Abstract
The stomatogastric nervous system (STNS) is a premiere model for studying modulation of motor pattern generation. Whereas the cellular and network responses to monoamines have been particularly well characterized electrophysiologically, the transduction mechanisms that link the different monoaminergic signals to specific intracellular responses are presently unknown in this system. To begin to elucidate monoaminergic signal transduction in pyloric neurons, we used a bioinformatics approach to predict the existence of 18 monoamine receptors in arthropods, 9 of which have been previously cloned in Drosophila and other insects. We then went on to use the two existing insect databases to clone and characterize the 10th putative arthropod receptor from the spiny lobster, Panulirus interruptus. This receptor is most homologous to the 5-HT2 subtype and shows a dose-dependent response to 5-HT but not to any of the other monoamines present in the STNS. Through a series of pharmacological experiments, we demonstrate that this newly described receptor, 5-HT2βPan, couples with the traditional Gq pathway when expressed in HEK293 cells, but not to Gs or Gi/o. Moreover, it is constitutively active, because the highly conserved DRY motif in transmembrane region 3 has evolved into DRF. Site-directed mutagenesis that reverts the motif back to DRY abolishes this agonist-independent activity. We further demonstrate that this receptor most likely participates in the modulation of stomatogastric motor output, because it is found in neurites in the synaptic neuropil of the stomatogastric ganglion as well as in the axon terminals at identified pyloric neuromuscular junctions.
Keywords: STG, constitutive, Panulirus interruptus , Gq, neuromodulator, monoamine, immunocytochemistry, PLC, signal transduction, PKC, IP3, cAMP, serotonin, stomatogastric, motor pattern, CPG, GPCR
Introduction
The 14 neuron pyloric network in the stomatogastric ganglion (STG) of Decapod crustaceans is a useful model of a highly modulated neural circuit for which all the major cells, their patterns of connection, and their biophysical properties are well understood (Harris-Warrick et al., 1992b; Nusbaum and Beenhakker, 2002). More than 20 different substances modulate the pyloric circuit, with monoamines figuring prominently among them (Harris-Warrick et al., 1992a; Pulver et al., 2003; Richards et al., 2003). Monoamines can alter the strength of synaptic interactions between pyloric neurons and/or their intrinsic properties (Harris-Warrick et al., 1998). Each monoamine can modify different currents to change the same intrinsic property in a given cell (Harris-Warrick and Flamm, 1987), and the effects of a particular monoamine on a given current are cell-type specific (Harris-Warrick et al., 1995a,b; Kloppenburg et al., 1999; Peck et al., 2001).
The wealth of information on the electrophysiological responses to aminergic neuromodulation contrasts sharply with the dearth of information on signal transduction mechanisms in pyloric neurons. There has been only a single attempt to characterize monoamine G-protein-coupled receptors (GPCRs) in pyloric neurons, and the data suggest that there are a minimum of three 5-HT receptors (Zhang and Harris-Warrick, 1994). Little is known about the signaling cascades operating in stomatogastric neurons (Flamm et al., 1987; Hempel et al., 1996; Scholz et al., 1996, 2001), and there is no information linking any crustacean monoamine receptor to a specific second messenger pathway in any cell type.
In contrast, the field of GPCR signal transduction is exploding with new data that challenge the tenets of traditional receptor theory. It has recently been shown that GPCRs can participate in G-protein-independent signaling (Marrero et al., 1995; Guillet-Deniau et al., 1997) and can exist as homodimers and heterodimers or higher-order structures (Angers et al., 2002; Canals et al., 2003). Over 40% of GPCRs can exhibit constitutive activity (Seifert and Wenzel-Seifert, 2002). Moreover, a single GPCR subtype can couple with multiple signaling pathways in a cell, and a given agonist will differentially activate a subset of pathways (Berg et al., 1998; Pauwels, 2000).
Perhaps the most exciting discovery regarding signal transduction in recent years is that GPCRs exist as part of a large multi-protein signaling complex composed of receptors, effectors, cytoskeletal elements, and signaling molecules (Hall and Lefkowitz, 2002; Bockaert et al., 2003; Rebois and Hebert, 2003). By organizing signaling cascades into physically and functionally distinct units, the cell ensures proper localization of microdomains and optimizes specificity, selectivity, and the time course of signaling. There are several examples of GPCR multi-protein complexes, with increasingly more examples of receptors coupling with ion channels (Liu et al., 2000; Davare et al., 2001; Lavine et al., 2002; Lee et al., 2002). The data suggest that ion channels and GPCRs cannot be regarded as isolated proteins. They exist within dynamic complexes, the composition of which will influence several properties including ion channel function and signal compartmentalization. Understanding how ion channels and GPCRs are organized into functional units in stomatogastric neurons is a prerequisite for comprehending how neuromodulators sculpt an adaptive output from stomatogastric nervous system (STNS) networks. In an effort to describe the GPCRs that exist in stomatogastric networks, we have used a bioinformatics approach to identify novel arthropod monoamine receptors and, for the first time, clone and characterize one of the monoaminergic receptors expressed in pyloric neurons.
Materials and Methods
Cloning and mutagenesis of the 5-HT2βPan receptor. Total RNA was extracted from the Panulirus interruptus nervous system using Trizol (Ambion, Austin, TX) according to the manufacturer's instructions. RNA quality was assessed with denaturing gel electrophoresis and quantified with a biophotometer (Fisher Scientific, Houston, TX). Multiple RNA extractions were performed. When necessary, mRNA was isolated with an oligotex kit (Qiagen, Chatsworth, CA). cDNA was obtained from total and/or mRNA preparations by performing reverse transcription reactions using Superscript II (Invitrogen, San Diego, CA) according to the manufacturer's instructions. The following degenerate primers (written 5′ to 3′) were designed based on conserved regions of the Drosophila and Anopheles orthologs of a putative monoamine receptor, as described in Results: 5a, TGGATITGYYTIGAYGTNYTNTTYTG; 5b, TITTYTGYACIGCIWSNATNATG; 5c, ACIGCIWSATNATGCAYYTNTGYAC; 3a, GGIATRTARAARCAIACIATNSWNCC; 3b, CATIACICCNARNGGNATRTARAARCA; 3c, TAIGTIARIARCATIACNCCNARNGG.
Fragments of the lobster ortholog were amplified from multiple cDNA preparations using various combinations of the degenerate primers in nested PCR experiments (only two degenerate primers for any given PCR), as described previously (Baro et al., 1994). Nested PCR products were size fractionated on acrylamide gels. Appropriate bands of expected size were gel isolated. DNA was eluted (Ausubel et al., 1990) and cloned using a TA cloning kit (Invitrogen). Forty-four independent clones were sequenced (Georgia State University Biotechnology Facility). Twenty-five clones from nine independent nested PCR experiments representing multiple cDNA templates fell into a single contig that displayed strong amino acid identity with the previously identified Drosophila protein that served as the template for the design of the degenerate primers. A DNA fragment from these 25 clones was amplified with specific primers in a standard PCR. The resulting fragment was gel isolated and used as the template in a primer extension reaction containing 32P, according to the manufacturer's instructions (ladderman kit; Takara, Kyoto, Japan). Un-incorporated nucleotides were removed from the reaction by ethanol precipitation, and the resulting product was used to probe five different P. interruptus nervous system cDNA libraries as described previously (Baro et al., 1996). The conventional library screen yielded five clones all missing the start methionine codon. DNA fragments containing the 5′ end of the full-length cDNA were obtained using a SMART RACE cDNA amplification kit (BD Biosciences Clontech, Cambridge, UK) according to the manufacturer's instructions. The following primers (written 5′ to 3′) were used in the reaction: GSPAM1, CGTGACGGCCAGGGAGAGCAGGAAGTAGTTG;NGSPAM1,GCCAGGATGAGGAGGATGTTGCCGAAGAGTGTC; ProbeAM1, CAACCTATCTTACGGGAGGGAGAACGAGACGT.
To guard against PCR-induced sequence errors, four different RNA preparations were used in separate experiments, and multiple, independent RACE (rapid amplification of cDNA ends) fragments were isolated. Fragments were cloned and sequenced as described above. Criteria used to identify clones containing the complete 5′ end of the ORF were: (1) the clone must be bounded by the primers used in the experiment and contain a region of overlap with the library clones; (2) the clone must contain multiple stop codons in all three reading frames 5′ to the start methionine in the N terminus region (sequence 5′ to TM1); and (3) the translation start site should conform to the Kozak consensus sequence. Five independent clones from multiple cDNA preparations contained the same DNA fragment representing the 5′ end of the transcript. The Kozak consensus sequence was recognizable but not optimal; nevertheless, protein expression levels were adequate in HEK293 cells using this endogenous site (see below). The full length of the isolated cDNA was 3.92 kb and included a polyA tail. Constructs containing the complete ORF (2.426 kb in length) were assembled using standard procedures (Ausubel et al., 1990). Both strands of the constructs were sequenced with the following primers (written 5′ to 3′): U1, TGTTTCCCCACTTACCCACCAG; U2, TGCGGAGGTCCCACTGTCG; U3, CTCCTGTGGGCTCCGTTCTTCATC; U4, TCGGAGTTGATGGCTTCTTGTG; U5, GAATTAGTGCTGTGCTCCGTGTG; U6, GCTGAGTTGTGCTTTGGAAGTGA; L1, ACACAGCTCCTTACCCAACTTCACAC; L2, GTTGACCATGGAGGAGGCGTAGC; L3, ACGAGGTCCGGGGTGGTTCTG; L4, TCGGTGGAGCGGGGAAGT; L5, TCGGTGATGGAGACGGCAGTG; L6, ATGAGGAGGATGTTGCCGAAGAGT; L7, GGAGGAGGCGTAGCCCAGCCAGGTCACCAAGTTGA.
Two independent constructs were used to establish permanent cell lines as described below. These constructs also served as templates in site-directed mutagenesis experiments that were performed with the Quickchange kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Both strands of multiple mutagenized clones were sequenced, and one clone was used to establish permanent cell lines as described below. Sequence data were analyzed and manipulated using Sequencher (Genecodes, Ann Arbor, MI) and Lasergene (DNAStar, Madison, WI) software.
Generation of HEK293 cell lines stably expressing 5-HT receptors. HEK293 cells were maintained in DMEM supplemented with 10% horse serum, 50 U/ml penicillin, and 50 μg/ml streptomycin at 37°C, and 5% CO2. Cells were grown to 90–95% confluency in 35 mm dishes and transfected with 2 μg of DNA using 10 μl of Lipofectamine in 100 μl of opti-MEM. After varying amounts of time (6–24 hr), the medium was replaced with DMEM supplemented with 10% horse serum, according to the manufacturer's suggestions. After 2 d in culture, cells expressing the transfected genes were selected in medium containing 10% dialyzed FBS and 500 μg/ml neomycin (Sigma, St. Louis, MO). Cells were maintained in selection media for ≥28 d, after which each plate was expanded to produce a cell line. Each cell line was assayed for receptor expression by performing Western blot experiments on proteins extracted from each cell line, using anti-5-HT2βCrust to probe the membranes (see below). All experiments reported here were conducted on the cell line with the highest 5-HT2βPan expression level. However, the same results were obtained with a second line. The same procedure was used to establish two 5-HT2βPan (F171Y) cell lines. All tissue culture lines and reagents were purchased from American Type Culture Collection (Manassas, VA), except the dialyzed serum, Lipofectamine, and opti-MEM (Invitrogen).
PKC assay. We used a modified version of a previously described procedure to fractionate cells and measure total PKC activity in each fraction (Setterblad et al., 1998). A 100 mm plate of confluent cells was trypsinized, and cells were recovered by centrifugation. The pellet was resuspended in DMEM, and cell numbers were determined. Aliquots of 5 × 105 resuspended cells were removed to separate 1.5 ml tubes and were exposed to varying concentrations of monoamines (usually 0–10–2 m) for 15 min at 37°C in a total volume of 1 ml of DMEM. Normally, one plate would yield enough cells for an entire concentration series for a given drug (i.e., nine aliquots ranging from 0 to 10–2 m). After exposure to the drug, cells were pelleted in a centrifuge for 2 min, the medium was removed and replaced with liquid nitrogen. Tubes were stored on dry ice and assayed immediately, or tubes could be stored at –70°C for up to 1 month with similar results in both cases. For the PKC assay, frozen pellets were resuspended in 500 μl of lysis buffer (25 mm Tris-HCl, 0.5 mm EDTA, 0.5 mm EGTA, 0.05% Triton X-100, 10 mm β-mercaptoethanol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 2 mm PMSF) and centrifuged at 100,000 × g for 1 hr. The supernatant (soluble fraction) was collected, and the pellet (membrane fraction) was resuspended in 500 μl of lysis buffer containing 1% Triton X-100. Total PKC activity in each fraction was determined using a PKC assay system (Promega, Madison, WI) that measures 32P phosphorylation of a PKC-specific substrate. Briefly, 5 nmol of a biotinylated PKC peptide substrate [neurogranin (28–43)] was mixed with 5 μl of a given cell lysate in the presence of 32P-ATP, activation buffer (1.6 mg/ml phosphatidylserine, 0.16 mg/ml diacylglycerol, 100 mm Tris-HCl, and 50 mm MgCl2), and coactivation buffer (1.25 mm EGTA, 2 mm CaCl2, and 0.5 mg/ml BSA). After a 5 min incubation at 30°C, the reaction was terminated by the addition of 7.5 m guanine hydrochloride. Samples were applied to streptavidin-coated discs, to which the biotinylated substrate is specifically bound. Excess free 32P-ATP and nonbiotinylated cellular components are removed by several washes in 2 m NaCl. A phosphorimaging system (Fuji Photo Film, Tokyo, Japan) was used to determine the radioactivity incorporated into the substrate. The signal was expressed in units of photo-stimulated luminescence (PSL). Protein concentrations in each cell fraction were determined using a BCA Protein Assay kit (Pierce, Rockford, IL) and the residual cell lysate from the fractionation procedure described above. The specific activity in each fraction was expressed as PSL/μg protein. The total specific activity for a given aliquot of 5 × 105 cells is equal to the specific activity of the membrane fraction plus the specific activity of the soluble fraction. In some experiments, 10–7 m PMA (Sigma), a PKC activator, was substituted for a monoamine, or 5-HT application was preceded by a 15 min application of 10 μm 1-O-Octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine (Et-18-OCH3; Calbiochem, La Jolla, CA), a phospholipase C (PLC) inhibitor, or before removing cells from the plate, a 24 hr application of 100 ng/ml pertussis toxin (PTX; Calbiochem), a Gi/o inhibitor.
Assay for inositol phosphate formation or phosphatidyl inositol hydrolysis. Measurement of inositol phosphate (IP) formation in cultured cells was performed using a slight modification of a previously described protocol (Li et al., 1995). Briefly, confluent cells in 24-well plates were labeled with [3H]-myoinositol (1.3 μCi/ml; Perkin-Elmer Life Sciences, Norwalk, CT) for 48 hr. They were washed with PBS and preincubated with 10 mm LiCl (Sigma) in PBS for 30 min. Cells were incubated an additional 60 min in either 10–3 m 5-HT, 20 mm NaF, or no drug (control). Cells were lysed by adding 0.75 ml of ice-cold 20 mm formic acid to the wells and incubating the plates at –20°C for 1 hr. The lysate was loaded onto AG1-X8 columns (Bio-Rad, Hercules, CA) that were pre-equilibrated with formic acid (20 mm). Care was taken not to disturb the membranes that remained attached to the plate. The columns were washed with 3 ml of 50 mm ammonium hydroxide. IP1 and IP2 were collected with 10 ml of 0.1 m formic acid/0.4 m ammonium formate. IP3 was eluted with 10 ml of 0.1 m formic acid/1 m ammonium formate. The IP fractions were then combined, and radioactivity in the samples was quantified by scintillation counting. The cell membranes attached to the bottom of the wells were dissolved in 1 m NaOH and counted as total phosphatidyl inositols (PIs), as described previously (Agretti et al., 2003). Results are expressed as the percentage of radioactivity incorporated in inositol phosphates (IP1 + IP2 + IP3) over the sum of radioactivity in IPs and PIs.
[cAMP] determinations. A total of 1 × 105 cells were plated in 35 mm dishes and grown to confluency. Cells were washed with 2 ml of PBS and preincubated at 37°C for 10 min in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (2.5 mm; Sigma). Cells were incubated an additional 30 min at 37°C with either 5-HT (10–3 m) or forskolin (2.5 μm) or forskolin and 5-HT. The medium was removed, and 0.5 ml of 0.1 m HCl with 0.8% Triton X-100 was added to the plates. After a 30 min incubation at room temperature, the lysate was removed from the plates and spun in a centrifuge for 2 min. Supernatant was collected and assayed for cAMP levels using a direct cAMP enzyme immunoassay kit (Assay Design, Ann Arbor, MI) according to the manufacturer's instructions. Protein concentrations in each sample were determined using a BCA Protein Assay kit (Pierce). For some experiments, cells were pretreated with PTX (100 ng/ml) for 24 hr before the experiment. Data are expressed as picomoles of cAMP/milligram of protein.
Antibody production. Two affinity-purified antibodies were synthesized by Bethyl Laboratories (Montgomery, TX) against sequences that are conserved across crustacean orthologs of the 5-HT2βPan receptor (P. interruptus and Machrobrachium rosenbergii) (M. A. Sosa and D. J. Baro, unpublished observations). Because these antibodies will recognize the 5-HT2βPan receptor in multiple species of Crustacea, we call them anti-5-HT2βCrust. Anti-5-HT2βCrust.A was made against the peptide DRFLSLRYPMKFGRHKTRRR. Anti-5-HT2βCrust.B was generated against the peptide DPHSTIVDGVCQIPVSLFQI. When necessary, a C was appended to the end of the sequence for conjugation to a carrier.
Protein extractions and Western blots. Protein extractions and Western blots were performed as described previously (Sosa et al., 2004). For preabsorption experiments, blots contained mirror images of the same protein extracts on either side of a prelabeled molecular weight marker (Bio-Rad). Blots were cut down the midline of the lane containing the visibly labeled molecular weight marker (Bio-Rad) and incubated with either the antibody or the antibody preabsorbed with its peptide antigen for ≥2 hr at room temperature. The ratio of peptide to antibody was 1:20 (w/w), respectively. Blots were reassembled just before chemiluminescent detection.
Immunocytochemistry. Whole-mount immunocytochemistry with stomatogastric ganglia was as described previously (Baro et al., 2000). Controls included preabsorption of the primary antibody with the peptide used to generate the antibody for 2 hr at room temperature before incubation with the preparation (peptide to antibody: 1:20, w/w) and omission of the primary antibody. In all cases, the 5-HT2βPan signals were lost, indicating specificity of the antibody (data not shown). To determine whether the receptor was present at neuromuscular junctions (NMJs), a double-label protocol involving anti-5HT2βCrust and antisynaptotagmin, which labels NMJs (Littleton et al., 1993; Cooper et al., 1995) and was a kind gift from Dr. Hugo Bellen (Baylor College of Medicine, Houston, TX), was performed on identified muscles [ventral pyloric dilator (PD), also known as cpv2 and P8) (Govind and Atwood, 1975). Muscles were dissected without the ganglion and fixed intact in 3.2% paraformaldehyde in PBS (0.14 m NaCl, 0.27 mm KCl, 10 mm Na2 HPO4, and 0.18 mm KH2 PO4, pH 7.3) for 2 hr at 4°C. The fixed muscle was then pulled into strips (and for the PD, cut in half to reduce the length) and washed in eight changes of PBST (PBS plus 0.3% Triton X-100) over 2–8 hr. The primary antibody was added (1:1000 dilution of rabbit anti-synaptotagmin in PBST containing 5% NGS) and incubated overnight at 4°C. The preparation was then washed in eight changes of PBST over 2–8 hr. The secondary antibody, goat anti-rabbit IgG Fab fragments conjugated to FITC or tetramethyl rhodamine (Jackson ImmunoResearch, West Grove, PA), was added at a dilution of 1:50 in PBST containing 5% NGS and incubated overnight at 4°C. The preparation was washed in eight changes of PBST over 2–8 hr. The second primary antibody was added (1–10 μg/ml rabbit anti-5-HT2βCrust in PBST containing 5% NGS), and the preparation was incubated overnight at 4°C. The primary antibody was washed out with eight changes of PBST over 2–8 hr, and a second secondary antibody was added [goat anti-rabbit IgG conjugated to Texas Red (Jackson ImmunoResearch) or Oregon Green 488 (Molecular Probes, Eugene, OR) in PBST containing 5% NGS] and incubated overnight at 4°C. The secondary antibody was washed out in eight changes of PBS over 2–8 hr. The preparation was placed on a poly-lysine-coated coverslip, dehydrated in an ethanol series, cleared in xylene, and mounted on a glass slide with DPX (Fluka, Buchs, Switzerland), as described previously (Baro et al., 2000). In some experiments, the order of the primary antibodies was reversed. Negative controls included preparations in which the second primary antibody (always rabbit anti-5-HT2βCrust) was omitted or preabsorbed for ≥2 hr with the peptide that served as the antigen in antibody production (antibody to antigen ratio: 20:1, w/w). Negative controls always included sequential addition of both secondary antibodies. In the negative controls, NMJs were still identified by robust synaptotagmin staining, but unlike the experimental preparations, the NMJs in the negative controls never showed double labeling. In addition, single-label experiments using only one primary antibody and one secondary antibody confirmed the findings of the double-label experiments. Similarly, double-label experiments were performed on the STG using the same protocol, except that the additional primary antibody was anti-shal 1.b, instead of anti-synaptotagmin. All images were obtained with a LSM510 confocal imaging system (Zeiss, Oberkochen, Germany) and manipulated with Adobe Photoshop 5.5 software (Adobe Systems, Mountain View, CA).
Experimental animals. Pacific spiny lobsters (P. interruptus) were obtained from Don Tomlinson Commercial Fishing (San Diego, CA). Lobsters were maintained at 16°C in constantly aerated and filtered seawater. All animals were anesthetized by cooling on ice before experiments.
Statistical analyses. Student's t tests were performed with Excel software (α = 0.05). Results are given as the mean ± SEM.
Results
Bioinformatics and cloning of 5-HT2βPan
We took advantage of the insect genome projects (Drosophila and Anopheles) to clone a novel arthropod 5-HT type 2 receptor. First, we mined the Drosophila database for potential monoamine GPCRs and identified several candidates, including nine previously cloned monoamine receptors (Table 1). We next used one of the uncloned candidates (accession number NP_731257) in a protein–protein blast against the Anopheles database to identify the mosquito ortholog. An alignment of the two protein sequences revealed highly conserved regions that were then used as templates in the design of degenerate primers. Nested PCRs with the degenerate primers and a spiny lobster cDNA template produced fragments of the lobster ortholog. These fragments were used as probes to screen five Panulirus cDNA libraries. We obtained five partial library clones, all missing the N terminus. RACE was used to obtain the missing 5′ ends.
Table 1.
Monoamine GPCRs in Drosophila
|
Protein accession number(s)a |
Subtype |
Original references |
Original name(s) |
Renamed according to the new nomenclature rules |
|---|---|---|---|---|
| NP_524548 | DA1 | Feng et al. (1996), Han et al.(1996) | DAMB, DopR99B | D1αDro |
| NP_733299 | DA1 | Gotzes et al. (1994), Sugamori et al. (1995) | Dmdop1, dDA1 | D1βDro |
| NP_477007 | DA2 | Hearn et al. (2002) | DD2R | D2Dro |
| NP_524419 | TYR | Arakawa et al. (1990), Saudou et al. (1990) | Dmoct/tyr | |
| NP_732541 | OCT | Han et al. (1998) | OAMB | |
| NP_524599 | 5-HT7 | Witz et al. (1990) | 5HT-dro1 | 5-HT7Dro (Colas et al., 1995) |
| NP_476802 | 5-HT1 | Saudou et al. (1992) | 5HT-dro2A | 5-HT1ADro (Colas et al., 1995) |
| 5-HT1αDro (present study) | ||||
| NP_523789 | 5-HT1 | Saudou et al. (1992) | 5HT-dro2B | 5-HT1BDro (Colas et al., 1995) |
| NP_524223 | 5-HT2 | Colas et al. (1995) | 5-HT2Dro | 5-HT1βDro (present study) |
| NP_731257, NP_649805 | 5-HT2 | Present study | 5-HT2βDro | 5-HT2αDro (present study) |
| NP_651057 | Putative | |||
| NP_650651 | Putative | |||
| NP_650652 | Putative | |||
| NP_650754 | Putative | |||
| NP_651772 | Putative | |||
| NP_647897 | Putative | |||
| NP_572358 | Putative | |||
|
NP_731719 to 731721, NP_650212 to 650213 |
Putative |
|
|
|
DA, Dopamine; TYR, tyramine; OCT, octopamine.
Additional accession numbers for a given receptor may exist in the databases.
The predicted protein sequence from the complete lobster cDNA (Fig. 1) was used in a stringent protein–protein blast against the Drosophila database. To our surprise, this returned two adjacent genes on chromosome arm 3R (FLYBASE, http://flybase.bio.indiana.edu). The first was our candidate gene (accession number NP_731257) situated at cytological location 85A5. The second gene (accession number NP_649805) mapped to cytological position 85A4 and was among the remaining genes from our mining expedition. Closer inspection of the two protein sequences revealed that transmembrane regions (TM) 3–7 and the C terminus of the receptor were contained in the original candidate gene, whereas the N terminus and TM1–2 were contained in the second candidate gene. Clearly, the algorithms used to annotate the database and identify introns and gene boundaries did not detect the very large intron between TM2 and TM3, because instead of identifying one large, sprawling gene, the program defined two smaller genes. As a result, we reanalyzed all candidate genes resulting from the mining expedition for the presence of all seven transmembrane regions. Complete predicted receptors currently represented by multiple genes in the databases are indicated as such in Table 1. This study makes two important points: (1) the existing genome projects (two or more) can be used effectively to clone orthologs from related species for which no sequence data are available; and (2) cloning genes from related organisms helps to annotate existing databases.
Figure 1.

Conservation of 5-HT receptors. The predicted protein sequences for the two arthropod 5-HT2β receptors are aligned with those predicted for the human type 2C receptor, the arthropod 5-HT2α paralog, and a lobster 5-HT type 1 receptor. Amino acids are numbered such that the start methionine is +1 in each sequence. The heavy black bars above the sequence approximate the seven transmembrane regions. Amino acids matching the consensus sequence are boxed. Amino acids discussed in the text or listed in Table 2 are highlighted. Highlighted circles represent N-linked glycosylation sites. The accession numbers are: 2Chum, NM_000868; 2αDro, NP_524223; 2βDro, NP_731257 plus NP_649805; 2βPan, AY550910; 1Pan, AY528822.
5-HT2βPan sequence analyses and comparisons
A protein–protein blast of the complete Genbank with the predicted amino acid sequence of the newly identified lobster monoamine receptor revealed that this novel receptor is a homolog of mammalian 5-HT2 receptors, with e-values ranging from e-33 to e-31 for mammalian type 2 receptors, e-26 for 5-HT2αDro (Colas et al., 1995), and e-23 for an arthropod 5-HT type 7 receptor. Arthropod 5-HT type 1 receptors were not listed in the results, but a paired protein–protein blast against 5-HT1αDro returned an e-value of 6e-16. Because one 5-HT type 2 receptor has already been identified in Drosophila (Colas et al., 1995), we named the two new proteins 5-HT2βPan (lobster) and 5-HT2βDro (fruit fly) according to a modification of the suggested nomenclature rules (Colas et al., 1995; Tierney, 2001). With this nomenclature, homology to the mammalian subtypes is indicated by a subscripted number immediately after 5-HT (i.e., 5-HT1–5-HT7). This number is meant to imply conservation between vertebrate and invertebrate receptors at the level of the DNA sequence and the signaling pathway. Thus, the arthropod 5-HT1 receptors, originally named 5-HT2A and 5HT2B (Table 1) (Saudou et al., 1992; Colas et al.,1995), are most homologous to the mammalian 5-HT1 receptors and all negatively couple with cAMP. Similarly, the arthropod 5-HT7 receptor, originally named 5-HTdro1 (Table 1) (Witz et al., 1990; Colas et al., 1995), positively couples with cAMP like its mammalian homologs. When there is more than one known gene within a subtype, individuals are represented by subscripted letters that immediately follow the number. At this point, we modified the original nomenclature scheme to include Greek letters for arthropod receptors (i.e., 5-HT1α, 5-HT1β, etc.), rather than the Roman letters used in the mammalian nomenclature (i.e., 5-HT1A, 5-HT1B, etc.). This is because the subscripted letter is not meant to imply orthology across vertebrate/invertebrate lines. It is generally accepted that the paralogs within a subtype (i.e., 5-HT1A, 5-HT1B, etc.) evolved independently for mammals and invertebrates (Tierney, 2001); thus, the arthropod 5-HT2β receptor has no real ortholog among the three vertebrate type 2 receptors: 2A, 2B, 2C. To emphasize this fact and thereby reduce confusion, here we indicate paralogs within a subtype using Greek letters for arthropods and Roman letters for vertebrates. Species is indicated immediately after the subscripted letter.
A prosite scan revealed that both arthropod 2β orthologs contain N-linked glycosylation sites in their N termini (Fig. 1) and multiple phosphorylation sites (data not shown). Figure 1 shows an alignment of the arthropod 5-HT2βPan receptors with a mammalian 5-HT2C receptor, as well as 5-HT2αDro and a 5-HT subtype 1 homolog from lobster (5-HT1Pan) (Sosa et al., 2004). Only the transmembrane regions were aligned, thereby emphasizing the differences in the lengths of the extracellular N termini, the third intracellular loops (i3), and the intracellular C termini. Alhough characteristic and expected, the lack of i3 conservation between 2β orthologs is somewhat baffling given that G-protein coupling and receptor targeting are conserved functions of the i3 domain (Kroeze et al., 2002). The other intracellular loops (i1 and i2) are well conserved between 2β orthologs, whereas the extracellular loops (e1–e3) are not. G-protein coupling is partially determined by i2 (Burns et al., 1997; Lembo et al., 1997; Niswender et al., 1999) and e2 functions in ligand selectivity (Kroeze et al., 2002), but the functions of the other loops are unknown.
The N termini of 5-HT receptors are usually not conserved in sequence or length, and their role remains undefined. The conserved proximal portion of the C terminus (2βPan, 706K–717K) is predicted to form a helix parallel to the membrane surface, and the adjacent conserved cysteine (2βPan, 718C) represents a putative palmitoylation site that may play a role in anchoring this presumed eighth cytoplasmic helix at the membrane surface (Palczewski et al., 2000; Kroeze et al., 2002). The terminal amino acids of the human 2C receptor form a type 1 PDZ domain that interacts with the MUPP1 scaffold protein to form a multiprotein signaling complex (Becamel et al., 2001, 2002). Similarly, this domain helps to determine functional activity and receptor trafficking of 5-HT2A receptors (Xia et al., 2003). In contrast, this domain is not present in the arthropod 2β orthologs, although the terminal amino acids of the lobster receptor form an atypical PDZ domain (Bezprozvanny and Maximov, 2001).
There is a great deal of conservation in the transmembrane regions, which function both in ligand binding and receptor activation. Residues known to be important to these functions are listed in Table 2. Amino acids in the transmembrane helices near the extracellular surface are thought to interact and form a binding pocket for ligands (Kroeze et al., 2002). Roughly half of the amino acids known to be involved in ligand binding are conserved between the human 2C and lobster 2β receptors (Table 2), which is consistent with the fact that pharmacological profiles are not fully preserved across species. It has been suggested that the cytoplasmic regions of TM3 and TM6 are closely associated in the inactive receptor but that these helices move apart in the activated receptor (Visiers et al., 2001; Kroeze et al., 2002; Shapiro et al., 2002). The highly conserved DRY motif in TM3 is partially responsible for mediating this effect. The conserved arginine residue (2βPan: 170R) is thought to interact with the neighboring conserved aspartate in TM3 (2βPan: 169D) and a conserved glutamate in TM6 (2βPan: 644E). Mutations that disrupt this triad cause constitutive activity in mammalian receptors. Although the tyrosine (Y) in the DRY motif is highly conserved among most monoamine receptors, to our knowledge, its function in receptor activation has not been characterized by mutagenic analyses. Interestingly, this residue is not conserved in the lobster 2β ortholog (2βpan: 171F).
Table 2.
5-HT2 receptor transmembrane region (TMR) amino acids with known functions
|
TMR |
Position in receptor |
Function |
Conserved |
References |
|---|---|---|---|---|
| TM1 | 2CHum55W | LB | No | Roth et al. (1997a,b) |
| TM2 | 2CHum104L | LB | No | Wang et al. (1993), Choudhary et al. (1995), Sealfon et al. (1995), Roth et al. (1997b), Manivet et al. (2002) |
| 2CHum111L | LB | No | ||
| 2CHum113A | LB | No | ||
| 2βPan118D | LB and G-protein coupling, binds to residue | Yes | ||
| 2BPan697N in TM7 | ||||
| TM3 | 2βPan152D | LB and membrane targeting | Yes | Kristiansen et al. (2000), Visiers et al. (2001), Kroeze et al. (2002), Manivet et al. (2002), Shapiro et al. (2002), present study |
| 2CHum138S | LB | No | ||
| 2βPan169D | Activation (see text) | Yes | ||
| 2βPan170R | Activation (see text) | Yes | ||
| 2βPan171F | Activation (see text) | No | ||
| TM5 | 2βPan236S | LB | Yes | Almaula et al. (1996a,b), Johnson et al. (1997), Shapiro et al. (2000), Manivet et al. (2002) |
| 2βPan240F | LB contributes to HBP | Yes | ||
| 2βPan242I | LB | Yes | ||
| 2CHum222A | LB | No | ||
| 2CHum224F | LB | No | ||
| 2CHum228T | LB | No | ||
| TM6 | 2CHum305N | Activation | No | Choudhary et al. (1993, 1995); Roth et al. (1997a,b), Shapiro et al. (2000), Visiers et al. (2001), Manivet et al. (2002) |
| 2CHum310S | Activation | No | ||
| 2βPan644E | ||||
| 2βPan645Q | ||||
| 2βPan646K | Activation (see text) | Yes | ||
| 2βPan650V | Activation | Yes | ||
| 2βPan651L | Activation | Yes | ||
| 2βPan654V | Activation | Yes | ||
| 2βPan662W | (Activation) | Yes | ||
| 2βPan664P | (Activation) | Yes | ||
| 2βPan665F | LB contributes to HBP | Yes | ||
| 2βPan666F | Activation: hinge allowing TM3/TM6 association—disassociation | Yes | ||
| 2βPan669N | LB contributes to HBP | Yes | ||
| TM7 | 2βPan688W | LB contributes to HBP | Yes | Sealfon et al. (1995), Roth et al. (1997a,b), Rosendorff et al. (2000), Manivet et al. (2002) |
| 2βPan691Y | LB contributes to HBP | Yes | ||
| 2CHum353F | LB | No | ||
| 2βPan697N | LB/coupling: interacts with 2Bpan118D | Yes | ||
|
|
2βPan701Y |
Activation |
Yes |
|
LB, Ligand binding; HBP, hydrophobic binding pocket.
The 5-HT2βPan signaling pathway: Gq to PLC to PKC
Whereas a 5-HT type 2 receptor has been cloned in Drosophila (Colas et al., 1995), its G-protein coupling(s) is unknown. Conservation of a given receptor subtype across species usually extends beyond the sequence to the primary signaling pathway. Traditionally, mammalian 5-HT type 2 receptors are thought to couple via Gq to phospholipase Cβ (PLC). Activated PLC hydrolyzes phosphatidylinositol 4,5 bisphosphate (PIP2), thereby producing IP3 and DAG. IP3 goes on to release calcium stores, and together, Ca2+ and DAG bind to the C2 and C1 sites on PKC, respectively, thereby linking PKC to the membrane where it is activated by association with phosphotidyl serine (Newton, 2001). It has now been shown that 5-HT2 receptors can additionally couple with multiple pathways (Berg et al., 1998; Pauwels, 2000; Kurrasch-Orbaugh et al., 2003) and that there are three subtypes of PKC isozymes that vary with respect to their substrates and requirements for DAG and Ca2+ (Way et al., 2000). In the next series of experiments, we investigated whether 5-HT2βPan is coupled with the Gq signaling pathway.
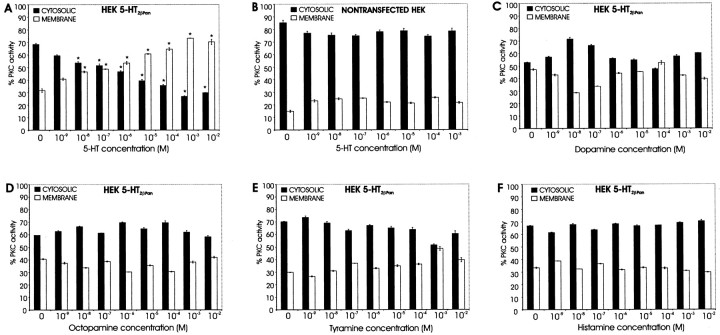
Using standard cloning techniques, we generated full-length constructs for 5-HT2βPan and used them to establish permanent human embryonic kidney (HEK) cell lines expressing the receptor (HEK 5-HT2βPan). We then assayed cells for PKC activation in response to increasing concentrations of 5-HT (Fig. 2). PKC translocates from the cytosol to the membrane where it is activated, and translocation is a standard assay for PKC activation in response to GPCR activity (Newton, 2001). Therefore, we exposed cells to 10–9 to 10–2 m 5-HT for 15 min, lysed and fractionated the cells (membrane vs cytosol), and then measured total PKC activity in each of the two fractions using an assay that detects phosphorylation of neurogranin, a substrate for conventional PKC isozymes that are sensitive to both [DAG] and [Ca2+] (Huang et al., 1993; Ramakers et al., 1999). Figure 2A demonstrates that for HEK 5-HT2βPan cells there is a significant dose-dependent translocation of PKC to the membrane in response to increasing concentrations of 5-HT. In contrast, the parental, nontransfected HEK cells show no response to 5-HT application (Fig. 2B). Because some monoamine receptors can be activated by multiple ligands (Hearn et al., 2002), we next asked whether other monoamines that normally function as modulators in the lobster nervous system could activate the 5-HT2βPan receptor. Neither dopamine (Fig. 2C), octopamine (Fig. 2D), tyramine (Fig. 2E) nor histamine (Fig. 2F) produced significant PKC translocation in HEK5-HT2βPan cells, even at concentrations as high as 10 mm. These results are consistent with the classification of our newly discovered GPCR as a 5-HT2 receptor.
Figure 2.
The effect of biogenic amines on PKC translocation. HEK 5-HT2βPan or nontransfected HEK cells were exposed to the indicated drug for 15 min. Cytosolic and membrane fractions were separated, and PKC-specific activity in each fraction was measured. The percentage of total PKC-specific activity associated with the cytosolic fractions (▪) versus membrane fractions (□) is indicated. Data are expressed as the mean ± SEM, and n = 3 for all experiments, except A and B, in which n = 8 for 0 5-HT. *p < 0.05, significantly different when compared with the same fraction in the absence of drug.
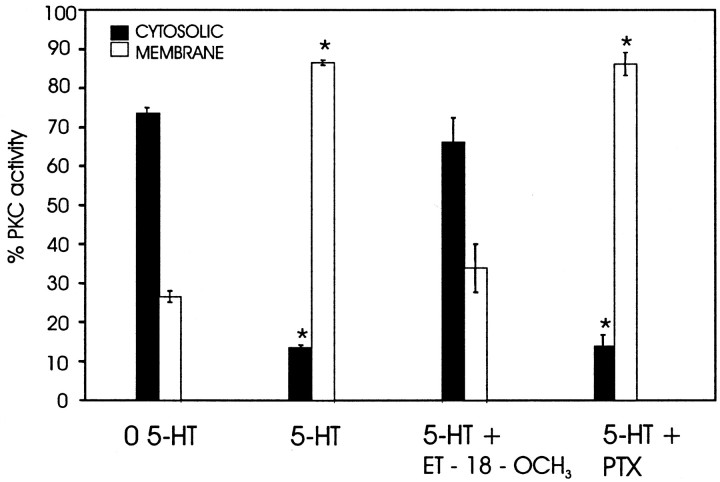
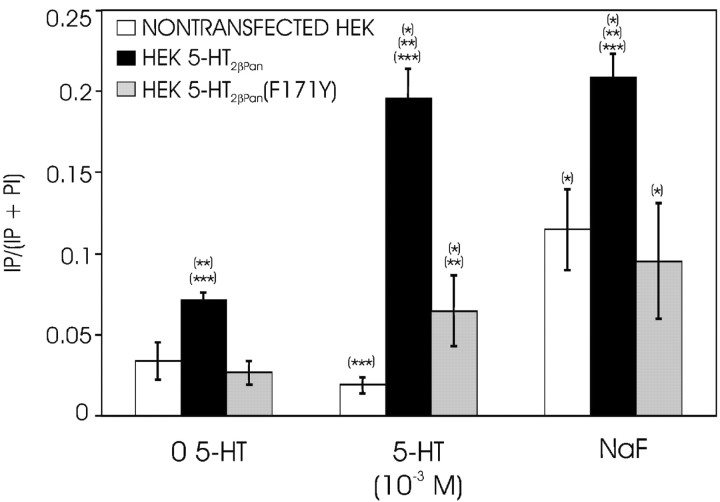
To show that PLC activation led to the previously observed PKC translocation in cells expressing the 5-HT2βPan receptor, we applied the PLC inhibitor ET-18-OCH3 15 min before incubation with 5-HT and assayed the cells for PKC translocation. Figure 3 shows that application of the PLC inhibitor precludes the 5-HT-evoked PKC translocation, suggesting that the signaling cascade activated by the 5-HT2βPan receptor includes PLC. To further test this hypothesis, we measured IP accumulation in response to 5-HT. Figure 4 illustrates that HEK 5-HT2βPan cells displayed a significant 2.75-fold increase in PI hydrolysis (or IP accumulation) in response to 10–3 m 5-HT (p < 0.01), whereas the parental HEK cells showed no significant change. However, both cell lines responded to the nonspecific activator of trimeric G-proteins, NaF, with approximately a threefold increase in PI hydrolysis. Collectively, these data suggest that the 5-HT2βPan receptor transduces signals via PLC.
Figure 3.
The PLC inhibitor ET-18-OCH3, but not PTX, blocks the 5HT2βPan receptor-mediated translocation of PKC. After pretreatment with the indicated inhibitor, cells were incubated an additional 15 min with 5-HT (10–3m). PKC activity was measured in the cytosolic and membrane fractions. Data are expressed as the mean ± SEM; n = 3 per condition. *p < 0.05, significantly different when compared with 0 5-HT.
Figure 4.
5-HT stimulates IP production in transfected cells expressing serotonin receptors but not in the parental HEK cell line. Total PI and IP were assayed in nontransfected HEK (□), HEK 5-HT2βPan (▪), and HEK 5-HT2βPan (F171Y) (▦) cells. Three conditions are shown: control (no drug), 5-HT (60 min exposure to 10–3 m 5-HT), and NaF (60 min exposure to the nonspecific trimeric G-protein activator 20 mm NaF). Results are expressed as a ratio of IP/(PI + IP). Data represent the mean ± SEM from three separate experiments. *Significantly different (p < 0.05) for drug versus control for a given cell line; **significantly different from HEK cells under the same condition (i.e., comparisons within each of the three conditions: control, 5-HT, NaF); ***significantly different from HEK 5-HT2βPan (F171Y) under the same condition.
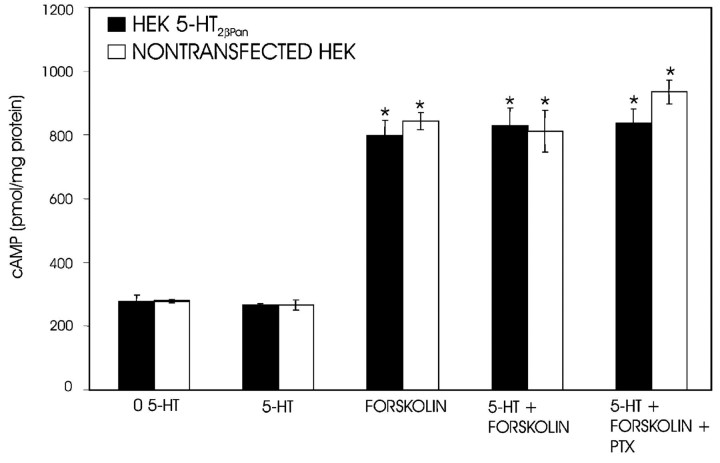
PLC can be activated by the α subunit of Gq or through the βγ subunits of other G-proteins. Three pieces of data suggest that PLC is activated via Gq. First, both nontransfected HEK and HEK 5-HT2βPan cells showed no increase in cAMP levels in response to 10–3 m 5-HT (Fig. 5). However, both cell lines responded to the adenylate cyclase activator forskolin with equivalent increases in [cAMP] (Fig. 5). These data suggest that 5-HT2βPan does not activate Gs, which positively couples with adenylate cyclase. Second, exposure to 5-HT did not significantly alter the forskolin response in either cell line, suggesting that Gi/o, which inhibits adenylate cyclase, was not activated by 5-HT in either cell line (Fig. 5). Third, PTX, which specifically blocks Gi/o dissociation into Gα and Gβγ subunits, had no significant effect on the 5-HT-evoked PKC translocation in HEK 5-HT2βPan cells (Fig. 3) or on levels of cAMP in cells exposed to both forskolin and 5-HT (Fig. 5). This further demonstrates that the βγ subunit of Gi/o does not activate PLC in response to 5-HT. Collectively, these data are consistent with a mode of action by which the 5-HT2βPan receptor couples with what has been designated as the traditional pathway for 5-HT2 receptors in mammals: Gq to PLC to PKC.
Figure 5.
cAMP levels do not change in response to 5-HT treatment. cAMP levels were measured in 5-HT2βPan (▪) and nontransfected HEK (□) cells or cells exposed to 5-HT (10–3 m), or forskolin (2.5 mm), or forskolin (2.5 mm) with 5-HT(10–3 m), or forskolin (2.5 mm) with 5-HT(10–3 m) after pretreatment with PTX. Results are expressed as picomoles of cAMP/per milligram of protein. Data are the mean ± SEM from three separate experiments. *p < 0.05, significantly different for drug versus control for a given cell line. There were no significant differences between any treatments containing forskolin within or between cell lines.
Agonist-independent activity of the 5-HT2βPan receptor
It is well documented that GPCRs can demonstrate agonist-independent activity (Seifert and Wenzel-Seifert, 2002; Mustard et al., 2003). This is usually detected as an increase in signaling pathway activity under baseline conditions (no agonist present) in cells expressing the GPCR (HEK 5-HT2βPan) relative to the nontransfected parental cell line (HEK). In all experiments, we noticed that in the absence of agonist, the PKC activity associated with the membrane fraction was significantly higher in HEK 5-HT2βPan cells relative to nontransfected HEK cells (p < 2 × 10–5; Fig. 2, compare the first set of bars in A,C–F with B). The average PKC activity associated with the membrane fraction was 11 ± 1.4% for nontransfected HEK cells and 35 ± 4.0% for HEK5-HT2βPan cells under baseline conditions. Because PKC activation is associated with translocation to the membrane, we conclude that there is approximately a threefold increase in the basal level of PKC activity in cells expressing the 5-HT2βPan receptor. Similarly, in the absence of agonist, we observed a significant 2.3-fold increase in IP levels in cells expressing the 5-HT2βPan receptor, relative to the parental cell line (Fig. 4) (p < 0.05). In contrast, baseline levels of cAMP were the same for both cell lines (Fig. 5). The most parsimonious interpretation of these data is that when the 5-HT2βPan receptor is expressed in HEK cells, it displays some agonist-independent activity.
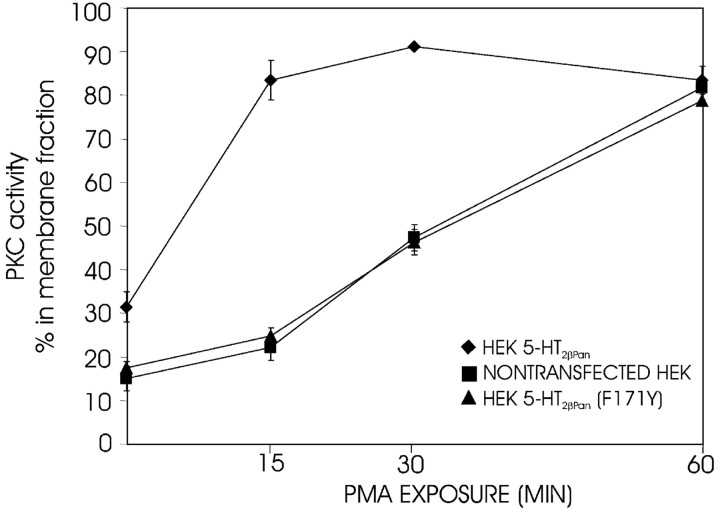
Phorbol esters, like PMA, can substitute for DAG, because they associate with the DAG binding site, C1, on the PKC enzyme. Thus, both PMA and DAG serve as hydrophobic anchors that can recruit conventional PKC isozymes to the membrane even in the absence of Ca2+ (Newton, 2001). A 15 min application of 0.1 μm PMA alone caused 90% of the PKC to translocate to the membrane in HEK 5-HT2βPan cells but not in the parental, nontransfected cells (Fig. 6). A 60 min exposure to PMA was required to obtain the same level of translocation in the parental cell line. The average PKC-specific activity was not significantly different between HEK 5-HT2βPan and HEK cells (0.11 ± 0.02 psl/μg vs 0.095 ± 0.03 psl/μg; p = 0.2). Thus, the average number of PKC molecules is probably similar in the two cell lines. Because the level of the membrane affinity of PKC is linearly related to the mol fraction of the C1 ligand in the bilayer (i.e., DAG and/or PMA), and because Ca2+ binding at the C2 site on conventional PKC enzymes acts synergistically with binding at the C1 site to facilitate PKC association with the membrane (Newton, 1995, 2001), the potentiated response of the transfected cells to PMA is consistent with the idea of a constitutively active receptor.
Figure 6.
The PMA response is potentiated in HEK 5-HT2βPan cells. The PKC activator PMA (0.1 μm) was applied for 15, 30, or 60 min to each of three cell lines [HEK 5-HT2βPan, ♦; HEK, ▪; HEK 5-HT2βPan (F171Y), ▴]. The PKC-specific activity associated with the cytosolic and membrane fractions was measured, and the percentage of the total specific activity associated with a given fraction was determined. The percentage of PKC-specific activity associated with the membrane is plotted for each time point in each cell line. Each data point represents the mean ± SEM; n = 3 per data point.
Blocking constitutive activity by restoring the DRY motif
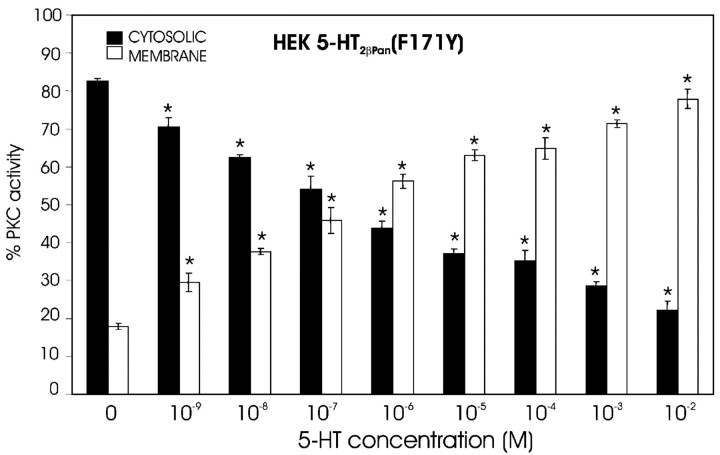
As discussed previously, it has been suggested that the highly conserved DRY motif in TM3 helps to mediate receptor activation, and mutation of the D and/or R in this motif is known to cause constitutive receptor activity in mammalian type 2 receptors (Kroeze et al., 2002; Shapiro et al., 2002). In the 5-HT2βPan receptor, the DRY motif has evolved into DRF (Fig. 1). A hydrophobic amino acid (F) replaces a polar residue (Y), producing a nonconservative change in the motif. Interestingly, this same alteration is also observed in the nematode receptor 5-HT2As (Huang et al., 2002). Restoration of the DRY motif in the nematode receptor caused an increase in its affinity for 5-HT, but the effect on activation was not considered. We hypothesized that the Y to F transition might contribute to the observed constitutive activity of the lobster receptor. To test this hypothesis, we used site-directed mutagenesis (F171Y) on our original construct to restore the DRY motif to the 5-HT2βPan receptor. We used this new construct to stably transfect HEK cells and establish a second cell line, HEK 5-HT2βPan(F171Y). We then assayed the cell line for PKC activity and PI hydrolysis as described previously. Figure 7 demonstrates that HEK 5-HT2βPan(F171Y) cells still show a dose-dependent translocation of PKC in response to increasing levels of 5-HT. However, in the absence of agonist, only 17 ± 2.0% of the total PKC-specific activity was now associated with the membrane fraction, which was not significantly different from the 11 ± 1.4% observed for the parental, nontransfected HEK cell line but was significantly different from the 35 ± 4.0% observed for the wild-type receptor containing the DRF motif (p < 0.002). Similarly, cells expressing the mutated receptor still responded to 10–3m 5-HT with a significant ∼2.4-fold increase in PI hydrolysis (Fig. 4) (p < 0.05). However, under baseline conditions, IP levels were not significantly different between HEK 5-HT2βPan(F171Y) and parental HEK cells (Fig. 4) but were significantly lower than for cells expressing the wild-type DRF receptor (p < 0.003). Finally, Figure 6 illustrates that restoration of the DRY motif abolishes the potentiated PMA response associated with the wild-type receptor. All of these results strengthen the idea that the wild-type 5-HT2βPan receptor is constitutively active when expressed in HEK cells and suggest that the residue at position 171 plays a role in receptor activation.
Figure 7.
Restoration of the DRY motif disrupts the agonist-independent activity associated with the 5-HT2βPan receptor but not the response to 5-HT. HEK 5-HT2βPan (F171Y) cells were assayed for PKC translocation after a 15 min exposure to varying concentrations of 5-HT, as indicated. Data are expressed as the mean ± SEM from three separate experiments, except for the 0 5-HT data point, in which n = 5. *p < 0.05 when compared with activity in the same fraction in the absence of drug treatment.
Interestingly, in addition to triggering constitutive activity, the Y to F transition at position 171 also seems to increase evoked PI hydrolysis. Figure 4 shows that PI hydrolysis in response to 5-HT was significantly higher in cells expressing the wild-type DRF versus the mutant DRY receptor. Similarly, the response of 5-HT2βPan cells to NaF was significantly greater than for either of the other two cell lines. Surprisingly, there did not appear to be a concomitant potentiation of the evoked PKC response. An interpretation of these data is confounded by the experimental protocols used. First, in our assay for PI hydrolysis, we measure total IP, which consists of IP1 + IP2, + IP3. Thus, an increase in IP accumulation may not explicitly translate into an increase in the second messengers that lead to PKC activation. Furthermore, the 5-HT exposure time varied between the two assays (15 min for the PKC assay vs 60 min for the IP assay), as did the state of the cells during exposure to the drug (resuspended vs plated, respectively).
5-HT2βPan localization in the STNS
In P. interruptus, stomatogastric neurons are modulated by bath application of 5-HT (Harris-Warrick, 1992a). Interestingly, there are no serotonergic input fibers to the STG. Instead, the ganglion is located in a blood vessel, and a short distance away is a neurohemal plexus that releases 5-HT into the hemolymph that constantly bathes the ganglion (Sullivan et al., 1977; Beltz et al., 1984; Beltz, 1999). The 5-HT receptors in P. interruptus have a high affinity for 5-HT and respond to physiological concentrations contained in the hemolymph (Beltz, 1999). Thus, it is generally thought that 5-HT acts as a neurohormone in this system. We were interested in whether 5-HT2βPan could potentially serve as a neurohormonal receptor mediating some of the actions of 5-HT on stomatogastric neurons. Therefore, we asked whether 5-HT2βPan was expressed in the STNS and, if so, where this potential neurohormone receptor might be located.
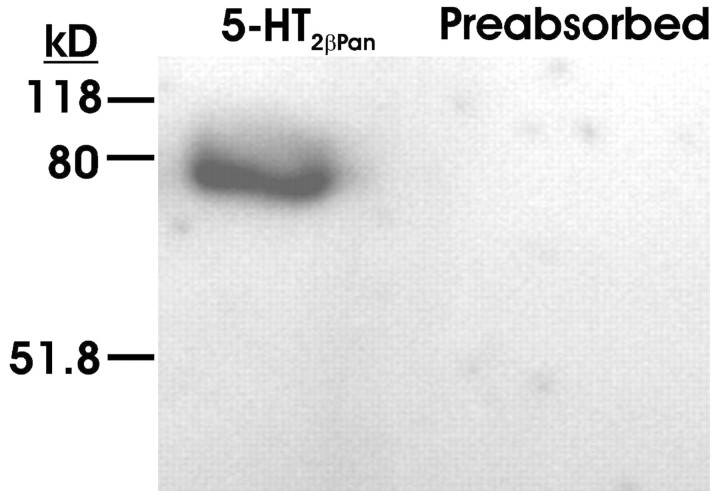
We generated affinity-purified antibodies against the receptor as detailed in Materials and Methods. The Western blot in Figure 8 shows that the antibody recognizes a single band around the predicted molecular weight of 79 kDa, and the signal is lost on preabsorption with the peptide used to generate the antibody, suggesting that the antibody is specific for the 5-HT2βPan protein. To determine whether this receptor is expressed in pyloric neurons, we used the antibody to perform whole-mount immunocytochemistry experiments on the STG and identified muscles, followed by confocal microscopy, as described previously (Baro et al., 2000).
Figure 8.
Anti-5HT2βCrust specifically recognizes the 5-HT2βPan receptor in lobster nervous tissue. A representative Western blot experiment is shown. The blot containing protein extracts from lobster nervous tissue was probed with an antibody against 5-HT2βPan (5-HT2βCrust) or the same antibody preabsorbed with the peptide antigen used to generate the antibody (preabsorbed). Molecular weight standards are indicated. The antibody produces a signal corresponding to the predicted size of the protein, which is lost on preabsorption.
The STG is a sphere containing a core of coarse neuropil, which is encompassed by a layer of fine neuropil, which in turn, is surrounded by the peripheral layer that contains neuronal cell bodies, nerve fibers, blood vessels, and blood cells interspersed among numerous glial elements. A perineural sheath covers the entire ganglion (for a more complete perspective, see Baro et al., 2000; Wilensky et al., 2003). A neuronal soma in the peripheral layer sends a primary process into the central coarse neuropil that then branches into secondary and tertiary neurites. Processes from the coarse neuropil enter and branch in the more peripheral fine neuropil, where synapses occur between stomatogastric neurons as well as modulatory input fibers. Afferent fibers mainly enter the ganglion through the peripheral layer and then go on to form synapses in the fine neuropil (Friend, 1976; King, 1976a,b). The stomatogastric nerve and dorsal ventricular nerve (dvn) are associated with the ganglion anteriorly and posteriorly, respectively. Most stomatogastric neurons are motoneurons that send an axon out the dvn, each of which goes on to innervate identified muscles.
Figure 9 shows receptor distribution in the STG. We found that the receptor was expressed in what appears to be the somatic endomembrane compartment of 98% of the neurons (n = 6 ganglia), but expression levels varied across neurons (Figs. 9A,B). In an attempt to determine whether the receptor might be in the somatodendritic plasma membrane, we performed double-label experiments (Fig. 9C) using anti-5HT2βCrust and a second antibody against the terminal amino acids of the K+ channel, Shal 1.b (Baro et al., 2001). Although shal channels have previously been shown to reside in the somatodendritic plasma membranes of stomatogastric neurons and glial cells (Baro et al., 2000), this particular isoform is expressed only in neurons (Krenz and Baro, unpublished observations). The typical neuronal shal channel profile, which includes an intense ring around the somata, can be seen in Figure 9C. This ring, which indicates that the protein is concentrated in the plasma membrane, was missing in the 5-HT2 receptor profile for all single- and double-label experiments. Thus, the 5-HT2 receptor does not appear to be well represented in the somatic plasma membrane relative to K+ channels. Similarly, an intense ring surrounding the large diameter process in the coarse neuropil was seen only in the K+ channel profiles (data not shown). In contrast, the receptor was observed in higher branch order processes of the fine neuropil and in what we interpret to be the endomembrane system of a fraction of fibers throughout the ganglion (Fig. 9A,B). The receptor was not observed in the membranes of axons leaving the ganglion via the dvn or in glial cells surrounding the neurons.
Figure 9.
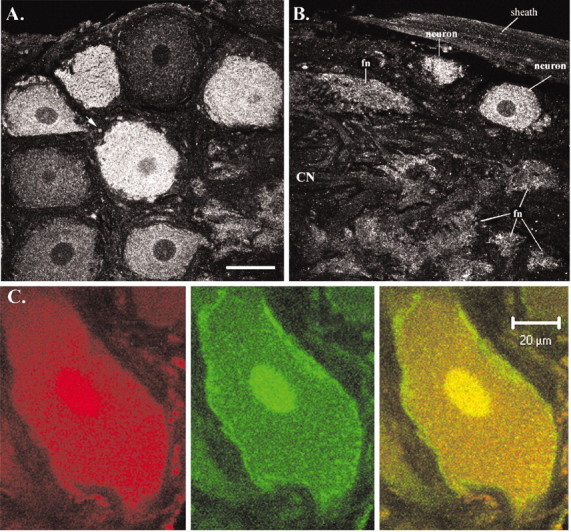
Most stomatogastric neurons express 5-HT2βPan to varying degrees. A, B, A 5.45 μm confocal slice from different representative ganglia are shown. Scale bar (in A), 50 μm. A, Anterior left quadrant ∼20 μm from the dorsalmost aspect of the ganglion. This slice depicts mostly the peripheral layer showing eight neuronal cell bodies; however, the fine, synaptic neuropil-containing tufts of neurites from stomatogastric and modulatory input neurons can be seen in the bottom right corner, just above the scale bar. The arrow points to one of many profiles most likely representing transport of the receptor in fibers that are entering/leaving the ganglion through the peripheral layer. B, Optical slice representing posterior left quadrant of the ganglion ∼35 μm from the ventralmost aspect. All layers of the ganglion appear in this slice: the triangular course neuropil (CN) jutting out from the left, surrounded by several tufts of fine neuropil (fn), surrounded by the peripheral layer containing somata, surrounded by the perineural sheath. C, Confocal projection representing ∼1.5μm in depth, showing a single neuron double-labeled for the 5-HT2βPan receptor (red) and the K+ channel shal 1.b (green). The right panel represents the merged image. Notice the ring of protein in the plasma membrane that is present in the K+ channel profile but absent in the receptor profile. In our hands, the nuclei always stained intensely in the double-label experiments (in both the receptor and channel profiles) but not in the single-label experiments (in both the receptor and channel profiles). We do not understand this technical artifact.
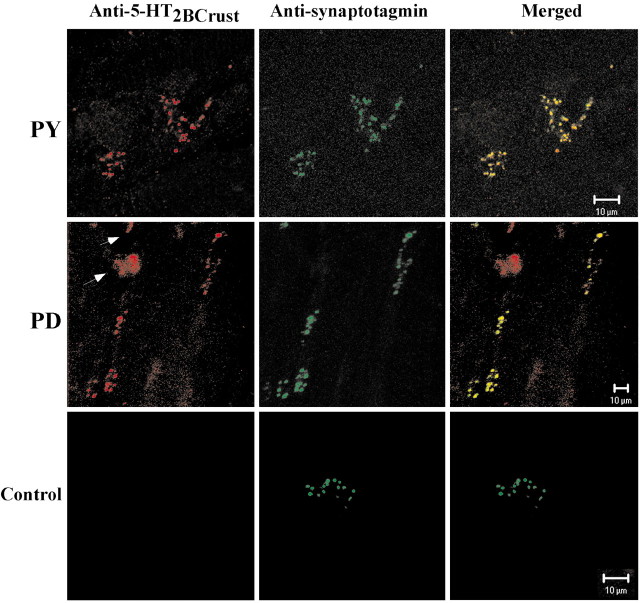
The signals detected in the endomembrane system most likely represent turnover, processing, and/or transport of the receptor protein. We asked whether pyloric neurons transport the receptors to axon terminals at NMJs by examining receptor distribution at two identified NMJs: the PD NMJ [PD neurons exclusively innervating the paired ventral PD muscles (cpv2) n = 5 animals] and the pyloric constrictor (PY) NMJ (PY neurons exclusively innervating the paired P8 muscles; n = 4 animals). In these experiments, we used a second antibody against synaptotagmin that has previously been used to identify the axon terminals at crustacean NMJs (Littleton et al., 1993; Cooper et al., 1995). Figure 10 shows that the 5-HT2βPan receptor is observed at both identified NMJs. The strong colocalization with synaptotagmin, an integral, synaptic vesicle membrane protein, suggests that the receptor is located presynaptically, which is consistent with the idea of receptor transport to axon terminals. We occasionally observed receptor staining without counterpart synaptotagmin staining (Fig. 10, arrows). We do not know whether this staining represents extrasynaptic receptors in the muscle and/or terminal or simply “nonspecific” sticking of the antibody; however, this staining was not observed when the anti-5-HT2βCrust antibody was preabsorbed. In addition, receptor staining at the PD NMJ was always robust, whereas at the PY NMJ, staining intensities varied with the animal. We do not know whether this variability was biological or technical, however, synaptotagmin staining at all NMJs was always robust in all animals.
Figure 10.
Colocalization of 5-HT2βPan receptors with synaptotagmin at identified NMJs. Double-label immunocytochemistry was performed on PD and PY muscles, using the antibodies indicated above the panels. Control represents the same double-label experiment, except that the anti-5HT2βCrust antibody was preabsorbed. Each row displays representative NMJs from one experiment, in which identified muscles and control are as indicated. Arrows point to 5-HT2βPan staining that is not localized to the synapse. The PD panels represent a projection of five confocal slices representing ∼5 μm in depth. Each set of PY and preabsorbed panels represent a single 1.0 μm confocal slice.
Discussion
We have successfully used a bioinformatics approach to clone a novel 5-HT receptor from arthropods and, in particular, from a crustacean that lacks a sequenced genome. This illustrates that the profusion of data existing for model genetic organisms, like Drosophila, can be exploited to the advantage of other important, but genetically intractable, arthropod models used to study physiological processes like motor pattern generation. The number of potential monoamine receptors identified by this approach suggests that a little over half of the existing arthropod monoamine receptors have been cloned and characterized (10 of the predicted 18) and that invertebrate receptor numbers may parallel those observed for vertebrates.
The 5-HT2β receptor that we identified in fly and spiny lobster is a paralog of the 5-HT2α receptor previously cloned from Drosophila (Colas et al., 1995). Thus, there are two known arthropod 5-HT2 genes to date. We have demonstrated, for the first time, that arthropod 5-HT2 receptors couple with the Gq signaling pathway, like their mammalian homologs. This is consistent with most comparisons of monoamine receptor subtypes across vertebrate/invertebrate lines (Blenau and Baumann, 2001; Tierney, 2001), and it upholds the emerging concept that for a given receptor subtype, both sequence and primary signaling pathways will be conserved across species, whereas pharmacological profiles may not.
Similar to many other GPCRs, the 5-HT2βPan receptor displays agonist-independent activity when heterologously expressed in a mammalian cell line. Our data suggest that the polar Y to hydrophobic F transition that evolved in the highly conserved TM3 DRY motif contributes to the observed agonist-independent activity and, therefore, this residue is implicated in receptor activation. Inverse agonists stabilize GPCRs in the inactive conformation and thereby reduce agonist-independent activity. Inverse agonists (synthetic and/or endogenous) have been identified for the majority of constitutively active GPCRs (Seifert and Wenzel-Seifert, 2002). Because endogenous inverse agonists exist (Ango et al., 2001; Seifert and Wenzel-Seifert, 2002), much of the constitutive activity displayed in heterologous systems may be absent in the native system in which the inverse agonists also reside. Still, there have been demonstrations of agonist-independent activity for endogenous GPCRs in native cells (Morisset et al., 2000; Seifert and Wenzel-Seifert, 2002), and it has been further shown that constitutive activity can be regulated by cellular processes. For example, neuronal excitation can disrupt the interaction between the metabotropic glutamate receptor and the scaffold protein/inverse agonist homer 3, thereby activating the associated G-protein in the absence of ligand with a time constant that is different from that of agonist-mediated stimulation (Ango et al., 2001). Important issues that we will address in future experiments include whether the 5-HT2βPan receptor can display agonist-independent activity in pyloric neurons, under what conditions, and how this might affect network properties and motor output.
The presence of 5-HT receptors in the STG supports two decades of electrophysiological and anatomical experimentation showing that 5-HT modulates neural circuits located in this ganglion (Beltz et al., 1984; Marder and Eisen, 1984; Flamm and Harris-Warrick, 1986a,b; Harris-Warrick and Flamm, 1987; Katz et al., 1989; Katz and Harris-Warrick, 1989, 1990; Johnson and Harris-Warrick, 1990; Kiehn and Harris-Warrick, 1992a,b; Meyrand et al., 1992; Johnson et al., 1993; Turrigiano and Marder, 1993; Zhang and Harris-Warrick, 1994, 1995; Christie et al., 1995; Zhang et al., 1995; Hempel et al., 1996; Ayali and Harris-Warrick, 1999; Kilman et al., 1999; Tierney et al., 1999; Peck et al., 2001; Birmingham et al., 2003; Richards et al., 2003). To date, we have localized two serotonin receptors to the STG: 5-HT2βPan and 5-HT1Pan (Baro and M. C. Clark, unpublished observations). Another three arthropod 5-HT receptors have been cloned and characterized in insects, and it may be the case that other uncharacterized arthropodal 5-HT receptors exist (Table 1). Whether all or only a subset of arthropod 5-HT receptors are expressed in the STG has yet to be determined, but our results suggest that a single cell can express multiple 5-HT receptors and that cells differentially express a given receptor, which is consistent with previously demonstrated cell-specific responses to the neuromodulator.
Stomatogastric neurons do not target detectable numbers of 5-HT2βPan receptors to the plasma membrane surrounding the somatic compartment and the large diameter, lower branch order processes in the coarse neuropil, although receptors are seen in the endomembrane system throughout the cells and in axon terminals. The latter finding is consistent with a previous study showing that 5-HT can regulate the amplitude of nerve-evoked, stomatogastric muscle contractions (Jorge-Rivera et al., 1998) and other pharmacological and electrophysiological studies demonstrating the existence of presynaptic 5-HT2 receptors at crustacean NMJs (Dixon and Atwood, 1989; Tabor and Cooper, 2002). Although nearly all stomatogastric neurons express the 5-HT2βPan receptor, we do not know whether they target it to the plasma membranes encompassing the axon terminals, the distal neurites in the fine neuropil, and/or the spike initiation zones (sizs) (Raper, 1979; Miller, 1980; Meyrand et al., 1992; Bucher et al., 2003). It is possible that all stomatogastric neurons exclusively target 5-HT2βPan to the NMJ and that most ganglionic, plasma membrane-bound 5-HT2βPan receptors are located in modulatory neurons that synapse on stomatogastric neurites in the fine neuropil. Indeed, we observed profiles that are consistent with receptor transport in modulatory neurons. We may be able to address the likelihood of this possibility once we have the pharmacological profile for this receptor in hand.
Recent studies suggests that ion channels and GPCRs can be physically organized into functional units. One question to emerge from these studies is whether GPCRs modulate only those ion channels that exist in the same multiprotein complex, or whether, by diffusional mechanisms, they act on distant channels throughout the cell. The answer has important implications for how pyloric neurons operate, because distinct tasks such as non-spiking dendrodendritic graded synaptic transmission, axonal spike, and somal voltage oscillation generation may reside in discrete cellular compartments that are differentially decorated with receptors. 5-HT is known to differentially modulate pyloric neurons. It alters the firing properties of all pyloric neurons, except the PDs and PYs, and the efficacies of many chemical and electrical synapses, including a weakening of the chemical synapse between PD (presynaptic) and PY (postsynaptic) neurons (Flamm and Harris-Warrick, 1986b; Johnson and Harris-Warrick, 1990; Harris-Warrick et al., 1992a; Johnson et al., 1993, 1995). Thus, 5-HT alters PD and/or PY synapses but not their firing properties, whereas it alters the synapses and/or firing properties of all other pyloric neurons. This differential modulation of pyloric neurons may reflect differences in receptor distributions. For example, 5-HT receptors could be located near the siz in all but the PD and PY motoneurons. This has been shown for primate motoneurons, for example, in which 5-HT1A receptors are concentrated at the axon hillock (Kheck et al., 1995). The sizs for stomatogastric neurons are thought to reside in the large diameter processes in the coarse neuropil (Raper, 1979; Miller, 1980). We have not yet performed a careful study to determine whether 5-HT2βPan receptors are concentrated at discrete locations representing stomatogastric sizs; however, as a general rule, this receptor is not obviously expressed throughout neuronal plasma membranes in the coarse neuropil, which is consistent with the fact that a diffusionally restrictive glial sheath encases stomatogastric neurons, except at points of synaptic contact (Friend 1976; King 1976a,b).
The aforementioned differential modulation could also reflect differences in the numbers and types of 5-HT receptor multi-protein complexes that exist in a cell. For example, by including or excluding signal-terminating molecules, such as phosphodiesterases for cyclic nucleotide pathways or DAG kinase for PLC pathways, multi-protein complexes containing the same receptor could modulate ion channels locally or globally, respectively. Interestingly, a cAMP imaging study showed that stimulation of modulatory inputs caused an initial accumulation of second messenger in the fine neurites that could eventually diffuse to the somata of stomatogastric neurons (Hempel et al., 1996). Moreover, bath application of dopamine or serotonin to the STG alters the biophysical properties of ion channels located in pyloric somata (Hartline et al., 1993; Harris-Warrick et al., 1995a,b; Kloppenburg et al., 1999; Peck et al., 2001). However, puffing monoamines onto the somata of these neurons elicits no response. These data suggest that ion channels participating in a compartmentalized cellular task, restricted to the soma or lower branch order processes, for example, can be regulated by distant GPCRs via voltage-independent mechanisms. In this case, 5-HT2βPan receptors in the fine neurites of pyloric neurons could modulate firing properties and/or synaptic efficacies depending on the other components of the multi-protein complex. Future work will be aimed at elucidating the different types and distributions of GPCR multi-protein complexes that exist in pyloric neurons.
In summary, we predict that there are ∼18 monoamine receptors in arthropods, including multiple dopamine and serotonin receptors. We cloned and characterized a novel arthropodal metabotropic 5-HT receptor, 5-HT2βPan, that signals via Gq. There is clear evidence that the receptor can be constitutively active and that this agonist-independent activity in HEK cells is dependent on an evolutionary alteration to the monoaminergic signature sequence, DRY. This receptor is localized to the synaptic neuropil of the STG and axon terminals of stomatogastric neurons and, therefore, is most likely involved in modulating the motor output of stomatogastric networks.
Footnotes
This work was supported by National Institutes of Health Grants NS38770, RCMI G12RR03051, MBRS S06 GM008224 and NS33697, and by National Science Foundation Grants IBN-9904017 and IBN-0349042. We thank Drs. Lee Morris and Harold Atwood for useful discussions on stomatogastric muscle anatomy and Dr. Hugo Bellen for providing an anti-synaptotagmin antibody.
Correspondence should be addressed to Dr. Deborah J. Baro, Department of Biology, Georgia State University, P.O. Box 4010, Atlanta, GA 30303. E-mail: dbaro@gsu.edu.
DOI:10.1523/JNEUROSCI.0062-04.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/243421-15$15.00/0
References
- Agretti P, De Marco G, Collecchi P, Chiovato L, Vitti P, Pinchera A, Tonacchera M (2003) Proper targeting and activity of a nonfunctioning thyroid-stimulating hormone receptor (TSHr) combining an inactivating and activating TSHr mutation in one receptor. Eur J Biochem 270: 3839–3847. [DOI] [PubMed] [Google Scholar]
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, Sealfon SC (1996a) Contribution of a helix 5 locus to selectivity of hallucinogenic and non-hallucinogenic ligands for the human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors: direct and indirect effects on ligand affinity mediated by the same locus. Mol Pharmacol 50: 34–42. [PubMed] [Google Scholar]
- Almaula N, Ebersole BJ, Zhang D, Weinstein H, Sealfon SC (1996b) Mapping the binding site pocket of the serotonin 5-hydroxytryptamine2A receptor. Ser3.36(159) provides a second interaction site for the protonated amine of serotonin but not of lysergic acid diethylamide or bufotenin. J Biol Chem 271: 14672–14675. [DOI] [PubMed] [Google Scholar]
- Angers S, Salahpour A, Bouvier M (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435. [DOI] [PubMed] [Google Scholar]
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L (2001) Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965. [DOI] [PubMed] [Google Scholar]
- Arakawa S, Gocayne JD, McCombie WR, Urquhart DA, Hall LM, Fraser CM, Venter JC (1990) Cloning, localization, and permanent expression of a Drosophila octopamine receptor. Neuron 4: 343–354. [DOI] [PubMed] [Google Scholar]
- Ausubel A, Brent R, Kinston R, Moore D, Seidman J, Smith JA, Struhl K (1990) Current protocols in molecular biology. New York: Greene Publishing Associates and Wiley Interscience.
- Ayali A, Harris-Warrick RM (1999) Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci 19: 6712–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Cole CL, Zarrin AR, Hughes S, Harris-Warrick RM (1994) Shab gene expression in identified neurons of the pyloric network in the lobster stomatogastric ganglion. Receptors Channels 2: 193–205. [PubMed] [Google Scholar]
- Baro DJ, Coniglio LM, Cole CL, Rodriguez HE, Lubell JK, Kim MT, Harris-Warrick RM (1996) Lobster shal: comparison with Drosophila shal and native potassium currents in identified neurons. J Neurosci 16: 1689–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Ayali A, French L, Scholz NL, Labenia J, Lanning CC, Graubard K, Harris-Warrick RM (2000) Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system. J Neurosci 20: 6619–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baro DJ, Quinones L, Lanning CC, Harris-Warrick RM, Ruiz M (2001) Alternate splicing of the shal gene and the origin of I(A) diversity among neurons in a dynamic motor network. Neuroscience 106: 419–432. [DOI] [PubMed] [Google Scholar]
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, Lubbert H, Ullmer C (2001) Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem 276: 12974–12982. [DOI] [PubMed] [Google Scholar]
- Becamel C, Alonso G, Galeotti N, Demey E, Jouin P, Ullmer C, Dumuis A, Bockaert J, Marin P (2002) Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J 21: 2332–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz B, Eisen JS, Flamm R, Harris-Warrick RM, Hooper SL, Marder E (1984) Serotonergic innervation and modulation of the stomatogastric ganglion of three decapod crustaceans (Panulirus interruptus, Homarus americanus and Cancer irroratus). J Exp Biol 109: 35–54. [DOI] [PubMed] [Google Scholar]
- Beltz BS (1999) Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc Res Tech 44: 105–120. [DOI] [PubMed] [Google Scholar]
- Berg KA, Maayani S, Goldfarb J, Clarke WP (1998) Pleiotropic behavior of 5-HT2A and 5-HT2C receptor agonists. Ann NY Acad Sci 861: 104–110. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Maximov A (2001) Classification of PDZ domains. FEBS Lett 509: 457–462. [DOI] [PubMed] [Google Scholar]
- Birmingham JT, Billimoria CP, DeKlotz TR, Stewart RA, Marder E (2003) Differential and history-dependent modulation of a stretch receptor in the stomatogastric system of the crab, Cancer borealis. J Neurophysiol 90: 3608–3616. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A (2001) Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol 48: 13–38. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Marin P, Dumuis A, Fagni L (2003) The “magic tail” of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett 546: 65–72. [DOI] [PubMed] [Google Scholar]
- Bucher D, Thirumalai V, Marder E (2003) Axonal dopamine receptors activate peripheral spike initiation in a stomatogastric motor neuron. J Neurosci 23: 6866–6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB (1997) Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature 387: 303–308. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, De Benedetti P, Goldberg SR, Fuxe K, Agnati LF, Woods AS, Ferre S, Lluis C, Bouvier M, Franco R (2003) Adenosine A2A-dopamine D2 receptor-receptor heteromerization. Qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741–46749. [DOI] [PubMed] [Google Scholar]
- Choudhary MS, Craigo S, Roth BL (1993) A single point mutation (Phe340→ Leu340) of a conserved phenylalanine abolishes 4-[125I]iodo-(2,5-dimethoxy)phenylisopropylamine and [3H]mesulergine but not [3H]ketanserin binding to 5-hydroxytryptamine2 receptors. Mol Pharmacol 43: 755–761. [PubMed] [Google Scholar]
- Choudhary MS, Sachs N, Uluer A, Glennon RA, Westkaemper RB, Roth BL (1995) Differential ergoline and ergopeptine binding to 5-hydroxytryptamine2A receptors: ergolines require an aromatic residue at position 340 for high affinity binding. Mol Pharmacol 47: 450–457. [PubMed] [Google Scholar]
- Christie AE, Skiebe P, Marder E (1995) Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J Exp Biol 198: 2431–2439. [DOI] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Kellermann O, Rosay P, Maroteaux L (1995) Drosophila 5-HT2 serotonin receptor: coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci USA 92: 5441–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Hampson DR, Atwood HL (1995) Synaptotagmin-like expression in the motor nerve terminals of crayfish. Brain Res 703: 214–216. [DOI] [PubMed] [Google Scholar]
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW (2001) A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293: 98–101. [DOI] [PubMed] [Google Scholar]
- Dixon D, Atwood HL (1989) Phosphatidylinositol system's role in serotonin-induced facilitation at the crayfish neuromuscular junction. J Neurophysiol 62: 239–246. [DOI] [PubMed] [Google Scholar]
- Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM (1996) Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci 16: 3925–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM (1986a) Aminergic modulation in lobster stomatogastric ganglion. I. Effects on motor pattern and activity of neurons within the pyloric circuit. J Neurophysiol 55: 847–865. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM (1986b) Aminergic modulation in lobster stomatogastric ganglion. II. Target neurons of dopamine, octopamine, and serotonin within the pyloric circuit. J Neurophysiol 55: 866–881. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Fickbohm D, Harris-Warrick RM (1987) cAMP elevation modulates physiological activity of pyloric neurons in the lobster stomatogastric ganglion. J Neurophysiol 58: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Friend B (1976) Morphology and location of dense-core vesicles in the stomatogastric ganglion of the lobster, Panulirus interruptus. Cell Tissue Res 175: 369–390. [DOI] [PubMed] [Google Scholar]
- Gotzes F, Balfanz S, Baumann A (1994) Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels 2: 131–141. [PubMed] [Google Scholar]
- Govind CK, Atwood HL (1975) Innervation and neuromuscular physiology of intrinsic foregut muscles in the blue crab and spiny lobster. J Comp Physiol 96: 185–204. [Google Scholar]
- Guillet-Deniau I, Burnol AF, Girard J (1997) Identification and localization of a skeletal muscle secrotonin 5-HT2A receptor coupled to the Jak/STAT pathway. J Biol Chem 272: 14825–14829. [DOI] [PubMed] [Google Scholar]
- Hall RA, Lefkowitz RJ (2002) Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 91: 672–680. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL (1996) DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron 16: 1127–1135. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL (1998) A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci 18: 3650–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Flamm RE (1987) Multiple mechanisms of bursting in a conditional bursting neuron. J Neurosci 7: 2113–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Nagy F, Nusbaum MP (1992a) Neuromodulation of stomatogastric networks by identified neurons and transmitters. In: Dynamic biological networks: the stomatogastric nervous system (Harris-Warrick RM, Marder E, Selverston AI, Moulins M, eds), pp 87–137. Cambridge, MA: MIT.
- Harris-Warrick RM, Marder E, Selverston AI, Moulins M, eds (1992b) Dynamic biological networks: the stomatogastric nervous system. Cambridge, MA: MIT.
- Harris-Warrick RM, Coniglio LM, Levini RM, Gueron S, Guckenheimer J (1995a) Dopamine modulation of two subthreshold currents produces phase shifts in activity of an identified motoneuron. J Neurophysiol 74: 1404–1420. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Coniglio LM, Barazangi N, Guckenheimer J, Gueron S (1995b) Dopamine modulation of transient potassium current evokes phase shifts in a central pattern generator network. J Neurosci 15: 342–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J (1998) Distributed effects of dopamine modulation in the crustacean pyloric network. Ann NY Acad Sci 860: 155–167. [DOI] [PubMed] [Google Scholar]
- Hartline DK, Gassie DV, Jones BR (1993) Effects of soma isolation on outward currents measured under voltage clamp in spiny lobster stomatogastric motor neurons. J Neurophysiol 69: 2056–2071. [DOI] [PubMed] [Google Scholar]
- Hearn MG, Ren Y, McBride EW, Reveillaud I, Beinborn M, Kopin AS (2002) A Drosophila dopamine 2-like receptor: molecular characterization and identification of multiple alternatively spliced variants. Proc Natl Acad Sci USA 99: 14554–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel CM, Vincent P, Adams SR, Tsien RY, Selverston AI (1996) Spatiotemporal dynamics of cyclic AMP signals in an intact neural circuit. Nature 384: 166–169. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL, Chen HC (1993) Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch Biochem Biophys 305: 570–580. [DOI] [PubMed] [Google Scholar]
- Huang X, Xiao H, Rex EB, Hobson RJ, Messer WS, Komuniecki PR, Komuniecki RW (2002) Functional characterization of alternatively spliced 5-HT2 receptor isoforms from the pharynx and muscle of the parasitic nematode, Ascaris suum. J Neurochem 83: 249–258. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Harris-Warrick RM (1990) Aminergic modulation of graded synaptic transmission in the lobster stomatogastric ganglion. J Neurosci 10: 2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM (1993) Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol [A] 172: 715–732. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Peck JH, Harris-Warrick RM (1995) Distributed amine modulation of graded chemical transmission in the pyloric network of the lobster stomatogastric ganglion. J Neurophysiol 74: 437–452. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Wainscott DB, Lucaites VL, Baez M, Nelson DL (1997) Mutations of transmembrane IV and V serines indicate that all tryptamines do not bind to the rat 5-HT2A receptor in the same manner. Brain Res Mol Brain Res 49: 1–6. [DOI] [PubMed] [Google Scholar]
- Jorge-Rivera JC, Sen K, Birmingham JT, Abbott LF, Marder E (1998) Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J Neurophysiol 80: 2559–2570. [DOI] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM (1989) Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. II. Rapid nicotinic and prolonged modulatory effects on neurons in the stomatogastric ganglion. J Neurophysiol 62: 571–581. [DOI] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM (1990) Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. J Neurosci 10: 1495–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Eigg MH, Harris-Warrick RM (1989) Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol 62: 558–570. [DOI] [PubMed] [Google Scholar]
- Kheck NM, Gannon PJ, Azmitia EC (1995) 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J Comp Neurol 355: 211–220. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM (1992a) Serotonergic stretch receptors induce plateau properties in a crustacean motor neuron by a dual-conductance mechanism. J Neurophysiol 68: 485–495. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM (1992b) 5-HT modulation of hyperpolarization-activated inward current and calcium-dependent outward current in a crustacean motor neuron. J Neurophysiol 68: 496–508. [DOI] [PubMed] [Google Scholar]
- Kilman V, Fenelon VS, Richards KS, Thirumalai V, Meyrand P, Marder E (1999) Sequential developmental acquisition of cotransmitters in identified sensory neurons of the stomatogastric nervous system of the lobsters, Homarus americanus and Homarus gammarus. J Comp Neurol 408: 318–334. [DOI] [PubMed] [Google Scholar]
- King DG (1976a) Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol 5: 207–237. [DOI] [PubMed] [Google Scholar]
- King DG (1976b) Organization of crustacean neuropil. II. Distribution of synaptic contacts on identified motor neurons in lobster stomatogastric ganglion. J Neurocytol 5: 239–266. [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM (1999) Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J Neurophysiol 81: 29–38. [DOI] [PubMed] [Google Scholar]
- Kristiansen K, Kroeze WK, Willins DL, Gelber EI, Savage JE, Glennon RA, Roth BL (2000) A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT(2A) serotonin receptor but does not participate in activation via a “salt-bridge disruption” mechanism. J Pharmacol Exp Ther 293: 735–746. [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, Roth BL (2002) Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem 2: 507–528. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE (2003) Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther 304: 229–237. [DOI] [PubMed] [Google Scholar]
- Lavine N, Ethier N, Oak JN, Pei L, Liu F, Trieu P, Rebois RV, Bouvier M, Hebert TE, Van Tol HH (2002) G protein-coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem 277: 46010–46019. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F (2002) Dual regulation of NMDA receptor functions by direct protein–protein interactions with the dopamine D1 receptor. Cell 111: 219–230. [DOI] [PubMed] [Google Scholar]
- Lembo PM, Ghahremani MH, Morris SJ, Albert PR (1997) A conserved threonine residue in the second intracellular loop of the 5-hydroxytryptamine 1A receptor directs signaling specificity. Mol Pharmacol 52: 164–171. [DOI] [PubMed] [Google Scholar]
- Li XC, Giot JF, Kuhl D, Hen R, Kandel ER (1995) Cloning and characterization of two related serotonergic receptors from the brain and the reproductive system of Aplysia that activate phospholipase C. J Neurosci 15: 7585–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Bellen HJ, Perin MS (1993) Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development 118: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB (2000) Direct protein–protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature 403: 274–280. [DOI] [PubMed] [Google Scholar]
- Manivet P, Schneider B, Smith JC, Choi DS, Maroteaux L, Kellermann O, Launay JM (2002) The serotonin binding site of human and murine 5-HT2B receptors: molecular modeling and site-directed mutagenesis. J Biol Chem 277: 17170–17178. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS (1984) Electrically coupled pacemaker neurons respond differently to same physiological inputs and neurotransmitters. J Neurophysiol 51: 1362–1374. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein KE (1995) Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature 375: 247–250. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Weimann JM, Marder E (1992) Multiple axonal spike initiation zones in a motor neuron: serotonin activation. J Neurosci 12: 2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JP (1980) Mechanisms underlying pattern generation in the lobster stomatogastric ganglion. PhD thesis, University of California, San Diego.
- Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM (2000) High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature 408: 860–864. [DOI] [PubMed] [Google Scholar]
- Mustard JA, Blenau W, Hamilton IS, Ward VK, Ebert PR, Mercer AR (2003) Analysis of two D1-like dopamine receptors from the honey bee Apis mellifera reveals agonist-independent activity. Brain Res Mol Brain Res 113: 67–77. [DOI] [PubMed] [Google Scholar]
- Newton AC (1995) Protein kinase C: structure, function, and regulation. J Biol Chem 270: 28495–28498. [DOI] [PubMed] [Google Scholar]
- Newton AC (2001) Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev 101: 2353–2364. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Copeland SC, Herrick-Davis K, Emeson RB, Sanders-Bush E (1999) RNA editing of the human serotonin 5-hydroxytryptamine 2C receptor silences constitutive activity. J Biol Chem 274: 9472–9478. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP (2002) A small-systems approach to motor pattern generation. Nature 417: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289: 739–745. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ (2000) Diverse signalling by 5-hydroxytryptamine (5-HT) receptors. Biochem Pharmacol 60: 1743–1750. [DOI] [PubMed] [Google Scholar]
- Peck JH, Nakanishi ST, Yaple R, Harris-Warrick RM (2001) Amine modulation of the transient potassium current in identified cells of the lobster stomatogastric ganglion. J Neurophysiol 86: 2957–2965. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Thirumalai V, Richards KS, Marder E (2003) Dopamine and histamine in the developing stomatogastric system of the lobster Homarus americanus. J Comp Neurol 462: 400–414. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, Gerendasy DD, de Graan PN (1999) Substrate phosphorylation in the protein kinase Cgamma knockout mouse. J Biol Chem 274: 1873–1874. [DOI] [PubMed] [Google Scholar]
- Raper JA (1979) Nonimpulse-mediated synaptic transmission during the generation of a cyclic motor program. Science 205: 304–306. [DOI] [PubMed] [Google Scholar]
- Rebois RV, Hebert TE (2003) Protein complexes involved in heptahelical receptor-mediated signal transduction. Receptors Channels 9: 169–194. [PubMed] [Google Scholar]
- Richards KS, Simon DJ, Pulver SR, Beltz BS, Marder E (2003) Serotonin in the developing stomatogastric system of the lobster, Homarus americanus. J Neurobiol 54: 380–392. [DOI] [PubMed] [Google Scholar]
- Rosendorff A, Ebersole BJ, Sealfon SC (2000) Conserved helix 7 tyrosine functions as an activation relay in the serotonin 5HT(2C) receptor. Brain Res Mol Brain Res 84: 90–96. [DOI] [PubMed] [Google Scholar]
- Roth BL, Shoham M, Choudhary MS, Khan N (1997a) Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine2A receptors. Mol Pharmacol 52: 259–266. [DOI] [PubMed] [Google Scholar]
- Roth BL, Choudhary MS, Khan N, Uluer AZ (1997b) High-affinity agonist binding is notsufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther 280: 576–583. [PubMed] [Google Scholar]
- Saudou F, Amlaiky N, Plassat JL, Borrelli E, Hen R (1990) Cloning and characterization of a Drosophila tyramine receptor. EMBO J 9: 3611–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R (1992) A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J 11: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz NL, Goy MF, Truman JW, Graubard K (1996) Nitric oxide and peptide neurohormones activate cGMP synthesis in the crab stomatogastric nervous system. J Neurosci 16: 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz NL, de Vente J, Truman JW, Graubard K (2001) Neural network partitioning by NO and cGMP. J Neurosci 21: 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealfon SC, Chi L, Ebersole BJ, Rodic V, Zhang D, Ballesteros JA, Weinstein H (1995) Related contribution of specific helix 2 and 7 residues to conformational activation of the serotonin 5-HT2A receptor. J Biol Chem 270: 16683–16688. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K (2002) Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol 366: 381–416. [DOI] [PubMed] [Google Scholar]
- Setterblad N, Onyango I, Pihlgren U, Rask L, Andersson G (1998) The role of protein kinase C signaling in activated DRA transcription. J Immunol 161: 4819–4824. [PubMed] [Google Scholar]
- Shapiro DA, Kristiansen K, Kroeze WK, Roth BL (2000) Differential modes of agonist binding to 5-hydroxytryptamine(2A) serotonin receptors revealed by mutation and molecular modeling of conserved residues in transmembrane region 5. Mol Pharmacol 58: 877–886. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Kristiansen K, Weiner DM, Kroeze WK, Roth BL (2002) Evidence for a model of agonist-induced activation of 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. J Biol Chem 277: 11441–11449. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Spitzer N, Edwards DE, Baro DJ (2004) A crustacean serotonin receptor: Cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J Comp Neurol, in press. [DOI] [PubMed]
- Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB (1995) A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett 362: 131–138. [DOI] [PubMed] [Google Scholar]
- Sullivan RE, Friend BJ, Barker DL (1977) Structure and function of spiny lobster ligamental nerve plexuses: evidence for synthesis, storage, and secretion of biogenic amines. J Neurobiol 8: 581–605. [DOI] [PubMed] [Google Scholar]
- Tabor JN, Cooper RL (2002) Physiologically identified 5-HT2-like receptors at the crayfish neuromuscular junction. Brain Res 932: 91–98. [DOI] [PubMed] [Google Scholar]
- Tierney AJ (2001) Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol A Mol Integr Physiol 128: 791–804. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Godleski MS, Rattananont P (1999) Serotonin-like immunoreactivity in the stomatogastric nervous systems of crayfishes from four genera. Cell Tissue Res 295: 537–551. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Marder E (1993) Modulation of identified stomatogastric ganglion neurons in primary cell culture. J Neurophysiol 69: 1993–2002. [DOI] [PubMed] [Google Scholar]
- Visiers I, Hassan SA, Weinstein H (2001) Differences in conformational properties of the second intracellular loop (IL2) in 5HT(2C) receptors modified by RNA editing can account for G protein coupling efficiency. Protein Eng 14: 409–414. [DOI] [PubMed] [Google Scholar]
- Wang CD, Gallaher TK, Shih JC (1993) Site-directed mutagenesis of theserotonin 5-hydroxytrypamine2 receptor: identification of amino acids necessary for ligand binding and receptor activation. Mol Pharmacol 43: 931–940. [PubMed] [Google Scholar]
- Way KJ, Chou E, King GL (2000) Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci 21: 181–187. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Baldwin DH, Christie AE, Graubard K (2003) Stereotyped neuropil branching of an identified stomatogastric motor neuron. J Comp Neurol 466: 554–563. [DOI] [PubMed] [Google Scholar]
- Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, Hen R (1990) Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci USA 87: 8940–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL (2003) A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem 278: 21901–21908. [DOI] [PubMed] [Google Scholar]
- Zhang B, Harris-Warrick RM (1994) Multiple receptors mediate the modulatory effects of serotonergic neurons in a small neural network. J Exp Biol 190: 55–77. [DOI] [PubMed] [Google Scholar]
- Zhang B, Harris-Warrick RM (1995) Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. I. Calcium current and its modulation by serotonin. J Neurophysiol 74: 1929–1937. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wootton JF, Harris-Warrick RM (1995) Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. II. Calcium-activated slow inward current. J Neurophysiol 74: 1938–1946. [DOI] [PubMed] [Google Scholar]