Abstract
An interaction with the GABA type A (GABAA) receptor has long been recognized as one of the main neurochemical mechanisms underlying many of the pharmacological actions of ethanol. However, more recent data have suggested that certain behavioral and electrophysiological actions of ethanol are mediated by an increase in brain concentration of neuroactive steroids that results from stimulation of the hypothalamic-pituitary-adrenal (HPA) axis. Neuroactive steroids such as 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP) are, in fact, potent and efficacious endogenous positive modulators of GABAA receptor function. Because neurosteroids can be synthesized de novo in the brain, we have investigated whether ethanol might affect both neurosteroid synthesis and GABAA receptor function in isolated rat hippocampal tissue. Here, we show that ethanol increases the concentration of 3α,5α-THP as well as the amplitude of GABAA receptor-mediated IPSCs recorded from CA1 pyramidal neurons in isolated hippocampal slices. These effects are shared by the neurosteroid precursor progesterone, the peripheral benzodiazepine receptor-selective agonist CB34, and γ-hydroxybutyrate, all of which are known to increase the formation of neuroactive steroids in plasma and in the brain. The action of ethanol on GABAA receptor-mediated IPSC amplitude is biphasic, consisting of a rapid, direct effect on GABAA receptor activity and an indirect effect that appears to be mediated by neurosteroid synthesis. Furthermore, ethanol affects GABAA receptor activity through a presynaptic action, an effect that is not dependent on neurosteroid formation. These observations suggest that ethanol may modulate GABAA receptor function through an increase in de novo neurosteroid synthesis in the brain that is independent of the HPA axis. This novel mechanism may have a crucial role in mediating specific central effects of ethanol.
Keywords: GABAA receptors; ethanol; neurosteroids; 3α,5α-THP; hippocampus; CA1 pyramidal cells; patch clamp; mIPSC; peripheral benzodiazepine receptor; γ-hydroxybutyrate (GHB)
Introduction
Ethanol, a widely consumed and abused recreational drug, exerts several CNS-depressant actions, including anxiolytic, sedative-hypnotic, anticonvulsant, and muscle relaxant effects similar to those induced by different modulators of the GABA type A (GABAA) receptor such as benzodiazepines, barbiturates, and neurosteroids such as the progesterone metabolite 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP or allopregnanolone) (Brot et al., 1995; Grobin et al., 1998; Koob et al., 1998; VanDoren et al., 2000). Evidence indicates that GABAA receptors are indeed targets for the acute as well as chronic actions of ethanol. Several behavioral effects of this drug are thus enhanced by GABAA receptor agonists and are attenuated by receptor antagonists or inverse agonists (Martz et al., 1983). Furthermore, ethanol potentiates GABA-mediated inhibitory neurotransmission in a manner dependent on dose and brain region or neuronal cell type (Grobin et al., 1998; VanDoren et al., 2000; Roberto et al., 2003).
The acute action of ethanol at GABAA receptors was recently proposed to be mediated by the peripheral secretion of neuroactive steroids (Morrow et al., 1999). Acute ethanol administration indeed increases the concentrations of 3α,5α-THP in the plasma, cerebral cortex, and hippocampus of rats (Barbaccia et al., 1999; Morrow et al., 2001). 3α,5α-THP modulates the opening of the GABAA receptor-associated Cl- channel at nanomolar concentrations in vitro (Harrison and Simmonds, 1984; Majewska et al., 1986; Majewska, 1992; Lambert et al., 2001). The anxiolytic and anticonvulsant properties of progesterone are mostly attributable to its conversion to 3α,5α-THP (Smith et al., 1987; Kokate et al., 1994; Bitran et al., 1995) (see Fig. 1). Furthermore, pretreatment of animals with the 5α-reductase inhibitor finasteride, which inhibits the biosynthesis of 3α,5α-THP (see Fig. 1), reduced the extent of the ethanol-induced increase in the cerebrocortical concentration of 3α,5α-THP and prevented certain neurochemical, electrophysiological, and behavioral effects of ethanol (VanDoren et al., 2000). The ability of ethanol to promote 3α,5α-THP biosynthesis is thought to be dependent on its stimulatory action on the hypothalamic-pituitary-adrenal (HPA) axis (Ellis, 1966; Rivier et al., 1984; Rivier, 1996; Ogilvie et al., 1997; Khisti et al., 2003a). In fact, in adrenalectomized rats, ethanol failed to increase the plasma or brain level of 3α,5α-THP and to induce some of its pharmacological effects (Khisti et al., 2002, 2003b).
Figure 1.
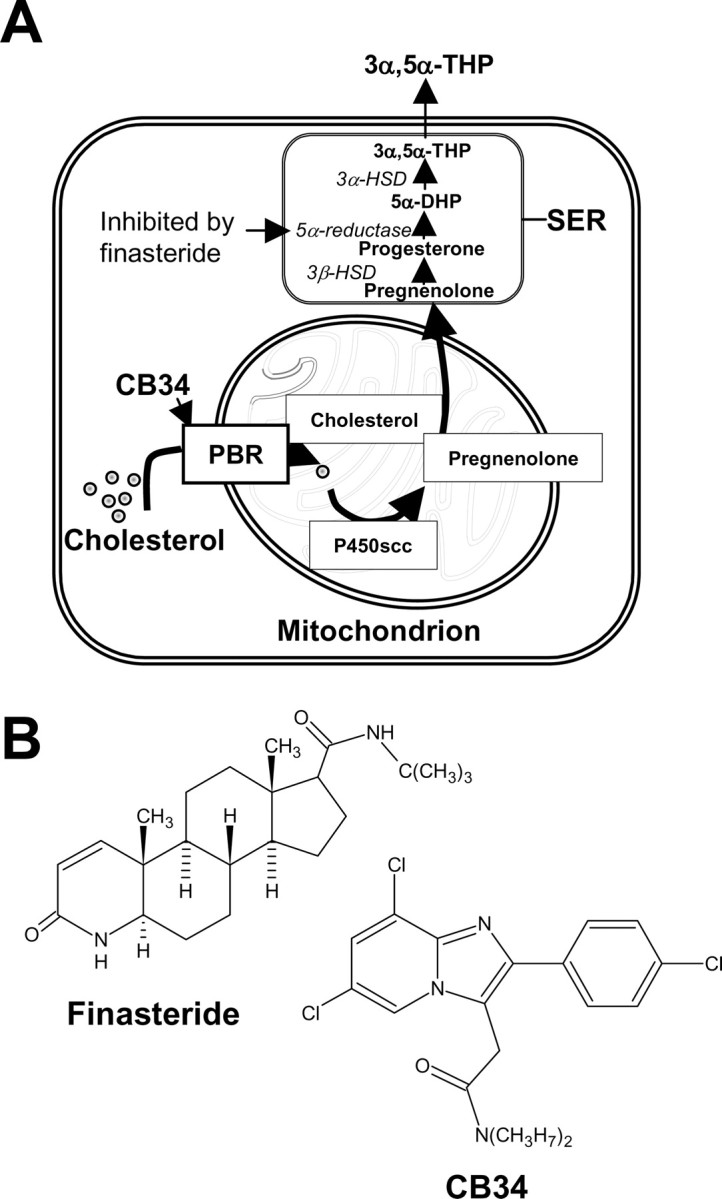
A, Pathway in glial cells of the hippocampus for the biosynthesis of the neurosteroid 3α,5α-THP (3α-hydroxy-5α-pregnan-20-one or allopregnanolone) from cholesterol. Translocation of cholesterol on the inner part of mitochondria, the first rate-limiting step in neurosteroid synthesis, is performed by several proteins, among others, the PBR, which in our experimental protocol was activated by the selective agonist CB34 (Serra et al., 1999). Finasteride, also used in our experiments, inhibits the enzyme 5α-reductase, thus blocking the conversion of progesterone to 5α-DHP and the formation of 3α,5α-THP. P450scc, Mitochondrial cholesterol side-chain cleavage enzyme; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 5α-DHP, 5α-dihydroprogesterone; 3α-HSD, 3α-hydroxysteroid dehydrogenase; SER, smooth endoplasmic reticulum. B, Chemical structure of finasteride and CB34.
Together, these observations support the notion that certain effects of ethanol on brain GABAA receptors are mediated by neuroactive steroids produced in peripheral organs, or by peripheral precursors that are converted to neurosteroids by brain cells. Given that neurosteroids are produced de novo in the brain independently of precursors derived from peripheral organs (Hu et al., 1987; Mathur et al., 1993), we have now evaluated the ability of ethanol to stimulate neurosteroidogenesis in isolated brain tissue. We compared the effects of ethanol with those of the neurosteroid precursor progesterone and of CB34 (see Fig. 1), a selective and high-affinity agonist of the peripheral benzodiazepine receptor (PBR) and stimulator of neurosteroidogenesis (Serra et al., 1999). In fact, PBRs are found primarily on the outer mitochondrial membrane, where they promote neurosteroidogenesis by facilitating cholesterol translocation into the mitochondria and are extremely abundant in steroidogenic endocrine tissues, including the brain (Gavish et al., 1999) (Fig. 1).
Moreover, we examined the role of local 3α,5α-THP synthesis induced by ethanol as well as other drugs on GABAA receptor function by incubating hippocampal slices with finasteride.
Materials and Methods
Animals. Male Sprague Dawley CD rats (Charles River, Como, Italy) were studied at 20-30 d of age (body mass, 70-90 gm). After arrival at the animal facility, rats were allowed to acclimatize to the new housing conditions for at least 1 week. They were housed six per cage under an artificial 12 hr light/dark cycle (lights on from 8:00 A.M. to 8:00 P.M.) and at a constant temperature of 22 ± 2°C and a relative humidity of 65%. They had ad libitum access to water and standard laboratory food at all times. Animal care and handling throughout the experimental procedures were in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC). The experimental protocols were also approved by the Animal Ethics Committee of the University of Cagliari.
Preparation of rat hippocampal minces. Rat hippocampal minces were prepared as described (Roscetti et al., 1998). In brief, the brain was excised rapidly (within 1 min) from the skull, and the hippocampus was dissected on ice and cut along two orthogonal planes into blocks (300 × 300 μm) with a McIlwain tissue chopper. The minced tissue from each rat was allowed to stabilize for 1 hr at 37°C with at least five changes of Krebs-bicarbonate buffer, pH 7.4, comprising (in mm) 118 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 NaH2PO4, 1.2 MgCl2, 25 Na2HPO4, and 22.2 d-glucose, saturated with 95% O2 and 5% CO2. It was then incubated for 30 min at 34°C with 25, 50, and 100 mm ethanol, 1 μm progesterone, 30 μm CB34, 300 μm γ-hydroxybutyrate (GHB), or vehicle. For the time course of the effect of ethanol (25, 50, and 100 mm), the incubation was stopped immediately after the addition of ethanol (0 min) or 10, 20, or 30 min later. In all cases, the incubation was stopped by heating the samples at 95°C for 10 min in a water bath.
Neurosteroid extraction and assay. Neurosteroids were extracted and purified as described previously (Barbaccia et al., 1996). In brief, hippocampal minces (110-130 mg of tissue in 3 ml of PBS, pH 7.0) were homogenized with a Polytron tissue disrupter (Kinematica, Lucerne, Switzerland), and 3α,5α-THP was extracted from the homogenate three times with ethyl acetate. The combined organic phases were dried under vacuum, the resulting residue was dissolved in 5 ml of n-hexane and applied to a SepPak silica cartridge (Waters Associates, Milford, MA), and components were eluted with n-hexane and 2-propanol (7:3, v/v). Fractions containing 3α,5α-THP were further purified by HPLC on a 5 μm Lichrosorb-diol column (250 × 4 mm) (Phenomenex, Belmont, CA) with a discontinuous gradient of 2-propanol (0-30%) in n-hexane. The recovery (70-80%) of 3α,5α-THP through the extraction and purification procedures was monitored by adding a trace amount of tritiated standard (6000-8000 cpm; ∼80 Ci/mmol) to the tissue homogenate. 3α,5α-THP was quantitated by RIA with specific antibodies that were generated in sheep and characterized as described previously (Barbaccia et al., 1996).
Hippocampal slice preparation. Rats were anesthetized by intraperitoneal injection of ketamine (250 mg/kg body mass), and the brain was removed rapidly into ice-cold cutting solution (in mm: 220 sucrose, 2 KCl, 1.3 NaH2PO4, 12 MgSO4, 0.2 CaCl2, 10 glucose, and 2.6 NaHCO3, pH 7.3, equilibrated with 95% O2 and 5% CO2). Coronal slices (thickness, 300 μm) of the hippocampus were cut with a Vibratome 1000 plus (Vibratome, St. Louis, MO) and then incubated in artificial cerebrospinal fluid (ACSF) containing (in mm) 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 2 CaCl2, 10 glucose, and 26 NaHCO3, pH 7.3, equilibrated with 95% O2 and 5% CO2, first for 40 min at 34°C and then for 30 min at room temperature before beginning experiments.
Whole-cell patch-clamp recording. Tissue slices were transferred to a chamber perfused with ACSF at a rate of ∼2 ml/min at room temperature. Whole-cell patch-clamp electrophysiological recordings from CA1 pyramidal neurons were performed with an Axopatch 200-B amplifier (Axon Instruments, Union City, CA) and an infrared-differential interference contrast microscope. Patch microelectrodes (boro-silicate capillaries with a filament; outer diameter, 1.5 μm; Sutter Instruments, Novato, CA) were prepared with a two-step vertical puller (Sutter Instruments) and had a resistance of 4-6 MΩ. Spontaneous miniature IPSCs (mIPSCs) as well as evoked IPSCs were recorded at a holding potential of -60 mV with an internal solution containing (in mm) 140 CsCl, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES-CsOH, pH 7.3, 2 adenosine triphosphate (disodium salt), and 5 QX-314 (lidocaine N-ethyl bromide). Access resistance varied between 20 and 40 MΩ; if it changed by >20% during an experiment, the recording was discarded. Currents through the patch-clamp amplifier were filtered at 2 kHz and digitized at 5.5 kHz with commercial software (pClamp 8.2; Axon Instruments).
In one set of experiments, we recorded spontaneous GABAA receptor-mediated mIPSCs in ACSF containing 600 nm TTX and 1 mm kynurenic acid. When indicated, bicuculline methiodide (20 μm), progesterone (1 μm), CB34 (10 or 30 μm), ethanol (25, 50, 100, or 150 mm), GHB (100, 300 μm, 1 or 10 mm), or finasteride (1 μm) was added to ACSF containing both TTX and kynurenic acid. Spontaneous mIPSCs were recorded during a fixed time (3 min) and were sampled every 10 min before (two samplings) and after (four samplings) the start of perfusion with drug for 30 min as well as after drug washout (one sampling). When the effect of finasteride was determined, tissue slices were perfused with this drug for 10-20 min before the onset of perfusion with finasteride together with other agents. The mIPSCs were analyzed with Mini Analysis 5.4.17 software (Synaptosoft, Decatur, GA). The detection threshold was set at 1.5 times the baseline noise. Each event identified was confirmed by visual inspection for each experiment. We evaluated the effects of the various drugs on the amplitude and frequency of mIPSCs in individual neurons by cumulative probability analysis, with statistical significance determined with the Kolmogorov-Smirnov nonparametric two-sample test. Decay time constants (fast and slow decay components) were determined on averaged mIPSCs by using a two-exponential function (Mini Analysis 5.4.17 software). The weighted decay time constant (τw) was calculated as τw = (A1 × τ1 + A2 × τ2)/(A1 + A2), where τ1 and τ2 are the time constants of the first and second exponential functions, respectively, and A1 and A2 are the current amplitudes measured at time t, equal to τ1 and τ2, respectively (Banks et al., 1998; Vicini et al., 2001).
In another set of experiments, we recorded synaptically evoked, GABAA receptor-mediated IPSCs in ACSF containing 1 mm kynurenic acid. IPSCs were evoked at 30 sec intervals with a concentric bipolar stainless steel and platinum-iridium electrode placed in the stratum radiatum (constant current stimulation with 100-500 μA for 50 μsec). Access resistance was monitored continuously by evaluating, after each stimulation, the transient current generated in response to a 20 mV hyperpolarizing pulse. Evoked IPSCs were recorded continuously both before (for ∼10 min) and after the onset of perfusion with ethanol (50 or 100 mm). When the effect of finasteride was determined, tissue slices were perfused for 10 min with this drug alone before the onset of perfusion with finasteride and ethanol. The paired-pulse facilitation (PPF) protocol was also used with an interstimulus interval of 100 msec, and this double stimulation was repeated at 30 sec intervals. The amplitude ratio of the second to the first IPSCs evoked by paired-pulse (PP) stimulation was defined as the PP ratio and was calculated on the average of groups of seven consecutive sweeps recorded before (control), at different times (0, 10, 20, and 30 min) during ethanol perfusion, and 10 min after washout.
Evoked IPSCs were analyzed by Clampfit 8.2 software (Axon Instruments).
Statistical analysis. Statistical comparisons of pooled data were performed by one-way ANOVA, followed by Scheffe's post hoc test. In all cases, a p value of <0.05 was considered statistically significant.
Results
Ethanol, progesterone, CB34, and GHB stimulate 3α,5α-THP production in isolated hippocampal tissue
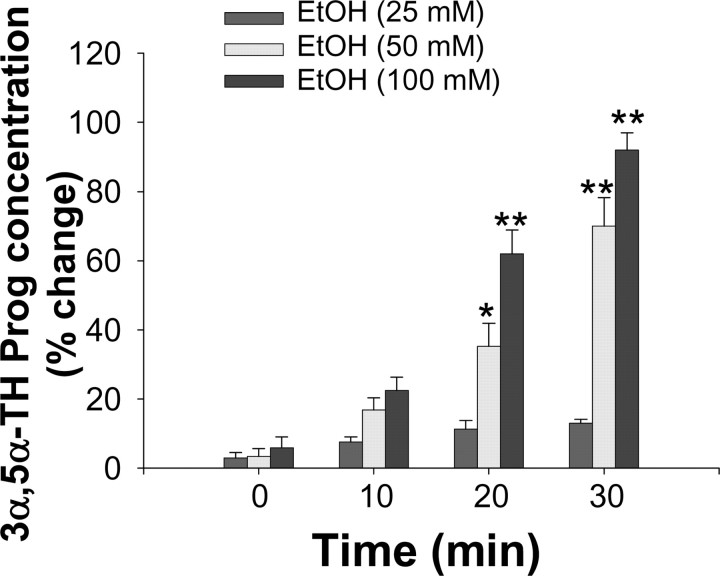
We first tested the ability of ethanol to stimulate neurosteroid biosynthesis in rat hippocampal minces. Incubation with 50 and 100 mm ethanol for 30 min at 34°C resulted in a 70 ± 8% (F(1,14) = 17.88; p < 0.0007) and a 92 ± 5% (F(1,13) = 19.33; p < 0.0007) increase, respectively, in the tissue content of 3α,5α-THP (basal value, 0.5 ± 0.04 ng/gm tissue) (Table 1). Incubation of 25 mm ethanol failed to significantly affect 3α,5α-THP concentrations. A time course study revealed that the effect of 50 and 100 mm, but not 25 mm, ethanol on hippocampal 3α,5α-THP content was apparent with an onset of ∼20 min and increased further after 30 min of incubation (Fig. 2).
Table 1.
Effects of ethanol, progesterone, CB34, and GHB on 3α, 5α-THP concentrations in isolated rat hippocampal minces after a 30 min incubation
|
|
Control |
|
|---|---|---|
| Treatment |
ng/gm−1 tissue |
Percentage of change |
| Vehicle | 0.50 ± 0.04 | |
| Ethanol | ||
| 25 mm | 0.56 ± 0.03 | 13 ± 6.1 |
| 50 mm | 0.85 ± 0.04* | 70 ± 8.3* |
| 100 mm | 0.96 ± 0.02* | 92 ± 5.0* |
| Progesterone (1 μm) | 1.05 ± 0.03* | 110 ± 7.1* |
| CB34 (30 μm) | 1.22 ± 0.04* | 145 ± 7.8* |
| GHB (300 μm) |
1.37 ± 0.04*
|
175 ± 8*
|
Minces of freshly isolated rat hippocampus (see Materials and Methods) were incubated for 30 min in the presence of the different drugs or vehicle. Data are expressed as ng/gm−1 tissue ± SEM obtained from hippocampal minces of eight rats per group of treatment and also as percentage of change ± SEM from the value measured in vehicle-treated minces. *p < 0.01 versus vehicle.
Figure 2.
Time course for the stimulating effect of ethanol (EtOH) on 3α,5α-THP formation inisolated rat hippocampal tissue. Fresh hippocampal minces were incubated in the presence of ethanol (25, 50, and 100 mm) or vehicle at 34°C for various times (0-30 min), as described in Materials and Methods. Data are expressed as percentage change in 3α,5α-THP concentration from control value ± SEM obtained from hippocampal minces of eight rats per group of treatment or time point. *p < 0.05; **p < 0.01 versus control.
The effect of ethanol on 3α,5α-THP was mimicked by 1 μm progesterone (F(1,14) = 18.21; p < 0.0005), by 30 μm CB34 (F(1,14) = 18.45; p < 0.0006), and by 300 μm GHB (F(1,14) = 19.89; p < 0.0004) (Table 1). The effects of ethanol, progesterone, CB34, and GHB were prevented by pretreatment of the tissue with 1 μm finasteride for 10 min (data not shown).
GABAA receptor-mediated mIPSCs recorded from CA1 neurons
We next applied the patch-clamp technique to assess the effects of ethanol as well as other drugs on GABAA receptor function in CA1 pyramidal neurons present in hippocampal slices prepared from 20- to 30-d-old rats. Spontaneous mIPSCs were apparent in CA1 neurons under whole-cell voltage-clamp conditions (holding potential, -60 mV) in the presence of TTX (600 nm) and kynurenic acid (1 mm), a broad-spectrum ionotropic glutamate receptor antagonist (Fig. 3A, Table 2). Inward mIPSCs (attributable to symmetrical Cl- concentrations) were completely suppressed by the GABAA receptor antagonist bicuculline methiodide (20 μm) (Fig. 3A).
Figure 3.
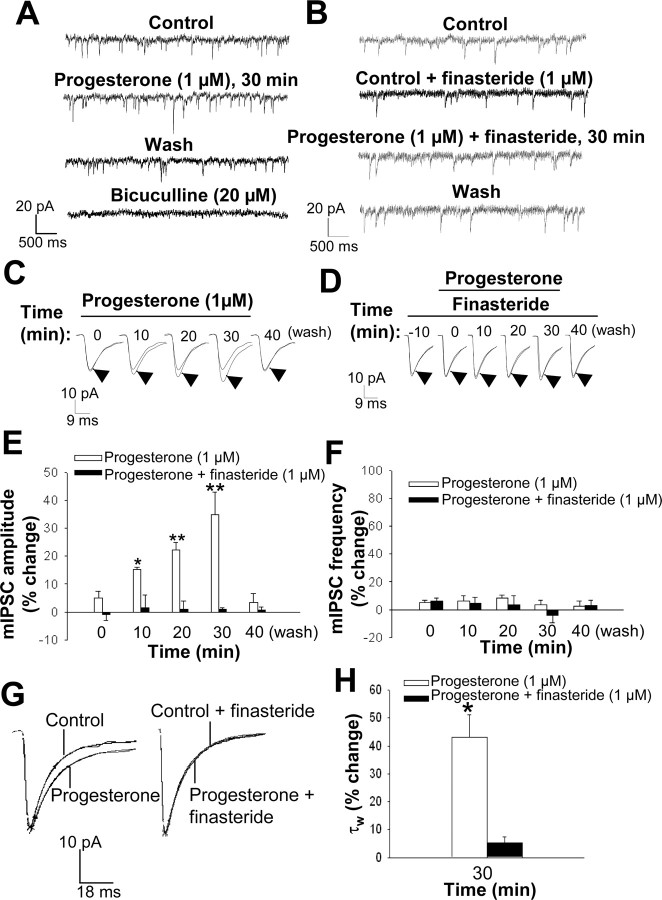
Modulation of GABAA receptor-mediated mIPSCs in CA1 pyramidal neurons in hippocampal slices by progesterone. A, Representative mIPSC recordings obtained before (control) and during (30 min) bath application of 1 μm progesterone as well as 10 min after drug washout and in the presence of 20 μm bicuculline. B, Representative mIPSC recordings obtained in the presence of 1 μm finasteride alone, 30 min after application of 1 μm progesterone in the presence of finasteride, and 10 min after washout of progesterone. C, Averaged mIPSC traces recorded at various times during bath application of 1 μm progesterone for 30 min as well as 10 min after drug washout; the time 0 trace was recorded during the initial 3 min of progesterone application. Each experimental averaged trace (arrowhead) is compared with the control trace. D, Averaged mIPSC traces recorded in the presence of 1 μm finasteride alone, at various times during the coapplication (10 min later) of 1 μm progesterone and finasteride for 30 min and 10 min after washout of progesterone. E, Percentage change in mean mIPSC amplitude at various times during bath application of 1 μm progesterone for 30 min in the absence or presence of 1 μm finasteride. *p < 0.05; **p < 0.01 versus control (n = 8-12 cells). F, Percentage change in mean mIPSC frequency at various times during bath application of 1 μm progesterone for 30 min in the absence and presence of 1 μm finasteride (n = 8-12 cells). G, Normalized average mIPSC traces showing that bath application of 1 μm progesterone for 30 min increases the current decay time (left), an effect that is reversed in the presence of 1 μm finasteride (right). H, Percentage change in mean mIPSC decay time constant τw after 30 min of bath application of 1 μm progesterone in the absence and presence of 1 μm finasteride. *p < 0.01 versus control (n = 8-12 cells)
Table 2.
mIPSC characteristics in CA1 pyramidal neurons
|
Peak amplitude (pA) |
25.7 ± 0.5 |
| Frequency (Hz) | 1.67 ± 0.05 |
| Rise time (msec) | 2.55 ± 0.02 |
| Decay time, tw (msec) |
31.6 ± 4.3 |
Values are means ±SE (n = 55).
Effects of progesterone on GABAA receptor-mediated mIPSCs recorded from CA1 neurons
To examine whether local synthesis of neuroactive steroids by hippocampal cells was able to alter mIPSC characteristics, we exposed hippocampal slices to progesterone (1 μm) for 30 min, during which period mIPSCs were recorded over 3 min epochs at 10 min intervals. Bath application of progesterone induced a marked increase in the mean amplitude of mIPSCs. This effect was time dependent, being first apparent (+15 ± 1%) after 10 min and maximal (+35 ± 8%) after 30 min, and it was fully reversible within 10 min after drug washout (Fig. 3A,C,E). Progesterone did not affect mIPSC frequency (Fig. 3F) but significantly increased the decay time constant τw (Fig. 3G,H). To determine whether the effect of progesterone on mIPSC amplitude and decay time was mediated by its metabolism to 3α,5α-THP, we exposed hippocampal slices to 1 μm finasteride both for 10 min before and during the 30 min application of progesterone. Finasteride, which per se failed to affect mIPSC amplitude or decay time, prevented the increase in these parameters induced by progesterone (Fig. 3B,D,E,G,H). Given that progesterone does not activate GABAA receptors directly (Lambert et al., 2001), these results suggest that the exogenously applied hormone is converted by hippocampal cells to active metabolites (Smith et al., 1987), which are then released into the extracellular medium in amounts sufficient to influence the amplitude of GABAA receptor-mediated mIPSCs.
Effects of CB34 on GABAA receptor-mediated mIPSCs recorded from CA1 neurons
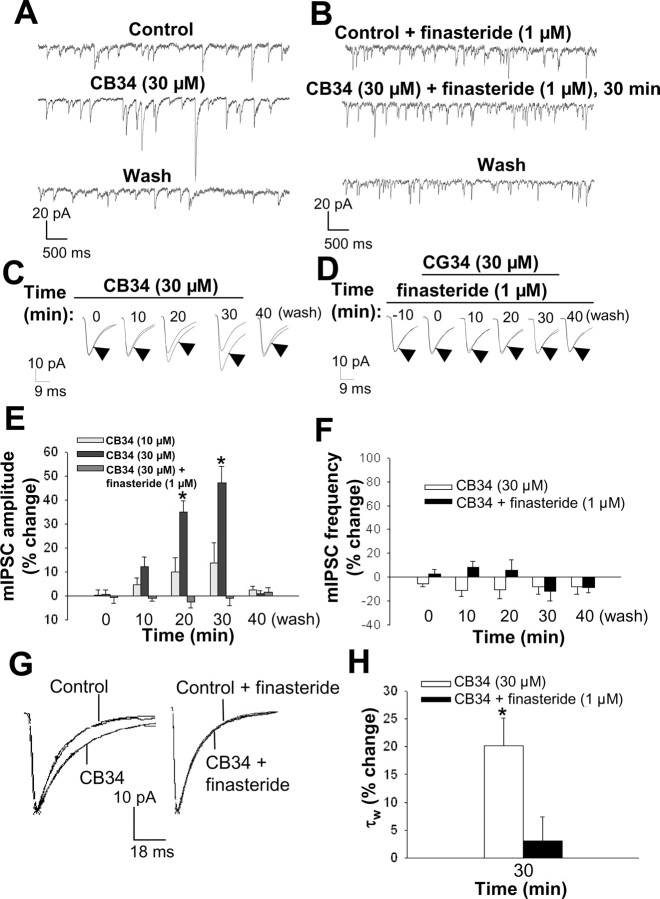
We next examined whether the effect of progesterone on mIPSCs was mimicked by activation of neurosteroid biosynthesis through selective stimulation of the PBR (Papadopoulos, 1993). Exposure of hippocampal slices to the PBR-selective agonist CB34, the systemic administration of which elicits a marked increase in both the plasma and brain concentrations of neuroactive steroids in rats (Serra et al., 1999), resulted in a time- and concentration-dependent increase in mIPSC amplitude (Fig. 4A,C,E). At a concentration of 30 μm, but not 10 μm, this compound increased mIPSC amplitude by 35 ± 6% and 47 ± 7% 20 and 30 min after its bath application, respectively. The effect of CB34 was completely reversed 10 min after its washout. CB34 (30 μm), like progesterone, did not affect mIPSC frequency (Fig. 4F), but it induced a marked increase in the decay time constant τw (Fig. 4G,H). Finasteride (1 μm) completely blocked the effect of 30 μm CB34 on both mIPSC amplitude and decay time (Fig. 4B,D,E,G,H).
Figure 4.
Modulation of GABAA receptor-mediated mIPSCs in CA1 pyramidal neurons in hippocampal slices by the PBR agonist CB34. A, Representative mIPSC recordings obtained before (control) and during (30 min) bath application of 30 μm CB34 as well as 10 min after drug washout. B, Representative mIPSC recordings obtained in the presence of 1 μm finasteride alone, 30 min after application of 30 μm CB34 in the presence of finasteride, and 10 min after washout of CB34. C, Averaged mIPSC traces recorded at various times during bath application of 30 μm CB34 for 30 min as well as 10 min after drug washout; the time 0 trace was recorded during the initial 3 min of CB34 application. Each experimental averaged trace (arrowhead) is compared with the control trace. D, Averaged mIPSC traces recorded in the presence of 1 μm finasteride alone, at various times during the coapplication (10 min later) of 30 μm CB34 and finasteride for 30 min and 10 min after washout of CB34. E, Percentage change in mean mIPSC amplitude at various times during bath application of 10 or 30 μm CB34 for 30 min in the absence or presence of 1 μm finasteride. *p < 0.05 versus control (n = 12-18 cells). F, Percentage change in mean mIPSC frequency at various times during bath application of 30 μm CB34 for 30 min in the absence and presence of 1 μm finasteride (n = 12-14 cells). G, Normalized average mIPSC traces showing that bath application of 30 μm CB34 for 30 min increases the current decay time (left), and the reversal by 1 μm finasteride (right). H, Percentage change in mean mIPSC decay time constant τw after 30 min of bath application of 30 μm CB34 in the absence and presence of 1 μm finasteride. *p < 0.01 versus control (n = 12-18 cells).
Effects of lorazepam on GABAA receptor-mediated mIPSCs recorded from CA1 neurons
As a control, we also tested the effect of lorazepam, a positive allosteric modulator of the GABAA receptor. This drug (3 μm) induced a rapid and transient increase (+41 ± 5%) in mIPSC amplitude as well as in the decay time constant τw (Fig. 5A-C); these effects were no longer evident 10 min after the onset of drug perfusion and were not altered by preincubation of the tissue with finasteride (Fig. 5A-C).
Figure 5.
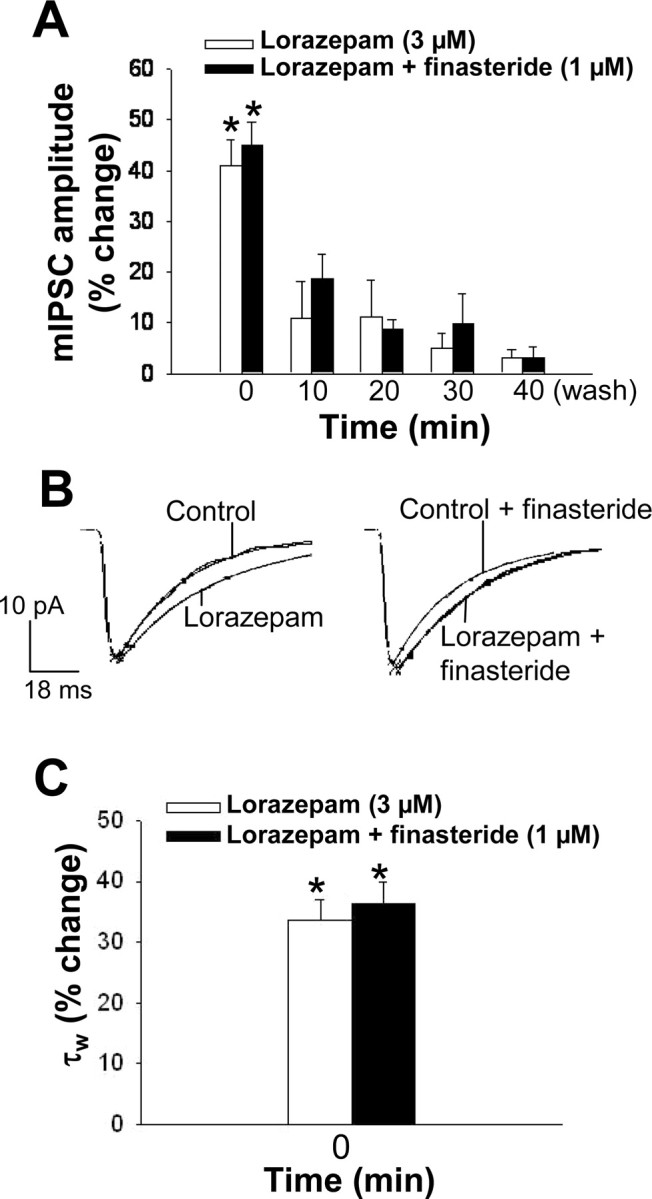
A, Modulation of GABAA receptor-mediated mIPSCs in CA1 pyramidal neurons in hippocampal slices by the benzodiazepine lorazepam. Data represent the percentage change in mIPSC amplitude at various times during bath application of 3 μm lorazepam for 30 min in the absence or presence of 1 μm finasteride. *p < 0.05 versus control (n = 3-6 cells). B, Normalized average mIPSC traces showing that 3 μm lorazepam during the initial 3 min (time 0) of its bath application increases the current decay time (left), and this effect is not affected by 1 μm finasteride (right). C, Percentage change in mean mIPSC decay time constant τw during the initial 3 min (time 0) of bath application of 3 μm lorazepam in the absence and presence of 1 μm finasteride. *p < 0.05 versus control (n = 3-6 cells).
Effects of ethanol on GABAA receptor-mediated mIPSCs recorded from CA1 neurons
We next examined the effect of bath application of ethanol on GABAA receptor-mediated mIPSCs. Ethanol (25, 50, 100, and 150 mm) increased mIPSC amplitude in a time- and concentration-dependent manner (see Fig. 7A,C,E,I). At a concentration of 100 mm, ethanol induced a 28 ± 7% increase in mIPSC amplitude during the initial 3 min of perfusion (time 0). This effect was reduced in extent, although still significant, after 10 min of ethanol exposure but was increased in extent after 30 min to +36 ± 5%. The ethanol-induced increase in mIPSC amplitude was completely reversed 10 min after drug washout. The decay time constant τw was unaffected by ethanol during the initial 3 min of perfusion, but a significant increase resulted after 30 min of ethanol exposure (Fig. 6G,H). In contrast to progesterone and CB34, ethanol also increased mIPSC frequency, an effect evident after 10 min (+43 ± 26%) and maximal at 20 min (+85 ± 49%) (Fig. 6F). To determine whether the increased synthesis of neurosteroids induced by ethanol was responsible for the effects of this drug on mIPSC characteristics, we exposed hippocampal slices to finasteride (1 μm) before and during application of ethanol (100 mm). Again, although pretreatment with finasteride for 10 min failed to prevent the initial increase in mIPSC amplitude induced by ethanol, it completely inhibited the effect of ethanol measured 10, 20, or 30 min after the onset of its application (Fig. 6B,D,E). Finasteride also blocked the delayed increase of ethanol on the decay time constant τw measured at 30 min of exposure (Fig. 6G,H). The ethanol-induced increase in mIPSC frequency was not blocked by finasteride (Fig. 6 F), suggesting that this action is not mediated by neurosteroids.
Figure 7.
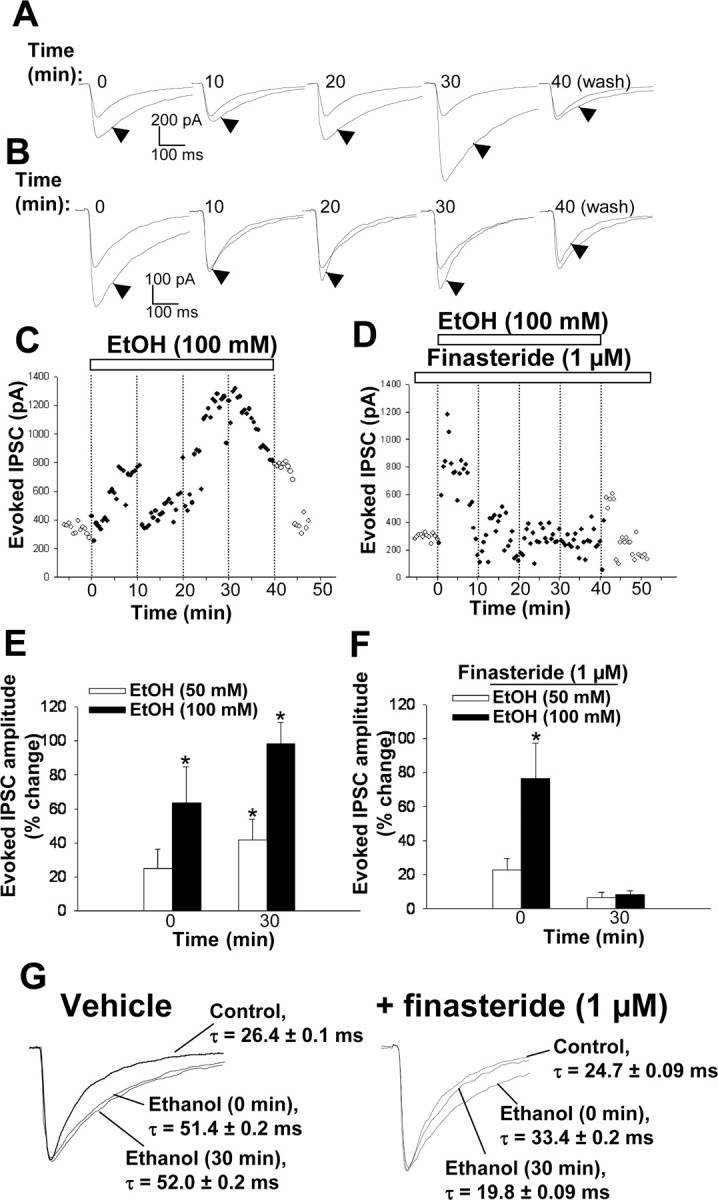
Modulation of synaptically evoked, GABAA receptor-mediated IPSCs by ethanol (EtOH). A, Representative recordings of synaptically evoked IPSCs obtained at various times during bath application of 100 mm ethanol for 30 min and 10 min after drug washout. The evoked IPSCs in the presence of ethanol (arrowheads) are compared with the control IPSC. B, Representative recordings of evoked IPSCs obtained at various times during bath application of 100 mm ethanol and 1 μm finasteride for 30 min (after pretreatment with finasteride alone for 10 min) as well as 10 min after washout of ethanol. C, Representative recording of IPSCs evoked at 30 sec intervals before, during, and after bath application of ethanol (100 mm) for 40 min. D, Representative recording of IPSCs evoked at 30 sec intervals in the presence of 1 μm finasteride before, during, and after its coapplication with 100 mm ethanol for 40 min. E, Percentage change in mean amplitude of evoked IPSCs induced by ethanol (50 or 100 mm) immediately (time 0) and 30 min after its bath application. *p < 0.05 versus control (n = 10-12 cells). F, Percentage change in evoked IPSC amplitude induced by ethanol (50 or 100 mm) immediately (time 0) and 30 min after its bath application in the presence of 1 μm finasteride (after pretreatment for 10 min with finasteride alone). *p < 0.05 versus control (n = 8-13 cells). G, Normalized evoked IPSCs obtained at time 0 and 30 min of 100 mm ethanol exposure either in the absence or presence of 1 μm finasteride, with the indication of the decay time constant values.
Figure 6.
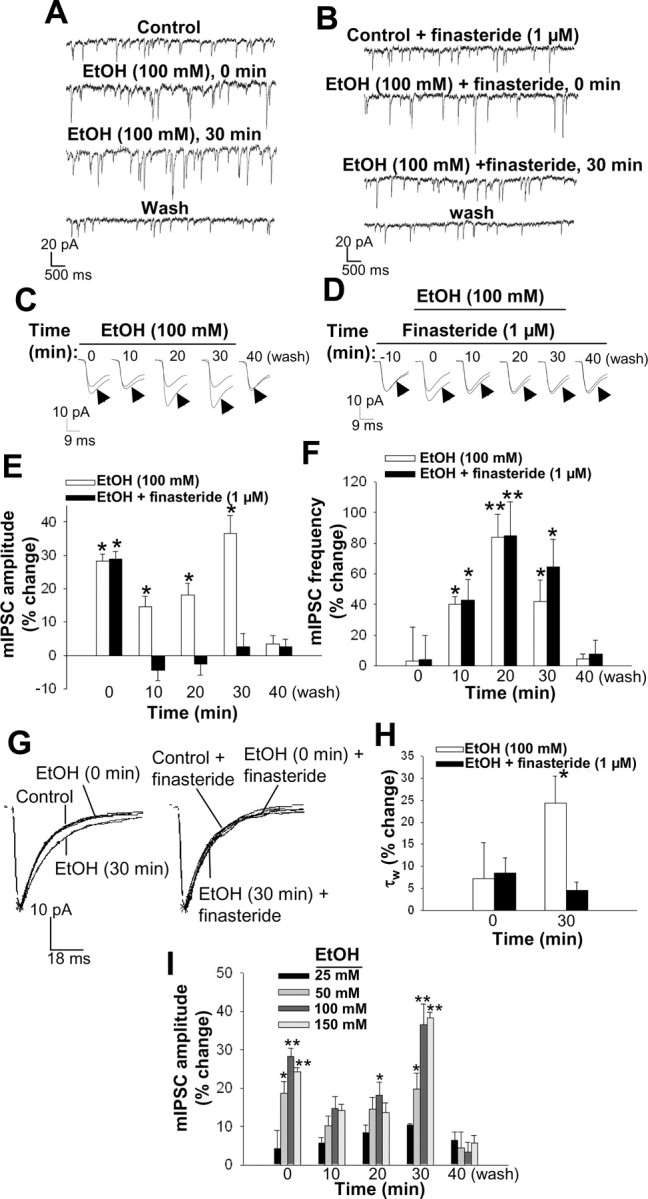
Modulation of GABAA receptor-mediated mIPSCs by ethanol. A, Representative mIPSC recordings obtained before (control), during the initial 3 min (time 0), and at the end of a 30 min bath application of 100 mm ethanol (EtOH), and 10 min after drug washout. B, Representative mIPSC recordings obtained in the presence of 1 μm finasteride alone, at the onset and end of a 30 min application of both finasteride and ethanol (100 mm) and 10 min after washout of ethanol. C, Averaged mIPSC traces recorded at various times during bath application of 100 mm ethanol for 30 min as well as 10 min after drug washout. Each experimental averaged trace (arrowhead) is compared with the control trace. D, Averaged mIPSC traces recorded in the presence of 1 μm finasteride alone, at various times during its application (10 min later) together with ethanol (100 mm) for 30 min and 10 min after washout of ethanol. E, Percentage change in mean mIPSC amplitude induced by bath application of 100 mm ethanol in the absence or presence of 1 μm finasteride. *p < 0.05 versus control (n = 18-24 cells). F, Percentage change in mean mIPSC frequency induced by bath application of 100 mm ethanol in the absence or presence of 1 μm finasteride. *p < 0.05; **p < 0.01 versus control (n = 10-16 cells). G, Normalized average mIPSC traces showing the effect of bath application of 100 mm ethanol during the initial 3 min (time 0) and after 30 min on the current decay time in the absence (left) and presence (right) of 1 μm finasteride. H, Percentage change in mean mIPSC decay time constant τw during the initial 3 min (time 0) and after 30 min of bath application of 100 mm ethanol in the absence and presence of 1 μm finasteride. *p < 0.05 versus control (n = 18-24 cells). I, Concentration-response curve for the effect of ethanol on GABAA receptor-mediated mIPSCs in CA1 pyramidal cells. Data are presented as percentage change in mIPSC amplitude induced by bath application of various concentrations (25-150 mm) of ethanol ± SEM. *p < 0.05; **p < 0.01 versus control (n = 5-24 cells).
Effects of ethanol on GABAA receptor-mediated synaptically evoked IPSCs recorded from CA1 neurons
To investigate further the effect of ethanol on GABAA receptor responses, we recorded synaptically evoked IPSCs from CA1 pyramidal cells in hippocampal slices incubated in the presence of kynurenic acid (1 mm). The amplitude of evoked IPSCs was markedly increased immediately after ethanol perfusion (Fig. 7 A, C,E). The extent of this effect of ethanol declined after 10 min and then increased again, becoming maximal between 20 and 40 min. The decay time constant was also significantly enhanced by ∼90% both immediately and after 30 min of ethanol perfusion (Fig. 7G). Pretreatment with finasteride (1 μm) did not affect the rapid increase in IPSC amplitude and decay time induced by ethanol but abolished the secondary increase of both parameters apparent between 20 and 40 min (Fig. 7 B, D, F, G).
Effects of ethanol on PPF in CA1 pyramidal cells
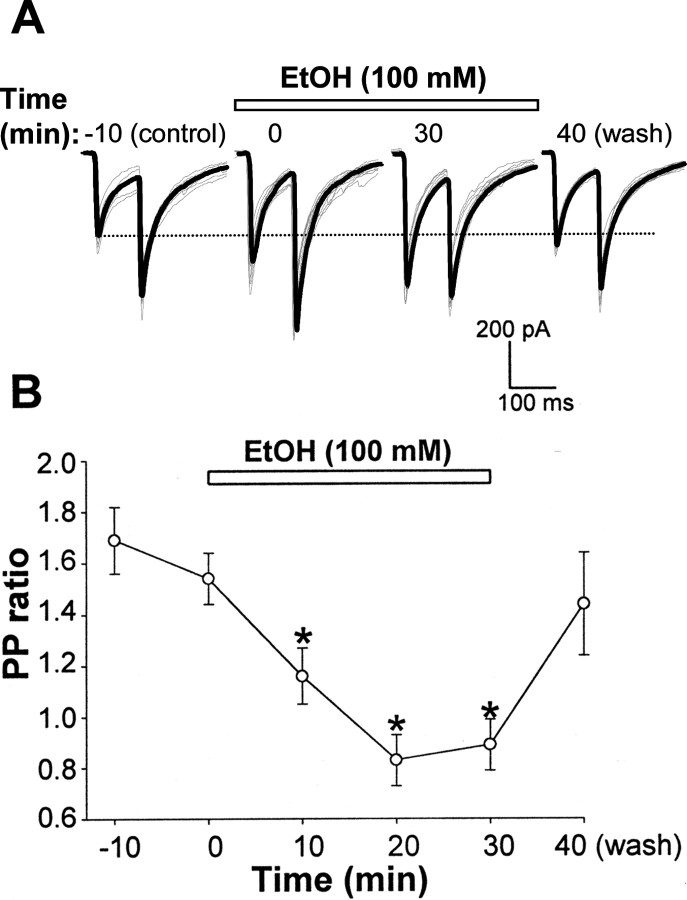
The increase in mIPSC frequency induced by ethanol suggested that this drug might also act at the presynaptic level. Thus, to better evaluate whether incubation of hippocampal slices with ethanol alters the probability of GABA release at CA1 pyramidal neurons, we performed a PPF experiment, based on the facts that a reduction of PPF is associated with an increased probability of transmitter release (Andreasen and Hablitz, 1994; Roberto et al., 2003). Perfusion for 30 min of 100 mm ethanol significantly reduced and reversed the PP ratio from 1.69 ± 0.13 (control) to 1.16 ± 0.11, 0.83 ± 0.1, and 0.89 ± 0.1 after 10, 20 and 30 min, respectively. This effect was promptly reversed following 10 min washout (Fig. 8).
Figure 8.
Bath application of ethanol (EtOH) reduces the PPR in CA1 pyramidal cells. A, Representative synaptically evoked IPSC traces, obtained with an interstimulus interval of 100 msec, recorded before, during the initial 3 min (time 0), and after 30 min of bath application of 100 mm ethanol and after washout. Averaged (from 7 sweeps/3 min; gray traces) currents are superimposed (black trace). B, Change in PPR versus time for the bath application of 100 mm ethanol. Data are the average ± SEM obtained from five different cells. *p < 0.05 versus control.
Effects of GHB on GABAA receptor-mediated synaptically evoked IPSCs recorded from CA1 neurons
Finally, we tested the effect of GHB on GABAA receptor function. The systemic administration of this compound, like that of ethanol, increases the plasma and brain concentrations of neuroactive steroids in rats (Barbaccia et al., 2002). Bath application of GHB (0.1-10 mm) induced a time- and concentration-dependent increase in mIPSC amplitude (Fig. 9A,C,E,I). In contrast to ethanol, but consistent with its lack of direct activity at the GABAA receptor (Serra et al., 1991), GHB failed to affect mIPSC amplitude during the first 3 min of bath application. The modulatory effect of GHB became evident, however, after perfusion for ∼10 min. At a concentration of 300 μm, GHB increased mean mIPSC amplitude by 32 ± 4% after perfusion for 30 min (Fig. 9E). The effect of 300 μm GHB was completely reversed after drug washout for 10 min (Fig. 9A,C,E,I). GHB (300 μm) did not affect mIPSC frequency (Fig. 9F) but significantly increased the decay time constant τw (Fig. 9G,H). Pretreatment of hippocampal slices with 1 μm finasteride also prevented the GHB-induced increase in mIPSC amplitude and decay time constant (Fig. 9B,D,E,G,H).
Figure 9.
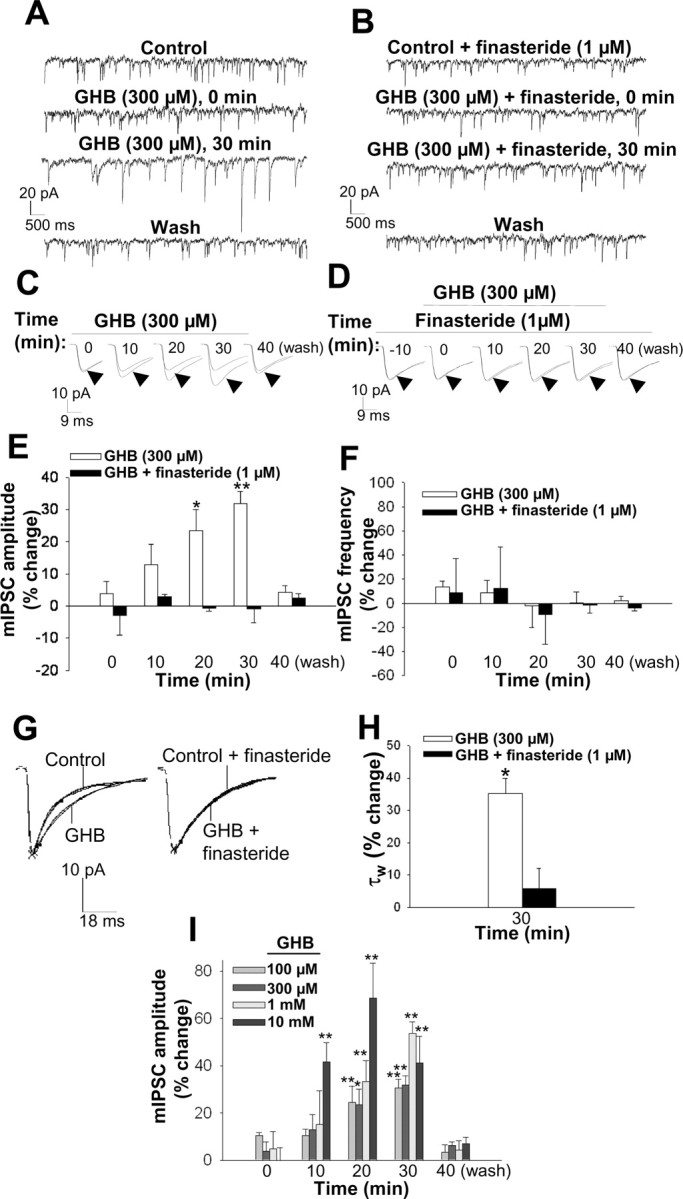
Modulation of GABAA receptor-mediated mIPSCs by GHB. A, Representative mIPSC recordings obtained before (control), at the onset and end of a 30 min bath application of 300 μm GHB, and 10 min after drug washout. B, Representative recordings of mIPSCs obtained in the presence of 1 μm finasteride alone, at the onset and end of a 30 min application of both finasteride and GHB (300 μm), and 10 min after washout of GHB. C, Averaged mIPSC traces recorded at various times during bath application of 300 μm GHB for 30 min as well as 10 min after drug washout. The mIPSCs in the presence or GHB (arrowheads) are compared with the control IPSC. D, Averaged mIPSC recordings obtained at various times during bath application of 300 μm GHB in the presence of 1 μm finasteride for 30 min (after pretreatment for 10 min with finasteride alone) as well as after washout of GHB. E, Percentage change in mean mIPSC amplitude induced by bath application of 300 μm GHB for the indicated times in the absence or presence of 1 μm finasteride. *p < 0.05; **p < 0.01 versus control (n = 15-24 cells). F, Percentage change in mean mIPSC frequency induced by bath application of 300 μm GHB in the absence or presence of 1 μm finasteride (n = 8-15 cells). G, Normalized average mIPSC traces showing the effect of 30 min bath application of 300 μm GHB on the current decay time in the absence (left) and presence (right) of 1 μm finasteride. H, Percentage change in the mean mIPSC decay time constant τw after 30 min of bath application of 300 μm GHB in the absence and presence of 1 μm finasteride. *p < 0.05 versus control (n = 15-24 cells). I, Concentration-response curve for the effect of GHB on GABAA receptor-mediated mIPSCs in CA1 pyramidal cells. Data are presented as percentage change in mIPSC amplitude at various times during bath application of the indicated concentrations of GHB for 30 min. *p < 0.05; **p < 0.01 versus control (n = 5-24 cells).
Similar results on the effects of progesterone, CB34, ethanol, and GHB on GABAA receptor-mediated mIPSCs were obtained in hippocampal slices prepared from adrenalectomized/castrated male rats (our unpublished observations).
Discussion
In this study, we demonstrated a previously uncharacterized effect of ethanol, acting by increasing the local biosynthesis of the neurosteroid 3α,5α-THP in isolated hippocampal tissue. This effect results in a marked increase in the amplitude and decay prolongation of GABAA receptor-mediated miniature or evoked IPSCs. Progesterone, CB34, and GHB also each increased the amplitude and the decay time of GABAA receptor-mediated mIPSCs. Because these latter drugs also increase 3α,5α-THP biosynthesis, these results further support the role of brain steroid metabolism in shaping GABAA receptor-mediated inhibition in a discrete brain area (Belelli and Herd, 2003).
Progesterone is metabolized by neurons and glial cells to 3α,5α-THP (Hu et al., 1987; Bitran et al., 1995; Follesa et al., 2000), whereas CB34 is a selective agonist of the PBR and stimulates steroidogenesis in the brain and peripheral organs (Serra et al., 1999). The capacity of GHB to increase both plasma and brain levels of 3α,5α-THP as well as other neurosteroids after systemic administration has been proposed to involve GABAB receptors (Barbaccia et al., 2002). Although the precise mechanism by which ethanol directly stimulates brain steroidogenesis remains to be established, the inhibition by finasteride of the effects of ethanol on the amplitude of mIPSCs or evoked IPSCs suggests that these effects are mediated in part by an increased production of 3α,5α-THP in hippocampal tissue. The time course of the effect of ethanol, as well as that of progesterone and CB34, on GABAA receptor-mediated mIPSCs in brain slices is compatible with that (20-30 min) of their stimulatory action on 3α,5α-THP production in hippocampal minces. However, it is important to establish whether the levels of 3α,5α-THP measured in hippocampal tissue are indeed relevant for modulating GABAA receptor function. Morrow et al. (1987, 1990) have shown that cerebral-cortical concentration of 3α,5α-THP above 50 nm (or ∼6 ng/gm tissue) potentiated GABAA receptor-mediated chloride flux. In our study, the exposure of hippocampal tissue resulted in a stimulation of 3α,5α-THP concentration to ∼1 ng/gm tissue. However, it should be pointed out that, although this concentration of 3α,5α-THP was measured in whole tissue homogenate, it is conceivable that in the slice preparation, the concentration of 3α,5α-THP released locally by hippocampal cells may be much higher at the synaptic level in the proximity of the CA1 pyramidal neuron under examination.
Unlike progesterone, CB34, and GHB, however, ethanol increases the amplitude of both spontaneous mIPSCs and synaptically evoked IPSCs by two distinct actions. In addition to the finasteride-sensitive, delayed effect that is likely mediated by an increased local biosynthesis of 3α,5α-THP, ethanol also induces a rapid effect that is finasteride insensitive and is likely attributable to direct interaction with GABAA receptors. This latter direct effect of ethanol on GABAA receptors appeared to undergo some process of tolerance, seen within 10 min of ethanol exposure, a phenomenon that was observed for both mIPSCs and evoked IPSCs. This effect may be attributable to receptor desensitization occurring as a result of the prolonged presence of ethanol and may involve processes such as phosphorylation of receptor subunits. On the contrary, progesterone, CB34, and GHB mimicked only the finasteride-sensitive, delayed action of ethanol, consistent with their inability to interact directly with the GABAA receptor (Serra et al., 1991, 1999; Lambert et al., 2001).
Another strong evidence that the delayed, but not the immediate, action of ethanol, as well as progesterone, CB34, and GHB, may be mediated by the increased synthesis of 3α,5α-THP is that their effect on current amplitude is accompanied by an enhancement of the decay time of both mIPSCs and evoked IPSCs. Accordingly, steroids have been shown previously to prolong GABAA receptor IPSCs (Harrison et al., 1987; Zhu and Vicini, 1997). Furthermore, prolongation of current decay time by ethanol, progesterone, CB34, and GHB was abolished by finasteride, again supporting the role of 3α,5α-THP in the modulatory action of these drugs in hippocampal pyramidal cells.
It should also be noted that ethanol increased the mean evoked IPSC decay time during its initial 3 min bath application, but a similar immediate effect was not measured on mIPSCs. Although this apparent discrepancy is difficult to explain, we should also consider that the immediate effect of 100 mm ethanol on the amplitude of evoked IPSCs (63.4 ± 21%) (Fig. 7) was comparatively much greater than that on mIPSCs (28.2 ± 2.1%) (Fig. 6).
In contrast to progesterone and CB34, ethanol, consistent with previous data (Roberto et al., 2003), also increased mIPSC frequency, and this presynaptic action of ethanol was not inhibited by finasteride, suggesting that this effect does not involve 3α,5α-THP. The reduction and reversal of PPF induced by ethanol also suggested that the increased mIPSC frequency is attributable to an increased probability of GABA release from the synaptic site (Andreasen and Hablitz, 1994; Roberto et al., 2003).
Finally, the failure of finasteride to antagonize the effect of lorazepam on mIPSC amplitude further suggests the specificity of action of this 5α-reductase inhibitor.
Our results demonstrate for the first time that ethanol promotes brain steroidogenesis by a local action independent of the HPA axis. This action of ethanol, together with or independent of stimulation of HPA axis activity, might thus be important in mediating some of the central effects of this drug of abuse. Finally, this novel mechanism may be important in mediating the effects of ethanol in such physiological and pathological conditions as menstrual cycle, pregnancy, menopause, premenstrual syndrome, and a variety of neurological or psychiatric disorders in which the steroidogenic machinery undergoes dramatic functional changes (Barbaccia et al., 1996; Bicikova et al., 1998; Concas et al., 1998; Genazzani et al., 1998; Biggio and Purdy, 2001).
Footnotes
This work was supported by Grant CE00042735 (Project Center of Excellence for the Neurobiology of Dependence, D.M. 21 January 2001) and PRIN Grants 2002053959-001 and 2001055774 from the Ministry of Instruction, University and Research, Italy; by National Institute on Alcohol Abuse and Alcoholism Grant U01AA13641; by the Sardinian Health Ministry; and in part by the GIO.I.A. Foundation (Pisa, Italy). We thank C.F. Valenzuela and M. Carta for helpful discussions.
Correspondence should be addressed to Dr. Enrico Sanna, Department of Experimental Biology, Section of Neuroscience, University of Cagliari, Cittadella Universitaria, 09123 Cagliari, Italy. E-mail: esanna@unica.it.
Copyright © 2004 Society for Neuroscience 0270-6474/04/246521-10$15.00/0
References
- Andreasen M, Hablitz JJ (1994) Paired-pulse facilitation in the dentate gyrus: a patch-clamp study in rat hippocampus in vitro. J Neurophysiol 72: 326-336. [DOI] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA (1998) The synaptic basis of GABAA,slow J Neurosci 18: 1305-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G (1996) Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology 63: 166-172. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL (1999) Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol 384: R1-R2. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Colombo G, Affricano D, Carai MA, Vacca G, Melis S, Purdy RH, Gessa GL (2002) GABA(B) receptor-mediated increase of neurosteroids by gamma-hydroxybutyric acid. Neuropharmacology 42: 782-791. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB (2003) The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci 23: 10013-10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicikova M, Dibbelt L, Hill M, Hampl R, Starka L (1998) Allopregnanolone in women with premenstrual syndrome. Horm Metab Res 30: 227-230. [DOI] [PubMed] [Google Scholar]
- Biggio G, Purdy RH, eds (2001) Neurosteroids and brain function, Vol 46. San Diego: Academic. [Google Scholar]
- Bitran D, Shiekh M, McLeod M (1995) Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol 7: 171-177. [DOI] [PubMed] [Google Scholar]
- Brot MD, Koob GF, Britton KT (1995) Anxiolytic effects of steroid hormones during the estrous cycle. Interactions with ethanol. Recent Dev Alcohol 12: 243-259. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G (1998) Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA 95: 13284-13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis FW (1966) Effect of ethanol on plasma corticosterone levels. J Pharmacol Exp Ther 153: 121-127. [PubMed] [Google Scholar]
- Follesa P, Serra M, Cagetti E, Pisu MG, Porta S, Floris S, Massa F, Sanna E, Biggio G (2000) Allopregnanolone synthesis in cerebellar granule cells: roles in regulation of GABA(A) receptor expression and function during progesterone treatment and withdrawal. Mol Pharmacol 57: 1262-1270. [PubMed] [Google Scholar]
- Gavish M, Bachman I, Shoukrun R, Katz Y, Veenman L, Weisinger G, Weizman A (1999) Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev 51: 629-650. [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M (1998) Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab 83: 2099-2103. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL (1998) The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 139: 2-19. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA (1984) Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res 323: 287-292. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL (1987) A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci 7: 604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ZY, Bourreau E, Jung-Testas I, Robel P, Baulieu EE (1987) Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci USA 84: 8215-8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, Kralic JE, VanDoren MJ, Morrow AL (2002) Adrenalectomy attenuates increase in cortical allopregnanolone and behavioral effects induced by acute ethanol administration. Alcohol Clin Exp Res 26: 103A. [Google Scholar]
- Khisti RT, Kumar S, Morrow AL (2003a) Ethanol rapidly induces steroidogenic acute regulatory protein expression and translocation in rat adrenal gland. Eur J Pharmacol 473: 225-227. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, O'Buckley T, Morrow AL (2003b) Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res 980: 255-265. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA (1994) Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 270: 1223-1229. [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F (1998) Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res 22: 3-9. [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG (2001) Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev 37: 68-80. [DOI] [PubMed] [Google Scholar]
- Majewska MD (1992) Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol 38: 379-395. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232: 1004-1007. [DOI] [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA (1983) Behavioral evidence for the involvement of gamma-aminobutyric acid in the actions of ethanol. Eur J Pharmacol 89: 53-62. [DOI] [PubMed] [Google Scholar]
- Mathur C, Prasad VV, Raju VS, Welch M, Lieberman S (1993) Steroids and their conjugates in the mammalian brain. Proc Natl Acad Sci USA 90: 85-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM (1987) Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol 142: 483-485. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM (1990) Characterization of steroid interactions with gamma-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol 37: 263-270. [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA (1999) Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res 23: 1933-1940. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB (2001) The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev 37: 98-109. [DOI] [PubMed] [Google Scholar]
- Ogilvie KM, Lee S, Rivier C (1997) Role of arginine vasopressin and corticotropin-releasing factor in mediating alcohol-induced adrenocorticotropin and vasopressin secretion in male rats bearing lesions of the paraventricular nuclei. Brain Res 744: 83-95. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V (1993) Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr Rev 14: 222-240. [DOI] [PubMed] [Google Scholar]
- Rivier C (1996) Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res 20: 240-254. [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W (1984) Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther 229: 127-131. [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA 100: 2053-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscetti G, Del Carmine R, Trabucchi M, Massotti M, Purdy RH, Barbaccia ML (1998) Modulation of neurosteroid synthesis/accumulation by l-ascorbic acid in rat brain tissue: inhibition by selected serotonin antagonists. J Neurochem 71: 1108-1117. [DOI] [PubMed] [Google Scholar]
- Serra M, Sanna E, Foddi C, Concas A, Biggio G (1991) Failure of gamma-hydroxybutyrate to alter the function of the GABAA receptor complex in the rat cerebral cortex. Psychopharmacology (Berl) 104: 351-355. [DOI] [PubMed] [Google Scholar]
- Serra M, Madau P, Chessa MF, Caddeo M, Sanna E, Trapani G, Franco M, Liso G, Purdy RH, Barbaccia ML, Biggio G (1999) 2-Phenylimidazo[1,2-a]pyridine derivatives as ligands for peripheral benzodiazepine receptors: stimulation of neurosteroid synthesis and anticonflict action in rats. Br J Pharmacol 127: 177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ (1987) Locally applied progesterone metabolites alter neuronal responsiveness in the cerebellum. Brain Res Bull 18: 739-747. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL (2000) Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci 20: 1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE (2001) GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci 21: 3009-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S (1997) Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci 17: 4022-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]