Abstract
Hox genes are critical for establishing the segmental pattern of the vertebrate hindbrain. Changes in their expression can alter neural organization of hindbrain segments and may be a mechanism for brain evolution. To test the hypothesis that neurons induced through changes in Hox gene expression can integrate into functional neural circuits, we examined the roles of ectopic Mauthner cells (M-cells) in the escape response of larval zebrafish. The activity of the paired Mauthner cells in rhombomere 4 (r4) has been shown to be critical for generating a high-performance startle behavior in response to stimulation of the tail (Liu and Fetcho, 1999). Previous studies have found that misexpression of particular Hox genes causes ectopic M-cells to be generated in r2 in addition to the r4 cells (Alexandre et al., 1996; McClintock et al., 2001). With calcium imaging, we found that the homeotically transformed neurons respond to startle stimuli. To determine the roles of ectopic and endogenous M-cells in the behavior, we lesioned the r2, r4, or both M-cells with cell-specific laser lesion and examined the effect on startle performance. Lesion of the normal M-cells did not decrease escape performance when the ectopic cells were present. These results indicate that the homeotically transformed Mauthner cells are fully functional in the escape circuit and are functionally redundant with normal M-cells. We suggest that such functional redundancy between neurons may provide a substrate for evolution of neural circuits.
Keywords: hindbrain, Hox genes, homeosis, Mauthner cell, startle, escape response
Introduction
Hox genes are critical for appropriate segmental patterning of the anteroposterior axis of the hindbrain. Alteration of Hox gene expression in the early embryo can result in changes in hindbrain segment identity and may be a mechanism for brain evolution (for review, see Lumsden and Krumlauf, 1996; Moens and Prince, 2002). Although the morphology of homeotically induced neurons has been examined, the functions of such cells have just begun to be determined. Recent work (del Toro et al., 2001) has demonstrated that in Hoxa1–/– mutant mice, loss of cells in the respiratory rhythm circuit is compensated in part by ectopic neurons that are integrated into novel breathing circuits. These data indicate that the brain can incorporate Hox-induced morphological changes at many levels of organization to generate a functioning organism.
Here, we used Hox gene misexpression to investigate the function of homeotically induced reticulospinal neurons and the integration of normal and ectopic cells in neural circuits. Ectopic expression of the zebrafish hoxb1b gene causes a posteriorizing transformation of the segmentally organized hindbrain at the level of rhombomere 2 (r2) (Alexandre et al., 1996; McClintock et al., 2001). In response to ectopic hoxb1b, r2 takes on multiple aspects of the character of the more posterior r4. These include expression of r4 marker genes and alterations in segment-specific neuronal identity. In particular, the large paired r4-characteristic Mauthner cells (M-cells) are duplicated in r2 (Alexandre et al., 1996; McClintock et al., 2001).
The bilateral Mauthner cells play a dedicated role in the initiation of the escape response (for review, see Zottoli and Faber, 2000). The M-cells and the startle behavior they elicit have provided a useful model for examining neural control of movement. The excitation of this circuit results in strong muscle activity contralateral to the stimulus and inhibition of ipsilateral activity (Fetcho and Faber, 1988). The resulting behavior is a rapid, forceful bend of the body and tail into a characteristic C-shape, turning away from the stimulus direction. We examined the effect of homeotic transformations in the escape circuit on function to investigate how new cells are integrated into pre-existing neural circuits.
We selectively lesioned the r2, the r4, or both M-cells on one side of the brain and examined the effect of the lesion on the kinematics of startle behavior. We found that the ectopic r2 M-cell can elicit a normal, high-performance startle response in the absence of the r4 M-cell. The presence of both cells did not confer higher startle performance than either r2 or r4 M-cells alone. Thus, the r2 and r4 Mauthner cells appear to be functionally redundant, suggesting that a homeotic transformation may add operative cells to a pre-existing neural circuit without changing the functionality of that circuit. We suggest that such cells may provide a substrate for circuit evolution.
Materials and Methods
Hoxb1b mRNA and embryo injection. Synthetic capped hoxb1b mRNA was synthesized from linearized pCS2hoxb1b DNA template (McClintock et al., 2001) using the Ambion (Austin, TX) Megascript kit according to the instructions of the manufacturer. mRNA was microinjected into one to two cell-stage zebrafish embryos at 50 ng/μl in phenol red buffer (0.25% phenol red, 120 mm KCl, and 20 mm HEPES–NaOH, pH 7.5). Fish were maintained in embryo medium (until ∼48 hr) and 10% HBSS (after ∼48 hr).
Labeling Mauthner neurons in larval zebrafish. At 3 d postfertilization (dpf), zebrafish were visually screened for morphological abnormalities that occasionally result from early embryonic injections. Fish that appeared normal were injected with 50% fluorescein dye [molecular weight of 10,000 (MW); Molecular Probes, Eugene, OR] in 10% HBSS for lesioning experiments or with a combination of 44% calcium green dextran (MW of 10,000; Molecular Probes) and 5.5% Texas Red dextran (MW of 10,000; Molecular Probes) for calcium imaging. The injection procedure followed that of Fetcho and O'Malley (1995). Fish were anesthetized with 0.02% 3-aminobenoic acid ethyl ester (Sigma, St. Louis, MO). A fine-tipped microelectrode connected to a Picospritzer (General Valve, Fairfield, NJ) was used to pressure inject dye into the caudoventral spinal cord to hit the M-cell axons. After injection, fish were returned to 10% HBSS to recover. At 4 dpf, fish were screened for labeled M-cells in r2 and r4. Fish with either unilateral or bilateral M-cells in r2 were selected for physiological and behavioral experiments. Note that because our retrograde labeling was performed with an unusually fine-tipped microelectrode to avoid morphological damage, fish with apparent unilateral duplications may have had bilateral duplication that was not detected.
Calcium imaging. The activity of r2 M-cells in response to startle stimuli was assessed by calcium imaging on the confocal microscope (Fetcho and O'Malley, 1995) in five fish. At 5 dpf, fish were anesthetized as described previously, rinsed in 10% HBSS, and embedded in a 1.2% agar solution dorsal-side down onto a coverslip that formed the floor of a 3.5 cm Petri dish. The live mounted fish was covered with HBSS to prevent drying. The dish was placed on the stage of an inverted confocal microscope (model 510; Zeiss, Thornwood, NY), and a fine glass tapper actuated with an electric current was positioned against the side of the body on the ipsilateral side to the M-cells being investigated, as described previously (Liu et al., 2003). A time series of images was recorded beginning before application of the startle stimulus and lasting until the fluorescence levels in the cell returned to baseline. By simultaneously examining both the response of calcium-sensitive dye and the response of the calcium-insensitive Texas Red dextran, we could correct the change in fluorescence for any movement artifact during the response.
Laser lesioning of Mauthner cells. To lesion M-cells, fish were first anesthetized and embedded in a light agar solution, as described for calcium imaging. The general lesion protocol of Liu and Fetcho (1999) was used. An argon laser at full strength was focused on the middle of the r2 or r4 M-cell with a 63× water objective. The laser scanned for 10–13 min per cell. Longer scanning times were necessary when the fish was heavily pigmented over the hindbrain. After scanning, the fish was gently removed from the agar with fine forceps and held in HBSS overnight before the postlesion behavior was conducted. The success of cell lesions was confirmed after the postlesion behavioral trials using the confocal imaging procedures described above.
Recording startle behavior. Startle behavior of individual fish was recorded before and after cell lesion. At 5 dpf, prelesion behavioral trials were performed, and at 6 dpf, postlesion trials were performed using the general protocol of Liu and Fetcho (1999) and Liu et al. (2003). A touch to the side of the body was used as the startle stimulus. Fish were placed in 3.5 cm Petri dish in filtered 10% HBSS and filmed with a video camera mounted onto a dissecting microscope. High-speed video (1000 Hz; PCI 2000S; Redlake, San Diego, CA) was used to record behavioral responses to touching the tail with a blunt-tipped glass microelectrode. We used a tail stimulus in these experiments, because Liu and Fetcho (1999) found that the M-cells are critical for generating a high-performance tail-elicited escape. Responses to touching both the right and left sides of the tail were recorded to compare between turns to lesioned and nonlesioned sides of the body. There was no significant decrease in performance across the responses of each fish, indicating that the animal did not fatigue or habituate to the stimulus during filming. The same protocol was used to record responses after M-cell lesions were performed (see below).
Behavioral analysis. Startle responses of zebrafish were analyzed with an automated digitizing program custom designed by Dr. J. R. Fetcho (State University of New York at Stony Brook, Stony Brook, NY) as a LabView (National Instruments, Austin, TX) virtual instrument. Trials were analyzed at 1000 frames per second of temporal resolution. We examined several parameters identified previously as being determined in part by M-cell activity (Liu and Fetcho, 1999). In particular, we focused on the angle of head movement in the initial body bend and the peak angular velocity of this movement. Three trials per direction were examined for each of five fish in each condition: r2 M-cell lesion, r4 M-cell lesion, and r2 plus r4 M-cell lesion. Prelesion and postlesion trials were compared, as well as trials to the intact and the lesioned side. In addition, control M-cell lesions in normal fish were conducted on four individuals (three trials per individual for each stimulus, left and right side prelesion, left and right side postlesion). We used an ANOVA with repeated measures (JMP 3.1.6; SAS Institute, Cary, NC) to analyze the results of the lesion experiments.
Results
Ectopic gene expression as an approach for examining neural circuit function
Methodological approaches pioneered for studies of developmental genetics can provide powerful tools for altering nervous system morphology and function. Here, we made use of an ectopic gene expression strategy to produce altered brain anatomy such that ectopic Mauthner cells form in r2, simulating homeosis. We then used imaging and lesioning approaches to examine the consequences of altering neuronal disposition on both neural circuitry and behavior. In combination, these approaches not only provide insight into homeotic processes but also a simple model for examining basic principles of neural circuit organization and function.
Ectopic expression of zebrafish hoxb1b has been shown previously to result in duplication of the M-cells and several other neurons typical of r4 in r2 (Fig. 1A,B) (Alexandre et al., 1996; McClintock et al., 2001). No other phenotypic consequences have been reported in response to ectopic expression of hoxb1b (Alexandre et al., 1996; McClintock et al., 2001). Using retrograde labeling, we found both unilateral and bilateral M-cell duplications in r2, as reported previously. We found that the ectopic M-cells are very similar morphologically to the r4 M-cells. Thus, a single cell lies in a hemisegment of r2, with laterally and ventrally projecting dendrites and a large commissural axon that descends the full length of the spinal cord. Using retrograde labeling, we found that the r2 and r4 Mauthner axons lie in close proximity to one another in the ventral spinal cord (Fig. 1C, arrows), suggesting that the ectopic r2 M-cells may be integrated into functional circuits.
Figure 1.
Morphology of ectopic Mauthner cells. A, B, Reticulospinal neurons in the hindbrain of normal (A) and hoxb1b mRNA-injected (B) larval zebrafish. The large, paired Mauthner neurons are labeled bilaterally in r4. After injection of hoxb1b mRNA into the single-cell embryo, an additional Mauthner cell has formed in r2; in the image to the right, the r2 M-cell is labeled on the left side of the hindbrain. Scale bar, 50 μm. C, Axons of r2 and r4 Mauthner cells (blue arrows) in the trunk region of the spinal cord. Scale bar, 5 μm.
The rhombomere 2 Mauthner cell responds to startle stimuli
Before examining the possible role of r2 Mauthner cells in startle behavior, we used calcium imaging to determine whether the r2 M-cell was active in response to a startle stimulus (a tail touch) that is known to elicit a response in an r4 M-cell. We retrograde labeled M-cells with calcium-sensitive dye and recorded their activity in response to a touch–startle stimulus using time-lapse confocal imaging (n = 5 fish). Figure 2A shows the typical response of an r2 M-cell to a startle stimulus; fluorescence in the cell increased by nearly 40% during the response (Fig. 2B), indicating that the neuron had fired an action potential. The ability of ectopic r2 M-cells to respond to the same startle stimulus as r4 M-cells shows that they receive appropriate sensory input and suggests that they may play a role in the behavioral response.
Figure 2.
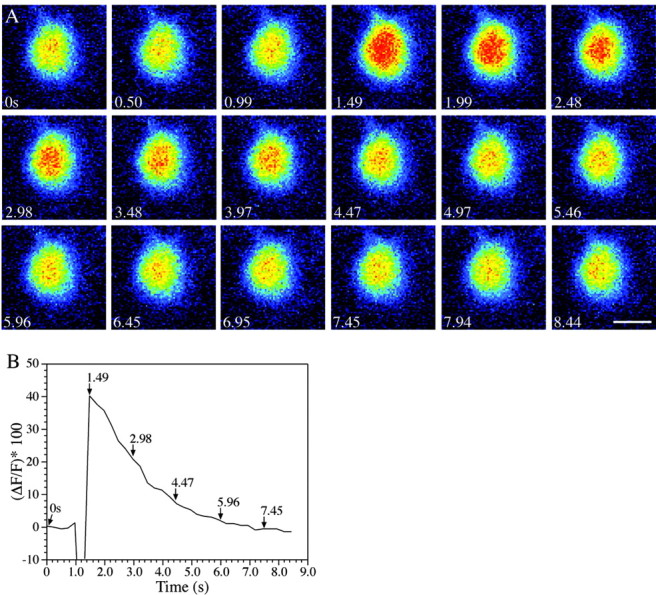
An ectopic r2 Mauthner cell responds to startle stimuli. A, A time series of images progressing left to right across rows beginning at time point 1 shows that the cell responds (1.49 sec), as indicated by increasing orange and red pixels; a pseudocolor demonstration of increasing brightness of fluorescence was applied by LSM 510 software (Zeiss). After the response, calcium is sequestered, and the cell returns to its resting fluorescence levels, as shown in the second and third rows. Scale bar, 10 μm. B, The r2 Mauthner cell responds to startle stimulation. Calcium green dextran (MW of 10,000; Molecular Probes) was used to visualize the response of the r2 M-cell to a touch–startle stimulus. The graph shows the percentage change in cell brightness through the duration of the response (time in seconds). Times indicated by arrows refer to image times in Figure 1C. The initial decrease in brightness after the stimulus was attributable to movement artifact from the attempted startle. There is an ∼40% change in the brightness of the r2 Mauthner cell in response to touch–startle stimulation.
Larval zebrafish with ectopic r2 Mauthner cells show a normal startle behavior
Because misexpression of hoxb1b causes major changes in zebrafish hindbrain organization (McClintock et al., 2001), we investigated whether the homeotically transformed larvae show a normal startle response. We compared the behavioral responses of normal larval zebrafish with those with ectopic r2 M-cells and found that both performed a normal startle behavior, consisting of a rapid turn away from the stimulus and subsequent burst swimming (Fig. 3A). We further examined both the angle that the head turned through during the initial body bend and the peak angular velocity of that turn, because these two parameters have been shown previously to be significantly reduced in response to Mauthner cell lesion in normal larvae (Liu and Fetcho, 1999). We found that head movement angle and peak angular velocity did not differ significantly between normal zebrafish larvae and those with M-cell duplications resulting from hoxb1b misexpression (Table 1). Our results show that larvae with ectopic r2 M-cells show startle performance equivalent to that of normal larvae. We conclude that startle behavior is neither negatively nor positively impacted by the presence of ectopic r2 M-cells.
Figure 3.
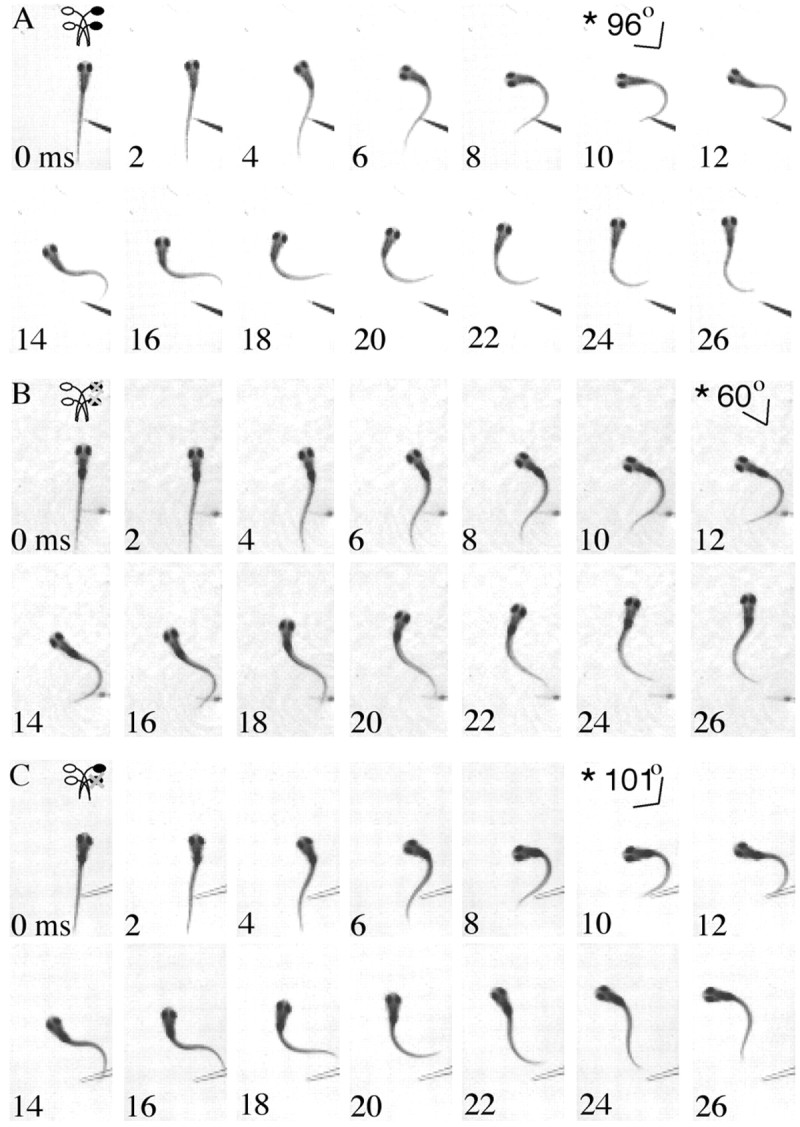
Time series illustrating postablation startles of larval zebrafish. The angle of the head in frames indicated by an asterisk shows the peak angle at the end of the initial lateral bend compared with the position of the body just before movement (0 msec). Fish are startled with a fine glass probe, and their behavior is recorded with high-speed video at 1000 Hz. A, A time series of images illustrating startle in a fish with both r2 and r4 M-cells. The fish turns ∼96° at 10 msec. The initial body bend is followed by continued swimming movements reflective of a typical larval zebrafish startle. B, Startle after both the r2 and r4 Mauthner cells are lesioned. The fish turns away from the tactile stimulus, forming a peak angle of 60° at 12 msec. The startle is followed by rhythmic swimming. C, The same pattern of startle behavior is seen in fish after the r4 Mauthner cell is ablated with a turn away from the stimulus. However, the angle at the end of the initial bend is higher than that seen with both cells lesioned, 101° at 10 msec. The startle is followed by rhythmic swimming.
Table 1.
Kinematic parameters of startle behavior before and after Mauthner cell ablation
|
|
Head angle (°) |
Head angular velocity (°/msec) |
|---|---|---|
| Ectopic M-cells in R2a | ||
| R2 and R4 ablation | ||
| Preablation | ||
| Intact side | 119.12 (5.80)c | 19.34 (1.02) |
| Ablation side | 113.76 (4.07) | 18.15 (0.79) |
| Postablation | ||
| Intact side | 111.28 (4.42) | 18.02 (1.02) |
| Ablation side | 88.10 (4.49) | 14.28 (0.86) |
| R2 ablation | ||
| Preablation | ||
| Intact side | 118.59 (6.01) | 20.83 (0.74) |
| Ablation side | 112.44 (5.95) | 19.00 (0.89) |
| Postablation | ||
| Intact side | 122.48 (4.91) | 20.96 (0.92) |
| Ablation side | 122.74 (4.85) | 19.31 (0.89) |
| R4 ablation | ||
| Preablation | ||
| Intact side | 108.71 (6.18) | 19.24 (0.73) |
| Ablation side | 118.27 (3.22) | 20.50 (0.81) |
| Postablation | ||
| Intact side | 109.02 (5.43) | 18.62 (1.16) |
| Ablation side | 108.69 (6.23) | 17.47 (0.80) |
| Normal fishb | ||
| R4 ablation | ||
| Preablation | ||
| Intact side | 123.76 (4.44) | 19.85 (0.42) |
| Ablation side | 113.36 (4.51) | 18.01 (1.25) |
| Postablation | ||
| Intact side | 108.59 (6.48) | 19.93 (0.67) |
| Ablation side |
80.96 (2.97) |
14.56 (0.64) |
Angle and angular velocity of head movement are shown before and after ablation in Mauthner cells in rhombomeres 2 and 4, rhombomere 2, or rhombomere 4, and before and after rhombomere 4 M-cell ablation in normal fish. The ablation side refers to startles toward the side of the body to which the targeted neurons project, whereas the intact side refers to turns to the opposite side of the body.
Fish with ectopic Mauthner cells: n = 5 fish per ablation regime; three trials per treatment per fish.
Normal fish: n = 3 fish; three trials per treatment per fish.
Mean ± SEM.
Simultaneous lesion of both r2 and r4 M-cells disrupts the startle response
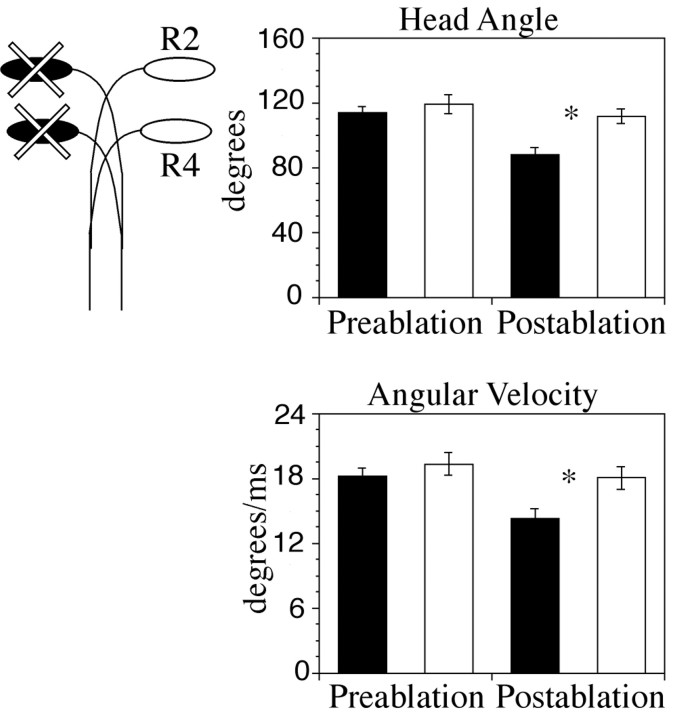
To begin to explore the functionality of the M-cells, we used a laser lesion strategy to remove labeled M-cells while leaving surrounding cells intact and assessed how this deletion affected performance. To establish baseline responses for each individual before cell lesions, we recorded startle behaviors at 5 dpf (Table 1). We then lesioned both the r2 and the r4 M-cells from one side of the hindbrain. After recovery overnight, we assessed the startle behavior of each larva. As a control for developmental changes between 5 and 6 dpf, we compared prelesion and postlesion startles toward the intact side. We found no significant difference in either head angle or angular velocity (p = 0.143 and p = 0.2852, respectively). In contrast, we recorded significantly reduced head angle (p < 0.007) and peak angular velocity (p < 0.003) in turns to the side of the body normally controlled by the lesioned cells compared with postlesion trials of turns to the opposite side of the body, which should not be affected by the lesion (Figs. 3B, 4). We found similar differences comparing the postlesion turns to the side of the body normally controlled by the ablated cells with prelesion turns to that same side [p < 0.02 (angle) and p < 0.0008 (angular velocity)].
Figure 4.
Startle kinematics before and after r2 and r4 Mauthner cell lesions. On the left, diagrams of Mauthner cell arrangement illustrate which cell or cells were lesioned. Turns toward the side that normally receives input from the ablated side are illustrated with black bars; turns toward the opposite side are illustrated with white bars. After ablation of both r2 and r4 Mauthner cells, there was a significant decrease (indicated by asterisks) in both head angle and angular velocity in turns toward the side of the body that would normally receive input from the side of the hindbrain in which cells were ablated. Means and SE for treatment are shown in Table 1.
We compared head angle and angular velocity of these experimental larvae with results from normal larvae in which an r4 M-cell was ablated to compare the roles of M-cells in the startles of normal zebrafish and zebrafish with M-cells in r2 and r4. We found no significant difference between these populations for either turn angle (p = 0.84) or angular velocity (p = 0.28) (Table 1). We conclude that lesion of both r2 and r4 M-cells in our experimental animals disrupts the startle response in a similar manner to lesion of the r4 M-cell in a normal larva.
Both r2 and r4 M-cells can mediate startle responses
To further explore the roles of the r2 and r4 M-cells in behavior, we selectively lesioned r2 or r4 M-cells to investigate their individual functions in the startle circuit. When we lesioned an r2 M-cell from a homeotically transformed larva, leaving intact the r4 M-cell, we found no significant difference (p > 0.05) in head angle or angular velocity when compared between prelesion and postlesion trials or between postlesion turns to the intact and ablated sides (Fig. 5A). The unaltered startle response of these larvae in the absence of the ectopic r2 M-cell demonstrated that the r4 M-cell functions normally within the transformed hindbrain.
Figure 5.
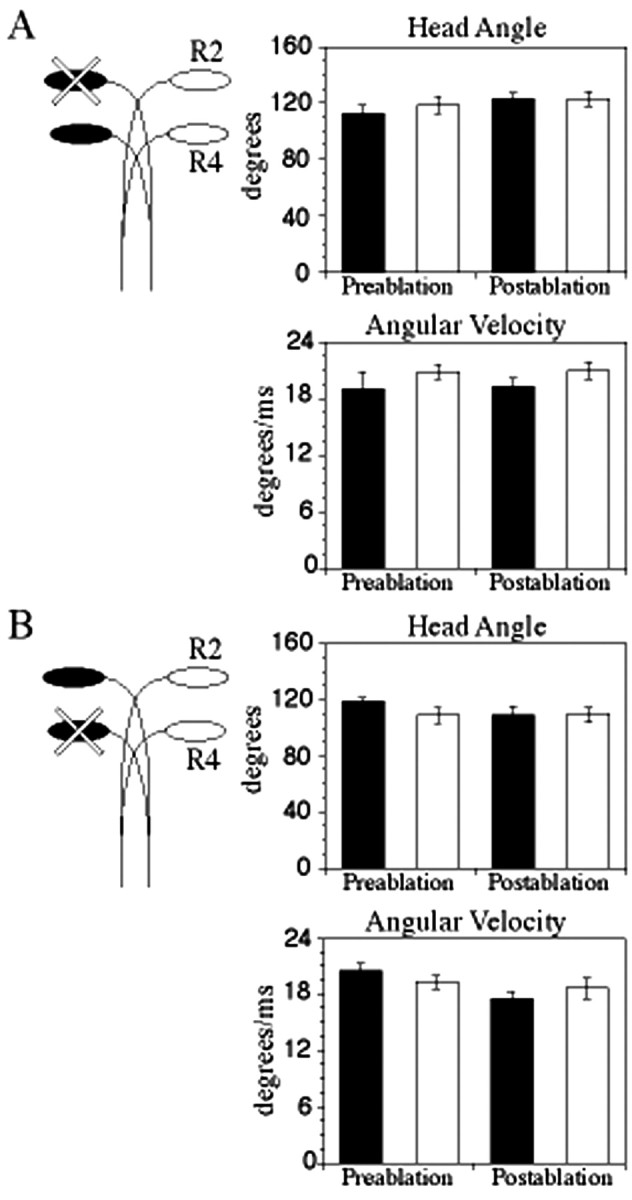
Startle kinematics before and after either r2 or r4 Mauthner cell lesions. On the left, diagrams of Mauthner cell arrangement illustrate which cell or cells were lesioned. Turns toward the side that normally receives input from the ablated side are illustrated with black bars; turns toward the opposite side are illustrated with white bars. There was no significant difference in postablation turns when either the r2 or the r4 Mauthner cell was ablated. Means and SE for treatments are shown in Table 1.
We next investigated function of ectopic r2 M-cells in the absence of r4 cells by lesioning the r4 Mauthner cell. In normal larvae, this manipulation resulted in a decrease in head angle and angular velocity (Table 1) (Liu and Fetcho, 1999). In contrast, in fish with ectopic r2 M-cells, lesion of r4 M-cells caused no significant decrease (p > 0.05) in either head angle or angular velocity when comparing postlesion with prelesion trials or postlesion turns to the ablated and intact side (Figs. 3C, 5B). These results demonstrated that activity of the ectopic r2 M-cell was sufficient to initiate a normal startle response, revealing that the ectopic M-cell had integrated into a fully functional neural circuit. We conclude that the functions of ectopic r2 and endogenous r4 M-cells are redundant: the r2 M-cell can elicit a startle behavior with performance parameters equivalent to those elicited by coactive r2 and r4 M-cells or the r4 M-cell alone.
Discussion
We combined Hox gene misexpression with neural imaging, cell-specific lesion, and behavioral analysis to investigate how duplicated neurons are integrated into neural circuits and behaviors and, more specifically, how neurons generated by homeotic processes function. We demonstrate that a combination of molecular genetic approaches with neural and behavioral analysis provides a powerful approach for the examination of neural circuit organization and evolution. We find that alteration of the circuit by addition of a duplicated M-cell in r2 neither increases nor decreases startle performance. Rather, we demonstrated that the functions of the r2 and r4 M-cells are redundant, with either cell independently able to drive a normal startle. Our results indicate that major changes to the organization of a neural circuit need not be deleterious to its function. We suggest that cell duplication during evolution may be a mechanism for the diversification of neural circuits.
The role of multiple Mauthner cells in startle behavior
Our finding that startle behavior is not affected in zebrafish with ectopic Mauthner cells is consistent with previous work on zebrafish deadly seven mutants (desb420 and destp37) (van Eeden et al., 1996), which develop up to eight Mauthner cells on each side of the hindbrain in rhombomere 4 (Gray et al., 2001). Liu et al. (2003) found that in the desb420, all M-cells appear to respond to startle stimuli, and they respond simultaneously, suggesting that they are receiving similar sensory input. In addition, although multiple M-cells were active in response to a startle stimulus, the additional activity did not enhance behavioral performance (Liu et al., 2003). We found similar results for fish with ectopic M-cells generated by hoxb1b misexpression; although both r2 and r4 M-cells were active in response to startle stimuli, behavioral performance remained the same.
Liu and Fetcho (1999) found that the performance of startles elicited by a tail-directed stimulus decreased after a single M-cell was lesioned, demonstrating that the M-cell is critical for a high-performance startle under this stimulus regime. Using protocols for behavioral tests modeled after Liu and Fetcho (1999), we found a similar decrease in startle performance after lesion of both the r2 and r4 M-cells on one side of the hindbrain. We also found that the activity of each M-cell alone is sufficient to drive a high-performance startle response. We conclude that the homeotically induced cells in these fish do not need to function together to maintain a normal, high-performance startle response.
In deadly seven mutants, each of the multiple M-cells is thought to have only a limited output in the spinal cord; thus, individual M-cells may not be able to elicit a startle response at peak performance (Liu et al., 2003). Reduced output of individual M-cells is suggested by correlation between an increased number of Mauthner cells (three to six cells) and a decreased number of Mauthner axon collaterals (Liu et al., 2003). These collaterals are the sites of contact to spinal cord interneurons and motoneurons in the startle circuit (Fetcho and Faber, 1988). Furthermore, the M-cells of the destp3 mutant tend to have collaterals with nonoverlapping distributions, indicating that the multiple M-cells are dividing their input among the startle interneurons and motoneurons in the spinal cord (Liu et al., 2003). Our contrasting finding, that lesioning one of two M-cells does not decrease performance, suggests that when there are only two M-cells on one side of the hindbrain, there is not significantly reduced output in the spinal cord. In support of this idea, Liu et al. (2003) noted that the one destp37 mutant with two M-cells that they examined had axon collateral numbers at the low end of the range of collateral numbers for fish with one M-cell.
Homeosis as a mechanism for brain evolution
The term homeosis was coined by Bateson (1894) to describe naturally occurring phenotypes in which one body segment takes on the likeness of another. Subsequent work from Lewis (1978) revealed that homeotic phenotypes in the fruit fly (Drosophila melanogaster) can be generated by mutations in the homeotic (Hox) genes. Hox genes have since been isolated from a wide range of animal phyla and appear to represent an ancient and well conserved mechanism to regionalize the developing primary body axis (de Rosa et al., 1999). Comparative analysis of arthropods has allowed differences in Hox gene expression or function to be correlated with major alterations to the body plan. For example, changes in regulation of the Hox genes Ubx and AbdA correlate with evolution of modified feeding appendages in crustacea (Averof and Patel, 1997). Similarly, changes in the protein structure of Ubx correlate with the transition from multilimbed crustacea to hexapod insects (Galant and Carroll, 2002; Ronshaugen et al., 2002). Furthermore, comparative analysis within vertebrates has revealed correlations between Hox gene expression domains and vertebral morphology (Burke et al., 1995). Such studies suggest that evolutionary changes in Hox genes could play a major role in the establishment of different body plans.
Although convincing links have been made between gene expression and morphology, the subsequent step of determining how homeotic changes in morphology affect animal function is just beginning to be addressed. del Toro et al. (2001) examined the respiratory circuit of Hoxa1–/– mice and found that in animals lacking Hoxa1 function, a novel neural circuit took over respiratory rhythm generation. This work demonstrates that novel circuits specified by Hox gene expression can function in behavior. We have now shown that a homeotic transformation in the zebrafish produces a functional segment: a transformed hindbrain segment generates a functional neural circuit, redundant with pre-existing neural circuits, that is capable of mediating behavior. Similar to del Toro et al. (2001), we suggest that production of localized novel circuitry may provide a neural substrate for evolution.
The reticulospinal neurons that lie in each zebrafish rhombomere have unique segment-specific morphologies and functions (Mendelson et al., 1986a,b; Hanneman et al., 1988). However, it has been suggested previously that the nonequivalent segments of the hindbrain evolved from a reiterated series of equivalent or “homonomous” segments (Hanneman et al., 1988; Lee and Eaton, 1991). In the ancestral condition, each rhombomere would have contained equivalent neurons, possibly with M-cell-like properties. Thus, our homeotically transformed animals may not represent a novel morphology but rather an atavastic change toward a more primitive condition.
Duplicated circuitry may represent a novel condition or an atavistic change, but in either case, we suggest that redundancy in function of circuits could facilitate functional specialization. In our specific case of duplicated M-cells, modification of either the r2 or r4 M-cell circuit would not decrease the startle performance. Thus, one of the duplicate circuits is freed to evolve a novel role, or alternatively, the two circuits could evolve complementary, nonredundant functions within the startle behavior. Such mechanisms may have led to the specialization of neural circuits. Although the evolution of neural circuits is thought to be highly constrained (Nishikawa et al., 1992; Tierney, 1996), we suggest that neuron duplications, which could arise from homeotic transformation, provide a viable mechanism by which large-scale changes in brain morphology and organization may occur.
Footnotes
This work was supported by a Brain Research Foundation grant (M.E.H.), National Institutes of Health Grant NS043977 (M.E.H.), and National Science Foundation Grant IBN0091101 (V.E.P.). We thank Drs. J. Clarke, M. Feder, R. Ho, M. LaBarbera, N. Patel, C. Ragsdale, and M. Westneat for helpful suggestions on this manuscript. We thank Dr. J. Fetcho for providing software for behavioral analysis.
Correspondence should be addressed to Melina E. Hale, University of Chicago, Organismal Biology and Anatomy, 1027 East 57th Street, Chicago, IL 60637. E-mail: mhale@uchicago.edu.
DOI:10.1523/JNEUROSCI.5624-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/243070-07$15.00/0
References
- Alexandre D, Clarke JD, Oxtoby E, Yan YL, Jowett T, Holder N (1996) Ectopic expression of Hoxa-1 in the zebrafish alters the fate of the mandibular arch neural crest and phenocopies a retinoic acid-induced phenotype. Development 122: 735–746. [DOI] [PubMed] [Google Scholar]
- Averof M, Patel NH (1997) Crustacean appendage evolution associated with changes in Hox gene expression. Nature 388: 682–686. [DOI] [PubMed] [Google Scholar]
- Bateson W (1894) Materials for the study of variation. London: Macmillan.
- Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121: 333–346. [DOI] [PubMed] [Google Scholar]
- del Toro ED, Borday V, Davenne M, Neun R, Rijli FM, Champagnat J (2001) Generation of a novel functional neuronal circuit in Hoxa1 mutant mice. J Neurosci 21: 5637–5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rosa R, Grenier JK, Andreeva T, Cook CE, Adoutte A, Akam M, Carroll SB, Balavoine G (1999) Hox genes in brachiopods and priapulids and protostome evolution. Nature 399: 772–776. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, Faber DS (1988) Identification of motoneurons and interneurons in the spinal network for escapes initiated by the Mauthner cell in goldfish. J Neurosci 8: 4192–4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho JR, O'Malley DM (1995) Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophysiol 73: 399–406. [DOI] [PubMed] [Google Scholar]
- Galant R, Carroll SB (2002) Evolution of a transcriptional repression domain in an insect Hox protein. Nature 415: 910–913. [DOI] [PubMed] [Google Scholar]
- Gray M, Moens CB, Amacher SL, Eisen JS, Beattie CE (2001) Zebrafish deadly seven functions in neurogenesis. Dev Biol 237: 306–323. [DOI] [PubMed] [Google Scholar]
- Hanneman E, Trevarrow B, Metcalfe WK, Kimmel CB, Westerfield M (1988) Segmental pattern of development of the hindbrain and spinal cord of the zebrafish embryo. Development 103: 49–58. [DOI] [PubMed] [Google Scholar]
- Lee RK, Eaton RC (1991) Identifiable reticulospinal neurons of the adult zebrafish, Brachydanio rerio J Comp Neurol 304: 34–52. [DOI] [PubMed] [Google Scholar]
- Lewis EB (1978) Gene complex controlling segmentation in Drosophila Nature 276: 565–570 [DOI] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR (1999) Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron 23: 325–335. [DOI] [PubMed] [Google Scholar]
- Liu KS, Gray M, Otto SJ, Fetcho JR, Beattie CE (2003) Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit. J Neurosci 23: 8159–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden A, Krumlauf K (1996) Patterning the vertebrate neuraxis. Science 274: 1109–1115. [DOI] [PubMed] [Google Scholar]
- McClintock JM, Carlson R, Mann DM, Prince VE (2001) Consequences of Hox gene duplication in the vertebrates: an investigation of the zebrafish Hox paralogue group 1 genes. Development 128: 2471–2484. [DOI] [PubMed] [Google Scholar]
- Mendelson B (1986a) Development of reticulospinal neurons of the zebrafish. I. Time of origin. J Comp Neurol 251: 160–167. [DOI] [PubMed] [Google Scholar]
- Mendelson B (1986b) Development of reticulospinal neurons of the zebrafish. II. Early axonal outgrowth and cell body position. J Comp Neurol 251: 172–184. [DOI] [PubMed] [Google Scholar]
- Moens CB, Prince VE (2002) Constructing the hindbrain: insights from the zebrafish. Dev Dyn 224: 1–17. [DOI] [PubMed] [Google Scholar]
- Nishikawa KC, Anderson CW, Deban SM (1992) The evolution of neural circuits controlling feeding behavior in frogs. Brain Behav Evol 40: 125–140. [DOI] [PubMed] [Google Scholar]
- Ronshaugen M, McGinnis N, McGinnis W (2002) Hox protein mutation and macroevolution of the insect body plan. Nature 415: 914–917. [DOI] [PubMed] [Google Scholar]
- Tierney AJ (1996) Evolutionary implications of neural circuit structure and function. Behav Processes 35: 173–182. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Wienberg ES, Nusslein-Volhard C (1996) Mutations affecting somite formation and patterning in the zebrafish, Danio rerio Development 123: 153–164. [DOI] [PubMed] [Google Scholar]
- Zottoli SJ, Faber DS (2000) The Mauthner cell: what has it taught us? Neuroscientist 6: 25–37. [Google Scholar]