Abstract
The GABAA receptor γ2 subunit mutation R43Q is an autosomal dominant mutation associated with childhood absence epilepsy and febrile seizures. Previously, we demonstrated that homozygous α1β3γ2L(R43Q) receptor whole-cell currents had reduced amplitude with unaltered time course, suggesting reduced cell surface expression of functional receptors. In human embryonic kidney 293-T cells, we demonstrate that both heterozygous and homozygous α1β2γ2S(R43Q) GABAA receptor current amplitudes were reduced when receptors were assembled from coexpressed α1, β2, and γ2S subunits and from β2-α1 tandem subunits coexpressed with the γ2L subunit. Using fluorescence confocal microscopy, we demonstrated that mutant receptors containing enhanced yellow fluorescent protein-tagged γ2S subunits had reduced surface expression and were retained in the endoplasmic reticulum. In addition, using biotinylation of surface receptors and immunoblotting, we confirmed that α1β2γ2S(R43Q) receptors had reduced surface expression. These results provide evidence that the γ2S(R43Q) mutation impaired GABAA receptor function by compromising receptor trafficking and reducing surface expression.
Keywords: GABAA receptor, R43Q mutation, endoplasmic reticulum, receptor trafficking, childhood absence epilepsy, febrile seizures
Introduction
A GABAA receptor γ2 subunit missense mutation (R43Q) was identified in a large family with autosomal dominant childhood absence epilepsy and febrile seizures (Wallace et al., 2001). We demonstrated that homozygous expression of α1β3γ2L(R43Q) receptors in human embryonic kidney (HEK) 293-T cells had substantially reduced peak current amplitudes with no alteration in current time course or single-channel properties (Bianchi et al., 2002), but the basis for the current reduction was unclear. Native GABAA receptors likely contain a single γ2 subunit (Chang et al., 1996), which would result in a mixture of wild-type and mutant receptors in heterozygous patients. However, the heterozygous receptor current phenotype has not been characterized. It is not known to what extent this mutation affects receptor assembly and trafficking to the cell surface. It is possible that there could be a compensatory increase of the wild-type γ2 ternary receptors with 2α2β1γ stoichiometry or increased expression of receptors with 2α3β stoichiometry, possibly resulting from impaired interaction of the mutant γ2 subunit with αβ subunit dimers during receptor assembly.
To determine the heterozygous receptor current phenotype and the effect of the γ2S(R43Q) mutation on cell surface expression, we coexpressed either human α1, β2, and γ2S or α1, β2, and γ2S(R43Q) subunits in HEK293-T or Cos-7 cells to form α1β2γ2S or α1β2γ2S(R43Q) receptors. The α1β2γ2S receptors were expressed using wild-type γ2S subunit cDNA, mutant (homozygous) γ2S subunit cDNA, or an equal mixture of wild-type and mutant (heterozygous) γ2S subunit cDNA. In addition, to rule out a contribution of αβ receptor expression with cotransfection of α, β, and γ subunits (Angelotti and Macdonald, 1993), we used “tethered” (forced) assembly of receptors with a 2α2β1γ stoichiometry by expressing a rat β2-α1 subunit tandem cDNA construct (Baumann et al., 2001) with either wild-type or mutant rat γ2L(R43Q) subunit cDNAs. We determined functional receptor surface expression in HEK293-T cells of wild-type α1β2γ2S receptors and heterozygous and homozygous α1β2γ2S(R43Q) receptors by the following: (1) recording peak whole-cell currents, (2) expressing receptors with an enhanced yellow fluorescent protein (EYFP)-tagged γ2S subunit followed by confocal microscopy, and (3) performing a biotinylation assay.
Materials and Methods
Expression vectors with GABAA receptor subunits. Human α1, β2, and γ2S GABAA receptor subunit subtype cDNAs were subcloned into expression vector pcDNA3.1(+). EYFP was inserted between amino acids 4 and 5 of the γ2S cDNA. R43Q mutations in the γ2S subunit and γ2S-EYFP construct were made using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) and were confirmed by DNA sequencing. Cell membrane (Mem) marker enhanced cyan fluorescent protein (pECFP)-membrane and endoplasmic reticulum (ER) marker pECFP-ER (calreticulin and KDEL) plasmids were obtained from BD Biosciences (Palo Alto, CA). All rat wild-type and mutant γ2L subunits and β2-α1 construct were subcloned into the plasmid expression vector pCMV.
Electrophysiology. HEK293-T cells were cotransfected with 4 μg of each subunit plasmid and 2 μg of the pHook-1 cDNA (Invitrogen, Carlsbad, CA) using a modified calcium phosphate precipitation method and selected 24 hr after transfection by magnetic hapten-coated beads. Whole cells were lifted and recorded under previously described conditions (Bianchi et al., 2002). Cells with membrane capacitance >40 pF were excluded, because we demonstrated previously that cells with larger size and capacitance >40 pF had decreased contribution of the fastest phase of desensitization (Hinkle and Macdonald, 2003). Whole cells were voltage clamped at -50 mV. A multibarrel fast application device was used to switch solutions (Hinkle et al., 2003).
Live cell confocal microscopy and fluorescence quantification. Live cell confocal microscopy was performed using an inverted Zeiss (model 510; Thornwood, NY) laser scanning microscope with a 63 × 1.4 numerical aperture oil immersion lens, 2-2.5× zoom, and multitrack excitation. Both HEK293-T and Cos-7 cells were plated on poly-d-lysine-coated, glass-bottom imaging dishes at the density of 1-2 × 105 cells and cotransfected with 1 μg each of human subunit plasmid and pECFP-ER with either calcium phosphate precipitation or Lipofectamine Plus re-agents using the suggestions of the manufacturer. Cell membrane lipophilic styryl dye FM4-64 (10 μm; Molecular Probes, Eugene, OR), which has been demonstrated not to stain the ER (Bolte et al., 2004), was applied to cells immediately before experiments, and images were obtained within 20 min. Cells were examined with excitation at 458 nm for ECFP, 514 nm for EYFP, and 543 nm for FM4-64. Because of expression variation, fluorescence quantification in single cells was averaged. Fluorescence intensities of both cell surface and ER areas were determined using MetaMorph imaging software by colocalizing each specific area with FM4-64 for the plasma membrane or pECFP-ER for the ER, and the fluorescence intensities were measured in the EYFP channel, which was for γ2S-EYFP tagged receptors. Only cells with all three colors detectable with detector gain under 800 were included. All images were single confocal sections averaged from 8 to 16 times to reduce noise, except when specified otherwise.
Biotinylation, immunoprecipitation, and Western blot analysis. For cell surface receptor biotinylation, live transfected cells were washed with PBS containing 0.1 mm CaCl2 and 1 mm MgCl2, pH 7.4, and then incubated with sulfo-N-hydroxysuccinimide (NHS) biotin for 1 hr at 4°C. The sulfo-NHS biotin was quenched with PBS containing 0.1 mm glycine. Cells were lysed in radioimmunoprecipitation assay buffer (20 mm Tris, 20 mm EGTA, 1 mm DTT, 1 mm benzamidine), supplemented with 0.01 mm PMSF, 0.005 μg/ml leupeptin, and 0.005 μg/ml pepstatin for 1 hr at 4°C. The extracted supernatant was then incubated with immobilized streptavidin for 1 hr at room temperature. The biotinylated proteins were eluted from the streptavidin by incubation with 2× sample buffer at 70°C for 15 min. For immunoprecipitation, whole-cell lysates were incubated overnight with a GABAA receptor α1 subunit-specific antibody (bd24; Chemicon, Temecula, CA) or an antibody to green fluorescent protein (GFP) (Zymed, San Francisco, CA) at a volume ratio of 1:500 at 4°C and then precipitated by incubating with 50 μl of protein A-Sepharose under gentle rotation for 1 hr. The bound protein was then eluted by 2× sample buffer at 90°C for 10-15 min and then subjected to Western blot analysis. Each sample (30 μg/lane) was then subjected to 12.5% SDS-PAGE. After transfer, membranes were incubated with primary mouse monoclonal antibodies against α1 subunit and GFP and rabbit polyclonal γ2 (Alpha Diagnostic International, San Antonio, TX) subunits (1:1000). The monoclonal anti-β-actin (mouse IgG2a isotype; 1:5000) and pECFP-Mem were applied as internal controls. After washing, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG, 1:2000; goat anti-rabbit IgG, 1:2000; Upstate Biotechnology, Lake Placid, NY). Antibody-reactive bands were revealed by chemiluminescence.
Data analysis. Macroscopic currents were low-pass filtered at 2 kHz, digitized at 10 kHz, and analyzed using pClamp9 software suite (Axon Instruments, Foster City, CA). Numerical data were expressed as mean ± SEM. Statistical significance, using Student's unpaired t test (GraphPad Prism; GraphPad Software, San Diego, CA), was taken as p < 0.05.
Results
Heterozygous and homozygous α1β2γ2S(R43Q) GABAA receptors had unaltered benzodiazepine sensitivity
There has been some controversy concerning the sensitivity of α1β2γ2S(R43Q) currents to benzodiazepines (Bianchi et al., 2002; Bowser et al., 2002). We determined the effect of the γ2S subunit R43Q mutation on benzodiazepine sensitivity with receptors formed using both free and forced assembly. Diazepam significantly and reversibly enhanced wild-type and heterozygous and homozygous α1β2γ2S(R43Q) currents with both free (Fig. 1A) and forced assembly. The extent of diazepam enhancement of the heterozygous or homozygous receptors with free (α1β2γ2S) and forced (β2-α1/γ2L) assembly did not differ from enhancement of wild-type receptor currents (Fig. 1B).
Figure 1.

Heterozygous and homozygous α1β2γ2S(R43Q) receptors had unaltered diazepam sensitivity. A, Whole-cell recordings were made from lifted HEK293-T cells expressing wild-type (wt) hα1β2γ2S or heterozygous (het) or homozygous (hom) hα1β2γ2S(R43Q) receptors. An approximate EC20 value of GABA concentration (2 μm) was applied for 6 sec to cells voltage clamped at -50 mV, and 1 μm diazepam was coapplied with GABA. B, Diazepam enhancement is shown as a percentage of control current (average peak current before and after diazepam coapplication). The γ2S(R43Q) mutation did not alter the magnitude of diazepam enhancement for either heterozygous or homozygous expression with both free hα1β2γ2S assembly and forced rβ2-α1/γ2L assembly (n = 6 for each group).
Heterozygous and homozygous α1β2γ2S(R43Q) GABAA receptor currents were reduced
We previously demonstrated that homozygous rat α1β3γ2L (R43Q) currents were reduced relative to wild-type currents (Bianchi et al., 2002). However, the composition of GABAA receptors following cotransfection with α, β, and γ subunits has been questioned. It has been suggested that cotransfection of HEK293-T cells with α, β, and γ subunits leads primarily to αβγ receptors (Angelotti and Macdonald, 1993) or may lead to formation of αβ as well as αβγ receptors (Boileau et al., 2003). To interpret the effect of the γ2S(R43Q) mutation, it is important to record only from αβγ receptors. To ensure assembly of only α1β2γ2L or α1β2γ2L(R43Q) receptors, we coexpressed a rat β2-α1 tandem construct with rat γ2L and/or rat γ2L(R43Q) subunits. Expression of the β2-α1 tandem alone failed to form functional channels in oocytes (Baumann et al., 2001) or in transfected HEK293-T cells (data not shown). We therefore coexpressed the β2-α1 tandem construct with the γ2L subunit to form a forced 2α2β1γ ternary stoichiometry. Currents recorded from α1β2γ2S (free, untethered assembly) and β2-α1/γ2L (forced, tethered assembly) receptors had similar time courses (Fig. 2A) and amplitudes (Fig. 2B). Heterozygous and homozygous expression of α1β2γ2S(R43Q) and β2-α1/γ2L(R43Q) receptors had time courses similar to wild-type currents (Fig. 2A). Heterozygous α1β2γ2S(R43Q) and β2-α1/γ2L(R43Q) receptor peak current amplitudes (Fig. 2B) and the current amplitudes relative to cell membrane capacitance (Fig. 2C) were significantly smaller than those of wild type but were significantly larger than those of homozygous peak current amplitudes. The cell membrane capacitance and the receptor desensitization rates did not differ significantly among these groups. The extent of desensitization was slightly, but nonsignificantly, enhanced in both heterozygous and homozygous receptors in these four batches of cells (supplemental material, available at www.jneurosci.org).
Figure 2.
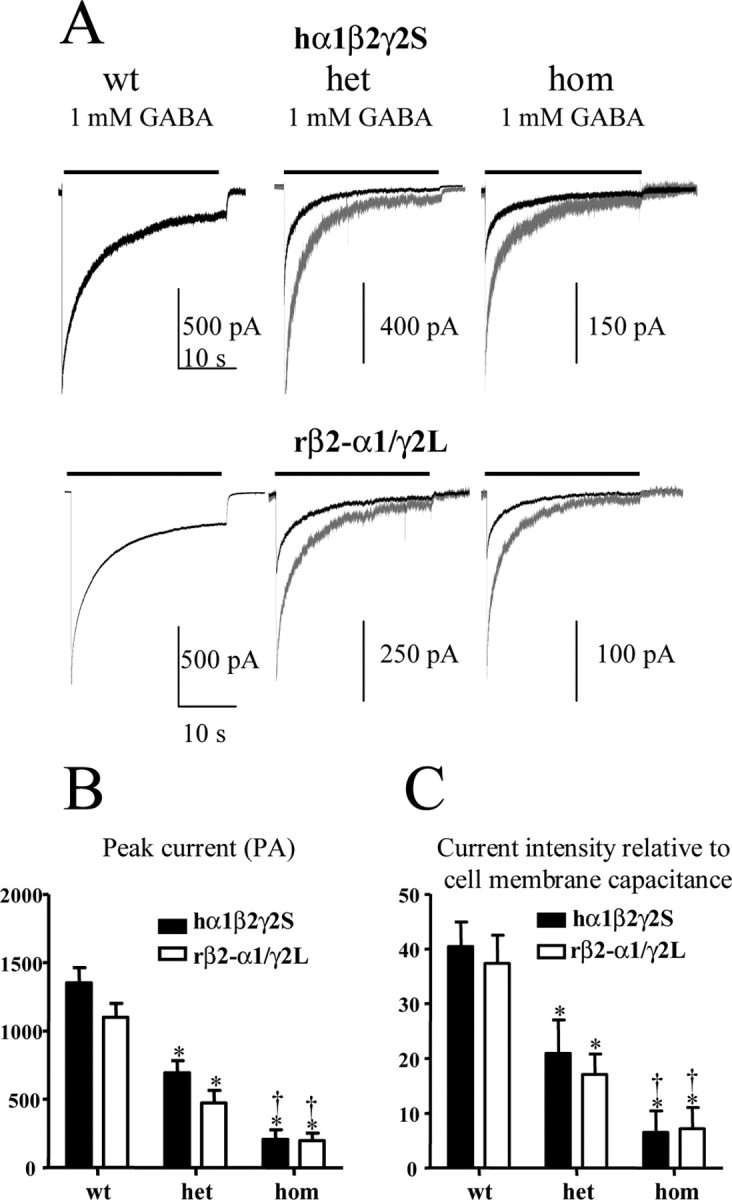
Heterozygous and homozygous α1β2γ2S(R43Q) receptors had reduced current amplitudes. A, Whole-cell currents were obtained from either hα1β2γ2S (wt), hα1β2γ2S(R43Q) (het), or hα1β2γ2S (R43Q) (hom) receptors in free assembly or forced rβ2-α1γ2L assembly. GABA (1 mm) was applied for 28 sec. Currents are shown to scale (dark traces) and normalized to wild-type currents (gray traces). The time scale for the first trace applies to all traces. B, C, Peak amplitudes (B) and relative current intensities (C) of heterozygous and homozygous hα1β2γ2S(R43Q) and rβ2-α1γ2L(R43Q) receptor currents were significantly reduced with both free and forced assembly (*p < 0.01 vs wild type; †p < 0.01 vs heterozygous; data are from 9-17 patches from 4 batches of cells).
α1β2γ2S(R43Q) receptors were trapped in intracellular compartments
To determine the cellular fate of α1β2γ2S(R43Q) receptors, N-terminal EYFP-tagged wild-type and R43Q mutant γ2S subunits were cotransfected with α1 and β2 subunits into HEK293-T or Cos-7 cells. The surface and intracellular distribution of α1β2γ2S-EYFP receptors were determined under confocal microscopy by colabeling with pECFP-ER and membrane-selective dye FM4-64 to mark the plasma membrane. FM4-64 is a selective fluorescent dye that can insert into one leaflet of a membrane lipid bilayer via their lipophilic tails (two aliphatic chains) with the pyridinium dicationic head anchored at the membrane surface. In live Cos-7 cells, a significant portion of wild-type α1β2γ2S-EYFP receptor fluorescence had a smooth distribution with some clusters that could be detected both on the surface and in intracellular compartments (Fig. 3A, wt). Heterozygous and homozygous expression of α1β2γ2S(R43Q)-EYFP receptors also resulted in cell surface and intracellular fluorescence (Fig. 3A, het, hom), but homozygous and heterozygous α1β2γ2S(R43Q)-EYFP cell surface fluorescence was reduced (Fig. 3B). We also used an EYFP-9E10-γ2S double epitope-tagged construct combined with anti-9E10 conjugated with cyanine 5 (Cy5) antibody labeling under a nonpermeabilized condition and achieved consistent results (data not shown).
Figure 3.

Heterozygous and homozygous hα1β2γ2S(R43Q) receptors were trapped in the ER. A, Representative confocal fluorescence images of Cos-7 cells transfected with hα1β2γ2S-EYFP or hα1β2γ2S(R43Q)-EYFP receptors are presented. R, Receptor; Co, colocalized image. Wt α1β2γ2S-EYFP receptors were primarily in the cell membrane. Heterozygous (het) α1β2γ2S(R43Q)-EYFP receptors were found in both membrane and intracellular compartments. Homozygous (hom)α1β2γ2S(R43Q)-EYFP receptors were found primarily in intracellular compartments with minimal cell surface localization. Both heterozygous and homozygous receptors had a fluorescence pattern that was similar in distribution to the pECFP-ER fluorescence pattern (supplemental material, available at www.jneurosci.org). In B, the relative membrane/ER fluorescence intensity ratios for heterozygous and homozygous receptors were significantly reduced compared with that for the wild-type receptors (see Materials and Methods) (for each group, 9-11 randomly chosen cells were measured from 5 batches; *p < 0.01 vs wild type).
The ER marker, pECFP-ER, contains sequences for both ER targeting (calreticulin) and ER retrieval (KDEL) and functions as a tag to localize the ER. Transfection of pECFP-ER into HEK293-T or Cos-7 cells resulted in an irregular, discontinuous, clumped, or mesh-like perinuclear fluorescence pattern (supplemental material, available at www.jneurosci.org). Cotransfection of pECFP-ER with α1β2γ2S-EYFP receptor cDNA demonstrated minimal colocalization (Fig. 3A, wt) (supplemental material, available at www.jneurosci.org). Substantial blue (ECFP) and green (EYFP) or faint aqua fluorescence can be seen indicating only minimal colocalization. When images were scanned in a series of slices with confocal microscopy, this colocalization appeared in the middle and was absent in the top and bottom slices, confirming the presence of some of the receptors in subcellular structures (data not shown). Coexpression of both heterozygous (het) and homozygous (hom) α1β2γ2S(R43Q)-EYFP receptors with the ER marker resulted in substantial colocalization of pECFP-ER and receptor fluorescence (Fig. 3A) (supplemental material, available at www.jneurosci.org). The ratio of plasma membrane and ER fluorescence intensity was significantly reduced for both heterozygous and homozygous compared with the wild-type receptors (Fig. 3B). The fluorescence ratio for heterozygous receptors was higher than that for homozygous receptors but did not reach statistical significance (Fig. 3B). We also used the live cell membrane potential indicator dye di-8 Anepps (Molecular Probes), membrane marker pECFP-Mem, and Golgi marker pECFP-Golgi (human β 1,4 GT; BD Biosciences) to investigate further the spatial distribution of the receptors. Consistent with the above findings, a majority of the heterozygous and homozygous α1β2γ2S(R43Q)-EYFP fluorescence signal showed a perinuclear localization and retention in the ER (data not shown).
Reduced surface expression of heterozygous and homozygous α1β2γ2S(R43Q) receptors
To determine directly the effect of the γ2S R43Q mutation on surface expression of α1β2γ2S receptors, we performed biotinylation followed by Western blot analyses using HEK293-T cells transfected with wild-type and heterozygous and homozygous α1β2γ2S(R43Q) receptors. The membrane-bound proteins were first precipitated by streptavidin and then visualized by Western blots. For detection, we used a monoclonal antibody targeted against the N terminus of the human α1 subunit (Fig. 4A), a monoclonal mouse antibody to GFP and its variants for the γ2S-EYFP chimera (Fig. 4B), and a polyclonal rabbit antibody against the extracellular domain (189-299 amino acid) of the human γ2 subunit (Fig. 4C). We used antibodies to human α1 subunit or to GFP to immunoprecipitate the α1β2γ2S or α1β2γ2S-EYFP receptors. When detected with the monoclonal antibody against human α1 subunits, a specific immunoreactive band for the α1 subunit was visualized at 51 kDa (Fig. 4A). No change was seen in total cell α1 subunit protein expression with wild-type or with heterozygous or homozygous α1β2γ2S(R43Q) receptors (Fig. 4A, total). However, there was reduced α1 subunit protein expression for heterozygous α1β2γ2S receptors on the cell surface relative to wild-type receptors and reduced α1 subunit protein expression for homozygous α1β2γ2S receptors on the cell surface relative to heterozygous receptors (Fig. 4A, surface). After cell transfection with wild-type and heterozygous and homozygous α1β2γ2S(R43Q)-EYFP receptors, similar results were obtained using an antibody against GFP and its variants. A specific band at ∼75 kDa was detected for γ2S-EYFP, in agreement with a previous study (Kittler et al., 2000). No change was seen in total cell γ2 subunit protein expression with wild-type or with heterozygous or homozygous α1β2γ2S(R43Q) receptors (Fig. 4B, total). However, a significant reduction of surface expression of α1β2γ2S-EYFP receptors was seen with both heterozygous and homozygous α1β2γ2S-EYFP(R43Q) receptors relative to wild-type receptors, and expression of heterozygous receptors was higher than that of homozygous receptors (Fig. 4B, surface). To further confirm the effect of the R43Q γ2 subunit mutation on protein expression, we detected receptor expression with an anti-γ2 antibody in HEK293-T cells using immunoprecipitation with either anti-α1 or anti-GFP antibodies (data not shown) and then detected the γ2S subunit protein using a rabbit antibody against the γ2 subunit. With human α1β2γ2S or heterozygous or homozygous α1β2γ2S-EYFP receptors, total receptor protein was similar (Fig. 4C, total). To demonstrate that an equal amount of protein was loaded, the transferred membrane was reblotted with a β-actin antibody (Fig. 4C, β-actin). In the cell surface fraction, both heterozygous and homozygous α1β2γ2S(R43Q) receptors demonstrated reduced surface protein expression when detected using the human γ2 antibody. To exclude variability of receptor expression in different transfection groups, we cotransfected the membrane marker pECFP-Mem with the α1β2γ2S receptor. Blotting with GFP antibody for pECFP-Mem revealed a similar protein expression for each group (Fig. 4C, pECFP-Mem). Quantification of the Western blots revealed that there was no difference in the relative intensity of total protein among groups (data not shown). However, on the cell surface, heterozygous receptor protein intensities were lower than wild-type intensities but higher than homozygous receptors intensities for each group (Fig. 4D).
Figure 4.

Heterozygous and homozygous hα1β2γ2S(R43Q) receptors had reduced surface expression on HEK293-T cells. A, B, HEK293-T cells transfected with wild-type (wt) or heterozygous (het) or homozygous (hom) hα1β2γ2S(R43Q) receptors were biotinylated and immunoblotted with antibodies against the α1 subunit (A) and GFP variant EYFP (B). Expression of heterozygous or homozygous α1β2γ2S(R43Q) receptors resulted in similar levels of whole-cell protein expression (total) but reduced cell surface protein expression (surface) compared with wild-type receptors. C, HEK293-T cells transfected with wild-type or heterozygous or homozygous α1β2S(R43Q) receptors and pECFP-Mem were biotinylated, and whole-cell protein was precipitated with antibody against the human α1 subunit and detected by antibody against the γ2 subunit. Heterozygous and homozygous α1β2γ2S(R43Q) receptors revealed a similar protein expression in whole-cell level (total) but a reduced protein expression in the cell surface (surface). β-Actin was used as a control to demonstrate that equal amounts of protein were loaded (β-actin). Similarly, pECFP-Mem protein expression was also similar in each group (pECFP-Mem). D, The optical absorbency of Western blots was quantified with Bio-Rad QuantifyOne. In each group, heterozygous protein intensities were lower than wild type but higher than homozygous receptors (*p < 0.05 vs wild type; †p < 0.05 vs heterozygous; data are from 5 experiments).
Discussion
The functional consequence of the GABAA receptor γ2 subunit R43Q mutation has been reported to not change (Bianchi et al., 2002), abolish (Wallace et al., 2001), or reduce (Bowser et al., 2002) diazepam potentiation of α1β2γ2 currents without altering GABA EC50 value or current amplitude. Consistent with our previous study, we demonstrate here that with both free and forced assembly, heterozygous or homozygous expression of the R43Q mutation did not significantly reduce diazepam enhancement of α1β2γ2S receptor current.
We also reported that homozygous expression of the R43Q mutation reduced receptor current, but we did not determine the effect of heterozygous expression or the basis for the reduced current. Here, we demonstrate that heterozygous α1β2γ2S (R43Q) currents had unaltered time course and were reduced relative to wild-type currents but were larger than homozygous currents. Furthermore, our use of forced α1β2γ2 receptor assembly directly demonstrated that the current reduction was not attributable to failure of the mutant γ2 subunit to assemble with α1β2 subunits.
These results suggest that the γ2S(R43Q) mutation interferes with some aspect of receptor expression, folding, assembly, trafficking, or stability. Interestingly, it is known that N-terminal sequences are critical for proper assembly of GABAA receptors (Klausberger et al., 2000), and an arginine was reported to regulate ER export of GluR5 kainate receptors (Ren et al., 2003). However, the flanking sequence around the R43 residue has not been identified to be critical for receptor assembly or trafficking. The absolute conservation of an arginine residue at this location in all sequenced species from Caenorhabditis elegans to humans across subunits suggests that it may play an important role in receptor assembly, trafficking, or surface expression (which are required of all subunits) rather than with benzodiazepine modulation (which is highly subunit subtype selective). Several other human diseases have also been linked to mutations in conserved arginines (among other residues) that result in altered protein folding and subsequent degradation (Bross et al., 1999). However, the effect of the R43Q mutation on protein folding, assembly trafficking, and targeting needs to be further characterized. The basis for the discrepancy between our findings and those of Bowser et al. (2002), who reported large currents recorded from HEK293 cells expressing the mutant channels, remains unclear. A possible explanation may be that cells were incubated at a permissive temperature (30°) for 24-96 hr before recording in Bowser's study, whereas in our study, a nonpermissive physiological temperature (37°) was used. These different incubation conditions may have resulted in different temperature-related enzyme activity, protein folding, heat-denatured glycoproteins, and intracellular trafficking, thus producing different levels of surface expression.
Use of peak current amplitude is an indirect approach to study receptor surface expression. To address this issue more directly, we used confocal microscopy to localize fluorescent marker-tagged receptors. We determined the trafficking and cellular fate of wild-type and heterozygous and homozygous α1β2γ2S (R43Q)-EYFP receptors in live cells (Carter and Sorkin, 1998; Connolly et al., 1999). Insertion of GFP (EYFP) in the N terminus downstream from the signal peptide in γ2 subunits did not alter receptor function (Kittler et al., 2000). Wild-type receptors exhibited a smooth and continuous fluorescence that was detected primarily in the cell membrane with relatively weak expression in subcellular structures. The cell surface and subcellular colocalizations were confirmed by the FM4-64 membrane dye, pECFP-Mem membrane marker, pECFP-Golgi marker, and pECFP-ER marker. In contrast, with heterozygous and homozygous α1β2γ2S(R43Q)-EYFP expression, cell surface expression was weak. Instead, a clumpy or strongly convoluted, reticular fluorescent pattern colocalizing with the ER marker was observed. This reticular pattern was very similar to that obtained by only expressing the ECFP-ER marker (supplemental material, available at www.jneurosci.org). Previous work on the nonsense mutation γ2S(Q351X), which results in truncation of the subunit 78 amino acids from the C terminus, demonstrated a similar pattern of intracellular compartment localization (Harkin et al., 2002).
Thus, our study suggests that with heterozygous expression, the γ2S(R43Q) mutation may result in impaired receptor trafficking and increased retention of the receptor in intracellular compartments, including the ER. This reduced cell surface expression would result in decreased inhibitory GABAA receptor current in vivo, and consequently, an increase in neuronal excitability and epilepsy. The observations reported here used a heterologous expression system, and thus this result must be confirmed after expression in neurons. GABAA receptors exist in synapses as clusters and require interactions with other neuronal-specific trafficking proteins or accessory proteins like gephyrin. Future studies using neuronal preparations and knock-in animals may help better elucidate the effect of this mutation in a neuronal milieu.
Footnotes
This research was supported by National Institutes of Health Grant R01 NS33300. We give special thanks to Dr. Matt Bianchi for his help with manuscript revision and Dr. Erwin Sigel for providing the tandem construct.
Correspondence should be addressed to Dr. Robert L. Macdonald, Vanderbilt University Medical Center, 6140 Medical Research Building III, 465 21st Avenue South, Nashville, TN 37232-8552. E-mail: robert.macdonald@vanderbilt.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/248672-06$15.00/0
References
- Angelotti TP, Macdonald RL (1993) Assembly of GABAA receptor subunits: α1β1 and α1β1γ2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci 13: 1429-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann SW, Baur R, Sigel E (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem 276: 36275-36280. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Song L, Zhang H, Macdonald RL (2002) Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci 22: 5321-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA (2003) Effects of γ2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology 44: 1003-1012. [DOI] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, Satiat-Jeunemaitre B (2004) FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J Microsc 214: 159-173. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Wagner DA, Czajkowski C, Cromer BA, Parker MW, Wallace RH, Harkin LA, Mulley JC, Marini C, Berkovic SF, Williams DA, Jones MV, Petrou S (2002) Altered kinetics and benzodiazepine sensitivity of a GABAA receptor subunit mutation [γ2(R43Q)] found in human epilepsy. Proc Natl Acad Sci USA 99: 15170-15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bross P, Corydon TJ, Andresen BS, Jorgensen MM, Bolund L, Gregersen N (1999) Protein misfolding and degradation in genetic diseases. Hum Mutat 14: 186-198. [DOI] [PubMed] [Google Scholar]
- Carter RE, Sorkin A (1998) Endocytosis of functional epidermal growth factor receptor-green fluorescent protein chimera. J Biol Chem 273: 35000-35007. [DOI] [PubMed] [Google Scholar]
- Chang Y, Wang R, Barot S, Weiss DS (1996) Stoichiometry of a recombinant GABAA receptor. J Neurosci 16: 5415-5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CN, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ (1999) Cell surface stability of γ-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem 274: 36565-36572. [DOI] [PubMed] [Google Scholar]
- Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S (2002) Truncation of the GABA(A)-receptor γ2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet 70: 530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle DJ, Macdonald RL (2003) β Subunit phosphorylation selectively increases fast desensitization and prolongs deactivation of α1β1γ2L and α1β3γ2L GABAA receptor currents. J Neurosci 23: 11698-11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle DJ, Bianchi MT, Macdonald RL (2003) Modifications of a commercial perfusion system for use in ultrafast solution exchange during patch clamp recording. Biotechniques 35: 472-476. [PubMed] [Google Scholar]
- Kittler JT, Wang J, Connolly CN, Vicini S, Smart TG, Moss SJ (2000) Analysis of GABAA receptor assembly in mammalian cell lines and hippocampal neurons using γ2 subunit green fluorescent protein chimeras. Mol Cell Neurosci 16: 440-452. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Fuchs K, Mayer B, Ehya N, Sieghart W (2000) GABA(A) receptor assembly. Identification and structure of γ(2) sequences forming the intersubunit contacts with α(1) and β(3) subunits. J Biol Chem 275: 8921-8928. [DOI] [PubMed] [Google Scholar]
- Ren Z, Riley NJ, Needleman LA, Sanders JM, Swanson GT, Marshall J (2003) Cell surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal. J Biol Chem 278: 52700-52709. [DOI] [PubMed] [Google Scholar]
- Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF (2001) Mutant GABA(A) receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet 28: 49-52. [DOI] [PubMed] [Google Scholar]


