Abstract
AMP-activated protein kinase (AMPK) is tightly regulated by the cellular AMP:ATP ratio and plays a central role in the regulation of energy homeostasis and metabolic stress. A pharmacological activator of AMPK, 5-amino-4-imidazole carboxamide riboside (AICAR) inhibited lipopolysaccharide (LPS)-induced expression of proinflammatory cytokines (tumor necrosis factor α, interleukin-1β, and interleukin-6) and inducible nitric oxide synthase in primary rat astrocytes, microglia, and peritoneal macrophages. AICAR attenuates the LPS-induced activation of nuclear factor κB via downregulation of IκB kinase α/β activity. It also inhibits nuclear translocation of CCAAT/enhancer-binding protein (C/EBP) transcription factor by inhibiting the expression of C/EBP-δ in brain glial cells. The dominant negative form of AMPKα2 (D157A) and its antisense documents a possible role of AMPK in the regulation of the cellular proinflammatory process. AICAR also inhibited the production of inflammatory mediators in serum and their expression in CNS of rats injected with a sublethal dose of LPS by intraperitoneal injection. These observations in cultured cells as well as in the animal model suggest that AICAR may be of therapeutic value in treating inflammatory diseases.
Keywords: glia, AICAR, NF-κB, inflammation, C/EBP, AMPK
Introduction
AMP-activated protein kinase (AMPK) is a multisubstrate protein kinase that appears to play a central role in response to metabolic stress (Hardie et al., 1998). It has been suggested that the AMPK system evolved to protect the cell against ATP depletion by inhibiting biosynthetic pathways and stimulating energy-generating pathways (Hardie and Carling, 1997; Hardie et al., 1998; Winder and Hardie, 1999). AMPK plays a major role in the regulation of lipid metabolism and phosphorylates key regulatory enzymes, including 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) reductase, acetyl CoA carboxylase, and hormone-sensitive lipase (Hardie, 1992). The activity of AMPK is regulated in a complex manner by adenine nucleotides as well as by an upstream kinase, AMPK kinase (AMPKK), and becomes active when the AMP levels are high relative to ATP (Hardie et al., 1998). 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR)has been used extensively to activate AMPK in various cell types (Sullivan et al., 1994; Corton et al., 1995). Once inside the cell, AICAR is phosphorylated to AICA riboside monophosphate (ZMP) and mimics the multiple effects of AMP on the allosteric activation of AMPK without altering the levels of nucleotides (Corton et al., 1995). It not only induces allosteric activation, but also promotes phosphorylation and activation of the upstream kinase, AMPK kinase (Moore et al., 1991; Sullivan et al., 1994). The expression of AMPKα1 and α2 catalytic subunits have been reported in the developing mouse brain (Turnley et al., 1999), but their function has yet to be explored.
The predominant inflammatory mediators are multifunctional cytokines that play important roles in normal CNS development as well as in the pathophysiology of the brain in response to injury (Saliba and Henrot, 2001). Inflammation is common to a number of diseases, such as multiple sclerosis, Parkinson's disease, Alzheimer's disease, acquired immunodeficiency syndrome dementia complex, viral/bacterial encephalitis, trauma, and stroke (Bauer et al., 2001; Gebicke-Haerter, 2001). Interleukin-1β, tumor necrosis factor α, interferon-γ, and interleukin-6 are among the best characterized early response proinflammatory cytokines and are synthesized and secreted by several CNS cell types, including microglia, astrocytes, and neurons (Benveniste, 1997; Bauer et al., 2001). Biological effects of these cytokines could influence the progression of injury in the brain, including stimulating the synthesis of other cytokines and neuronal injury mediators such as the expression of inducible nitric oxide synthase (iNOS) and adhesion molecules, leukocyte infiltration, and glial gene expression, finally leading to cell death (Smith et al., 1999; Torreilles et al., 1999).
Recently, statins (inhibitors of HMG-CoA reductase) have been shown to be anti-inflammatory and immunomodulators (Pahan et al., 1997; Kwak et al., 2000). Because AMPK also inhibits HMG-CoA reductase activity by phosphorylation (Hardie, 1992), we investigated the effect of AICAR on the inflammatory response in glial cells. We observed that activation of AMPK by AICAR attenuates the production of proinflammatory cytokines and mediators. Unlike statins, its anti-inflammatory effect is independent of the mevalonate pathway. The present study suggests a possible role of AMPK in the regulation of expression of proinflammatory cytokines, and iNOS and AICAR may be used as therapeutic agents.
Materials and Methods
Cell culture and reagents. Primary rat astrocytes and microglia were prepared from 1- to 3-d-old postnatal Sprague Dawley rat pups (McCarthy and de Vellis, 1980) and maintained in DMEM (4.5 gm glucose/l) with 10% fetal bovine serum (FBS) and antibiotics. On the basis of glial fibrillary acidic protein and macrophage 1 staining, astrocytes, and microglia were >95% pure. Peritoneal macrophages were isolated and cultured in RPMI-1640 supplemented with heat-inactivated 1% FBS medium (Pahan et al., 1997). BV2 is a microglia cell line derived from murine primary microglia provided by Dr. Michael McKinney (Mayo Clinic, Jacksonville, FL) and maintained in DMEM (4.5 gm glucose/l) supplemented with 10% FBS and antibiotics. DMEM (4.5 gm glucose/l), RPMI-1640 medium, fetal bovine serum, and HBSS were from Invitrogen (Grand Island, NY). Lipopolysaccharide (LPS) (Escherichia coli, serotype 055:B5), geranylgeranyl pyrophosphate (GGPP), farnesyl pyrophosphate (FPP), AICAR, mevalonate, and protease inhibitor mixture were from Sigma (St. Louis, MO). Antibodies against iNOS were obtained from Upstate Biotechnology (Waltham, MA). [γ-32P] ATP (3000 Ci/mmol) and [γ-32P]dCTP(3000 Ci/mmol) were from PerkinElmer Life Sciences (Boston, MA). Antibodies for p65; p50; IκB kinase (IKK) α/β; CCAAT/enhancer-binding protein (C/EBP)-α, -β, -δ, and -ϵ; and oligonucleotides for NF-κB and C/EBP were from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant tumor necrosis factor (TNF) α, interleukin (IL)-1β, interferon (IFN)-γ, and ELISA kits for TNFα, IL-1β, IL-6, and IFN-γ were from R & D Systems (Minneapolis, MN). Trizol and transfection reagents (Lipofectamine-2000, Lipofectamine-Plus, and Oligofectamine) were from Invitrogen (Carlsbad, CA). Chloramphenicol acetyltransferase ELISA, β-galactosidase (β-gal), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), and lactic dehydrogenase(LDH) kits were obtained from Roche (Nutley, NJ). The enhanced chemiluminescence-detecting reagents were purchased from Amersham Biosciences (Arlington Heights, IL). The luciferase assay system was from Promega (Madison, WI). Gene expression arrays for inflammatory cytokines were from Superarray (Bethesda, MD). Antibodies against phosphospecific as well as nonphospho-p42/44, -p38, cJun NH(2)-terminal kinase (JNK1/2), and -AMPK were from Cell Signaling (Beverly, MA). NF-κB-luciferase, iNOS-luciferase (3.2 kb), and AMPKα2-dominant negative expression vector (D157A) were provided by Dr. Georges Rawadi (Institut Pasteur, Paris, France), Dr. Hanfang Zhang (Medical College of Georgia, Augusta, GA), and Dr. David Carling (MRC Clinical Sciences Centre, London, UK), respectively. The expression vector for hemagglutinin (HA)-IKKβ was a gift from Dr. Zheng-Gang Liu. The iNOS (-1486/+145)-luciferase and iNOS-C/EBPdel-luciferase were provided by Dr. Bruce C. Kone (Houston). The iNOS (-234/+31)-luciferase and iNOS (-331/+31NF-κB mutated)-luciferase were a gift from Dr. Mark A. Perrella (Boston). Pyrazolo[3,4-d]pyrimidenes riboside (IC-51) was a gift from Dr. Howard B. Cottam (University of California San Diego, La Jolla, CA).
Nitrite concentration. Synthesis of NO was determined by assay of culture supernatants for nitrite, a stable reaction product of NO with molecular oxygen as reported previously (Pahan et al., 1997; Giri et al., 2002). Briefly, supernatants were mixed with an equal volume of the Griess reagent in 96 well plates, gently shaken, and read in microplate reader at 570 nm. Nitrite concentrations were calculated from a standard curve derived from the reaction of NaNO2 in the assay.
Immunoblot analysis. Cells were harvested in ice-cold lysis buffer (50 mm Tris-HCl, pH 7.4, containing 50 mm NaCl, 1 mm EDTA, 0.5 mm EGTA, 10% glycerol, and protease inhibitor mixture) and protein was estimated using Bradford reagent (Bio-Rad, Hercules, CA). Fifty micrograms of total protein per lane was separated by SDS-PAGE and blotted to nitrocellulose (Amersham Biosciences). Blots were blocked for 1 hr in 5% nonfat dry milk-TBS-0.1% Tween 20 and incubated overnight with primary antibody (1:1000) in 5% BSA-TBS-0.1% Tween 20 at 4°C. This was followed by incubation for 1 hr with appropriate secondary peroxidase-conjugated antibody (1:10,000; Sigma). Immunoreactivity was detected using the enhanced chemiluminescence detection method according to the manufacturer's instructions (Amersham Biosciences) and subsequent exposure of the membrane to x-ray film.
Fatty acid and cholesterol biosynthesis. Astrocytes grown in a six well plate (∼80% confluency) and preincubated in serum-free media with AICAR for 2 hr received [2-14C] acetate (5 μCi per well). After 2 hr, the cells were washed twice with PBS and scraped off. The incorporation of labeled acetate in fatty acid and cholesterol was analyzed as mentioned previously (Khan et al., 2000).
Antisense experiments. To decrease the levels of endogenous AMPK, astrocytes were pretreated for 48 hr with 25 μm phosphothiorated antisense (AS) oligonucleotide (5′CGCCCGTCGTCGTGCTTCTGC3′) directly against both the α1- and α2-subunits of AMPK (Culmsee et al., 2001). Α missense (MS) oligonucleotide (5′CTCCCGGCTTGCTGCCGT3′) was used in control cultures. Oligonucleotides were transfected with Oligofectamine reagent per the manufacturer's instructions.
AMPK and IKKα/β assays. AMPK activity was assayed in primary rat astrocytes as described previously (Kim et al., 2001). For IKKα/β assays, primary astrocytes were pretreated with AICAR (1 mm) and then stimulated with LPS (1 μg/ml) for 30 min. Cells were washed with cold PBS and lysed in lysis buffer (50 mm Tris-HCl, pH 7.4, containing 50 mm NaCl, 1 mm EDTA, 0.5 mm EGTA, 10% glycerol, and protease inhibitor mixture). Approximately 200 μg of cell extracts was incubated with anti-IKKα/β antibody (Santa Cruz Biotechnology) for 2 hr, then 30 μl of protein A/G plus agarose was added and incubated for an additional 1 hr at 4°C. The immune complexes were washed twice in lysis buffer and twice in kinase buffer (20 mm HEPES, pH 7.5, 10 mm MgCl2) and incubated at 30°C in 30 μl of kinase buffer containing 20 mm β-glycerophosphate, 20 mm p-nitrophenyl phosphate, 1 mm dithiothreitol, 50 μm Na3VO4, 20 μm ATP, and 5 μCi of [γ-32P] ATP. Approximately, 2 μg of GST-IκBα fusion protein (Santa Cruz Biotechnology) was used as the substrate in each reaction. Reactions were stopped after 30 min by denaturation in SDS loading buffer. Proteins were resolved by SDS-PAGE, and substrate phosphorylation was visualized by autoradiography.
Electrophoretic mobility shift assay. Nuclear extracts from stimulated or unstimulated astrocytes were prepared, and electrophoretic mobility shift assay (EMSA) was performed as described previously (Giri et al., 2002) with NF-κB and C/EBP consensus sequences, which were end-labeled with [γ-32P] ATP. Nuclear extracts were normalized on the basis of protein concentration and equal amounts of protein (5 μg) were loaded. DNA-protein complexes were resolved on 5% nondenaturing PAGE in Tris-borate-EDTA [45 mm Tris, 45 mm boric acid, 1 mm EDTA (0.5× TBE)] and run at 11 V/cm. The gels were dried and then autoradiographed at -70°C using x-ray film.
Northern blot, gene array analysis, and reverse transcription-PCR for cytokine expression. Total RNA was extracted with Trizol (Invitrogen, Gaithersburg, MD) according to the manufacturer's protocol. Northern blot for iNOS was performed with 15 μg of RNA per reaction as described previously (Pahan et al., 1997). β-Actin was used as a control for RNA loading. A gene expression array for inflammatory cytokines (TNFα, IL-1β, IL-6, and IFN-γ) was used according to the manufacturer's protocol (Superarray). Signal quantitation was determined using imaging densitometer (Bio-Rad). For reverse transcription (RT)-PCR, RNA was isolated from treated rat brain cerebral cortex by extracting in Trizol as above. cDNA was prepared from 5 μg of total RNA using poly deoxynucleotidyl transferase as a primer and Moloney murine leukemia virus reverse transcriptase (Promega) per the manufacturer's instructions. Two microliters of cDNA was used to amplify the following products [given as product name, expected size, and forward (F) and reverse (R) primers used]: iNOS, 730 bp, (F) 5′CTCCTTCAAAGAGGCAAAAATA3′, (R) 5′CACTTCCTC CAGGATGTTGT3′; IL-1β, 623 bp, (F) 5′GCTGACAGACCCCAAAAGATT3′, (R) 5′TGTGCAGACTCAAACTCCACTT3′; TNF-α, 473 bp, (F) 5′CAGGGCAATGAT CCCAAAGTA3′, (R) 5′GCAGTGAGATCATCTTCTCGA3′; GAPDH, 528 bp, (F) 5′ACCACCATGGAGAAGGCTGG3′, (R) 5′CTCAGTGTAGCCCAGGATGC3′. PCR products were visualized by electrophoresis in a 1.2% agarose gel containing 0.5 μg ethidium bromide and photographed with the Ultra Violet Products Bio-doc system (Upland, CA).
Cytokine assay. The levels of TNFα, IL-1β, and IFN-γ were measured in culture supernatant as well as in serum by ELISA using protocols supplied by the manufacturer (R & D Systems).
Transcriptional assays. Primary astrocytes or the microglial cell line BV2 were transiently transfected with NF-κB- or iNOS-luciferase reporter gene with β-galactosidase in the presence or absence of dominant negative AMPKα2 or HA-IKKβ by Lipofectamine-2000 (astrocytes) and Lipofectamine-Plus (BV2; Invitrogen) according to the manufacturer's instructions. pcDNA3 was used to normalize all groups to equal amounts of DNA. Luciferase activity was determined using a luciferase kit (Promega).
Animals and LPS treatment. The use of animals was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (publication number 86-23) and the protocol was approved by Medical University of South Carolina, Institutional Animal Care and Use Committee. Female Sprague Dawley rats (200-250 gm; The Jackson Laboratory, Bar Harbor, ME) were group housed at room temperature under 12 hr light/dark conditions with food and water available ad libitum. Animals were injected with AICAR (100 mg/kg body weight, i.p.) 30 min before LPS treatment (0.5 mg/kg body weight) dissolved in 0.9% saline or 0.9% sterile saline alone. After 6 hr, the cerebral cortex was isolated and frozen in liquid nitrogen and stored at -70°C until later use.
Cell viability. Cytotoxic effects of treatments were determined by measuring the metabolic activity of cells with MTT and LDH release assay (Roche).
Statistical analysis. Results shown represent means ± SD. Statistical analysis was performed by ANOVA by the Student-Neumann-Keuls test using GraphPad InStat software (San Diego, CA).
Results
AICAR downregulates LPS-induced expression of proinflammatory cytokines in brain glial cells and peritoneal macrophages
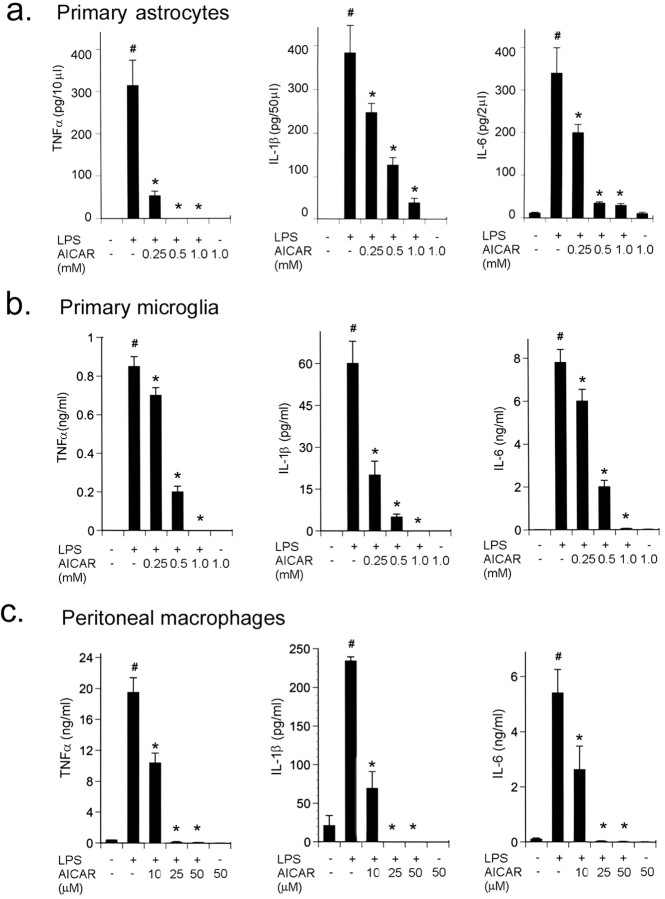
Activated astrocytes, microglia, and macrophages are the major sources of NO and cytokines production and actively participate in inflammatory disease (Benveniste, 1997). Rat primary astrocytes, microglia, and peritoneal macrophages were pretreated with different concentrations of AICAR and then exposed to LPS (1 μg/ml). Bacterial LPS markedly induced the production of proinflammatory cytokines (TNFα, IL-1β, and IL-6) in astrocytes (Fig. 1a), microglia (Fig. 1b), and macrophages (Fig. 1c) determined by ELISA. AICAR alone had no effect on the production of cytokines; however, it strongly inhibited the LPS-induced production of TNFα, IL-1β, and IL-6 in the supernatants of these cells in a dose-dependent manner (Fig. 1). Inhibition in cytokine production was accompanied by decreased mRNA expression (data not shown). Interestingly, macrophages were more sensitive to AICAR because a micromolar concentration of AICAR was sufficient to inhibit proinflammatory cytokine production compared with astrocytes and microglia (Fig. 1c). AICAR or LPS had no effect on cell viability as tested by LDH and MTT assays (data not shown).
Figure 1.
AICAR inhibits LPS-induced cytokine synthesis in a dose-dependent manner. Primary rat astrocytes (a), primary microglia (b), and peritoneal macrophages (c) were incubated for 2 hr with different concentrations of AICAR as indicated, followed by LPS (1 μg/ml) treatment for 24 hr. We measured the concentration of TNFα (left), IL-1β (middle), and IL-6 (right) released in the medium using ELISA. For TNFα levels, media were taken out at 6 hr of LPS treatment, whereas for IL-1β and IL-6, media were taken out at 24 hr. Results are the means ± SD of four determinations. *p < 0.001 compared with LPS treatment; #p < 0.001 compared with control.
AICAR inhibits LPS-induced NO production and iNOS gene expression in brain glial cells
Along with the production of proinflammatory cytokines, NO production in response to cytokines has been shown to be important in the pathophysiology of a number of inflammatory diseases (Smith et al., 1999). Rat primary astrocytes, microglia, and macrophages were pretreated with different concentrations of AICAR and then exposed to LPS (1 μg/ml). LPS induced NO production (measured as nitrite) 10-fold higher compared with untreated cells. AICAR treatment inhibited LPS-induced nitrite production in primary astrocytes, microglia, and peritoneal macrophages in a dose-dependent manner (Fig. 2a,b). Similar to cytokine production, macrophages were more sensitive to AICAR treatment compared with primary astrocytes and microglia (Fig. 1c).
Figure 2.
AICAR inhibits the expression of iNOS in primary astrocytes, microglia, and peritoneal macrophages. a, b, NO was measured in the supernatant of primary astrocytes, microglia (a), and peritoneal macrophages (b) after 24 hr of LPS/AICAR treatment. Data are means ± SD of four experiments. *p < 0.001 compared with LPS treatment; #p < 0.001 compared with control. c, For the detection of iNOS protein expression by immunoblot in response to AICAR treatment, cell lysate from astrocytes was prepared after 24 hr with LPS treatment. Blots are the representation of three experiments. d, For the detection of the iNOS message, RNA was isolated from astrocytes 6 hr after treatment with LPS and processed for Northern blot analysis as discussed in Materials and Methods. *p < 0.001 compared with LPS treatment; #p < 0.001 compared with control. Blots are representative of three experiments. e, Primary astrocytes were transiently transfected with Lipofectamine-2000 reagent with 1.5 μg per well iNOS-luciferase reporter vector along with 0.1 μg per well of plasmid cytomegalovirus (pCMV)-β-gal. After 24 hr of transfection, cells were pretreated with the indicated concentration of AICAR for 2 hr, followed by LPS (1 μg/ml) for 4 hr. Cells were lysed and processed for luciferase activity (Promega) and β-galactosidase (Invitrogen). The luciferase activity was normalized with respect to β-galactosidase activity and expressed relative to the activity of the control. Data are means ± SD of three values. ***p < 0.001 compared with control; @p < 0.001 compared with LPS treatment. f, Primary astrocytes were transiently transfected as discussed in Materials and Methods, and cells were treated with GGPP (10 μm), FPP (10 μm), mevalonate (10 mm), AICAR (1 mm), and LPS (1 μg/ml) as indicated, and luciferase activity was determined. Results are means ± SD of three values. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment; NS, not significant compared with LPS treatment; !p > 0.05 (not significant) compared with LPS/AICAR treatment.
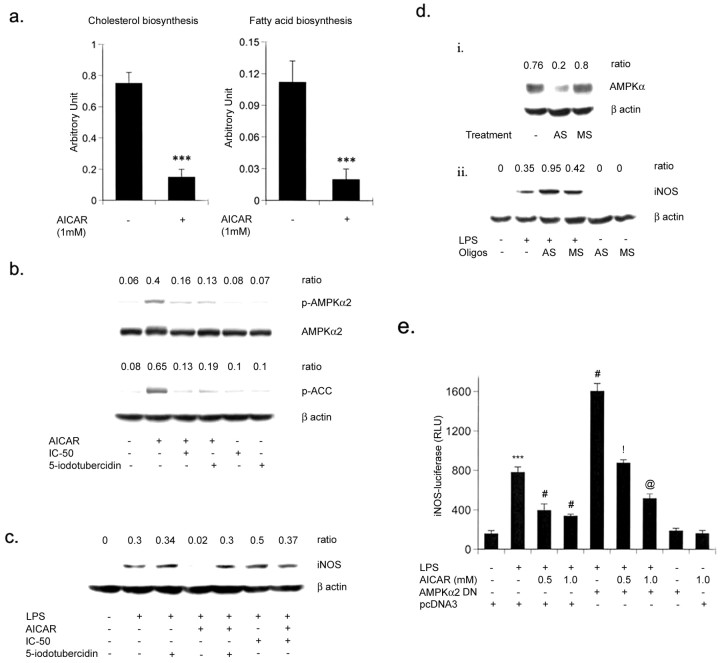
To understand the inhibitory mechanism of AICAR on LPS-mediated nitrite production, the effect of AICAR on iNOS protein and the mRNA level in primary rat astrocytes was examined. Consistent with the production of nitrite, the LPS-induced expression of iNOS was inhibited by AICAR at the mRNA as well as the protein level (Fig. 2c,d). We next examined the activation of iNOS promoter in primary astrocytes in response to LPS treatment and the effect of the AICAR on that activity. A plasmid containing a 3.2 kb portion of the rat iNOS promoter attached to the luciferase gene (iNOS-luc) was introduced into subconfluent cultures of primary astrocytes by transient transfection. After 24 hr, the cultures were pretreated with different concentrations of AICAR (0.25-1 mm) followed by LPS treatment for an additional 6 hr (Fig. 2e). The iNOS promoter activity was substantially (2.5-fold) stimulated on incubation with LPS (Fig. 2e). However, it was significantly inhibited by AICAR treatment in a dose-response manner. Previous reports (Hardie, 1992) as well as our experiment demonstrate that AICAR significantly inhibits cholesterol biosynthesis in primary astrocytes (Fig. 3a). Furthermore, we investigated whether any intermediate(s) or metabolite(s) of cholesterol biosynthesis pathway may be responsible for the anti-inflammatory effect of AICAR. The addition of mevalonate, GGPP and FPP did not reverse the inhibitory effect of AICAR on LPS-induced iNOS-luciferase activity in primary astrocytes (Fig. 2f).
Figure 3.
AICAR inhibits NO production and iNOS gene expression in glial cells via activation of AMPK. a, Primary astrocytes were pretreated with AICAR (1 mm) for 2 hr followed by [14C]-acetate pulse for 2 hr. Lipids were isolated and incorporation of labeled acetate in cholesterol and fatty acids was assayed by HP-TLC. Data are means ± SD of three values. ***p < 0.001 compared with untreated cells. b, Inhibitors of adenosine kinase (5′-iodotubercidin, and IC-51, 0.1 μm) were preincubated for 30 min before the addition of AICAR (1 mm). After 2 hr of incubation with AICAR, primary astrocytes were processed for the detection of p-AMPKα (p-Thr 172), AMPKα, p-ACC, and β-actin (for equal loading) by immunoblot as discussed in Materials and Methods. Densitometry analysis was performed to estimate the ratio of p-AMPKα and AMPKα or p-ACC and β-actin. Blots are representative of two experiments. c, The expression of iNOS protein was determined in cell lysate at 24 hr in astrocytes, after treating cells with 5′-iodotubercidin/IC-51/AICAR with or without LPS (1 μg/ml). Blots are representative of two experiments. di, Primary rat astrocytes were incubated for 48 hr with an antisense or missense oligo (25 μm) along with Oligofectamine transfection reagent and AMPKα levels were determined by immunoblot analysis. dii, Cells were treated with LPS (1 μg/ml) and lysed for the detection of iNOS and AMPKα protein by immunoblot as discussed in Materials and Methods. Densitometry analysis was performed to estimate the ratio of AMPKα or iNOS and β-actin. Blots are representatives of two experiments. e, Microglial cells (BV2) were transiently transfected with Lipofectamine-Plus with iNOS-luciferase with β-gal in the presence or absence of DN AMPKα2 (0.5 μg/ml) as discussed in Materials and Methods. pcDNA3 empty vector was used to normalize the total DNA content in cotransfection studies. After 48 hr of transfection, cells were treated with AICAR (1 mm) and LPS (1 μg/ml) as indicated, and luciferase activity was determined after 6 hr of LPS stimulation. Luciferase activity was normalized with respect to β-galactosidase activity. Data are means ± SD of three values. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment; !p < 0.001 compared with LPS/AICAR (0.5 mm) treatment; @p < 0.01 compared with LPS/AICAR (1 mm) treatment.
AICAR inhibits iNOS gene expression via activation of AMPK
AICAR mediates its effects by activating AMPK; it was imperative to establish the role of AMPK in the regulation of the inflammatory process. Once activated, AMPK downregulates ATP-consuming pathways such as fatty acid and cholesterol synthesis by phosphorylating acetyl CoA-carboxylase and HMG-CoA reductase. We observed the same when treatment of primary astrocytes with AICAR (1 mm) resulted in a significant inhibition of cholesterol and fatty acid biosynthesis (∼80%) (Fig. 3a). Treatment of astrocytes with AICAR also induced the phosphorylation of ACC (Fig. 3b), demonstrating that AICAR induced AMPK activity. AMPK is not only allosterically activated by AMP, but it is also the target of the upstream kinase AMPKK, and the phosphorylation of AMPK by AMPKK is necessary for its full activity (Moore et al., 1991; Hawley et al., 1996; Hardie et al., 1998; Stein et al., 2000). Phosphorylation of AMPK in AICAR-treated astrocytes indicated that AICAR not only induced AMPK activity (induced phosphorylation of ACC) but also induced upstream kinase (AMPKK) activity (to induce the phosphorylation of AMPK) (Fig. 3b). The treatment of astrocytes with AICAR induced the phosphorylation of AMPK and ACC (Fig. 3b), which was blocked by 5-iodotubercidin and IC-51, inhibitors of adenosine kinase (ADK) that blocks the formation of ZMP (5′-phosphorylated form of AICAR) from AICAR. ZMP mimics the effect of AMP in the allosteric activation of AMPK without altering the levels of AMP, ADP, and ATP (Corton et al., 1995). AICAR-induced phosphorylation of AMPK can be blocked by ADK inhibitors (Fig. 3b) and in turn reverse the inhibitory effect of AICAR on LPS-induced iNOS protein expression (Fig. 3c).
To further elucidate the role of AMPK in regulating iNOS expression, we used an antisense oligonucleotide against a sequence near the translation initiation site of the mRNA encoding the catalytic subunits (AMPKα1 and α2) of AMPK (Culmsee et al., 2001). Transfection with 25 μm ΑΜPΚα AS of primary astrocytes decreased the catalytic subunit AMPKα, whereas MS had no effect (Fig. 3di). Next, we examined the effect of the AMPKα subunit antisense oligonucleotide on LPS-mediated iNOS gene expression. AMPKα subunit antisense treatment alone had no effect on iNOS expression, but it significantly induced the LPS-mediated iNOS protein expression in primary astrocytes (Fig. 3dii). These experiments provide clear evidence of the involvement of AMPK in the regulation of inflammatory responses. To define a direct role of AMPK in the inflammatory process and to decipher the molecular mechanism/pathway of AMPK in this function, the microglial cell line BV2 was used. This cell line, derived from mouse primary microglia, permits ease of transfection and produces the proinflammatory cytokines and mediators in response to LPS (Kim et al., 2002; Su et al., 2003). In transient transfection studies, LPS significantly induced (approximately fourfold) iNOS-luc activity in the BV2 cell line, and the pretreatment of AICAR in a dose-dependent manner decreased the luciferase activity (Fig. 3e). In cotransfection experiments, we tested the effect of expression of dominant negative (DN) AMPKα2 (D157A) (Stein et al., 2000) on iNOS promoter activity. DN AMPKα2 not only resulted in a significant increase in LPS-induced iNOS-luc activity but also significantly reversed the AICAR-induced inhibition in iNOS-luc activity (Fig. 3e). These experiments clearly demonstrate a correlation between AMPK and the expression of inflammatory mediators in brain glial cells.
Mitogen-activated protein kinases (ERK1/2, p38, and JNK1/2) are known to play a regulatory role in the expression of proinflammatory mediators (Arbabi and Maier, 2002). Therefore, the effect of AICAR on the LPS-mediated activation of ERK1/2, JNK1/2, and p38 MAPKs in rat primary astrocytes was investigated. Consistent with the documented role of MAPKs, LPS induced the phosphorylation of all three MAPKs, whereas AICAR inhibited the LPS-mediated phosphorylation of these kinases by 40-60% (Fig. 4). AICAR alone had no effect on the phosphorylation of these MAP kinases.
Figure 4.
AICAR inhibits LPS-induced mitogen-activated protein kinases (ERK1/2, p38, and JNK1/2) in primary astrocytes: Primary astrocytes were incubated with different concentration of AICAR (0.5 to1 mm) for 2 hr, followed by LPS treatment (1 μg/ml) for 30 min. Cells were washed with chilled PBC and scraped in lysis buffer as discussed in Materials and Methods. Fifty milligrams of total protein was loaded on SDS-PAGE followed by immunoblot analysis with phosphor-specific antibodies against p42/44, JNK1/2, and p38. The same blot was stripped and reprobed with pan antibodies of p42/44, JNK1/2, and p38 for equal loading. Blots are representative of two experiments.
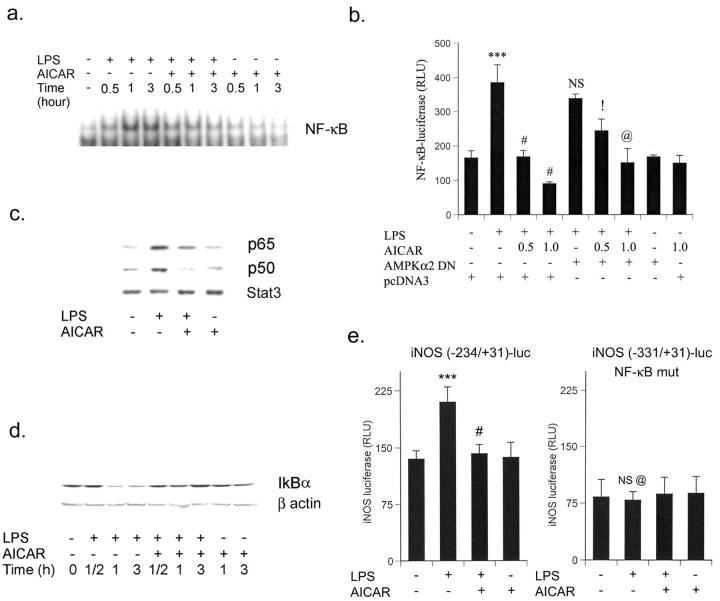
AICAR attenuates the inflammatory response by inhibiting nuclear translocation of LPS-induced NF-κB and C/EBP
To understand the mechanism of AICAR-mediated downregulation of the inflammatory process, we investigated the effect of AICAR on LPS-mediated NF-κB activation. In unstimulated cells, NF-κB consists of a p65/p50 heterodimer and is retained in cytoplasm by its association with IκB. After stimulation of cells with various agents, the cytosolic NF-κB/IκB complex dissociates and free NF-κB translocates to the nucleus and regulates the transcription of various genes. Phosphorylation of IκBα by the upstream kinase IKK is essential for the dissociation of IκBα from NF-κB and its degradation (Ghosh and Karin, 2002). The activation of NF-κB has been shown to be critical for the expression of iNOS and proinflammatory cytokines (TNFα and IL-6) (Zagariya et al., 1998; Zhang et al., 1998; Hu et al., 2000). The role of AICAR was investigated in LPS-mediated activation of NF-κB in primary astrocytes. As shown in Figure 5a, LPS treatment activates and translocates NF-κB into the nucleus within 30 min; this is sustained for up to 3 hr after treatment. Pretreatment with AICAR significantly reduced the LPS-induced DNA-binding activity of NF-κB (Fig. 5a). The possible role of AMPK in the regulation of NF-κB transcriptional activity was investigated by cotransfecting a NF-κB-dependent transcriptional reporter (3xNF-κB-luc) with an expression vector encoding dominant negative AMPK in BV2 cells. As depicted in Figure 5b, AICAR pretreatment significantly inhibited LPS-induced NF-κB-luc activity. Although the dominant negative AMPKα2 had no effect on LPS-induced NF-κB-luc activity, it significantly reversed the AICAR-induced inhibition (Fig. 5b). AICAR-induced inhibition in LPS-mediated NF-κB nuclear translocation was consistent with the results of immunoblot analysis of nuclear extracts for p65 and p50 (members of the NF-κB family) (Fig. 5c). Moreover, these conclusions are further supported by the inhibition of degradation of IκBα by AICAR treatment (Fig. 5d).
Figure 5.
AICAR inhibits LPS-induced NF-κB transcriptional response in primary astrocytes and BV2 cells. a, Nuclear extract was prepared from LPS/AICAR-treated primary astrocytes as indicated and analyzed by EMSA for NF-κB. EMSA data are representative of two experiments. b, Microglial cells (BV2) were transiently cotransfected with 1.5 μg of p(NF-κB)3Ldluc along with 0.5 μg of AMPKα2 dominant negative or pcDNA3, followed by stimulation for 4 hr with the indicated treatment with AICAR (1 mm) and LPS. Luciferase activity was normalized with respect to β-galactosidase activity. Data are means ± SD of three values. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment; !p < 0.05 compared with LPS/AICAR (0.5 mm) treatment; @p < 0.05 compared with LPS/AICAR (1 mm) treatment; NS, not significant compared with LPS treatment. c, Immunoblot was performed for p65 and p50 in nuclear extract from primary astrocytes stimulated with LPS with or without AICAR. Blots are representative of two experiments. d, Total cell lysate of primary astrocytes was processed for the detection of IκBα by immunoblot at the indicated time. Blots are representatives of two experiments. e, Microglial cells (BV2) were transiently transfected with 1.5 μg of iNOS (-234/+31)-luciferase or iNOS (-331/+31 NF-κB mutated)-luciferase followed by stimulation for 4 hr with the indicated treatment with AICAR (1 mm) and LPS. Luciferase activity was normalized with respect to β-galactosidase activity. Data are means ± SD of four values. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment; NS, not significant compared with control; @p < 0.001 compared with LPS treatment (iNOS (-234/+31)-luciferase transfected cells).
In addition to this, microglial cells (BV2) were transfected with the iNOS-luc (-234/+31) vector, a construct strictly dependent on NF-κB activation. As Figure 5e shows, AICAR completely abolished the luciferase activity induced by LPS treatment. Interestingly, the use of a fragment of the NOS-2 promoter deleted in the κB sites [iNOS(-331/+31)-luc] completely abolished the activity of the promoter, reflecting the necessity of this motif for expression of the reporter gene in response to LPS stimulation (Fig. 5e) and AICAR mediates its effect by downregulating the NF-κB pathway.
IκBα is phosphorylated by the IKK complex containing catalytic subunits (IKKα and β) and the IKKγ or NF-κB essential modulator regulatory subunits at sites that trigger its ubiquitin-dependent degradation (Ghosh and Karin, 2002). To determine whether IKKα/β could be the target for AICAR action, astrocytes were preincubated with AICAR (1 mm), followed by LPS treatment. As documented in Figure 6a, LPS stimulated the IKKα/β activity, and this stimulation was significantly blocked by AICAR treatment. To confirm this observation, we cotransfected wild-type expression vector of IKKβ with NF-κB-luc in microglial cell (BV2) and primary astrocytes cells. AICAR treatment significantly inhibited IKKβ-mediated NF-κB-luc activity in BV2 cells and primary astrocytes (Fig. 6b,c). These observations clearly demonstrate that AICAR inhibits NF-κB DNA binding as well as its transcriptional activity by inhibiting some unknown upstream molecule(s) of IKKs.
Figure 6.
AICAR inhibits LPS-induced IKKα/β activity and IKKβ-mediated NF-κB-luciferase activity in primary astrocytes and BV2 cells. a, Primary astrocytes cells were incubated with AICAR (1 mm) before LPS (1 μg/ml). After 30 min, IKKα/β activity was measured as discussed in Materials and Methods. Densitometry analysis was performed and expressed as arbitrary units. Data are means ± SD of three values. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment. b, c, Microglial cells (BV2) and primary astrocytes were transiently cotransfected with 1.5 μg of p(NF-κB)3L dluc along with 0.5 μg of HA-IKKβ or pcDNA3 and 0.1 μg of plasmid cytomegalovirus-β-gal per well. Luciferase and β-galactosidase activities were done as mentioned previously. Data are means ± SD of three experiments. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment; !p < 0.001 compared with LPS-treated and IKKβ-transfected cells.
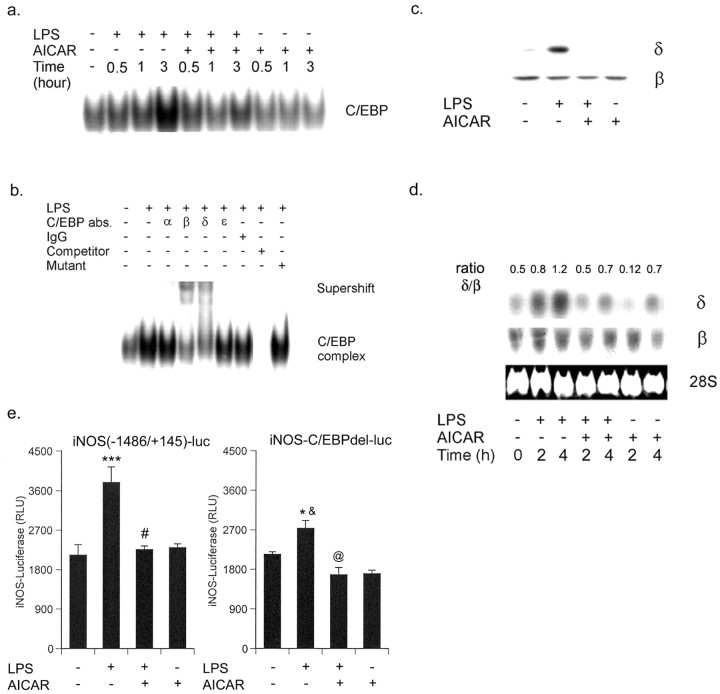
In addition to NF-κB, C/EBP-binding motifs have been identified in the functional regulatory regions of various proinflammatory genes, such as IL-6, IL-1β, TNFα, IL-8, IL-12, granulocyte colony-stimulating factor, iNOS, lysozyme, myeloperoxidase, neutrophil elastase, and granulocyte-macrophage receptor (Poli, 1998). Therefore, C/EBP DNA binding activity was examined by EMSA at different times (varying from 0.5 to 3 hr) in primary astrocytes treated with LPS and/or AICAR. LPS induced the nuclear translocation of C/EBP and AICAR abolished the LPS-induced C/EBP DNA binding activity, whereas AICAR alone had no effect on the nuclear translocation of C/EBP (Fig. 7a). Furthermore, to identify the C/EBP protein(s) responsible for the C/EBP complex, supershift assays with antibodies specific for C/EBP-α,-β,-δ, and -ϵ were performed. Only the IgGs specific for C/EBP-β and -δ significantly supershifted C/EBP complex (Fig. 7b). The nuclear translocation of C/EBP-β and -δ was examined by immunoblot of nuclear extracts of LPS and LPS/AICAR-treated primary astrocytes. C/EBP-β was constitutively expressed and localized in the nucleus of untreated cells and its level was not modulated with LPS and/or AICAR (Fig. 7c). However, high levels of C/EBP-δ were observed in the nuclear extract of LPS-treated cells compared with untreated cells and the translocation of C/EBP-δ was completely inhibited by AICAR treatment (Fig. 7c). It was of interest to examine whether AICAR inhibited the translocation of C/EBP-δ into the nucleus or its expression in primary rat astrocytes. For this, we examined the expression of C/EBP-δ in primary astrocytes treated with LPS and AICAR. LPS-induced mRNA expression of C/EBP-δ and AICAR attenuated the LPS-mediated C/EBP-δ expression (Fig. 7d). To demonstrate that C/EBP plays an important role in the regulation of iNOS gene expression in glial cells, we used iNOS2 promoter lacking the -150 to -142 C/EBP box (iNOS-C/EBPdel-luc). Microglial cells (BV2) transfected with iNOS-luc and treated with LPS exhibited a significant increase in normalized luciferase activity, and AICAR treatment completely abolished this luciferase activity (Fig. 7e). In contrast, cells transfected with iNOS-C/EBPdel-luc generated a slight increase in normalized luciferase activities after LPS stimulation (Fig. 7e). These results indicated the role of C/EBP in regulation of iNOS gene expression in response to LPS.
Figure 7.
AICAR inhibits LPS-induced nuclear translocation of C/EBP by downregulating the expression of C/EBP-δ. a, Nuclear extracts were prepared from LPS/AICAR-treated primary astrocytes as indicated and analyzed by EMSA for C/EBP. EMSA data are representative of two experiments. b, Polyclonal IgGs specific for C/EBP -α, -β, -δ, and -ϵ were used in supershift experiments with nuclear extracts from LPS-treated (3 hr) primary rat astrocytes and the 32P-labeled C/EBP oligomer. Autoradiograms are representative of two independent experiments performed on separate preparations of nuclear extracts. c, Nuclear extracts prepared from various treatments were subjected to immunoblot for C/EBP-β and -δ proteins. d, Primary astrocytes were incubated with LPS (1 μg/ml) with or without treatment of 1 mm of AICAR. At the defined time, RNA was isolated for Northern blot analysis for C/EBP-β and -δ. Blots are representative of two experiments. e, Microglial cells (BV2) were transiently transfected with 1.5 μg of iNOS (-1486/+145)-luciferase or iNOS-C/EBPdel-luciferase followed by stimulation for 4 hr with the indicated treatment with AICAR (1 mm) and LPS. Luciferase activity was normalized with respect to β-galactosidase activity. Data are means ± SD of four values. ***p < 0.001 compared with control; #p < 0.001 compared with LPS treatment; *p < 0.05 compared with control; @p < 0.05 compared with LPS treatment; &p < 0.01 compared with LPS treatment (iNOS (-1486/+145)-luciferase transfected cells).
Together, the observations document that AICAR inhibited the LPS-induced C/EBP nuclear translocation by downregulating the expression of C/EBP-δ.
AICAR inhibits the production of proinflammatory cytokines and nitrite in LPS-treated rats
Because AICAR exhibited anti-inflammatory properties in cultured cell (by inhibiting the nuclear translocation of NF-κB and C/EBP), it was of additional interest to examine the same effect of AICAR in vivo. It is well established that expression of proinflammatory cytokines, such as TNFα, IL-1β, and IFN-γ, are induced by the intraperitoneal injection of LPS in vivo (Hesse et al., 1988), Therefore, we examined the effect of AICAR on serum cytokines levels in LPS-injected rats. The levels of TNFα, IL-1β, and IFN-γ were measured in serum 6 hr after LPS injection. As shown in Figure 8a, LPS efficiently induced proinflammatory cytokines (TNFα, IL-1β, and IFN-γ), whereas pretreatment with AICAR almost abolished LPS-mediated increased levels of IL-1β and IFN-γ in serum. However, it had no effect on the levels of TNFα. AICAR treatment also significantly inhibited the LPS-induced expression of iNOS in peritoneal macrophages isolated from these animals (Fig. 8b). Furthermore, we examined the effect of AICAR on the expression of these cytokines in spleen by gene array analysis. Similar to the observations in serum, the intraperitoneal injection of LPS significantly induced the expression of TNFα, IL-1β, and IFN-γ message in spleen (Fig. 8c). The mRNA expression of IL-1β and IFN-γ was significantly decreased by AICAR, whereas no significant change was observed in the expression of TNFα in spleen (Fig. 8c). Neither saline nor AICAR alone induced a detectable signal for these cytokines.
Figure 8.
AICAR inhibits the expression of proinflammatory mediators in serum and brain cerebral cortex of LPS-injected rats. a, Rats were given saline intraperitoneally with or without AICAR (100 mg/kg) 1 hr before LPS administration (0.5 mg/kg). Blood and organs were taken out 6 hr after LPS injection. The levels of NO (i), TNFα (ii), IFNγ (iii), and IL-1β (iv) were measured in serum by ELISA as discussed in Materials and Methods. Results are the means ± SD of six determinations. *p < 0.001 compared with LPS treatment; #p < 0.001 compared with control; NS, not significant. b, Immunoblot was performed for iNOS protein in peritoneal macrophages isolated at 6 hr. c, For determination of expression of cytokines, spleen was isolated from treated rats and total RNA was isolated by Trizol reagent (Invitrogen) for gene array (Superarray). Results are the representation of two independent experiments. The cerebral cortex was isolated from treated rats and total RNA was isolated as mentioned before. d, The expression of iNOS, TNFα, and IL-1β was examined by RT-PCR as discussed in Materials and Methods. The blots are representative of two experiments.
Models of peripheral immune challenge or peripheral inflammation have been shown to induce the expression of proinflammatory cytokines within the brain (Pitossi et al., 1997); therefore, we examined the expression of TNFα, IL-1β, and iNOS in the cerebral cortex of LPS-injected rats treated or not treated with AICAR. LPS induced the expression of TNFα, IL-1β, and iNOS in the cerebral cortex, whereas AICAR treatment significantly reduced the expression of these molecules (Fig. 8d). These findings document that similar to cultured glial cells, AICAR was also effective in attenuating the expression of proinflammatory molecules (except TNFα) in an animal model (Fig. 8).
Discussion
AMPK was originally identified through its ability to phosphorylate and inhibit the key enzymes involved in biosynthetic pathways, such as acetyl CoA-carboxylase (fatty acid synthesis) and HMG CoA-reductase (isoprenoid and cholesterol biosynthesis) (Moore et al., 1991; Vincent et al., 1991; Hardie and Carling, 1997; Hardie et al., 1998; Winder and Hardie, 1999). Because cholesterol metabolites have been reported recently to attenuate the inflammatory process (Pahan et al., 1997; Kwak et al., 2000), we examined the possible role of AMPK in the induction of the inflammatory process in cultured cells as well as in LPS-injected animals. Several lines of evidence presented in this study clearly support the conclusion that activation of AMPK by AICAR downregulates LPS-mediated induction of proinflammatory cytokines, iNOS, and nitric oxide (NO) production in rat primary astrocytes, microglia, and peritoneal macrophages by inhibiting the nuclear translocation of NF-κB and C/EBP transcription factors, thereby demonstrating the involvement of AMPK in the regulation of expression of inflammatory mediators. This study also suggests the therapeutic use of AICAR or other pharmacological activators of AMPK for inflammatory diseases. Although AICAR inhibits proinflammatory cytokines in tissue culture and the CNS of LPS-injected rats but why it did not affect LPS-induced TNFα levels in serum, cannot be explained at this time.
AICAR induced the phosphorylation and activation of AMPK and inhibition of ACC and HMG-CoA reductase, suggesting the activation of AMPK and its upstream kinase AMPKK. HMG-CoA reductase inhibitors such as statins have been reported to be immunomodulatory and anti-inflammatory (Pahan et al., 1997; Kwak et al., 2000). However, we found that the mechanism of action of AICAR/AMPK is not through the mevalonate pathway because the addition of mevalonate and other metabolites did not reverse the inhibitory effect of AICAR on LPS-induced NO production. Because MAPKs are known to play an important role in the expression of proinflammatory molecules such as TNFα, IL-1β, IL-6, IL-8, cyclo-oxygenase-2, and iNOS (Arbabi and Maier, 2002), the observed inhibition of LPS-induced activation of all three MAPKs (ERK1/2, p38, and JNK1/2) by AICAR indicates a role for AMPK in the regulation of these signaling pathways. AMPK has been reported to regulate the endothelial growth factor- and insulin growth factor-mediated ERK pathway by the phosphorylation of Raf-1 Ser621 (Sprenkle et al., 1997; Kim et al., 2001). In contrast with our observations, the activity of p38 MAPK has been shown to be activated by AMPK in a rat-liver-derived nontransformed cell line (Xi et al., 2001). This may be one of the cell-specific functions of AMPK.
In our experimental conditions, the specificity of AICAR to activate AMPK is documented by a number of experiments, as follows: (1) The dominant negative form of AMPKα2 reversed the AICAR-induced inhibition in iNOS- and NF-κB-luciferase activity. (2) Inhibitors of adenosine kinase (5′-iodotubercidin and IC-51) were not only able to reverse the inhibition induced by AICAR on iNOS protein expression, but they also inhibited the AICAR-induced phosphorylation of AMPK and ACC. (3) Downregulation of catalytic subunits of AMPK by antisense oligonucleotides induced the expression of iNOS protein levels in primary astrocytes. Recently, AMPKα2 knock-out mice have been reported (Viollet et al., 2003), and studies in these mice will define the role of AMPK in the regulation of inflammatory cytokines.
The possibility that AMPK is a component of the transcriptional regulatory complexes has yet to be explored. Recently, p300, a transcriptional coactivator, has been reported to be a substrate of AMPK in vivo and in vitro, and on phosphorylation its interaction with other nuclear receptors such as peroxisome proliferator-activated receptor-γ, thyroid receptor, retinoic acid receptor, and retinoid-X receptor were dramatically reduced (Yang et al., 2001). Our study clearly demonstrated that AMPK regulates the transcriptional activity of NF-κB and C/EBP. The activation of AMPK inhibits nuclear translocation as well as the transcriptional activity of NF-κB by inhibiting LPS-induced IKKα/β activity and phosphorylation/degradation of IκBα, indicating that AMPK targets the NF-κB pathway upstream of IKKs. However, AICAR not only inhibited the nuclear translocation of of C/EBP but also downregulated the LPS-induced expression of C/EBP-δ in primary astrocytes. These observations identify the C/EBP pathway as one of the potential candidates for therapeutics against inflammatory disease because C/EBP is known to regulate the expression of TNFα, IL-1β, IL-6, iNOS, IL-8, IL-12, and granulocyte-macrophage colony-stimulating factor (Poli, 1998).
Because proinflammatory cytokines (TNFα, IL-1β, and IL-6) and NO have been implicated in the pathogenesis of demyelinating and neurodegenerative diseases (Benveniste, 1997; Smith et al., 1999; Torreilles et al., 1999; Bauer et al., 2001), our results provide a potentially important mechanism whereby an activator of AMPK may prevent or ameliorate neural injury. AMPK is a heterotrimeric protein kinase consisting of a catalytic α-subunit and noncatalytic β- and γ-subunits (Hardie and Carling, 1997; Hardie et al., 1998; Winder and Hardie, 1999). There are different isoforms for each subunit, termed α1 or α2, β1 or β2, and γ1, γ2, or γ3 that have been described previously (Kemp et al., 1999). Immunostaining for AMPK documented that the expression of a α2 AMPK subunit in the brain was confined mainly to neurons and white matter astrocytes (Turnley et al., 1999). Normally, most astrocytes express low levels of AMPK, but its expression increases (mainly α2 and β2) when there is an increase in metabolic activity, such as during reactive gliosis (Turnley et al., 1999), in which astrocytes become enlarged, migrate to the site of injury, and release a variety of cytokines and growth factors. The observed higher expression of AMPK in reactive astrocytes and identification of colocalization of AMPK isoforms in the nucleus indicate that AMPK may also have functions in addition to the regulation of energy metabolism (Salt et al., 1998; Turnley et al., 1999). These findings are consistent with the role of AMPK in the inflammatory process reported in this study. AMPK has been reported recently to have a protective function during glucose deprivation in neurons (Culmsee et al., 2001) and has been shown to protect astrocytes (Blazquez et al., 2001) and thymocytes (Stefanelli et al., 1998) from apoptosis and necrosis. Recently, a novel function of AMPK in neurodegeneration and APPL/APP processing has been demonstrated in Drosophila, which could be mediated through HMG-CoA reductase and cholesterol ester (Tschape et al., 2002). All of these previous studies and our study suggest a critical role for AMP in the CNS that has yet to be established. It strongly indicates that AMPK plays an important role as an anti-inflammatory molecule and may be exploited as a target molecule for anti-inflammatory drugs such as AICAR. Moreover, AICAR has been used previously as a drug for treating Lesch-Nyhan syndrome at a relatively high dose (100 mg/kg body weight) safely and with no side effects (Page et al., 1994). The safety, tolerance, and pharmacokinetics of intravenous doses of 10-100 mg/kg of AICAR in health men have been reported previously (Dixon et al., 1991). AICAR has a high clearance and is poorly bioavailable with oral administration.
In summary, the studies described here document a novel role of AMPK in inflammatory disease. AMPK may be an interesting target for neuroprotective drugs in inflammatory conditions such as multiple sclerosis, Alzheimer's disease, stroke, and other neurodegenerative diseases.
Footnotes
This work was supported by National Institutes of Health Grants NS-22576, NS-34741, NS-40810, NS 37766, and NS-40144. We thank Dr. Georges Rawadi, Dr. Hanfang Zhang, Dr. David Carling, Dr. Zheng-Gang Liu, Dr. Bruce C. Kone, Dr. Mark A. Perrella, and Dr. Howard B. Cottam for constructs and IC-51, Hope Terry for secretarial assistance, and Joyce Bryan for technical assistance.
Correspondence should be addressed to Dr. Inderjit Singh, 316 Clinical Science Building, Medical University of South Carolina, Charleston, SC 29425. E-mail: singhi@musc.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/240479-09$15.00/0
References
- Arbabi S, Maier RV (2002) Mitogen-activated protein kinases. Crit Care Med 30: S74-S79. [PubMed] [Google Scholar]
- Bauer J, Rauschka H, Lassmann H (2001) Inflammation in the nervous system: the human perspective. Glia 36: 235-243. [DOI] [PubMed] [Google Scholar]
- Benveniste EN (1997) Cytokines: influence on glial cell gene expression and function. Chem Immunol 69: 31-75. [DOI] [PubMed] [Google Scholar]
- Blazquez C, Geelen MJ, Velasco G, Guzman M (2001) The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett 489: 149-153. [DOI] [PubMed] [Google Scholar]
- Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229: 558-565. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP (2001) AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17: 45-58. [DOI] [PubMed] [Google Scholar]
- Dixon R, Gourzis J, McDermott D, Fujitaki J, Dewland P, Gruber H (1991) AICA-riboside: safety, tolerance, and pharmacokinetics of a novel adenosine-regulating agent. J Clin Pharmacol 31: 342-347. [DOI] [PubMed] [Google Scholar]
- Gebicke-Haerter PJ (2001) Microglia in neurodegeneration: molecular aspects. Microsc Res Tech 54: 47-58. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell [Suppl] 109: S81-S96. [DOI] [PubMed] [Google Scholar]
- Giri S, Jatana M, Rattan R, Won JS, Singh I, Singh AK (2002) Galactosylsphingosine (psychosine)-induced expression of cytokine-mediated inducible nitric oxide synthases via AP-1 and C/EBP: implications for Krabbe disease. FASEB J 16: 661-672. [DOI] [PubMed] [Google Scholar]
- Hardie DG (1992) Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochim Biophys Acta 1123: 231-238. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D (1997) The AMP-activated protein kinase-fuel gauge of the mammalian cell? Eur J Biochem 246: 259-273. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67: 821-855. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879-27887. [DOI] [PubMed] [Google Scholar]
- Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino Jr MA, Cerami A, Shires GT, Lowry SF (1988) Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet 166: 147-153. [PubMed] [Google Scholar]
- Hu HM, Tian Q, Baer M, Spooner CJ, Williams SC, Johnson PF, Schwartz RC (2000) The C/EBP bZIP domain can mediate lipopolysaccharide induction of the proinflammatory cytokines interleukin-6 and monocyte chemoattractant protein-1. J Biol Chem 275: 16373-16381. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA (1999) Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci 24: 22-25. [DOI] [PubMed] [Google Scholar]
- Khan M, Contreras M, Singh I (2000) Endotoxin-induced alterations of lipid and fatty acid compositions in rat liver peroxisomes. J Endotoxin Res 6: 41-50. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, Choi YK, Ha J (2001) Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway. J Biol Chem 276: 19102-19110. [DOI] [PubMed] [Google Scholar]
- Kim OS, Park EJ, Joe EH, Jou I (2002) JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem 277: 40594-40601. [DOI] [PubMed] [Google Scholar]
- Kwak B, Mulhaupt F, Myit S, Mach F (2000) Statins as a newly recognized type of immunomodulator. Nat Med 6: 1399-1402. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85: 890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F, Weekes J, Hardie DG (1991) Evidence that AMP triggers phosphorylation as well as direct allosteric activation of rat liver AMP-activated protein kinase. A sensitive mechanism to protect the cell against ATP depletion. Eur J Biochem 199: 691-697. [DOI] [PubMed] [Google Scholar]
- Page T, Barshop B, Yu AL, Nyhan WL (1994) Treatment of Lesch-Nyhan syndrome with AICAR. Adv Exp Med Biol 370: 353-356. [DOI] [PubMed] [Google Scholar]
- Pahan K, Sheikh FG, Namboodiri AM, Singh I (1997) Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest 100: 2671-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitossi F, del Rey A, Kabiersch A, Besedovsky H (1997) Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res 48: 287-298. [DOI] [PubMed] [Google Scholar]
- Poli V (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem 273: 29279-29282. [DOI] [PubMed] [Google Scholar]
- Saliba E, Henrot A (2001) Inflammatory mediators and neonatal brain damage. Biol Neonate 79: 224-227. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG (1998) AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J 334: 177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Kapoor R, Felts PA (1999) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 9: 69-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenkle AB, Davies SP, Carling D, Hardie DG, Sturgill TW (1997) Identification of Raf-1 Ser621 kinase activity from NIH 3T3 cells as AMP-activated protein kinase. FEBS Lett 403: 254-258. [DOI] [PubMed] [Google Scholar]
- Stefanelli C, Stanic I, Bonavita F, Flamigni F, Pignatti C, Guarnieri C, Caldarera CM (1998) Inhibition of glucocorticoid-induced apoptosis with 5-aminoimidazole-4-carboxamide ribonucleoside, a cell-permeable activator of AMP-activated protein kinase. Biochem Biophys Res Commun 243: 821-826. [DOI] [PubMed] [Google Scholar]
- Stein SC, Woods A, Jones NA, Davison MD, Carling D (2000) The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345: 437-443. [PMC free article] [PubMed] [Google Scholar]
- Su Y, Ganea D, Peng X, Jonakait GM (2003) Galanin down-regulates microglial tumor necrosis factor-alpha production by a post-transcriptional mechanism. J Neuroimmunol 134: 52-60. [DOI] [PubMed] [Google Scholar]
- Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK (1994) Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353: 33-36. [DOI] [PubMed] [Google Scholar]
- Torreilles F, Salman-Tabcheh S, Guerin M, Torreilles J (1999) Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev 30: 153-163. [DOI] [PubMed] [Google Scholar]
- Tschape JA, Hammerschmied C, Muhlig-Versen M, Athenstaedt K, Daum G, Kretzschmar D (2002) The neurodegeneration mutant lochrig interferes with cholesterol homeostasis and Appl processing. EMBO J 21: 6367-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72: 1707-1716. [DOI] [PubMed] [Google Scholar]
- Vincent MF, Marangos PJ, Gruber HE, Van den Berghe G (1991) Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes 40: 1259-1266. [DOI] [PubMed] [Google Scholar]
- Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S (2003) The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111: 91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder WW, Hardie DG (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol 277: E1-10. [DOI] [PubMed] [Google Scholar]
- Xi X, Han J, Zhang JZ (2001) Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem 276: 41029-41034. [DOI] [PubMed] [Google Scholar]
- Yang W, Hong YH, Shen XQ, Frankowski C, Camp HS, Leff T (2001) Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J Biol Chem 276: 38341-38344. [DOI] [PubMed] [Google Scholar]
- Zagariya A, Mungre S, Lovis R, Birrer M, Ness S, Thimmapaya B, Pope R (1998) Tumor necrosis factor alpha gene regulation: enhancement of C/EBPβ-induced activation by c-Jun. Mol Cell Biol 18: 2815-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen X, Teng X, Snead C, Catravas JD (1998) Molecular cloning and analysis of the rat inducible nitric oxide synthase gene promoter in aortic smooth muscle cells. Biochem Pharmacol 55: 1873-1880. [DOI] [PubMed] [Google Scholar]