Abstract
δ Subunit-containing GABAA receptors are located predominantly at nonsynaptic sites in the dentate gyrus where they may play important roles in controlling neuronal excitability through tonic inhibition and responses to GABA spillover. Immunohistochemical methods were used to determine whether δ subunit expression was altered after pilocarpine-induced status epilepticus in C57BL/6 mice in ways that could increase excitability of the dentate gyrus. In pilocarpine-treated animals, the normal diffuse labeling of the δ subunit in the dentate molecular layer was decreased by 4 d after status epilepticus (latent period) and remained low throughout the period of chronic seizures. In contrast, diffuse labeling of α4 and γ2 subunits, potentially interrelated GABAA receptor subunits, was increased during the chronic period. Interestingly, δ subunit labeling of many interneurons progressively increased after pilocarpine treatment. Consistent with the observed changes in δ subunit labeling, physiological studies revealed increased excitability in the dentate gyrus of slices obtained from the pilocarpine-treated mice and demonstrated that physiological concentrations of the neurosteroid tetrahydrodeoxycorticosterone were less effective in reducing excitability in the pilocarpine-treated animals than in controls. The findings support the idea that alterations in nonsynaptic δ subunit-containing GABAA receptors in both principal cells and interneurons could contribute to increased seizure susceptibility in the hippocampal formation in a temporal lobe epilepsy model.
Keywords: tonic inhibition, neurosteroids, dentate gyrus, hippocampus, pilocarpine, immunohistochemistry
Introduction
Studies of GABAergic inhibition in epilepsy have generally been focused on synaptic events and synaptically localized GABAA receptor subunits. Yet, nonsynaptic GABAA receptors could be equally important because of the key role that such receptors may play in regulating neuronal excitability (Otis et al., 1991; Brickley et al., 1996; Mody, 2001; Semyanov et al., 2004).
δ Subunit-containing GABAA receptors are critical mediators of nonsynaptic inhibition in the dentate gyrus and are ideally suited for this role because they have a particularly high affinity for GABA and a slow rate of desensitization (Saxena and Macdonald, 1994; Haas and Macdonald, 1999). Thus, these receptors are capable of responding to low concentrations of GABA and are appropriately positioned for such responses. The δ subunit is localized exclusively at extrasynaptic sites in the cerebellum (Nusser et al., 1998b) and is most highly concentrated at perisynaptic sites on granule cell dendrites in the dentate gyrus (Wei et al., 2003). In both locations, δ subunit-containing receptors could be activated by ambient levels of GABA in the neuropil or by spillover of GABA after its synaptic release (Otis et al., 1991; Rossi and Hamann, 1998; Wei et al., 2003).
In addition, δ subunit-containing receptors are quite sensitive to modulation by neurosteroids. Recent studies of cells expressing the δ subunit have demonstrated that the presence of the δ subunit substantially increases the GABA receptors' responses to neurosteroids (Adkins et al., 2001; Belelli et al., 2002; Wohlfarth et al., 2002). In vitro studies of the dentate gyrus have also demonstrated a strong enhancement of tonic inhibition, mediated by δ subunit-containing receptors, by tetrahydrodeoxycorticosterone (THDOC), a neuroactive metabolite of cortisone (Stell et al., 2003a).
Two other subunits, α4 and γ2, are of particular interest because of their relationship to the δ subunit in the normal assembly of GABAA receptors. The α4 subunit is considered a major partner of the δ subunit in the forebrain (Sur et al., 1999), whereas the γ2 subunit is generally excluded from GABAA receptors that contain δ subunits (Quirk et al., 1995; Araujo et al., 1998; Jechlinger et al., 1998). Interrelated changes in expression among the three subunits have been suggested by studies of δ subunit-deficient mice, in which expression of the γ2 and α4 subunits is altered in precisely the same regions that normally express high levels of the δ subunit (Tretter et al., 2001; Peng et al., 2002a).
The importance of δ subunit-containing receptors in tonic inhibition and their potential involvement in epilepsy have led to the present studies of δ subunit expression in a pilocarpine model of recurrent seizures in C57BL/6 mice. The specific goals were to characterize the changes in δ subunit expression after pilocarpine-induced status epilepticus (SE), to determine whether δ subunit changes were accompanied by altered expression of the α4 and γ2 subunits, and to find out whether neurosteroid modulation of excitability of the dentate gyrus was altered in the epileptic mice. Preliminary studies have been reported previously in abstract form (Peng et al., 2002b; Stell et al., 2003b).
Materials and Methods
Animals and pilocarpine treatment. Young adult (6-8 weeks of age) C57BL/6 male mice (20-27 gm; Harlan, Indianapolis, IN) were used in this study. Sustained seizures were induced in experimental animals by the administration of pilocarpine, a muscarinic cholinergic agonist, and the injection protocols were similar to those used previously by our group in rats (Obenaus et al., 1993; Esclapez and Houser, 1999). Thirty minutes before pilocarpine administration, animals were injected with a low dose of the cholinergic antagonist methyl scopolamine nitrate (1 mg/kg, i.p.) to reduce peripheral cholinergic effects. Animals in the experimental group then received an injection of pilocarpine hydrochloride (320-340 mg/kg, i.p.; Sigma, St. Louis, MO) to induce status epilepticus. Diazepam (5 mg/kg, i.p.; Abbott Laboratories, Chicago, IL) was administered to the animals 3 hr after the onset of status epilepticus to reduce behavioral seizures. Control animals received an identical series of injections, except that the pilocarpine was replaced with a similar volume of sterile saline. After the pilocarpine injection, experimental animals were monitored for a minimum of 5 hr to assess the severity and length of the behavioral seizures.
All experimental animals that developed status epilepticus after pilocarpine administration were used in either the histological or electrophysiological studies (n = 44), and control mice were included in all experiments (n = 26). Histological studies included animals at 1, 4, 7, 14, 30, and 60 d after pilocarpine or control treatment. Electrophysiological studies were performed on pilocarpine and control animals at 14-21 d after treatment.
After recovery from status epilepticus, pilocarpine-treated mice were videotaped to monitor the development and occurrence of spontaneous seizures. For six mice, the videotape recordings began the day after pilocarpine injections and continued for 1 week. For the other animals, videotape recordings were performed at least three times each week for 6-8 hr per session. In addition, the mice were videotaped during the 24 hr before perfusion to determine whether spontaneous seizures occurred during this period. All animal use protocols conformed to National Institutes of Health guidelines and were approved by the University of California, Los Angeles, Chancellor's Animal Research Committee.
Behavioral outcomes. Spontaneous behavioral seizures were observed in all but one of the animals that were used at survival times of 14 d or longer after the initial period of status epilepticus (n = 25 of 26). The spontaneous seizures typically consisted of periods of freezing, clonic movements of the forelimbs, rearing, or rearing and falling (stage 3-5 limbic seizures) (Racine, 1972), either followed or preceded by a brief (10-20 sec) generalized motor seizure.
A subgroup of the mice was used for densitometry studies of the time course of receptor subunit changes, and the times of spontaneous seizure onset were of particular interest in this group. No behavioral seizures (excluding occasional body jerks) were observed in the mice included in the 4 and 7 d groups and in one of the mice in the 14 d group. Spontaneous motor seizures were documented in all other mice in the 14-60 d groups.
Tissue preparation. After survival periods of 1, 4, 7, 14, 30, and 60 d, the mice were deeply anesthetized with sodium pentobarbital (90 mg/kg, i.p.) and perfused through the ascending aorta with 4% paraformaldehyde in 0.12 m phosphate buffer, pH 7.3. At least two control and three pilocarpine-treated mice were studied at each time point. After perfusion, the brains were maintained in situ at 4°C for 1 hr and then removed from the skull and postfixed in the same fixative for 1 hr. After thorough rinsing in phosphate buffer, the brains were cryoprotected in a 30% sucrose solution, blocked in the coronal plane, frozen on dry ice, and sectioned at 30 μm on a cryostat. Forebrain sections containing the rostral half of the hippocampus were sectioned in the coronal plane. Near the middle of the hippocampus (∼2.18 mm posterior to bregma) (Franklin and Paxinos, 1997), the brain blocks were reoriented, and the caudal half of the hippocampus was sectioned horizontally. Sections at 300 μm intervals were mounted on slides and stained with cresyl violet for general morphological study. The remaining sections were stored in a cryoprotectant solution at -20°C until processing.
Antisera. Subunit-specific antisera that recognize the δ, α4, and γ2 subunits of the GABAA receptor were produced in rabbit to the following synthetic peptide sequences: δ (1-44) (Sperk et al., 1997); α4 (379-421) (Bencsits et al., 1999); and γ2 (319-366) (Tretter et al., 1997). The specificity of the affinity-purified antisera has been demonstrated previously in immunochemical (Jechlinger et al., 1998) and immunohistochemical (Sperk et al., 1997; Peng et al., 2002a) studies. These antisera were kindly provided by W. Sieghart (University of Vienna, Vienna, Austria).
Antiserum to the α1 subunit was produced in guinea pig to the specific synthetic peptide sequence α1 (1-16) and was generously provided by J.-M. Fritschy (University of Zurich, Zurich, Switzerland). The specificity of the antiserum has been demonstrated previously (Fritschy and Mohler, 1995).
Immunohistochemistry. Before immunohistochemical processing, sections were incubated in 1% H2O2 for 30 min to reduce endogenous peroxidase-like activity. After a rinse in 0.1 m Tris-buffered saline (TBS), pH 7.3, the sections were processed with water bath antigen-retrieval methods (Jiao et al., 1999; Peng et al., 2002a). Free-floating sections were incubated in 0.05 m sodium citrate solution, pH 8.6, for 30 min at room temperature (RT) and then heated in a water bath in the same solution at 90°C for 70 min. The sections were allowed to cool at RT for 30 min and then were rinsed in TBS.
Free-floating sections were processed for immunohistochemistry with avidin-biotin-peroxidase methods (Vectastain Elite ABC; Vector Laboratories, Burlingame, CA). Sections were incubated in 10% normal goat serum (NGS) in TBS containing 0.3% Triton X-100 and avidin (200 μl/ml) for 3-4 hr to reduce nonspecific binding. The sections were incubated with primary antiserum (anti-δ, 1:4000; anti-α4, 1:1500; anti-γ2, 1:2000) diluted in TBS containing 2% NGS and biotin (200 μl/ml) overnight at RT. After rinsing, the sections were incubated in biotinylated goat anti-rabbit IgG (1:1000) at RT for 1 hr. After a thorough rinse, the sections were incubated in avidin-biotin-peroxidase complex (1:200 in TBS, pH 7.3) for 1 hr. To reveal the peroxidase labeling, some sections were processed with 0.06% diaminobenzidine tetrahydrochloride (DAB) and 0.006% H2O2 diluted in 0.075 m PBS for 10-15 min, and immunolabeling was enhanced by incubation in 0.003% osmium tetroxide in PBS for 30 sec. Other sections were processed with a glucose oxidase-DAB-nickel method (Shu et al., 1988) to intensify the labeling. After rinsing, sections were mounted on gelatin-coated slides, dehydrated, and coverslipped.
Sections were also processed for double immunofluorescence labeling of δ and α1 subunits. After water bath antigen-retrieval treatment, the sections were treated with 10% NGS in TBS at RT for 1 hr and then incubated in a solution containing guinea pig anti-α1 (1:50,000) and rabbit anti-δ (1:3000) in TBS with 2% NGS at RT for 3 d. After thorough rinsing in TBS, sections were incubated in a mixture of goat anti-guinea pig IgG conjugated to Alexa Fluor 488 and goat anti-rabbit IgG labeled with Alexa Fluor 594 (both 1:500; Molecular Probes, Eugene, OR) at RT for 4 hr. Sections were then rinsed in TBS for at least 20 min, mounted on slides, and coverslipped with Prolong antifade medium (Molecular Probes). Sections were analyzed with a Zeiss (Thornwood, NY) LSM 410 confocal microscope.
Densitometric analyses. Expression levels of δ, α4, and γ2 subunits in control and pilocarpine-treated animals were evaluated with densitometry to determine the extent and patterns of change over time. Sections used for these analyses were obtained from three pilocarpine-treated animals and two controls for each time point (1, 4, 7, 14, 30, and 60 d) after pilocarpine treatment. For each subunit, sections from animals at all time points were processed identically in the same immunohistochemical experiment. Such experiments were repeated at least three times for each subunit to ensure the reliability of the results. Digital images of immunolabeling in the dentate gyrus were obtained with a Zeiss Axio-plan 2 microscope equipped with an AxioCam digital camera system and AxioVision 3.0 software. Images to be included in the same analysis were photographed under identical conditions on the same day with stabilized light levels. The densities of labeling were then analyzed with morphometric AxioVision software (version 3.0; Zeiss).
For determining the levels of diffuse immunolabeling for each subunit in the molecular layer, the sections were photographed with a 10× objective, and gray level values were obtained from three rectangular areas (75 × 200 μm) within the molecular layer of each dentate gyrus (regions at middle of upper blade, apex, and middle of lower blade). Thus, six measurements were obtained for each animal. All values were corrected for background labeling by subtracting the gray level values of the corpus callosum in the same section.
The intensity of δ subunit labeling in interneurons in the molecular layer and subgranular region was also assessed by densitometry in control and pilocarpine-treated animals at 1 month after pilocarpine treatment (n = 2 animals per group; two dentate gyri per animal). All δ subunit-labeled interneurons with a well-defined nucleus were photographed using a 100× objective. Gray level values were measured in three small areas (2 μm2) within the cytoplasmic regions of the interneurons. The nucleus of each interneuron was selected as the background reference region, and the gray level value in this region was subtracted from the mean gray level value obtained for the cytoplasmic labeling of the same interneuron.
The densitometry measurements for diffuse labeling in the molecular layer were analyzed with a repeated measures ANOVA (general linear model, including subject as a within subject factor) using SPSS software (version 12.0; SPSS, Chicago, IL). Interneuron measurements were analyzed with Student's t test. For all analyses, p < 0.05 was considered significant. Graphs were prepared with Origin 7.5 software (OrigenLab, Northampton, MA).
Extracellular field recordings. Mice were anesthetized with halothane according to a protocol approved by the University of California, Los Angeles Chancellor's Animal Research Committee. The brains were removed and placed in ice-cold artificial CSF (ACSF) containing the following (in mm): 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4,26 NaHCO3, 10 d-glucose, pH 7.3-7.4 when bubbled with 95% O2 and 5% CO2. In ACSF containing 5 μm GABA, field potentials were evoked (paired pulses 20 msec apart; 0.05 Hz) by stimulating the medial perforant path. Bipolar electrodes delivered a constant current stimulus (A365; World Precision Instruments, Sarasota, FL). At a stimulus width (W) of 60 μsec, the intensity was increased until a threshold response was collected over a 10 min stable baseline. The W was then varied (PG4000; Neurodata Instruments, New York, NY) to create stimulus-response curves by delivering two stimulation trials (10 stimuli each), with W ranging from 20 to 240 μsec (in 20 and 40 μsec increments). After the control trial, THDOC (10 nm) was perfused for 20 min before generating a second pair of stimulus-response curves.
Data were filtered between 0.10 and 3 KHz, and an in-house data analysis package (EVAN, version 1.3.9) was used to fit a straight line to the initial rising phase of the excitatory postsynaptic field potential (fEPSP). The slope of the line was then used to represent the magnitude of the fEPSP and plotted against W to obtain stimulus-response curves. Stimulus-response curves were fit to a Boltzman equation of the form f(W) = [-MAX/(1 + exp[(W - W50)/k]) + MAX], where W is stimulus width, MAX is the maximum response relative to the response elicited by the largest W (240 μsec) under control conditions, k is a slope factor, and W50 is the stimulus width that elicits 50% of MAX (Microsoft Excel 2003; Microsoft, Seattle, WA). Differences were considered significant at p < 0.05, as determined by Student's t test.
Results
Neuronal loss in pilocarpine-treated mice
Although C57/BL/6 mice are apparently resistant to neuronal damage after kainate-induced seizures (Schauwecker and Steward, 1997), this strain is susceptible to cell loss after pilocarpine-induced status epilepticus (Houser et al., 2002). In the present mouse model, the patterns of neuronal loss in the hippocampal formation were similar to those observed in pilocarpine-treated rats (Obenaus et al., 1993). Extensive neuronal loss was found in the hilus and CA3 of all pilocarpine-treated mice in this study. In contrast, dentate granule cells were generally well preserved. Neuronal loss in CA1 was variable but did not appear to affect the patterns of receptor subunit labeling in the dentate gyrus.
Decreased diffuse δ subunit labeling in pilocarpine-treated mice
In normal animals, diffuse δ subunit labeling is abundant in several forebrain regions that include many thalamic nuclei, the caudate-putamen, outer layers of the cerebral cortex, and the molecular layer of the dentate gyrus (Fig. 1A). In pilocarpine-treated animals at 2 weeks after status epilepticus, the most noticeable and consistent change was a decrease in diffuse δ subunit labeling in the molecular layer of the dentate gyrus (Fig. 1B). In some mice, mild decreases in diffuse δ subunit labeling were also present in the cerebral cortex. No changes in δ subunit immunoreactivity were evident in the thalamus (Fig. 1B), and this suggested that the decreased labeling in the dentate gyrus was not a result of global decreases in δ subunit labeling in all brain regions. The decrease in diffuse δ subunit labeling in the molecular layer was evident in caudal as well as rostral regions of the dentate gyrus (Fig. 1C,D). In some animals, the diffuse δ subunit labeling was also decreased in CA1 and the entorhinal cortex (Fig. 1D). However, decreases in diffuse δ subunit immunoreactivity were found most consistently in the dentate gyrus, and this region was the focus of the present study. The decrease in diffuse labeling was presumably located on dendrites of granule cells in the molecular layer and around the cell bodies of these neurons in the granule cell layer (Figs. 2A,B, 3A,B).
Figure 1.
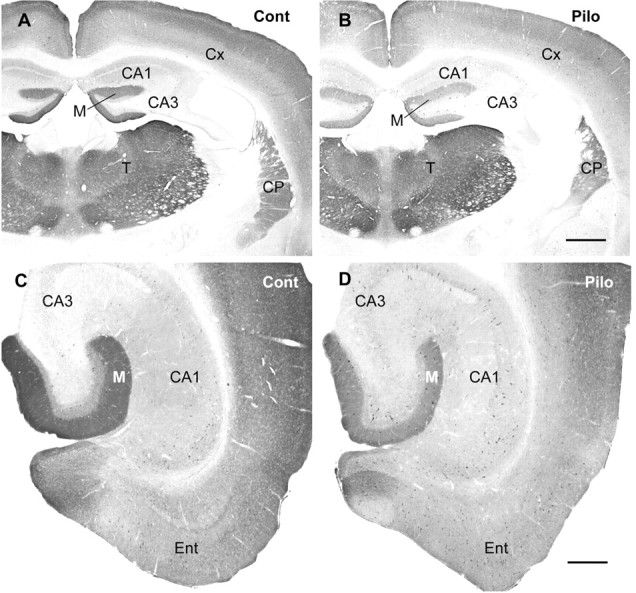
Comparison of immunohistochemical labeling for the δ subunit in control (Cont) (A, C) and pilocarpine-treated (Pilo) (B, D) mice at 2 months after status epilepticus. A, B, In coronal sections of the forebrain, decreased δ subunit labeling is most striking in the molecular layer (M) of the dentate gyrus in the pilocarpine-treated animal (B). Labeling is also moderately decreased in the neocortex (Cx) and slightly decreased in the caudate-putamen (CP) in this animal. No changes in δ subunit immunoreactivity are evident in the thalamus (T). C, D, In horizontal sections from the same animals, δ subunit immunoreactivity is also decreased in the molecular layer of the dentate gyrus at caudal levels (D), and the normally light labeling in CA1 (C) is further decreased in the pilocarpine-treated mouse (D). Decreased labeling of the δ subunit is also evident in the entorhinal cortex (Ent). Scale bars: A, B, 600 μm; C, D, 300 μm.
Figure 2.
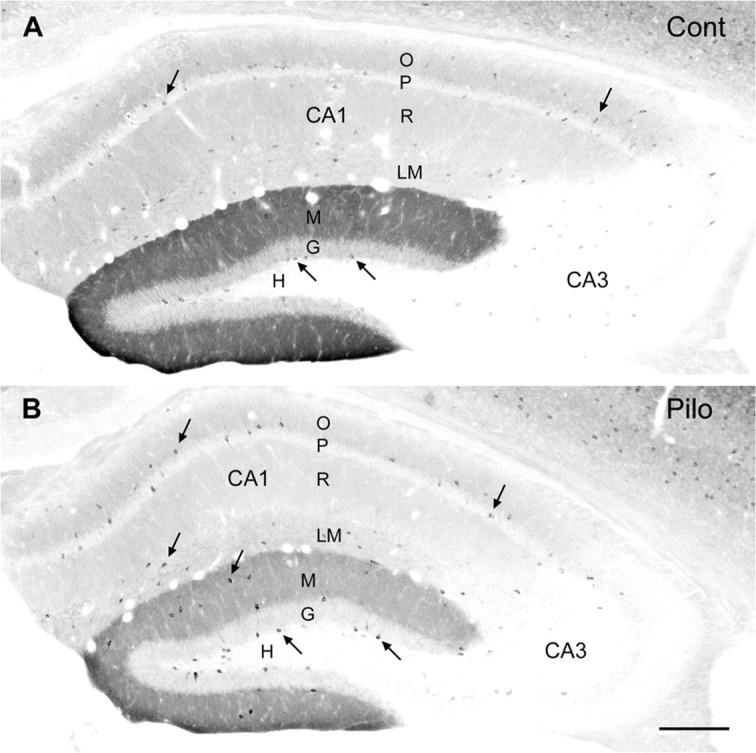
Alterations in δ subunit labeling in the hippocampal formation after pilocarpine-induced status epilepticus. A, In a control mouse, diffuse δ subunit labeling is high in the molecular layer (M) of the dentate gyrus. Moderate levels of diffuse labeling are evident in strata oriens (O), radiatum (R), and lacunosum-moleculare (LM) of CA1. Immunolabeling is low in CA3. Some moderately labeled interneurons (arrows) are evident along the base of the granule cell layer (G) and within CA1 where they are most numerous near the pyramidal cell layer (P). Very few δ subunit-labeled interneurons are present in the hilus (H). B, In a pilocarpine-treated mouse at 2 weeks after status epilepticus, diffuse δ subunit labeling in the molecular layer is substantially decreased. Diffuse labeling is also slightly decreased in CA1. In contrast, labeling of many interneurons (arrows) is increased. Strongly labeled interneurons are prominent along the base of the granule cell layer, within the molecular layer, and in strata pyramidale (P) and lacunosum-moleculare of CA1. Both sections were processed with a nickel-intensified labeling method. Scale bar: A, B, 200 μm.
Figure 3.
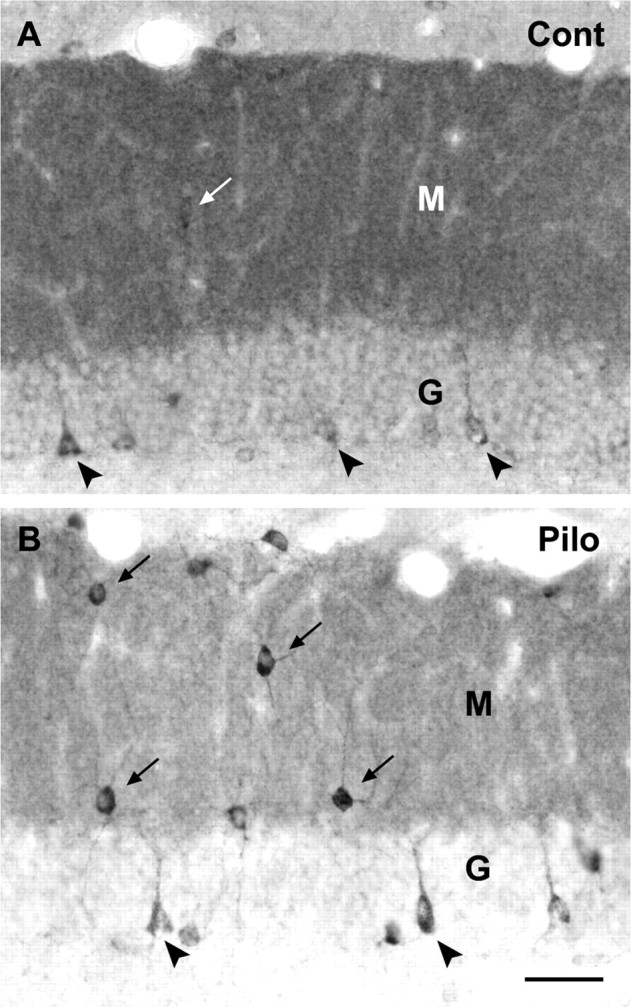
Comparison of δ subunit-labeled interneurons in the dentate gyrus of control (Cont) (A) and pilocarpine-treated (Pilo) (B) mice. A, In control tissue, only lightly or moderately labeled interneurons can be detected within the molecular layer (M; arrow) and along the inner border of the granule cell layer (G; arrowheads). B, In a pilocarpine-treated animal at 60 d after status epilepticus, increased numbers of darkly labeled interneurons are evident in the molecular layer (arrows) and along the base of the granule cell layer (arrowheads) where many resemble basket cells. Immunolabeling is quite dense within the cytoplasm and extends into the proximal dendrites in many of the labeled interneurons. A decrease in diffuse labeling is evident within both the molecular and granule cell layers in the pilocarpine-treated animal. Scale bar: A, B, 40 μm.
Increased δ subunit labeling of interneurons in pilocarpine-treated animals
In control mice, some moderately to lightly labeled interneurons were dispersed throughout most regions of the hippocampal formation (Fig. 2A). Such neurons were most noticeable along the base of the granule cell layer, within or near the pyramidal cell layer of CA1, and in stratum lacunosum-moleculare of CA1. In pilocarpine-treated animals at 2 weeks or longer after status epilepticus, the interneurons in these locations appeared more strongly labeled than in control mice (Fig. 2B).
At higher magnification, δ subunit-labeled neurons could be detected along the base of the granule cell layer in control animals, but most of these interneurons exhibited only moderate or light labeling (Fig. 3A). A few moderately labeled interneurons were also detected within the molecular layer (Fig. 3A). In pilocarpine-treated animals at 2 weeks or longer after status epilepticus, δ subunit labeling was increased substantially within the cell bodies and proximal dendrites of the interneurons (Fig. 3B). Greater numbers of strongly labeled interneurons were evident along the base of the granule cell layer and within the molecular layer than in these regions of control animals (Fig. 3A,B).
The interneurons in the molecular layer were of particular interest because they were difficult to detect in normal animals but were quite evident in the pilocarpine-treated animals. To verify that the increased visibility of these neurons was attributable to increased δ subunit expression within the interneurons rather than to decreased diffuse labeling within the neuropil, the density of labeling in interneurons within the molecular layer was determined in control and pilocarpine-treated animals. Densitometric measurements demonstrated significantly stronger labeling in the cytoplasm of interneurons in the pilocarpine-treated animals than in the control animals (control gray level value, 14.3 ± 1.0, n = 13; pilocarpine gray level value, 27.1 ± 1.0, n = 37; p < 0.01). The number of labeled interneurons that could be detected within the molecular layer was also significantly larger in pilocarpine-treated animals than in controls. This was consistent with increased δ subunit expression in many interneurons that normally expressed low levels of the δ subunit and thus could not be visualized readily. Many of the labeled interneurons in the molecular layer were probably molecular layer perforant path (MOPP) cells that have extensive axonal arborizations in the outer two-thirds of the molecular layer and are ideally positioned to modulate the effects of perforant path input through feedforward inhibition of the dentate granule cells (Halasy and Somogyi, 1993; Freund and Buzsaki, 1996).
Interneurons along the base of the granule cell layer also showed significantly higher levels of labeling in the pilocarpine-treated mice (control gray level value, 18.6 ± 1.1, n = 29; pilocarpine gray level value, 38.2 ± 1.2, n = 45; p < 0.01). Many of these interneurons were presumed to be basket cells that form synaptic contacts with the cell bodies and proximal dendrites of the granule cells (Ribak and Seress, 1983).
In confocal microscopy studies of δ subunit labeling of interneurons in the dentate molecular layer, the neurons were independently identified by immunohistochemical labeling of the α1 subunit of the GABAA receptor. Labeling of the δ subunit in these neurons was then compared in control and pilocarpine-treated animals. Distinct α1 subunit labeling of many interneurons was present in control mice, and similar labeling was observed in the pilocarpine-treated animals (Fig. 4A,D) (Z.P. and C.R.H., unpublished findings). In control mice, α1 subunit-labeled interneurons in the molecular layer showed a low level of δ subunit labeling within the cytoplasm (Fig. 4B). In contrast, virtually all α1 subunit-labeled interneurons in the molecular layer of pilocarpine-treated animals exhibited strong δ subunit labeling within the cytoplasm (Fig. 4E). Labeling for both the α1 and δ subunits was present on the surface of many of the interneurons in the pilocarpine-treated animals, suggesting that the δ subunit was increased along the plasma membrane as well as within the cytoplasm of the neurons (Fig. 4F).
Figure 4.
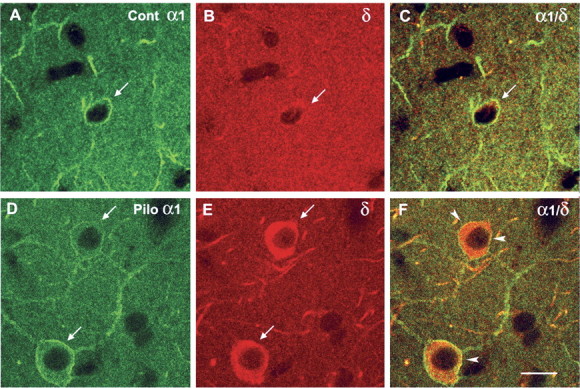
Comparisons of δ subunit immunolabeling in α1 subunit-expressing interneurons in the molecular layer of control (Cont) (A-C) and pilocarpine-treated (Pilo) (D-F) mice with confocal microscopy. A-C, In a control animal, strong α1 immunolabeling is evident around the soma (arrow) and dendritic processes within the neuropil (A). Immunolabeling for the δ subunit is relatively low in the cytoplasm of the interneuron (B, arrow), and no δ subunit labeling can be detected on the surface of the interneuron (C). D-F, In a pilocarpine-treated animal, distinct α1 subunit labeling is evident on the surface of interneurons (arrows) in the molecular layer, and lighter labeling is present within the cytoplasm (D). Strong immunolabeling for the δ subunit is present in the cytoplasm of the same interneurons (E, arrows), and overlapping labeling for the δ and α1 subunits is present on the surface of the neurons (F, arrowheads). Scale bar: A-F, 10 μm.
Interestingly, interneurons in the dentate gyrus did not appear to be labeled for α4 in sections from either experimental or control animals. This observation and the frequent finding of double labeling of interneurons for the α1 and δ subunits raise the possibility that the δ subunit may be associated predominantly with the α4 subunit in principal cells, as is generally recognized, but with the α1 subunit in interneurons.
Progression of δ subunit changes
To determine the time course of the δ subunit changes, sections from animals at several time points between 1 and 60 d were processed in parallel in the same immunohistochemical experiments. By 24 hr after status epilepticus, little change in diffuse δ subunit labeling was evident within the molecular layer (Fig. 5, compare A and B). However, at this early time point, labeling of interneurons along the base of the granule cell layer appeared to be decreased (Fig. 5B). At 4 d after status epilepticus, diffuse labeling in the molecular layer was decreased below control levels, and labeling of interneurons in the dentate gyrus also remained decreased (Fig. 5C). At 1 week after status epilepticus, diffuse labeling in the molecular layer was lower, but the δ subunit-labeled interneurons along the base of the granule cell layer and within the molecular layer were more evident than at earlier time points (4 d) or in control tissue (Fig. 5D). At 14, 30, and 60 d after pilocarpine-induced seizures, diffuse labeling in the molecular layer remained substantially lower than that in control animals, and strongly labeled interneurons continued to be observed at these later time points (Fig. 5E,F).
Figure 5.
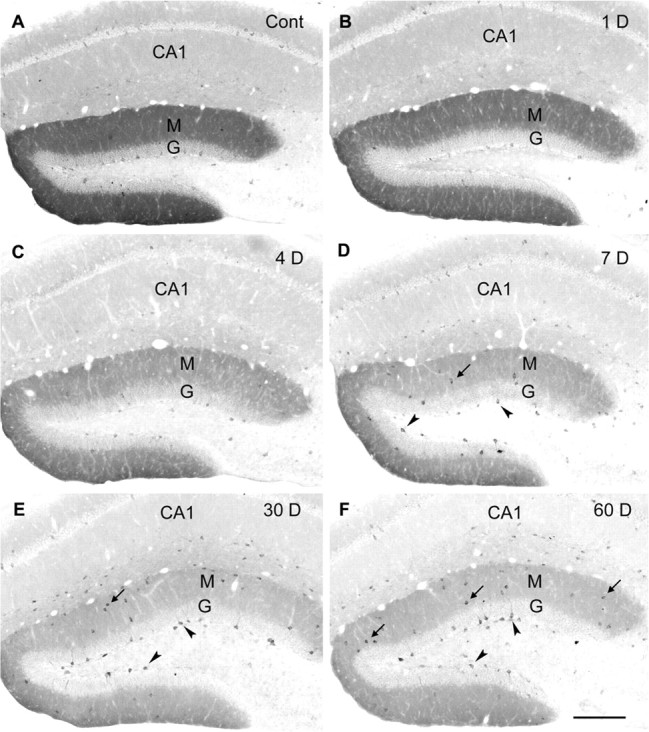
Progressive changes in immunohistochemical labeling of the δ subunit in the dentate gyrus after pilocarpine-induced status epilepticus. In comparison with that in a control (Cont) mouse (A), diffuse immunolabeling is slightly decreased in the molecular (M) and granule cell (G) layers of the dentate gyrus at 1 d after status epilepticus (B) and decreases further by 4 d after pilocarpine treatment (C). At these early times, labeling of interneurons is also generally decreased (B, C). At 7 d (D), 30 d (E), and 60 d (F) after status epilepticus, the diffuse δ subunit immunoreactivity is substantially decreased throughout the molecular and granule cell layers of the dentate gyrus. In contrast, δ subunit labeling of many interneurons is increased, and labeled interneurons become particularly evident in the subgranular region (arrowheads) and molecular layer of the dentate gyrus (arrows). Scale bar: A-F, 200 μm.
Progression of α4 and γ2 subunit changes
The identification of progressive decreases in diffuse δ subunit labeling over time led to questions about changes in potentially interrelated GABAA receptor subunits during the same period. Thus, immunolabeling for the α4 and γ2 subunits was studied in the same groups of animals and at the same time points as those used in the δ subunit studies.
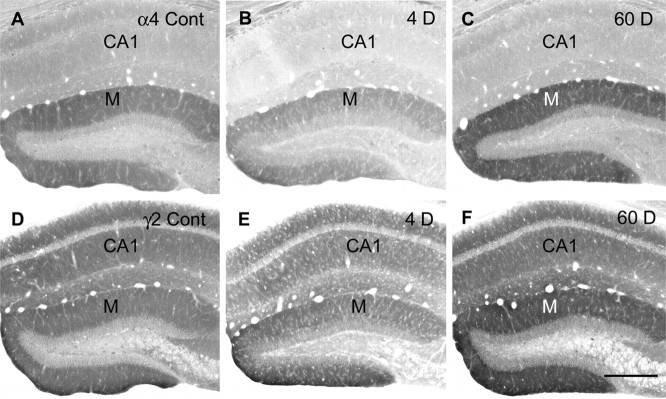
In control animals, diffuse α4 subunit labeling was moderately high throughout the molecular layer of the dentate gyrus (Fig. 6A). In pilocarpine-treated animals, α4 labeling was decreased by 24 hr and appeared even lower at 4 d after status epilepticus (Fig. 6B). By 7 d after pilocarpine-induced seizures, α4 labeling was only slightly lower than that in control animals. However, by 30-60 d after status epilepticus, α4 subunit labeling in the molecular layer was higher than that in control animals (Fig. 6C).
Figure 6.
Comparisons of immunolabeling for the α4 (A-C) and γ2 (D-F) subunits in the dentate gyrus in control (Cont) and pilocarpine-treated mice at 4 d (B, E) and 60 d (C, F) after status epilepticus. A, In a control mouse, labeling of the α4 subunit is prominent in the molecular layer (M) of the dentate gyrus. B, At 4 d after SE, α4 labeling is decreased in the molecular layer as well as in CA1. C, At 60 d after SE, α4 subunit labeling in the molecular layer is stronger than that in the control (compare C and A). D, In a control mouse, strong labeling for the γ2 subunit is evident in the molecular layer of the dentate gyrus and CA1. E, At 4 d after pilocarpine treatment, γ2 labeling is slightly decreased throughout the hippocampal formation. F, At 60 d after SE, γ2 labeling in the molecular layer is increased and is stronger than that in the control mouse (compare F and D). Illustrated sections were processed in parallel for each subunit, and sections for both subunits are from the same animal. Scale bar: A-F, 300 μm.
Immunolabeling of the γ2 subunit was distributed throughout the hippocampal formation in control animals and was moderately strong within the molecular layer of the dentate gyrus and dendritic regions of CA1. In pilocarpine-treated animals, little change in the level of labeling was evident at 24 hr, and only a slight decrease in labeling was present at 4 d (Fig. 6E). From 7-60 d after status epilepticus, increased γ2 subunit labeling was observed throughout the hippocampal formation. The increases were most pronounced in the molecular layer of the dentate gyrus (Fig. 6F). Increased immunolabeling of the γ2 subunit was observed in most animals studied during the chronic period, but the extent of the increase varied among animals at the same poststatus interval. The γ2 labeling in Figure 6F is from an animal with a comparatively large increase in γ2 subunit expression.
Changes in intensity of labeling for δ, α4, and γ2 subunits
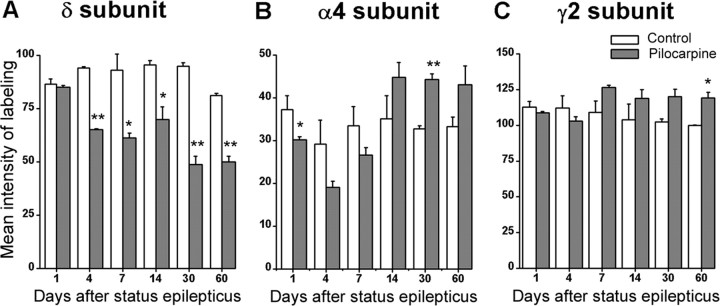
Densitometry measurements were conducted to provide a semi-quantitative description of the changes in the three GABAA receptor subunits over time. The results were consistent with the qualitative analysis of each subunit, and similar results were obtained among the different time course experiments for each subunit.
The density of immunolabeling for the δ subunit was significantly decreased by 4 d after status epilepticus and continued to be lower than control values at all later time points. Differences were statistically significant at all times after 1 d (Fig. 7A).
Figure 7.
Comparisons of the mean intensity of immunolabeling for the α4, γ2, and δ subunits in control and pilocarpine-treated animals at selected times after status epilepticus. **p < 0.01; *p < 0.05.
Density measurements of α4 subunit labeling confirmed an initial decrease in the intensity of α4 labeling in pilocarpine-treated animals compared with their paired controls at 1 and 4 d after status epilepticus (Fig. 7B). Differences were less marked at 7 d, suggesting a return toward control values (Fig. 7B). The intensity of labeling in the pilocarpine-treated animals was increased above control levels at 2 weeks, and the increased intensity of labeling continued to be present at 30 and 60 d after status epilepticus. Differences were statistically significant at 1 d (p < 0.05) and 30 d (p < 0.01).
Density measurements of γ2 subunit labeling showed mild to moderate increases in labeling over time in the pilocarpine-treated animals. No significant differences were found at 1 and 4 d after status epilepticus (Fig. 7C). The density of γ2 subunit labeling in pilocarpine-treated animals was higher than that of paired control animals at all later times, from 7 to 60 d (Fig. 7C). The differences were statistically significant at 60 d (p < 0.05).
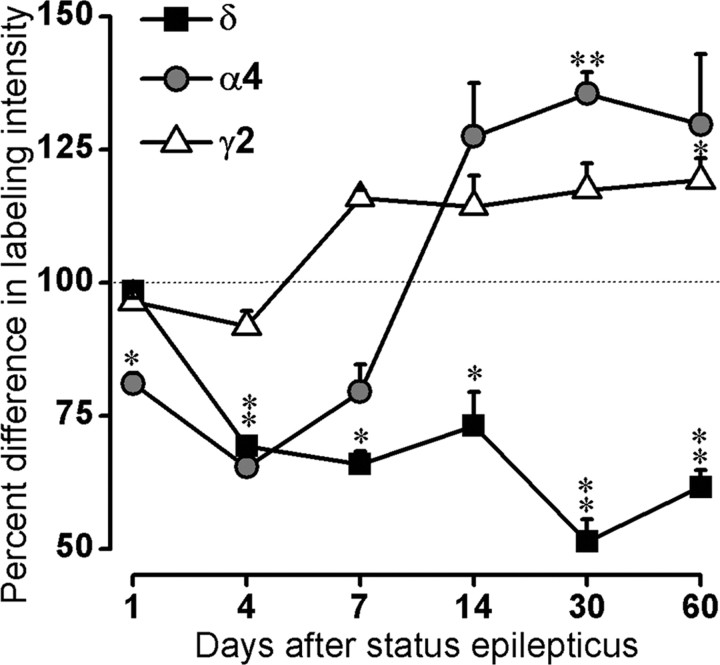
To facilitate comparisons of the patterns of change for the three subunits over time, densitometric values from the pilocarpine-treated animals were converted to percentages of control values at each time point, with control values represented by 100%. The patterns are described graphically in Figure 8.
Figure 8.
Comparisons of percentage differences in intensity of labeling for δ, α4, and γ2 subunits in pilocarpine-treated animals compared with controls at 1-60 d after status epilepticus. Control values are represented as 100% (dotted line) for all subunits. Intensity of δ subunit labeling is below control values at 4-60 d after status epilepticus. In contrast, after initial small decreases, the intensity of both α4 and γ2 labeling increases above control values and remains elevated through the remainder of the study. **p < 0.01; *p < 0.05.
In summary, the density of δ subunit labeling decreased in pilocarpine-treated mice and remained significantly lower than control values from 4 to 60 d after status epilepticus. Although labeling for the α4 subunit decreased initially, it then increased above control levels and remained increased from 7 to 60 d after pilocarpine treatment. The γ2 subunit showed a general, although comparatively small, increase above control values that was maintained from 7 to 60 d after treatment. At 60 d after status epilepticus, the latest time point studied, δ subunit labeling was decreased by 38.4%, whereas the labeling for the α4 and γ2 subunits was increased by 29.7 and 19.2%, respectively (Table 1).
Table 1.
Comparisons of mean intensity of labeling for δ, α4, and γ2 subunits in the molecular layer of control and pilocarpine-treated mice between 1 and 60 d after status epilepticus
|
|
|
Intensity of labeling ±SEM |
Percentage of difference in pilocarpine versus control mice |
|
|
||
|---|---|---|---|---|---|---|---|
| Subunit |
Time (d) |
Control |
Pilo |
F value |
p value |
||
| δ | 1 | 86.4 ± 2.5 | 85.1 ± 0.9 | −1.6 | 0.39 | n.s. | |
| 4 | 94.0 ± 0.7 | 65.1 ± 0.4 | −30.7 | 1413.25 | <0.01 | ||
| 7 | 93.0 ± 7.5 | 61.3 ± 2.3 | −34.1 | 25.14 | <0.05 | ||
| 14 | 95.5 ± 2.1 | 69.8 ± 6.1 | −26.9 | 10.22 | <0.05 | ||
| 30 | 94.9 ± 1.6 | 48.7 ± 4.0 | −48.6 | 76.75 | <0.01 | ||
| 60 | 81.2 ± 0.9 | 50.1 ± 2.5 | −38.4 | 86.60 | <0.01 | ||
| α4 | 1 | 37.3 ± 3.3 | 30.2 ± 0.7 | −19.0 | 14.69 | <0.05 | |
| 4 | 29.2 ± 5.6 | 19.1 ± 1.4 | −34.6 | 4.97 | n.s. | ||
| 7 | 33.5 ± 4.5 | 26.6 ± 1.7 | −20.6 | 2.97 | n.s. | ||
| 14 | 35.1 ± 5.4 | 44.8 ± 3.5 | +27.5 | 2.54 | n.s. | ||
| 30 | 32.7 ± 0.7 | 44.4 ± 1.3 | +35.5 | 41.51 | <0.01 | ||
| 60 | 33.3 ± 2.3 | 43.1 ± 4.4 | +29.7 | 2.77 | n.s. | ||
| γ2 | 1 | 112.9 ± 3.9 | 108.8 ± 0.8 | −3.6 | 1.75 | n.s. | |
| 4 | 112.1 ± 8.5 | 102.9 ± 3.2 | −8.2 | 1.49 | n.s. | ||
| 7 | 109.2 ± 7.9 | 126.6 ± 1.6 | +15.9 | 7.80 | n.s. | ||
| 14 | 104.0 ± 11.1 | 118.8 ± 6.1 | +14.3 | 1.69 | n.s. | ||
| 30 | 102.4 ± 2.1 | 120.2 ± 5.1 | +17.4 | 6.86 | n.s. | ||
|
|
60 |
100.0 ± 0.3 |
119.2 ± 4.1 |
+19.2 |
13.18 |
<0.05 |
|
Pilo, Pilocarpine; n.s., not significant.
Reduced neurosteroid modulation and increased excitability in slices from pilocarpine-treated mice
Because the expression of δ subunit-containing GABAA receptors is necessary for modulation of dentate gyrus granule cells by physiological concentrations of neurosteroids (Stell et al., 2003a), the decreased expression of δ subunit-containing receptors in granule cells of pilocarpine-treated mice should result in decreased neurosteroid modulation of these cells. To test this, evoked fEPSPs were used to generate stimulus-response curves before and after application of 10 nM THDOC to slices from control and pilocarpine-treated mice. After application of the neurosteroid, curves generated in control mice were shifted to the right (control W50, 143.9 ± 8.4 μsec vs control plus THDOC W50, 153.8 ± 12.0 μsec; n = 5; p < 0.05) (Fig. 9A), indicating that THDOC decreased the excitability of dentate gyrus granule cells in control mice (consistent with Stell et al., 2003a). However, the stimulus-response curves generated in slices from pilocarpine-treated animals were unaffected by THDOC (pilocarpine W50, 122.1 ± 4.4 μsec vs pilocarpine plus THDOC W50, 117.9 ± 5.0 μsec, n = 6; p > 0.05) (Fig. 9A). Furthermore, analysis of stimulus-response curves from both the control and pilocarpine-treated animals under control conditions indicated that slices from control animals were less excitable than slices from pilocarpine-treated animals (control W50, 143.9 ± 8.3 μsec vs pilocarpine W50, 122.1 ± 4.4 μsec; p < 0.05) (Fig. 9A,B). Immunohistochemical analyses of slices from animals used in the physiological studies confirmed lower levels of δ subunit labeling of the dentate molecular layer in all pilocarpine-treated animals than in their paired controls. The physiological findings indicate that the decrease in δ subunit-containing receptors in pilocarpine-treated mice substantially reduced the neurosteroid modulation of the dentate gyrus and could also have contributed to the increase in general excitability of this region.
Figure 9.
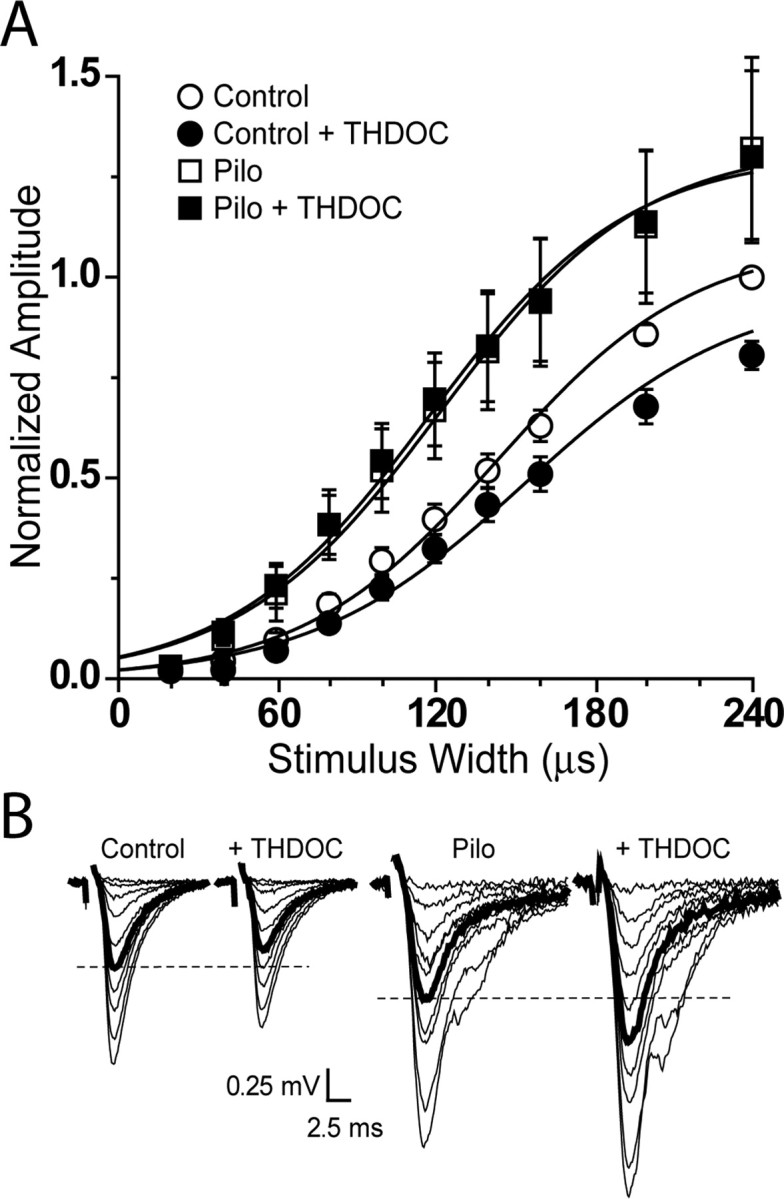
A physiological concentration of THDOC (10 nm) does not affect fEPSP slope in slices from pilocarpine-treated animals. A, Stimulus-response curves from control (○, •) and pilocarpine-treated animals (□, ▪) in control conditions (○, □) and after a 20 min perfusion of THDOC (•, ▪). Data (±SEM) are normalized to the slope of the EPSP240 (evoked by stimulus width, 240 μsec) under control conditions (○). Lines represent averages of Boltzman function generated by the averages of the parameters fitted to individual experiments. B, Representative traces from individual experiments. Bolded traces are approximate W50 responses (W50, stimulus width required to elicit a half-maximal response), and dashed lines are drawn to facilitate comparison of W50 responses.
Discussion
Our major finding is that expression of the δ subunit of the GABAA receptor was altered in ways that could contribute to increased excitability of the dentate gyrus in a mouse model of temporal lobe epilepsy. Diffuse labeling of the δ subunit on dendrites of dentate gyrus granule cells was decreased, whereas δ subunit expression in interneurons was increased during the chronically epileptic period. Consistent with these findings, the excitability of the dentate gyrus was increased in extracellular field recordings of pilocarpine-treated animals, and neurosteroids were less effective in reducing excitability in the pilocarpine-treated animals than in controls.
The findings are unique in the following ways: (1) focusing attention on GABAA receptor subunit alterations involved in nonsynaptic, tonic inhibition; (2) demonstrating differential changes in GABAA receptor subunit expression in principal cells and interneurons; and (3) suggesting that the observed subunit changes can limit the effectiveness of neurosteroids in enhancing inhibition in an epilepsy model.
Subunits involved in nonsynaptic GABAA receptor function could be altered in epilepsy
A decrease in diffuse labeling of the δ subunit in the dentate molecular layer occurred consistently during the chronic period. These results are in agreement with some but not all previous reports. Decreased expression of the δ subunit mRNA and protein was found at several intervals from 12 hr to 30 d after kainate-induced seizures in rats (Schwarzer et al., 1997; Tsunashima et al., 1997). Likewise, a recent microarray analysis identified the δ subunit mRNA as one of a group of mRNAs that was significantly decreased at 2 weeks after status epilepticus (Elliott et al., 2003).
In contrast, single-cell mRNA amplification methods in dissociated granule cells have shown a significant increase in δ subunit mRNA during the chronic period in the rat pilocarpine model (Brooks-Kayal et al., 1998). Currently, there is no explanation for the differences between the latter findings and those of the present study and other previous reports, but the discrepancies do not appear to be attributable to major variables such as the animal model or species.
Interestingly, mutations of the gene for the δ subunit of the GABAA receptor have recently been reported in humans with two forms of generalized epilepsy (Dibbens et al., 2004). Recombinant receptors with at least one of the identified mutations have decreased GABAA receptor current amplitudes, suggesting that the mutated δ subunit could contribute to increased neuronal excitability (Dibbens et al., 2004).
Although the δ subunit is the major subunit associated with tonic inhibition in the dentate gyrus, the α5 subunit may be involved in nonsynaptic inhibition in CA1 (Crestani et al., 2002; Caraiscos et al., 2004), and decreased labeling of the α5 subunit mRNA and protein in CA1 was previously observed in a rat pilocarpine model of recurrent seizures in which pyramidal cells were preserved (Houser and Esclapez, 2003). The decreased expression of these two putative nonsynaptic subunits contrasts with the more frequent finding of increased expression of other GABAA receptor subunits in several epilepsy models (Schwarzer et al., 1997; Tsunashima et al., 1997; Brooks-Kayal et al., 1998; Nusser et al., 1998a; Fritschy et al., 1999) and human temporal lobe epilepsy (Loup et al., 2000).
Differential changes in δ subunit expression in principal cells and interneurons could impair inhibition of dentate granule cells
In this study, δ subunit expression was differentially altered in granule cells and interneurons, and we hypothesize that these changes could converge to increase excitability in this mouse model of temporal lobe epilepsy.
A decrease in δ subunit expression in the dentate granule cells, presumably at perisynaptic and extrasynaptic locations, could lead to reduced responsiveness to GABA spillover or a reduction in tonic inhibition. Such alterations could directly reduce the effectiveness of the dentate “gate” that normally limits the amount of excitatory input that enters the hippocampus (Lothman et al., 1992). Indeed, in the normal dentate gyrus, a pharmacologically induced reduction in tonic inhibition was particularly effective in allowing excitation through the perforant path to invade the hippocampus (Carlson et al., 2003).
Increased expression of δ subunits in interneurons could also have powerful effects on excitability within the dentate gyrus if the changes were to increase the tonic inhibition of inhibitory interneurons. Other investigators have demonstrated substantial tonic GABAA receptor conductances in interneurons in CA1 of the normal guinea pig hippocampus and have emphasized the potential importance of such inhibition in regulating excitability within the hippocampus (Semyanov et al., 2003, 2004). Strong tonic inhibition has also been found in some interneurons in the dentate gyrus of normal mice (W. Wei and I.M., unpublished findings), and studies of tonic inhibition in interneurons of pilocarpine-treated mice are planned.
δ subunit labeling was increased in several classes of interneurons that most likely included MOPP cells in the molecular layer and basket cells along the base of the granule cell layer. Increased tonic inhibition of these and other interneurons throughout the hippocampus could decrease their basal levels of activity and also make them less responsive to excitatory afferents, including those of the perforant path. Both functional changes could compromise inhibitory control of the principal cells.
Responses to neurosteroids are decreased in pilocarpine-treated mice
The decreased responsiveness of the dentate gyrus to neurosteroid modulation in the pilocarpine-treated mice strongly suggests that the altered δ subunit expression has functional consequences. In the normal dentate gyrus, the enhancement of tonic inhibition by physiological concentrations of neurosteroids is mediated primarily by δ subunit-containing GABAA receptors (Stell et al., 2003a). Accordingly, physiological concentrations of THDOC decreased the excitability of the dentate gyrus in control animals but were essentially ineffective in reducing excitability in slices from the pilocarpine-treated mice.
These findings are consistent with a previous report of diminished allopregnanolone sensitivity of GABAA receptor currents in acutely isolated granule cells from chronically epileptic rats (Mtchedlishvili et al., 2001). At the time of the study, the relationship between the δ subunit and neurosteroid actions was unclear, but it now appears quite possible that a decrease in δ subunit expression could have been responsible for the reduced response to the neurosteroid.
Alterations of δ, α4, and γ2 subunit expression could be interrelated
In the current study, decreased labeling of the δ subunit was accompanied by increased expression of the α4 and γ2 subunits. Similar increases in α4 and γ2 subunit expression have been observed in other temporal lobe epilepsy models. In kainate-treated rats, changes in these subunits as well as the δ subunit were very similar to those in the present study (Schwarzer et al., 1997). Other studies have reported increases in either the γ2 or α4 subunit (Brooks-Kayal et al., 1998; Fritschy et al., 1999).
Similar, potentially interrelated changes in the δ, α4, and γ2 subunits have also been observed in a chronic, intermittent, ethanol model of alcohol withdrawal (Cagetti et al., 2003) that exhibits decreased inhibition and a decreased threshold for pentylenetetrazol seizures (Kokka et al., 1993; Kang et al., 1998; Liang et al., 2004). Thus, the profile of decreased δ and increased α4 and γ2 subunit expression may predominate in several animal models with increased seizure susceptibility.
It has been suggested that the δ and γ2 subunits may compete with each other for partnership with the α4 subunit in the forebrain and the α6 subunit in the cerebellum (Tretter et al., 2001; Peng et al., 2002a). In our epilepsy model, δ subunit expression decreased progressively in the days after status epilepticus, whereas, after initial mild decreases, the α4 and γ2 subunits increased over the same time course. Such patterns are consistent with the previously proposed model of subunit assembly and with altered subunit composition of some GABAA receptors in epilepsy (Coulter, 2001; Fritschy and Brünig, 2003).
δ subunit alterations could contribute to increased seizure susceptibility We suggest that changes in δ subunit expression could contribute to increased seizure susceptibility in the present model of temporal lobe epilepsy, and the time course of the changes supports this suggestion. Significant changes in δ subunit expression were not observed at 24 hr but developed during the first 2 weeks after status epilepticus. Importantly, the δ subunit changes developed before the occurrence of spontaneous behavioral seizures, consistent with the δ subunit changes contributing to (rather than resulting from) the development of spontaneous seizures.
The δ subunit-containing receptors are particularly intriguing targets for alterations that could contribute to epilepsy, because they can be affected by modulators such as neurosteroids (Adkins et al., 2001; Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002; Stell et al., 2003a), ethanol (Wallner et al., 2003), and pH (Feng and Macdonald, 2004). Decreased δ subunit expression on principal cells could lead to less effective enhancement of inhibition by such modulators. Furthermore, alterations in the normal dynamic regulation of δ subunit-containing receptors by these modulators could contribute to the fluctuations in seizure susceptibility that characterize many forms of epilepsy.
Conclusion
Additional studies are necessary to demonstrate directly that tonic GABAergic inhibition is decreased in this epilepsy model and that such deficits are not compensated for by other GABAA receptors or non-GABAergic mechanisms, such as occurs in the cerebellum after loss of both the δ and α6 subunits (Brickley et al., 2001). However, the present findings support the enticing possibility that alterations in subunits associated with nonsynaptic inhibition may contribute to temporal lobe epilepsy, despite compensatory changes in subunits normally associated with phasic inhibition.
Footnotes
This work was supported by National Institutes of Health Grant NS35985 to R. W. Olsen, I.M., and C.R.H. and Veterans Affairs Medical Research Funds to C.R.H. We thank Drs. Werner Sieghart and Jean-Marc Fritschy for generously providing antisera to the GABAA receptor subunits, Dr. Richard W. Olsen for many helpful discussions, Dr. Lynn Fairbanks for assistance with the statistical analyses, and Mahsan Rafizadeh and John Feng for technical assistance.
Correspondence should be addressed to Carolyn R. Houser, Department of Neurobiology, CHS 73-235, David Geffen School of Medicine at University of California, Los Angeles, 10833 Le Conte Avenue, Los Angeles, CA 90095-1763. E-mail: houser@mednet.ucla.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248629-11$15.00/0
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB (2001) α4β3δ GABAA receptors characterized by FRET-derived measurements of membrane potential. J Biol Chem 276: 38934-38939. [DOI] [PubMed] [Google Scholar]
- Araujo F, Ruano D, Vitorica J (1998) Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol 347: 347-353. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ (2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43: 651-661. [DOI] [PubMed] [Google Scholar]
- Bencsits E, Ebert V, Tretter V, Sieghart W (1999) A significant part of native γ-aminobutyric acidA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem 274: 19613-19616. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M (1996) Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 497: 753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M (2001) Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409: 88-92. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA (1998) Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 4: 1166-1172. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136: 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW (2003) Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63: 53-64. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, Macdonald JF, Orser BA (2004) Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101: 3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GC, Liang S, Coulter DA (2003) Extrasynaptic GABAA receptors control dentate gyrus throughput in hippocampus. Soc Neurosci Abstr 29: 374.12. [Google Scholar]
- Coulter DA (2001) Epilepsy-associated plasticity in γ-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol 45: 237-252. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U (2002) Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA 99: 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Feng H-J, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC (2004) GABRD encoding a protein for extra- or perisynaptic GABAA receptors is a susceptibility locus for generalised epilepsies. Hum Mol Genet 13: 1315-1319. [DOI] [PubMed] [Google Scholar]
- Elliott RC, Miles MF, Lowenstein DH (2003) Overlapping microarray profiles of dentate gyrus gene expression during development- and epilepsy-associated neurogenesis and axon outgrowth. J Neurosci 23: 2218-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Houser CR (1999) Up-regulation of GAD65 and GAD67 in remaining hippocampal GABA neurons in a model of temporal lobe epilepsy. J Comp Neurol 412: 488-505. [PubMed] [Google Scholar]
- Feng H-J, Macdonald RL (2004) Proton modulation of α1β3δ GABAA receptor channel gating and desensitization. J Neurophysiol 92: 1577-1585. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. San Diego: Academic.
- Freund TF, Buzsaki G (1996) Interneurons of the hippocampus. Hippocampus 6: 347-470. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Brünig I (2003) Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharm Ther 98: 299-323. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Mohler H (1995) GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154-194. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Kiener T, Bouilleret V, Loup F (1999) GABAergic neurons and GABAA-receptors in temporal lobe epilepsy. Neurochem Int 34: 435-445. [DOI] [PubMed] [Google Scholar]
- Haas K, Macdonald RL (1999) GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol (Lond) 514: 27-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasy K, Somogyi P (1993) Subdivision in the multiple GABAergic innervation of granule cells in the dentate gyrus of the rat hippocampus. Eur J Neurosci 5: 411-429. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M (2003) Downregulation of the α5 subunit of the GABAA receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus 13: 633-645. [DOI] [PubMed] [Google Scholar]
- Houser CR, Huang C, Peng Z (2002) Patterns of neuronal loss following pilocarpine-induced seizures in C57BL/6 mice. Epilepsia [Suppl 43] 7: 16. [Google Scholar]
- Jechlinger M, Pelz R, Tretter V, Klausberger T, Sieghart W (1998) Subunit composition and quantitative importance of hetero-oligomeric receptors: GABAA receptors containing α6 subunits. J Neurosci 18: 2449-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A (1999) A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods 93: 149-162. [DOI] [PubMed] [Google Scholar]
- Kang MH, Spigelman I, Olsen RW (1998) Alteration in the sensitivity of GABAA receptors to allosteric modulatory drugs in rat hippocampus following chronic intermittent ethanol treatment. Alcohol Clin Exp Res 22: 2165-2173. [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW (1993) The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res 17: 525-531. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I (2004) Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther 310: 1234-1245. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH (1992) The dentate gyrus as a control point for seizures in the hippocampus and beyond. In: The dentate gyrus and its role in seizures (Ribak CE, Gall CM, Mody I, eds), pp 301-313. Amsterdam: Elsevier. [PubMed]
- Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy J-M (2000) Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J Neurosci 20: 5401-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I (2001) Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res 26: 907-913. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Bertram EH, Kapur J (2001) Diminished allopregnanolone enhancement of GABAA receptor currents in a rat model of chronic temporal lobe epilepsy. J Physiol (Lond) 537 2: 453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Hájos N, Somogyi P, Mody I (1998a) Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395: 172-177. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P (1998b) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Comp Neurol 18: 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenaus A, Esclapez M, Houser CR (1993) Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci 13: 4470-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Staley K, Mody I (1991) Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res 545: 142-150. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR (2002a) GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol 446: 179-197. [DOI] [PubMed] [Google Scholar]
- Peng Z, Olsen RW, Houser CR (2002b) Altered expression of the delta subunit of the GABAA receptor in the dentate gyrus in a mouse model of temporal lobe epilepsy. Soc Neurosci Abstr 28: 433.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk K, Whiting PJ, Ragan CI, McKernan RM (1995) Characterisation of δ-subunit containing GABAA receptors from rat brain. Eur J Pharmacol 290: 175-181. [DOI] [PubMed] [Google Scholar]
- Racine RJ (1972) Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281-294. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L (1983) Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J Neurocytol 12: 577-597. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M (1998) Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron 20: 783-795. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL (1994) Assembly of GABAA receptor subunits: role of the δ subunit. J Neurosci 14: 7077-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, Steward O (1997) Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA 94: 4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G (1997) GABAA receptor subunits in the rat hippocampus. II. Altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience 80: 1001-1017. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM (2003) GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484-490. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262-269. [DOI] [PubMed] [Google Scholar]
- Shu S, Ju G, Fan L (1988) The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett 85: 169-171. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs J, Sieghart W (1997) GABAA receptor subunits in the rat hippocampus. I. Immunocytochemical distribution of 13 subunits. Neuroscience 80: 987-1000. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003a) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100: 14439-14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Peng Z, Houser CR, Mody I (2003b) Physiological concentrations of neurosteroids modulate excitability via a δ-subunit dependent GABAA conductance. Soc Neurosci Abstr 29: 374.7. [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM (1999) Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol 56: 110-115. [DOI] [PubMed] [Google Scholar]
- Tretter V, Ehya N, Fuchs K, Sieghart W (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci 17: 2728-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Höger H, Homanics GE, Somogyi P, Sieghart W (2001) Targeted disruption of the GABAA δ subunit gene leads to an up-regulation of γ2 subunit-containing receptors in cerebellar granule cells. J Biol Chem 276: 10532-10538. [DOI] [PubMed] [Google Scholar]
- Tsunashima K, Schwarzer C, Kirchmair E, Sieghart W, Sperk G (1997) GABAA receptor subunits in the rat hippocampus. III. Altered messenger RNA expression in kainic acid-induced epilepsy. Neuroscience 80: 1019-1032. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW (2003) Ethanol enhances α4β3δ and α6β3δ GABAA receptors at low concentrations known to have effects in humans. Proc Natl Acad Sci USA 100: 15218-15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003) Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23: 10650-10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL (2002) Enhanced neurosteroid potentiation of ternary GABAA receptors containing the delta subunit. J Neurosci 22: 1541-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]