Abstract
Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays a critical role in synaptic plasticity and memory formation in a variety of learning systems and species. The present experiments examined the role of CaMKII in the circuitry underlying pavlovian fear conditioning. First, we reveal by immunocytochemical and tract-tracing methods that αCaMKII is postsynaptic to auditory thalamic inputs and colocalized with the NR2B subunit of the NMDA receptor. Furthermore, we show that fear conditioning results in an increase of the autophosphorylated (active) form of αCaMKII in lateral amygdala (LA) spines. Next, we demonstrate that intra-amygdala infusion of a CaMK inhibitor, 1-[NO-bis-1,5-isoquinolinesulfonyl]-N-methyl-l-tyrosyl-4-phenylpiperazine, KN-62, dose-dependently impairs the acquisition, but not the expression, of auditory and contextual fear conditioning. Finally, in electrophysiological experiments, we demonstrate that an NMDA receptor-dependent form of long-term potentiation at thalamic input synapses to the LA is impaired by bath application of KN-62 in vitro. Together, the results of these experiments provide the first comprehensive view of the role of CaMKII in the amygdala during fear conditioning.
Keywords: CaMKII, fear, phosphorylation, LTP, plasticity, translocation
Introduction
Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) has been widely implicated in synaptic plasticity (for review, see Fukunaga and Miyamoto, 2000; Fink and Meyer, 2002; Lisman et al., 2002). During a transient elevation of intracellular Ca2+ through the NMDA receptor, CaMKII translocates to the synapses of pyramidal neurons (Shen and Meyer, 1999; Shen et al., 2000) where it can interact with and modulate a number of proteins at the postsynaptic density (PSD) that are critical for synaptic plasticity, including AMPA (Barria et al., 1997; Derkach et al., 1999) and NMDA receptors (Buchs and Muller, 1996; Strack and Colbran, 1998; Leonard et al., 1999; Strack et al., 2000). The association of CaMKII with the NR2B subunit of the NMDA receptor appears to be particularly important; previous work has demonstrated that an interaction between CaMKII and NR2B may be critical for synaptic plasticity (Omkumar et al., 1996; Strack and Colbran, 1998; Bayer et al., 2001; Mayadevi et al., 2002). During activation by CaM binding, CaMKII undergoes autophosphorylation at a critical threonine residue (Thr286) that transforms it to persistently active, calcium-independent state (Soderling et al., 2001; Hudmon and Schulman, 2002). The autophosphorylation of CaMKII on Thr286 and binding to NR2B work in tandem to regulate synaptic strength (Bayer et al., 2001).
CaMKII consists of a family of isoforms, with the α and β isoforms being the most prominent (Bennett et al., 1983; Miller and Kennedy, 1985). In particular, αCaMKII has been implicated as a major contributor to synaptic plasticity and memory formation. For example, transgenic mice with a deletion of the αCaMKII gene display deficits in hippocampal long-term potentiation (LTP) and spatial memory (Silva et al., 1992a,b). Similarly, the CaMKII inhibitor KN-62 (1-[NO-bis-1,5-isoquinolinesulfonyl]-N-methyl-l-tyrosyl-4-phenylpiperazine) (Hidaka and Yokokura, 1996) blocks the induction of LTP in hippocampal area CA1 (Ito et al., 1991; Stanton and Gage, 1996; Broutman and Baudry, 2001) and impairs hippocampal-dependent learning and memory (Tan and Liang, 1996). Moreover, the disruption of dendritic translation of αCaMKII causes a reduction of the kinase in PSDs and late-phase LTP, in addition to impairments in spatial memory, object recognition, and associative fear conditioning (Miller et al., 2002). A reversible deficit in fear conditioning has also been observed after induced overexpression of active αCaMKII by a transgene that replaces Thr286 with an aspartate residue in the lateral amygdala (LA) and striatum (Mayford et al., 1996).
To date, the precise role of CaMKII in fear conditioning and LTP in the LA, the putative locus of synaptic plasticity underlying fear conditioning (but see Cahill et al., 1999), has not been thoroughly investigated. In the present study, we address this question using a combination of anatomical, behavioral, and electrophysiological methods. We show that αCaMKII autophosphorylation at Thr286 is regulated at LA synapses by fear conditioning and that memory formation of fear conditioning and synaptic plasticity in the LA are impaired by pharmacological inhibition of CaMKII. Collectively, the results of this study indicate that CaMKII in the LA plays a crucial role in memory formation and synaptic plasticity at synapses involved in fear conditioning.
Materials and Methods
Subjects. Adult male Sprague Dawley rats (Hilltop, Scottdale, PA) were housed individually in plastic Nalgene cages on a 12 hr light/dark cycle. Food and water were provided ad libitum throughout the experiment. All procedures were approved by the New York University Animal Care and Use Committee.
Anatomical experiments. For light microscopy (LM) experiments, LA tissue was processed immunocytochemically as described previously (Rodrigues et al., 2002) using mouse monoclonal antisera directed against αCaMKII (1:120,000; Upstate Biotechnology, Lake Placid, NY) or rabbit polyclonal antisera directed against the autophosphorylation of CaMKII at Thr286 (pCAMKII-Thr286) (1:2000; Promega, Madison, WI) and standard ABC/DAB methods (Vector Laboratories, Burlingame, CA). Some tissue sections were then processed for electron microscopy (EM) as described previously (Rodrigues et al., 2002). For double-label fluorescence experiments, sections were incubated overnight in a mixture of anti-αCaMKII (1:1000) and anti-NR2B (1:500; rabbit polyclonal; Upstate Biotechnology) and visualized using Alexa 484 and 594 secondary antibodies (1:200; Molecular Probes, Eugene, OR), respectively.
Anterograde transport studies and EM were conducted as described previously (Rodrigues et al., 2002). For tract-tracing experiments, biotinylated dextran amine (BDA) was injected into the medial division of the medial geniculate body (MGm) and posterior intralaminar nucleus (PIN). For triple-label EM experiments, preembedding gold was used to label αCaMKII. Tissue sections containing the LA from BDA-injected animals were incubated overnight at room temperature with anti-αCaMKII (1:1000) and anti-NR2B (1:500). The following day, the NR2B antigen was visualized using ABC/DAB methods. Tissue was then incubated in goat anti-mouse conjugated to 0.6 nm gold (ultrasmall gold; Aurion, Wageningen, The Netherlands) for 2 hr, silver-enhanced with Aurion for 25 min, and placed in Amersham Bioscience (Arlington Heights, IL) Silver Enhancement solutions for 2 min. Tissue was then processed for EM as described previously (Rodrigues et al., 2002).
Western blotting. Rats were habituated to handling and to the conditioning chamber for 3 d before training. Paired rats received five conditioning trials consisting of a 20 sec, 5 kHz, 75 dB tone that coterminated with a 0.5 sec, 1.0 mA footshock. Unpaired controls also received a series of shocks and tones over the same time duration but in a noncontingent, explicitly unpaired manner. Here, the unconditioned stimulus (US) shock preceded the tone conditioned stimulus (CS) by 60 sec, and at least 120 sec were allowed to pass between a tone CS presentation and the next trial. Fifteen minutes after training, paired (n = 8) and unpaired (n = 8) rats were deeply anesthetized with pentobarbital (100 mg/kg, i.p.) and decapitated. Brains were frozen and stored at –80°C. Amygdala punches were obtained with a 1 mm punch tool (Fine Science Tools, Foster City, CA) from 400-μm-thick sections taken on a sliding freezing microtome. The punches included the LA and the basal nucleus and possibly portions of the lateral central nucleus and cortical tissue directly lateral to the external capsule. Punches were briefly sonicated in 100–200 μl of ice-cold buffer (20 mm Tris-HCl, pH 7.5, 1 mm EGTA, 1 mm EDTA, 25 μg/ml aprotinin, 25 μg/ml leupeptin, 1 mm sodium pyrophosphate, 500 μm phenylmethylsulfonyl fluoride, 4 mm para-nitrophenyl-phosphate, and 1 mm sodium orthovanadate). Sample buffer was immediately added to the homogenates, and the samples were boiled at 95°C for 5 min. Homogenates were electrophoresed on 10% SDS-polyacrylamide gels and blotted to Immobilon-P (Millipore, Bedford, MA). Western blots were blocked in TTBS buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.05% Tween 20) with 5% bovine serum albumin and then incubated with anti-pCAMKII-Thr286antibody (Promega). Blots were then incubated with anti-rabbit conjugated to horseradish peroxidase (Cappel, West Chester, PA) and developed using enhanced chemiluminescence (Amersham Biosciences). Densitometry was conducted using Intelligent Quantifier software (BioImage, Ann Arbor, MI).
Immunogold labeling of autophosphorylated αCaMKII. Rats (n = 3 per group) were given five tone–shock pairings or five unpaired presentations of tone and shock as in the Western blotting experiments. An additional control group of rats was handled and exposed to the conditioning box for an equivalent amount of time but were not exposed to tones or shocks. Rats were perfused 15 min after the end of the training protocol with saline followed by 1% glutaraldehyde–4% paraformaldehyde made in 0.1 m phosphate buffer (PB). After perfusions, the brains were removed, sectioned immediately at 40 μm on a vibratome, and washed with 1% sodium borohydride for 30 min as described previously. The tissue was stored in 0.05% sodium azide–PBS at 4°C until immunocytochemistry was performed on the tissue.
Five tissue sections from each brain, containing the amygdala from comparable rostrocaudal levels, were incubated for 4 d at 4°C inthe same pCAMKII-Thr286 antibody (1:375) used for LM. After incubation, the tissue was rinsed and incubated overnight at room temperature in goat anti-rabbit IgG conjugated to 0.8 nm gold (1:75; ultrasmall; Aurion). Primary and secondary antibodies were made in 1% bovine serum albumin and distributed equally to each of the nine samples.
Tissue was then rinsed in PBS, incubated in 2% glutaraldehyde–PB for 10 min, rinsed in PB, followed by PBS and conditioning solution (Aurion), and placed in silver-enhancement solution (Aurion R-Gent SEEM) for 60 min. After silver enhancement, the tissue was rinsed in conditioning solution and PBS and processed using the osmium-free method (Phend et al., 1995) to prevent loss of silver-enhanced gold particles. The tissue was then dehydrated and flat embedded and capsule embedded in Epon as described previously (Farb and LeDoux, 1997). Camera lucida drawings were made from each block from which 85 nm ultrathin sections were collected. Twenty-four blocks were sectioned from the most dorsal portion of the LA (LAd) (two to three blocks from each condition), and 8–15 grids were collected from each block. Care was taken to sample exactly the same region of the LA for each block of the corresponding rostrocaudal level. The tissue was then examined on a JEOL (Peabody, MA) 1200EXII electron microscope.
Initial experiments involved processing tissue for postembedding immunogold to avoid problems associated with penetration of gold particles. However, in our hands, this method did not provide consistent results using the pCaMKII-Thr286 antibody, and we ultimately abandoned this method because of the reliability of the preembedding method.
Sampling of immunogold-labeled tissue. Because pCAMKII-Thr286 immunoreactivity (IR) resides at the surface-most portion of the vibratome section, only tissue within the 3 μm of the surface was analyzed. For quantitative EM analysis, each labeled spine encountered within 3 μmof the surface was photographed digitally using a CCD camera (Advanced Microscopy Techniques, Danvers, MA) at a magnification of 30,000–40,000×. These micrographs represented 9.9–13.1 μm2 and provided adequate resolution of subcellular organelles and identification of the presynaptic and postsynaptic membranes. Given that most thalamic afferents synapse onto dendritic spines (LeDoux et al., 1991) and the vast majority of thalamo-amygdala synapses are asymmetric (excitatory), quantitative analysis of immunogold was confined to those dendritic spines with identifiable asymmetric densities. Previous work has demonstrated that the immunoperoxidase method, the most sensitive method of immunolabeling, labels only two-thirds of CaMKII dendritic spines (McDonald et al., 2002). Because the immunogold method label would be expected to label a lower proportion of spines, our analysis was limited to those spines that contained immunogold.
Behavioral experiments. Rats were implanted bilaterally with guide cannulas (28 gauge; Plastics One, Roanoke, VA) positioned just above the LA, as described previously (Rodrigues et al., 2001, 2002). After 5 d of recovery, they were habituated, conditioned, and tested for short-term memory (STM) (1 hr) and long-term (LTM) (24 hr) for auditory and contextual fear conditioning as described previously (Rodrigues et al., 2001, 2002).
In the first experiment, rats received infusions of vehicle or multiple doses of KN-62 (34 or 340 ng) 30 min before conditioning (five pairings of a 20 sec, 5 kHz, 80 dB tone with a 0.5 sec, 0.5 mA footshock). In the second experiment, they received vehicle or KN-62 (340 ng) 30 min before testing. For all drug infusions, a total volume of 0.5 μl of KN-62–2-hydroxypropyl-β-cyclodextrin (HBC) complex (catalog #K-109; Sigma, St. Louis, MO) or vehicle (45% w/v HBC) was infused bilaterally into the amygdala. HBC was used to solubilize KN-62 in aqueous solution at sufficient concentrations for delivery into the brain (Yaksh et al., 1991).
Rats were carefully observed throughout the training procedure. No differences in reactivity to the shock US were detected. Rats were observed to run, jump, and/or vocalize normally to the shock (data not shown).
At the end of behavioral experimentation, brains were examined using Nissl staining and light microscopy for injector tip penetration into the amygdala.
Slice electrophysiology. Electrophysiological experiments in amygdala slices were conducted as described previously (Weisskopf et al., 1999; Bauer et al., 2002; Rodrigues et al., 2002). The LTP induction protocol consisted of a 30 Hz tetanus (100 pulses, given twice with a 20 sec interval at test intensity). KN-62 (catalog #I2142; Sigma) was dissolved in DMSO and diluted in artificial CSF, yielding a final concentration of 3 μm. In all experiments, the slope of the EPSP was measured, and LTP for each time point was expressed as a percentage of the preinduction baseline. For analysis, the values for the initial slope of the EPSP were recorded during the last 5 min of the recording session (min 55–60) and were averaged into a single score for each cell. The amount of potentiation was analyzed by comparing these values with the preinduction values and testing with a paired Student's t test. To analyze the effects of KN-62 on transmission, we compared the values at 20–30 min with predrug baseline values and tested with a paired Student's t test.
Results
αCaMKII is distributed throughout the LA, postsynaptic to auditory thalamic afferents, and colocalized with NR2B
Previous studies have examined the distribution of αCaMKII in the amygdala (McDonald et al., 2002). However, whether αCaMKII is present postsynaptic to auditory inputs in the LA has not been established. The aim of the first set of experiments was twofold. First, we used electron microscopy methods to determine whether CAMKII was present in sites postsynaptic to BDA-labeled terminals whose cells originate in auditory thalamus (MGm–PIN). Second, we examined the colocalization of αCaMKII with the NR2B subunit of the NMDA receptor using a combination of LM and electron microscopy. The NR2B subunit, which has been implicated recently in synaptic plasticity in the LA and in fear conditioning (Rodrigues et al., 2001; Bauer et al., 2002), has been closely linked to CaMKII activity at the PSD (Buchs and Muller, 1996; Omkumar et al., 1996; Strack and Colbran, 1998; Strack et al., 2000). Here, we used triple-labeling methods and EM to examine the colocalization of NR2B and αCaMKII in LA spines postsynaptic to auditory thalamic inputs.
Light microscopic experiments revealed the presence of αCaMKII throughout the LA (Fig. 1A), expressed in cytoplasm, proximal dendrites, and punctate processes (Fig. 1A, inset). Immunoreactivity appeared particularly robust in the LAd, which plays an especially important role in fear conditioning (Repa et al., 2001). Double-labeling experiments revealed that a large number of αCaMKII-expressing cells in the LAd also expressed NR2B (Fig. 1B).
Figure 1.

Distribution of αCaMKII in the LA and its colocalization with NR2B. A, Low-power micrograph (4×) illustrates the distribution of αCaMKII in the LA and adjacent regions. Immunoreactivity appeared to be most robust in the LAd but was also strongly present in all subregions of the LA [ventrolateral LA (LAvl); ventromedial LA (LAvm)] and other amygdaloid nuclei [central nucleus (CE); basal nucleus(B)]. Labeling was also observed in the nearby amygdala-striatal transition zone (AST), striatum (CPu), and cortex (Cx). Inset, High-power micrograph (40×) of αCaMKII in the LA. B, High-power fluorescent micrograph (40×) of double label for αCaMKII (green) and NR2B (red) in the LAd. C, Electron micrograph of an LAd spine double labeled for the NR2B receptor (peroxidase) and αCaMKII (silver-enhanced gold; arrowheads) that is postsynaptic to a BDA-labeled thalamic terminal. The BDA terminal also synapses on a spine that expresses only CaMKII (silver-enhanced gold; arrowheads) and receives an asymmetric synapse (asterisks) from an unlabeled terminal (ut1). Also shown is an unlabeled terminal (ut2) forming an asymmetric synapse on a spine expressing NR2B. Scale bar, 200 nm.
At the EM level, αCaMKII-IR was localized to perikarya with the morphological characteristics of projection cells. It was also observed in large and small dendritic shafts, spines, and axon terminals. In addition, αCaMKII-IR was colocalized to NR2B-IR spines in the LA that are postsynaptic to auditory thalamic afferents (Fig. 1C). Thus, αCaMKII, along with NR2B, is anatomically well situated within the fear conditioning circuitry to mediate synaptic plasticity and memory formation.
Fear conditioning regulates the autophosphorylation of αCaMKII at LA synapses
We next determined whether fear conditioning regulates autophosphorylation of αCaMKII in the LA. To address this issue, we used antibodies that specifically recognize αCaMKII phosphorylated on its Thr286 residue. In biochemical experiments, binding assays show that the association of αCaMKII to PSDs is rapid and reaches saturation at 5–15 min (Strack et al., 1997). Likewise, high-frequency stimulation of area CA1 leads to significant increases in pCaMKII-Thr286 5–15 min after stimulation (Barria et al., 1997; Giovannini et al., 2001). In addition, application of glutamate to or electrical stimulation of cultured hippocampal CA1-CA3 pyramidal neurons results in the fast translocation of αCaMKII to the synapse (Shen and Meyer, 1999; Shen et al., 2000), and pCaMKII-Thr286 prolongs the association of the enzyme with the PSD (Shen and Meyer, 1999).
In our experiments, pCaMKII-Thr286 was abundantly expressed in the amygdala and surrounding regions under basal conditions (Fig. 2A,B). At the EM level, pCaMKII-Thr286 was observed to have a very similar distribution to that of total αCaMKII, notably in large and small dendritic shafts, spines, and axon terminals (Fig. 2C).
Figure 2.
Distribution of pCaMKII-Thr286 in the LA and its regulation after fear conditioning. A, Low-power micrograph (4×) illustrates the distribution of pCaMKII-Thr286 in the LA and adjacent regions. The distribution was similar to that of total αCaMKII (for details and abbreviations, see Fig. 1). B, Higher-power micrograph (20×) of pCaMKII-Thr286 in the LA. C, Electron micrograph of pCaMKII-Thr286 peroxidase label in the LA. Labeled dendrites (LD) and labeled terminals (LT) are shown. Inset, An unlabeled terminal (ut) is shown synapsing onto three pCaMKII-Thr286-immunoreactive spines [labeled spines (LSp)]. Scale bars, 500 nm. D, Percentage of freezing in rats given either five paired or unpaired presentations of a tone (80 dB, 20 sec, 5 kHz) and shock (1.0 mA, 0.5 sec). Each group was tested at 24 hr after conditioning. E, Western immunoblotting of LA homogenates reveals no significant difference in pCaMKII-Thr286 expression between paired and unpaired animals killed 15 min after fear conditioning. F, Sample immunogold staining for pCaMKII-Thr286 in LA spine of a fear-conditioned animal. Scale bar, 160 nm. G, Average number of pCaMKII-Thr286 immunogold particles in LA spines of paired, unpaired (UnP), and control (Cont.) animals. *p < 0.001, relative to unpaired and control groups.
To examine the regulation of pCaMKII-Thr286 by fear conditioning, rats were given five CS–US pairings (5 kHz, 80 dB tone paired with a 1.0 mA, 0.5 sec footshock) or five unpaired presentations of tone and shock (Fig. 2D) and killed 15 min later. In our initial experiments, we used Western blotting techniques to examine the regulation of pCaMKII-Thr286 in whole amygdala homogenates. The findings of those experiments indicated that there was no significant difference in pCaMKII-Thr286 expression between paired and unpaired animals (Fig. 2E). This pattern of results, however, is not unexpected given the very high level of pCaMKII-Thr286 in the LA under basal conditions. If αCaMKII is regulated in an anatomically restricted manner during fear conditioning, e.g., within spines, such a small regulation would not be expected to be easily detected using whole amygdala extracts. To examine this question more directly, we used immunogold EM methods to quantitatively examine the total number of pCaMKII-Thr286 particles within LA spines taken from paired and unpaired animals (Fig. 2F). Rats were trained as before and perfused 15 min after conditioning (for details, see Materials and Methods). A third group of animals that received no stimulation (other than handling) served as a control.
A total of nine rats were examined, three for each condition. For each animal, two to three vibratome sections were examined by EM, such that the total number of tissue sections analyzed was 24. The distribution of immunogold labeling of pCaMKII-Thr286 at the EM level was consistent with the pattern seen by light microscopy when immunoperoxidase was used. Immunogold label was seen in somata, large and small dendrites, dendritic spines, and axon terminals (data not shown). Approximately 30 micrographs were taken from each one of the 24 blocks that was examined, and, from these micrographs, ∼45 spines were analyzed at a final magnification of 50,000×.
Approximately 350 spines were examined from each of the control, unpaired, and paired groups for a total of 1047 spines culled from >10,500 μm2 of tissue. All gold particles within each labeled dendritic spine were counted. Results, presented in Figure 2G, indicated that fear conditioning led to significant elevations (∼30%) in pCaMKII-Thr286 labeling in LA spines in paired animals relative to unpaired (t(697) = 5.01; p < 0.001; two-tailed) or handled (t(688) = 3.37; p < 0.001; two-tailed) controls. No significant differences were observed between unpaired rats and handled controls (p > 0.05). Thus, fear conditioning, but not unpaired presentation of tone and shock, appears to regulate autophosphorylation of αCaMKII at LA synapses.
Intra-amygdala infusion of KN-62 dose dependently impairs the acquisition but not the expression of short-term and long-term fear memories
Previous experiments using both molecular–genetic and pharmacological methods have demonstrated that αCaMKII plays a prominent role in memory formation in a variety of kinds of memory, including memory for fear conditioning (Silva et al., 1992b; Mayford et al., 1996; Tan and Liang, 1996; Giese et al., 1998; Frankland et al., 2001; Elgersma et al., 2002; Miller et al., 2002). However, the contribution of CaMKII in the LA to fear conditioning has not been examined. Here, we used intra-LA infusions of the CaMK inhibitor KN-62 to examine the role of CaMKII in the acquisition and expression of fear conditioning.
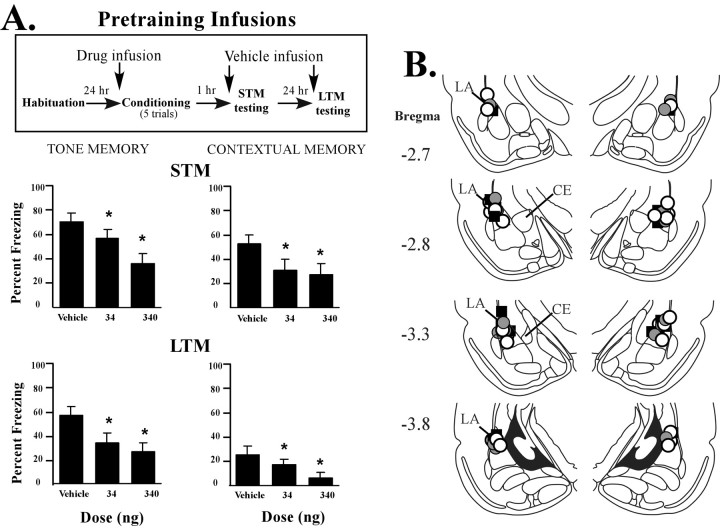
Pretraining infusions
Intra-amygdala infusions of KN-62 before conditioning produced a dose-dependent impairment in freezing for both auditory and contextual STM at 1 hr after conditioning (Fig. 3A). The ANOVA for tone STM showed a significant effect for group (F(2,29) = 27.11; p < 0.001), and post hoc t tests showed that both the low-dose and high-dose groups that received KN-62 froze significantly less than the vehicle group (p = 0.05). Moreover, a dose-dependent effect of KN-62 was evident. The ANOVA for contextual STM revealed significant effect for group (F(2,29) = 9.41; p < 0.01), and post hoc t tests revealed that both low and high doses of KN-62 produced a significant decrease in freezing behavior (p < 0.01) compared with vehicle controls (Fig. 3A, top). Although a dose-dependent effect is suggested by the data, there was no significant difference between the low-dose and high-dose drug groups (p > 0.05). However, this is likely attributable to the fact that contextual fear memory is generally a weaker type of training compared with cued fear memory (Phillips and LeDoux, 1992), which makes it more susceptible to manipulation with lower drug doses.
Figure 3.
Effects of pretraining intra-amygdala infusions of KN-62 on STM and LTM. A, Top, Outline of general behavioral procedures for pretraining intra-amygdala infusions of KN-62 for STM testing followed by LTM testing. Bottom, Mean ± SE percentage freezing for tone and contextual STM and LTM in rats given bilateral intra-amygdala infusions of vehicle (n = 11), 34 ng of KN-62 (n = 9), or 340 ng of KN-62 (n = 12) before training. B, Cannula tip placements from rats infused with vehicle (black squares), 34 ng of KN-62 (gray circles), or 340 ng of KN-62 (white circles) before training.
LTM tests performed 24 hr after conditioning also revealed a dose-dependent impairment in auditory and contextual fear memory (Fig. 3A, bottom). The ANOVA for tone LTM freezing scores displayed a significant effect for group (F(2,29) = 35.76; p < 0.05), and post hoc t tests showed that both doses of KN-62 caused an impairment of freezing relative to controls. Similarly, the ANOVA for contextual LTM displayed a significant effect for group (F(2,29) = 4.10; p < 0.05), and t tests showed that both doses of KN-62 significantly disrupted contextual fear conditioning (p < 0.05). A comparison of the LTM freezing scores for the low-dose and high-dose groups revealed a dose-dependent effect of KN-62.
Pretesting infusions
Unlike pretraining infusions, intraamygdala infusions of KN-62 before testing did not produce a significant effect on freezing for either tone or contextual conditioning (Fig. 4A). This was the case at both 1 and 24 hr after training. Thus, the ANOVAs for both tone and context STM and LTM showed no significant effects of the drug on memory retrieval (p > 0.05). Therefore, KN-62 appears to affect the acquisition but not expression of auditory and contextual fear conditioning. This is significant because it shows that the drug does not block the basic ability of the animal to hear the tone or to express the fear–freezing response.
Figure 4.
Effects of pretesting intra-amygdala infusions of KN-62 on STM and LTM. A, Top, Outline of general behavioral procedures for pretesting intra-amygdala infusions of KN-62 for STM testing followed by LTM testing. Bottom, Mean ± SE percentage freezing for tone and contextual STM and LTM in rats given bilateral intra-amygdala infusions of vehicle (n = 7) or 340 ng of KN-62 (n = 8) before testing. B, Cannula tip placements from rats infused with vehicle (black squares) or 340 ng of KN-62 (white circles) before testing. CE, Central nucleus.
Histology
Cannula placements are shown in Figures 3B and 4B for the pretraining and pretesting infusion experiments, respectively. Cannula injector tips were observed throughout the rostrocaudal extent of LA, and only rats with cannula tips at or within the boundaries of LA were included in the data analysis.
In vitro application of KN-62 to amygdala slices impairs NMDA receptor-dependent LTP in the LA without affecting routine synaptic transmission
Previous studies using both pharmacological and molecular–genetic methods have shown that αCaMKII is required for LTP in both the hippocampus (Silva et al., 1992a) and cortex (Frankland et al., 2001). The role of CaMKII in amygdala LTP, however, has not been examined. To this end, we applied KN-62 (3 μm) to amygdala slices before delivering a 30 Hz tetanus, a type of LTP induction protocol that, in the LA, is known to be NMDA receptor dependent and NR2B dependent (Bauer et al., 2002). In these experiments, we stimulated fibers coursing through the ventral striatum just medial to the LA that contain, in part, projections from the auditory thalamus (Fig. 5A) (Weisskopf et al., 1999).
Figure 5.
Impaired amygdala LTP by KN-62. A, Schematic of the amygdala slice preparation, showing placement of stimulating and recording electrodes. Recordings were made just below the site of termination of auditory thalamic fibers terminating in the LAd. IC, Internal capsule; OT, optictract; EC, external capsule. B, Mean ± SE percentage EPSP slope (% of baseline) incells (n = 6) before and after treatment with KN-62 (3 μm; solid bar). Traces from an individual experiment before and 25 min after application of KN-62 are shown in the inset. C, Mean ± SE percentage EPSP slope (relative to baseline) in cells treated with vehicle (n = 10; black squares) or 3μm KN-62 (n = 7; black triangles). Traces from an individual experiment before and 50 min after tetanic stimulation are shown in the inset.
Results showed that KN-62 impaired LTP at thalamic inputs to the LA. The control group showed 150 ± 11% potentiation, which was significantly different from baseline (t(9) = 4.49; p < 0.01). Cells treated with 3 μm KN-62 showed 102 ± 9.5% potentiation, which was not significantly different from baseline (t(6) = 0.36; p > 0.05) but was significantly different from controls (t(15) = 3.22; p < 0.01) (Fig. 5C).
To determine whether the drug affected baseline synaptic transmission in the LA, we examined the effects of 3 μm KN-62 on the initial slope of EPSPs induced by thalamic stimulation (the rationale for focusing on the initial slope is described by Weisskopf et al., 1999). KN-62 was added to the bath after a baseline period of at least 10 min. An analysis of the initial slope of the EPSPs 25–30 min after KN-62 application showed no significant effect of the drug (97 ± 5.7% of baseline), which was not significantly different from baseline (t(5) = 0.52; p > 0.05) (Fig. 5B). Thus, KN-62 impairs LTP at thalamic inputs to the LA, and, at this concentration and at this stimulation intensity, appears to be without effect on routine synaptic transmission.
Discussion
CaMKII appears to be essential for synaptic plasticity and learning in a variety of learning paradigms, including fear conditioning. However, no study to date has examined the role of CaMKII specifically in the LA, the putative locus of the plasticity underlying fear conditioning (Maren, 1999; Schafe et al., 2001). In the present experiments, we used a combination of light and electron microscopy and behavioral and electrophysiological methods to address the role of CAMKII in the LA. Findings showed that fear conditioning regulates the autophosphorylation of αCaMKII at Thr286 in LA spines and that pharmacological blockade of CaMKII activation impairs both memory formation of fear conditioning and synaptic plasticity in the LA.
Consistent with previous findings (McDonald et al., 2002), light microscopy verified the distribution of αCaMKII throughout the LA and adjacent areas. αCaMKII was highly concentrated throughout the LA, particularly in the dorsal subdivision, and was observed in LAd spines postsynaptic to terminals of projections that originate in the auditory thalamus. Furthermore, fear conditioning led to a significant increase in the autophosphorylation of αCaMKII at Thr286 in LA spines shortly after training. These findings represent the first demonstration, of which we are aware, of the regulation of αCaMKII at the synaptic level by behavioral stimulation. They are consistent with the findings of other studies that have demonstrated a role of excitatory αCaMKII autophosphorylation in synaptic plasticity and learning and memory. For example, synaptic activity results in the accumulation and translocation of CaMKII to postsynaptic sites (Strack et al., 1997; Leonard et al., 1999; Shen and Meyer, 1999; Shen et al., 2000), and autophosphorylation of the enzyme at Thr286 has been shown to prolong the binding or “trapping” of CaMKII at the PSD to allow its persistent activity at the synapse (Strack et al., 1997; Strack and Colbran, 1998; Shen et al., 2000). This has important implications behaviorally because transgenic blockade of the CaMKII autophosphorylation at Thr286, but not its CaM activity, results in the absence of NMDA receptor-dependent LTP and spatial learning (Giese et al., 1998). In addition, a mutational substitution of Thr286 with aspartate, a replacement that mimics autophosphorylation and converts αCaMKII to a persistently active, Ca2+-independent form, results in deficits of hippocampal LTP, spatial memory, and fear conditioning (Mayford et al., 1996).
Interestingly, no significant changes in the amount of pCaMKII-Thr286 were observed in the LA of rats in the unpaired group, which, under normal circumstances, might be expected to acquire significant contextual fear. In our experiment, however, it is likely that contextual learning was greatly minimized by the extensive preexposure to the conditioning apparatus (3 d) before conditioning [for a comparable lack of ERK/MAPK (extracellular signal-regulated kinase/mitogen-activated protein kinase) labeling in the LA after unpaired tone–shock presentations, see Schafe et al., 2000]. It is also conceivable that the acquisition of fear to contextual cues may follow a different biochemical time course than that of fear learning to discrete stimuli. Another possibility is that pCaMKII-Thr286 might be regulated in other brain regions that are thought to be more directly involved in the rapid acquisition of contextual fear learning, including the basal and accessory basal nuclei of the amygdala and the dorsal hippocampus. Future experiments will be needed to address these issues more thoroughly.
In the present experiments, double-label and EM experiments showed that, within the LA, αCaMKII was colocalized with the NR2B subunit of the NMDA receptor and within cell bodies and dendritic spines. This finding is consistent with previous experiments that have shown that NR2B is a critical substrate of CaMKII during synaptic plasticity and learning. Their association with each other occurs during Ca2+ increases in the cell (Omkumar et al., 1996), and catalytic site-mediated binding of CaMKII displays specificity for the phosphorylation site of NR2B (Strack et al., 2000; Mayadevi et al., 2002). Autophosphorylation at Thr286 of CaMKII is associated with high-affinity binding of the enzyme to NR2B, whereas there is little or no binding of CaMKII to NR1 or NR2A subunits by this mechanism (Strack and Colbran, 1998). Furthermore, CaMKII binding to NR2B locks the enzyme into an active conformation and suppresses inhibitory phosphorylation (Bayer et al., 2001). Our results indicate that, within LA, NR2B and CaMKII are anatomically situated at LA spines postsynaptic to auditory thalamic inputs. These results, together with our previous findings that showed NR2B to be critical to fear conditioning and synaptic plasticity in the LA (Rodrigues et al., 2001; Bauer et al., 2002), suggest that CaMKII activation via association with NR2B may play an integral part in the acquisition of fear memories in the LA.
CaMKII exists in four known main isoforms: α, β, δ, and δ (Bennett et al., 1983; Bennett and Kennedy, 1987; Hanley et al., 1987; Lin et al., 1987; Tobimatsu et al., 1988; Tobimatsu and Fujisawa, 1989). The α and β isoforms of the kinase exist in nervous tissue (Bennett et al., 1983; Erondu and Kennedy, 1985) and have opposing effects on synaptic strength (Thiagarajan et al., 2002). Because there is no isoform-specific antagonist for CaMKII, we cannot conclude unambiguously that our pharmacological manipulations were specific to the α isoform. However, because αCaMKII couples positively to synaptic strengthening and NMDA receptor activity, whereas βCaMKII does not (Thiagarajan et al., 2002), the effects of KN-62 in the present experiments are most likely attributable to effects on αCaMKII, especially in light of our EM data.
Importantly, KN-62 has no effect on protein kinase A, protein kinase C, or NMDA receptors (Hidaka and Yokokura, 1996), excluding the possibility that we produced our effects by influencing these other memory molecules. However, KN-62 affects all isoforms of CaMK, including CaMKIV (Hidaka and Yokokura, 1996), which has recently been implicated in synaptic plasticity and memory formation (Kang et al., 2001; Wei et al., 2002). Therefore, we cannot distinguish which isoform of CaMK was affected in the present experiments. We believe, however, that our results cannot be exclusively attributable to blockade of CaMKIV activity because STM (tested within hours of training) and LTM (tested 24 hr after training) were both affected. In contrast, transgenic mice lacking CaMKIV do not display deficits in auditory and contextual fear memories until 24 hr after training (Wei et al., 2002). In another study, the memory deficit in CaMKIV mutants emerged only at 7 d after training, with memory at 1 and 24 hr being intact (Kang et al., 2001). Collectively, these findings suggest that the absence of CaMKIV results in a deficit in the ability to form and/or maintain LTM (24 hr or later) but does not influence acquisition and initial storage of memory.
In our experiments, auditory and contextual fear memories were not completely eliminated by the pretraining infusions of the highest dose of KN-62. A higher dose might be more effective at impairing fear conditioning, but the relatively poor solubility of KN-62, even in HBC, makes testing this hypothesis difficult. It is also likely that other rapidly activated intracellular cascades participate in fear learning and that the partial retention of fear acquisition under the influence of KN-62 reflects the participation of these proteins.
It has been shown recently that inhibitory autophosphorylation of CaMKII (on Thr305) also plays a key function in PSD association, hippocampal LTP threshold, and hippocampal-dependent learning, including contextual fear conditioning (Elgersma et al., 2002). Persistent excitatory autophosphorylation results in increases of CaMKII at the PSD, facilitated LTP induction, and generalized learning. Therefore, the activation and deactivation of CaMKII after synaptic activity through electrophysiological stimulation and learning involves a complicated balance between the two to fine-tune the appropriate connectivity. The role of CaMKII inhibitory autophosphorylation in the LA has not been explored and would be an interesting avenue for future research.
The findings of the present experiments indicate that CaMK activity, and specifically the autophosphorylation of αCaMKII at Thr286, plays a critical role in synaptic plasticity and fear conditioning in the LA. These findings lay the groundwork for future experiments aimed at understanding the detailed mechanisms of how this multifunctional enzyme and its substrates mediate the learning and consolidation of emotional memories in the amygdala.
Footnotes
This work was supported in part by National Institute of Mental Health Grants MH 46516, MH 00956, MH 39774, MH 11902, and MH 570161. The work was also supported by a grant from the W. M. Keck Foundation to New York University. We thank Dr. Coleen Atkins for helpful discussions about this work.
Correspondence should be addressed to Dr. Joseph E. LeDoux, Center for Neural Science, New York University, 4 Washington Place, Room 809, New York, NY 10003. E-mail: ledoux@cns.nyu.edu.
DOI:10.1523/JNEUROSCI.5303-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/243281-08$15.00/0
References
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276: 2042–2045. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE (2002) NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci 22: 5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801–805. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Kennedy MB (1987) Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci USA 84: 1794–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Erondu NE, Kennedy MB (1983) Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem 258: 12735–12744. [PubMed] [Google Scholar]
- Broutman G, Baudry M (2001) Involvement of the secretory pathway for AMPA receptors in NMDA-induced potentiation in hippocampus. J Neurosci 21: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchs PA, Muller D (1996) Induction of long-term potentiation is associated with major ultrastructural changes of activated synapses. Proc Natl Acad Sci USA 93: 8040–8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Weinberger NM, Roozendaal B, McGaugh JL (1999) Is the amygdala a locus of “conditioned fear”? Some questions and caveats. Neuron 23: 227–228. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR (1999) Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96: 3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ (2002) Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron 36: 493–505. [DOI] [PubMed] [Google Scholar]
- Erondu NE, Kennedy MB (1985) Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci 5: 3270–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb CR, LeDoux JE (1997) NMDA and AMPA receptors in the lateral nucleus of the amygdala are postsynaptic to auditory thalamic afferents. Synapse 27: 106–121. [DOI] [PubMed] [Google Scholar]
- Fink CC, Meyer T (2002) Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol 12: 293–299. [DOI] [PubMed] [Google Scholar]
- Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ (2001) Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature 411: 309–313. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Miyamoto E (2000) A working model of CaM kinase II activity in hippocampal long-term potentiation and memory. Neurosci Res 38: 3–17. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ (1998) Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 279: 870–873. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Morrison JH, Iyengar R, Landau EM (2001) Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J Neurosci 21: 7053–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley RM, Means AR, Ono T, Kemp BE, Burgin KE, Waxham N, Kelly PT (1987) Functional analysis of a complementary DNA for the 50-kilodalton subunit of calmodulin kinase II. Science 237: 293–297. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Yokokura H (1996) Molecular and cellular pharmacology of a calcium/calmodulin-dependent protein kinase II (CaM kinase II) inhibitor, KN-62, and proposal of CaM kinase phosphorylation cascades. Adv Pharmacol 36: 193–219. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H (2002) Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J 364: 593–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito I, Hidaka H, Sugiyama H (1991) Effects of KN-62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, on long-term potentiation in the rat hippocampus. Neurosci Lett 121: 119–121. [DOI] [PubMed] [Google Scholar]
- Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S (2001) An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell 106: 771–783. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Milner TA (1991) Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res 85: 577–586. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW (1999) Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA 96: 3239–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CR, Kapiloff MS, Durgerian S, Tatemoto K, Russo AF, Hanson P, Schulman H, Rosenfeld MG (1987) Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci USA 84: 5962–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190. [DOI] [PubMed] [Google Scholar]
- Maren S (1999) Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci 22: 561–567. [DOI] [PubMed] [Google Scholar]
- Mayadevi M, Praseeda M, Kumar KS, Omkumar RV (2002) Sequence determinants on the NR2A and NR2B subunits of NMDA receptor responsible for specificity of phosphorylation by CaMKII. Biochim Biophys Acta 1598: 40–45. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER (1996) Control of memory formation through regulated expression of a CaMKII transgene. Science 274: 1678–1683. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F (2002) GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol 446: 199–218. [DOI] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M (2002) Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36: 507–519. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB (1985) Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem 260: 9039–9046. [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB (1996) Identification of a phosphorylation site for calcium/calmodulindependent protein kinase II in the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 271: 31670–31678. [DOI] [PubMed] [Google Scholar]
- Phend KD, Rustioni A, Weinberg RJ (1995) An osmium-free method of epon embedment that preserves both ultrastructure and antigenicity for post-embedding immunocytochemistry. J Histochem Cytochem 43: 283–292. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285. [DOI] [PubMed] [Google Scholar]
- Repa JC, Muller J, Apergis J, Desrochers TM, Zhou Y, LeDoux JE (2001) Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nat Neurosci 4: 724–731. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE (2001) Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci 21: 6889–6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE (2002) The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci 22: 5219–5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE (2000) Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci 20: 8177–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nader K, Blair HT, LeDoux JE (2001) Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci 24: 540–546. [DOI] [PubMed] [Google Scholar]
- Shen K, Meyer T (1999) Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284: 162–166. [DOI] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T (2000) Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci 3: 881–886. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y (1992a) Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 201–206. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S (1992b) Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257: 206–211. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Chang B, Brickey D (2001) Cellular signaling through multi-functional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 276: 3719–3722. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Gage AT (1996) Distinct synaptic loci of Ca2+/calmodulin-dependent protein kinase II necessary for long-term potentiation and depression. J Neurophysiol 76: 2097–2101. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ (1998) Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 273: 20689–20692. [DOI] [PubMed] [Google Scholar]
- Strack S, Choi S, Lovinger DM, Colbran RJ (1997) Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J Biol Chem 272: 13467–13470. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ (2000) Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-d-aspartate receptor. J Biol Chem 275: 23798–23806. [DOI] [PubMed] [Google Scholar]
- Tan SE, Liang KC (1996) Spatial learning alters hippocampal calcium/calmodulin-dependent protein kinase II activity in rats. Brain Res 711: 234–240. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW (2002) Alpha- and beta-CaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron 36: 1103–1114. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H (1989) Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem 264: 17907–17912. [PubMed] [Google Scholar]
- Tobimatsu T, Kameshita I, Fujisawa H (1988) Molecular cloning of the cDNA encoding the third polypeptide (gamma) of brain calmodulin-dependent protein kinase II. J Biol Chem 263: 16082–16086. [PubMed] [Google Scholar]
- Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M (2002) Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci 5: 573–579. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE (1999) L-Type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci 19: 10512–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Jang JD, Nishiuchi Y, Braun KP, Ro SG, Goodman M (1991) The utility of 2-hydroxypropyl-beta-cyclodextrin as a vehicle for the intracerebral and intrathecal administration of drugs. Life Sci 48: 623–633. [DOI] [PubMed] [Google Scholar]