Abstract
Overwhelming evidence indicates that the effects of β-amyloid (Aβ) are dose dependent both in vitro and in vivo, which implies that Aβ is not directly detrimental to brain cells until it reaches a threshold concentration. In an effort to understand early Alzheimer's disease (AD) pathogenesis, this study focused on the effects of subthreshold soluble Aβ and the underlying molecular mechanisms in murine microglial cells and an AD transgenic mouse model. We found that there were two phases of dose-dependent Aβ effects on microglial cells: at the threshold of 5 μm and above, Aβ directly induced tumor necrosis factor-α (TNF-α) release, and at subthreshold doses, Aβ indirectly potentiated TNF-α release induced by certain G-protein-coupled receptor (GPCR) activators. Mechanistic studies revealed that subthreshold Aβ pretreatment in vitro reduced membrane GPCR kinase-2/5 (GRK2/5), which led to retarded GPCR desensitization, prolonged GPCR signaling, and cellular hyperactivity to GPCR agonists. Temporal analysis in an early-onset AD transgenic model, CRND8 mice, revealed that the membrane (functional) GRK2/5 in brain cortices were significantly reduced. More importantly, such a GRK abnormality took place before cognitive decline and changed in a manner corresponding with the mild to moderate soluble Aβ accumulation in these transgenic mice. Together, this study not only discovered a novel link between subthreshold Aβ and GRK dysfunction, it also demonstrated that the GRK abnormality in vivo occurs at prodromal and early stages of AD.
Keywords: G-protein coupled receptor, GPCR kinase, Alzheimer's disease, hyperactivity, β-amyloid, thrombin
Introduction
Understanding early Alzheimer's disease (AD) pathogenesis holds the key for establishing effective prevention and early treatments for the disease. Multiple lines of evidence indicate that, whether resulting from an inherent genetic mutation or caused by an as yet insufficiently understood environmental insult(s), over-accumulation of β-amyloid (Aβ) in AD brains appears to be at least one of the common points in which multiple initiating pathways may converge (Hardy and Selkoe, 2002). In this regard, most previous studies used highly concentrated fibrillary Aβ to reveal its direct cellular effects, such as cytotoxicity and glial activation (Yankner et al., 1989; Iversen et al., 1995; Meda et al., 1995; Suo et al., 1997), whereas the recent trend appears to favor a pathogenic role of soluble Aβ (Walsh et al., 1997, 2002; Crawford et al., 1998b; Hartley et al., 1999; Lue et al., 1999).
Transgenic (Tg) mice that overproduce Aβ have been shown to mimic AD-like cognitive impairment and most AD-like pathologies, except for formation of typical neurofibrillary tangles (NFTs) (van Leuven, 2000; Richardson and Burns, 2002). Moreover, aside from some divergent evidence to the contrary (Moechars et al., 1999), many Tg mouse and human studies indicate that, although Aβ levels in AD or Tg mouse brains are significantly elevated long before the disease onset (cognitive decline) compared with their corresponding controls, the cognitive impairment does not necessarily occur until Aβ accumulates to a certain level. Therefore, we propose that there exists a “threshold” for Aβ to produce direct detrimental effects in the brain, whereas investigation of effects of subthreshold Aβ on various brain cells may reveal pathogenetic mechanisms at prodromal or early stages of AD. In addition, because Aβ is generated in soluble forms, principally composed of oligomers (Walsh et al., 2002), soluble Aβ, rather than fibrillary Aβ, should be used to study early AD pathogenesis.
In support of our hypothesis, previous studies have revealed two distinct phases of dose-dependent effects of Aβ on vascular tissues. For example, with a minimum effective concentration (ECmin) of ∼4 μm, Aβ dose dependently increased vascular cytotoxicity in hours or days (Suo et al., 1997, 1998b; Wang et al., 2000; Mok et al., 2002). On the other hand, although nanomolar (subthreshold) Aβ had no direct effects, it sensitized vascular cells in a dose-dependent manner (with an EC range of 20–1000 nm) so that vasoconstrictions induced by phenylephrine or endothelin-1 (ET-1) were strongly potentiated (Thomas et al., 1996; Crawford et al., 1997, 1998a; Paris et al., 2000). Moreover, in vivo studies indicated that Aβ vasoactivity may be associated with the cerebral blood flow reduction frequently observed in early stages of AD patients (Suo et al., 1998a, 2000; Niwa et al., 2002). Therefore, armed with the knowledge about effects of subthreshold Aβ on vascular tissues, this study attempted to characterize effects of subthreshold Aβ on microglia and to investigate the underlying molecular mechanisms with hope to gain some insights into the pathogenesis relevant to early stages of AD.
Materials and Methods
Materials. Previously, we found that Aβ peptides from different suppliers have different solubility and produce significantly variable data (Suo et al., 1997, 1998b; Crawford et al., 1998b). Our results in these studies also suggested that Aβ peptides from BioSource (Camarillo, CA) consistently show the greatest solubility in aqueous solution and form a mixture of soluble Aβ primarily composed of Aβ conformational intermediates, as measured by circular dichroism spectrum (Crawford et al., 1998b), or soluble Aβ oligomers, as analyzed by Western blot (WB) analysis (our unpublished data). Therefore, synthetic Aβ1-40, Aβ1-42, and Aβ40-1 used in this study were purchased from BioSource and prepared as described previously (Suo et al., 1998b). Other reagents and their corresponding suppliers are listed below: human α-thrombin (Hematologic Technologies, Essex Junction, VT); polyclonal antibodies (pAbs) to protease-activated receptors (PARs), PAR1 and PAR4, and G-protein coupled receptor (GPCR) kinase 2 (GRK2) and GRK5 (Santa Cruz Biotechnology, Santa Cruz, CA); phospho-specific and total p44/42 MAPKs (mitogen-activated protein kinases) pAbs (Cell Signaling Technology, Beverly, MA); Griffonia simplicifolia isolectin B4-FITC, glutamate, lipopolysaccharide (LPS), poly-l-lysine, and alkaline phosphatase-conjugated secondary antibodies (Abs) (Sigma, St. Louis, MO); Fluo-4, phalloidin-FITC, fluorescent-conjugated secondary antibodies, and dyes (Molecular Probes, Eugene, OR); mouse tumor necrosis factor-α (TNF-α) ELISA kit (R & D Systems, Minneapolis, MN); precast SDS-PAGE gels (Novex, San Diego, CA); the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL); and polyvinylidene difluoride membranes (Millipore, Bedford, MA). Cell culture supplies, Iscoves modified Delbecco's medium (IMDM), DMEM, fetal bovine sera (FBS), and other routinely used reagents were from Sigma, Invitrogen (Carlsbad, CA), and Fisher Scientific (Pittsburgh, PA).
Tg-CRND8 mice on a C57BL/6 × C3H/He mixed genetic background (Chishti et al., 2001) were weaned, genotyped, and single housed by age 4–5 weeks before they were entered into this study. Care and use of these mice in this study were in compliance with National Institutes of Health regulations.
Cell culture and treatments. Primary microglial cultures were prepared from neonatal mouse (C57BL/6) brains as described previously (Suo et al., 2002, 2003a) and maintained in DMEM with F-12 nutrient mixture containing 10% FBS and antibiotics. N9 clonal murine microglial cells, a gift from Drs. Bottani and Ricciardi-Castagnoli (Università Degli Studi Di Milano-Bicocca, Milan, Italy), were maintained in IMDM containing 5% heat-inactivated FBS, 2 mm l-glutamine, 50 μm 2-mercaptoethanol, and antibiotics. Treatment of both primary and N9 microglial cells were performed under serum-free conditions. Unless specified, cells were always pretreated with Aβ for 5 min and then followed by challenge with α-thrombin (100 nm or as indicated), glutamate (2 mm), or LPS (5 ng/ml).
Ca2+ imaging. We measured [Ca2+]i in microglial cells using Fluo-4 (2 μm) with the aid of a Nikon Bio-Rad (Hercules, CA) Microradiance Plus triple laser scanning confocal microscope. Image collection, quantification, and data analysis were performed as described previously (Suo et al., 2002, 2003a).
Immunoprecipitation and Western blot analysis. Cultured cells were lysed in prechilled 2× immunoprecipitation (IP) buffer I (20 mm Tris, pH 7.4, 300 mm NaCl, 2 mm EDTA, 2 mm EGTA, pH 8.0, 0.4 mm sodium orthovanadate, 2% Triton X-100, 1% NP-40, and 0.4 mm PMSF) supplemented with both phosphatase and protease inhibitor mixtures. After centrifugation, the supernatants were collected, and total protein concentrations were determined by BCA assay. Equal amounts (250 μg) of cell lysates from each group were mixed with 20 μl (∼5 μg) of either PAR1 or PAR4 antibodies and brought to a total volume of 1 ml in 1× IP buffer. After a 1 hr incubation at 4°C, appropriate secondary Ab-agarose conjugates were added, followed by incubation (30 min with agitation), centrifugation, and sufficient washes. The pellets were resuspended in 30 μl of electrophoresis sample buffer and boiled for 5 min. The supernatants were directly used for SDS-PAGE and WB analysis. To prepare subcellular fractions of brain samples, we homogenized fresh or fresh-frozen tissues in Tris-buffered saline solution (in mm: 10 Tris, pH 7.4, 150 NaCl, 1 EDTA, 1 EGTA, pH 8.0, and 0.2 sodium orthovanadate) supplemented with protease inhibitor mixtures. After centrifugation (12,000 × g at 4°C for 15 min), the supernatants were collected as cytosolic fractions. The pellets were resuspended in 1× IP buffer containing protease inhibitor mixture. After brief sonication and centrifugation (12,000 × g at 4°C for 15 min), the supernatants were collected as membrane fractions. Both antibodies to GRK2 and GRK5 (Santa Cruz Biotechnology) were diluted 1:500 for WB. WB for total and phospho-specific p44/42 MAPK and other routine procedures were performed as described previously (Suo et al., 2002, 2003a). For semiquantitative analysis of protein band density, image of the blot was imported into NIH Image software, and the protein band density was analyzed with gel plotting macros. The raw data were expressed as mean density and then standardized against untreated control or otherwise specified separately. The analyzed data were expressed as fold changes over the standardized control and plotted into a bar graph.
Measurements of TNF-α production. TNF-α production in microglial culture media was measured and standardized as described previously using a mouse TNF-α ELISA kit (Suo et al., 2002, 2003a).
Immunocytochemistry and semiquantification of GRK subcellular distribution. Microglial cells were seeded onto poly-l-lysine-coated eight-chamber slides at a density of 1 × 104 per well. After treatments, cells were immediately fixed with prechilled (4°C) 5% acetic acid in methanol for 45 min at 4°C, followed by washing with PBS. The fixed cells were blocked and double stained with phalloidin-FITC (1:1000) and anti-GRK5 (1:500) or GRK2 (1:500). Secondary Ab-Cy3 conjugate (1:500) staining, washing, mounting, and confocal microscopic visualization were performed routinely as described previously (Suo et al., 2002, 2003a).
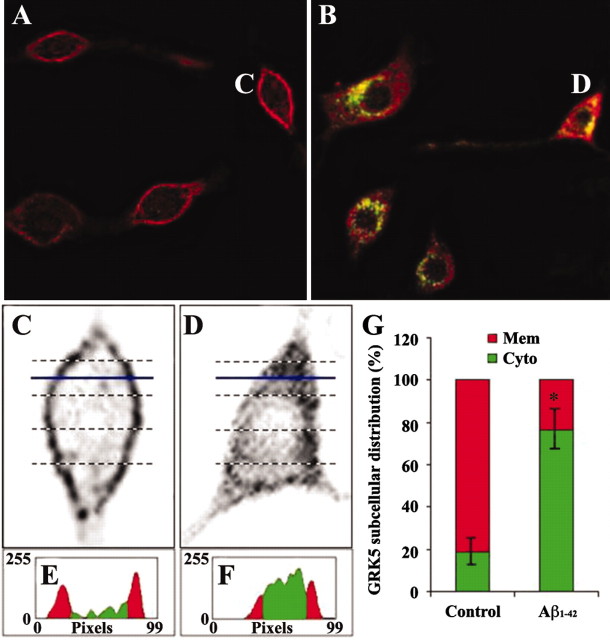
GRK subcellular distribution in Aβ-treated microglial cells was semi-quantified according to a previously published method (Barak et al., 1999) with slight modifications. Figure 4, C and D, shows the selected cells that were chosen to elucidate the semiquantification. Briefly, the selected cell image corresponding to GRK staining (red) was copied, enlarged, inverted, and rotated to an appropriate position for quantification with NIH Image. Linear slices (5–10 per each cell) across the cell were profiled to estimate GRK subcellular distribution. Figure 4, E and F, shows the examples of profiles for the blue-line indicated levels in C and D, respectively. The red and green areas represent the membrane and cytosol, respectively. The sum of areas for the membrane and cytosol from all slices in each cell represents GRK subcellular distribution in this particular cell. For each treatment, 20 randomly selected cells were quantified, and the average data were shown.
Figure 4.
Subthreshold soluble Aβ induced rapid GRK5 translocation from the membrane to the cytosol. N9 microglial cells were treated with Aβ1-42 (500 nm) for 5 min, followed by immunofluorescent staining with a GRK5 Ab (red) and phalloidin-FITC (green). A, C, and E show representative resting (untreated control) microglial cells and corresponding semiquantitative analysis; B, D, and F display representative microglial cells treated with Aβ and corresponding examples of the analysis as described in Materials and Methods. For each group, 20 randomly selected cells were quantified for GRK5 subcellular distribution, and the average data were plotted and shown in G. *p < 0.001 compared with untreated controls (t test). Mem, Membrane; Cyto, cytosol.
Statistical analysis. All qualitative experiments [i.e., WB, IP, and immunocytochemistry (ICC)] were repeated at least three times for each sample or treatment, and all WBs and ICC for GRK5 and GRK2 were also subjected to semiquantitative analysis to ensure maximal accuracy of the conclusion drawn from these data. Calcium imaging data were averages taken from three separate experiments. TNF-α measurements were repeated once with a total of n = 6 for each treatment. Quantitative data are expressed as mean ± SEM and analyzed by ANOVA using StatView 6.0 (Abacus Concepts, Mountain View, CA). Post hoc comparisons of means were made using Scheffe's or Tukey's methods when appropriate.
Results
Subthreshold Aβ increases microglial sensitivity to GPCR activators
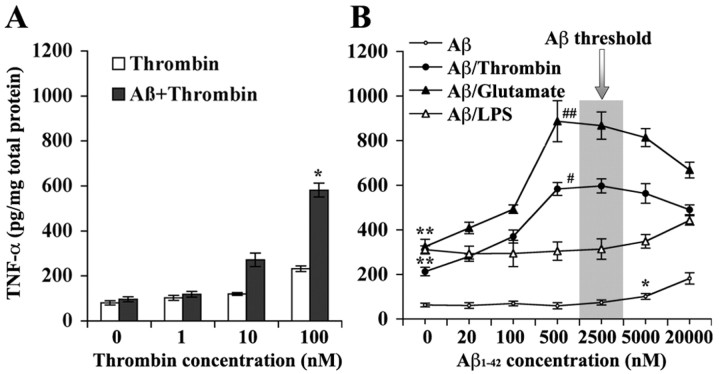
To determine whether Aβ also has two different phases of dose-dependent effects on microglial cells, as it does in vascular cells, we first tested the effects of 500 nm freshly solubilized Aβ1-42 on TNF-α secretion induced by increasing doses of thrombin in primary mouse brain microglial cultures (Suo et al., 2002, 2003a). As shown in Figure 1A, Aβ alone did not induce TNF-α increase compared with the untreated control. Cells pretreated with Aβ for 5 min, followed by increasing thrombin exposure for 24 hr produced significantly more TNF-α than those without Aβ pretreatment (p < 0.01; Aβ pretreatment status vs thrombin dose by two-way ANOVA). These data suggest that Aβ, at a dose that does not have a direct effect (subthreshold), can indirectly potentiate thrombin-induced TNF-α release in microglia. This encouraged us to further characterize the dose-dependent effects of Aβ on microglial cells. As shown in Figure 1B, when a series doses of Aβ1-42 were exposed to N9 clonal microglial cell line (Suo et al., 2002, 2003a) for 24 hr, a dose-dependent effect of Aβ on TNF-α release became significant (p < 0.05; Aβ dose by one-way ANOVA) only when the doses within the nanomolar range were omitted. The ECmin for Aβ to produce the direct effect was 5 μm (p < 0.05; post hoc comparisons of the means by Scheffe's test). Nevertheless, although nanomolar Aβ had no direct effects on TNF-α release, pretreatment of the cells with the nanomolar Aβ for 5 min strongly and dose dependently (p < 0.01; Aβ dose vs thrombin status by two-way ANOVA) potentiated TNF-α release induced by thrombin with an ECmin of 20 nm and ECmax of 500 nm. These results indicate that there are indeed two phases of dose-dependent Aβ effects on microglial cells. One phase reveals the direct Aβ effects, the other underlies the indirect Aβ effects on microglial cells, and the threshold to distinguish these two phases is within a few micromolar for Aβ1-42.
Figure 1.
Subthreshold soluble Aβ induced microglial hyper-reactivity to GPCR activators. A, Primary mouse brain microglial cultures were pretreated (5 min) with Aβ1-42 (500 nm), followed by increasing doses of thrombin (24 hr) as indicated (n = 4). *p < 0.01, Aβ pretreatment status versus thrombin dose by two-way ANOVA. B, N9 clonal murine microglial cells were exposed to increasing doses of Aβ1-42 as indicated for 24 hr or for 5 min, followed by a single dose of thrombin (100 nm), glutamate (2 mm), and LPS (5 ng/ml), respectively, for an additional 24 hr (n = 6). Compared with the untreated control, a single dose of thrombin (**p < 0.001), glutamate (**p < 0.001), or LPS (**p < 0.001) significantly stimulated TNF-α release. Aβ1-42 alone can directly induce TNF-α release dose dependently but with doses higher than 5 μm (threshold; *p < 0.05 compared with untreated control). Moreover, subthreshold Aβ1-42 pretreatment (without direct effect) dose dependently potentiated effects of thrombin (#p < 0.01, Aβ vs Aβ/thrombin by ANOVA) and glutamate (##p < 0.001, Aβ vs Aβ/glutamate by ANOVA) but not LPS (p > 0.05, Aβ vs Aβ/LPS by ANOVA) on TNF-α production in N9 microglial cells.
In addition to thrombin, we also tested effects of pretreatment with Aβ on TNF-α release induced by glutamate (2 mm) and LPS (5 ng/ml) in N9 microglial cells (Fig. 1B). We found that both glutamate and LPS alone strongly stimulated TNF-α release (p < 0.001 for both glutamate and LPS compared with untreated control by post hoc mean comparisons), whereas pretreatment with Aβ further increased TNF-α release induced by glutamate (p < 0.001; Aβ dose vs glutamate status by two-way ANOVA) but not by LPS. Similar to that for thrombin, potentiation of Aβ on glutamate-induced TNF-α release was also dose dependent, with an ECmin of 20 nm and ECmax of 500 nm. Aβ doses higher than 500 nm remained significantly synergistic with both thrombin and glutamate (i.e., p < 0.05 for 20 μm Aβ vs thrombin; p < 0.01 for 20 μm Aβ vs glutamate) but tended to decrease with increasing Aβ concentrations (i.e., p < 0.05 for thrombin and p < 0.01 for glutamate when comparing 20 μm with 500 nm Aβ effects). Analysis of the interactions between Aβ and LPS indicated that there were no significant interactions between subthreshold (nanomolar) Aβ and LPS, whereas results with micromolar Aβ and LPS were additive rather than synergistic. It is known that both thrombin and glutamate can act through GPCRs (Noda et al., 2000; Suo et al., 2002, 2003a), whereas LPS activates non-GPCR Toll-like receptors (Hajjar et al., 2002). Therefore, these results together infer that the subthreshold Aβ may preferentially enhance GPCR-mediated effects in microglial cells.
Aβ pretreatment induces prolongation of thrombin signaling
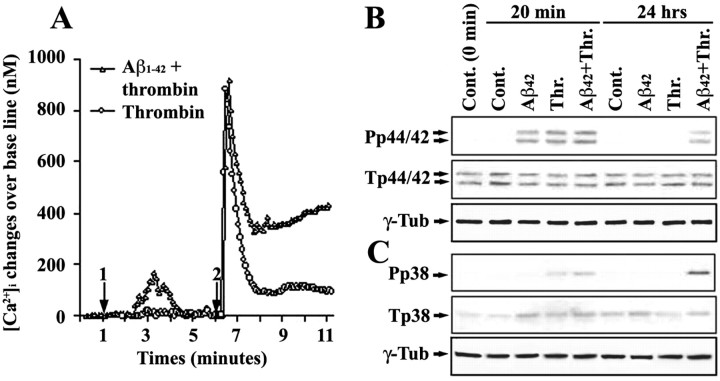
To understand the molecular mechanisms by which subthreshold Aβ sensitizes microglial activation induced by thrombin, we examined changes in thrombin signaling in Aβ-pretreated microglial cells. We found that Aβ1-42 (500 nm) induced a slow and moderate increase of free [Ca2+]i that returned to basal levels within 4 min (Fig. 2A). Thrombin alone induced a steep increase of [Ca2+]i that rapidly decreased to ∼100 nm above basal levels and remained for at least 4 min. In contrast, in Aβ-pretreated cells, thrombin induced a similar [Ca2+]i increase that quickly decreased to ∼350 nm above baseline but then slowly climbed to >450 nm without a subsequent decrease within the time observed. These data suggest that Aβ pretreatment resulted in prolonged calcium signaling triggered by thrombin in microglial cells. In addition, Western blots for total and phospho-specific p44/42 (Fig. 2B) and p38 (Fig. 2C) MAPKs revealed increased phosphorylation of p44/42 and p38 MAPKs 20 min after Aβ (much less for p38 than p44/42), 15 min after thrombin, and Aβ pretreatment (5 min), followed by thrombin treatment (15 min). By 24 hr, both p38 and p44/42 MAPK phosphorylation in Aβ or thrombin alone-treated microglia returned to basal levels. However, phosphorylation of the p38 and p44/42 MAPKs remained elevated in cells pretreated with Aβ, followed by thrombin. These data indicate that Aβ pretreatment also resulted in prolongation of thrombin-induced MAPK activation in addition to the prolonged calcium signaling in microglial cells. Together, these results suggest that pretreatment with subthreshold Aβ can lead to prolongation of thrombin signaling in microglial cells. Moreover, the effects of subthreshold Aβ take place in minutes (as indicated by the prolonged calcium signaling) and remain until the end of the experiments for at least 24 hr (as indicated by the MAPK changes and TNF-α release).
Figure 2.
The effect of subthreshold Aβ pretreatment on prolonging thrombin signaling. A, N9 microglial cells were pretreated with 500 nm Aβ1-42 (arrow 1) for 5 min, followed by treatment with 100 nm thrombin (arrow 2). Changes in [Ca2+]i were measured from three separate experiments, as described previously (Suo et al., 2002, 2003a). Mean values at each point were plotted. Error bars were omitted to make the figure more legible, but the SDs were all <46.7 nm. For B and C, N9 microglial cells were pretreated with Aβ1-42 (500 nm) for 5 min, followed by thrombin (Thr.; 100 nm) treatment for either 20 min or 24 hr as indicated. Western blots were performed as described in Materials and Methods with antibodies against phospho-specific and total p44/42 MAPKs (Pp44/42 and Tp44/42; B) and p38 MAPK (Pp38 and Tp38; C). Reprobe of the membranes with anti-γ-tubulin (γ-Tub) was used as an unrelated protein control. Cont., Control.
Aβ disrupts binding of GRKs to activated GPCRs
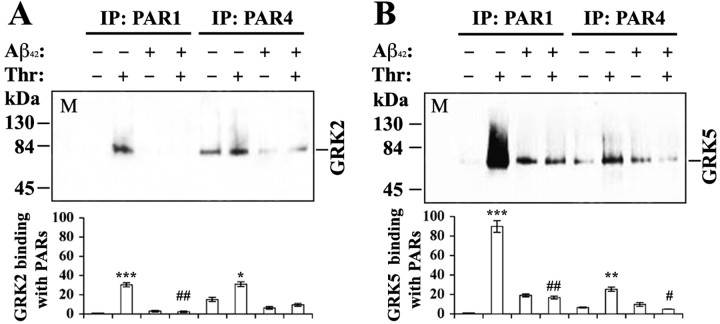
One possible mechanism leading to cellular hyperactivity to GPCR agonists is impaired GPCR desensitization. Because GRKs are primarily responsible for initiating GPCR desensitization (Premont et al., 1995; Hisatomi et al., 1998; Pitcher et al., 1998), we questioned whether subthreshold Aβ has any impact on GRKs. Although regulation of many individual GPCRs by specific GRKs is still under investigation, previous studies have established that PAR1 is desensitized primarily by GRK5 at least in endothelial cells (Tiruppathi et al., 2000), whereas GRK2 may participate in PAR1 desensitization as well (Hammes et al., 1999). Therefore, to address whether subthreshold Aβ may interfere with interactions between GPCRs and GRKs, we first examined potential effects of Aβ on binding of activated thrombin receptors (both PAR1 and PAR4) to GRK5 and GRK2. For this purpose, we treated N9 microglial cells with α-thrombin (100 nm) for 30 sec in the absence or presence of pretreatment with Aβ1-42 (500 nm) for 5 min. Equivalent amounts (250 μg) of the cell lysates from different groups were used for IP with PAR1 and PAR4 Abs, followed by WB with GRK5 and GRK2 Abs. Compared with untreated microglial cells, 30 sec treatment with thrombin alone increased binding of PAR1 to GRK5 and GRK2, as well as PAR4 binding to GRK5 and GRK2 by ∼90-, 30-, 4-, and 2-fold, respectively (Fig. 3A,B). Compared with the groups in the absence of Aβ pretreatment, the thrombin-induced increase in binding of both PAR1/4 to GRK2/5 was essentially abolished in the Aβ-pretreated cells, although Aβ pretreatment itself induced a significant increase of nonspecific PAR1–GRK5 binding. The differential binding between different isoforms of GRKs and PARs in the cells without Aβ pretreatment agreed with those published previously in other cell types (Shapiro et al., 2000; Tiruppathi et al., 2000), suggesting that microglial PAR1 is also desensitized primarily by GRK5 and to a lesser extent by GRK2, whereas PAR4 is barely under significant control by either GRK5 or GRK2. In the Aβ-pretreated cells, the disrupted binding of GRKs to PARs strongly suggests that subthreshold Aβ can severely impair GRK–PAR (GPCR) interactions.
Figure 3.
Effects of subthreshold Aβ pretreatment on the binding of GRKs to PARs. N9 cells were pretreated with Aβ1-42 (500 nm) for 5 min and then challenged with thrombin (Thr, 100 nm) for 30 sec as in Materials and Methods. Cells were immediately lysed for IP and WB analysis. The bar graphs represent averages of three repeats of the same experiments as analyzed by densitometry. For both A (probed for GRK2) and B (probed for GRK5), lane M is the marker; the others are as indicated. The number on both y-axes indicates fold changes of the mean densities as standardized against the untreated control of IP with PAR1 for each blot. Separate two-way ANOVA followed by post hoc comparisons of the means (Scheffe's test) were made. #p < 0.01 and ##p < 0.001 indicate the significance of the groups in the presence of Aβ pretreatment compared with the groups in the absence of Aβ pretreatment. *p < 0.05, **p < 0.01, and ***p < 0.001 are the levels of significance over the untreated controls in each corresponding measurement.
Aβ inhibits GRK–GPCR binding by reducing availability of membrane GRKs
The physical location of GRKs, which is determined at least in part by counterbalance between their binding factors in the membrane (i.e., phospholipids and Gβγ, etc.) and cytosol (i.e., calcium sensor proteins and cytoskeleton proteins, etc.) (Pitcher et al., 1998; Sallese et al., 2000; Kohout and Lefkowitz, 2003), is a critical factor that regulates interactions (including binding) between GRKs and GPCRs. To determine whether subthreshold Aβ alters GRK subcellular distributions, we treated primary murine microglial cells cultured in serum-free medium with Aβ1-42 (500 nm) for 5 min, followed by double-fluorescent staining with phalloidin-FITC and Abs to GRK2 or GRK5. As shown in Figure 4, 5 min Aβ treatment induced a rapid translocation of GRK5 from the membrane to the cytosol. Moreover, Aβ also caused rapid formation of fibrillar actin (F-actin) as indicated by phalloidin staining, and the translocated cytosolic GRK5 partially colocalized with F-actin. In comparison, staining with GRK2 Ab confirmed its cytosolic location but did not reveal noticeable changes in response to the Aβ treatment (data not shown).
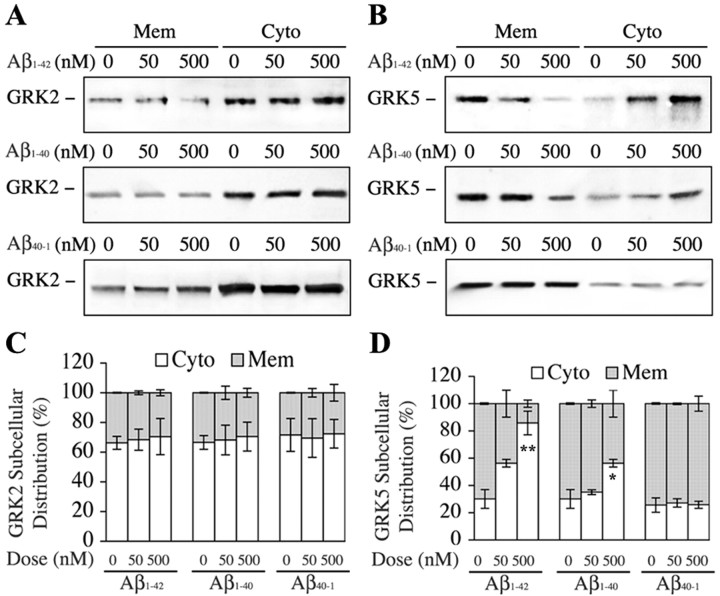
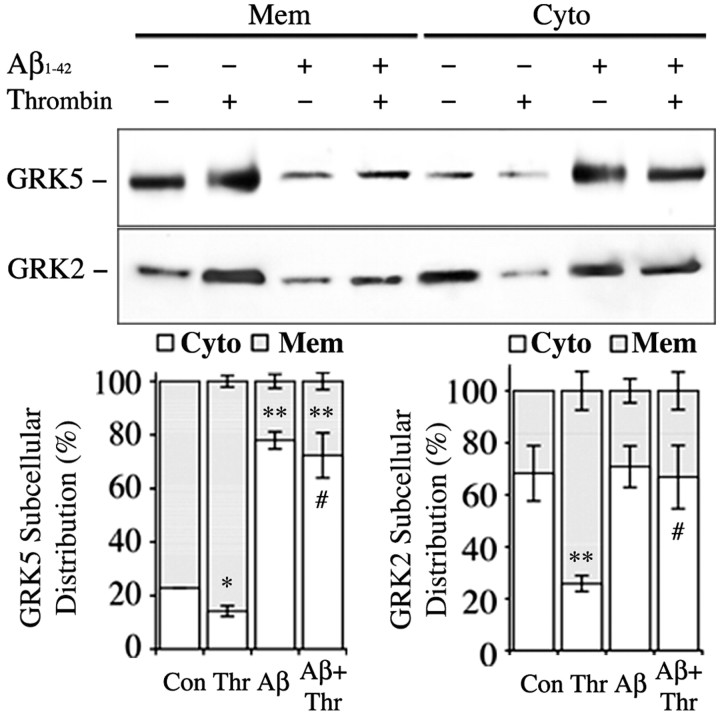
In a parallel experiment, we treated N9 microglial cells with Aβ1-42 (0, 50, and 500 nm), Aβ1-40 (0, 50, and 500 nm), and Aβ40-1 (0, 50, and 500 nm) for 5 min, and the cell homogenates were separated into the membrane and cytosol fractions for Western blotting analysis of GRK2 and GRK5. We found that both Aβ1-40 (p < 0.05 by one-way ANOVA) and Aβ1-42 (p < 0.002 by one-way ANOVA) but not Aβ40-1 induced dose-dependent translocation of GRK5 from the membrane to the cytosol, whereas none of the Aβ peptides had significant effects on GRK2 subcellular distributions (Fig. 5). In addition, when N9 microglial cells were challenged with thrombin (100 nm) for 30 sec in the absence or presence of pretreatment with Aβ1-42 (500 nm) for 5 min, we found that, in the absence of Aβ pretreatment, thrombin induced significant translocation of both GRK2 (p < 0.001; mean comparisons) and GRK5 (p < 0.05; mean comparisons) from the cytosol to the membrane. However, in the presence of Aβ pretreatment, thrombin failed (p < 0.01; Aβ pretreatment status vs thrombin by two-way ANOVA) to induce significant translocation for both GRK2 and GRK5 from the cytosol to the membrane (Fig. 6). Together, these results suggest that the subthreshold Aβ cannot only induce translocation of GRK5 (but not GRK2) from the membrane to the cytosol but also strongly inhibit the reverse translocation of both GRK2 and GRK5 back to the membrane when cells are challenged with a GPCR activator. In other words, the subthreshold Aβ can reduce availability of the membrane GRKs, which may contribute to the inhibited binding of PARs (GPCRs) to GRKs after challenging with thrombin in the presence of Aβ pretreatment.
Figure 5.
Dose-dependent, subthreshold soluble Aβ induction of GRK5, but not GRK2, translocation from the membrane (Mem) to the cytosol (Cyto). N9 microglial cells were treated with increasing doses of Aβ1-40, Aβ1-42, and Aβ40-1 for 5 min as indicated. The cell homogenates were separated into membrane and cytosol fractions and then subjected to WB analysis. A and B show examples of the effects of Aβ1-42, Aβ1-40, and Aβ40-1 (from top to bottom) on translocation of GRK2 and GRK5, respectively. C and D are semiquantitative analysis (n = 3) for effects of all tested Aβ peptides on GRK2 and GRK5 translocation, respectively. Statistical analysis (one-way ANOVA; Aβ dose vs GRK subcellular distribution) indicated that none of the Aβ peptides had significant effects on GRK2 translocation, whereas both Aβ1-40 (*p < 0.05) and Aβ1-42 (**p < 0.002), but not Aβ40-1, dose dependently induced GRK5 translocation from the membrane to the cytosol.
Figure 6.
Subthreshold Aβ pretreatment reduced availability of the membrane GRKs to activated PARs. N9 microglial cells were challenged with thrombin (100 nm) for 30 sec in the absence or presence of pretreatment with Aβ1-42 (500 nm) for 5 min, and then cell homogenates were fractionated and analyzed by WB as in Materials and Methods. The top panels are representative WBs for GRK2 and GRK5 as indicated. The bar graphs show the averages of three repeats of the same experiments after semiquantitative analysis. Two-way ANOVA followed by post hoc comparisons of the means (Scheffe's test) were made. #p < 0.01 indicates the significance of the groups in the presence of Aβ pretreatment compared with the groups in the absence of Aβ pretreatment. *p < 0.05 and **p < 0.001 indicate the significance of the means compared with the untreated controls.
GRK abnormality occurs before cognitive decline in Tg-CRND8 mice
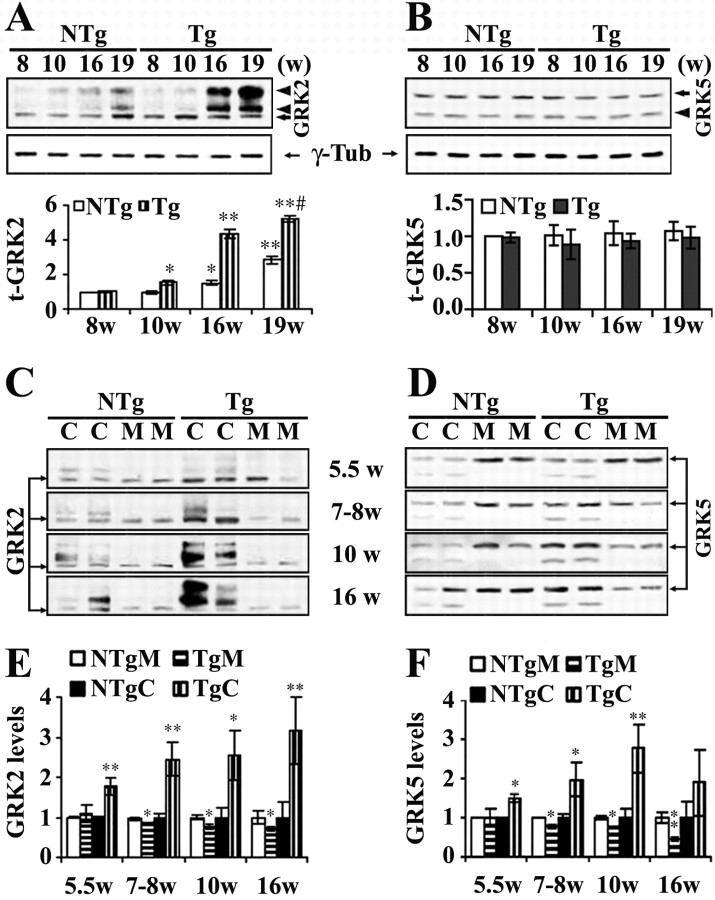
Armed with the novel finding that subthreshold Aβ can strongly affect GRK subcellular distributions in vitro, we sought to further assess significance of the subthreshold Aβ-induced GRK abnormality in vivo with particular interest of its significance in early AD pathogenesis. This was accomplished by analyzing the temporal changes in subcellular distribution of GRK2 and GRK5 in an early-onset AD transgenic mouse line, the Tg-CRND8 mice (Chishti et al., 2001). Cortical (including hippocampal) homogenates of the Tg-CRND8 mice and their nontransgenic littermates (NTg) were separated into the membrane and cytosol fractions and analyzed by WB along with their total cortical extracts. We found that total brain GRK2 levels increased in mice in an age-dependent manner (within the age groups examined) for both the Tg and NTg animals. However, they were significantly higher in the Tg than in the NTg (p < 0.01) (Fig. 7A). Post hoc comparison of the means indicated that the total GRK2 levels in the Tg mice became significantly higher than that in the NTg at 10 weeks of age (p < 0.05) and remained significantly upregulated at least until 19 weeks of age (p < 0.01). Total brain GRK5 levels showed no statistically significant changes for either the age dependency or between the Tg and NTg mice (Fig. 7B). The distinct age dependency for the total GRK2 and GRK5 levels in mouse brain agreed with that in peripheral tissues (Schutzer et al., 2001), whereas a greater level of increase in the total GRK2 was genotype dependent. Of interest, the increased GRK2 in the Tg mice displayed primarily as high-molecular-weight bands (arrowheads) rather than the monomer (arrow). Moreover, such changes appeared to be age dependent and became more apparent in post-plaque (16 and 19 week groups) Tg-CRND8 mouse brains. In contrast to GRK2, low-molecular-weight, rather than high-molecular-weight, GRK5 bands (arrowhead) appeared consistently in all groups examined without apparent differences in their intensity (Fig. 7B).
Figure 7.
Expression and subcellular distribution of GRK2 and GRK5 in Tg-CRND8 mouse brains. Cortical tissues of Tg-CRND8 mice and their NTg littermates (n ≥ 4 for each group) were used for WB analysis as in Materials and Methods. A and B show the total brain GRK2 and GRK5 levels, respectively. Top blots represent examples of GRK2 and GRK5 WBs. The same blots were stripped and reprobed with anti-γ-tubulin (γ-Tub) as shown below. Bar graphs show the corresponding semiquantitative analysis (mean ± SE). #p < 0.01 indicates the significance of the Tg age groups compared with the NTg age groups by two-way ANOVA. *p < 0.05 and **p < 0.01 indicate the significance of the mean compared with the corresponding 8 week groups. C, D (representative blots) and E, F (corresponding semiquantitative analysis) show analysis of subcellular distribution of GRK2 and GRK5 in the Tg-CRND8 and NTg mice. Arrows indicates monomeric GRK2 or GRK5; arrowheads point to the non-monomeric GRKs (high-molecular-weight bands for GRK2 and low-molecular-band for GRK5). M, Membrane; C, cytosol. For semiquantitative analysis, GRK levels in different subcellular fractions and each individual age group were standardized against the means of the corresponding NTg and were expressed as fold changes over the means of NTg. *p < 0.05 and **p < 0.01 indicate the significance levels of the means compared with the corresponding NTg fraction.
When subcellular fractions were analyzed, we found that, compared with the NTg mice, both GRK2 and GRK5 in the membrane fraction were significantly decreased in Tg-CRND8 mice beginning as early as 7–8 weeks of age (p < 0.05 for both GRK2 and GRK5), whereas increase of GRK2 (p < 0.01) and GRK5 (p < 0.05) in the cytosol was found even earlier, beginning at 5.5 weeks of age (Fig. 7C–F). Moreover, this reverse correlation of GRK subcellular distribution in the membrane and cytosol fractions persisted in later age groups. In addition, analysis of the subcellular fractions also indicated that both the high-molecular-weight GRK2 and the low-molecular-weight GRK5 bands that were observed in the total cortical protein extracts appeared limited in the cytosolic fraction. In other words, the membrane GRKs appeared to remain monomeric all of the time, and the reduction of the membrane GRKs in the Tg mice, although later than the increase of the cytosolic GRKs, may have more important functional implications, at least in the sense of GPCR desensitization.
Tg-CRND8 mice encode a double-mutant form of APP695 (amyloid precursor protein) (KM670/671NL + V717F) under the control of the PrP (prion protein) gene promoter and are a model of early-onset AD (Chishti et al., 2001). These mice display increased Aβ accumulation in brains with significant elevation beginning at 5.5 weeks of age for both PBS- and SDS-soluble Aβ1-42 and for SDS-soluble Aβ1-40, although PBS-soluble Aβ1-40 becomes significantly elevated at 10–11 weeks of age (G. T. Wong, unpublished data). Approximately 1 week after a sharp increase of brain Aβ1-42 at 10 weeks of age, the cognitive decline (“disease” onset) becomes significant, whereas consistent appearance of amyloid plaques in all animals is even later, at ∼13 weeks of age (Chishti et al., 2001). As shown above, the abnormal GRK subcellular distribution took place as early as 5.5 weeks in Tg-CRND8 mice and became more skewed with increased age, suggesting that the GRK abnormality observed in vivo was associated with the very early increase of brain-soluble Aβ levels. Moreover, because changes in both Aβ levels and GRK abnormality in the Tg mice occurred before the cognitive decline (disease onset at ∼11 weeks of age), these in vivo observations imply that the GRK abnormality may be an early pathogenetic event manifesting at prodromal and early stages of AD that is closely associated with very early accumulation of brain-soluble Aβ.
Discussion
This study was initiated by revealing that two phases of dose-dependent Aβ effects existed in cultured microglial cells. One phase involves Aβ, in the micromolar range, directly inducing microglial TNF-α release as demonstrated previously (Meda et al., 1995). In addition to this known effect produced directly by Aβ, we discovered that, in the subthreshold nanomolar range, soluble Aβ, although insufficient to directly induce TNF-α release, can potentiate, in a dose-dependent manner, TNF-α release induced by other microglial activators, preferentially those that do so via GPCRs. Microglia-mediated inflammation is an important component of AD pathology (McGeer and McGeer, 2001). We showed recently that the ultimate coagulation factor, thrombin, a serine protease with elevated levels in AD brains (Akiyama et al., 1992), can activate microglial cells (as demonstrated by TNF-α induction, inducible nitric oxide synthase, and CD40 upregulation, etc.) via activation of G-protein-coupled PARs (Suo et al., 2002, 2003a). In addition, relevant to AD, the excitotoxic neurotransmitter glutamate can also activate microglial cells (Taylor et al., 2002), likely primarily through activation of G-protein-coupled metabotropic glutamate receptors (mGluRs), because we found that MCPG [(±)-amino-4-carboxy-methyl-phenylacetic acid] (a nonselective mGluR antagonist) reduced glutamate-induced TNF-α by 83.7 ± 12.1%, whereas MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate] (an NMDA receptor antagonist) reduced glutamate-induced TNF-α by 19.4 ± 8.3% in N9 microglial cells (our unpublished data). Therefore, although many other factors (i.e., cytokines, chemokines, and CD40 ligand, as well as anti-inflammatory agents, etc.) may also influence microglial activation status, the use of thrombin and glutamate to study the Aβ-potentiated microglial activation not only provides an in vitro model for GPCR-related mechanistic studies but also has direct relevance to further understanding the AD pathogenesis.
Previous studies pointed out that the signal transduction system in AD brain displays extensive hyperactivity (Saitoh et al., 1993), particularly related to various GPCRs (Joseph et al., 1993). More specifically, several authors indicated that the locus of the signal transduction deficits in AD appears to be at the “receptor–G protein interface” (Joseph et al., 1993; Fowler et al., 1996), although particular responsible molecules remain to be identified. In this study, we demonstrated that subthreshold Aβ pretreatment in vitro induced membrane (functional) GRK reduction and cytosol GRK accumulation, which led to retarded GPCR desensitization and prolonged GPCR signaling, and resulted in cellular hyperactivity to GPCR agonists. Moreover, we found that, in the Tg-CRND8 mouse brains, although total GRK5 levels did not change significantly, total GRK2 levels increased age dependently in both the Tg and NTg brains. Moreover, the increase in total GRK2 levels in the Tg mice was more dramatic than that in the NTg mice and became statistically significant at 10 weeks of age, which was 1 week before the cognitive decline or “disease onset.” Furthermore, both GRK2 and GRK5 in the Tg mice displayed abnormal subcellular distributions compared with that in the NTg mice. The abnormality mainly appeared as reduced membrane GRK2/5 and increased cytosol GRK2/5. More importantly, the abnormal GRK subcellular distribution occurred several weeks before the cognitive decline, and the GRK changes appeared to be closely associated with very early accumulation of soluble Aβ in the brains.
Aβ is generated in soluble forms, principally composed of oligomers (Walsh et al., 2002). As characterized previously, freshly solubilized Aβ peptides used in previous and the current study were primarily composed of soluble Aβ oligomers or Aβ conformational intermediates (Crawford et al., 1998b). Therefore, the effects and the molecular mechanisms revealed here may mainly apply to soluble Aβ oligomers or protofibrils rather than insoluble Aβ aggregates. In agreement with the in vitro findings, the GRK abnormality revealed in the Tg-CRND8 mice was closely associated with the mild to moderate Aβ accumulation at the preplaque stage (Chishti et al., 2001), suggesting that the GRK abnormality is associated with early pathogenic events in the disease process.
GRKs belong to a relatively new family of protein kinases, and most of the members were identified in the last decade or so (Hisatomi et al., 1998; Pitcher et al., 1998). Of the seven known GRK isoforms, GRK1 and GRK7 are expressed almost exclusively in retina and GRK4 appears limited to testis, whereas GRK2/3 and GRK5/6 are ubiquitously expressed, including the brain (Pitcher et al., 1998; Kohout and Lefkowitz, 2003). This study focused on the two most widely studied isoforms, GRK2 and GRK5, and it provided evidence of their abnormality in AD. However, whether other isoforms, such as GRK3 and GRK6, are also disturbed in AD remains to be clarified. In addition, the specificity of GRKs in the regulation of desensitization of a large variety of GPCRs has been a common concern in the field of GRKs (Kohout and Lefkowitz, 2003). The current understanding is that, although there are only seven known GRKs and thousands of GPCRs, desensitization of GPCRs by GRKs is specifically regulated in an agonist-dependent manner (Hisatomi et al., 1998; Pitcher et al., 1998). In addition to GRKs, other protein kinases, such as cAMP-dependent protein kinase and protein kinase C, can directly regulate GPCR desensitization as well (Kohout and Lefkowitz, 2003). More convincing evidence that supports the relative specificity of GRKs to GPCRs came from genetically altered mice. For example, GRK5-deficient mice display phenotypes mainly relevant to an impaired desensitization in cholinergic but not dopaminergic neuronal activities (Gainetdinov et al., 1999), whereas GRK6-deficient mice primarily show phenotypes relevant to an impaired desensitization in dopaminergic but not cholinergic neuronal activities (Gainetdinov et al., 2003). Therefore, dysfunction of a particular GRK member is likely to cause impaired desensitization of a specific spectrum of GPCRs rather than all or most GPCRs nonspecifically.
As mentioned above, we used the thrombin-induced microglial TNF-α release as an in vitro model to study the mechanisms underlying the effects of subthreshold soluble Aβ. There is no doubt that thrombin, and its several PARs, are beginning to be viewed as important injury and proinflammatory modulating factors that interact and intersect with inflammatory cascades in the CNS (Festoff, 2003; Suo et al., 2004). However, our finding that subthreshold soluble Aβ induced a GRK abnormality and cellular hyperactivity to GPCR activators may not be limited to thrombin signaling on microglial cells. In addition to microglial cells, glutamate is known to be directly toxic to HT22 hippocampal neuronal cells via activation of its metabolic GPCRs (Stanciu et al., 2000). We found recently that thrombin can induce rapid tau hyperphosphorylation and aggregation and delayed cell death in the HT22 hippocampal neurons through activation of G-protein-coupled PAR1 and PAR4 (Suo et al., 2003b). When the HT22 cells are pretreated with a nontoxic subthreshold dose (500 nm) of soluble Aβ1-42 for 5 min, both glutamate- and thrombin-induced HT22 cell death can be strongly potentiated (our unpublished data). Moreover, transgenic mice overproducing Aβ have increased vulnerability to glutamate cytotoxicity (Sandhu et al., 1993) and traumatic brain injury (Smith et al., 1998). Therefore, additional investigations should be able to determine whether the subthreshold Aβ-induced GRK abnormality plays a significant role in mediating the increased neuronal vulnerability to glutamate–thrombin toxicity induced by Aβ. Furthermore, we showed previously that subthreshold soluble Aβ enhances vasoconstriction induced by phenylephrine or ET-1 (Thomas et al., 1996; Crawford et al., 1998a), and this Aβ vasoactivity in vivo may be associated with the cerebral blood flow reduction frequently observed in early stages of AD patients (Suo et al., 1998a, 2000; Niwa et al., 2002). Because receptors for both phenylephrine and ET-1 are also GPCRs, it would be interesting to know whether the Aβ-impaired GPCR desensitization by GRKs also underlies the Aβ vasoactivity. Together, the GRK abnormality found in AD could have a fundamental impact on many important aspects of AD pathogenesis, including NFT formation, neurotoxicity, brain inflammation, and cerebral hypoperfusion, etc. Therefore, the potential importance of the GRK abnormality warrants additional investigations to specifically address the above-mentioned questions.
As discussed, although many questions remain to be answered, the fact that the GRK abnormality exists before the disease onset in AD indicates that one of the most important differences between AD and normal individuals may be that AD patients are more vulnerable to a variety of insults, including brain inflammation, stress, and trauma, etc. In other words, as a multifactorial causal disorder, the mild to moderate accumulation of soluble Aβ oligomers at prodromal and early stages of AD may function as the primary pathogenetic factor to cause GRK abnormality and cellular hyperactivity, which predisposes individuals to a vulnerable state without clinical phenotypes. Other factors, such as insoluble Aβ fibrils themselves (induce chemokines), brain trauma (activates thrombin), and stress (releases excess neurotransmitters such as glutamate or vasoconstrictors such as ET-1), as well as subclinical cerebral vascular complications, etc., then function as the secondary insults to trigger the disease onset and/or exaggerate the disease progression. Therefore, the immediate implication for AD prevention and therapy from this study is not only to reduce Aβ accumulation but also to avoid all such secondary insults.
Footnotes
This work was supported by the following: grants to Z.S., B.A.C., and B.W.F. from the Medical Research and Development Service, Department of Veterans Affairs; a grant to Z.S. from the Missouri Alzheimer's Research Fund; a grant to B.A.C. from the Alzheimer's Association; and resources from the Midwest Biomedical Research Foundation. We acknowledge Dr. Robert Lefkowitz (Howard Hughes Medical Institute, Durham, NC) for his critical review of this manuscript. We thank both Drs. Robert Lefkowitz and Jeffrey Benovic (Thomas Jefferson University, Philadelphia, PA) for providing helpful advice for experiments related to GRK analysis. We also thank Drs. Bottani and Ricciardi-Castagnoli (Università Degli Studi Di Milano-Bicocca, Milan, Italy) for their generosity in providing us the N9 microglial cell line.
Correspondence should be addressed to Zhiming Suo, Laboratory for Alzheimer's Disease and Aging Research, Veterans Affairs Medical Center, 4801 Linwood Boulevard, Kansas City, MO 64128. E-mail: zsuo@kumc.edu.
DOI:10.1523/JNEUROSCI.4856-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/243444-09$15.00/0
References
- Akiyama H, Ikeda K, Kondo H, McGeer PL (1992) Thrombin accumulation in brains of patients with Alzheimer's disease. Neurosci Lett 146: 152–154. [DOI] [PubMed] [Google Scholar]
- Barak LS, Warabi K, Feng X, Caron MG, Kwatra MM (1999) Real-time visualization of the cellular redistribution of G protein-coupled receptor kinase 2 and beta-arrestin 2 during homologous desensitization of the substance P receptor. J Biol Chem 274: 7565–7569. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zucker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morrisette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, et al. (2001) Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of APP695. J Biol Chem 15: 21562–21570. [DOI] [PubMed] [Google Scholar]
- Crawford F, Suo Z, Fang C, Sawar A, Su G, Arendash G, Mullan M (1997) The vasoactivity of A beta peptides. Ann NY Acad Sci 826: 35–46. [DOI] [PubMed] [Google Scholar]
- Crawford F, Suo Z, Fang C, Mullan M (1998a) Characteristics of the in vitro vasoactivity of beta-amyloid peptides. Exp Neurol 150: 159–168. [DOI] [PubMed] [Google Scholar]
- Crawford F, Soto C, Suo Z, Fang C, Parker T, Sawar A, Frangione B, Mullan M (1998b) Alzheimer's beta-amyloid vasoactivity: identification of a novel beta-amyloid conformational intermediate. FEBS Lett 436: 445–448. [DOI] [PubMed] [Google Scholar]
- Festoff BW (2003) Proteinase-activated receptors (PARs) in the nervous system: roles in neuroplasticity and neurotrauma. Drug Dev Res 60: 58–64. [Google Scholar]
- Fowler CJ, Garlind A, O'Neill C, Cowburn RF (1996) Receptor-effector coupling dysfunctions in Alzheimer's disease. Ann NY Acad Sci 786: 294–304. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT (1999) Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron 24: 1029–1036. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, Cyr M, Laakso A, Macrae AD, Torres GE, Kim KM, Lefkowitz RJ, Caron MG, Premont RT (2003) Dopaminergic supersensitivity in g protein-coupled receptor kinase 6-deficient mice. Neuron 38: 291–303. [DOI] [PubMed] [Google Scholar]
- Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI (2002) Human toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol 3: 354–359. [DOI] [PubMed] [Google Scholar]
- Hammes SR, Shapiro MJ, Coughlin SR (1999) Shutoff and agonist-triggered internalization of protease-activated receptor 1 can be separated by mutation of putative phosphorylation sites in the cytoplasmic tail. Biochemistry 38: 9308–9316. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19: 8876–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatomi O, Matsuda S, Satoh T, Kotaka S, Imanishi Y, Tokunaga F (1998) A novel subtype of G-protein-coupled receptor kinase, GRK7, in teleost cone photoreceptors. FEBS Lett 424: 159–164. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Mortishire-Smith RJ, Pollack SJ, Shearman MS (1995) The toxicity in vitro of beta-amyloid protein. Biochem J 311: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Cutler R, Roth GS (1993) Changes in G protein-mediated signal transduction in aging and Alzheimer's disease. Ann NY Acad Sci 695: 42–45. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lefkowitz RJ (2003) Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol Pharmacol 63: 9–18. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG (2001) Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging 22: 799–809. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos Jr L, Baron P, Villalba M, Ferrari D, Rossi F (1995) Activation of microglial cells by beta-amyloid protein and interferongamma. Nature 374: 647–650. [DOI] [PubMed] [Google Scholar]
- Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274: 6483–6492. [DOI] [PubMed] [Google Scholar]
- Mok SS, Turner BJ, Beyreuther K, Masters CL, Barrow CJ, Small DH (2002) Toxicity of substrate-bound amyloid peptides on vascular smooth muscle cells is enhanced by homocysteine. Eur J Biochem 269: 3014–3022. [DOI] [PubMed] [Google Scholar]
- Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C (2002) Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol 283: H315–H323. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N (2000) AMPA-kainate sub-types of glutamate receptor in rat cerebral microglia. J Neurosci 20: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D, Town T, Mori T, Parker TA, Humphrey J, Mullan M (2000) Soluble beta-amyloid peptides mediate vasoactivity via activation of a proinflammatory pathway. Neurobiol Aging 21: 183–197. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67: 653–692. [DOI] [PubMed] [Google Scholar]
- Premont RT, Inglese J, Lefkowitz RJ (1995) Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J 9: 175–182. [DOI] [PubMed] [Google Scholar]
- Richardson JA, Burns DK (2002) Mouse models of Alzheimer's disease: a quest for plaques and tangles. ILAR J 43: 89–99. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Horsburgh K, Masliah E (1993) Hyperactivation of signal transduction systems in Alzheimer's disease. Ann NY Acad Sci 695: 34–41. [DOI] [PubMed] [Google Scholar]
- Sallese M, Iacovelli L, Cumashi A, Capobianco L, Cuomo L, De Blasi A (2000) Regulation of G protein-coupled receptor kinase subtypes by calcium sensor proteins. Biochim Biophys Acta 1498: 112–121. [DOI] [PubMed] [Google Scholar]
- Sandhu FA, Porter RH, Eller RV, Zain SB, Salim M, Greenamyre JT (1993) NMDA and AMPA receptors in transgenic mice expressing human beta-amyloid protein. J Neurochem 61: 2286–2289. [DOI] [PubMed] [Google Scholar]
- Schutzer WE, Reed JF, Bliziotes M, Mader SL (2001) Upregulation of G protein-linked receptor kinases with advancing age in rat aorta. Am J Physiol Regul Integr Comp Physiol 280: R897–R903. [DOI] [PubMed] [Google Scholar]
- Shapiro MJ, Weiss EJ, Faruqi RT, Coughlin SR (2000) Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J Biol Chem 275: 25216–25221. [DOI] [PubMed] [Google Scholar]
- Smith DH, Nakamura M, McIntosh TK, Wang J, Rodriguez A, Chen XH, Raghupathi R, Saatman KE, Clemens J, Schmidt ML, Lee VM, Trojanowski JQ (1998) Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol 153: 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri MR, Johnson JW, DeFranco DB (2000) Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem 275: 12200–12206. [DOI] [PubMed] [Google Scholar]
- Suo Z, Fang C, Crawford F, Mullan M (1997) Superoxide free radical and intracellular calcium mediate A beta(1–42) induced endothelial toxicity. Brain Res 762: 144–152. [DOI] [PubMed] [Google Scholar]
- Suo Z, Humphrey J, Kundtz A, Sethi F, Placzek A, Crawford F, Mullan M (1998a) Soluble Alzheimers beta-amyloid constricts the cerebral vasculature in vivo. Neurosci Lett 257: 77–80. [DOI] [PubMed] [Google Scholar]
- Suo Z, Crawford F, Fang C, Paris D, Parker T, Placzek A, Humphrey J, Mullan M (1998b) Phenyl-N-tert-butyl nitrone neutralizes the activities of β-amyloid peptides in both cultured cells and isolated vessels. Alzheimers Rep 1: 381–387. [Google Scholar]
- Suo Z, Su G, Placzek A, Kundtz A, Humphrey J, Crawford F, Mullan M (2000) A beta vasoactivity in vivo. Ann NY Acad Sci 903: 156–163. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW (2002) Participation of protease-activated receptor-1 in thrombin-induced microglial activation. J Neurochem 80: 655–666. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Citron BA, Gao C, Festoff BW (2003a) Persistent protease-activated receptor 4 signaling mediates thrombin-induced microglial activation. J Biol Chem 278: 31177–31183. [DOI] [PubMed] [Google Scholar]
- Suo Z, Wu M, Citron BA, Palazzo RE, Festoff BW (2003b) Rapid tau aggregation and delayed hippocampal neuronal death induced by persistent thrombin signaling. J Biol Chem 278: 37681–37689. [DOI] [PubMed] [Google Scholar]
- Suo Z, Citron BA, Festoff BW (2004) Thrombin: a proinflammatory mediator of central nervous system trauma and neurodegenerative disorders. Current Drug Targets Inflam Allerg, in press. [DOI] [PubMed]
- Taylor DL, Diemel LT, Cuzner ML, Pocock JM (2002) Activation of group II metabotropic glutamate receptors underlies microglial reactivity and neurotoxicity following stimulation with chromogranin A, a peptide up-regulated in Alzheimer's disease. J Neurochem 82: 1179–1191. [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas G, McLendon C, Sutton T, Mullan M (1996) beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380: 168–171. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Yan W, Sandoval R, Naqvi T, Pronin AN, Benovic JL, Malik AB (2000) G protein-coupled receptor kinase-5 regulates thrombin-activated signaling in endothelial cells. Proc Natl Acad Sci USA 97: 7440–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leuven F (2000) Single and multiple transgenic mice as models for Alzheimer's disease. Prog Neurobiol 61: 305–312. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB (1997) Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem 272: 22364–22372. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539. [DOI] [PubMed] [Google Scholar]
- Wang Z, Natte R, Berliner JA, van Duinen SG, Vinters HV (2000) Toxicity of Dutch (E22Q) and Flemish (A21G) mutant amyloid beta proteins to human cerebral microvessel and aortic smooth muscle cells. Stroke 31: 534–538. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Dawes LR, Fisher S, Villa-Komaroff L, Oster-Granite ML, Neve RL (1989) Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science 245: 417–420. [DOI] [PubMed] [Google Scholar]