Abstract
To study the functions of 5-HT4 receptors, a null mutation was engineered in the corresponding gene. 5-HT4 receptor knock-out mice displayed normal feeding and motor behaviors in baseline conditions but abnormal feeding and locomotor behavior in response to stress and novelty. Specifically, stress-induced hypophagia and novelty-induced exploratory activity were attenuated in the knock-out mice. In addition, pentylenetetrazol-induced convulsive responses were enhanced in the knock-out mice, suggesting an increase in neuronal network excitability. These results provide the first example of a genetic deficit that disrupts the ability of stress to reduce feeding and body weight and suggest that 5-HT4 receptors may be involved in stress-induced anorexia and seizure susceptibility.
Keywords: anorexia, stress, locomotion, epilepsy, 5-HT4 receptor, knock-out
Introduction
Serotonin (5-HT) modulates feeding behavior as shown by the potent anorectic properties of 5-HT releasers (Bonasera and Tecott, 2000). The most widely prescribed drug for obesity was fenfluramine, a 5-HT uptake inhibitor and releaser, until negative cardiovascular side effects were reported. Fenfluramine exerts potent anorectic effects because of an indirect activation of 5-HT1B and 5-HT2C receptors in rodents (Lucas et al., 1998; Vickers et al., 1999, 2001). This conclusion was reached using both pharmacological and knock-out strategies. The 5-HT1B receptor-null mice are insensitive to fenfluramine-induced anorexia (Lucas et al., 1998), and the 5-HT2C receptor-null mice show reduced sensitivity to the same treatment (Vickers et al., 1999, 2001). It has been suggested recently that the lack of response to fenfluramine of 5-HT1B knock-out mice may be attributable to a downregulation of 5-HT2C receptors (Hewitt et al., 2002). The hypophagic action of the hallucinogenic drug 1-(2, 5-Dimethoxy-4-iodophenyl)-2-aminopropane appears to be mediated by the activation of 5-HT2C as well as 5-HT2A receptors (Schechter and Simansky, 1988). The important contribution of 5-HT2C receptors in feeding behavior is also supported by the obesity of 5-HT2C receptor-null mice (Tecott et al., 1995).
Less investigated is the phenomenon of stress-induced hypophagia. Hypothalamic-pituitary-adrenal axis hormones have been suggested to be involved in this stress response, at least in part, via the release of 5-HT in the medial prefrontal cortex, nucleus accumbens, amygdala, and dorsal hippocampus (Inoue et al., 1994; Ge et al., 1997; Konstandi et al., 2000). Only one study proposed that 5-HT2A/2C receptors could be involved in this cascade of events (Grignaschi et al., 1993).
5-HT4 receptors are well known for their peripheral effects on the gastrointestinal tract, where they may serve as targets for therapeutic drugs used to treat dyspepsia, gastroesophageal reflux disease, gastroparesis (Eglen et al., 1995), or irritable bowel syndrome (Callahan, 2002). They are also expressed in limbic brain structures (hypothalamus, nucleus accumbens, amygdala), olfactory tubercles, hippocampus, basal ganglia (striatum, globus pallidus), and substantia nigra. Their involvement in learning and memory is well documented (Eglen et al., 1995; Bockaert et al., 1998). However, nothing is known about their contribution to feeding behavior and, furthermore, in stress-induced anorexia. Given the fact that knock-out phenotypes are often best revealed after an environmental or pharmacological challenge, we decided to investigate a series of behaviors after such challenges. Several studies have revealed disorders in novelty-induced locomotion in 5-HT receptor knock-out mice: 5-HT1A (Ramboz et al., 1998), 5-HT1B (Brunner et al., 1999), 5-HT2C (Rocha et al., 2002), and 5-HT5A (Grailhe et al., 1999); whereas these mutant mice did not exhibit any locomotion impairments in their home cage, reinforcing the view that (1) adaptive mechanisms are limited in such mice and (2) serotoninergic systems modulate stress responsiveness in situations such as reactivity to a novel environment.
Deficits in adaptation to stress and novel environments may be related to change in neuronal network excitability. Interestingly, 5-HT2C receptor-null mice exhibit an increased sensitivity to convulsant pentylenetetrazol (PTZ), which induces seizures (Tecott et al., 1995). This suggests that 5-HT inhibits neuronal network excitability, as reported previously (Jobe et al., 1973; Sparks and Buckholtz, 1985; Dailey et al., 1992). We generated mice lacking the gene encoding 5-HT4 receptors by homologous recombination to study the various functions of this receptor.
Materials and Methods
5-HT4 gene targeting. The gene-encoding 5-HT4 receptors was cloned from a 129 Sv-J genomic library (Genome Systems, St. Louis, MO) using mouse 5-HT4 cDNA probe. DNA PstI fragments from 5-HT4 receptor gene were then cloned in pGEM-5Zf(-). Genes encoding neomycin phosphotransferase (Neo), under the control of the phosphoglycerate kinase I promoter, were inserted into a created XbaI site in the exon encoding the third transmembrane domain. The DNA construct was transfected in W9.5 embryonic stem cells (800 V and 3 μF; Gene Pulser; Bio-Rad, Hercules, CA) with 30 μg of the targeting construct. The transfected embryonic stem cells were then plated onto mitomycin C-treated mouse embryonic fibroblasts for 1 week in the presence of G418 (150 μg/ml of active substance). The G418-resistant clones were screened by Southern blot with an AseI digest and a 32P-labeled outside probe (300 bp BglII/PstI fragment). Positive cells for the targeting event were injected into C57BL6/J blastocysts and implanted in B6CBAF1/J foster mothers, which gave birth to chimeric mice. Chimeras were mated with 129/Sv females to generate heterozygous mutant (+/-) mice on a pure 129/Sv genetic background. The resulting heterozygous mice were bred and generated 18% homozygous mutant mice (Table 2). The experiments were performed in accordance with the National Institutes of Health's Guide for Care and Use of Laboratory Animals.
Table 2.
Ratio of wild-type, heterozygote, and homozygote mutants derived from heterozygote crossings
|
Wild types |
Heterozygotes |
Homozygotes |
|---|---|---|
| 36% (414) |
46% (532) |
18% (209) |
Animals. All experiments were performed on male wild-type and 5-HT4 receptor knock-out mice (4-6 months of age) on a 129/Sv genetic background. All were obtained from heterozygous breeding at the transgenic animal facility of Columbia University and Unité Propre de Recherche Centre National de la Recherche Scientifique 2580. Each mouse was identified using PCR technique. Mice were housed with food and water available ad libitum (n = 5 per cage) and maintained in a temperature-controlled environment on a 12 hr light/dark cycle with light onset at 6 A.M. For each experimental paradigm, we used different groups of wild-type and mutant mice.
Feeding paradigms test. In a first set of experiments, the body weight of mice of both genotypes was measured when housed in their home cage (n = 5), from days 21 to 52 after birth. A second group of mice (4 months of age) was housed individually to measure body weight, food intake, and metabolism parameters.
In a third set of experiments, a new set of mice was used to analyze their feeding responses after the restraint stress. On the basis of a previous study of rats (Rybkin et al., 1997), each experiment was divided into three periods: baseline (7 d), day of restraint stress, and recovery period (10 d after stress). Food intake (normal chow) and body weight of isolated mice were measured daily at 9:00 A.M., 2 hr after the start of light cycle for both periods. In each experiment, mice were divided into control and restraint groups matched for average weight. On the day of stress, day 8, control wild-type mice (n = 17) and 5-HT4 receptor-null animals (n = 13) were individually placed in regular cages without any food or water for 110 min. Thirteen additional wild-type mice and 15 mutant animals were restrained in ventilated tubes of 50 ml of polypropylene. An interval of 3 min was maintained between each mouse to measure its body weight, and the stress was applied when necessary. After 110 min of a stress period, restrained animals returned to their regular cages with free access to classic food (16.5% crude proteins, 3.6% crude fat, 4.6% crude fiber, 5.2% Ash) and water. Food and water were provided to unrestrained animals 110 min after their manipulation.
Novelty tests. Open-field test: mice were tested for 30 min on three consecutive test days. The open-field test environment is a square chamber with an inside area that measures 43.2 × 43.2 × 30.5 cm. Mice were placed in the center and monitored with 32 infrared light sources spaced 0.5 inches apart (1.25 cm) (Med Associates, Georgia, VT) specifically adapted to record the location and the traveled path length.
Pentamethylenetetrazol-induced seizure. After a systemic injection of PTZ (60 mg/kg, 10 ml/kg) in mice of both genotypes, a trained observer evaluated their seizure profile and recorded the latency-to-seizure: latency to the first twitch, clonic-tonic seizure >5 sec with a loss of righting reflex, full tonic seizure. Finally, the duration to death was recorded. Seizure profiles were assessed through 30 min after PTZ dosing (no response, ear and facial twitching, myoclonic body jerks, clonic forelimb, generalized convulsions with tonic extension episode, status epilepticus). A score to each seizure was assigned (0, no motor seizure; 1, prone stretch posture, ataxia, tremors, immobile with hindlimb splay; 2, head search, twitches, tonic tremors, immobility; 3, slight clonic-tonic seizure <5 sec, chomping; 4, pawing, praying, trumpet, pop slight clonic-tonic seizures, increased frequency and duration; 5, clonic-tonic seizure >5 sec with loss of righting reflex; 6, crazy and tonic seizures with extension of hindlimbs, death). The occurrence of death was recorded during 30 and 60 min after injection.
Autoradiography. Frontal brain sections (15 μm) from adult (60 d) wild-type, heterozygous, and 5-HT4 receptor-null mice (n = 6 mice per genotype) were thaw-mounted on gelatin-coated slides and stored (-80°C). 5-HT4 receptor sites were labeled using the specific [3H]-GR113808 5-HT4 receptor antagonist, as reported previously (Compan et al., 1996). Briefly, sections were incubated for 30 min at 37°C in the following medium: [3H]-GR113808 (Amersham Biosciences, Little Chalfont, UK; specific activity, 83 Ci/mmol; final concentration, 0.1 nm), 50 mm HEPES, pH 7.4, 10 μm pargyline (Sigma, St. Louis, MO), and 0.01% ascorbic acid. Nonspecific binding was determined on consecutive sections incubated in the presence of 10 μm 5-HT (Sigma). The labeled sections were exposed to [3H]-Hyperfilms alongside tritiated polymer standards (Amersham Biosciences). The films were developed in Kodak (Rochester, NY) D-19 after a 2 month exposure at 4°C. Sections from all genotypes were processed together to obtain corresponding radiograms on the same films.
Radioimmunoassay: corticosterone. Animals were singly housed for 4 d. A first blood sample was collected after a small tail incision on the fourth day. After 24 hr, the same animals were subjected to the elevated plus maze (EPM; square center platform and four arms, 30 cm above the floor), and, 30 min later, a second blood sample was taken from the incised tail over 10 min period. Blood samples were immediately centrifuged (4°C), and plasma samples were stored in -80°C until use. The concentration of corticosterone was measured using appropriate [125I]-radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA). In parallel, two other groups of mice were used to evaluate the amount of food consumed after a simple handling (Procedure 1) or handling and EPM (Procedure 2). Food consumption was also measured 3 hr after the beginning of the EPM (2.30 hr after the second tail incision; Procedure 3).
Data analysis. Data were analyzed using Statview 5 (Abacus Concepts, Calabasas, CA). A repeated measures ANOVA was performed on data that were obtained in multiple sessions over time. Genotype and stress were used as independent variables, if necessary. Food intake, body weight, or parameters from the elevated plus maze or open-field test session were used as dependant variables. If significant effects of genotype or stress or a genotype x treatment interaction, overtime or not, were found, the independent variables were split for a two-way (genotype and stress) or one-way ANOVA (genotype or treatment) analysis. For multiple comparisons, we used the Scheffé F test with a probability of 0.01 and 0.05 as a significant difference.
Results
Generation of 5-HT4 receptor-null mice
We generated 5-HT4 receptor-null mice following standard procedures (Fig. 1). In this study, we used only male mice (4-6 months of age) born from couples of heterozygous mice. To verify that 5-HT4 receptors were absent in homozygous mutant animals, we performed coronal brain sections from wild-type, heterozygous, and homozygous mutant (null) mice using the selective 5-HT4 antagonist [3H]GR113808. In wild-type mice, a heterogeneous distribution of [3H]GR113808-binding sites was observed in the limbic system, hippocampal formation, or basal ganglia, in agreement with previous observations (Waeber et al., 1994; Compan et al., 1996). No specific binding site was found in 5-HT4 receptor-null mice, confirming the absence of receptor proteins (Fig. 2). In heterozygous mice, the density of [3H]GR113808-binding sites was decreased in all examined brain structures when compared with control animals (Table 1). In the absence of compensations in the heterozygous mice, we would expect 50% of mRNA, as described in the 5-HT1A receptor knock-out mice (Ramboz et al., 1998). This 50% of mRNA may translate in 50% of protein (ventral pallidum, rostral striatum). However, in some structures, such as the hippocampus, the values are considerably lower. This result may be explained by nonlinear changes in conformation of the receptor (such as dimer formation) or by a nonlinear trafficking of the receptor to various intracellular compartments (axons vs dendrites), as described previously for the 5-HT1B receptors (Ghavami et al., 1999).
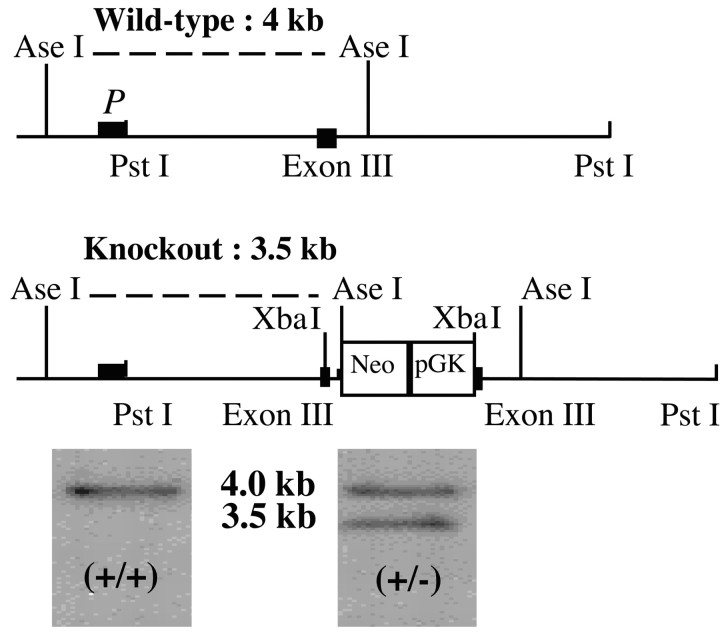
Figure 1.
Targeted mutation of the 5-HT4 receptor gene. a, Schematic diagram of a 6.5 kb 5-HT4 genomic fragment encoding for the transmembrane domains II and III of 5-HT4 receptors (exon III). The solid line (P) represents the external probe to detect Ase I-hybridizing restriction fragments (4 kb) from wild-type genomic DNA. b, Schematic representation of the 5-HT4-targeting vector. The neomycin phosphotransferase gene (Neo) under the control of the phosphoglycerate kinase I promoter (pGK) is inserted in an engineered-created XbaI site localized in the DNA sequence encoding for the transmembrane domain III. The size of Ase I-hybridizing restriction fragments is 3.5 kb to identify the knock-out genomic DNA. c, Southern blot of genomic DNA from embryonic stem cells digested with Ase I and hybridized by the external probe.
Figure 2.
Radiograms of 5-HT4 receptor-binding sites labeled with [3H]GR113808 in the frontal brain sections from wild-type, heterozygoous, and 5-HT4 receptor-null mice. In wild-type animals, the [3H]GR113808 binding is more intense in the nucleus accumbens shell than the core, as analyzed previously in rat24. At the same levels, the olfactory tubercles and fundus striati, like the striatum tail in a next posterior level, exhibit strong labeling. The various hypothalamic nuclei show moderate density (medial preoptic area). The concentration of binding sites covered an intermediate range, although a classic distinct laminar pattern is observed in the hippocampal formation: it is weak in the dorsal and medial raphe nuclei.
Table 1.
Densities of specific binding sites for [3H] GR113808 (0.1 nm) in adult wild-type and 5-HT4 receptor heterozygote mice
|
|
Bound receptor (mean ± SEM) in fmol/mg protein* |
||
|---|---|---|---|
| Regions |
Wild types |
Heterozygotes |
|
| Olfactory tubercles | 201 ± 34 | 66 ± 16 (67%) | |
| Pallidum ventral | 171 ± 37 | 53 ± 21 (70%) | |
| Nucleus accumbens | 116 ± 28 | 36 ± 16 (68%) | |
| Rostral striatum | 115 ± 15 | 48 ± 11 (48%) | |
| Caudal striatum | 142 ± 25 | 18 ± 10 (85%) | |
| Globus pallidus | 90 ± 20 | 14 ± 8 (85%) | |
| Hippocampus |
104 ± 11 |
9 ± 8 (91%) |
|
Assuming an even concentration of 1 mg of protein per 10 mg of tissue throughout the brain.
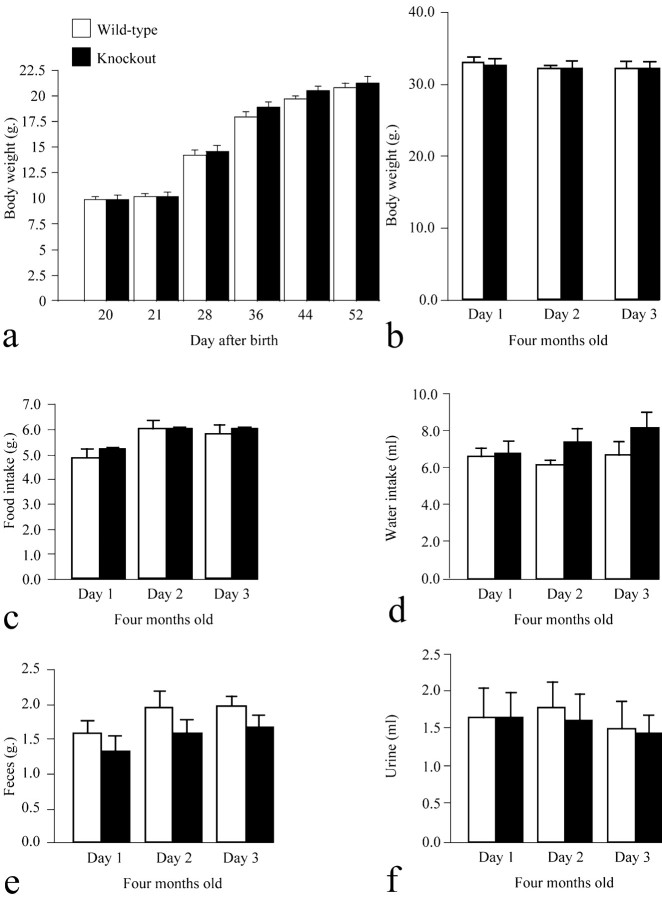
A small Mendelian bias against null mutant mice was observed over several generations, with only 18% of the offspring of heterozygous crossings resulting in homozygous mutant mice, over a 3 year period (Table 2). As yet, we have no explanation for this unexpected non-Mendelian ratio. A cardiovascular or respiratory problem may have occurred (Edwards and Paton, 1999; Eftekhari et al., 2001; Manzke et al., 2003). Despite this bias, the mice lacking 5-HT4 receptors appeared to develop normally. When grouped in their home cages, no difference in body weight was detected between wild-type and null mice during development (Fig. 3a) or at 4 months of age (Fig. 3b). In their home cage, no differences in eating (Fig. 3c), drinking (Fig. 3d), or metabolism (Fig. 3e,f) have been found between mice of both genotypes at 4 months of age. In addition, no differences in social behavior, aggression, or sleep have been observed between mice of both genotypes (data not shown).
Figure 3.
The body weight of 5-HT4 receptor-null and wild-type mice is similar during development. a, Data are mean ± SEM in body weight gain for a group of 20 wild-type and knock-out animals. Repeated measures ANOVA revealed no significant difference in body weight between mice of both genotypes from day 21 to 52 after birth. b-f, No significant difference between wild-type (n = 14) and 5-HT4 receptor-null (n = 13) mice has been found at 4 months of age in body weight (b), feeding (c), water intake (d), or metabolism (e,f).
Abnormal consumption of food after stress in 5-HT4 receptor-deficient mice
We tested the motivation of wild-type and 5-HT4 receptor-null mice for foods after restraint stress, a proposed animal model of anorexia nervosa (Rybkin et al., 1997). Daily total food intake and body weight were measured during 8 d before the stress control period (habituation) and 10 d recovery period. The restraint stress was applied for duration of 110 min at the end of the habituation period. For all the following experiments, the animals were separated from their congeners and housed in individual cages.
Although food intake in 5-HT4 receptor-null mice did not significantly differ from wild-type mice during the habituation period, they gained less body weight than wild-type mice over this same period (F(6,360) = 2.46; p < 0.03). Separate ANOVAs confirmed that body weight gain was significant for the wild-type mice (F(6, 174) = 4.45; p < 0.001) but did not reach significance for 5-HT4 receptor-null mice (F(6,186) = 0.94) (Fig. 4c,d). Discrepancy between food intake and body weight, as reported previously for mice (Yamada et al., 2000), may be associated with changes in metabolism, resulting in an alteration in fat content. In a series of new experiments, perigonadic adipose tissue was carefully dissected. We found that, after stress, the ratio of white adipose tissue weight/body weight was lower in null than wild-type mice (-38%; 0.021 ± 0.001 vs 0.013 ± 0.013; F(1,23) = 15.92; p < 0.001), again with no difference in their total body weight (wild-type, 27.32 ± 0.649, n = 16; knock-out, 26.38 ± 0.853, n = 9).
Figure 4.

In 5-HT4 receptor-null mice, the restraint stress lost its ability to decrease food intake and body weight. Data are means ± SEM daily total food intake and body weight gain or loss for groups of 14 wild-type (WT) and 18 WT mice plus stress (a,c), 17 knock-out (KO), and 16 KO animals plus stress (b,d). Data were measured from the first day (9:00 A.M. in the light cycle) for the habituation (8 d) and recovery periods (10 d). The 110 min acute restraint stress or immobilization was applied on day 8 (arrow). Repeated measures ANOVA indicated significant effects of stress on food intake over the recovery period (F(9,567) = 13.99; p < 0.0001) and an interaction of genotype and time (F(9,549) = 2.2; p < 0.05). Subsequently, ANOVA analysis for each genotype and day of experiment revealed a significant difference in food intake between genotypes 24 hr after stress (F(1,61) = 6.73; p < 0.05). a, Stress-induced anorexia in WT animals for the 48 hr recovery period. b, This effect is less marked in KO mice. In parallel, repeated measures ANOVA revealed significant effects of stress on body weight gain over the recovery period (F(9,522) = 11.33; p < 0.0001) and an interaction of genotype and time (F(9, 522) = 2.38; p < 0.05). c, d, Stress-induced, significant marked decreases in body weight gain in WT mice (c) but less or not effective in KO mice (d). Significant differences between restrained and unrestrained animals are marked (§§§p < 0.0001; §§p < 0.001; §p < 0.05). Significant genotype effect is noted (*p < 0.05), and significant genotype x stress interaction is noted (#p < 0.05).
Restraint stress induced a marked decrease of food intake in wild-type mice, as compared with their unrestrained genotype counterparts (-36.4%) (Fig. 4a); however, this effect was lost, in part, in mutant animals (-19.6%) (Fig. 4b). A post hoc comparison between mice of both genotypes revealed that 5-HT4 receptor-null mice consumed significantly more food than wild-type animals during the first 24 hr after the restraint stress (+24%; p < 0.05). No significant effect on food intake was detected in 5-HT4 receptor-null mice 48 hr after restraint stress as compared with their baseline level (Fig. 4b).
Restraint stress also provoked a significant body weight loss in wild-type animals for the first 4 d of the recovery period (Fig. 4c). In contrast, restraint stress did not induce body weight loss in 5-HT4 receptor-null mice (Fig. 4d). They were not totally insensitive to immobilization, because a significant difference between the body weight changes of restrained and unrestrained mutant mice was detected 24 hr after stress (Fig. 4d). This was because of a slight body weight gain of unrestrained mutant animals, which was also found in unrestrained wild-type mice (Fig. 4c,d). This effect may be related to the 110 min of food deprivation in both restrained and unrestrained animals (see Materials and Methods).
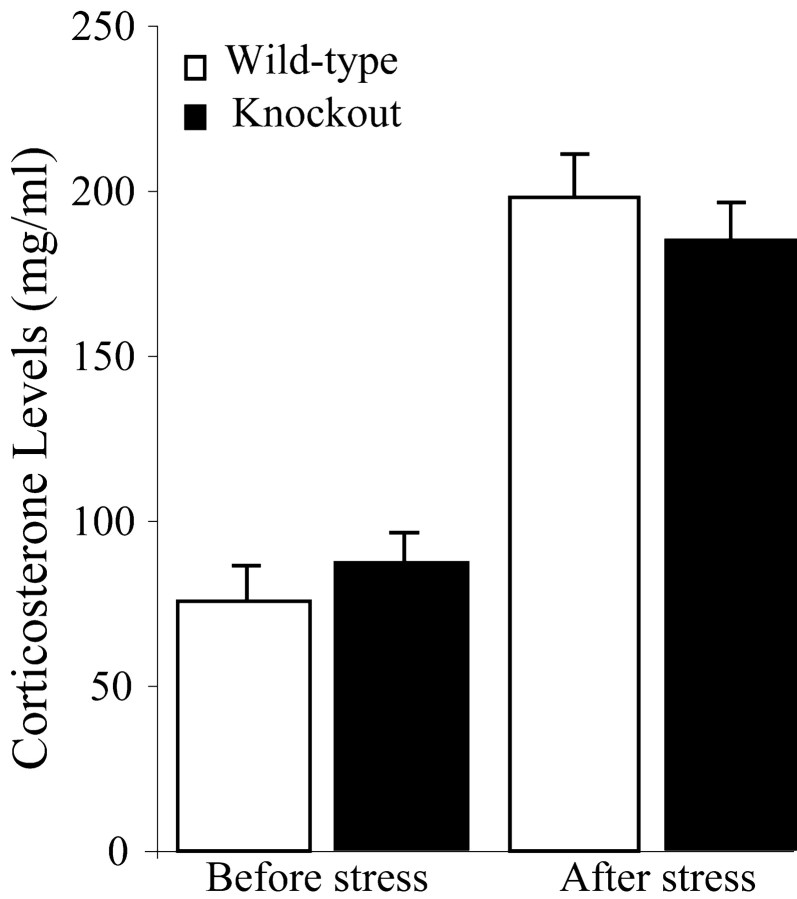
The reduced sensitivity of 5-HT4 receptor-null mice to stress-induced anorexia was also found in a gradual stressful task (handling, elevated plus maze combined, or not, with a small tail incision) (Table 3). We then tested whether the lack of 5-HT4 receptors would alter the activity of the hypothalamo-pituitary axis in response to stress. However, no difference in corticosterone levels was found between wild-type and mutant mice before or after the elevated plus maze (Fig. 5).
Table 3.
Feeding responses after a gradual stressful task
|
|
Procedure 1 |
Procedure 2 |
Procedure 3 |
|---|---|---|---|
| Wild type | 0.82 ± 0.07 (n = 9) | 0.64 ± 0.07 (n = 9) | 0.20 ± 0.08 **,*** (n = 8) |
| Knock-out mice |
0.91 ± 0.08 (n = 10) |
0.89 ± 0.09 * (n = 10) |
0.45 ± 0.09 *,**,*** (n = 10) |
Data are mean ± SEM of total food intake, measured 3 hr after the beginning of adverse procedures using wild-type and 5-HT4 receptor null mice. ANOVA revealed a significant effect of genotype (F(1,50) = 8.18; p < 0.01) and procedure (F(2,50) = 21.81; p < 0.0001). A gradual stressful paradigm (Procedure 1, isolation for 3 hr; Procedure 2, elevated plus maze, EPM 5 min and isolation for 3 hr; Procedure 3, isolation for 96 hr, small tail incision, saline injection, EPM) significantly reduced food intake in wild-type mice (**p<0.01), whereas the anorectic effect was reduced in 5-HT4 receptor null mice. The significance between Procedures 1 and 2 or 3 are respectively marked (**p<0.01; ***p<0.01). A significant genotype effect is noted (*p<0.05).
Figure 5.
The absence of 5-HT4 receptors did not alter a stress-induced increase in corticosterone levels. Data are mean ± SEM corticosterone levels in wild-type (n = 9) and mutant mice (n = 12) 24 hr before and 30 min after a 5 min trial in the elevated plus maze.
5-HT4 receptor-null mice are less reactive in novel environments
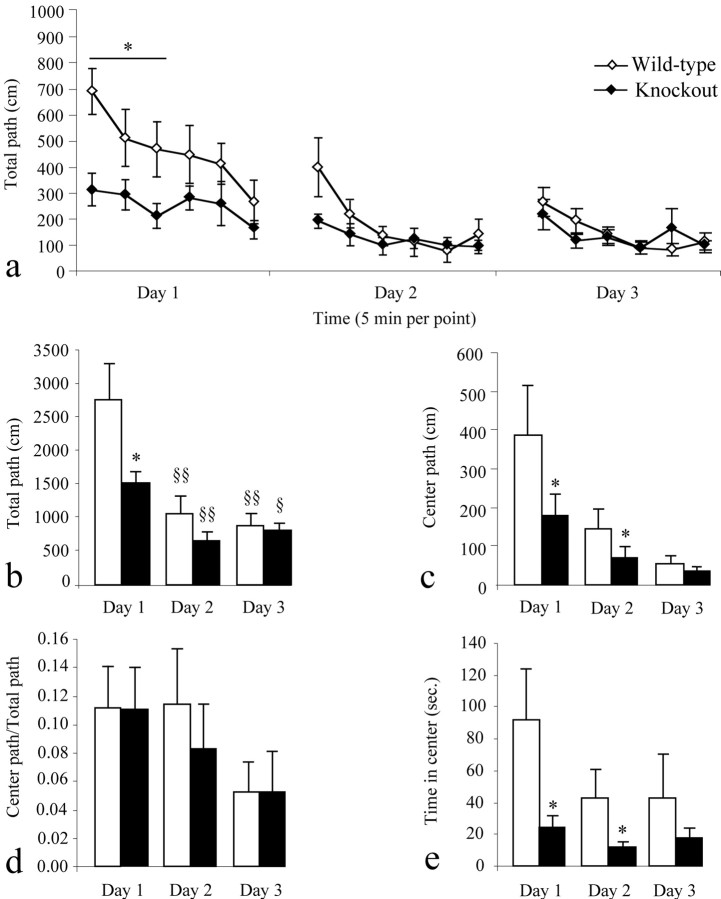
5-HT4 receptor-null mice were placed into an open field, and their locomotion was monitored for 30 min. The test was repeated for three consecutive days to evaluate habituation to a novel environment. The mutant mice displayed an overall decrease in the traveled path length on the first day of exposure, as compared with wild-type animals (-45%; F(1,14) = 6.76; p < 0.05) (Fig. 6a,b) in both the periphery and center of the open field (Fig. 6b,c,d). During the second and third day, the horizontal activity of 5-HT4 receptor-null and wild-type mice was similar. Less reactivity in 5-HT4 receptor-null mice has also been found in the elevated plus maze and alley tests (data not shown). In contrast, there was no difference in locomotion between the mutant and wild-type animals in their home cages (data not shown). In addition, the mutant mice did not exhibit impairment in the rotarod test, which demonstrates that there is no deficit in motor coordination in 5-HT4 receptor-null mice (data not shown). Together, these results suggest that the 5-HT4 receptor-null mice display a decreased reactivity to novelty rather than a locomotor impairment.
Figure 6.
5-HT4 receptor-null mice are less reactive in the open field than wild-type animals. a-e, Naive wild-type (open bars or points) and mutant (filled bars or points) animals were placed in identical open fields, and their horizontal activity (a-d) and time in the center (e) were monitored for 30 min (5 min per point) for three consecutive days. a, The mutant males were less active in the open field for the first 15 min of day 1 in the open field as compared with wild-type mice, whereas this effect was not detected over the following days. b, In the same line, the total distance traveled was lower in mutant animals than wild-type mice on the first day of exposure but not day 2 or 3. c, d, The absence of significant difference in the center/total path ratio between mice of both genotypes (d) indicated that this lower activity was detected in any part of the open field, as illustrated for the center (c). The mutant mice spent less time in the center during the first two consecutive days (e), which suggests a slight hyperanxiety-like behavior in the absence of 5-HT4 receptors. Significant genotype effect is noted (*p < 0.05). Significant differences in path between days for the wild-type or null mice are noted (§§p < 0.01; §p < 0.05).
5-HT4 receptor-null mice are hypersensitive to pentylenetetrazol-induced seizures
Beyond the possibility that inabilities to adapt may be related to impairments in neuronal network excitability, we noticed that 5-HT2C receptor (Tecott et al., 1995), norepinephrine (Thomas and Palmiter, 1997; Szot et al., 1999), and NPY (Erickson et al., 1996; Baraban et al., 1997) null mice exhibit both hypersensitivity to convulsants and changes in appetite. A better understanding of such coexisting mechanisms may provide clues to help prevent the increase in body weight often observed in humans treated with anticonvulsants (for review, see Jallon and Picard, 2001).
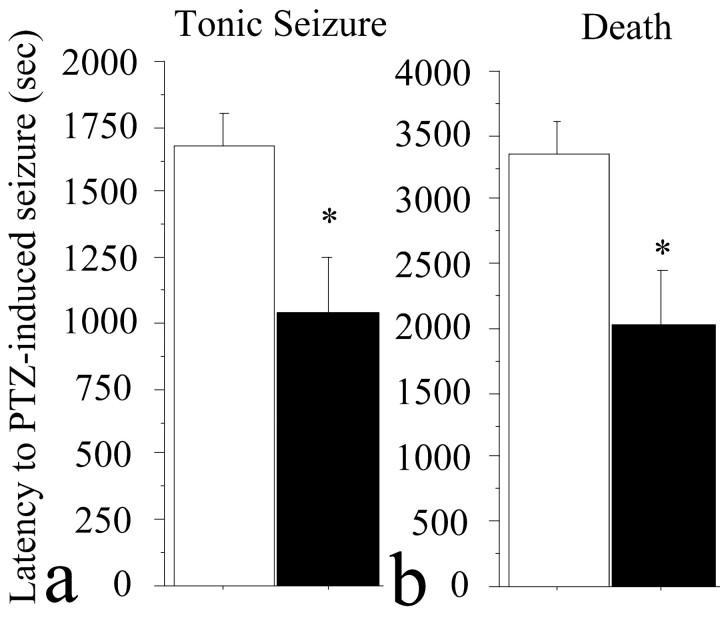
To analyze the seizure susceptibility of 5-HT4 receptor-null mice, we used the response to the convulsant PTZ (a GABAA receptor antagonist) as an overall index of neuronal network excitability. The 5-HT4 receptor-null mice were more sensitive to PTZ-induced seizures compared with wild-type animals, as shown in an increased number of deaths at 60 min after treatment (Fisher exact test; p < 0.04) and decreased latency to both death and tonic seizure (F(1,27) = 6.44; p < 0.02) (Fig. 7).
Figure 7.
The 5-HT4 receptor-null mice are hypersensitive to the convulsant GABAA receptor PTZ compared with wild-type animals (open bars). a, b, Data are means ± SEM latency (s.) after the PTZ administration-induced tonic seizures (a) and death (b) in mice of both genotypes. Significant genotype effect is noted (*p < 0.05).
Discussion
A number of studies suggest that an increase in 5-HT neuromodulation may contribute to stress-induced anorexia. Stress-related behavioral paradigms, such as conditioned fear, increase 5-HT metabolism and release in the medial prefrontal cortex, nucleus accumbens, amygdala, and dorsal hippocampus (Inoue et al., 1994; Ge et al., 1997; Konstandi et al., 2000). In particular, restraint stress increases 5-HT turnover in the hypothalamus and amygdala in mice and rats (Konstandi et al., 2000). However, the identities of the 5-HT receptors involved in mediating stress-induced decreases in food intake are primarily unknown. This study provides the first evidence that stress-induced hypophagia may be mediated by 5-HT4 receptors.
The ability of stress to decrease food intake is not only attributable to an increase in the activity of the serotonergic systems but also to the hyperactivity of hypothalamo-pituitary adrenal (HPA) axis (Beck, 2000). Peptides of the corticotropin-releasing hormone (CRH) family, such as stresscopin or urocortin, induce decreases in food intake (Momose et al., 1999). The reciprocal influences between serotonergic systems and HPA axis (Lopez et al., 1999) have made it difficult to identify a clear neurochemical cascade underlying the influence of stress on feeding behavior. A working hypothesis would be that an increase in the activity of the HPA axis could induce an elevation in 5-HT, which, in combination with stress hormones, induces a decrease in food intake. In keeping with this hypothesis, CRH has been shown to stimulate the activity of serotoninergic neurons (Kirby et al., 2000; Lowry et al., 2000). In addition, repeated injections of corticosterone enhance the excitatory effect of 5-HT4 agonists on hippocampal CA1 neurons (Zahorodna et al., 2000). However, despite of the ability of CRH to decrease food intake (Momose et al., 1999), mutant mice lacking CRH are still sensitive to stress-induced hypophagia (Weninger et al., 1999). This finding suggests that other mechanisms are involved. We found that the absence of 5-HT4 receptors did not affect the increase in corticosterone levels after stress. Our results could be explained by two related mechanisms. First, stress induces an increase in the levels of 5-HT that activates 5-HT4 receptors and decreases food intake. Secondly, stress induces an increase in stress hormones, which indirectly increases the activity of 5-HT4 receptors, which in turn results in a decrease in food intake. Numerous studies indicate that corticosterone levels can modify the density, as well as the mRNA levels of 5-HT1A and 5-HT1B receptors. In particular, one recent study indicates that rats chronically treated with corticosterone develop some extra sensitivity of presynaptic 5-HT1B receptors in the hypothalamus (Gur et al., 2001).
Other 5-HT receptors have also been shown to regulate feeding and body weight. For example, the body weight of adult 5-HT2C receptor-null mice is greater than that of wild-type animals (Tecott et al., 1995). These results suggest the presence of at least two modes of action of 5-HT to regulate body weight. In baseline conditions, the body weight is primarily regulated via the 5-HT2C receptors, whereas after an unusual stressful event, 5-HT4 receptors may become involved.
We found that 5-HT4 receptor-null mice are less reactive to different novel environments, whereas their locomotor activity was not altered in their home cages. These results suggest that the absence of 5-HT4 receptors results in an attenuation of the motor responses induced by novel environments. 5-HT1A receptor-null mice are also less reactive to novelty-induced locomotion, whereas it is the inverse for 5-HT1B, 5-HT2C, or 5-HT5A receptor-null mice (Brunner et al., 1999; Grailhe et al., 1999; Rocha et al., 2002). These data suggest that a permanent absence of the genes encoding 5-HT1A, 5-HT1B, 5-HT2C 5-HT5A, or 5-HT4 receptors provokes either a hyporeactivity or hyperreactivity to novelty. Interestingly, a lesion of serotonergic neurons enhanced novelty-induced locomotion (Geyer et al., 1976). However, various modes of 5-HT depletion induce different changes in the density of 5-HT1B and 5-HT2A/2C receptors in the rat basal ganglia, nucleus accumbens, and frontoparietal cortex (Compan et al., 1998a,b), which may in turn induce different motor outcomes. Together, these findings suggest that 5-HT4 receptors modulate the activity of neuronal circuits involved in the response to stress and novelty.
Similar to the 5-HT2C receptor-null mice (Tecott et al., 1995), we found that 5-HT4 receptor-null mice were more sensitive to PTZ-induced seizure. The GABAA receptor antagonist PTZ blocks the neurotransmission of GABA, which in turn increases the excitability of neuronal networks. This result suggests a tonic inhibitory influence of 5-HT4 receptors on the excitability of neurons either in adulthood or during development. Several studies have described a 5-HT4 receptor-mediated increase in neuronal excitability via the blockade of K+ channels in cortical and hippocampal neurons (Dumuis et al., 1988; Bockaert et al., 1990; Chaput et al., 1990; Fagni et al., 1992; Monferini et al., 1993; Ansanay et al., 1995). This could ultimately contribute to the facilitation of neuromediator release. Because 5-HT4 receptors are localized on neurons expressing GABA (Siarey et al., 1995; Compan et al., 1996), decreased GABA release in 5-HT4 receptor-null mice is likely. Indeed, activation of 5-HT4 receptors increases the frequency of postsynaptic GABAA and GABAB IPSP recorded in the hippocampal dentate gyrus (Bijak and Misgeld, 1997). It is difficult to correlate a general decrease in GABA transmission and absence of 5-HT4 receptors all over the brain and feeding behaviors, because stimulation of GABA receptors inhibits or activates food intake when respectively injected in the lateral hypothalamus (Maldonado-Irizarry et al., 1995) or in the shell of the nucleus accumbens and in the paraventricular nucleus of the hypothalamus (Stratford and Kelley, 1997; Pu et al., 1999). Decreased GABA transmission is not always correlated with increased body weight and eating, because hyperanxiety, related to a decrease in GABA transmission, coexists with both anorexia and bulimia (Chesters et al., 1998). This point opens the possibility that the inability of null mice to adapt to the restraint stress may be related to a higher level of anxiety after stress.
Our results suggest that 5-HT4 receptors modulate the activity of neuronal circuits involved in stress-induced hypophagia, reactivity to novelty, and PTZ-induced seizures. It is therefore conceivable that 5-HT4-specific ligands will be useful in treating eating disorders.
Footnotes
This study was supported by Roche Bioscience Grant SSYNTEXCU 53124401 and grants from Columbia University, Centre National de la Recherche Scientifique, and Burgundy University. V.C. was supported in part by a grant from Association Française pour la Recherche Thérapeutique and Roche Bioscience. We are extremely grateful to S. Charriot, S. Claeysen, D. Cockayne, I. Giroux, and M. Sebben for their help. We also greatly appreciate the assistance of K. Holick and Dr. M. Knight in editing this manuscript.
Correspondence should be addressed to Valérie Compan, Unité Propre de Recherche Centre National de la Recherche Scientifique 2580, Génomique fonctionnelle, 141 rue de la Cardonille, Montpellier 34094, France. E-mail: Valerie.Compan@ccipe.cnrs.fr.
Copyright © 2004 Society for Neuroscience 0270-6474/04/240412-08$15.00/0
References
- Ansanay H, Dumuis A, Sebben M, Bockaert J, Fagni L (1995) A cyclic AMP-dependent, long-lasting inhibition of a K+ current in mammalian neurons. Proc Natl Acad Sci USA 92: 6635-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD (1997) Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci 17: 8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B (2000) Neuropeptides and obesity. Nutrition 16: 916-923. [DOI] [PubMed] [Google Scholar]
- Bijak M, Misgeld U (1997) Effects of serotonin through serotonin1A and serotonin4 receptors on inhibition in the guinea-pig dentate gyrus in vitro Neuroscience 78: 1017-1026. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Sebben M, Dumuis A (1990) Pharmacological characterization of 5-HT4 receptors positively coupled to adenylate cyclase in adult guinea pig hippocampal membranes: effect of substituted benzamide derivatives. Mol Pharmacol 37: 408-411. [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Sebben M, Dumuis A (1998) 5-HT4 receptors: gene, transduction and effects on olfactory memory. Ann NY Acad Sci 861: 1-15. [DOI] [PubMed] [Google Scholar]
- Bonasera SJ, Tecott LH (2000) Mouse models of serotonin receptor function: toward a genetic dissection of serotonin systems. Pharamcol Ther 88: 133-142. [DOI] [PubMed] [Google Scholar]
- Brunner D, Buhot MC, Hen R, Hofer M (1999) Anxiety, motor activation, and maternal-infant interactions in 5HT1B knock-out mice. Behav Neurosci 113: 587-601. [DOI] [PubMed] [Google Scholar]
- Callahan MJ (2002) Irritable bowel syndrome neuropharmacology. A review of approved and investigational compounds. J Clin Gastroenterol 35: S58-S67. [DOI] [PubMed] [Google Scholar]
- Chaput Y, Araneda RC, Andrade R (1990) Pharmacological and functional analysis of a novel serotonin receptor in the rat hippocampus. Eur J Pharmacol 182: 441-456. [DOI] [PubMed] [Google Scholar]
- Chesters MH, Monsell S, Cooper PJ (1998) The disorder salient stroop effect as measure psychopathology eating disorders. Int J Eat Disord 24: 65-82. [DOI] [PubMed] [Google Scholar]
- Compan V, Daszuta A, Salin P, Sebben M, Bockaert J, Dumuis A (1996) Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci 8: 2591-2598. [DOI] [PubMed] [Google Scholar]
- Compan V, Ségu L, Buhot M-C, Daszuta A (1998a) Selective increases in serotonin 5-HT1B/1D and 5-HT2A/2C binding sites in adult rat basal ganglia following lesions of serotonergic neurons. Brain Res 793: 103-111. [DOI] [PubMed] [Google Scholar]
- Compan V, Ségu L, Buhot MC, Daszuta A (1998b) Laminar distribution of changes in neuropeptide Y immunoreactivity and 5-HT1A, 5-HT1B/1D, 5-HT2 serotonin receptors in the rat cortex after various modes of serotonergic depletion. Brain Res 795: 264-276. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Mishra PK, Ko KH, Penny JE, Jobe PC (1992) Serotonergic abnormalities in the central nervous system of seizure-naive genetically epilepsy-prone rats. Life Sci 50: 319-326. [DOI] [PubMed] [Google Scholar]
- Dumuis A, Bouhelal R, Sebben M, Cory R, Bockaert J (1988) A non classical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol Pharmacol 34: 880-887. [PubMed] [Google Scholar]
- Edwards E, Paton JF (1999) 5-HT(4) receptors in nucleus tractus solitarii attenuate cardiopulmonary reflex in anesthetized rats. Am J Physiol 277: H1914-H1923. [DOI] [PubMed] [Google Scholar]
- Eftekhari P, Roegel JC, Lezoualc'h F, Fischmeister R, Imbs JL, Hoebeke J (2001) Induction of neonatal lupus in pups of mice immunized with synthetic peptides derived from amino acid sequences of the serotoninergic 5-HT4 receptor. Eur J Immunol 31: 573-579. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Wong EH, Dumuis A, Bockaert J (1995) Central 5-HT4 receptors. Trends Pharmacol Sci 16: 391-398. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD (1996) Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381: 415-421. [DOI] [PubMed] [Google Scholar]
- Fagni L, Dumuis A, Sebben M, Bockaert J (1992) The 5-HT4 receptor subtype inhibits K+ current in colliculi neurons via activation of a cyclic AMP-dependent protein kinase. Br J Pharmacol 105: 973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Barnes NM, Costall B, Naylor RJ (1997) Effect of aversive stimulation on 5-hydroxytryptamine and dopamine metabolism in the rat brain. Pharmacol Biochem Behav 58: 775-783. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Puerto A, Menkes DB, Segal DS, Mandell AJ (1976) Behavioral studies following lesions of the mesolimbic and mesostriatal serotonergic pathways. Brain Res 106: 257-269. [DOI] [PubMed] [Google Scholar]
- Ghavami A, Stark KL, Jareb M, Ramboz S, Segu L, Hen R (1999) Differential addressing of 5-HT1A and 5-HT1B receptors in epithelial cells and neurons. J Cell Sci 112: 967-976. [DOI] [PubMed] [Google Scholar]
- Grailhe R, Waeber C, Dulawa SC, Hornung JP, Zhuang X, Brunner D, Geyer MA, Hen R (1999) Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor. Neuron 22: 581-591. [DOI] [PubMed] [Google Scholar]
- Grignaschi G, Mantelli B, Samanin R (1993) The hypophagic effect of restraint stress in rats can be mediated by 5-HT2 receptors in the paraventricular nucleus of the hypothalamus. Neurosci Lett 152: 103-106. [DOI] [PubMed] [Google Scholar]
- Gur E, Dremencov E, Lerer B, Newman ME (2001) Functional effects of corticosterone on 5HT(1A) and 5-HT(1B) receptor activity in rat brain: in vivo microdialysis studies. Eur J Pharmacol 411: 115-122. [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Lee MD, Dourish CT, Clifton PG (2002) Serotonin 2C receptor agonists and the behavioural satiety sequence in mice. Pharmacol Biochem Behav 71: 691-700. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tsuchiya K, Koyama T (1994) Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol Biochem Behav 49: 911-920. [DOI] [PubMed] [Google Scholar]
- Jallon P, Picard F (2001) Bodyweight gain and anticonvulsants: a comparative review. Drug Saf 24: 969-978. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Picchioni AL, Chin L (1973) Role of brain 5-hydroxytryptamine in audiogenic seizure in the rat. Life Sci 13: 1-13. [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ (2000) Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology 22: 148-162. [DOI] [PubMed] [Google Scholar]
- Konstandi M, Johnson E, Lang MA, Malamas M, Marselos M (2000) Noradrenaline, dopamine, serotonin: different effects of psychological stress on brain biogenic amines in mice and rats. Pharmacol Res 41: 341-346. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ (1999) Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100, 907. Neural circuits mediating stress. Biol Psychiatry 46: 1461-1471.10599476 [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD (2000) Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci 20: 7728-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J, Yamamoto A, Scearce-Levie K, Saudou F, Hen R (1998) Absence of fenfluramine-induced anorexia and reduced C-Fos induction in the hypothalamus and center amygdaloid complex of serotonin 1B receptor knock-out mice. J Neurosci 18: 5537-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15: 6779-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW (2003) 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226-229. [DOI] [PubMed] [Google Scholar]
- Momose K, Inui A, Asakawa A, Ueno N, Nakajima M, Fujimiya M, Kasuga M (1999) Intracerebroventricularly administered corticotropin-releasing factor inhibits food intake and produces anxiety-like behaviour at very low doses in mice. Diabetes Obes Metab 5: 281-284. [DOI] [PubMed] [Google Scholar]
- Monferini E, Gaetani P, Rodriguez y Baena R, Giraldo E, Parenti M, Zocchetti A, Rizzi CA (1993) Pharmacological characterization of the 5-hydroxytrytamine receptor coupled to adenylyl cyclase stimulation in human brain. Life Sci 52: 61-65. [DOI] [PubMed] [Google Scholar]
- Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP (1999) Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology 140: 933-940. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R (1998) Serotonin receptor 1A knock-out: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA 95: 14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Goulding EH, O'Dell LE, Mead AN, Coufal NG, Parsons LH, Tecott LH (2002) Enhanced locomotor, reinforcing, and neurochemical effects of cocaine in serotonin 5-hydroxytryptamine 2C receptor mutant mice. J Neurosci 22: 10039-10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB (1997) Effect of restraint stress on food intake and body weight is determined by time of day. Am J Physiol 273: R1612-R1622. [DOI] [PubMed] [Google Scholar]
- Schechter LE, Simansky KJ (1988) 1-(2, 5-Dimethoxy-4-iodophenyl)-2-aminopropane (DOI) exerts an anorexic action that is blocked by 5-HT2 antagonists in rats. Psychopharmacology 94: 342-346. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Andreasen M, Lambert JD (1995) Serotoninergic modulation of excitability in area CA1 of the in vitro rat hippocampus. Neurosci Lett 199: 211-214. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Buckholtz NS (1985) Combined inhibition of serotonin uptake and oxidative deamination attenuates audiogenic seizures in DBA/2J mice. Pharmacol Biochem Behav 23: 753-757. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17: 4434-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Weinshenker D, White SS, Robbins CA, Rust NC, Schwartzkroin PA, Palmiter RD (1999) Norepinephrine-deficient mice have increased susceptibility to seizure-inducing stimuli. J Neurosci 19: 10985-10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D (1995) Eating disorder and epilepsy in mice lacking 5-HT2C serotonin receptors. Nature 374: 542-546. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Palmiter RD (1997) Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387: 94-97. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH (1999) Reduced satiating effect of d-fenfluramine in serotonin 5-HT2C receptor mutant mice. Psychopharmacology 143: 309-314. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Dourish CT, Kennett GA (2001) Evidence that hypophagia induced by d-fenfluramine and d-norfenfluramine in the rat is mediated by 5-HT2C receptors. Neuropharmacology 41: 200-209. [DOI] [PubMed] [Google Scholar]
- Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A (1994) Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology 33: 527-541. [DOI] [PubMed] [Google Scholar]
- Weninger SC, Muglia LJ, Jacobson L, Majzoub JA (1999) CRH-deficient mice have a normal anorectic response to chronic stress. Regul Pept 84: 69-74. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ohki-Hamzaki H, Wada K (2000) Differential effects of social isolation upon body weight, food consumption, and responsiveness to novel and social environment in bombesin receptor subtype-3 (BRS-3) deficient mice. Physiol Behav 68: 555-561. [DOI] [PubMed] [Google Scholar]
- Zahorodna A, Tokarski K, Bijak M (2000) Repeated corticosterone administration increases excitatory effect of 5-HT4 receptor agonist in the rat hippocampus. Pol J Pharmacol 52: 107-109. [PubMed] [Google Scholar]