Abstract
In the mature cochlea, inner hair cells (IHCs) transduce acoustic signals into receptor potentials, communicating to the brain by synaptic contacts with afferent fibers. Before the onset of hearing, a transient efferent innervation is found on IHCs, mediated by a nicotinic cholinergic receptor that may contain both α9 and α10 subunits. Calcium influx through that receptor activates calcium-dependent (SK2-containing) potassium channels. This inhibitory synapse is thought to disappear after the onset of hearing [after postnatal day 12 (P12)]. We documented this developmental transition using whole-cell recordings from IHCs in apical turns of the rat organ of Corti. Acetylcholine elicited ionic currents in 88-100% of IHCs between P3 and P14, but in only 1 of 11 IHCs at P16-P22. Potassium depolarization of efferent terminals caused IPSCs in 67% of IHCs at P3, in 100% at P7-P9, in 93% at P10-P12, but in only 40% at P13-P14 and in none of the IHCs tested between P16 and P22. Earlier work had shown by in situ hybridization that α9 mRNA is expressed in adult IHCs but thatα10 mRNA disappears after the onset of hearing. In the present study, antibodies toα10 and to the associated calcium-dependent (SK2) potassium channel showed a similar developmental loss. The correlated expression of these gene products with functional innervation suggests that Alpha10 and SK2, but not Alpha9, are regulated by synaptic activity. Furthermore, this developmental knock-out of α10, but not α9, supports the hypothesis that functional nicotinic acetylcholine receptors in hair cells are heteromers containing both these subunits.
Keywords: IHC, mammalian cochlea, cholinergic, efferent innervation, α9α10 nAChR, transient synapse, Ca2+-activated K+ channel, neonatal development
Introduction
During postnatal development of the mammalian cochlea, afferent and efferent nerve fibers only gradually take up their final positions on inner hair cells (IHCs) and outer hair cells (OHCs) (Pujol et al., 1998). In particular, IHCs initially are contacted by olivocochlear efferent fibers that “wait” under the IHC region before targeting OHCs (Simmons et al., 1996b). This transition occurs around the onset of hearing, and adult IHCs are innervated mainly by afferent fibers, having few if any remaining efferent contacts (Liberman et al., 1990). In contrast, adult OHCs are the principal targets of cholinergic olivocochlear efferents (Guinan, 1996).
Recordings from the organ of Corti demonstrated cholinergic postsynaptic currents in IHCs, showing that efferent contacts on neonatal IHCs are indeed functional (Glowatzki and Fuchs, 2000). As reported for chick short hair cells (Fuchs and Murrow, 1992) and mammalian OHCs (Blanchet et al., 1996; Dulon and Lenoir, 1996; Evans, 1996; Fuchs, 1996; Oliver et al., 2000), efferent inhibition of IHCs is mediated by a nicotinic acetylcholine receptor (nAChR) through which calcium enters and activates calcium-dependent SK potassium channels (Glowatzki and Fuchs, 2000).
Two nicotinic subunits, α9 and α10, are expressed in cochlear hair cells (Elgoyhen et al., 1994, 2001; Park et al., 1997; Luo et al., 1998; Morley et al., 1998; Morley and Simmons, 2002). Although α10 fails to make functional receptors on its own in Xenopus oocytes, α9α10 receptors generate 100-fold larger currents than those of homomeric α9. Also, whereas homomeric α9 and heteromeric α9α10 nAChRs have similar pharmacological profiles (Elgoyhen et al., 1994, 2001; Erostegui et al., 1994; Chen et al., 1996; Verbitsky et al., 2000), some biophysical characteristics of the α9α10 heteromer (Elgoyhen et al., 2001; Weisstaub et al., 2002) resemble more closely those of the native hair cell nAChRs (Blanchet et al., 1996; Dulon and Lenoir, 1996; Evans, 1996; McNiven et al., 1996). Thus, the functional hair cell nAChR may include both α9 and α10 nicotinic subunits.
Both α9 and α10 mRNA are expressed by OHCs in the adult cochlea. However, IHCs cease to express α10 mRNA at about the onset of hearing (Elgoyhen et al., 2001; Morley and Simmons, 2002) while continuing to express α9 mRNA into adulthood (Elgoyhen et al., 1994; Luo et al., 1998; Simmons and Morley, 1998; Morley and Simmons, 2002). We have charted the cholinergic sensitivity of cochlear IHCs throughout the first three postnatal weeks to evaluate the requirement for α10 in functional nAChRs. We show that IHCs become increasingly responsive to acetylcholine (ACh) from postnatal day 3 (P3) to P10. However, by P16 cholinergic sensitivity is severely reduced, and efferent synaptic activity is undetectable. Thus, native hair cell nAChRs, like those expressed in Xenopus oocytes, require the α10 subunit for optimal function.
Materials and Methods
Animal procedures. Sprague Dawley (Charles River, Wilmington, MA) rats at different postnatal ages (day of birth was considered P0) were anesthetized with an intraperitoneal injection of sodium pentobarbital. Animals were decapitated after assessing that a deep anesthetic state had been obtained by observing a lack of tail flick after tail pinch and lack of eye blink response after a corneal touch. All experimental protocols were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications number 80-23), revised in 1978.
Electrophysiological experiments. Apical turns of the organ of Corti were excised from rats at P3 to P22 and used within 3 hr. Cochlear preparations were mounted under an Axioskope microscope (Zeiss, Oberkochen, Germany) and viewed with differential interference contrast (DIC) using a 63× water immersion objective and a camera with contrast enhancement (C2400-07; Hamamatsu, Tokyo, Japan). Methods to record from IHCs were as described before (Glowatzki and Fuchs, 2000). Briefly, IHCs were identified visually, by the size of their capacitance (7-12 pF) and by their characteristic voltage-dependent Na+ and K+ currents, including at older ages a fast-activating K+-conductance (Kros et al., 1998). Some cells were removed to access IHCs but mostly the pipette moved through the tissue under positive pressure. The extracellular solution was as follows (in mm): 155 NaCl, 5.8 KCl, 1.3 CaCl2, 0.9 MgCl2, 0.7 NaH2PO4, 5.6 d-glucose, and 10 HEPES buffer, pH 7.4. The pipette solution was (in mm): 150 KCl, 3.5 MgCl2, 0.1 CaCl2, 6 EGTA, 5 HEPES buffer, 2.5 Na2ATP, pH 7.2 (KCl-EGTA saline); for some experiments KCl and EGTA in the pipette solution were replaced by CsCl and bis(2-aminophenoxy)ethane-N, N,N′,N′-tetra-acetic acid (BAPTA) (CsCl-BAPTA saline). Glass pipettes, 1.2 mm inner diameter, had resistances of 7-10 MΩ. Solutions containing ACh or elevated potassium (40 mm K+) were applied by a gravity-fed multichannel glass pipette (∼150 μm tip diameter) positioned ∼300 μm from the recorded IHC. Spontaneous synaptic currents were recorded immediately after rupturing into the cell, in the extracellular saline containing 1.3 mm Ca2+ and 0.9 mm Mg2+. All working solutions containing either ACh or elevated K+ or both, were made up in a saline containing low Ca2+ (0.5 mm) and no Mg2+ to optimize the experimental conditions for measuring currents flowing through the α9α10 receptors (Weisstaub et al., 2002).
Currents in IHCs were recorded in the whole-cell patch clamp mode (Axopatch 200B amplifier), low-pass filtered at 2-10 kHz, and digitized at 5-20 kHz with a Digidata 1200 board (Axon Instruments, Union City, CA). Data were stored and analyzed with PClamp8 software (Axon Instruments). Recordings were made at room temperature (22-25°C). Reversal potentials were measured by applying ACh (100 μm) at different potentials (-100 to +60 mV, 10 mV steps) replacing KCl by CsCl in the patch pipette, and using either a CsCl-EGTA or a CsCl-BAPTA saline to obtain the combined (nAChR + SK) or the isolated (nAChR) I-V curve, respectively. For I-V curves, voltages were corrected for liquid junction potential (-4 mV). Voltages were not corrected for the voltage drop across the uncompensated series resistance. IPSCs were analyzed with Minianalysis (Synaptosoft, Jaejin Software, Leonia, NJ). IPSCs were identified using a search routine for event detection and confirmed by eye. τdecay values were fit with a monoexponential. For further analysis IgorPro software (Wavemetrics, Lake Oswego, OR) was used. Statistical analyses were performed by ANOVA followed by a Tukey′s test for multiple comparisons (when three populations of data where compared) or by a two-tailed Student′s t test (when two populations of data were compared). In both cases p < 0.05 was considered significant. Chemicals were bought from Sigma (St. Louis, MO).
Immunocytochemistry. Rat cochleas (P3-P15) were isolated, dissected, and fixed as described (Knipper et al., 2000). Cochleas were decalcified after fixation for 1-5 min in Rapid Bone Decalcifier (Eurobio catalog #904687; Fisher Scientific, Houston, TX). After overnight incubation, cochleas were embedded in O.C.T. compound (Miles Laboratories, Elkhart, IN), frozen at -80°C, and serially sectioned at -25°C (10 μm). Sections were collected on SuperFrost Plus microscope slides and stored at -20°C. Cochlear sections were thawed, permeabilized, with 0.1% Triton X-100 in PBS for 3 min at room temperature, and preblocked with 1% bovine serum albumin in PBS. Immunosera against the potassium channel SK2 (P0483; polyclonal anti-rabbit; Sigma) were used in a dilution of 1:50. Anti-α10 nAChR, raised in rabbits, was applied in a dilution of 1:50. For monitoring innervation to IHCs, anti-NF200 (PH187; polyclonal anti-sheep; The Binding Site, Heidelberg, Germany) or anti-synaptophysin (PH510; polyclonal anti-sheep; The Binding Site) were used. Antibodies were incubated overnight at 4°C. Goat anti-rabbit Cy3-conjugated antibody (catalog #305-165-008; 1:1500; Jackson ImmunoResearch, West Grove, PA) was used as a secondary IgG antibody for revealing immunoreactivity of α10 nAChR or SK2 channel and Alexa Fluor 488-conjugated antibodies (catalog #A-11015; 1:2000; Molecular Probes, Eugene, OR) for detecting immunoreactivity of NF200 and synaptophysin. Sections were embedded with Vectashield mounting medium with DAPI (catalog #H-1200; Vector Laboratories, Burlingame, CA) and photographed using an Olympus AX70 microscope equipped with epifluorescence illumination.
Results
Variation of ACh-evoked currents and K+-evoked synaptic currents during development
To chart changes in sensitivity to ACh during development, IHCs from the following ages were tested: P3, P7-P9, P10-P12, P13-P14, and P16-P22. Ach-activated currents are mediated by a combined flux of cations through the α9α10-containing nAChR and a K+ flux through calcium-dependent SK potassium channels activated by Ca2+ entering through the nAChR channel (Fuchs and Murrow, 1992; Evans, 1996; Dulon et al., 1998, Blanchet et al., 1996; Glowatzki and Fuchs, 2000; Oliver et al., 2000). Therefore, at voltages negative to EK (-82 mV), the ACh-activated inward current flows through both the nAChR channel and the SK channel (Fig. 1, nAChR + SK).
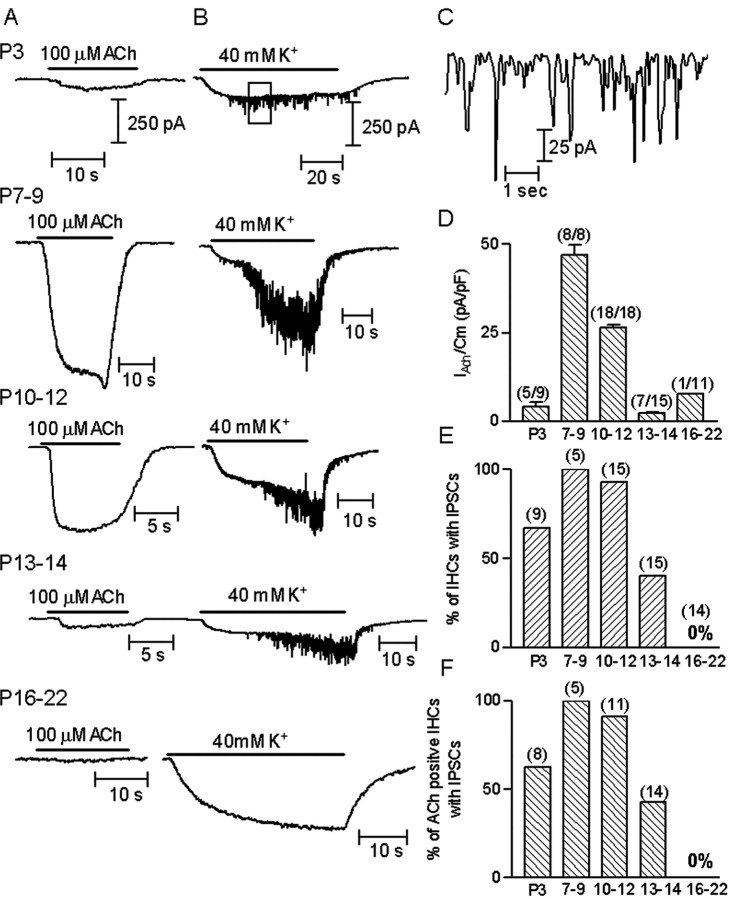
Figure 1.
ACh-evoked currents and K+-evoked IPSCs in IHCs throughout development. A, Representative records of currents (nAChR + SK) evoked by 100 μm ACh in IHCs voltage-clamped at -90 mV at the different postnatal ages. B, Representative records of currents evoked by 40 mm external K+ (at -90 mV). Note that at P16-P22, 40 mm K+ produced the expected change in the holding current but no IPSCs. Responses shown in A and B at each age were taken from the same IHC. C, Synaptic currents enclosed by box shown in B (top record), plotted at a slower time scale. D, Bar diagram illustrating the mean amplitude ± SEM of the currents evoked by 100 μm ACh, normalized to the mean capacitance (Cm) at each age group. Numbers between brackets are the number of cells in which 100 μm ACh elicited a current per number of cells tested. The mean amplitude includes only the ACh-positive cells. Amplitudes at P3 and at P13-P14 were significantly different from those at P7-P9 (p < 0.0019). Mean capacitance values used to normalize amplitudes were (in picofarads, mean ± SEM): P3, 10.4 ± 0.7; P7-P9, 9.5 ± 0.4; P10-P12, 10.0 ± 0.4; P13-P14, 12.6 ± 0.6 and P16-P22, 13.0 ± 1.0. E, Bar diagram representing the number of IHCs that had IPSCs after superfusing the preparation with saline containing 40 mm K+. Numbers between brackets are the number of cells studied at each age group. F, Bar diagram illustrating the fraction of Ach-sensitive IHCs that also had K+-evoked IPSCs at the different postnatal ages studied. Numbers between brackets are the number of cells positive to ACh that were superfused with high potassium to evaluate synaptic activity. In all cases the intracellular solution contained KCl and EGTA.
From P3 to P14, IHCs were responsive to 100 μm ACh (Fig. 1A). At P16 this treatment was ineffective, with the exception of a small response in one IHC of 11 cells tested. As shown in the bar diagram of Figure 1D and represented in the traces in A, response amplitudes (normalized by whole-cell capacitance to account for any systematic changes in cell size) were small at P3 (4.2 ± 1.2 pA/pF; n = 5), peaked at P7-P9 (47 ± 7.7 pA/pF; n = 8), started to decrease again at P10-P12 (26.3 ± 4.2 pA/pF; n = 18), and had strongly diminished by P13-P14 (2.3 ± 0.4 pA/pF; n = 7). The amplitude of the current observed in the only responsive IHC from the P16-P22 age group was 7.6 pA/pF.
Spontaneous IPSCs caused by efferent synaptic transmission were occasionally observed in IHCs. These IPSCs were identical to the efferent synaptic currents characterized in earlier work (Glowatzki and Fuchs, 2000) and, like the response to exogenous ACh, included current flowing through both the nAChR and calcium-dependent SK channels. Because spontaneous IPSCs occurred rarely, transmitter release from efferent endings was accelerated by depolarization using 40 mm external potassium saline. When the preparation was superfused with 40 mm potassium saline, a steady inward current was evoked in IHCs (Fig. 1B). This current at -90 mV probably flows through a resting potassium conductance as EK changes from -82 to -33 mV. In addition, IPSCs were observed because of the release of ACh from depolarized efferent endings (Fig. 1B, box in top panel is shown at a faster time scale in C).
As illustrated in Figure 1, B and E, the fraction of cells, in which synaptic activity could be elicited with K+ depolarization, also varied with age. At postnatal days 7-9 and 10-12 almost all (100 and 93%, respectively) of the IHCs tested showed synaptic currents. At P3 only 67% of the IHCs had synaptic currents, by P13-P14 the fraction of responsive IHCs was even smaller (40%), and synaptic responses were not found at P16-P22.
To increase the likelihood of activating nonresponsive cells, we raised external K+ to 40 mm to increase driving force at -90 mV. As a result, Ach-evoked currents in responsive cells increased approximately threefold over control (2.87 ± 1.57; n = 9). In addition, we increased ACh from 100 μm to 1 mm (EC50 ∼70 μm in P9-P11 rats) (M. E. Gómez-Casati, A. B. Elgoyhen, and E. Katz, unpublished observations). However, neither manipulation revealed any response to ACh in hair cells at P16-P22 (n = 4; data not shown). Likewise, IHCs at P16-P22 had no IPSCs (Fig. 1B). On the other hand, 1 mm ACh did increase the fraction of responsive cells at P3 from 67 to 89% (n = 4 cells) and at P13-P14 (40-93%; n = 7 cells).
Figure 1F illustrates a correlation between the expression of functional nAChRs and synaptic activity. As shown in the bar diagram, at P3 only ∼50% of ACh-positive cells had IPSCs, suggesting that in many cells functional receptors are present before efferent innervation. From P7 to P12, almost all cells had both ACh-evoked currents and IPSCs. By P13, however, only 40% of the ACh-positive IHCs had IPSCs, suggesting that efferent synapses ceased to operate before all functional nAChRs had disappeared from IHCs.
Expression of the α10 nAChR subunit throughout development
Previous reports have shown that the α9 subunit is expressed in both IHCs and OHCs of the mammalian cochlea from late embryonic stages through adulthood (Elgoyhen et al., 1994, 2001; Park et al., 1997; Luo et al., 1998; Morley et al., 1998; Morley and Simmons, 2002). However, although α10 is expressed in both OHCs and IHCs during late embryonic development, only OHCs, but not IHCs, do express α10 into adulthood (Elgoyhen et al., 2001; Morley and Simmons, 2002). The level of α10 mRNA peaks at P10 and remains high into adulthood in OHCs of the basal turn of the rat cochlea (Morley and Simmons, 2002). In contrast, the level of α10 mRNA expression peaks at approximately P1 in IHCs, and by P15 the signal has returned to background levels (Morley and Simmons, 2002).
Consistent with the expression of the α10 gene, immunocytochemistry experiments with an α10 nAChR antibody of the cochlea showed that at P10, the α10 nAChR protein is expressed in IHCs (Fig. 2A), whereas by P15, these cells have ceased to express this subunit (Fig. 2B). Thus, the loss of α10 subunit expression correlates well with the loss of electrophysiological responses to both exogenously applied ACh and K+-induced synaptic release of neurotransmitter from efferent terminals. α10 immunoreactivity was seen in discrete puncta around the basolateral margin of the IHCs, as well as within the super nuclear cytoplasm.
Figure 2.
Expression of the α10 nAChR subunit before and after the onset of hearing. α10 nAChR (red, closed arrow) in IHCs at P10 (A) and P15 (B) of the middle cochlear turn. The section is counterstained with synaptophysin to demarcate the efferent fibers (syn, green, open arrow). The IHC is delineated with a dotted line. Note the disappearance of the α10 nAChR on P15. Scale bars, 5 μm.
Coupling of the nAChR response to the activation of the SK channel
The inhibitory nature of the olivocochlear synapse in both OHCs and IHCs is caused by the activation of an SK channel after Ca2+ influx through the α9α10-containing nAChR (Fuchs and Murrow, 1992; Blanchet et al., 1996; Evans, 1996; Dulon et al., 1998; Glowatzki and Fuchs, 2000; Oliver et al., 2000). During development of rat OHCs, however, this coupling between the nAChR and the SK channel first appears at approximately P8. Before that age cholinergic responses are composed only of currents through the nAChR (Dulon and Lenoir, 1996). In contrast, neonatal gerbil OHCs have cholinergic responses that always include the activation of an outward K+ current (He and Dallos, 1999). Thus, we designed experiments to detect a putative SK component in postnatal rat IHCs. If sufficient SK gating occurs, then outward potassium current should be evident at membrane potentials positive to EK. Under our control conditions (CsCl-EGTA intracellular saline and 5.8 mm external potassium) the reversal potential (Erev) of the combined nAChR plus SK current was -67.6 ± 4.2 mV (n = 5; P9-P13), therefore, responses should be inward at -90 mV and outward at voltages more positive than -60 mV (see also Glowatzki and Fuchs, 2000). If there is little or no coupling to the SK channel, and the ACh-evoked responses result only from current through the nAChRs, current-voltage relations should reverse close to 0 mV, and currents should be inward at negative membrane potentials (Erev for the isolated nAChR current was -14.5 ± 0.7 mV; n = 2; P9-P11) (see also Glowatzki and Fuchs 2000). As illustrated in Figure 3A, ACh-activated currents were inward at -90 mV, but outward at -50 mV, consistent with the activation of a SK channel at all time points examined (P3, P9, P13; n = 3-6 cells each, only P3 and P13 are illustrated). Thus, for prolonged (10-20 sec) applications of exogenous ACh, SK channel activation can be demonstrated throughout the first two postnatal weeks.
Figure 3.
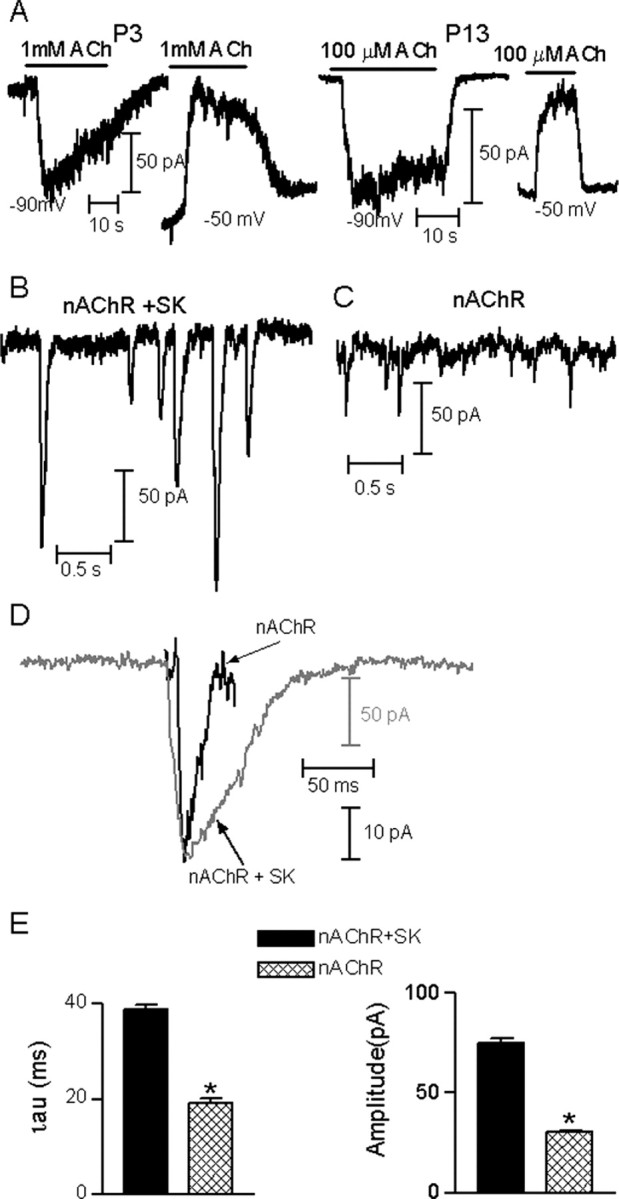
Coupling of the nAChR current to the activation of SK channels. A, Representative recordings of the currents evoked by ACh in IHCs at P3 and P13, voltage-clamped at the holding potentials indicated under each record. In all cases the intracellular solution contained KCl and EGTA. Notice that currents were inward at -90 mV and outward at -50 mV, indicating that SK potassium currents were present. Illustrated records are representative of those found in three to six different IHCs at each age. B, Representative traces of K+-evoked combined nAChR plus SK IPSCs at P9-P11 by 40 mm K+ (EK, -35 mV). C, Representative traces of K+-evoked isolated nAChR synaptic currents at P9-P11 by 40 mm K+ (EK, -35 mV). D, Representative records of one combined (nAChR + SK) IPSC (gray trace) and one isolated (nAChR) postsynaptic current (black trace) superimposed to illustrate the much slower kinetics of the combined nAChR plus SK IPSC. E, bar diagrams showing that combined nAChR plus SK IPSCs have a larger amplitude and slower kinetics than those of the isolated nAChR postsynaptic currents (p < 0.0001). In B, recordings were made with a KCl-EGTA intracellular solution; in C, recordings were made with an intracellular solution in which EGTA was replaced by BAPTA to prevent the activation of SK channels. Recordings in B-D were made at a Vhold of -90 mV.
It is worth asking if the much more rapid synaptic currents also maintain their component conductances. To evaluate this question, we first examined the amplitude and kinetics of K+-evoked synaptic currents in IHCs from P9-P11 rats under two conditions: with or without the SK channel component by using EGTA or BAPTA in the intracellular solution, respectively. Figure 3B (left panel) illustrates a representative trace of K+-evoked synaptic currents, recorded with EGTA in the pipette solution. Under these conditions synaptic currents at -90 mV are composed of inward currents through both the nAChR and the SK channel. On the other hand, synaptic currents recorded with pipette solutions containing BAPTA to prevent the activation of the SK channel (Fig. 3B, right panel) should be solely composed by currents through the nAChR. Consequently, both the amplitude and kinetics of the synaptic currents mediated by nAChRs plus SK channels were significantly larger and slower than those carried by the nAChR current alone (nAChR plus SK, amplitude = -74.8 ± 2.9 pA, τdecay = 40.7 ± 0.9 msec, 213 events, 10 cells; nAChR, amplitude =-34.87 ± 1.2 pA, τdecay = 23.08 ± 1 msec, 256 events, four cells; p < 0.0001) (Fig. 3C,D). These results are consistent with the notion that SK channel gating is slow compared with that of the nAChRs and that SK channel gating determines the time course of IPSCs (Oliver et al., 2000).
Analysis of the synaptic current waveform therefore is a qualitative measure to determine whether SK currents are activated during synaptic release. We evaluated the amplitudes and kinetic parameters of the K+-evoked IPSCs at the different postnatal ages spanning the onset of hearing using EGTA in the pipette solution (Fig. 4A,B). Under these experimental conditions, we found no significant difference in the waveform of K+-evoked IPSCs (nAChR plus SK channels) comparing postnatal days P3 to P11 (P3, amplitude = -81.6 ± 2.9 pA, τdecay = 40.7 ± 0.9 msec, 225 events, 5 cells; P9-P11, -74.8 ± 2.9 pA, τdecay = 40.7 ± 0.9 msec, 213 events, 10 cells). However, at P13-P14, there was a significant, ∼25% decrease in IPSC amplitude and decay time constants compared with younger ages (-61.3 ± 1.65 pA, τdecay = 27.7 ± 0.58 msec, 365 events, six cells, compared with values at P3 and P9-P11; p < 0.001).
Figure 4.
Amplitude and kinetics of IPSCs during development. A, Representative traces of K+-evoked (40 mm K+) combined nAChR plus SK IPSCs throughout development. Insets at the right of each trace are the average of 30-40 individual IPSCs from each record. B, Bar diagrams illustrating that both the amplitude and kinetics of nAChR + SK IPSCs remained constant from P3 to P11 and that at P13-P14, both parameters decreased by ∼25% with respect to previous ages (p < 0.001). P3, 225 events, 5 cells; P9-P11, 213 events, 10 cells; P13-P14, 365 events, 6 cells. Amplitudes are represented in absolute values.
Expression of the SK2 potassium channel throughout development
Expression of SK2 mRNA has been demonstrated by in situ hybridization in OHCs of the rat cochlea, but was not detected in IHCs (Dulon et al., 1998). Given the present electrophysiological evidence for an SK-like current in IHCs, SK2 channel expression in IHCs was re-examined using an antibody selective for the SK2 protein. With this label it was possible to demonstrate SK2 expression in IHCs during postnatal weeks 1-2 (Fig. 5), as well as in adult OHCs (data not shown). The antibody revealed numerous discrete clusters (∼0.1 μm diameter) around the basolateral surface of IHCs. These clusters of immunoreactivity were visible at P3 through P9, were weaker at P12, and disappeared completely by P15. Thus, immunolabeling with an SK2-specific antibody showed that this channel is present in neonatal IHCs but is downregulated after the onset of hearing, corresponding closely with developmental changes in membrane currents.
Figure 5.
Expression of the SK2 channel during postnatal development. SK2 channels (red, closed arrow) in IHCs at P3 (A), P9 (B), P12 (C), and P15 (D) of the middle cochlear turn. The section is counterstained with neurofilament-200 (green, open arrow) and DAPI (nuclear staining, blue). The IHC is delineated with a dotted line. Note the disappearance of SK2 from the basolateral pole of IHCs between P9 and P15. Scale bars, 5 μm.
Discussion
Variation of the cholinergic responses in IHCs during development
Whole-cell recordings of rat IHCs from P3 through P22 showed that efferent olivocochlear-IHC synapses are functional as of P3 and continue transmitting through P13-14. After P14, IHCs did not respond to exogenous or synaptic ACh, suggesting the loss of functional nAChRs. The developmental time course of cholinergic activity in IHCs found here by electrophysiological methods is in good agreement with the maturation of cochlear morphology; immature at birth but adult-like at approximately P16. This time course correlates with developmental changes in efferent innervation to IHCs in rats (Lenoir et al., 1980; Gil-Loyzaga and Pujol, 1988; Knipper et al., 1995), cats (Ginzberg and Morest, 1984; Liberman et al., 1990), hamsters (Simmons et al., 1996a), and mice (Emmerling et al., 1990). Light and electron microscopy studies (Lenoir et al., 1980; Liberman, 1980; Ginzberg and Morest, 1984; Gil-Loyzaga and Pujol, 1988; Emmerling et al., 1990; Knipper et al., 1995; Simmons et al., 1996a) suggest that fibers from the medial olivocochlear system wait under the IHC region before targeting OHCs (Simmons et al., 1996b).
Our study now presents evidence that as early as P3, nAChR and SK channel are already coupled and mediate inhibitory synaptic responses. From P3 through P13-P14, there were significant changes in the amplitude of the ACh-evoked responses and in the fraction of IHCs with functional synaptic contacts, indicative of synaptic remodeling during this period. Both the fraction of responsive cells and their response amplitudes were significantly smaller at P3 and P13-P14 than at intermediate stages (P7-P9 and P10-P12).
Coupling of the nAChR response to the activation of SK channels
The kinetics, reversal potential and pharmacology of spontaneous and K+-evoked IPSCs in IHCs (Glowatzki and Fuchs, 2000) and OHCs (Oliver et al., 2000) are consistent with the activation of both the α9α10-containing nAChR and a SK channel. Indeed, the principal decay rate of these IPSCs is thought to be determined by the closing rate of the SK channels (Oliver et al., 2000). Therefore, examining the kinetics of IPSCs could provide more detailed insights into developmental changes in the individual components, or their coupling. Because the cholinergic response of the hair cell involves SK channel activation as well as gating of nAChRs, it is also possible that changes in the coupling between these channels occur during development. IPSC waveforms were identical until P13, when faster IPSCs might result from the possible loss of SK channel activity. During the development of the mammalian cochlea, the timing of functional coupling between nAChRs and SK channels appears to vary among species. Thus, cholinergic responses of developing gerbil OHCs are coupled to the activation of a Ca+-activated K+ channel from the first age at which ACh-evoked currents are detectable (P6) (He and Dallos, 1999). In contrast, during OHC maturation in the rat, the earliest responses result from nAChR gating only, and coupling to the SK channels appears only in the second postnatal week (Dulon and Lenoir, 1996). In the present work we show that ACh-evoked currents in IHCs always involved both nAChRs and SK channels between P3 and P14, as judged by the reversal potential of the combined current. The possibility remains that cholinergic responses at ages earlier than P3 are not coupled to the activation of a SK channel.
Although the steady-state responses to applied ACh show some contribution from the SK current at all ages studied, synaptic IPSCs provide a hint that diminished cholinergic sensitivity in the second postnatal week might result from reduced coupling to SK channels. At P13-P14, K+-evoked IPSCs were not only smaller but also the decay times were significantly faster than those from earlier ages. These changes could result from a reduced contribution of the more slowly gating SK channels to the combined current because SK channel gating is rate-limiting (Oliver et al., 2000). The loss of SK current could result from less effective calcium signaling and/or from reduced expression of SK channels.
IHC nAChR function is correlated with expression of the α10 subunit during postnatal maturation
IHCs express mRNA for both α9 and α10 nAChR subunits beginning in late embryogenesis (Elgoyhen et al., 1994, 2001; Park et al., 1997; Luo et al., 1998; Morley et al., 1998; Morley and Simmons, 2002). At approximately the second postnatal week the expression of α10, but not that of α9, drops to undetectable levels (Elgoyhen et al., 1994, 2001; Morley and Simmons, 2002). The key role of the α9 subunit for hair cell cholinergic sensitivity has been demonstrated by the generation of α9 knock-out mice (Vetter et al., 1999). The present experiments now demonstrate that the α10 subunit is also a main component of this receptor, because lack of responses of IHCs to ACh correlates with the disappearance of α10 gene expression. The fact that cholinergic function of IHCs correlates with expression of mRNA for α10, but not with that of α9 is intriguing. Homomeric α9 nAChRs are functional in oocytes, but with far lower efficiency than expression of related genes, such as α7 (Bertrand et al., 1992; Séguéla et al., 1993, Elgoyhen et al., 1994) and with a 100-fold lower efficiency compared with the α9α10 heteromer. We did not see any currents in response to ACh in mature IHCs that do express α9 mRNA. The lack of current could be attributable to the α9 protein not being synthesized or incorporated into the hair cell membrane. Alternatively, we may not be able to discriminate currents mediated by α9 homomeric channels in the noise of the IHC whole-cell recording. Another possibility is that α9 assumes an alternative, not purely “ionotropic” function in mature IHCs.
Efferent synaptic activity and functional α9α10-containing nAChR
As discussed above, our results show that by P16 IHCs lack functional nAChRs and this correlates with the downregulation of α10 gene expression. Moreover, light and electron microscopy studies show that after P16, IHCs have very few, if any, axosomatic efferent synapses (Liberman et al., 1990; Simmons, 2002). Is there a causal relationship between α10 receptor expression and efferent innervation? As seen in Figure 1F, in at least some IHCs, functional nAChRs are present both before, and after synaptic activity can be detected. The implication is that α10-containing nAChRs are present in the membrane before efferent synapse formation, mimicking the well established pattern of synapse development in skeletal muscle (Schuetze and Role, 1987; Role and Berg, 1996). Thus, native α9α10-containing nAChRs might also be involved in other functions, such as regulation of gene expression, neuronal pathfinding, and synapse formation and stabilization, as postulated for other nAChRs (Schuetze and Role, 1987; Role and Berg, 1996).
The persistence of functional nAChRs after IPSCs are lost implies that something other than receptor expression regulates the maintenance of this efferent synapse. Efferent innervation to IHCs is lost soon after the onset of hearing at P12. This is a time of marked changes for the IHC because it begins transmitting continuously to the CNS and it is characterized by dramatic modifications in the expression of a variety of ion channels. In particular, voltage-gated calcium and sodium channels are downregulated (Beutner and Moser, 2001; Marcotti et al., 2003b), and a prominent voltage-gated potassium current appears that flows through large-conductance, calcium-activated potassium channels (Kros et al., 1998; Marcotti et al., 2003a). As a result, the IHC ceases to generate calcium action potentials and becomes more linearly responsive to activation by sounds. Such pronounced changes in excitability seem likely candidates to regulate synaptic function of the hair cell. Interestingly, it has been shown that in mice lacking the calcium channel Cav1.3, IHCs do not generate Ca2+ action potentials, lack functional large-conductance Ca2+-activated K+ channels, and efferent synaptic activity persists up to 4 weeks after birth (Brandt et al., 2003).
Electrical activity during development and denervation of both muscle and neurons can regulate the expression pattern of nAChR subunits (Hall and Sanes, 1993; Corriveau et al., 1995; Zoli et al., 1995). In developing chick ciliary ganglion, for example, the level of expression of the α3, α5, and β4 nAChR subunits is strongly reduced in the absence of innervation (Levey et al., 1995). This seems to be the case of the α10 subunit, because its expression in IHCs is severely reduced after the withdrawal of active efferent synaptic contacts.
The developmental changes in key components of this efferent synapse, namely, the expression of a functional cholinergic receptor, functional SK channels coupled to the activation of the α9α10-containing nAChR, and the presence of active axosomatic synaptic contacts, are matched by changes in ultrastructural features such as the synaptic cisterns. The postsynaptic specializations in IHCs, although less regular than in OHCs (Sobkowicz, 1992), are present at early postnatal ages and disappear after the onset of hearing (Ginzberg and Morest, 1984). It will be of interest, therefore, to learn whether the expression of other components of this synapse, such as proteins of the synaptic cistern, also vary with the developmental loss of efferent inhibition.
Footnotes
This work was supported by an International Research Scholar Grant from the Howard Hughes Medical Institute, a John Simon Guggenheim Memorial Foundation Fellowship, and a Research Grant from ANPCyT (Argentina) to A.B.E., National Institutes of Health Research Grant R03TW006247 to P.A.F. and A.B.E. from the Fogarty International Center and the National Institute on Deafness and Other Communication Disorders (NIDCD), NIDCD Grant DC01508 to P.A.F., a Research Grant from Fundación Antorchas to A.B.E. and D.V., and a grant from the Interdisciplinary Center of Clinical Research Tübingen (IZKF) as well as by the Deutsche Forschungsgemeinschaft SFB 430/Kni-B3 to M.K. D.V. is a Smith Family New Investigator Awards Program Researcher (formally The Chestnut Hill Charitable Foundation). The α10 antibody was a generous gift from Prof. B. Fakler (Department of Physiology, University Freiburg).
Correspondence should be addressed to Dr. Eleonora Katz, Instituto de Investigaciones en Ingeniería Genética y Biología Molecular, Vuelta de Obligado 2490, 1428 Buenos Aires, Argentina.
Copyright © 2004 Society for Neuroscience 0270-6474/04/247814-07$15.00/0
References
- Bertrand D, Devillers-Thiery A, Revah F, Galzi JL, Hussy N, Mulle C, Bertrand S, Ballivet M, Changeux JP (1992) Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci USA 89: 1261-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Moser T (2001) The presynaptic function of mouse cochlear inner hair cells during development of hearing. J Neurosci 21: 4593-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Erostegui C, Sugasawa M, Dulon D (1996) Acetylcholine-induced potassium current of guinea pig outer hair cells: its dependence on a calcium influx through nicotinic-like receptors. J Neurosci 16: 2574-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T (2003) CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci 23: 10832-10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, LeBlanc C, Bobbin RP (1996) Differences in cholinergic responses from outer hair cells of rat and guinea pig. Hear Res 98: 9-17. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Romano SJ, Conroy WG, Oliva L, Berg DK (1995) Expression of neuronal acetylcholine receptor genes in vertebrate skeletal muscle during development. J Neurosci 15: 1372-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Lenoir M (1996) Cholinergic responses in developing outer hair cells of the rat cochlea. Eur J Neurosci 8: 1945-1952. [DOI] [PubMed] [Google Scholar]
- Dulon D, Luo L, Zhang C, Ryan AF (1998) Expression of small-conductance calcium-activated potassium channels (SK) in outer hair cells of the rat. Eur J Neurosci 10: 907-915. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Johnson DS, Boulter J, Vetter DE, Heinemann S (1994) a9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell 79: 705-715. [DOI] [PubMed] [Google Scholar]
- Elgoyhen AB, Vetter D, Katz E, Rothlin C, Heinemann S, Boulter J (2001) Alpha 10: A determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci USA 98: 3501-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling MR, Sobkowicz HM, Levenick CV, Scott GL, Slapnick SM, Rose JE (1990) Biochemical and morphological differentiation of acetylcholinesterase-positive efferent fibers in the mouse cochlea. J Electron Microsc Tech 15: 123-143. [DOI] [PubMed] [Google Scholar]
- Erostegui C, Norris CH, Bobbin RP (1994) In vitro pharmacologic characterization of a cholinergic receptor on outer hair cells. Hear Res 74: 135-147. [DOI] [PubMed] [Google Scholar]
- Evans MG (1996) Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol (Lond) 491: 563-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P (1996) Synaptic transmission at vertebrate hair cells. Curr Opin Neurobiol 6: 514-519. [DOI] [PubMed] [Google Scholar]
- Fuchs PA, Murrow BW (1992) Cholinergic inhibition of short (outer) hair cells of the chick's cochlea. J Neurosci 12: 800-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Loyzaga P, Pujol R (1988) Synaptophysin in the developing cochlea. Int J Dev Neurosci 6: 155-160. [DOI] [PubMed] [Google Scholar]
- Ginzberg RD, Morest DK (1984) Fine structure of cochlear innervation in the cat. Hear Res 14: 109-127. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA (2000) Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 288: 2366-2368. [DOI] [PubMed] [Google Scholar]
- Guinan JJ (1996) Efferent physiology. In: The cochlea (Dallos P, Popper AN, Fay RR, eds), pp 435-502. New York: Springer.
- Hall ZW, Sanes JR (1993) Synaptic structure and development: the neuro-muscular junction. Cell 72 [Suppl]: 99-121. [DOI] [PubMed] [Google Scholar]
- He DZ, Dallos P (1999) Development of acetylcholine-induced responses in neonatal gerbil outer hair cells. J Neurophysiol 81: 1162-1170. [DOI] [PubMed] [Google Scholar]
- Knipper M, Zimmermann U, Rohbock K, Kopschall I, Zenner HP (1995) Synaptophysin and GAP-43 proteins in efferent fibers of the inner ear during postnatal development. Brain Res Dev Brain Res 89: 73-86. [DOI] [PubMed] [Google Scholar]
- Knipper M, Zinn C, Maier H, Praetorius M, Rohbock K, Kopschall I, Zimmermann U (2000) Thyroid hormone deficiency before the onset of hearing causes irreversible damage to peripheral and central auditory systems. J Neurophysiol 83: 3101-3112. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rusch A (1998) Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394: 281-284. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Schnerson A, Pujol R (1980) Cochlear receptor development in the rat with emphasis on synaptogenesis. Anat Embryol 160: 253-262. [DOI] [PubMed] [Google Scholar]
- Levey MS, Brumwell CL, Dryer SE, Jacob MH (1995) Innervation and target tissue interactions differentially regulate acetylcholine receptor subunit mRNA levels in developing neurons in situ. Neuron 14: 153-162. [DOI] [PubMed] [Google Scholar]
- Liberman MC (1980) Efferent synapses in the inner hair cell area of the cat cochlea: An electron microscopic study of serial sections. Hear Res 3: 189-204. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S (1990) Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol 301: 443-460. [DOI] [PubMed] [Google Scholar]
- Luo L, Bennett T, Jung HH, Ryan A (1998) Developmental expression of alpha 9 acetylcholine receptor mRNA in the rat cochlea and vestibular inner ear. J Comp Neurol 393: 320-331. [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Holley MC, Kros CJ (2003a) Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol (Lond) 548: 383-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Johnson SL, Ruesch A, Kros CJ (2003b) Sodium and Calcium Currents Shape Action Potentials in Immature Mouse Inner Hair Cells. J Physiol (Lond) 552-3: 743-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven AI, Yuhas WA, Fuchs PA (1996) Ionic dependence and agonist preference of an acetylcholine receptor in hair cells. Aud Neurosci 2: 63-77. [Google Scholar]
- Morley BJ, Simmons DD (2002) Developmental mRNA expression of the alpha10 nicotinic acetylcholine receptor subunit in the rat cochlea. Brain Res Dev Brain Res 139: 87-96. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Li HS, Hiel H, Drescher DG, Elgoyhen AB (1998) Identification of the subunits of the nicotinic cholinergic receptors in the rat cochlea using RT-PCR and in situ hybridization. Brain Res Mol Brain Res 53: 78-87. [DOI] [PubMed] [Google Scholar]
- Oliver D, Klocker N, Schuck J, Baukrowitz T, Ruppersberg JP, Fakler B (2000) Gating of Ca2+-activated K+ channels controls fast inhibitory synaptic transmission at auditory outer hair cells. Neuron 26: 595-601. [DOI] [PubMed] [Google Scholar]
- Park HJ, Niedzielski AS, Wenthold RJ (1997) Expression of the nicotinic acetylcholine receptor subunit, alpha9, in the guinea pig cochlea. Hear Res 112: 95-105. [DOI] [PubMed] [Google Scholar]
- Pujol R, Lavigne-Rebillard M, Lenoir M (1998) Development of sensory and neural structures in the mammalian cochlea. In: Development of the auditory system (Rubel EW, Popper AN, Fay RR, eds), pp 146-192. New York: Springer.
- Role LW, Berg DK (1996) Nicotinic receptors in the development and modulation of CNS synapses. Neuron 16: 1077-1085. [DOI] [PubMed] [Google Scholar]
- Schuetze SM, Role LW (1987) Developmental regulation of nicotinic acetylcholine receptors. Annu Rev Neurosci 10: 403-457. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci 13: 596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD (2002) Development of the inner ear efferent system across vertebrate species. J Neurobiol 53: 228-250. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Morley BJ (1998) Differential expression of the alpha 9 nicotinic acetylcholine receptor subunit in neonatal and adult cochlear hair cells. Brain Res Mol Brain Res 56: 287-292. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Moulding HD, Zee D (1996a) Olivocochlear innervation of inner and outer hair cells during postnatal maturation: an immunocytochemical study. Brain Res Dev Brain Res 95: 213-226. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Mansdorf NB, Kim JH (1996b) Olivocochlear innervation of inner and outer hair cells during postnatal maturation: evidence for a waiting period. J Comp Neurol 370: 551-562. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM (1992) The development of innervation in the organ of Corti. In: Development of auditory and vestibular systems (Romand, ed), pp 59-100. Amsterdam: Elsevier.
- Verbitsky M, Rothlin C, Katz E, Elgoyhen AB (2000) Mixed nicotinicmuscarinic properties of the α9 nicotinic cholinergic receptor. Neuro-pharmacology 39: 2515-2524. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Liberman MC, Mann J, Barhanin J, Boulter J, Brown MC, Saffiote-Kolman S, Heinemann SF, Elgoyhen AB (1999) Role of alpha9 nicotinic ACh receptor subunits in the development and function of cochlear efferent innervation. Neuron 23: 93-103. [DOI] [PubMed] [Google Scholar]
- Weisstaub N, Vetter DE, Elgoyhen AB, Katz E (2002) The alpha9alpha10 nicotinic acetylcholine receptor is permeable to and is modulated by divalent cations. Hear Res 167: 122-135. [DOI] [PubMed] [Google Scholar]
- Zoli M, Le Novere N, Hill Jr JA, Changeux JP (1995) Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci 15: 1912-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]