Abstract
α-Ketoglutarate dehydrogenase (α-KGDH), a key enzyme in the Krebs' cycle, is a crucial early target of oxidative stress (Tretter and Adam-Vizi, 2000). The present study demonstrates that α-KGDH is able to generate H2O2 and, thus, could also be a source of reactive oxygen species (ROS) in mitochondria. Isolated α-KGDH with coenzyme A (HS-CoA) and thiamine pyrophosphate started to produce H2O2 after addition of α-ketoglutarate in the absence of nicotinamide adenine dinucleotide-oxidized (NAD+). NAD+, which proved to be a powerful inhibitor of α-KGDH-mediated H2O2 formation, switched the H2O2 forming mode of the enzyme to the catalytic [nicotinamide adenine dinucleotide-reduced (NADH) forming] mode. In contrast, NADH stimulated H2O2 formation by α-KGDH, and for this, neither α-ketoglutarate nor HS-CoA were required. When all of the substrates and cofactors of the enzyme were present, the NADH/NAD+ ratio determined the rate of H2O2 production. The higher the NADH/NAD+ ratio the higher the rate of H2O2 production. H2O2 production as well as the catalytic function of the enzyme was activated by Ca2+. In synaptosomes, using α-ketoglutarate as respiratory substrate, the rate of H2O2 production increased by 2.5-fold, and aconitase activity decreased, indicating that α-KGDH can generate H2O2 in in situ mitochondria. Given the NADH/NAD+ ratio as a key regulator of H2O2 production by α-KGDH, it is suggested that production of ROS could be significant not only in the respiratory chain but also in the Krebs' cycle when oxidation of NADH is impaired. Thus α-KGDH is not only a target of ROS but could significantly contribute to generation of oxidative stress in the mitochondria.
Keywords: mitochondria, synaptosome, α-ketoglutarate dehydrogenase, hydrogen peroxide, oxidative stress, NADH/NAD ratio
Introduction
Involvement of mitochondria in tissue damage generated by reactive oxygen species (ROS) has been implicated in the pathogenesis of many diseases (Beal, 1996; Halliwell and Gutteridge, 1998). Mitochondria are considered crucial targets of free radical-mediated damage (Kowaltowski and Vercesi, 1999), because both the mitochondrial electron transport chain (Tretter et al., 1987; Bindoli, 1988) and enzymes of the citric acid cycle (Patel et al., 1996; Andersson et al., 1998; Humphries and Szweda, 1998) are vulnerable to oxidative insults. As a result, vital mitochondrial functions, including energy production (Otani et al., 1984; Fiskum, 1985), maintenance of plasma membrane potential (Tretter and Adam-Vizi, 1996), and cellular ionic homeostasis (Chinopoulos et al., 2000), are impaired in the early phase of oxidative stress. Oxidative insults might also induce secondary events leading to apoptosis (Liu et al., 1996).
In contrast, mitochondria are also considered a major site of ROS production (Chance et al., 1979). Operation of the mitochondrial electron transport system leads to formation of superoxide (Loschen et al., 1971; Boveris and Cadenas, 1975), which is avidly dismutated to H2O2 by the superoxide dismutases (Fridovich, 1995). Restriction of electron flow in the respiratory chain resulting from inhibition of complex III (Loschen et al., 1973) or complex I (Votyakova and Reynolds, 2001; Starkov and Fiskum, 2003) favors the formation of superoxide and H2O2. Recently, on synaptosomes, we established that the mitochondrial H2O2 generation caused by complex I inhibition could be physiologically more important than that induced by inhibition of complex III and IV in situ (Sipos et al., 2003).
We demonstrated previously that α-ketoglutarate dehydrogenase (α-KGDH) is sensitive to inhibition by H2O2, and impaired function of this enzyme plays a key role in limiting the generation of nicotinamide adenine dinucleotide-reduced (NADH) in the Krebs' cycle during the early phase of oxidative stress in synaptosomes (Tretter and Adam-Vizi, 2000). α-KGDH in heart and microglia mitochondria is also sensitive to inhibition by H2O2 (Nulton-Persson et al., 2003) and by peroxynitrite (Park et al., 1999), respectively.
α-KGDH is heavily regulated, as reviewed recently (Gibson et al., 2000), adjusting the rate of the Krebs' cycle to metabolic demand. Isolated dihydrolipoyl dehydrogenase, a subunit of α-KGDH, is able to catalyze NADH oxidation by oxygen with the concomitant formation of H2O2 (Huennekens et al., 1955; Gazaryan et al., 2002). Studies on flavoenzymes have demonstrated the possibility of H2O2 formation. However, in the case of the dehydrogenases, the reoxidation of reduced enzyme by O2 was found to be slow (Massey et al., 1969). Very recently, radical species as side products in the 2-oxo acid dehydrogenase reaction were detected by spin trapping, accompanied by inactivation of the enzyme (Bunik and Sievers, 2002). However, it is not known under what conditions α-KGDH, a key dehydrogenase in the Krebs' cycle, could generate measurable amounts of ROS during its catalytic function.
The present work demonstrates that isolated α-KGDH is able to produce ROS, which is regulated by the NADH/nicotinamide adenine dinucleotide-oxidized (NAD+) ratio. Thus, α-KGDH is not only a crucial target of ROS but could significantly contribute to generation of oxidative stress in the mitochondria.
Materials and Methods
Preparation of synaptosomes. Isolated nerve terminals (synaptosomes) were prepared from brain cortex of guinea pigs as detailed previously (Chinopoulos et al., 2000). The pellet obtained after centrifugation at 20,000 × g for 20 min was suspended in 0.32 m sucrose (∼20 mg/ml of protein) and kept on ice. For additional manipulations, aliquots from this suspension were used. Incubations were performed in standard medium containing the following (in mm): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 PIPES, pH 7.38, at 37°C as described below.
Measurement of H2O2 release from synaptosomes. The assay is based on the detection of H2O2 in the medium using the Amplex Red fluorescent dye (Mohanty et al., 1997). In the presence of horseradish peroxidase, the Amplex Red reagent reacts with H2O2 with a 1:1 stoichiometry producing highly fluorescent resorufin. Synaptosomes (0.5 mg/ml) were incubated for 1 hr in the standard medium containing no substrate, or 10 mm glucose, or 5 mm α-ketoglutarate (α-KG) (see Fig. 1 for details) and then horseradish peroxidase (1 U per 2 ml) and Amplex Red reagent (1 μm) were added. H2O2 released from synaptosomes was detected at 37°C using a Photon Technology International (PTI; Lawrenceville, NJ) Deltascan fluorescence spectrophotometer; the excitation wavelength was 550 nm, and the fluorescence emission was detected at 585 nm. A calibration curve was generated with known amounts of H2O2 at the end of each experiment.
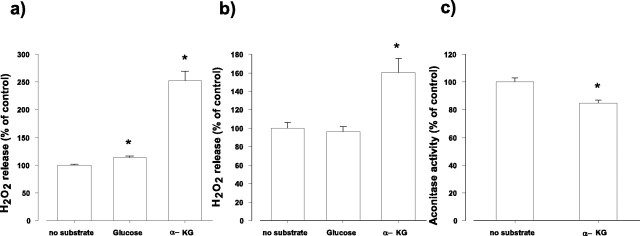
Figure 1.
H2O2 release from synaptosomes as measured by a direct assay with Amplex Red (a, b) or by the activity of endogenous aconitase (c). a, Synaptosomes (0.5 mg of protein/ml) were incubated for 1 hr in glucose-free medium, and then Amplex Red and HRP were added. The rate of H2O2 release was measured after addition of glucose (10 mm) or α-KG (5 mm). The results are expressed as percentage of the rate of H2O2 release from synaptosomes observed in the absence of added substrates (12.7 ± 0.17 pmol/min/mg synaptosomal protein; ±SEM; n = 4). b, Synaptosomes were incubated for 1 hr in glucose-free medium (control) or in the presence of glucose (10 mm) or α-KG (5 mm), and then the rate of H2O2 release was measured. Bars indicate the rate of H2O2 release as percentage of control (12.1 ± 0.77 pmol/min/mg synaptosomal protein; ±SEM; n = 4). c, Synaptosomes were incubated in the standard medium for 1 hr in the absence (control) or presence of α-KG (5 mm), and then the activity of aconitase was measured as described in Materials and Methods. Aconitase activity is expressed as percentage of control (6.3 ± 0.19 nmol/min/mg synaptosomal protein; ±SEM; n = 5). Asterisk indicates significant difference from the corresponding controls.
Assay for aconitase activity. Aconitase was assayed as described by Hausladen and Fridovich (1996) and detailed previously (Tretter and Adam-Vizi, 2000). Briefly, synaptosomes (1 mg/ml protein) were incubated in the absence or presence of α-ketoglutarate (5 mm) for 1 hr at 37°C and then aliquots of synaptosomes (0.4 mg of protein) were transferred to an assay medium containing the following (in mm): 50 Tris-HCl, 0.6 MnCl2, 30 sodium citrate, 0.2% Triton X-100, 2 U/ml isocitrate dehydrogenase (NADP+ dependent), and 1 U/ml catalase at 37°C, pH 7.4. The reaction was initiated by the addition of 0.2 mm NADP+. The fluorescence intensity was determined with a PTI Deltascan fluorescence spectrophotometer using 344 nm excitation and 460 nm emission wavelength, respectively. Changes in NADPH concentration were quantified using a calibration curve with known amounts of NADPH.
Parallel assay of α-KGDH activity and H2O2 generation by α-KGDH. Experiments on isolated α-KGDH were done with the commercially available enzyme isolated from porcine hearts (lots 58H7440 and 32K7445; Sigma, St. Louis, MO). The Sigma preparation is a solution of purified α-KGDH in 50% glycerol containing 10 mg/ml of bovine serum albumin, 30% sucrose, 2.5 mm EGTA, 2.5 mm 2-mercaptoethanol, 0.5% Triton X-100, 0.0055% sodium azide, and 25 mm potassium phosphate, pH 6.8. According to the specifications of the manufacturer, the enzyme activity was 9.4 U/ml for lot 58H7440 and 6 U/ml for lot 32K7445.
α-Ketoglutarate dehydrogenase was assayed similarly as described previously (Lai and Cooper, 1986). Aliquots of α-ketoglutarate dehydrogenase (2 μl from lot 58H7440 or 3 μl from lot 32K7445, giving similar results) were added to a medium containing 50 mm potassium phosphate, 0.2 mm thiamine pyrophosphate, 0.5 mm MgCl2, 0.4 mm ADP, 1 mm NAD+ where indicated, and 0.1 mm EGTA, pH 7.4. The reaction was initiated by the addition of 0.12 mm coenzyme A (HS-CoA) and 1 mm α-ketoglutarate. NADH and Amplex fluorescence (detailed above) was measured simultaneously using 340 and 550 nm excitation and 466 and 585 nm emission wavelengths, respectively. The assays were performed at 37°C. The two lots of Sigma α-KGDH used throughout the experiments gave essentially similar results.
For measurements on the Ca2+ dependency of α-KGDH, different Ca2+ concentrations in the medium were established. For this, aliquots from a calcium calibration buffer kit were added to the ADP and Mg2+ free assay medium, and free ionized [Ca2+] was measured by unesterified Fura-2 and Fura-6F (1 μM) at 340 and 380 nm excitation and 510 nm emission wavelength, respectively. Free calcium concentrations were calculated from the Grynkiewicz equation (Grynkiewicz et al., 1985).
Statistics. Results are expressed either as original traces or as mean ± SEM values. Statistical differences were calculated using one-way ANOVA. Differences were considered significant at a level of p < 0.05.
Materials. Standard laboratory chemicals were obtained from Sigma. Special peroxide-free and carbonyl-free Triton X-100 (Sigma) was used throughout the experiments for disrupting synaptosomal membranes. The Amplex Red reagent, Fura-6F, and the calcium calibration buffer kit were from Molecular Probes (Eugene, OR).
Results
Enhanced H2O2 generation in synaptosomes in the presence of α-ketoglutarate
We reported previously that a significant amount of H2O2 is produced in synaptosomes incubated in normal glucose-containing medium under resting, nonstimulated conditions (Sipos et al., 2003). Here, we investigated whether H2O2 production is different when α-KG, an alternative substrate, maintains the metabolism in intact synaptosomes. It has been established that α-KG is taken up by synaptosomes with high- and low-affinity carriers (Shank and Campbell, 1984; Willoughby et al., 1989), and by directly fueling a key regulatory enzyme, α-KGDH in the Krebs' cycle supports respiration in the in situ mitochondria (Willoughby et al., 1989). We also controlled that under conditions used for Figure 1a (see below), addition of α-KG (5 mm) to the medium indeed resulted in an immediate increase in the oxygen consumption (data not shown), reinforcing the role of α-KG as a substrate for in situ mitochondria. With Amplex Red, H2O2 released from synaptosomes was measured, the fluorescence being proportional to the generation of H2O2. However, a part of the oxidant was eliminated intracellularly by glutathione peroxidase and catalase (Chen et al., 2003) and remains undetected.
H2O2 generation was compared in glucose and α-KG-containing medium immediately after addition of the substrates (Fig. 1a) or after incubation for 1 hr (Fig. 1b). The rate of H2O2 release from synaptosomes incubated for 1 hr in the absence of added substrates was 12.7 ± 0.17 pmol/min/mg protein. After addition of glucose (10 mm) to the medium, H2O2 release increased by 13.6 ± 3.1% (Fig. 1a). When instead of glucose, α-KG (5 mm) was given to synaptosomes, the increase in H2O2 release was 2.5-fold (from 12.7 ± 0.17 to 32.0 ± 2.2 pmol/min/mg) (Fig. 1a). A remarkable increase in H2O2 generation by α-KG was also measured after incubation for 1 hr in the presence of this substrate (Fig. 1b). When glucose was present in the medium for 1 hr, no significant difference in the H2O2 release was found (Fig. 1b). The effect of α-KG on the H2O2 generation was confirmed by measuring the activity of endogenous aconitase. This enzyme is highly sensitive to different ROS, and decrease in the activity of aconitase is a marker of an enhanced ROS production (Patel et al., 1996; Liang et al., 2000; Sipos et al., 2003). It is demonstrated in Figure 1c that the activity of the endogenous aconitase was significantly decreased in synaptosomes (16 ± 2.4%) incubated in the presence of α-KG for 1 hr. α-KG had no direct effect on aconitase, as controlled in a separate assay (data not shown). These results indicate that H2O2 production is elevated in intact synaptosomes in the presence of α-KG. This somewhat unexpected result raised the possibility that α-KG could directly contribute to H2O2 generation. Three important enzymes could use α-KG as a substrate: glutamate dehydrogenase, transaminases, and α-KGDH. We controlled with isolated glutamate dehydrogenase that the enzyme, when α-KG is the substrate, produces no H2O2. Similarly, no H2O2 formation was detected in the reaction catalyzed by isolated glutamate-oxaloacetate transaminase (data not shown). In addition, preincubation of synaptosomes with the transaminase inhibitor d,l-vinylglycine (Lai and Cooper, 1986) was without effect on the H2O2 generation observed in the presence of α-KG (data not shown).
H2O2 generation by isolated α-KGDH
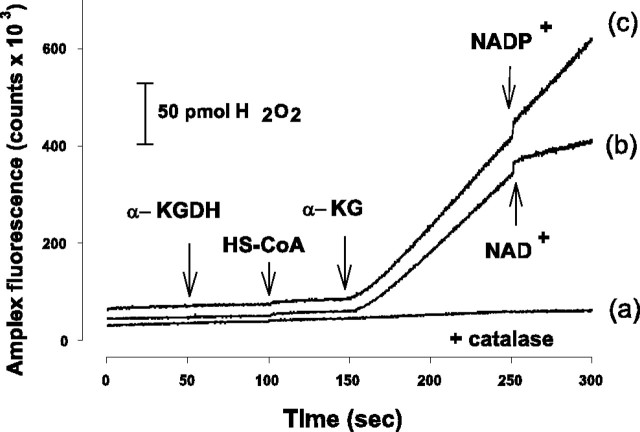
To test the possibility that H2O2 derives from the α-KGDH-catalyzed reaction, we measured the rate of H2O2 production in a standard assay medium used for measuring the activity of isolated α-KGDH. In the presence of α-KGDH and HS-CoA (0.12 mm), addition of α-KG (1 mm) induced an abrupt increase in the Amplex Red fluorescence (Fig. 2). The fluorescence signal did not increase further when superoxide dismutase was given after the addition of α-KG (data not shown) but was completely prevented when catalase was also present in the medium (Fig. 2, trace a); thus, the fluorescence increase could be attributed to H2O2 production. After addition of 5 μm NAD+, H2O2 production was inhibited, whereas NADP+ was without effect (Fig. 2, trace b,c). These experiments indicate that in the presence of its substrates (α-KG, HS-CoA), but in the absence of NAD+, α-KGDH is able to produce ROS. In the standard assay medium, the rate of H2O2 generation was 1.07 ± 0.07 pmol/sec (n = 16). We could also detect superoxide formation in this reaction as measured with the reduction of the acetylated cytochrome c assay (data not shown), which is in agreement with the observation made by Starkov et al. (2004) and Bunik and Sievers (2002). It was not possible to determine whether superoxide was quickly and spontaneously dismutated to H2O2, or whether both superoxide and H2O2 were generated in the enzyme reaction. In the present work, H2O2 generation was measured to characterize the α-KGDH-mediated ROS formation.
Figure 2.
H2O2 production by isolated α-KGDH. H2O2 formation was measured with Amplex Red as detailed in Materials and Methods. For a-c, α-KGDH, HS-CoA (0.12 mm), and α-KG (1 mm) were added as indicated. For a, catalase was given before α-KGDH. NAD+ (b) or NADP+ (c) (5 μm) was given as shown. Traces have been offset for clarity.
H2O2 production by α-KGDH is inhibited by NAD+
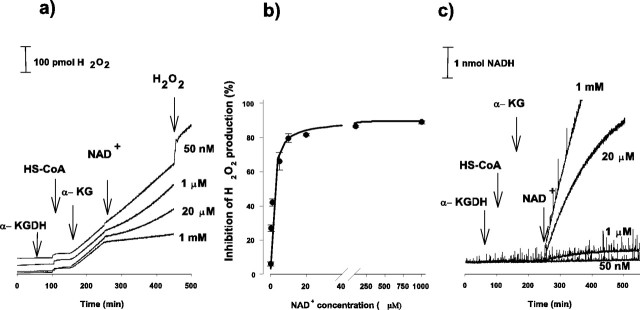
Representative traces showing the effect of NAD+ (50 nm to 1 mm) on the H2O2 production by the isolated α-KGDH are demonstrated in Figure 3a. Proportional with the increase in NAD+ concentration, the rate of H2O2 generation decreased and was almost completely eliminated in the presence of 1 mm NAD+. In this experiment, the rate of H2O2 formation decreased from 1.07 to 0.15 pmol/sec (86% inhibition) in response to the addition of 1 mm NAD+. Inhibition of the rate of H2O2 production as a function of NAD+ concentration is shown in Figure 3b. The ID50 (NAD+ concentration at which the rate of H2O2 generation initiated by 1 mm α-KG is inhibited by 50%) derived from Figure 3 is 1.58 ± 0.21 μm. We found that after addition of NAD+ in 1 or 20 μm concentrations, the rate of the diminished H2O2 generation was not linear but appeared to slowly recover (Fig. 3a) parallel with the conversion of NAD+ to NADH by α-KGDH (Fig. 3c).
Figure 3.
Inhibition of H2O2 production (a, b) and stimulation of NADH formation (c) by isolated α-KGDH in response to NAD+. α-KGDH, HS-CoA (0.12 mm),α-KG (1 mm), and NAD+ in different concentrations (50 nm to 1 mm) were applied as indicated. H2O2 and NADH formations were measured simultaneously in the same samples, as described in Materials and Methods. Traces have been offset for clarity. b, Inhibition of H2O2 generation is shown as a function of NAD+ concentrations. Points represent mean values from three experiments ±SEM. Rectangular hyperbola was fitted to the experimental points. H2O2 generation in the absence of NAD+ was 1.07 ± 0.01 pmol/sec (n = 6).
Stimulation by NADH of the α-KGDH-mediated H2O2 formation
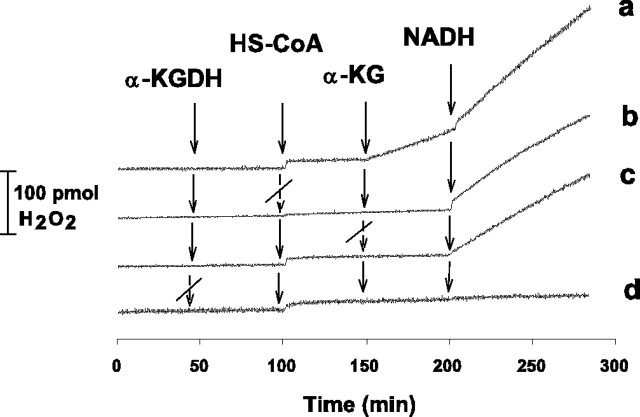
Next, we investigated the effect of NADH (without NAD+) on the H2O2 generation by α-KGDH. It is demonstrated in Figure 4a that 1 μm NADH, given 50 sec after addition of α-KG, further increased the rate of H2O2 formation (in this experiment, from 1.18 to 2.1 pmol/sec). It is also shown in Figure 4 that when HS-CoA or α-KGDH were not present, α-KG failed to initiate H2O2 generation (b, d). In contrast, NADH was able to induce H2O2 formation in the absence of HS-CoA (b) or α-KG (c) or both (data not shown). Only when α-KGDH was absent was NADH unable to stimulate H2O2 generation (d). These results clearly show that for the H2O2 generation initiated by α-KG, the substrates of α-KGDH (except for NAD+) are essential, whereas only the enzyme is necessary and sufficient for the NADH-induced H2O2 production. This indicates that the mechanism by which α-KGDH generates H2O2 in response to NADH is different from that initiated by α-KG in the presence of the substrates and cofactors of the enzyme but in the absence of NAD+.
Figure 4.
Stimulation of α-KGDH-mediated H2O2 production by NADH. α-KGDH, HS-CoA (0.12 mm), α-KG (1 mm), and NADH (1 μm) were applied as indicated. H2O2 production was measured as for Figures 2 and 3.
H2O2 generation by isolated α-KGDH as a function of α-KG concentration
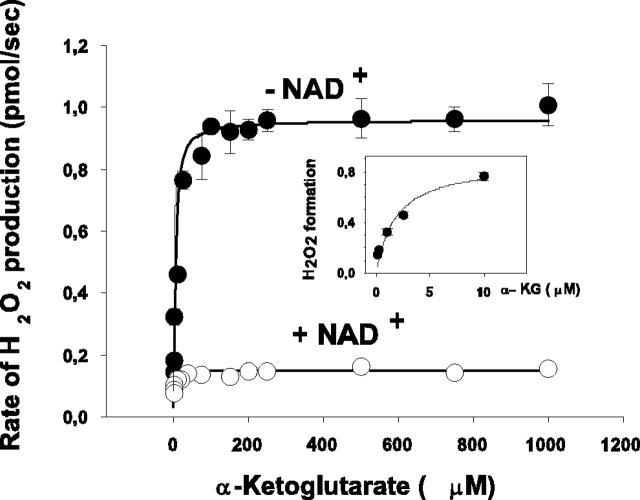
To further characterize the α-KGDH-mediated H2O2 production, the rate of H2O2 production was measured as a function of the concentration of α-KG (Fig. 5). When NAD+ (1 mm) was present, the rate of H2O2 formation was low; in the presence of 1 mm α-KG, the rate was 0.15 ± 0.02 pmol/sec. Under identical conditions, the rate of NADH formation was 44 ± 4.1 pmol/sec (n = 3; data not shown). H2O2 formation was found to be concentration dependent in the lower α-KG concentration range; the KM derived from the fitted rectangular hyperbola using the Michaelis equation was 3.8 ± 1.1 μm. In the absence of NAD+, as demonstrated in Figures 2 and 3, the rate of H2O2 formation was increased, but the dependence on α-KG concentrations remained the same (Fig. 5, inset), and the α-KG KM for H2O2 formation in the absence of NAD+ was not significantly different from that found in the presence of 1 mm NAD+. At 1 mm α-KG concentration, we found a seven times higher rate of H2O2 generation (1.05 ± 0.06 pmol/sec) in the absence of NAD+ compared with that measured in the presence of NAD+ (0.15 ± 0.02 pmol/sec).
Figure 5.
H2O2 formation by isolated α-KGDH as a function of α-KG. The experimental protocol was as for Figure 2, except that α-KG was added in different concentrations. The rate of the initial H2O2 formation was measured after addition of α-KG without (•) or with (○) subsequent application of NAD+ in 1 mm concentration. H2O2 formation at low concentrations of α-KG (1-10 μm) in the absence of NAD+ is shown in the inset. Points represent mean ± SEM from three experiments. SEM is within the size of symbols unless otherwise indicated.
H2O2 formation by α-KGDH at different NADH/NAD+ ratios
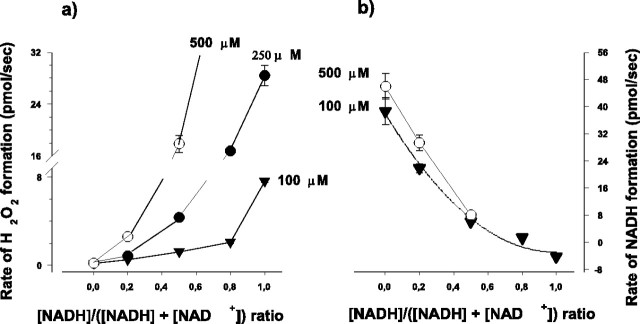
Given the opposite effect of NAD+ and NADH on the α-KGDH-mediated H2O2 generation, it is important to investigate the dependence of H2O2 formation on the NADH/NAD+ ratio. Total NADH plus NAD+ concentrations used in these experiments were 100, 250, or 500 μm, and at each concentration, different NADH/NADH plus NAD+ ratios were set. (At >500 μm NADH plus NAD+, when the NADH/NADH plus NAD+ ratio was >0.5, H2O2 formation was extremely rapid, making the calculation of the rate of H2O2 formation unreliable. Therefore, higher than 500 μm NADH plus NAD+ concentration was not used in these experiments). The rates of H2O2 and NADH formation after addition of NADH plus NAD+ were measured simultaneously (Fig. 6a,b). As a general tendency, it is apparent from Figure 6a that the higher the NADH/NAD+ ratio the higher the rate of H2O2 formation at each NADH plus NAD+ concentration. It is evident that the presence of NADH stimulated the H2O2 generation at a given NAD+ concentration. For example, the rate of H2O2 generation with 250 μm NAD+ (without NADH) was 0.16 ± 0.02 pmol/sec, but when the same amount of NADH was also present [NADH/(NADH plus NAD+) ratio 0.5, at 500 μm total NADH plus NAD+ concentration], the rate was 17.8 ± 1.3 pmol/sec. As expected, an increase in the NADH/NAD+ ratio decreased the normal catalytic activity (i.e., NADH formation by the enzyme) (Fig. 6b). With 100 μm NAD+, the enzyme is working close to its Vmax, and a similar curve describes the rate of NADH formation measured at 500 μm total [NADH plus NAD+]. Therefore, the decrease in the normal catalytic activity resulting from an increase in the NADH/NAD+ ratio parallels an enhancement in H2O2 generation. As shown in Table 1, the rate of NADH generation was 300 times larger than the rate of H2O2 generation when only NAD+ was present in the medium, whereas the rate of H2O2 generation in the α-KGDH catalyzed reaction increased by two orders of magnitude when the NADH/(NADH plus NAD+) ratio was 0.5 [total (NADH plus NAD+) was 500 μM]. It is known that NADH undergoes slow autoxidation and small concentrations of H2O2 are present in NADH solutions (Sawada and Yamazaki, 1973). In contrast, horseradish peroxidase is able to oxidize NADH at low pH with the concomitant formation of various oxidation-reduction intermediates in the active center of the enzyme (Yokota and Yamazaki, 1977). To rule out the possibility of artifacts in this study, control experiments were performed, and we found that the autoxidation of NADH in the presence of Amplex plus HRP is negligible compared with the oxidation in the presence of α-KGDH plus Amplex plus HRP. In fact, the presence of Amplex Red inhibited the HRP-stimulated oxidation of NADH (data not shown).
Figure 6.
The effect of NADH/NADH plus NAD+ ratio on the α-KGDH-mediated H2O2 and NADH formation. Experiments were performed as described for Figure 3; however, subsequent to α-KG, NADH plus NAD+ was added in different ratios shown in the abscissa at final concentrations of 100, 250, or 500 μm (a) and 100 or 500 μm (b) as indicated. The rate of H2O2 (a) and NADH formation (b) were measured after addition of NADH plus NAD+. Addition of NADH in this concentration range resulted in a sharp increase in the Amplex signal because of the presence of some H2O2 contamination always present in NADH solutions. Thereafter, the signal became linear, and the slope was taken as a measure of H2O2 generation under the indicated conditions. Catalytic activity of α-KGDH was followed by the reduction of NAD+, measuring absorbance changes at 340 nm with a GBC Scientific Equipment (Dandenong, Australia) UV-visible 920 spectrophotometer, using the extinction coefficient E340 = 6.22 mm-1*cm-1. Points represent mean values from three experiments (±SEM). SEM is within the size of symbols unless otherwise indicated.
Table 1.
The rate of NADH and H2O2 generation by α-KGDH at different NADH/NADH plus NAD+ ratios
|
NADH/NAD+ plus NADH |
NADH generation (pmol/sec) |
H2O2 generation (pmol/sec) |
NADH generation per H2O2 generation |
|---|---|---|---|
| 0 | 46 ± 2.8 | 0.15 ± 0.02 | 307 |
| 0.2 | 29.2 ± 2.3 | 2.58 ± 0.11 | 11.4 |
| 0.5 |
8.0 ± 0.19 |
17.8 ± 1.3 |
0.45 |
Experiments were performed as described for Figure 3, a and c, but NADH plus NAD+ was applied in different ratios at 500 μm NADH plus NAD+ concentration.
Values are mean ± SEM from three experiments.
The effect of Ca2+ on the α-KGDH-mediated H2O2 formation
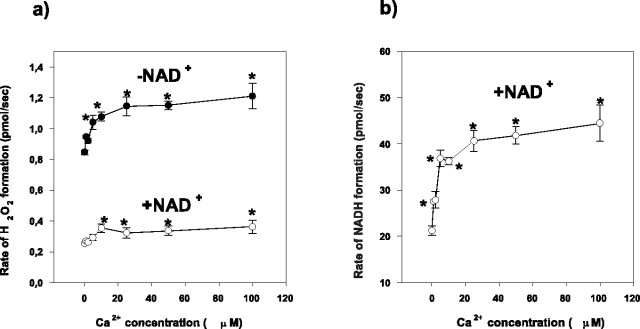
Ca2+ is known to be an important stimulator of the mitochondrial metabolism caused by activation of key dehydrogenases, among them, α-KGDH (McCormack and Denton, 1979; Lukacs et al., 1988; Panov and Scarpa, 1996). This activation could be demonstrated in our experiments by measuring the NADH formation by the isolated α-KGDH in the presence of different Ca2+ concentrations (Fig. 7b). In the same concentration range (0-100 μm), Ca2+ significantly increased the rate of H2O2 generation (Fig. 7a) both in the presence or absence of NAD+. These experiments were done in Mg2+ and ADP-free medium to exclude an effect of Mg2+ on the catalytic activity of the enzyme. The use of Mg2+-free medium explains the smaller catalytic activity of α-KGDH (Panov and Scarpa, 1996) measured in these experiments and also the smaller rate of H2O2 formation found in the absence of NAD+.
Figure 7.
a, b, The effect of free [Ca2+] on the H2O2 (a) and NADH formation (b) measured with isolated α-KGDH. Isolated α-KGDH was incubated in a buffer containing different concentrations of ionized Ca2+ (0-100 μm). Ca2+ concentrations were controlled as described in Materials and Methods. The experimental protocol was as described for Figure 3, but ADP and Mg2+ were not present in the medium. H2O2 formation was measured in the absence (•) or presence (○) of NAD+ (1 mm). Values represent mean ± SEM from three experiments. Asterisk indicates points significantly different from the control (measured in the absence of Ca2+).
Discussion
This study demonstrates that α-KGDH, a key NADH-generating enzyme in the Krebs' cycle, is able to produce ROS depending on the NADH/NAD+ ratio present in the medium. The results clearly show that in the absence of the physiological electron acceptor NAD+, electrons from the substrate eventually reduce oxygen mediated by α-KGDH. In contrast, with NADH, the enzyme produces H2O2 without the need of the substrates. It is also demonstrated that in the presence of the substrates, an increase in the NADH/NAD+ ratio, while inhibiting the physiological catalytic function of α-KGDH, favors the ROS generation by the enzyme. In addition, Ca2+, which is a physiological activator of the enzyme, also activates the H2O2 generation both in the absence and presence of NAD+.
α-KGDH is a complex enzyme catalyzing a key reaction in the Krebs' cycle: α-ketoglutarate plus HS-CoA plus NAD+ → succinyl-CoA plus CO2 plus NADH. The enzyme consists of the following three subunits: E1, a thiamine pyrophosphate-dependent dehydrogenase; E2, dihydrolipoamide succinyltransferase; and E3, dihydrolipoyl dehydrogenase (Sheu and Blass, 1999). Because HS-CoA is required for the α-KGDH-mediated H2O2 formation, the reactions should proceed via E1 and E2 components of the enzyme, and H2O2 is likely produced by the E3 subunit. Dihydrolipoyl dehydrogenase, the E3 component of α-KGDH, is a flavoprotein. Generally, in the reaction catalyzed by flavoproteins, O2 could be reduced to superoxide or H2O2 (Kakinuma et al., 1987). However, with dehydrogenases, the overall reoxidation of reduced enzyme by O2 is extremely slow, and the primary product is poorly defined (Massey, 1994). Superoxide production in the 2-oxo acid dehydrogenase reaction has been reported recently (Bunik and Sievers, 2002). The isolated lipoamide dehydrogenase was shown to exhibit NADH oxidase catalytic activity by which H2O2 is produced (NADH plus H+ plus O2 → NAD+ plus H2O2), and this reaction was accelerated by Zn2+ (Gazaryan et al., 2002).
In the α-KGDH-mediated reaction, both superoxide (data not shown) and H2O2 could be detected, but it is not possible to determine whether both are produced in the enzyme reaction as described for some flavoproteins (Badwey and Karnovsky, 1979) or whether H2O2 is generated by a very fast spontaneous dismutation of superoxide. It is most likely that both superoxide and H2O2 are generated in the α-KGDH-mediated reaction.
E3 is not unique to α-KGDH but is present in the pyruvate dehydrogenase, branched chain α-ketoacid dehydrogenase and glycine-cleavage system (Kochi et al., 1986). Our preliminary data, consistent with the results obtained by Starkov et al. (2004), show that H2O2 is also generated in the pyruvate dehydrogenase reaction (data not shown), which reinforces the suggestion that H2O2 is generated by the E3 subunit of α-KGDH.
Our experiments indicate that reducing equivalents for ROS formation by the E3 subunit of α-KGDH originate from substrate oxidation (forward reaction) when α-KG and HS-CoA are present and, in the absence of NAD+, reduce O2. In contrast, reducing equivalents for E3 could also be provided by NADH (reverse reaction), in agreement with the reaction described for the isolated lipoamid dehydrogenase (Gazaryan et al., 2002). The isolated α-KGDH (Sigma) used in our experiments is contaminated with pyruvate dehydrogenase subunits, among them, lipoamide dehydrogenase (Panov and Scarpa, 1996). This would not interfere with our assay where α-KG is the substrate, as established in a control assay where isolated pyruvate dehydrogenase was used (data not shown). When the effect of NADH was studied without α-KG present, the lipoamide dehydrogenase may have had some contribution to the H2O2 signal, consistent with the H2O2 formation by lipoamide dehydrogenase (Gazaryan et al., 2002).
With the isolated enzyme, NAD+ behaves as a switch accelerating the catalytic function of the enzyme by which the oxidation of α-KG results in the production of NADH. In contrast, in the absence of NAD+, substrate oxidation leads to ROS generation. When the substrate oxidation is stimulated by Ca2+, ROS generation is also accelerated. At 1 mm α-ketoglutarate concentration, the rate of ROS formation (1.05 ± 0.06 pmol H2O2/sec) is ∼40 times slower than the rate of NADH generation (44 ± 4.1 pmol/sec) measured in the presence of NAD+. In the mitochondrial matrix where both NAD+ and NADH are present, the NADH/NAD+ ratio could determine the rate of ROS generation by α-KGDH. This is indicated by the observation that H2O2 formation mediated by the isolated enzyme was strongly dependent on the NADH/NAD+ ratio. When the NADH/(NADH plus NAD+) ratio was >0.2, H2O2 generation was significantly increased. With 500 μm total NADH plus NAD+ concentration, an increase in the NADH/(NADH plus NAD+) ratio from 0.2 to 0.5 resulted in a 6.9 times increase in the rate of H2O2 generation by the isolated α-KGDH (Table 1). At the same time, the normal catalytic function (i.e., the rate of NADH generation) decreased from 29.2 ± 2.3 to 8.0 ± 0.19 pmol/sec. It is clear from Table 1 that at 0.2 NADH/(NADH plus NAD+) ratio, the rate of NADH generation is 11.4 times higher than the rate of H2O2 generation (29.2 ± 2.3 vs 2.58 ± 0.11 pmol/sec); however, when the NADH/(NADH plus NAD+) ratio is increased to 0.5, H2O2 generation by the enzyme becomes highly significant. Therefore, NADH/NAD+ ratio is critical to determine the rate of ROS formation in the reaction catalyzed by α-KGDH. Because of differences of the methods, available values in the literature for the ratio of pyridine nucleotides in different tissues or in isolated mitochondria cover a wide range (Siess et al., 1976; Siess et al., 1977; Shiino et al., 1999). However, the physiological NAD+ plus NADH concentration in isolated mitochondria appears to be in the millimolar range (Di Lisa et al., 2001). Data obtained from brain regions are consistent in indicating that the NADH/(NADH plus NAD+) ratio in the cortex, striatum, or hippocampus is ∼0.2 (Klaidman et al., 1995); our measurements done with synaptosomes indicated somewhat higher values. However, these measurements could provide information only for the whole tissue, and values for in situ mitochondria are not available and would be very difficult to give. Assuming that the NAD+ plus NADH concentration of in situ mitochondria is also in the millimolar range, and given the tendency shown in Figure 6, it can be suggested that an increase in the NADH/NAD+ ratio at this NAD+ plus NADH concentration could be even more significant in stimulating the H2O2 generation by α-KGDH.
ROS generation by α-KGDH in isolated mitochondria is demonstrated by Starkov et al. (2004), and results shown in our study indicate that it occurs also in the in situ mitochondria within isolated nerve terminals. The amount of ROS generated in situ is sufficient to inhibit endogeneous aconitase (Fig. 1). We reported previously that α-KGDH activity of in situ mitochondria in synaptosomes was 14.2 nmol/min/mg synaptosomal protein (Tretter and Adam-Vizi, 2000). Assuming that this activity is 300 times higher than the rate of H2O2 production (derived from data obtained in this study with the isolated enzyme; 46 ± 2.8 pmol NADH/sec vs 0.15 ± 0.02 pmol H2O2/sec), H2O2 generation mediated by α-KGDH in synaptosomes could be ∼50 pmol/min/mg. This is likely to be an underestimated value; when NAD+ and NADH were present, the rate of H2O2 generation mediated by the isolated α-KGDH was more significant.
The strong dependence of the H2O2 formation by α-KGDH on the NADH/NAD+ ratio has important physiological implications. When the reoxidation of NADH in the respiratory chain is impaired, the NADH/NAD+ ratio in the mitochondrial matrix is increased, thus inhibiting the NAD+-dependent dehydrogenases (Gomazkova and Krasovskaia, 1979; Lawlis and Roche, 1980). Under this condition, ROS production in the α-KGDH catalyzed reaction could become significant, contributing to the accumulation of ROS in cells. In particular, inhibition of complex I is important in this respect, because this is characteristically present in the degenerating substantia nigra obtained postmortem from patients suffering from Parkinson's disease (Schapira et al., 1989, 1990), and inhibition of complex I in vivo induces Parkinsonian syndromes (Betarbet et al., 2000). The experimental model of inhibition of complex I using rotenone revealed an enhanced H2O2 generation in both isolated (Votyakova and Reynolds, 2001) and in situ mitochondria present in isolated nerve terminals (Sipos et al., 2003), which was attributed to ROS generation at complex I in the respiratory chain. Given the observations in the present study, it is suggested that the stimulated ROS production caused by complex I inhibition could be attributed to, at least in part, the increased NADH/NAD+ ratio stimulating ROS generation in the α-KGDH-mediated reaction.
There are a few cases in which genetic defects of α-KGDH in humans have been reported (Gibson et al., 2000). When the E3 component was deficient, patients suffered from a progressive neural degeneration (Kohlschutter et al., 1982), pointing to a possible role of α-KGDH deficiency in the pathogenesis of neurodegenerative diseases. This enzyme was found to be inhibited in postmortem brain tissues from patients who suffered from Parkinson's or Alzheimer's disease (Gibson et al., 1988; Mizuno et al., 1990, 1994; Mastrogiacoma et al., 1996). It is tempting to speculate that with a deficient α-KGDH, in particular involving the E3 component, H2O2 generation by the enzyme could become dominant while losing a large part of the normal catalytic activity.
Footnotes
This work was supported by grants from the Hungarian Scientific Research Fund, Hungarian Academy of Sciences, and Hungarian Medical Research Council to V.A.-V. We are indebted to Melinda Miklos, Katalin Takács, and Andrea Várnagy for excellent assistance.
Correspondence should be addressed to Dr. Vera Adam-Vizi, Department of Medical Biochemistry, Semmelweis University, P.O. Box 262, Budapest H-1444, Hungary. E-mail: av@puskin.sote.hu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247771-08$15.00/0
References
- Andersson U, Leighton B, Young ME, Blomstrand E, Newsholme EA (1998) Inactivation of aconitase and oxoglutarate dehydrogenase in skeletal muscle in vitro by superoxide anions and/or nitric oxide. Biochem Biophys Res Commun 249: 512-516. [DOI] [PubMed] [Google Scholar]
- Badwey JA, Karnovsky ML (1979) Production of superoxide and hydrogen peroxide by an NADH-oxidase in guinea pig polymorphonuclear leukocytes. Modulation by nucleotides and divalent cations. J Biol Chem 254: 11530-11537. [PubMed] [Google Scholar]
- Beal MF (1996) Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol 6: 661-666. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci 3: 1301-1306. [DOI] [PubMed] [Google Scholar]
- Bindoli A (1988) Lipid peroxidation in mitochondria. Free Radic Biol Med 5: 247-261. [DOI] [PubMed] [Google Scholar]
- Boveris A, Cadenas E (1975) Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett 54: 311-314. [DOI] [PubMed] [Google Scholar]
- Bunik VI, Sievers C (2002) Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. Eur J Biochem 269: 5004-5015. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527-605. [DOI] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ (2003) Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278: 36027-36031. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Tretter L, Rozsa A, Adam-Vizi V (2000) Exacerbated responses to oxidative stress by an Na+ load in isolated nerve terminals: the role of ATP depletion and rise of [Ca2+]i. J Neurosci 20: 2094-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P (2001) Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem 276: 2571-2575. [DOI] [PubMed] [Google Scholar]
- Fiskum G (1985) Mitochondrial damage during cerebral ischemia. Ann Emerg Med 14: 810-815. [DOI] [PubMed] [Google Scholar]
- Fridovich I (1995) Superoxide radical and superoxide dismutases. Annu Rev Biochem 64: 97-112. [DOI] [PubMed] [Google Scholar]
- Gazaryan IG, Krasnikov BF, Ashby GA, Thorneley RN, Kristal BS, Brown AM (2002) Zinc is a potent inhibitor of thiol oxidoreductase activity and stimulates reactive oxygen species production by lipoamide dehydrogenase. J Biol Chem 277: 10064-10072. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P (1988) Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer's disease. Arch Neurol 45: 836-840. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Park LC, Sheu KF, Blass JP, Calingasan NY (2000) The alpha-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochem Int 36: 97-112. [DOI] [PubMed] [Google Scholar]
- Gomazkova VS, Krasovskaia OE (1979) Regulation of alpha-ketoglutarate dehydrogenase complex from pigeon breast muscle. Biokhimiia 44: 1126-1136. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440-3450. [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1998) Free radicals, other reactive species and disease. In: Free radicals in biology and medicine (Halliwell B, Gutteridge JMC, eds), pp 617-784. Oxford: Oxford UP.
- Hausladen A, Fridovich I (1996) Measuring nitric oxide and superoxide: rate constants for aconitase reactivity. Methods Enzymol 269: 37-41. [DOI] [PubMed] [Google Scholar]
- Huennekens FM, Basford RE, Gabrio BW (1955) An oxidase for reduced diphosphopiridine nucleotide. J Biol Chem 213: 951-967. [PubMed] [Google Scholar]
- Humphries KM, Szweda LI (1998) Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry 37: 15835-15841. [DOI] [PubMed] [Google Scholar]
- Kakinuma K, Fukuhara Y, Kaneda M (1987) The respiratory burst oxidase of neutrophils. Separation of an FAD enzyme and its characterization. J Biol Chem 262: 12316-12322. [PubMed] [Google Scholar]
- Klaidman LK, Leung AC, Adams Jr JD (1995) High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal Biochem 228: 312-317. [DOI] [PubMed] [Google Scholar]
- Kochi H, Seino H, Ono K (1986) Inhibition of glycine oxidation by pyruvate, alpha-ketoglutarate, and branched-chain alpha-keto acids in rat liver mitochondria: presence of interaction between the glycine cleavage system and alpha-keto acid dehydrogenase complexes. Arch Biochem Biophys 249: 263-272. [DOI] [PubMed] [Google Scholar]
- Kohlschutter A, Behbehani A, Langenbeck U, Albani M, Heidemann P, Hoffmann G, Kleineke J, Lehnert W, Wendel U (1982) A familial progressive neurodegenerative disease with 2-oxoglutaric aciduria. Eur J Pediatr 138: 32-37. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE (1999) Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 26: 463-471. [DOI] [PubMed] [Google Scholar]
- Lai JC, Cooper AJ (1986) Brain alpha-ketoglutarate dehydrogenase complex: kinetic properties, regional distribution, and effects of inhibitors. J Neurochem 47: 1376-1386. [DOI] [PubMed] [Google Scholar]
- Lawlis VB, Roche TE (1980) Effect of micromolar Ca2+ on NADH inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex and possible role of Ca2+ in signal amplification. Mol Cell Biochem 32: 147-152. [DOI] [PubMed] [Google Scholar]
- Liang LP, Ho YS, Patel M (2000) Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience 101: 563-570. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c Cell 86: 147-157. [DOI] [PubMed] [Google Scholar]
- Loschen G, Flohe L, Chance B (1971) Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett 18: 261-264. [DOI] [PubMed] [Google Scholar]
- Loschen G, Azzi A, Flohe L (1973) Mitochondrial H2O2 formation: relationship with energy conservation. FEBS Lett 33: 84-87. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Kapus A, Fonyo A (1988) Parallel measurement of oxoglutarate dehydrogenase activity and matrix free Ca2+ in fura-2-loaded heart mitochondria. FEBS Lett 229: 219-223. [DOI] [PubMed] [Google Scholar]
- Massey V (1994) Activation of molecular oxygen by flavins and flavoproteins. J Biol Chem 269: 22459-22462. [PubMed] [Google Scholar]
- Massey V, Strickland S, Mayhew SG, Howell LG, Engel PC, Matthews RG, Schuman M, Sullivan PA (1969) The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem Biophys Res Commun 36: 891-897. [DOI] [PubMed] [Google Scholar]
- Mastrogiacoma F, Lindsay JG, Bettendorff L, Rice J, Kish SJ (1996) Brain protein and alpha-ketoglutarate dehydrogenase complex activity in Alzheimer's disease. Ann Neurol 39: 592-598. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Denton RM (1979) The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem J 180: 533-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Suzuki K, Ohta S (1990) Postmortem changes in mitochondrial respiratory enzymes in brain and a preliminary observation in Parkinson's disease. J Neurol Sci 96: 49-57. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Matuda S, Yoshino H, Mori H, Hattori N, Ikebe S (1994) An immunohistochemical study on alpha-ketoglutarate dehydrogenase complex in Parkinson's disease. Ann Neurol 35: 204-210. [DOI] [PubMed] [Google Scholar]
- Mohanty JG, Jaffe JS, Schulman ES, Raible DG (1997) A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods 202: 133-141. [DOI] [PubMed] [Google Scholar]
- Nulton-Persson AC, Starke DW, Mieyal JJ, Szweda LI (2003) Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry 42: 4235-4242. [DOI] [PubMed] [Google Scholar]
- Otani H, Tanaka H, Inoue T, Umemoto M, Omoto K, Tanaka K, Sato T, Osako T, Masuda A, Nonoyama A, (1984) In vitro study on contribution of oxidative metabolism of isolated rabbit heart mitochondria to myocardial reperfusion injury. Circ Res 55: 168-175. [DOI] [PubMed] [Google Scholar]
- Panov A, Scarpa A (1996) Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+ Biochemistry 35: 427-432. [DOI] [PubMed] [Google Scholar]
- Park LC, Zhang H, Sheu KF, Calingasan NY, Kristal BS, Lindsay JG, Gibson GE (1999) Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J Neurochem 72: 1948-1958. [DOI] [PubMed] [Google Scholar]
- Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO (1996) Requirement for superoxide in excitotoxic cell death. Neuron 16: 345-355. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Yamazaki I (1973) One-electron transfer reactions in biochemical systems. VIII. Kinetic study of superoxide dismutase. Biochim Biophys Acta 327: 257-265. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1: 1269. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem 54: 823-827. [DOI] [PubMed] [Google Scholar]
- Shank RP, Campbell GL (1984) Alpha-ketoglutarate and malate uptake and metabolism by synaptosomes: further evidence for an astrocyte-to-neuron metabolic shuttle. J Neurochem 42: 1153-1161. [DOI] [PubMed] [Google Scholar]
- Sheu KF, Blass JP (1999) The alpha-ketoglutarate dehydrogenase complex. Ann NY Acad Sci 893: 61-78. [DOI] [PubMed] [Google Scholar]
- Shiino A, Haida M, Beauvoit B, Chance B (1999) Three-dimensional redox image of the normal gerbil brain. Neuroscience 91: 1581-1585. [DOI] [PubMed] [Google Scholar]
- Siess EA, Brocks DG, Wieland OH (1976) Subcellular distribution of key metabolites in isolated liver cells from fasted rats. FEBS Lett 69: 265-271. [DOI] [PubMed] [Google Scholar]
- Siess EA, Brocks DG, Lattke HK, Wieland OH (1977) Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J 166: 225-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos I, Tretter L, Adam-Vizi V (2003) Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem 84: 112-118. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G (2003) Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86: 1101-1107. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF (2004) Mitochondrial α-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24: 7779-7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V (1996) Early events in free radical-mediated damage of isolated nerve terminals: effects of peroxides on membrane potential and intracellular Na+ and Ca2+ concentrations. J Neurochem 66: 2057-2066. [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V (2000) Inhibition of Krebs' cycle enzymes by hydrogen peroxide: a key role of α-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J Neurosci 20: 8972-8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Szabados G, Ando A, Horvath I (1987) Effect of succinate on mitochondrial lipid peroxidation. II. The protective effect of succinate against functional and structural changes induced by lipid peroxidation. J Bioenerg Biomembr 19: 31-44. [DOI] [PubMed] [Google Scholar]
- Votyakova TV, Reynolds IJ (2001) DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79: 266-277. [DOI] [PubMed] [Google Scholar]
- Willoughby J, Craig FE, Harvey SA, Clark JB (1989) 2-Oxoglutarate: oxidation and role as a potential precursor of cytosolic acetyl-CoA for the synthesis of acetylcholine in rat brain synaptosomes. J Neurochem 52: 896-901. [DOI] [PubMed] [Google Scholar]
- Yokota K, Yamazaki I (1977) Analysis and computer simulation of aerobic oxidation of reduced nicotinamide adenine dinucleotide catalyzed by horseradish peroxidase. Biochemistry 16: 1913-1920. [DOI] [PubMed] [Google Scholar]