed] [Google Scholar]
- Meyer E, Muller CO, Fromherz P (1997) Cable properties of dendrites in hippocampal neurons of the rat mapped by a voltage-sensitive dye. Eur J Neurosci 9: 778–785. [DOI] [PubMed] [Google Scholar]
- Millard AC, Jin L, Lewis A, Loew LM (2003) Direct measurement of the voltage sensitivity of second-harmonic generation from a membrane dye in patch-clamped cells. Opt Lett 28: 1221–1223. [DOI] [PubMed] [Google Scholar]
- Moreaux L, Sandre O, Mertz J (2000) Membrane imaging by second-harmonic generation microscopy. J Opt Soc Am B 17: 1685–1694. [Google Scholar]
- Moreaux L, Sandre O, Charpak S, Blanchard-Desce M, Mertz J (2001) Coherent scattering in multi-harmonic light microscopy. Biophys J 80: 1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreaux L, Pons T, Dambrin V, Blanchard-Desce M, Mertz J (2003) Electro-optic response of second-harmonic generation membrane potential sensors. Opt Lett 28: 625–627. [DOI] [PubMed] [Google Scholar]
- Pons T, Moreaux L, Mongin O, Blanchard-Desce M, Mertz J (2003) Mechanisms of membrane potential sensing with second-harmonic generation microscopy. J Biomed Opt 8: 428–431. [DOI] [PubMed] [Google Scholar]
- Rink TJ, Montecucco C, Hesketh TR, Tsien RY (1980) Lymphocyte membrane potential assessed with fluorescent probes. Biochim Biophys Acta 595: 15–30. [DOI] [PubMed] [Google Scholar]
- Rohr S, Salzberg BM (1994) Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J 67: 1301–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubakhin SS, Li L, Moroz TP, Sweedler JV (1999) Characterization of the Aplysia californica cerebral ganglion F cluster. J Neurophysiol 81: 1251–1260. [DOI] [PubMed] [Google Scholar]
- So PTC (2003) Modern applications of 3-D optical microscopy in biology and medicine. Conference on Lasers and Electro-Optics–Quantum Electronics and Laser Science Conference, Baltimore, June.
- Stepnoski RA, LaPorta A, Raccuia-Behling F, Blonder GE, Slusher RE, Kleinfeld D (1991) Noninvasive detection of changes in membrane potential in cultured neurons by light scattering. Proc Natl Acad Sci USA 88: 9382–9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A (2003) In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B (1994) Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367: 69–72. [DOI] [PubMed] [Google Scholar]
- Szmacinski H, Gryczynski I, Lakowicz JR (1993) Calcium-dependent fluorescence lifetimes of Indo-1 for one- and two-photon excitation of fluorescence. Photochem Photobiol 58: 341–345. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Bacskai BJ (1995) Video-rate confocal microscopy. In: Handbook of biological confocal microscopy, Ed 2 (Pawley JB, ed), pp 459–477. New York: Plenum.
- Yuste R, Denk W (1995) Dendritic spines as basic functional units of neuronal integration. Nature 375: 682–684. [DOI] [PubMed] [Google Scholar]
- Zecevic D (1996) Multiple spike-initiation zones in single neurons revealed by voltage-sensitive dyes. Nature 381: 322–325. [DOI] [PubMed] [Google Scholar]
- Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic S, Zecevic D (2000) Imaging membrane potential with voltage-sensitive dyes. Biol Bull 198: 1–21. [DOI] [PubMed] [Google Scholar]