Abstract
The neuronal system signaling pain has often been characterized as a labeled line consisting of neurons in the pain-signaling pathway to the brain [spinothalamic tract (STT)] that respond only to painful stimuli. It has been proposed recently that the STT contains a series of analog labeled lines, each signaling a different aspect of the internal state of the body (interoception) (e.g., visceral-cold-itch sensations). In this view, pain is the unpleasant emotion produced by disequilibrium of the internal state. We now show that stimulation of an STT receiving zone in awake humans (66 patients) produces two different responses. The first is a binary response signaling the presence of painful stimuli. The second is an analog response in which nonpainful and painful sensations are graded with intensity of the stimulus. Compared with the second pathway, the first was characterized by higher pain ratings and stimulus-evoked sensations covering more of the body surface (projected fields). Both painful responses to stimulation were described in terms usually applied to external stimuli (exteroception) rather than to internal or emotional phenomena, which were infrequently evoked by stimulation of either pathway. These results are consistent with those of functional imaging studies that have identified brain regions activated in a binary manner by the application of a specific, painful stimulus while increases in stimulus intensity do not produce increased activation. Such binary pain functions could be involved in pain-related alarm-alerting functions, which are independent of stimulus amplitude.
Keywords: principal sensory nucleus of thalamus, human, microstimulation, pain, spinothalamic, thalamus
Introduction
The pain pathway has often been characterized as a labeled line consisting of neurons in the pain-signaling pathway to the brain [spinothalamic tract (STT)] that respond only to painful stimuli (Perl, 1984; Willis, 1985). It has been proposed recently that a series of labeled lines in the STT signal the internal state of the body (interoception) (Craig, 2003a,b) and that pain is the emotion produced by disequilibrium of that state. We now show that microstimulation (microampere thresholds) of the STT terminus produces two patterns of pain. The first is a binary pain response signaling the presence or absence of painful stimuli, consistent with an alerting-alarm function (Becker et al., 1993; Zaslansky et al., 1995). The second is an analog pathway in which activity is graded with intensity of the painful stimulus, consistent with STT neurons that encode the properties of external stimuli (Willis, 1985; Price et al., 2003). Both painful responses to stimulation were described in terms usually applied to external stimuli (exteroception) rather than to internal or emotional phenomena (interoception).
Materials and Methods
We stimulated groups of neuron-fibers and recorded single neurons in the thalamus during awake stereotactic procedures (see Figs. 1, 2, 3, 4) under a protocol approved by the local institutional review board (Lenz et al., 1993; Lee et al., 1999). All patients signed an informed consent for this protocol. The thalamic target was localized by magnetic resonance imaging and confirmed by single neuron recording and stimulation under local anesthesia with a high-impedance microelectrode (see Figs. 1, 4 A). The activity of isolated single neurons (see Fig. 4 A) was studied in response to stimuli including light touch, tapping or pressure to skin (i.e., cutaneous neurons) (Fig. 1C, sites 69-82), deep pressure to muscles or ligaments, and passive joint movement [i.e., deep neurons (51-66)] (Lee et al., 1999). The receptive field (RF) of cutaneous neurons was stimulated with a series of thermal (Peltier device: 39, 42, 45, 48, 51 from a baseline of 33°C) and mechanical (camel hair brush; large, medium, or small vessel clamp) stimuli, both of which were graded from the non-painful to the painful range (Lee et al., 1999).
Figure 1.
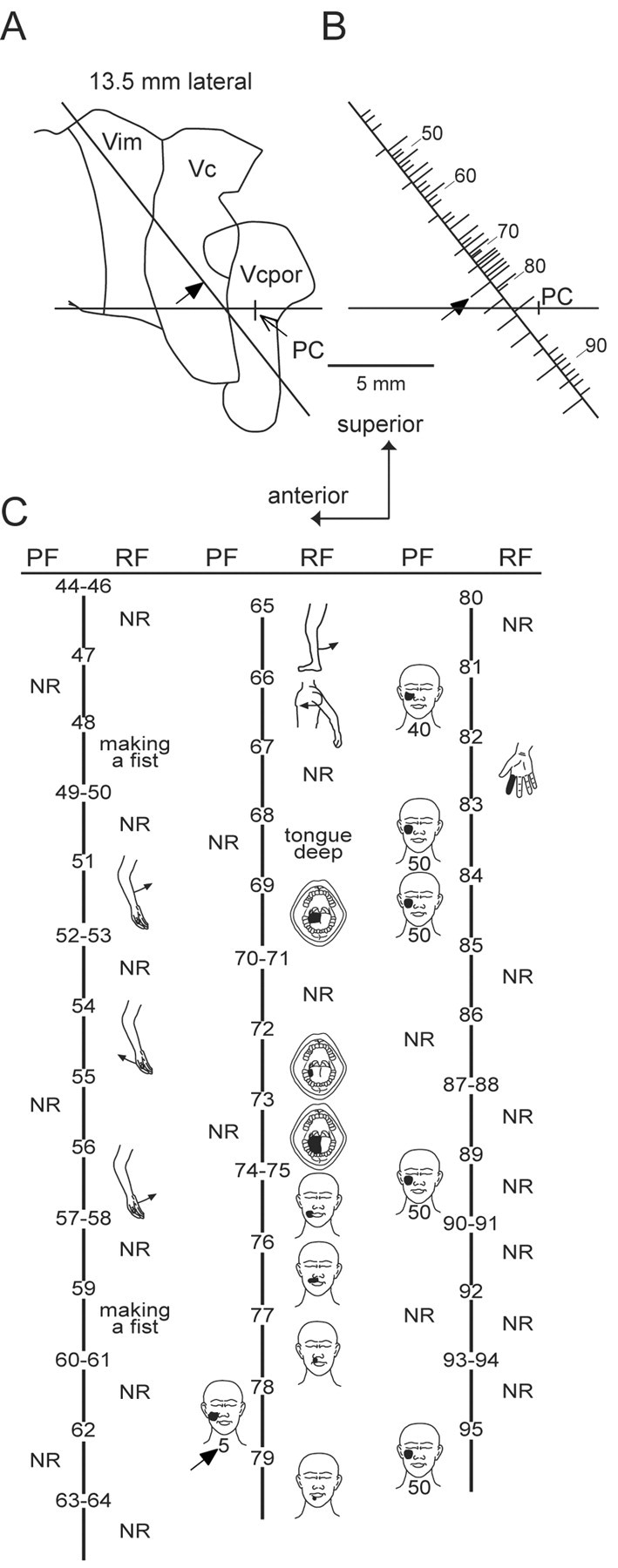
Thalamic map of a single patient with parkinsonian tremor. A, Position of the trajectory, indicated by the oblique line, relative to the anterior commissure-posterior commissure line [horizontal line, posterior commissure indicated] and the region of the human thalamic principle sensory nucleus [Vc and subnucleus, Vc portae (VCpor)], thalamic termini of the STT (Lenz et al., 1993). PC, Posterior commissure; Vim, ventral intermediate (Hirai and Jones, 1989).B, Locations of the neurons and stimulation sites along a trajectory displayed relative to the anterior commissure-posterior commissure line. Stimulation sites are indicated by ticks to the left of the trajectory. Long ticks indicate that a somatic sensation is evoked by stimulation in a part of the body (PF), and the filled arrow is the site illustrated in Figure 2 A, left. Neuronal recordings are located by ticks to the right of the trajectory. Long ticks indicate neurons responding to stimuli over an area on the body (RF). The anteroposterior location of the last neuron with such an RF (site 82) defined the posterior border of Vc. C, Each tick is numbered with the same number in B and C. C shows the site number and illustrations for RFs and PFs (threshold below in microamperes). NR, No response.
Figure 2.
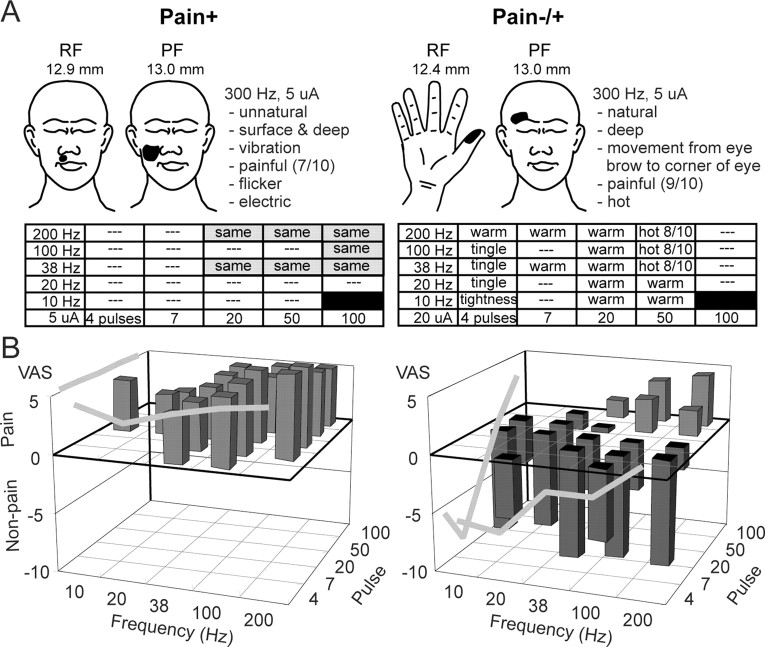
Pain+ and pain-/+ stimulation sites. Sensations evoked by threshold microstimulation were characterized by the PF, by descriptors from a validated questionnaire, and by a visual analog scale of intensity (Lenz et al., 1993, 1998; Lenz and Byl, 1999). A, Left, Site where stimulation at 300 Hz and 5 μA produced pain in the PF (shown in the illustration) and of the quality described. Pain identical to that evoked by 300 Hz was evoked at most sites with trains of ≥20 pulses and frequencies of ≥200 Hz (shaded rectangle; see Results). A, Right, Site where tightness was evoked in the first column at 10 Hz and then a tingle at 20, 38, and 100 Hz. At ≥200 Hz, warmth was evoked at each step in the staircase (except at 7 pulses of 10, 20, and 100 Hz) until 50 pulses, 38 Hz. At this step and further up the staircase, painful heat was evoked. B, Average VAS ratings across all pain+ and pain-/+ sites. Ratings were taken in response to pulse and frequency pairs ascending the staircase. The gray lines along the outside surfaces of the three-dimensional displays indicate the average VAS ratings across all sites by frequency and number of pulses.
Figure 3.
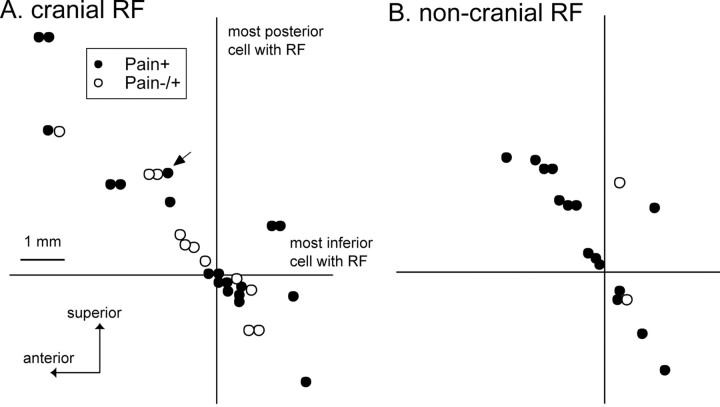
Location of pain+ and pain-/+ sites on sagittal planes through the thalamus. The location of pain+ and pain-/+ sites relative to the posterior (vertical line) and inferior (horizontal line) borders of Vc are identified by the most posterior and most inferior neuron with an RF (neuron 82 for both) (Fig. 1). The core of Vc is above this horizontal and anterior to this vertical. Sites in planes where the majority of cells have cranial and noncranial RFs are shown in A and B, respectively.
Figure 4.
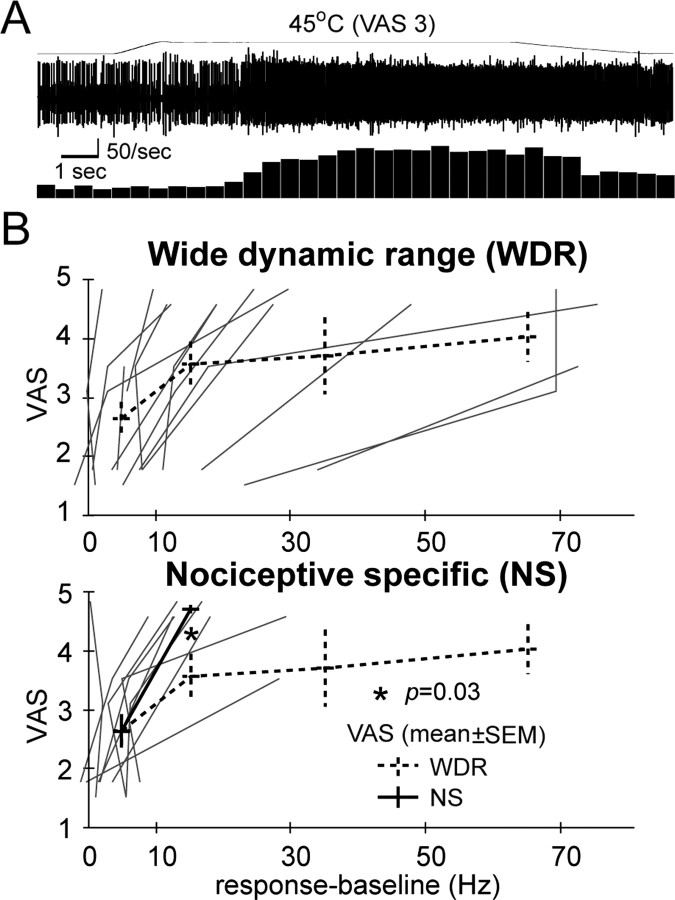
Activity of neurons in the region of Vc responding to painful thermal stimuli. The response to nonpainful and painful heat-mechanical stimuli applied within the RF (Lenz et al., 1994a; Lee et al., 1999) is compared with the VAS evoked by the same stimulus. A, The response of a cell (WDR) to painful heat. B, VAS and firing rates for the response to painful stimuli are plotted for NS cells that respond only to painful stimuli and WDR neurons that respond in a graded manner to nonpainful and painful stimuli. VAS scores by decade to 20 Hz and by 30 Hz steps from 20 to 80 Hz were compared by Mann-Whitney U test.
Candidate sites were stimulated with a 1 sec train of the 300 Hz bipolar stimulus in which a 0.2 msec anodal pulse was followed after 0.1 msec by a cathodal pulse of the same magnitude and duration. The detection threshold for current at 300 Hz was determined by the Method of Limits (Lenz et al., 1993) for sites with thresholds of 5-25 μA. Stimulation evoked sensations described by the projected field (PF), the quality, and the intensity of the sensation (see Fig. 2). Thalamic microstimulation- and cutaneous stimulation-evoked sensations were graded by a visual analog scale (VAS) anchored by the statement that “-10 is rated as no sensation, 0 is no pain or the most intense sensation that is nonpainful, and 10 is the most intense pain imaginable.”
The threshold current (at 300 Hz) was then applied at five frequencies (10, 20, 38, 100, and 200 Hz) in bursts of 4, 7, 20, 50, and 100 pulses in an ascending staircase protocol, the type of protocol commonly used in studies of pain (Gracely et al., 1988; Yarnitsky and Sprecher, 1994), including our studies (Greenspan et al., 2004). This protocol resulted in a factorial delivery of all pairs (steps on the staircase) of five frequencies and five numbers of pulses, except 10 Hz-100 pulses (see Fig. 2). Time constraints dictated that the staircase be delivered single blind, which is conventional in staircase protocols (i.e., the investigator knows that a staircase is being used) (Gracely et al., 1988). To assess the influence of the unblinded investigator, the stimulation paradigm at 13 sites was repeated with the investigator blinded to the order of the staircases during the repeat (i.e., random ordering of the columns in Fig. 2 A). No change in the classification of a site, related to randomization, was observed at the sites studied in this manner.
Results
During surgery on 66 patients with either movement disorders (n = 50) or chronic pain, somatic sensations were evoked by stimulation at 807 of 1406 stimulation sites. The staircase technique was applied at 189 of these sites, including all sites (n = 52) where pain was evoked. Current thresholds at 300 Hz did not vary significantly by type of patient, site, location, or any combination of these variables. The size of the PF changed with stimulus frequency or number of pulses (i.e., ascending the staircase) at only 9 of 189 sites, consistent with activation of the same population of neurons-fibers at each step of the staircase (Ranck, 1975).
We identified two types of response to stimulation. At pain+ sites, pain was evoked by stimulation at 300 Hz and at all steps in the staircase for which a sensation was evoked (n = 39) (Fig. 2A, left). At pain-/+ sites (n = 13) (Fig. 2A, right), stimulation evoked nonpainful sensations at threshold for numbers of pulses and frequency followed by painful sensations further up the staircase. Current (300 Hz; p = 0.46; t test), frequency (p = 0.39), and pulse thresholds (p = 0.53) for evoking pain were not significantly different between the two types of sites. Ratings of painful evoked sensations were higher for pain+ sites [4.9 ± 0.3 (mean ± SEM)] than for pain-/+ sites (3.7 ± 0.4; p = 0.01; post hoc Bonferroni t test after ANOVA by pulse number, frequency, and type of site). The number of pain+ sites (22 of 24; 92%) in patients with chronic pain was significantly higher than in those without chronic pain (17 of 28; 61%; p < 0.02; Fisher's test). The description, location, and intensity of the evoked pain and the locations of sites were not significantly different between patient groups.
Cutaneous sensations were commonly evoked at both pain+ (22 of 39; 56%) and pain-/+ (6 of 13; 46%) sites. Excluding the change from nonpainful to painful, the description-modality of the evoked sensation changed along the staircase for each pain-/+ site (13 of 13) but did not change for any pain+ site (0 of 39; p = 0.0001; Fisher's test). The incidence of particular descriptors, including the mechanical and thermal categories, did not vary significantly between types of patients, sites, locations, or any combination of these variables. Notably, itch (2 of 13 pain-/+ sites; 2 of 39 pain+ sites) was never evoked in isolation, and emotion descriptors were uncommonly endorsed at either pain+ (2 of 39) or pain-/+ sites (0 of 13) (cf. Lenz et al., 1995).
The parts of the body where stimulation evoked a sensation (PF) were analyzed, excluding chronic pain patients who commonly have altered PFs (Lenz et al., 1994b; Davis et al., 1998). PFs were classified as large if they included the entire limb below the elbow or knee, the hemicranium, or two body parts (e.g., face and arm). Large PFs were more common at pain+ sites (8 of 17; 47%) than at pain-/+ sites (1 of 11; 9%; p = 0.05; Fisher's test) (Lenz et al., 1993).
The dependence of sensory ratings on stimulus frequency and the number of pulses was different between the two types of sites. The slope of pain ratings versus stimulus frequency was higher for pain-/+ (0.026 VAS/Hz; r = 0.27; p = 0.004) than for pain+ sites (0.009 VAS/Hz; r = 0.26; p = 0.003). The slope of pain ratings versus number of pulses was higher for pain-/+ sites (0.107 VAS per number of pulses; r = 0.53; p = 0.00001) than for pain+ sites (-0.0002 VAS/number of pulses; r = -0.0036; p = 0.97). Overall, pain ratings above the detection threshold at pain+ sites were constant ascending the staircase, consistent with a binary function.
Sensations were not evoked at some steps in the staircase, despite stimulation-evoked sensations at surrounding steps. Therefore, the results of some staircases contained missing steps (e.g., Fig. 2A, left, gaps or white steps at 100 Hz for the columns indicating 20 or 50 pulses) in the expected top right rectangle of the staircase (e.g., Fig. 2A, left, shaded part of the staircase). Such gaps were found for 71% (37 of 52) of sites and were corrected by assumption of linearity, as usual (Gracely et al., 1988). The number of sites for which a gap was found was significantly higher for pain+ sites (32 of 39; 86%) than for pain-/+ sites (5 of 13; 38%; p = 0.005; Fisher's test).
Sites within the region of the thalamic principle sensory nucleus [monkey ventroposterior (VP), human ventrocaudal (Vc)] (Hirai and Jones, 1989) were classified by location in the parasagittal plane into the region where neurons respond to innocuous stimuli (Vc core) (legend to Fig. 3) or in the region behind it (right of the vertical). Additionally, location was classified at or medial to planes where neurons had cutaneous-mucosal RFs (Fig. 3A) on cranial structures (Fig. 1A) or lateral planes where noncranial RFs were found (Fig. 3B). There was a trend for pain-/+ sites (11 of 13; 83%) to be located in the medial plane more often than pain+ sites (19 of 34; 56%; p = 0.09; Fisher's test). Note that the total number of cells classified by mediolateral location is 47, less than the total number of pain sites (n = 52), because cutaneous cells were not found in the plane of five stimulation sites. No significant effects were observed along the anteroposterior axis medially, laterally, or overall.
Figure 4A shows the response of a single neuron with wide dynamic range (WDR) properties to a painful 45°C stimulus. Responses to painful stimuli were characterized by the mean firing rate during painful stimulation, recorded through the microelectrode (Fig. 4B, x-axis), and by VAS ratings of microstimulation-evoked pain (y-axis). There was a significantly steeper increase in VAS pain scores for the neurons that only responded to painful stimuli [nociceptive-specific (NS) neurons] than for WDR neurons.
Discussion
These studies demonstrate that stimulation at sites in the region of Vc evokes sensations consistent with one of two pathways, one binary and the other analog. Stimulation at pain+ sites evoked a constant high level of pain over large, often cutaneous PFs. These sites were characterized both by descriptors that did not change along the staircase and by more intense stimulation-evoked pain than that evoked at the pain-/+ sites. These results suggest that pain+ sites participate in a binary, exteroceptive, labeled line that signals the presence of a painful external stimulus. The steep initial increase in VAS with the firing rate of NS versus WDR neurons (Fig. 3B) is consistent with the shorter dynamic range of thalamic NS cells (Apkarian and Shi, 1994) and with the binary response to stimulation at pain+ sites (Fig. 2B).
These results might not be understood in terms of the activity of single cells or cell types, because stimulation at 5-25 μA activates multiple neurons and fibers over a radius of 50-200 μm (Ranck, 1975). However, STT fibers are clustered in Vc and in inferior Vc (Mehler, 1962) as well as in the corresponding monkey nuclei (Willis, 1985; Hirai and Jones, 1989). Cells responding to noxious stimuli are clustered both by location within Vc (Apkarian et al., 2000) and by location within somatotopically appropriate regions in the compartmentalized, innocuous mechanoreceptive representation of VP and Vc (Jones et al., 1982; Lenz et al., 1988). Cells responding to nociceptive stimuli are also clustered at the location of STT terminals within the inferior portion of VP (Apkarian and Shi, 1994), corresponding to the portion of Vc where most of these stimulations were performed (Fig. 3) (Hirai and Jones, 1989). Therefore, it is possible that stimulation-evoked pain was related to infrequent (52 of 704 sites with somatic sensory stimulation-evoked sensations) clusters of neural elements having similar nociceptive properties.
The thalamic stimulation thresholds for nonpainful and painful sensations are not significantly different (Lenz et al., 1993; Ohara and Lenz, 2003), suggesting that pain-/+ sites did not result from activation of the system transmitting nonpainful sensations (primarily medial lemniscal) before that transmitting painful sensations (primarily STT) (Willis, 1985). Finally, the equivalence of current, pulse, and frequency thresholds for pain at both types of sites predicts that the neural elements responsible for the two types of sites (i.e., possibly WDR and NS cells) should be activated together if they were found at the same site. Analog pain+ responses would be predicted to occur at such sites, because the combination of binary plus analog neural elements at a site will have analog properties, given the assumption of linearity. However, analog pain+ sites were not observed. For all of these reasons, it is plausible that our observations may be the result of selective activation of two functionally distinct pathways.
An analysis of location revealed only a nonsignificant trend for pain-/+ sites to be located medial to pain+ sites. There was no significant difference in types of sites by location along the anteroposterior axis. This suggests that the posterior part of the ventromedial nucleus is more likely to be involved in the analog pathway than in the predicted labeled-line pain pathway (Craig et al., 1994; Blomqvist et al., 2000; Craig, 2003a). Furthermore, we observed a binary pain labeled line (pain+) but not the predicted analog labeled line for pain (Craig, 2003a). The overlapping locations of pain+ and pain-/+ sites within and posterior to Vc (Fig. 3) may be consistent with the location of STT terminations in inferior Vc (Mehler, 1962), within Vc as dense bursts (Mehler, 1962; Willis, 1985), and with stimulation of fibers of passage (Ranck, 1975).
The present results are consistent with functional imaging studies that have identified brain regions where the application of a specific, painful stimulus activates the region but additional increases in stimulus intensity do not produce increased activation (Coghill et al., 1999; Bornhovd et al., 2002). These sites may serve as an alarm signal for the occurrence of a painful stimulus somewhere in a large area of the body in response to as few as four stimulation pulses (Fig. 2B, left) (Price et al., 2003). Such binary signals could be involved in pain-related alerting reactions produced by painful stimuli, which are independent of stimulus amplitude (Becker et al., 1993; Zaslansky et al., 1995; Bornhovd et al., 2002).
The pain-/+ sites have properties consistent with the pattern hypothesis of an exteroceptive pain pathway in which graded neuronal activity produces an analog, graded pain signal (Price et al., 2003). This hypothesis is supported by the finding that monkey dorsal horn WDR neurons but not NS neurons have electro-physiological properties (Price and Mayer, 1975) consistent with those of axons in the human pain-signaling STT pathway (Mayer et al., 1975). Sensations evoked by stimulation of the STT were described as a tingle, warm sensation, or cool sensation above detection threshold (-10 by our VAS) but below the pain threshold (0 by our VAS) (Mayer et al., 1975), as for our pain-/+ sites. Therefore, the present results suggest that pain-/+ sites signal the exteroceptive, sensory dimension of pain (Price et al., 2003).
The sensations evoked by stimulation at the pain-/+ sites were characterized by lower-intensity, smaller PFs, changes in pain descriptors, and fewer gaps ascending the staircase, suggesting more consistent detection of stimulation-evoked sensation. The pain-/+ sites have small PFs, consistent with the small RFs of thalamic WDR neurons, which are approximately the size of RFs for cells in monkey VP and human Vc that respond to innocuous stimuli (Casey and Morrow, 1983; Bushnell and Duncan, 1987; Apkarian and Shi, 1994; Lee et al., 1999). Thus, thalamic Vc WDR cellular RFs are much smaller than those of STT WDR cells (Chung et al., 1986). The sizes of the PFs of pain+ sites were larger than those observed for pain-/+ sites, consistent with the size of RFs for primate thalamic NS cells (Bushnell et al., 1993; Lee et al., 1999). Therefore, the pain+ and pain-/+ stimulation PFs are consistent with those predicted by the behavior of thalamic NS and WDR neurons.
In summary, pain+ and pain-/+ sites are consistent with exteroceptive pathways that signal, respectively, the presence of and the sensory features of painful, external somatic stimuli. Neither signals the internal sensations or emotional phenomena of the predicted interoceptive pain-signaling system in humans (Craig, 2003a,b). Overall, the present results suggest that many thalamic stimulation sites sound the alarm that a painful external stimulus has occurred somewhere, while others appear to indicate how much and where.
Footnotes
This work was supported by National Institutes of Health-National Institute of Neurological Disorders and Stroke Grants NS38493 and NS40059 (F.A.L.). We thank D. D. Price for critically reading a previous version of this manuscript and L. H. Rowland for excellent technical assistance.
Correspondence should be addressed to Frederick A. Lenz, Department of Neurosurgery, Johns Hopkins Hospital, Meyer Building 8-181, 600 North Wolfe Street, Baltimore, MD 21287-7713. E-mail: flenz1@jhmi.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/246540-05$15.00/0
References
- Apkarian AV, Shi T (1994) Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci 14: 6779-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Shi T, Bruggemann J, Airapetian LR (2000) Segregation of nociceptive and non-nociceptive networks in the squirrel monkey somatosensory thalamus. J Neurophysiol 84: 484-494. [DOI] [PubMed] [Google Scholar]
- Becker DE, Yingling CD, Fein G (1993) Identification of pain, intensity, and P300 components in the pain evoked potential. Electroencephalogr Clin Neurophysiol 88: 290-301. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Zhang ET, Craig AD (2000) Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain 123: 601-619. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C (2002) Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain 125: 1326-1336. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH (1987) Mechanical response properties of ventroposterior medial thalamic neurons in the alert monkey. Exp Brain Res 67: 603-614. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Tremblay N (1993) Thalamic VPM nucleus in the behaving monkey. I. Multimodal and discriminative properties of thermosensitive neurons. J Neurophysiol 69: 739-752. [DOI] [PubMed] [Google Scholar]
- Casey KL, Morrow TJ (1983) Ventral posterior thalamic neurons differentially responsive to noxious stimulation of the awake monkey. Science 221: 675-677. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Surmeier DJ, Sorkin LS, Kim J, Willis WD (1986) Response characteristics of neurons in the ventral posterior lateral nucleus of the monkey thalamus. J Neurophysiol 56: 370-390. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ (1999) Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 82: 1934-1943. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003a) Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 26: 1-30. [DOI] [PubMed] [Google Scholar]
- Craig AD (2003b) A new view of pain as a homeostatic emotion. Trends Neurosci 26: 303-307. [DOI] [PubMed] [Google Scholar]
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A (1994) A thalamic nucleus specific for pain and temperature sensation. Nature 372: 770-773. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kiss ZH, Luo L, Tasker RR, Lozano AM, Dostrovsky JO (1998) Phantom sensations generated by thalamic microstimulation. Nature 391: 385-387. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Lota L, Walter DJ, Dubner R (1988) A multiple random staircase method of psychophysical pain assessment. Pain 32: 55-63. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Ohara S, Sarlani E, Lenz FA (2004) Allodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individuals. Pain 109: 357-366. [DOI] [PubMed] [Google Scholar]
- Hirai T, Jones EG (1989) A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 14: 1-34. [DOI] [PubMed] [Google Scholar]
- Jones EG, Friedman DP, Hendry SH (1982) Thalamic basis of place- and modality-specific columns in monkey somatosensory cortex: a correlative anatomical and physiological study. J Neurophysiol 48: 545-568. [DOI] [PubMed] [Google Scholar]
- Lee J, Dougherty PM, Antezana D, Lenz FA (1999) Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comp Neurol 410: 541-555. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Byl NN (1999) Reorganization in the cutaneous core of the human thalamic principal somatic sensory nucleus (ventral caudal) in patients with dystonia. J Neurophysiol 82: 3204-3212. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Dostrovsky JO, Tasker RR, Yamashiro K, Kwan HC, Murphy JT (1988) Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol 59: 299-316. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Seike M, Richardson RT, Lin YC, Baker FH, Khoja I, Jaeger CJ, Gracely RH (1993) Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol 70: 200-212. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Rowland LH, Dougherty PM (1994a) A population of cells in the human thalamic principal sensory nucleus respond to painful mechanical stimuli. Neurosci Lett 180: 46-50. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Kwan HC, Martin R, Tasker R, Richardson RT, Dostrovsky JO (1994b) Characteristics of somatotopic organization and spontaneous neuronal activity in the region of the thalamic principal sensory nucleus in patients with spinal cord transection. J Neurophysiol 72: 1570-1587. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Romanoski AJ, Hope EJ, Rowland LH, Dougherty PM (1995) Stimulation in the human somatosensory thalamus can reproduce both the affective and sensory dimensions of previously experienced pain. Nat Med 1: 910-913. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Gracely RH, Baker FH, Richardson RT, Dougherty PM (1998) Reorganization of sensory modalities evoked by stimulation in the region of the principal sensory nucleus (ventral caudal - Vc) in patients with pain secondary to neural injury. J Comp Neurol 399: 125-138. [PubMed] [Google Scholar]
- Mayer DJ, Price DD, Becker DP (1975) Neurophysiological characterization of the anterolateral spinal cord neurons contributing to pain perception in man. Pain 1: 51-58. [DOI] [PubMed] [Google Scholar]
- Mehler WR (1962) The anatomy of the so-called “pain tract” in man: an analysis of the course and distribution of the ascending fibers of the fasciculus anterolateralis. In: Basic research in paraplegia (French JD, Porter RW, eds), pp 26-55. Springfield, IL: Thomas.
- Ohara S, Lenz FA (2003) Medial lateral extent of thermal and pain sensations evoked by microstimulation in somatic sensory nuclei of human thalamus. J Neurophysiol 90: 2367-2377. [DOI] [PubMed] [Google Scholar]
- Perl ER (1984) Pain and nociception. In: The nervous system: sensory processes, Pt 2 (Brookhart JM, Mountcastle VB, Darian-Smith I, Geiger SR, eds), pp 915-975. Bethesda, MD: American Physiological Society.
- Price DD, Mayer DJ (1975) Neurophysiological characterization of the anterolateral quadrant neurons subserving pain in M. mulatta Pain 1: 59-72. [DOI] [PubMed] [Google Scholar]
- Price DD, Greenspan JD, Dubner R (2003) Neurons involved in the exteroceptive function of pain. Pain 106: 215-219. [DOI] [PubMed] [Google Scholar]
- Ranck JB (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98: 417-440. [DOI] [PubMed] [Google Scholar]
- Willis WD (1985) The pain system. Basel: Karger.
- Yarnitsky D, Sprecher E (1994) Thermal testing: normative data and repeatability for various test algorithms. J Neurol Sci 125: 39-45. [DOI] [PubMed] [Google Scholar]
- Zaslansky R, Sprecher E, Tenke CE, Hemli JA, Yarnitsky D (1995) The P300 in pain evoked potentials. Pain 66: 39-49. [DOI] [PubMed] [Google Scholar]