Abstract
Our aim was to investigate the functional properties of the noradrenergic system in genetically modified mice lacking the norepinephrine transporter (NET). We measured the uptake and release of [3H]norepinephrine ([3H]NE) from hippocampal and cortical slices of NET-/- knock-out (KO) and NET+/+ wild-type (WT) mice and investigated the presynaptic α2-adenoceptor-mediated modulation of NE release in vitro and in vivo. The [3H]NE uptake was reduced to 12.6% (hippocampus) and 33.5% (frontal cortex) of WT control in KO mice. The neuronal component of this residual uptake was decreased by 79.4 and 100%, respectively, when a selective serotonin reuptake inhibitor (SSRI) citalopram was present during the loading. The more preserved neuronal release of [3H]NE (hippocampus, 28.1%; frontal cortex, 74.4%; compared with WT) almost completely disappeared in both regions (94.1 and 95.3% decrease compared with KO, respectively) in the presence of citalopram, suggesting that [3H]NE was taken up and released by serotonergic varicosities. This was further supported by the finding that the release of [3H]NE from hippocampal slices of KO mice was not modulated by the α2-adrenoceptor antagonist 7,8-(methylenedioxy)-14-α-hydroxyalloberbane HCl, whereas the endogenous release of NE measured by microdialysis was even more efficiently enhanced by this drug in NET-deficient mice. These experiments indicate that serotonergic varicosities can accumulate and release NE as a result of the heterologous uptake of transmitters. Because the diffusion of NE may be spatially limited by serotonin transporters, the SSRIs, despite their selectivity, might enhance not only serotonergic but also noradrenergic neurotransmission, which might contribute to their antidepressant action.
Keywords: NET KO mice, norepinephrine, serotonin, uptake, citalopram, depression
Introduction
According to the monoamine theory, the neurochemical background of depression is an impairment of noradrenergic and/or serotonergic neurotransmission and the concomitant decrease of biophase concentration of these transmitters (Hindmarch, 2001; Wong and Licinio, 2001). The extracellular concentration of monoamines is primarily determined by two factors: the release from terminals and varicosities, and the reuptake by monoamine transporters. Currently, the most common therapeutic approach for the treatment of depression is to increase the level of norepinephrine (NE) and/or serotonin (5-HT) by inhibition of neuronal reuptake; therefore, monoamine transporters have great clinical importance as primary targets for antidepressant drugs (Iversen, 2000; Vizi, 2000).
All of the monoamine uptake carriers belong to the family of Na+/Cl--dependent membrane transporters containing 12 transmembrane domains (Amara and Arriza, 1993). The cloning and sequencing of monoamine transporters in the early 1990s (Blakely et al., 1991; Giros et al., 1991, 1992; Pacholczyk et al., 1991; Ramamoorthy et al., 1993) revealed that these proteins show a very high degree of structural homology. Including the conservative substitutions, the amino acid sequence identity of the NE and dopamine transporters (NET and DAT) is >75% (Giros et al., 1992), whereas the 5-HT transporter (SERT) is also very similar, its homology with NET being >60% (Blakely et al., 1991). This high degree of homology may explain the accumulating observations suggesting that functional segregation of monoaminergic pathways is not as marked as it was assumed previously (Carboni et al., 1990, 2001; Cases et al., 1998; Rocha et al., 1998; Yamamoto and Novotney, 1998; Mundorf et al., 2001; Sora et al., 2001; Moron et al., 2002; Uhl et al., 2002).
Recent development of genetically modified mice lacking the NET (Wang et al., 1999; Xu et al., 2000) opened new perspectives for studying the function of different monoaminergic systems. Our aim was to investigate the alterations and possible interactions of noradrenergic and other monoaminergic systems in the NET-deficient mice using functional (uptake and release) experiments.
Materials and Methods
Animals
The original breeding pairs of NET knock-out (KO) and wild-type (WT) mice (C57BL/6J based) were transferred from the Duke University Medical Center (Durham, NC). The animals contained the DNA construct previously shown to produce genetic deletion of the NET (Xu et al., 2000). All of the experiments were performed on adult (3-5 months of age) homozygous (NET-/-) mice and their WT littermates (NET+/+) of both sexes weighing 20-29 gm. The line was maintained by crossing heterozygous animals. The animals were housed (three or four per cage) on a 12 hr light/dark cycle with ad libitum access to water and food. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the Institute of Experimental Medicine. All of the studies were conducted in compliance with National Institutes of Health guidelines for the care and use of animals.
[3H]Nisoxetine binding
Membrane preparation. Mice were decapitated, and the brain was removed over ice. The frontal cortex or the hippocampus was used in uptake and release experiments, while the rest of the cortex was kept at -70°C until use. The tissue was homogenized by Ultra-Turrax in ice-cold 50 mm Tris-HCl buffer, pH 7.4, containing 300 mm NaCl and 5 mm KCl, and centrifuged at 40,000 × g for 15 min. The pellet was washed three times with buffer by suspension and centrifugation. The final pellet was taken up in 30 vol (w/v) of buffer and used immediately for the binding experiment.
Saturation experiments. Aliquots of membrane preparation and different concentrations of [3H]nisoxetine (0.1-1.7 nm) were incubated at 4°C for 3 hr in a final volume of 250 μl of 50 mm Tris-HCl buffer, pH 7.4, containing 300 mm NaCl and 5 mm KCl. The nonspecific binding was determined in the presence of 10 μm desipramine. The binding was initiated by the addition of [3H]nisoxetine and was terminated by rapid vacuum filtration over GF/B filters soaked in 0.05% polyethylenimine. The protein concentration (0.8-1 mg/ml) was measured by the modified method of Lowry et al. (1951) using CuEDTA. The dissociation constant (Kd) and the receptor density (Bmax) were calculated by LIGAND program.
PCR analysis
The authenticity of WT, heterozygous, and NET KO mice was confirmed by PCR. Genomic DNA was isolated from the tails of young animals with DNeasy Tissue kit (Qiagen, Hilden, Germany), and the presence of NE transporter and eGFP (enhanced green fluorescent protein) gene was tested by PCR using gene-specific primers. PCR reactions were performed in a GeneAmp PCR System 2700 using Promega (Madison, WI) Taq polymerase (1 U per 30 μl reaction mixture). PCR temperature protocol consisted of initial activation at 94°C for 5 min, followed by 40 cycles at 94°C for 1 min, 57°C for 1 min, and 72°C for 1.5 min. A final elongation period of 10 min at 72°C was also included. PCR products were analyzed on 1% agarose gel stained with ethidium bromide.
[3H]NE uptake and release from brain slices.
Mice were killed by decapitation, and the brain was rapidly removed and immediately placed into ice-cold Krebs' solution [composition (in mm): 113 NaCl, 4.7 KCl, 1.2 MgSO4, 2.5 CaCl2, 25 NaHCO3, 1.2 KH2PO4, 115 glucose, 0.3 Na2EDTA, and 0.03 ascorbic acid] and continuously gassed with a mixture of 95% O2 and 5% CO2. The frontal cortex or hippocampus was prepared and sliced into 0.4 mm sections with a Bachofer tissue chopper. Slices were separated by gently shaking, washed with 5 ml of Krebs' solution, and loaded for 45 min with [3H]NE at a concentration of 10 μCi in 1 ml of Krebs' solution. When the involvement of 5-HT transporters was investigated, the selective serotonin reuptake inhibitor (SSRI) citalopram (1 μm) was present during the loading period and throughout the experiment. The nonspecific uptake and release was measured in the presence of citalopram, the selective DA uptake inhibitor 1-(2-[bis(4-fluorophenyl)methoxy]ethyl)-4-(3-phenylpropyl)piper-azine dihydro-chloride (GBR 12909), and the selective NE uptake inhibitor nisoxetine (1 μm each). The mixture of selective monoamine uptake inhibitors was present also during the loading period and throughout the experiment. After the loading period, the slices were washed three times with 10 ml of ice-cold oxygenated Krebs' solution and transferred into a four-channel microvolume (100 μl) perfusion system kept at 37°C. Four slices were put into each chamber and the preparation was superfused with Krebs' solution at a rate of 0.5 ml/min for 60 min (preperfusion period), and the effluent was discarded. After preperfusion, 19 3 min fractions were collected. Electrical stimulation (20 V; 2 Hz; 1 msec; 360 impulses) was applied during the third (S1) and the thirteenth sample (S2). When the effect of 7,8-(methylenedioxy)-14-α-hydroxyalloberbane HCl (CH-38083) was investigated, the drug was added to the Krebs' solution from the ninth fraction and was kept in the medium until the end of experiment. Then, the slices were removed from the chamber and homogenized in 5 ml of 10% trichloroacetic acid. A 0.5 ml aliquot of the supernatant was added to 2 ml of scintillation mixture (Ultima Gold; Packard, Meridian, CT), and the radioactivity was measured with a Packard 1900 TR liquid scintillation counter. Radioactivity was expressed in terms of disintegration per minute per gram of wet tissue (becquerels per gram). The [3H]NE uptake of slices was defined as the tissue content of radioactivity at the beginning (CB) of the perfusion period. This value was calculated according to the following equation: Σi = 1-19 FRi + CE = CB, where FRi is the released radioactivity in the fraction number i, and CE is the tissue content measured at the end of the experiment. The neuronal release of [3H]NE was measured by the integration of the surplus release over baseline in response to electrical stimulation. When the effect of CH-38083 was studied, the response to electrical stimulation in the presence (FRS2) and absence (FRS1) of the drug was compared (FRS2/FRS1 ratio). Data are the mean ± SEM of four independent experiments and were analyzed using two-tailed t test or ANOVA followed by Tukey-Kramer multiple comparison where appropriate (see Results).
In vivo microdialysis experiments
NET KO or WT mice of both sexes (28-30 gm) were anesthetized with pentobarbital (75 mg/kg, i.p.). The animals were placed in a stereotaxic frame and a homemade concentric dialysis probe (membrane, Spectrapor; permeability 6000 Da molecular weight; outer diameter, 0.2 mm; active length, 3.7 mm) was implanted into the right hippocampus (coordinates with respect to bregma: anteroposterior, -2.7; mediolateral, +3.5; dorsoventral, -4.8). The probe was perfused with modified Ringer's solution (in mm: 147 NaCl, 4 KCl, 1.2 CaCl2, 1.0 MgCl2) at a rate of 1.4 μl/min.
Sample collection was started 60 min after the probe insertion (equilibrium period), and the 15 min samples were analyzed immediately by the HPLC system. After the stabilization of NE release (typically after the collection of six to seven basal samples), animals received an intraperitoneal injection of CH-38083 (10 mg/kg), and the effect was studied in the next 60 min. In some experiments, tetrodotoxin (TTX, 1 μm) was added to the Ringer's solution after the stabilization of baseline and was perfused for 90 min to verify the neuronal origin of NE. To maintain anesthesia, supplemental pentobarbital (15-20 mg/kg) was applied 2 hr after the first injection. Body temperature was maintained at 37°C using a heating blanket. After the experiments, mice were killed, and the placement of the probe was verified by stereomicroscopic examination. In case of incorrect location, data were excluded from the analysis.
The NE content of samples was determined by an HPLC-electrochemical detector system consisting of a Shimadzu LC-10ADVP pump (Shimadzu, Kyoto, Japan), an electrically actuated injection valve (Valco International, Schenkon, Switzerland), and a Coulochem II electrochemical detector (ESA, Chelmsford, MA). The working potentials were set at -300 and +275 mV for the first and second cell, respectively. Separation was achieved on a reversed-phase column (Supelco LC-18-DB; Sigma, St. Louis, MO) with a mobile phase consisting of 0.15 m sodium dihydrogen phosphate, 1.6 mm sodium octanesulphonate, 1.0 mm EDTA, and 12% methanol, pH 3.7. Chromatograms were collected and analyzed by the MAXIMA 820 chromatography software. The detection limit of the assay was 0.2-0.3 pg/sample (on column).
Statistical analysis was performed on raw data (expressed as picograms per 20 μl sample; not corrected for probe recovery) or in some cases on normalized data, in which the average concentration of three samples before the injection of CH-38083 was taken as 100%, and all of the values were expressed relative to this control. Statistical analysis was performed using two-way ANOVA with repeated measures followed by Dunnett's or Tukey-Kramer multiple comparison test. When the area under the curve was compared (NET KO vs control), the response to CH-38083 was calculated as a surplus over the baseline and was analyzed by unpaired two-tailed t test. The level of significance was set to p < 0.05.
Materials
Citalopram HBr was a gift from Lundbeck (Copenhagen, Denmark) (GBR 12909 and nisoxetine HCl were obtained from Sigma). l-7,8-[3H]NE (specific activity, 30-50 Ci/mmol) and N-methyl-[3H] nisoxetine (specific activity, 84 Ci/mmol) were purchased from Amersham Biosciences (Little Chalfont, Buckinghamshire, UK). TTX was obtained from Alomone Labs (Jerusalem, Israel). CH-38083 was received from Chinoin Pharmaceutical (Budapest, Hungary). All of the other compounds were of analytical grade.
Results
Validation of the genetic deletion of NET: [3H]nisoxetine binding and PCR experiments
[3H]Nisoxetine binding
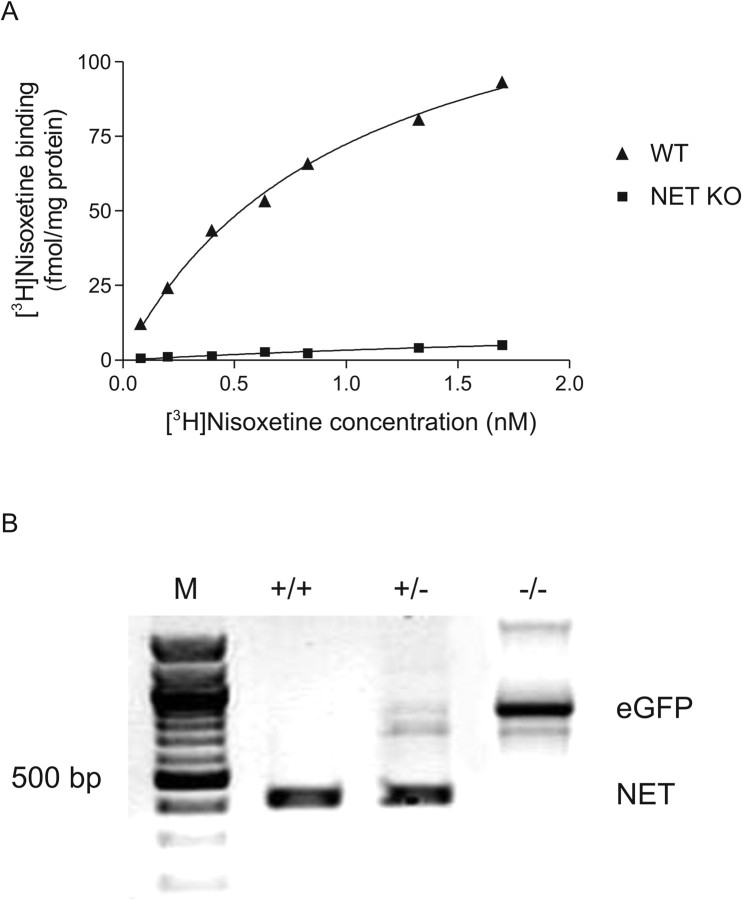
In our experiments, the functional properties of the cortical and hippocampal noradrenergic system were compared in NET KO and WT littermate mice. To control and verify the absence of NE transporters, the receptor binding of the highly selective NET ligand [3H]nisoxetine was investigated. Cortical membranes were obtained from the animals used in the release and uptake experiments (see Materials and Methods). In the WT mice, the specific binding of [3H]nisoxetine in cortical membranes was saturable with increasing concentrations of the ligand (Fig. 1A). The Bmax value was 156.1 ± 6.7 fmol/mg protein with a Kd of 1.01 ± 0.05 nm. In contrast, specific binding of [3H]nisoxetine was close to zero in the NET KO mice, verifying the absence of NET.
Figure 1.
Validation of the genetic deletion of NET. A, Saturation curves of specific binding of [3H]nisoxetine in WT and NET KO mice. The cortical membrane preparation was obtained from animals used in uptake and release experiments. B, PCR analysis of genomic DNA from WT (+/+), heterozygous (+/-), and homozygous (-/-) mice. The NET-specific band was detected as a 470 bp product, whereas the primers specific for the eGFP gene amplified a fragment of ∼700 bp. M, Molecular size markers.
PCR studies
The presence of NET was also tested by PCR analysis. In WT (+/+) mice, a 470 bp product representing the NET gene was detected. In heterozygous (+/-) animals, an additional band of ∼700 bp was also amplified with primers detecting the entire coding region of the eGFP gene that was also inserted into the genome during homologous recombination (Xu et al., 2000). In NET KO (-/-) mice, only the eGFP gene was amplified (Fig. 1B), showing the absence of NET in these animals.
Functional characterization of the noradrenergic system: [3H]norepinephrine uptake and release experiments
[3H]NE uptake
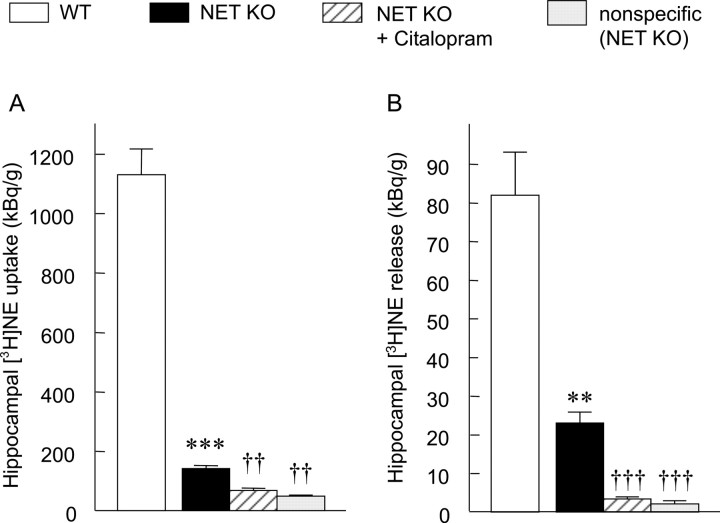
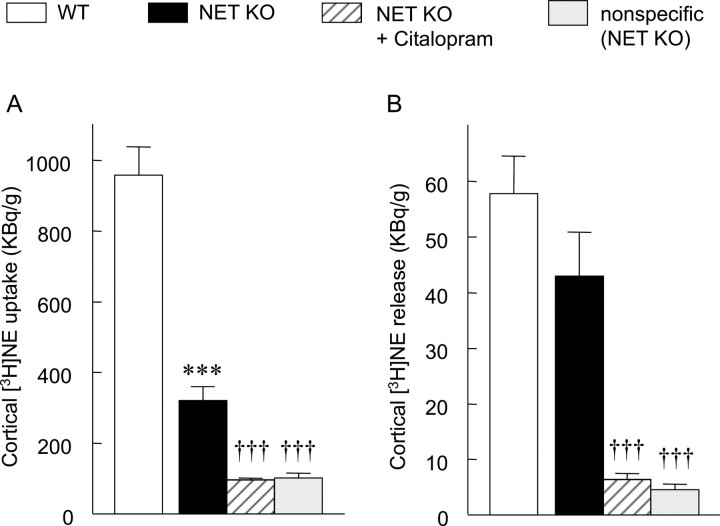
In the hippocampal slices of WT mice, the uptake of [3H]NE was 1130 ± 87 kBq/gm of wet tissue. Although in the NET KO mice the uptake was substantially decreased, it was still 142 ± 10 kBq/gm of wet tissue (12.6% of WT control; p < 0.001; two-tailed t test). The majority of this uptake proved to be neuronal, because in the presence of a mixture of selective monoamine uptake blockers (1 μm each of nisoxetine, citalopram, and GBR 12909), that is, under complete inhibition of neuronal monoamine transporters, the uptake further decreased to 50 ± 3 kBq/gm of wet tissue (here defined as nonspecific uptake; 35.2% of NET KO control; p < 0.01; ANOVA followed by Tukey-Kramer multiple comparison test). The SSRI citalopram (1 μm) alone resulted in a similar decrease (69 ± 8 kBq/gm of wet tissue; 48.5% of NET KO control; p < 0.01; not significantly different from nonspecific uptake), which corresponded to a 79.4% inhibition of the residual neuronal uptake (total - nonspecific) in NET KO mice (Fig. 2A). In the frontal cortex of WT mice, the uptake of [3H]NE was 958 ± 80 kBq/gm of wet tissue. This uptake was reduced to only one-third (321 ± 38 kBq/gm of wet tissue; 33.5% of the WT control; p < 0.01; two-tailed t test) in NET KO mice. Citalopram (1 μm) caused a substantial additional decrease (97 ± 4 kBq/gm of wet tissue; 30.2% of the NET KO control; p < 0.001; ANOVA followed by Tukey-Kramer multiple comparison test) (Fig. 3A). The citalopram-insensitive uptake was not attributable to any monoamine transporter activity, because the combination of citalopram, nisoxetine, and GBR 12909 (1 μm each) resulted in the same extent of inhibition (nonspecific uptake, 102 ± 14 kBq/gm of wet tissue; 31.8% of the NET KO control; p < 0.001; not significantly different from “citalopram alone” group) as citalopram alone (Fig. 3A). Thus, the residual neuronal [3H]NE uptake in NET KO mice completely disappeared (100% inhibition) in the presence of citalopram.
Figure 2.
Functional characterization of the monoaminergic systems in the hippocampus. Both the uptake (A) and release (B) of [3H]NE were measured in hippocampal slice preparation of WT and NET KO mice. When citalopram or a combination of citalopram, GBR 12909, and nisoxetine (nonspecific uptake and release) were used, the drugs were present at 1 μm throughout the experiment from the beginning of the loading period. The values represent the mean ± SEM of four to six independent experiments. WT and NET KO control data were compared by a two-tailed t test (***p < 0.001), whereas data of the NET KO groups were analyzed using ANOVA followed by Tukey-Kramer multiple comparison test (††p < 0.01; †††p < 0.001; compared with NET KO control).
Figure 3.
Functional characterization of the monoaminergic systems in the frontal cortex. Both the uptake (A) and release (B) of [3H]NE were measured in cortical slice preparation of WT and NET KO mice. When citalopram or a combination of citalopram, GBR 12909, and nisoxetine were used, the drugs were present at 1 μm throughout the experiment from the beginning of the loading period. The values represent the mean ± SEM of four to six independent experiments. WT and NET KO control data were compared by a two-tailed t test (***p < 0.001), whereas data of the NET KO groups were analyzed using ANOVA followed by Tukey-Kramer multiple comparison test (†††p < 0.001; compared with NET KO control).
[3H]NE release
In addition to the uptake, the other important factor that determines the extracellular concentration of monoamines is the neuronal release of transmitters. To test this function, the electrical-stimulation-evoked release of [3H]NE was investigated from hippocampal and cortical slices of KO and WT animals. In the hippocampus, the electrical-stimulation-evoked release was 82.05 ± 11.1 kBq/gm of wet tissue in WT animals. This value significantly decreased in the NET KO mice (23.05 ± 2.86 kBq/gm of wet tissue; 28.1% of WT control; p < 0.001; two-tailed t test) and the presence of citalopram (1 μm) caused an almost complete inhibition of this release (3.43 ± 0.52 kBq/gm of wet tissue; 14.9% of the NET KO control; p < 0.001; ANOVA followed by Tukey-Kramer multiple comparison test) (Fig. 2B). A very small electrical-stimulation-evoked release could be observed even in the presence of nisoxetine, citalopram, and GBR 12909 (2.19 ± 0.73 kBq/gm of wet tissue; 9.5% of NET KO control; p < 0.001; “nonspecific” release, not significantly different from the citalopram-alone group). Because in this case the neuronal uptake was fully inhibited, this residual release was regarded as a consequence of nonspecific accumulation of [3H]NE (e.g., passive diffusion through the membrane). The specific release (total - nonspecific) in the NET KO group almost completely disappeared in the presence of citalopram (94.1% decrease compared with the NET KO control). In the frontal cortex, the electrical-stimulation-evoked release was 57.82 ± 6.76 kBq/gm of wet tissue in WT mice. Interestingly, the neuronal [3H]NE release was not reduced significantly in the NET KO mice (43.02 ± 7.87 kBq/gm of wet tissue; 74.4% of WT control; two-tailed t test); however, in the presence of citalopram (1 μm), the decrease was dramatic (6.36 ± 1.09 kBq/gm of wet tissue; 14.8% of NET KO control; p < 0.001; ANOVA followed by Tukey-Kramer multiple comparison test). The nonspecific release in the presence of uptake inhibitor mixture was 4.57 ± 0.97 kBq/gm of wet tissue (10.6% of NET KO control; p < 0.001) in NET KO animals. The specific release, similarly to the hippocampus, almost completely disappeared (95.3% decrease, compared with NET KO control) after inhibition of 5-HT transporters by citalopram (Fig. 3B).
Study of the presynaptic modulation of norepinephrine release in vitro and in vivo: stimulation-evoked [3H]norepinephrine release and microdialysis experiments
The release of NE from noradrenergic varicosities is subject to presynaptic modulation by α2-adrenoceptors. This regulatory mechanism was investigated by using the selective α2-adrenoceptor antagonist CH-38083 (Vizi et al., 1986).
Electrical stimulation-evoked release of [3H]NE
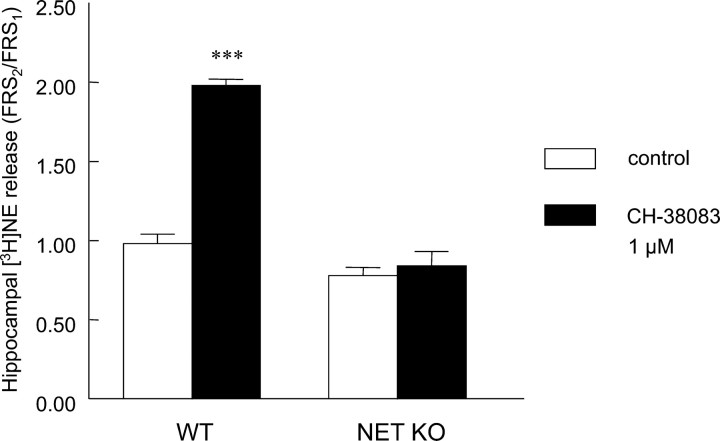
In control experiments, the FRS2/FRS1 ratio was close to 1 (0.98 ± 0.06; n = 4). In the NET KO group, this value was lower (0.78 ± 0.05; n = 4), but the difference was not significant. The α2-adrenoceptor antagonist CH-38083 (1 μm; applied before the second stimulation) significantly increased (p < 0.01, compared with WT control by ANOVA followed by Tukey-Kramer multiple comparison test) the release of NE in WT animals (1.98 ± 0.04; n = 4) but had no effect on the stimulation-evoked NE efflux in the NET KO mice (0.84 ± 0.09; n = 4) (Fig. 4).
Figure 4.
Presynaptic modulation of [3H]NE release from mouse hippocampal slices. The response to electrical stimulations in the presence (FRS2) and absence (FRS1) of the α2-adrenoceptor antagonist CH-38083 (1 μm) was compared (FRS2/FRS1 ratio) in WT and NET KO animals. Data are the mean ± SEM of four independent experiments. ANOVA followed by Tukey-Kramer multiple comparison test was used for statistical comparison (**p < 0.01; compared with WT control).
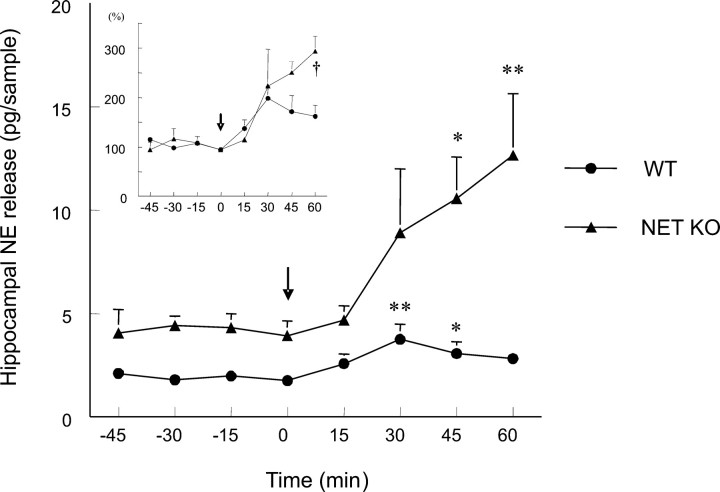
In vivo microdialysis experiments
The basal release of NE was significantly higher (p < 0.01; unpaired two-tailed t test) in the NET KO animals (4.13 ± 0.65 pg/20 μl sample; n = 6) than in the WT group (1.84 ± 0.20 pg/20 μl sample; n = 4). The basal release proved to be neuronal, because (in separate experiments) infusion of TTX (1 μm) for 90 min reduced the NE efflux by 65 and 85% in WT and NET KO mice, respectively (data not shown). The α2-adrenoceptor antagonist CH-38083 (10 mg/kg, i.p.) increased significantly the NE release in both groups (Fig. 5); however, the effect was stronger in the NET KO mice. This difference was not a simple consequence of the higher basal concentration, because the analysis of normalized data (Fig. 5, inset) showed that the surplus release over the baseline doubled (area under curve, 481.44 ± 81.66 vs 258.44 ± 53.93, in arbitrary units; p < 0.05; unpaired two-tailed t test), and the effect at 60 min was tripled (62% increase vs 193% increase; p < 0.05; two-way ANOVA with repeated measures followed by Tukey test) in the NET-deficient animals.
Figure 5.
Presynaptic modulation of endogenous NE release in the hippocampus of anesthetized mice. The microdialysis probes were implanted into the ventral hippocampus. After the stabilization of baseline, the animals received the α2-adrenoceptor antagonist CH-38083 (10 mg/kg, i.p.) at the time point 0, as indicated by the arrow, and then four 15 min samples were collected. Data are the mean ± SEM of four to six independent experiments and represent the concentration of NE (pg/20 μl sample) or the normalized values (percentage of baseline, inset). Two-way ANOVA with repeated measures followed by Dunnett's test (*p < 0.05; **p < 0.01; compared with corresponding baseline) or Tukey test (†p < 0.05; NET KO vs corresponding WT time points) was used for statistical comparison.
Discussion
In vitro release and uptake of [3H]norepinephrine
Our main goal was to investigate the changes in the function of the noradrenergic system induced by the genetic deletion of NET, and to explore the possible interaction between the noradrenergic and other monoaminergic systems. We studied, therefore, the uptake and electrical-stimulation-evoked release of [3H]NE in the hippocampus, which is innervated primarily by serotonergic and noradrenergic neurons (Mongeau et al., 1997; Vizi and Kiss, 1998) and in the frontal cortex, in which dopaminergic innervation is also present (Descarries et al., 1987; Doucet et al., 1988). Our most important finding is that, in the brain slices of NET-deficient mice, a variable portion of [3H]NE uptake persisted, and as it was evidenced by release experiments, a substantial part of [3H]NE was taken up by neurons. The use of the highly selective 5-HT uptake inhibitor citalopram provided convincing evidence that this uptake was mediated via SERT. The selectivity of citalopram is the greatest among SSRIs; the Ki values for 5-HT, NE, and DA are 14 nm, 32 μm, and 41 μm, respectively (Hyttel, 1977); therefore, at the concentration (1 μm) used in our experiments, only the SERT was effectively inhibited. The hippocampus is densely innervated by noradrenergic and serotonergic varicosities but contains only sparse dopaminergic innervation; the ratio is 30:25:1 for 5-HT/NE/DA (Feuerstein et al., 1986). The average densities of serotonergic (Oleskevich and Descarries, 1990) and noradrenergic (Oleskevich et al., 1989) varicosities are 2.7 and 2.1 million/mm3, respectively. Because of the genetic deletion of NET, only SERT is present in the hippocampus, which provides additional support for our conclusion that [3H]NE was taken up by serotonergic neurons. In the frontal cortex, there is also a significant dopaminergic innervation; however, the overall density of dopaminergic varicosities is only 0.25 million/mm3 (Doucet et al., 1988), whereas those of the noradrenergic and serotonergic varicosities are 1.2 and 5.8 million/mm3, respectively (Audet et al., 1988, 1989). In addition, immunocytochemical studies show that the density of transporters and monoaminergic varicosities is not necessarily proportional in a given region (Ciliax et al., 1995). In the prefrontal cortex, DAT immunoreactivity is observed only on the intervaricose segments but not on the varicosities, which suggests that the number of DAT might be very low in this region (Sesack et al., 1998). A recent observation suggests that DAT plays a minimal role in the clearance of DA in the frontal cortex, because the selective DA uptake inhibitor GBR 12909 fails to block the uptake of DA (Moron et al., 2002). Because the affinity of DAT for NE is much lower than for DA, elimination of [3H]NE by DAT in the cortex is not likely. Our data are in line with this finding, because citalopram, at a SERT-selective concentration, fully blocked the uptake of [3H]NE in the frontal cortex, and the combination of GBR 12909, nisoxetine, and citalopram resulted in the same inhibition as citalopram alone, suggesting that only SERT was responsible for the uptake of [3H]NE, and DAT was not involved in this process. The role of SERT in [3H]NE uptake is supported also by quantitative differences. In the hippocampus, in which the ratio of serotonergic and noradrenergic innervation (density of varicosities) is close to 1, only 12.6% of the uptake was preserved, whereas in the frontal cortex, in which the serotonergic innervation predominates (the ratio is >4), a higher uptake (33.5%) could be observed. The higher uptake resulted in a proportionally enhanced release, which underlines the functional significance of heterologous uptake of NE by serotonergic terminals and suggests that the firing rate of serotonergic neurons might influence the extracellular concentration of NE.
Presynaptic modulation of norepinephrine release
The hippocampal release of NE is inhibited by presynaptic α2-adrenoceptors located on noradrenergic varicosities (Vizi, 1979; Starke, 1981; Milusheva et al., 1994; Kiss et al., 1995). Although presynaptic α2-adrenoceptors can be found also on serotonergic terminals (Maura et al., 1982; Zhang et al., 1995; de Boer et al., 1996; Scheibner et al., 2001), it has been shown that the two α2-adrenoceptor populations are pharmacologically distinct (Frankhuyzen and Mulder, 1982; Mongeau et al., 1993). This difference might explain that the α2-adrenoceptors regulating 5-HT release are desensitized during chronic antidepressant (desipramine, nisoxetine) treatment (Blier and Bouchard, 1994; Mongeau et al., 1994; Yoshioka et al., 1995), but the autoreceptors on noradrenergic varicosities preserve their activity (Schoffelmeer and Mulder, 1982; Campbell and McKernan, 1986; Mongeau et al., 1997). Our microdialysis data show an elevated extracellular concentration of NE in NET KO mice; thus, the neurochemical conditions resemble a chronic antidepressant treatment, and the α2-adrenoceptors on serotonergic terminals are most likely desensitized. We observed that the electrical-stimulation-evoked [3H]NE release from hippocamapal slices was not modulated by α2-adrenoceptors in the NET KO mice, but the in vivo NE release could be enhanced by the α2-adrenoceptor antagonist CH-38083. This indicates that the source of [3H]NE release is the serotonergic varicosity, which accumulated the labeled transmitter via SERT, whereas in microdialysis experiments, we measure the endogenous NE release directly from the noradrenergic terminals where the efficacy of CH-38083 is even higher in the NET KO mice. This increased efficacy shows that the autoreceptors are not desensitized and the inhibitory tone on NE release is stronger because of the elevated extracellular concentration of NE. Our data on the presynaptic regulation, therefore, also support the assumption that [3H]NE is accumulated and released by serotonergic terminals.
Significance of the heterologous uptake of monoamines
Accumulating evidence indicates the existence of heterologous uptake among DAT, NET, and SERT. Early studies using selective uptake blockers and specific pathway lesions proved that [3H]DA could be taken up by noradrenergic and serotonergic neurons (Descarries et al., 1987). Later, microdialysis studies confirmed that NET can influence the dopaminergic neurotransmission, because NET inhibitors increased the extracellular DA concentration in the prefrontal cortex (Carboni et al., 1990; Yamamoto and Novotney, 1998). In voltammetry experiments, inhibition of NET significantly decreased DA clearance (Mundorf et al., 2001). Consistent with this finding, it has been proposed that clearance of DA by NET plays a role in cocaine self-administration in DAT KO mice (Carboni et al., 2001). The connection between dopaminergic and serotonergic uptake has also been described. It has been shown that dopaminergic terminals take up and release [3H]5-HT in the striatum and serotonergic varicosities take up and release [3H]DA in the hippocampus of rabbit (Feuerstein et al., 1986). A paper reported that cocaine place preference persists in DAT or SERT KO mice, but disappears in combined DAT/SERT KO animals, suggesting that both systems contribute to the rewarding effects of cocaine (Sora et al., 2001). Thus, the NET-DAT and the DAT-SERT interaction is well documented in the literature, but the functional connection between the NET and SERT, especially the fact that SERT may take up NE and serotonergic varicosities can release NE, is a novel finding. Our release experiments show that this heterologous uptake may have functional consequence, because the cortical release of [3H]NE was not decreased significantly in the NET-deficient animals; that is, the serotonergic system could substantially contribute to noradrenergic neurotransmission in NET KO animals. But what is the relevance of this finding in normal animals? An obvious difference is the presence of functional high-affinity NET on noradrenergic varicosities. In addition, the extracellular concentration of NE is much lower than in the NET-deficient animals. These circumstances reduce the chance of SERT being involved in noradrenergic neurotransmission to a similar extent as that observed in NET KO mice. Nevertheless, comprehensive data are not available on the density of NET in different brain regions; therefore, it is possible that certain areas receive noradrenergic and serotonergic projections but the density of NET is low, similarly to those conditions described in the prefrontal cortex in the case of dopaminergic pathways and DAT (Ciliax et al., 1995; Sesack et al., 1998). In such areas, the role of SERT might be important. In addition, the ability of 5-HT terminals to take up and release NE might be revealed under conditions when the function of NET is compromised by an endogenous or exogenous (e.g., drug effect) reason.
Conclusions
The functional interaction between noradrenergic and serotonergic varicosities in the CNS may have a great clinical importance, because these monoaminergic systems are involved in the pathophysiology of depression and their modulation is the major direction of recent antidepressant therapies. Our results provide evidence for the first time that, under certain conditions, serotonergic varicosities take up NE via SERT and might release NE in response to neuronal activity; thus, the serotonergic system might directly contribute to the regulation of extracellular NE concentration in the CNS. Because of this interaction, the SSRIs, despite their selectivity for SERT, might enhance not only the serotonergic but also the noradrenergic neurotransmission, at least in brain areas in which the density of SERT is higher than the density of NET or the function of NET expressed on noradrenergic varicosities is impaired. These findings may provide a better understanding of the functional properties of monoaminergic systems and the mechanism of action of antidepressant drugs.
Footnotes
This work was supported by grants from the Hungarian Research Fund (T 034622, TS 040736, T 046827) and from the Hungarian Medical Research Council (123/2003, 476/2003). We thank Dr. P. Varju for her valuable help in breeding of animals and PCR analysis. J.P.K. is a Janos Bolyai Research Fellow.
Correspondence should be addressed to Dr. E. Sylvester Vizi, Institute of Experimental Medicine, Hungarian Academy of Sciences, P.O. Box 67, H-1450 Budapest, Hungary. E-mail: esvizi@koki.hu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247888-07$15.00/0
References
- Amara SG, Arriza JL (1993) Neurotransmitter transporters: three distinct gene families. Curr Opin Neurobiol 3: 337-344. [DOI] [PubMed] [Google Scholar]
- Audet MA, Doucet G, Oleskevich S, Descarries L (1988) Quantified regional and laminar distribution of the noradrenaline innervation in the anterior half of the adult rat cerebral cortex. J Comp Neurol 274: 307-318. [DOI] [PubMed] [Google Scholar]
- Audet MA, Descarries L, Doucet G (1989) Quantified regional and laminar distribution of the serotonin innervation in the anterior half of adult rat cerebral cortex. J Chem Neuroanat 2: 29-44. [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau Jr RT, Caron MG, Peek MM, Prince HK, Bradley CC (1991) Cloning and expression of a functional serotonin transporter from rat brain. Nature 354: 66-70. [DOI] [PubMed] [Google Scholar]
- Blier P, Bouchard C (1994) Modulation of 5-HT release in the guinea-pig brain following long-term administration of antidepressant drugs. Br J Pharmacol 113: 485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IC, McKernan RM (1986) Clorgyline and desipramine alter the sensitivity of [3H]noradrenaline release to calcium but not to clonidine. Brain Res 372: 253-259. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G (1990) Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55: 1067-1070. [DOI] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G (2001) Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci 21: RC141(1-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Lebrand C, Giros B, Vitalis T, De Maeyer E, Caron MG, Price DJ, Gaspar P, Seif I (1998) Plasma membrane transporters of serotonin, dopamine, and norepinephrine mediate serotonin accumulation in atypical locations in the developing brain of monoamine oxidase A knock-outs. J Neurosci 18: 6914-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI (1995) The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci 15: 1714-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer TH, Nefkens F, van Helvoirt A, van Delft AM (1996) Differences in modulation of noradrenergic and serotonergic transmission by the alpha 2-adrenoceptor antagonists, mirtazapine, mianserin and idazoxan. J Pharmacol Exp Ther 277: 852-860. [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B (1987) Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience 21: 807-824. [DOI] [PubMed] [Google Scholar]
- Doucet G, Descarries L, Audet MA, Garcia S, Berger B (1988) Radioautographic method for quantifying regional monoamine innervations in the rat brain. Application to the cerebral cortex. Brain Res 441: 233-259. [DOI] [PubMed] [Google Scholar]
- Feuerstein TJ, Hertting G, Lupp A, Neufang B (1986) False labelling of dopaminergic terminals in the rabbit caudate nucleus: uptake and release of [3H]-5-hydroxytryptamine. Br J Pharmacol 88: 677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankhuyzen AL, Mulder AH (1982) Pharmacological characterization of presynaptic alpha-adrenoceptors modulating [3H]noradrenaline and [3H]5-hydroxytryptamine release from slices of the hippocampus of the rat. Eur J Pharmacol 81: 97-106. [DOI] [PubMed] [Google Scholar]
- Giros B, el Mestikawy S, Bertrand L, Caron MG (1991) Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett 295: 149-154. [DOI] [PubMed] [Google Scholar]
- Giros B, el Mestikawy S, Godinot N, Zheng K, Han H, Yang-Feng T, Caron MG (1992) Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol 42: 383-390. [PubMed] [Google Scholar]
- Hindmarch I (2001) Expanding the horizons of depression: beyond the monoamine hypothesis. Hum Psychopharmacol 16: 203-218. [DOI] [PubMed] [Google Scholar]
- Hyttel J (1977) Neurochemical characterization of a new potent and selective serotonin uptake inhibitor: Lu 10-171. Psychopharmacology (Berl) 51: 225-233. [DOI] [PubMed] [Google Scholar]
- Iversen L (2000) Neurotransmitter transporters: fruitful targets for CNS drug discovery. Mol Psychiatry 5: 357-362. [DOI] [PubMed] [Google Scholar]
- Kiss JP, Zsilla G, Mike A, Zelles T, Toth E, Lajtha A, Vizi ES (1995) Subtype-specificity of the presynaptic alpha 2-adrenoceptors modulating hippocampal norepinephrine release in rat. Brain Res 674: 238-244. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265-275. [PubMed] [Google Scholar]
- Maura G, Gemignani A, Raiteri M (1982) Noradrenaline inhibits central serotonin release through alpha 2-adrenoceptors located on serotonergic nerve terminals. Naunyn Schmiedebergs Arch Pharmacol 320: 272-274. [DOI] [PubMed] [Google Scholar]
- Milusheva E, Baranyi M, Zelles T, Mike A, Vizi ES (1994) Release of acetylcholine and noradrenaline from the cholinergic and adrenergic afferents in rat hippocampal CA1, CA3 and dentate gyrus regions. Eur J Neurosci 6: 187-192. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Blier P, de Montigny C (1993) In vivo electrophysiological evidence for tonic activation by endogenous noradrenaline of alpha 2-adrenoceptors on 5-hydroxytryptamine terminals in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol 347: 266-272. [DOI] [PubMed] [Google Scholar]
- Mongeau R, de Montigny C, Blier P (1994) Electrophysiologic evidence for desensitization of alpha 2-adrenoceptors on serotonin terminals following long-term treatment with drugs increasing norepinephrine synaptic concentration. Neuropsychopharmacology 10: 41-51. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Blier P, de Montigny C (1997) The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev 23: 145-195. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM (2001) Catecholamine release and uptake in the mouse prefrontal cortex. J Neurochem 79: 130-142. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L (1990) Quantified distribution of the serotonin innervation in adult rat hippocampus. Neuroscience 34: 19-33. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Descarries L, Lacaille JC (1989) Quantified distribution of the noradrenaline innervation in the hippocampus of adult rat. J Neurosci 9: 3803-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG (1991) Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 350: 350-354. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD (1993) Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA 90: 2542-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Fumagalli F, Gainetdinov RR, Jones SR, Ator R, Giros B, Miller GW, Caron MG (1998) Cocaine self-administration in dopamine-transporter knockout mice. Nat Neurosci 1: 132-137. [DOI] [PubMed] [Google Scholar]
- Scheibner J, Trendelenburg AU, Hein L, Starke K (2001) Alpha 2-adrenoceptors modulating neuronal serotonin release: a study in alpha 2-adrenoceptor subtype-deficient mice. Br J Pharmacol 132: 925-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Mulder AH (1982) 3H-Noradrenaline and 3H-5-hydroxytryptamine release from rat brain slices and its presynaptic alpha-adrenergic modulation after long-term desipramine pretreatment. Naunyn Schmiedebergs Arch Pharmacol 318: 173-180. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18: 2697-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, Wichems C, Lesch KP, Murphy DL, Uhl GR (2001) Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA 98: 5300-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K (1981) Presynaptic receptors. Annu Rev Pharmacol Toxicol 21: 7-30. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I (2002) Cocaine, reward, movement and monoamine transporters. Mol Psychiatry 7: 21-26. [DOI] [PubMed] [Google Scholar]
- Vizi ES (1979) Presynaptic modulation of neurochemical transmission. Prog Neurobiol 12: 181-290. [DOI] [PubMed] [Google Scholar]
- Vizi ES (2000) Role of high-affinity receptors and membrane transporters in nonsynaptic communication and drug action in the central nervous system. Pharmacol Rev 52: 63-89. [PubMed] [Google Scholar]
- Vizi ES, Kiss JP (1998) Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus 8: 566-607. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Harsing Jr LG, Gaal J, Kapocsi J, Bernath S, Somogyi GT (1986) CH-38083, a selective, potent antagonist of alpha 2-adrenoceptors. J Pharmacol Exp Ther 238: 701-706. [PubMed] [Google Scholar]
- Wang YM, Xu F, Gainetdinov RR, Caron MG (1999) Genetic approaches to studying norepinephrine function: knockout of the mouse norepinephrine transporter gene. Biol Psychiatry 46: 1124-1130. [DOI] [PubMed] [Google Scholar]
- Wong ML, Licinio J (2001) Research and treatment approaches to depression. Nat Rev Neurosci 2: 343-351. [DOI] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG (2000) Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci 3: 465-471. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Novotney S (1998) Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem 71: 274-280. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, Matsumoto M, Numazawa R, Togashi H, Smith CB, Saito H (1995) Changes in the regulation of 5-hydroxytryptamine release by alpha 2-adrenoceptors in the rat hippocampus after long-term desipramine treatment. Eur J Pharmacol 294: 565-570. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kindel GH, Wulfert E, Hanin I (1995) Effects of immobilization stress on hippocampal monoamine release: modification by mivazerol, a new alpha 2-adrenoceptor agonist. Neuropharmacology 34: 1661-1672. [DOI] [PubMed] [Google Scholar]