Abstract
The mechanisms of action of human synthetic and naturally secreted cell-derived amyloid β-peptide (Aβ)1–42 on the induction of long-term potentiation (LTP) were investigated in the medial perforant path to dentate granule cell synapses in hippocampal slices. Synthetic and cell-derived Aβ strongly inhibited high-frequency stimulation (HFS)-induced LTP at peak HFS and 1 hr after HFS. Cell-derived Aβ was much more potent than synthetic Aβ at inhibiting LTP induction, with threshold concentrations of ∼1 and 100–200 nm, respectively. The involvement of various kinases in Aβ-mediated inhibition of LTP induction was investigated by applying Aβ in the presence of inhibitors of these kinases. The c-Jun N-terminal kinase (JNK) inhibitor JNKI prevented the block of LTP induction by both synthetic and cell-derived Aβ. The block of LTP induced by synthetic Aβ was also prevented by the JNK inhibitor anthra[1,9-cd]pyrazol-6(2H)-one, the cyclin-dependent kinase 5 (Cdk5) inhibitors butyrolactone and roscovitine, and the p38 MAP kinase (MAPK) inhibitor 4-(4-fluorophenyl)-2-(4-methylsulfonylphenyl)-5-(4-pyridyl)-1H-imidazole but not by the p42–p44 MAP kinase inhibitor 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene. The group I–group II metabotropic glutamate receptor (mGluR) antagonist 2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-yl)propanoic acid and the mGluR5 antagonist methyl-6-(phenylethynyl)pyridine prevented the block of LTP induction by Aβ. However, theα7 nicotinic ACh receptor antagonist methylcaconatine did not prevent the inhibition of LTP induction by Aβ. These studies provide evidence that the Aβ-mediated inhibition of LTP induction involves stimulation of the kinases JNK, Cdk5, and p38 MAPK after the activation of both the Aβ receptor(s) and mGluR5.
Keywords: amyloid β-protein, LTP, metabotropic glutamate receptors, hippocampus, JNK, Cdk5, p38 MAPK, Alzheimer's disease
Introduction
Excessive cerebral accumulation of the amyloid β-peptide (Aβ), a cleavage product of the β-amyloid precursor protein (APP), has been strongly implicated as a causal factor in Alzheimer's disease (AD). Aβ can exist in a variety of different forms, including monomers, oligomers, protofibrils, and fibrils (Walsh et al., 1997; Bitan et al., 2003). Fibrillar Aβ has been linked to the pathogenesis of AD for many years and is known to have neurotoxic properties (Lorenzo and Yankner, 1994). However, recent studies have suggested a critical role for soluble diffusible forms of Aβ as key effectors of neuronal dysfunction in AD (Hartley et al., 1999; Walsh et al., 1999, 2002; Dahlgren et al., 2002; Selkoe, 2002; Kayed et al., 2003).
There is now considerable evidence that AD represents a synaptic failure, and in particular, that Aβ-induced dysfunction of synaptic plasticity contributes to early memory loss that precedes neuronal degeneration (Small et al., 2001; Selkoe, 2002). A prominent form of synaptic plasticity is long-term potentiation (LTP), a sustained increase in excitatory synaptic transmission. Certain species of synthetic Aβ, especially Aβ1–42, acutely inhibit the induction of LTP in the hippocampus without affecting basal synaptic transmission (Cullen et al., 1997; Lambert et al., 1998; Itoh et al., 1999; Chen et al., 2000; Stephan et al., 2001; Vitolo et al., 2002). In a recent study, we showed that LTP induction in the hippocampus in vivo was inhibited by a cell medium containing naturally secreted human Aβ oligomers but not monomers or fibrils (Walsh et al., 2002). APP transgenic mice also have deficient LTP induction (Chapman et al., 1999; Seabrook et al., 1999). The inhibition of LTP induction by Aβ may represent an early manifestation of AD and particularly its harbinger, minimal cognitive impairment (Cullen et al., 1997; Chapman et al., 1999; Selkoe, 2002). This hypothesis is supported by studies showing that infused synthetic Aβ peptides have an acute amnestic action (Flood et al., 1991; Maurice et al., 1996).
Little is known about the mechanisms involved in Aβ-mediated inhibition of LTP. However, there is evidence that synthetic Aβ can activate certain kinases that are known to be activated in the AD brain, including c-Jun N-terminal kinase (JNK) (Shoji et al., 2000; Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001; Zhu et al., 2001a), p38 MAP kinase (MAPK) (McDonald et al., 1998; Pyo et al., 1998; Hensley et al., 1999; Zhu et al., 2001b), and cyclin-dependent kinase 5 (Cdk5) (Alvarez et al., 1999; Patrick et al., 1999; Ahlijanian et al., 2000; Lee et al., 2000). Activation of metabotropic glutamate receptors (mGluRs) may also be involved in Aβ toxicity, because the mGluR5 antagonist methyl-6-(phenylethynyl)pyridine (MPEP) is protective against such toxicity (Bruno et al., 2000).
The aim of the present studies was twofold. First, we wanted to determine whether naturally secreted Aβ inhibited LTP induction in hippocampal slices and to compare the potency of naturally secreted and synthetic Aβ. Second, to understand further the mechanisms underlying the inhibition of LTP induction by Aβ, we examined the involvement of certain kinases and transmitter receptors, namely JNK, p38 MAPK, and Cdk5, as well as mGluRs.
Materials and Methods
Preparation of slices. All experiments were conducted on transverse slices of the rat hippocampus (males; 3–4 weeks of age; weight, 40–80 gm). The brains were rapidly removed after decapitation and placed in cold oxygenated (95% O2, 5% CO2) media. Slices were cut at a thickness of 350 μm using a Campden Instruments (Lafayette, IN) vibroslice and placed in a storage container with oxygenated medium at room temperature (20–22°C) for 1 hr. The slices were then transferred to a recording chamber for submerged slices and continuously superfused at a rate of 5–6 ml/min at 30–32°C. The control media contained the following (in mm): 120 NaCl, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2.0 MgSO4, 2.0 CaCl2, and 10 d-glucose. All solutions contained 100 μm picrotoxin (Sigma, St. Louis, MO) to block GABAA-mediated activity.
Agents. The following drugs were used: 2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), 1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene (U0126; Tocris Cookson, Ballwin, MO), synthetic human Aβ1–42 (Bachem, Bubendorf, Switzerland), JNKI, roscovitine (Calbiochem, La Jolla, CA), butyrolactone (Affiniti Research Products, Exeter, Devon, UK), 4-(4-fluorophenyl)-2-(4-methylsulfonylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580), anthra[1,9-cd]pyrazol-6(2H)-one (SP600125; Alexis, Grünberg, Germany), methylcaconatine (MLA), and MPEP (Sigma). LY341495, U0126, roscovitine, SP600125, butyrolactone, and SB203580 were dissolved in DMSO, with a maximum final concentration of 0.1% DMSO. JNKI, MPEP, and MLA were prepared in distilled water. Synthetic Aβ1–42 was prepared as a stock solution of 50 μm in ammonium hydroxide (0.1%), stored at –20°C, and then added to physiological medium immediately before each experiment. The actual concentration of Aβ1–42 from one batch of peptide was determined experimentally by quantitative amino acid analysis and found to be 33% lower than the nominal value.
Cell-derived and synthetic Aβ. Naturally secreted cell-derived human Aβ was obtained from cultures of Chinese hamster ovary (CHO) cells stably expressing human APP751, containing the Val717Phe familial AD mutation called 7PA2 cells. CHO and 7PA2 cells were cultured in DMEM with 10% fetal bovine serum, as described previously (Walsh et al., 2000). When confluent, the cells were washed with plain DMEM and then incubated in plain DMEM (4 ml/10 cm2 dish) for 16 hr. At the end of this period, media were harvested and cleared of cells by centrifugation at 500 × g for 10 min. Aliquots of the media were then removed, and the presence of both monomeric and oligomeric Aβ was assessed by immunoprecipitation–Western blotting (ip–wb) and ELISAs. ELISAs were performed as described previously (Walsh et al., 2000). Thus, ELISAs for Aβ1-total (all Aβ species beginning at Asp1) and Aβ1–42 were performed using 3D6 (which recognizes the extreme N terminus of Aβ) as the capture antibody and 6C6 (which binds to the midregion of Aβ) for detection. In detail, nearly confluent (95–100%) 10 cm2 dishes of 7PA2 cells and their corresponding untransfected parental CHO cell line were starved of methionine for 30 min and labeled with 750 μCi of [35S]methionine; their media were then harvested and immunoprecipitated. After electrophoresis on 16% tricine gels, bands were visualized by gel fluorography. For experiments examining the ability of Aβ oligomers to form in conditioned medium (CM) in the absence of cells, 7PA2 cells were pulsed with 1 mCi of [35S]methionine for 2 hr. The labeled medium was harvested, cleared of cells, incubated at either 4 or 37°C for 15 hr in the presence or absence of CHO cells and then immunoprecipitated with the polyclonal antibody R1282. As positive controls, 7PA2 cells were labeled for 17 hr, and their CM was immunoprecipitated as described above. To visualize steady-state levels of Aβ in human CSF and in cultures that were not radiolabeled, we devised an ip–wb protocol that allowed the highly sensitive detection of unlabeled Aβ species. Analysis of CM by ELISA and ip–wb revealed that our ip–wb protocol can readily detect as little as 200 pg of endogenously secreted Aβ. Samples were immunoprecipitated to avoid reconstitution procedures that might alter the assembly form or recovery of Aβ. After immunoprecipitation, samples were electrophoresed on 16% tricine gels and transferred onto 0.2 μm nitrocellulose membranes at 400 mA for 2 hr. Filters were boiled for 10 min in PBS and blocked overnight at 4°C with 5% fat-free milk in 20 mm Tris-HCl, pH 7.4, containing 150 mm NaCl and 0.05% Tween 20 (TBS-T). After washing the membranes in TBS-T, monoclonal antibody 6E10 or a combination of monoclonals 4G8 and 6C6 (each at 1 μg/ml) was used to probe the blots. Bound antibody was visualized using horse-radish peroxidase-conjugated anti-mouse Ig (at 1:40,000) (Jackson ImmunoResearch, West Grove, PA) and ECL Plus detection (Amersham Biosciences, Arlington Heights, IL).
Electrophysiological techniques. Standard electrophysiological techniques were used to record field potentials. Presynaptic stimulation was applied to the medial perforant pathway of the dentate gyrus using a bipolar insulated tungsten wire electrode, and field EPSPs were recorded at a control test frequency of 0.033 Hz from the middle one-third of the molecular layer of the dentate gyrus with a glass microelectrode. The outer blade of the dentate gyrus was used in all studies. In each experiment, an input–output curve (afferent stimulus intensity vs EPSP amplitude) was plotted at the test frequency. For all experiments, the amplitude of the test EPSP was adjusted to one-third of the maximum (∼1.2 mV). LTP was evoked by high-frequency stimulation (HFS) consisting of eight trains, eight of each stimuli at 200 Hz, and an intertrain interval of 2 sec, with the stimulation voltage increased during the HFS to elicit an initial EPSP of the train of double the normal test EPSP amplitude.
In experiments involving kinase inhibitors and receptor antagonists, the agents were preperfused over the slices for 60 min before HFS. Control (vehicle alone) and experimental levels of LTP were measured on slices prepared from the same hippocampus. In experiments involving kinase inhibitors, the effect of the kinase inhibitor alone and the effects of the kinase inhibitor applied together with Aβ were also assessed on slices from the same hippocampus.
Recordings were analyzed using pClamp software (Axon Instruments, Foster City, CA). Values are the means ± SEM for n slices. A two-tailed Student's t test was used for statistical comparison.
Results
Synthetic and cell-derived Aβ inhibits induction of LTP in hippocampal slices
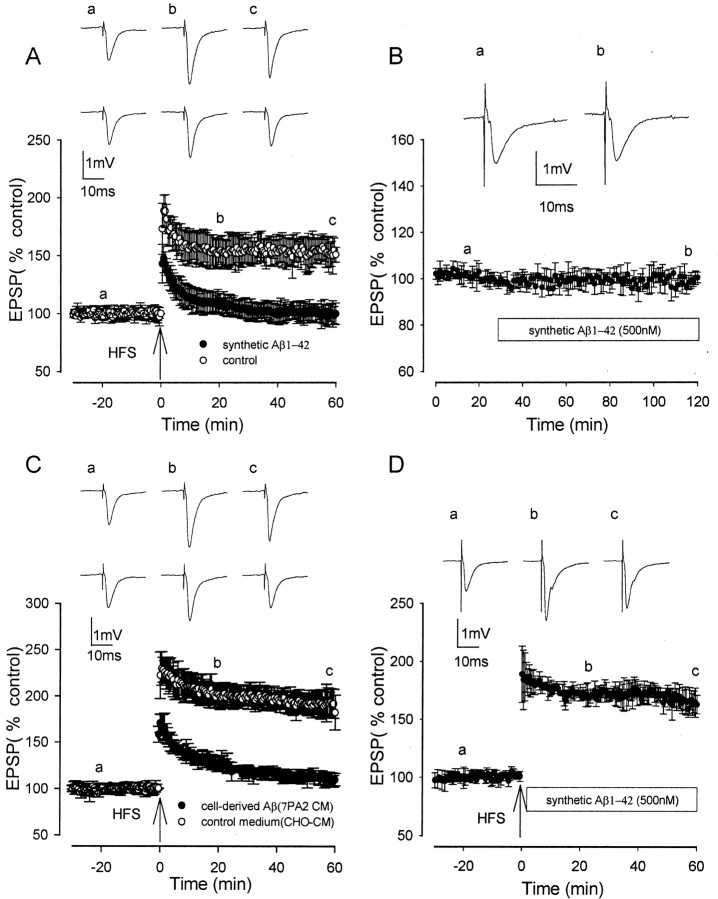
In rat hippocampal slices, HFS induced LTP under control conditions that reached a peak amplitude of ∼100% above baseline immediately after HFS and then slowly declined over the next hour to 50–80% above baseline. The averaged LTP ± SEM measured 188 ± 14, 155 ± 10, and 151 ± 6% at peak, 20 min after HFS, and 60 min after HFS, respectively (p < 0.005; n = 12) (Fig. 1 A).
Figure 1.
Synthetic and naturally secreted cell-derived human Aβ inhibits induction but not expression of LTP. A, Control LTP induced by a single brief HFS (open circles) and LTP induction in the presence of synthetic Aβ (500 nm), applied 60 min before HFS, were significantly reduced compared with controls (filled circles). B, The lack of effect of 500 nm synthetic Aβ on baseline EPSPs. C, Control LTP induction in CHO–CM (open circles) and LTP induction in the presence of 7PA2 CM containing cell-derived Aβ (filled circles) applied 60 min before HFS were significantly reduced compared with controls. D, LTP in experiments in which synthetic Aβ was applied immediately after HFS; LTP expression was not significantly reduced compared with controls. Traces a–c illustrate EPSPs before and 20 and 60 min after HFS, respectively. In A and C, the top set of traces shows the LTP induction in controls, and the bottom set of traces shows the inhibited LTP induction.
Both synthetic and cell-derived Aβ inhibited the induction of LTP, including the early and late phases of LTP. In the presence of synthetic Aβ1–42 (500 nm) perfused for 60 min before HFS, LTP measured 148 ± 12, 108 ± 9, and 100 ± 9% at peak, 20 min after HFS, and 60 min after HFS, respectively, with all three values significantly less than controls (p < 0.005; n = 8) (Fig. 1A). A two-way ANOVA comparing treatment with Aβ with time showed no significant interaction (F = 2.5; p < 0.05), demonstrating a constant inhibition with time. Additional experiments were conducted on the effects of 500 nm Aβ on LTP induction throughout the course of the present study. Three to six interleaved control experiments on the effect of Aβ on LTP induction were performed during the investigation of the effect of each agent on the Aβ-mediated block of LTP induction. These experiments showed an inhibitory effect of Aβ on LTP induction that was very similar to that seen for the initial set of controls, with LTP measuring 151 ± 12, 112 ± 8, and 100 ± 7% at peak, 20 min after HFS, and 60 min after HFS, respectively; in the presence of Aβ, all three values were significantly less than control LTP in the absence of Aβ (p < 0.005; n = 48) (data not shown). Synthetic Aβ (500 nm) did not alter baseline transmission, which measured 98 ± 2% after 90 min of perfusion (p > 0.05; n = 4) (Fig. 1B). A concentration of 200 nm synthetic Aβ also inhibited LTP induction, which measured 176 ± 8, 131 ± 6, and 115 ± 7% at peak, 20 min after HFS, and 60 min after HFS, respectively (p < 0.05; n = 5) (data not shown). However, 100 nm Aβ did not significantly inhibit LTP induction, with LTP measuring 192 ± 23, 155 ± 18, and 146 ± 10% at peak, 20 min after HFS, and 60 min after HFS, respectively (p > 0.05; n = 5) (data not shown).
Synthetic Aβ1–42 assembles into a variety of structures in aqueous buffers, including low N-oligomers, Aβ-derived diffusible neurotoxic soluble ligands (ADDLs), protofibrils, and fibrils (Walsh et al., 1997; Lambert et al., 1998; Bitan et al., 2003). The solutions of synthetic Aβ used in this study contained a mixture of these assemblies. To determine whether soluble forms of Aβ contributed to the inhibition of LTP in vitro, we tested the effect of 7PA2 CM, which contains only Aβ monomer and low N-oligomers and is free of fibrils and protofibrils (Walsh et al., 2002). Conditioned medium containing cell-derived Aβ was collected from 7PA2 cells. The concentration of Aβ peptide as measured by Aβ1-total ELISA (see Materials and Methods) in serum-free conditioned medium ranged from 3 to 6 pg/ml, in agreement with that found in our previous studies (Walsh et al., 2000, 2002). CHO–CM, which does not contain human Aβ, was used as a negative control (see Materials and Methods). All 7PA2 and CHO–CM samples were analyzed by immunoprecipitation–Western blotting and gave consistent results in which Aβ monomer and oligomers were detected in 7PA2 CM but absent in CHO–CM. The medium from the cell cultures was diluted with DMEM before perfusion onto the slices.
Control LTP in CHO–CM devoid of cell-derived Aβ was not significantly different from LTP in physiological medium, measuring 223 ± 18, 192 ± 13, and 188 ± 10% at peak, 20 min after HFS, and 60 min after HFS, respectively (Fig. 1C)(n = 5). However, in the presence of cell-derived naturally secreted Aβ diluted threefold in DMEM (measured Aβ concentration was 1.1 nm) and perfused for 60 min before HFS, LTP induction was inhibited, measuring 170 ± 10, 127 ± 6, and 110 ± 7% at peak, 20 min after HFS, and 60 min after HFS, respectively. All three values were significantly less than control LTP in normal bath media or CHO–CM (p < 0.005) (Fig. 1C)(n = 5). Cell-derived Aβ diluted fivefold also inhibited LTP induction, although to a lesser extent than the threefold dilution, measuring 192 ± 11, 153 ± 5, and 135 ± 7% at peak, 20 min after HFS, and 60 min after HFS, respectively (n = 5; p < 0.01) (data not shown).
To investigate the effect of Aβ on the expression of LTP, synthetic Aβ was perfused immediately after HFS. The expression of LTP after a single HFS was not inhibited by Aβ, with LTP measuring 163 ± 8% at 60 min after HFS (p > 0.05; n = 4) (Fig. 1D).
The inhibition of LTP by synthetic and cell-derived Aβ is prevented by inhibitors of JNK
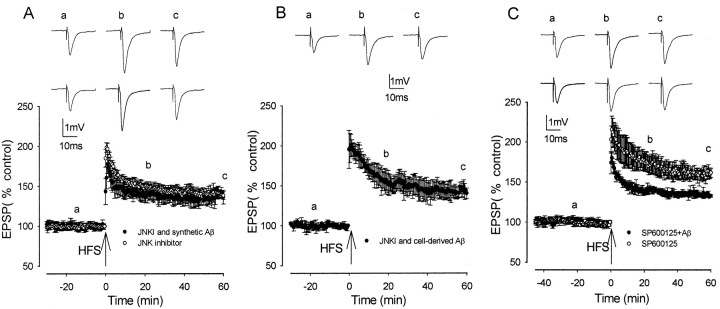
The JNK group of protein kinases is a subgroup of the MAP kinase family, which is known to be activated by cellular stresses, including synthetic Aβ (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001). Activation of JNK occurs in the AD brain (Shoji et al., 2000; Zhu et al., 2001a). To assess the involvement of JNK in the inhibitory effects of Aβ on LTP induction, two JNK inhibitors, JNKI and SP600125, were studied. The inhibitor JNKI, based on amino acids 143–163 of the JNK-binding domain of the JNK scaffolding protein JNK-interacting protein-1, has been shown to interact directly and inhibit JNK (IC50, ∼0.5 μm) but does not inhibit the activities of the related extracellular signal-regulated kinase (ERK) and p38 MAPKs (Bonny et al., 2001; Barr et al., 2002).
LTP induction was not altered by JNKI (2 μm) perfused alone, measuring 198 ± 11, 150 ± 4, and 138 ± 9% at peak, 20 min after HFS, and 60 min after HFS, respectively (n = 5; p > 0.05) (Fig. 2A). These values of LTP in JNKI are not significantly different from those of control LTP in the normal physiological medium. However, JNKI prevented the inhibition of LTP by synthetic Aβ, with LTP measuring 178 ± 12, 147 ± 7, and 136 ± 5% (n = 6) at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were significantly greater than the values of LTP induction in the presence of synthetic Aβ alone (p < 0.01) and were not significantly different from control LTP (p > 0.05) (Fig. 2A). JNKI also prevented the inhibition of LTP induction by cell-derived Aβ, with LTP measuring 200 ± 14, 154 ± 7, and 140 ± 7% (n = 5) at peak, 20 min after HFS, and 60 min after HFS, respectively; in the presence of JNKI, values were increased significantly compared with those in the presence of cell-derived Aβ alone (p < 0.01) and were not significantly different from control LTP in CHO–CM (p > 0.05) (Fig. 2B) (n = 5).
Figure 2.
Aβ-mediated inhibition of LTP induction is prevented by JNK inhibitors. A, LTP induction in JNKI (open circles) and in 500 nm synthetic Aβ plus JNKI (filled circles) was not significantly inhibited. B, LTP induction in naturally secreted cell-derived human Aβ plus JNKI was not significantly inhibited. C, LTP induction in the JNK inhibitor SP600125 (open circles) and in synthetic Aβ plus SP600125 (filled circles) was significantly increased compared with inhibited LTP induction in Aβ. Traces a–c illustrate EPSPs before and 20 and 60 min after HFS, respectively. In A and C, the top set of traces shows the LTP induction in the kinase inhibitor alone, and the bottom set of traces shows the LTP induction in the kinase inhibitor plus Aβ.
To confirm these results, a second JNK inhibitor was examined. SP600125 is a potent (Ki = 0.19 μm) ATP-competitive JNK inhibitor based on an anthrapyrazalone series with a >20-fold selectivity over a range of other kinases, including ERK, p38, PKA, and PKC (Bennett et al., 2001). Perfused alone, SP600125 (20 μm) did not significantly alter LTP induction, which measured 215 ± 17, 176 ± 10, and 161 ± 5% at peak, 20 min after HFS, and 60 min after HFS, respectively (n = 5; p > 0.05) (Fig. 2C). LTP induction was also not altered by 0.1% DMSO alone, the vehicle used for SP600125 and certain other compounds (see Materials and Methods), with LTP measuring 203 ± 11, 164 ± 17, and 152 ± 13% (n = 5; p > 0.05) at peak, 20 min after HFS, and 60 min after HFS, respectively (data not shown). Similar to JNKI, SP600125 prevented the inhibition of LTP by synthetic Aβ, with LTP measuring 180 ± 6, 137 ± 2, and 133 ± 2% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were increased significantly compared with the values of LTP induction in the presence of synthetic Aβ alone (n = 5; p < 0.05) (Fig. 2C). However, at the concentration used (20 μm), SP600125 did not completely reverse the inhibition of LTP by Aβ, with the values of LTP induction in SP600125 and Aβ being significantly lower than in SP600125 alone (p < 0.05).
The inhibition of LTP by synthetic Aβ is prevented by inhibitors of Cdk5
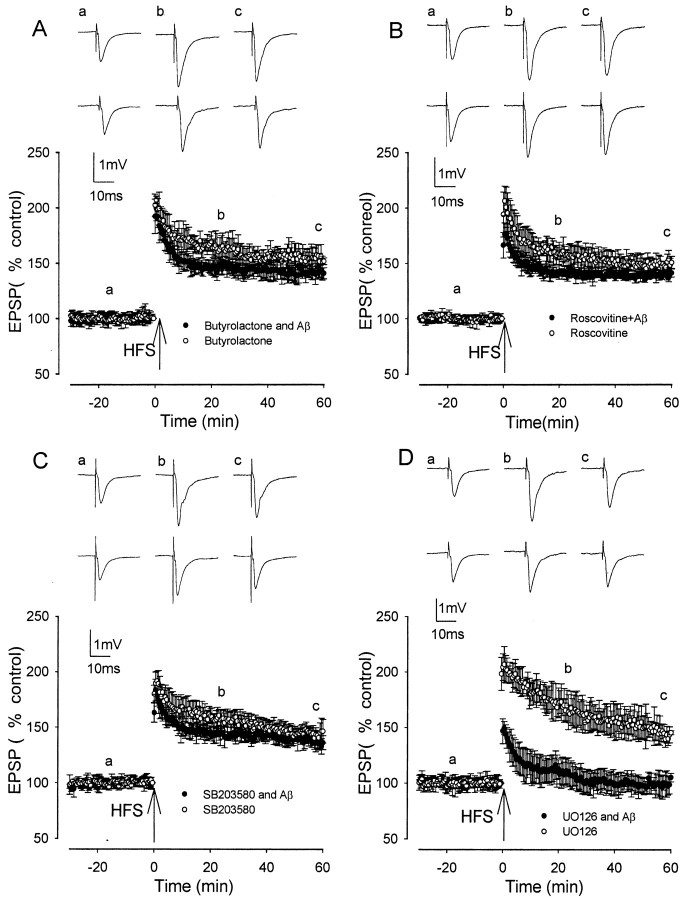
Cdk5 is a Ser–Thr kinase that has important general roles in phosphorylating cell cycle and cytoskeletal proteins. Activation of Cdk5 by binding to its regulatory subunit p35 to form the p35–Cdk5 complex is essential for neuronal development and plasticity (Dhavan and Tsai, 2001). There is compelling evidence that inhibition of the activity of Cdk is detrimental to survival of neurons (Nguyen et al., 2002), and Cdk5 has been implicated in Alzheimer's pathology (Patrick et al., 1999; Ahlijanian et al., 2000). To determine the involvement of Cdk5 in the inhibitory effects of Aβ on LTP induction, two inhibitors of Cdk5, butyrolactone and roscovitine, were studied. Butyrolactone and roscovitine are potent inhibitors of Cdk5, with IC50 values of 0.49 μm (Liu et al., 2001) and 0.16 μm (Knockaert et al., 2002), respectively.
Both butyrolactone and roscovitine reversed the inhibitory effect of synthetic Aβ on LTP induction. Butyrolactone alone, perfused 60 min before HFS, did not alter LTP induction, which measured 192 ± 9, 160 ± 7, and 147 ± 9% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were not significantly different from control LTP (n = 6; p > 0.01) (Fig. 3A). However, butyrolactone prevented the inhibition of LTP induction by synthetic Aβ. Thus, LTP induction measured 184 ± 10, 145 ± 6, and 136 ± 6% at peak, 20 min after HFS, and 60 min after HFS, respectively; in the presence of butyrolactone, values were increased significantly compared with those in the presence of synthetic Aβ alone (p < 0.05) and were not significantly different from control LTP (n = 6; p > 0.05) (Fig. 3A).
Figure 3.
The Aβ-evoked inhibition of LTP induction is prevented by the Cdk5 inhibitors butyrolactone and roscovitine and the p38 MAP kinase inhibitor SB203580 but not the p42–p44 MAP kinase inhibitor U0126. A, LTP induction in butyrolactone (open circles) and in the presence of butyrolactone plus 500 nm synthetic Aβ (filled circles) was not significantly inhibited. B, LTP induction in roscovitine (open circles) and in the presence of roscovitine plus 500 nm synthetic Aβ (filled circles) was not significantly reduced compared with controls. C, LTP induction in SB203580 (open circles) and in 500 nm synthetic Aβ plus SB203580 (filled circles) was not significantly reduced compared with controls. D, LTP induction in U0126 (open circles) and in 500 nm synthetic Aβ plus U0126 (filled circles) was significantly reduced compared with controls. Traces a–c illustrate EPSPs before and 20 and 60 min after HFS, respectively. The top set of traces shows the LTP induction in the kinase inhibitor alone, and the bottom set of traces shows the LTP induction in the kinase inhibitor plus Aβ.
Roscovitine alone, perfused 60 min before HFS, did not alter LTP induction, which measured 194 ± 20, 159 ± 10, and 150 ± 5% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were not significantly different from control LTP (p > 0.01; n = 6) (Fig. 3B). However, roscovitine prevented the inhibition of LTP induction by synthetic Aβ, with LTP induction measuring 176 ± 16, 142 ± 7, and 142 ± 4% (n = 6) at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were increased significantly compared with the values of LTP induction in the presence of synthetic Aβ alone (p < 0.05) and were not significantly different from LTP in roscovitine alone (p > 0.05) (Fig. 3B).
The inhibition of LTP by synthetic Aβ is prevented by inhibition of p38 MAP kinase but not p42–p44 MAP kinase
p38 MAPK and p42–p44 MAPK are distinct subgroups of the MAP kinase family. The p38 MAPK subgroup is well known to be involved in inflammation and cell death (Ono and Han, 2000), whereas p42–p44 MAPKs have been characterized extensively as a central component of signal transduction pathways stimulated by growth-related stimuli. To determine whether the inhibitory effects of Aβ on LTP induction are mediated via activation of p38 or p42–p44 MAPKs, the effect of applying synthetic Aβ in the presence of inhibitors of these kinases was determined.
SB203580 is a highly selective p38 MAP kinase inhibitor with an IC50 of 34 nm (Lee et al., 1994). Applied alone, SB203580 (1 μm) did not alter LTP induction, which measured 192 ± 9, 160 ± 7, and 147 ± 9% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were not significantly different from control LTP (n = 6; p > 0.005) (Fig. 3C). However, SB203580 prevented the inhibition of LTP induction by synthetic Aβ. Thus, LTP induction measured 184 ± 10, 145 ± 6, and 136 ± 6% at peak, 20 min after HFS, and 60 min after HFS, respectively; in the presence of SB203580, values were increased significantly compared with the values of LTP induction in the presence of synthetic Aβ alone (n = 6; p < 0.005) and were not significantly different from control LTP (p > 0.05) (Fig. 3C).
The effect of inhibition of p42–p44 MAP kinase was investigated using the MAP kinase kinase (MEK) inhibitor U0126 (5 μm). This concentration of U0126 is known to completely block both basal and stimulus-induced activation of p42–p44 MAP kinase in hippocampal slices (Roberson et al., 1999). Applied alone, U0126 did not alter LTP induction, which measured 207 ± 12, 172 ± 13, and 146 ± 7% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were not significantly different from those of control LTP induction (n = 5; p > 0.05) (Fig. 3D). Although LTP induced by a brief HFS in rat CA1 is inhibited by MEK inhibitors (Selcher et al., 2003), we find that in the rat dentate gyrus, only LTP induced by prolonged stimulation is sensitive to MEK inhibitors (our unpublished observations), which parallels the situation found in mice (Winder et al., 1999; Watabe et al., 2000), although LTP induced by prolonged stimulation is sensitive to such inhibitors (Winder et al., 1999; Watabe et al., 2000). U0126 did not prevent the inhibition of LTP by Aβ, with LTP measuring 152 ± 12, 106 ± 6, and 97 ± 3% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were not significantly different from those in Aβ alone (p > 0.05) (Fig. 3D).
The inhibition of LTP by Aβ involves activation of mGluR5 but not the α7 nicotinic receptor
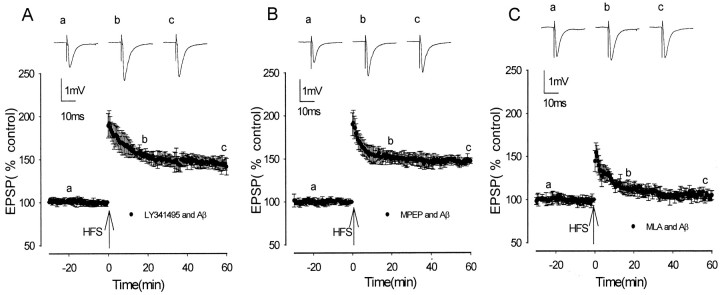
To identify whether the inhibitory action of Aβ on LTP induction is mediated via activation of the specific transmitter receptors mGluR5 or the α7 nicotinic ACh receptor (nAChR), synthetic Aβ was applied in the presence of selective antagonists of these receptors.
LY341495 has been shown to be a selective group I–group II mGluR antagonist (Fitzjohn et al., 1998; Kingston et al., 1998). This antagonist does not inhibit the induction of LTP in CA1 (Fitzjohn et al., 1998) or, as determined in previous experiments from this laboratory, in the medial perforant path of the dentate gyrus (Rush et al., 2002). LY341495 was found to prevent the Aβ-mediated inhibition of LTP induction. In the presence of LY341495 (10 μm) and Aβ, LTP measured 192 ± 14, 149 ± 5, and 147 ± 3% at peak, 20 min after HFS, and 60 min after HFS, respectively, with values significantly increased from the values in Aβ alone (n = 5; p < 0.05) but not significantly different from control LTP (p > 0.05) (Fig. 4A). The selective mGluR5 antagonist MPEP (5 μm) (Gasparini et al., 1999) also prevented the Aβ-mediated inhibition of LTP induction. In the presence of MPEP, LTP measured 192 ± 15, 155 ± 4, and 141 ± 9% at peak, 20 min after HFS, and 60 min after HFS, respectively, with values significantly increased from LTP in Aβ alone (n = 5; p > 0.01) but not significantly different from control LTP (p < 0.05) (Fig. 4 B).
Figure 4.
Activation of mGluR5 but not the α7 nAChR is required for synthetic Aβ inhibition of LTP induction. A, LTP induction in the presence of 500 nm synthetic Aβ plus the group I–II antagonist LY341495 was not significantly reduced compared with controls. B, LTP induction in the presence of 500 nm synthetic Aβ plus the mGluR5 antagonist MPEP was not significantly reduced compared with controls. C, LTP induction in the presence of the α7 nAChR antagonist MLA and 500 nm Aβ was significantly reduced compared with controls. Traces a–c illustrate EPSPs before HFS and 20 min and 60 min after HFS, respectively.
In view of reports that Aβ has been found to bind to and activate α7 nAChR at picomolar concentrations (Dineley et al., 2001a,b), the action of the selective α7 nAChR antagonist MLA was investigated regarding the ability of Aβ to inhibit LTP induction. MLA (1 μm) did not prevent the Aβ-mediated inhibition of LTP, with LTP measuring 154 ± 11, 113 ± 6, and 105 ± 5% at peak, 20 min after HFS, and 60 min after HFS, respectively, and values were not significantly different from those in Aβ alone (n = 5; p > 0.05) (Fig. 4D).
Discussion
In agreement with previous studies, we found that synthetic Aβ1–42 has a strong inhibitory effect on the induction of hippocampal LTP (Cullen et al., 1997; Lambert et al., 1998; Itoh et al., 1999; Chen et al., 2000; Stephan et al., 2001; Vitolo et al., 2002). In addition, we have also shown that cell-derived, naturally secreted Aβ inhibits LTP induction in vitro, extending our previous report on inhibition of LTP in CA1 in vivo (Walsh et al., 2002). When solubilized in aqueous buffers, Aβ1–42 assembles into a variety of structures, including low N-oligomers, ADDLs, protofibrils, and fibrils (Walsh et al., 1997; Lambert et al., 1998; Bitan et al., 2003). The solutions of synthetic Aβ used in this study contain a mixture of these different assemblies. In contrast, the Aβ present in the cell-derived 7PA2 CM is free of fibrils and protofibrils and contained only Aβ monomers and soluble low N-oligomers (Walsh et al., 2002). The finding that cell-derived Aβ inhibits LTP in vitro confirms that soluble assemblies of Aβ can inhibit LTP. Cell-derived, naturally secreted Aβ was much more potent than our synthetic Aβ preparation at inhibiting LTP induction. Thus, the threshold inhibitory concentration for cell-derived Aβ was calculated to be ∼0.7 nm, and that of synthetic Aβ was calculated to be between 100 and 200 nm (the values of the synthetic Aβ would be one-third lower than that stated if all batches of Aβ were identical to that determined; see Materials and Methods). The large difference in potency between cell-derived and synthetic Aβ may be explained by only a very low concentration of biologically active oligomers of Aβ being present in the synthetic Aβ solution.
It is of interest that the very early phase of LTP, including initial peak amplitude measured at 1 min after HFS, was inhibited by cell-derived Aβ in the present in vitro studies. This demonstrates that binding of Aβ to a substrate affects a very early stage of LTP induction, such as the stimulation of kinases involved in LTP induction or early stages of increased AMPA receptor trafficking. In our previous studies in vivo, we had shown that cell-derived Aβ only inhibited LTP beginning ∼1 hr after HFS. This difference between the in vitro and in vivo studies may be attributable to the longer period of pre-exposure to Aβ before HFS in vitro (60 min) compared with in vivo (10 min). Other possible explanations are a slower diffusion time of the Aβ after in vivo cannula injection compared with in vitro perfusion of Aβ or a concentration difference resulting from the fact that the Aβ injected in vivo is diluted by the CSF.
Few studies have explored the mechanisms underlying the Aβ-mediated inhibition of LTP, although recently, one study found that Aβ-mediated inhibition of LTP was reversed by rolipram and forskolin, drugs that enhance cAMP signaling (Vitolo et al., 2002). In the current study, we provide novel evidence that the Aβ-evoked inhibition of LTP is mediated via activation of the kinases JNK, Cdk5, and p38 MAPK. There is previous evidence for the involvement of JNK in the neuropathology of AD. JNK activation has been described around amyloid deposits in AD brains (Anderson et al., 1994, 1996; Shoji et al., 2000; Zhu et al., 2001a), and synthetic Aβ activated the JNK pathway in various neuronal systems (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001). Although Aβ increases expression of the stress-activated gene transcription factor c-Jun (Anderson et al., 1994; Estus et al., 1997), such actions are likely to be too slow to account for the rapid inhibition of LTP by Aβ observed in the present study. Rather, a local synaptic action is more likely to be responsible for the effect of JNK inhibitors on the block of LTP. JNK activation is known to have a local cytoplasmic action leading to inhibition of dendritic growth (Coffey et al., 2000; Savage et al., 2002), and it is possible that inhibition of LTP by Aβ-mediated activation of JNK is an initial stage preceding such inhibition of dendritic growth.
The present evidence for involvement of p38 MAP kinase in the Aβ-mediated inhibition of LTP parallels previous studies showing an increase in p38 MAP kinase activity affected by Aβ in cultured cells and also an increase in MAPK kinase 6, an upstream activator of p38, in susceptible neurons in AD brains (McDonald et al., 1998; Pyo et al., 1998). Activation of JNK and p38 MAPK is likely to result in the inhibition of LTP via inflammatory pathways. A growing body of evidence suggests that Aβ-mediated neurotoxicity involves the production of inflammatory cytokines such as tumor necrosis factor (TNF) and also free radicals and reactive oxygen species (Akama and Van Eldick, 2000). In this regard, activation of JNK and p38 MAPK is known to have a pivotal role in TNF signaling and cell death (Paul et al., 1997). The inhibition of LTP could be a very early indicator of the activation of inflammatory mediators. The Aβ-mediated block of LTP was not prevented by the p42–p44 MAP kinase inhibitor U0126, demonstrating a lack of involvement of p42–p44 MAP kinases. In fact, p42–p44 MAP kinase is known to be required for the induction rather than inhibition of LTP and to be involved in cell survival rather than cell death (Sweatt, 2001).
The present finding that the Cdk5 inhibitors butyrolactone and roscovitine prevent the Aβ-mediated inhibition of LTP parallels recent studies showing that Cdk inhibitors prevent Aβ-induced neurotoxicity (Alvarez et al., 1999; Milton, 2001). Cdk5 has been postulated to have a major role in AD and Aβ-induced neurodegeneration (Patrick et al., 1999; Ahlijanian et al., 2000), with Aβ causing mislocalization and deregulation of Cdk5 by increasing production of its pathogenic activator p25 (Patrick et al., 1999; Lee et al., 2000; Town et al., 2002).
The group I–II mGluR antagonist LY341495 and the mGluR5 antagonist MPEP were found to prevent the Aβ-mediated inhibition of LTP, thus demonstrating the involvement of mGluR5 in the Aβ-mediated inhibition of LTP. Selective antagonism of mGluR5 with MPEP has been shown previously to be neuroprotective against Aβ toxicity in cortical cultures (Bruno et al., 2000), emphasizing the parallels between Aβ-mediated inhibition of LTP and Aβ-mediated neurotoxicity. In addition, the membrane depolarization evoked by relatively high concentrations of synthetic Aβ1–42 was reported to involve activation of group I mGluRs (Blanchard et al., 2002).
A model for Aβ-mediated inhibition of LTP is proposed that involves activation of mGluRs and also the kinases JNK, Cdk5, and p38 MAPK. HFS is known to induce LTP via activation of NMDA receptors and influx of Ca2+. The inhibition of LTP is suggested to occur through a combination of the activation of an unidentified Aβ receptor(s) by Aβ and activation of mGluR5 by l-glutamate. Activation of mGluR5 could occur after l-glutamate is released from presynaptic terminals during HFS; mGluR5 is located perisynaptically on the postsynaptic cell and is known to be activated by spillover of glutamate during HFS. Alternatively, activation of mGluR5 could occur as a result of l-glutamate being released from glial cells, such as microglia after their activation by Aβ. The combined activation of mGluR5 and the Aβ receptor(s) is suggested to stimulate JNK, Cdk5, and p38 MAPK. There is well documented evidence that Aβ stimulates these kinases, as described above, and recent work has shown that group I mGluR activation stimulates Cdk5 (Liu et al., 2001) and p38 MAP kinase (Bolshakov et al., 2000; Rush et al., 2002). The requirement for combined activation of an Aβ receptor and mGluR5 may be necessary for sufficient stimulation of JNK, Cdk5, and p38 MAPK to produce inhibition of LTP. The mechanisms whereby these kinases inhibit LTP induction are unknown and are the subject of ongoing studies.
Antagonism of α7 nAChR did not prevent the Aβ block of LTP, demonstrating a lack of involvement of the α7 nAChR in Aβ-mediated block of LTP in our paradigm. Aβ has been found to bind to and activate α7 nAChR at picomolar concentrations (Dineley et al., 2001a,b). One possible reason for the lack of effect of blocking α7 nAChR on Aβ-mediated inhibition of LTP is that α7 nAChRs have been found to be located only at high density on interneurons in the dentate gyrus and only at very low density on granule cells (Dobelis et al., 2002).
In conclusion, our data demonstrate that Aβ-mediated inhibition of LTP arises from activation of mGluR5 and stimulation of three kinases, JNKI, Cdk5, and p38 MAP kinase. Synaptic plasticity, and especially LTP, is known to be a critical component of the neural mechanisms underlying certain types of learning and memory (for review, see Morris et al., 2003). Our study suggests that intervention using inhibitors of JNK, Cdk5, or p38 MAPK along with mGluR5 antagonists may turn out to ameliorate cognitive deficits in AD patients.
Footnotes
This work was supported by grants from the Wellcome Trust (R.A.) and the National Institutes of Health (D.J.S.).
Correspondence should be addressed to Roger Anwyl, Department of Physiology, Trinity College, Dublin 2, Ireland. E-mail: ranwyl@tcd.ie.
DOI:10.1523/JNEUROSCI.1633-03.2004
Copyright © 2004 Society for Neuroscience 0270-6474/04/243370-09$15.00/0
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD (2000) Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of Cdk5. Proc Natl Acad Sci USA 97: 2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Van Eldick L (2000) β-Amyloid stimulation of inducible nitric oxide synthase in astrocytes is interleukin-1-β and tumor necrosis factor-α-dependent. J Biol Chem 275: 7918–7924. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Toro R, Caceres A, Maccioni RB (1999) Inhibition of tau phosphorylating protein kinase Cdk5 prevents β-amyloid-induced cell death. FEBS Lett 459: 421–426. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, Cummings BJ, Cotman CW (1994) Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer's disease. Exp Neurol 125: 286–295. [DOI] [PubMed] [Google Scholar]
- Anderson AJ, Su JH, Cotman CW (1996) DNA damage and apoptosis in Alzheimer's disease: colocalization with c-Jun immunoreactivity, relationship to brain area, and effect of postmortem delay. J Neurosci 16: 1710–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RK, Kendrick TS, Bogoyevitch MA (2002) Identification of the critical features of a small peptide inhibitor of JNK activity. J Biol Chem 277: 10987–10997. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB (2003) Amyloid β-protein (Aβ) assembly: Aβ-40 and Aβ-42 oligomerize through distinct pathways. Proc Natl Acad Sci USA 7: 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BJ, Thomas VL, Ingram VM (2002) Mechanism of membrane depolarisation caused by the Alzheimer Aβ-42 peptide. Biochem Biophys Res Commun 293: 1197–1203. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, Carboni L, Cobb MH, Siegelbaum SA, Belardetti F (2000) Dual MAP kinase pathways mediate opposing forms of long-term plasticity at CA3-CA1 synapses. Nat Neurosci 3: 1107–1112. [DOI] [PubMed] [Google Scholar]
- Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF (2001) Cell-permeable peptide inhibitors of JNK: novel blockers of β-cell death. Diabetes 50: 77–82. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, O'Kane TM, Zhi-Liang W, Dobrzanski P, Murthy S, Vaught J, Scott R (2001) CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Aβ-induced cortical neuron apoptosis. J Neurochem 77: 849–863. [DOI] [PubMed] [Google Scholar]
- Bruno V, Ksiazek I, Battaglia G, Lukic S, Leonhardt T, Sauer D, Gasparini F, Kuhn R, Nicoletti F, Flor PJ (2000) Selective blockade of metabotropic glutamate receptor subtype 5 is neuroprotective. Neuropharmacology 39: 2223–2230. [DOI] [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TVP, Hyman BT, Younkin SG, Hsiao KK (1999) Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci 2: 271–276. [DOI] [PubMed] [Google Scholar]
- Chen QS, Kagan BL, Hirakura Y, Xie CW (2000) Impairment of hippocampal long-term potentiation by Alzheimer β-peptides. J Neurosci Res 60: 65–72. [DOI] [PubMed] [Google Scholar]
- Coffey ET, Hongisto V, Dickens M, Davis RJ, Courtney MJ (2000) Dual roles for c-Jun N-terminal kinase in developmental and stress responses in cerebellar granule neurons. J Neurosci 20: 7602–7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen WK, Suh Y-H, Anwyl R, Rowan MJ (1997) Block of LTP in rat hippocampus in vivo by β-amyloid precursor protein fragments. NeuroReport 8: 3213–3217. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine Jr WB, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem 277: 32046–32053. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH (2001) A decade of CDK5. Nat Rev Mol Cell Biol 10: 749–759. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe K, Sweatt JD (2001a) β-Amyloid activates the mitogen-activated protein kinase cascade via hippocampal α7 nicotinic acetylcholine receptors: in vitro and in vivo mechanisms related to Alzheimer's disease. J Neurosci 21: 4125–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KT, Bell K, Bui D, Sweatt JD (2001b) β-Amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem 277: 25056–25061. [DOI] [PubMed] [Google Scholar]
- Dobelis P, Staley KJ, Collins AC, Paylor R, Beaudet AL, Dunwiddie TV (2002) α-7 nicotinic-mediated responses in the mouse dentate gyrus. Soc Neurosci Abstr 28: 37.9. [Google Scholar]
- Estus S, Tucker HM, vanRoyen C, Wright S, Brigham EF, Wogulis M, Rydel RE (1997) Aggregated amyloid β-protein induces cortical neuronal apoptosis and concomitant apoptosis pattern of gene expression. J Neurosci 17: 7736–7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Bortolotto ZA, Palmer MJ, Doherty AJ, Ornstein PL, Schoepp DD, Kingston AE, Lodge D, Collingridge GL (1998) The potent mGluR receptor antagonist LY341495 identifies roles for both cloned and novel mGluR receptors in hippocampal synaptic plasticity. Neuropharmacology 37: 1455–1458. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E (1991) Amnestic effects in mice of four synthetic peptides homologous to amyloid β protein from patients with Alzheimer's disease. Proc Natl Acad Sci USA 88: 3363–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçelebi G, Kuhn R (1999) 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGluR5 receptor antagonist. Neuropharmacology 38: 1493–1503. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19: 8876–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye ON, Stewart CA, Geddes J, Markesberry WR, Patel E, Johnson GVW, Bing G (1999) p38 kinase is activated in Alzheimer's disease brain. J Neurochem 72: 2053–2058. [DOI] [PubMed] [Google Scholar]
- Itoh A, Akaike T, Sokabe M, Nitta A, Nabeshima T (1999) Impairment of long-term potentiation in hippocampal slices of β-amyloid-infused rats. Eur J Pharmacol 3: 167–175. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300: 486–489. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD (1998) LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37: 1–12. [DOI] [PubMed] [Google Scholar]
- Knockaert M, Greengard P, Meijer L (2002) Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol Sci 23: 417–425. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95: 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Green D, McNulty D, Blumenthal MJ, Heys RJ, Landvatter SW, Stricker JE, McLaughlin MM, Siemens I, Fisher S, Livi GP, White JR, Adams JL, Young PR (1994) Identification and characterization of a novel protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372: 739–746. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364. [DOI] [PubMed] [Google Scholar]
- Liu F, Ma X-H, Ule J, Bibb JA, Nishi A, DeMaggio AJ, Yan Z, Nairn AC, Greengard P (2001) Regulation of cyclin-dependent kinase 5 and casein kinase 1 by metabotropic glutamate receptors. Proc Natl Acad Sci USA 98: 11062–11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA (1994) β-Amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA 91: 12243–12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Lockart BP, Privat A (1996) Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res 706: 181–193. [DOI] [PubMed] [Google Scholar]
- McDonald DR, Bamberger ME, Combs CK, Landreth GE (1998) β-Amyloid fibrils activate parallel mitogen-activated protein kinase pathways in microglia and THP1 monocytes. J Neurosci 18: 4451–4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton NG (2001) Phosphorylation of amyloid-β at the serine 26 residue by human cdc2 kinase. NeuroReport 12: 3839–3844. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME (2001) β-Amyloid induced neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci 21: 7551–7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O'Carroll C (2003) Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci 358: 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S (2002) Innate immunity: the missing link in neuroprotection and neurodegeneration. Nat Rev Neurosci 3: 216–227. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J (2000) The p38 signal transduction pathway: activation and function. Cell Signal 12: 1–13. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622. [DOI] [PubMed] [Google Scholar]
- Paul A, Wilson S, Belham CM, Robinson CJM, Scott PH, Gould GW, Plevin R (1997) Stress-activated kinases: activation, regulation and function. Cell Signal 9: 403–410. [DOI] [PubMed] [Google Scholar]
- Pyo H, Jou I, Jung S, Hong S, Joe EH (1998) Mitogen-activated protein kinases activated by lipopolysaccharide and β-amyloid in cultured rat microglia. NeuroReport 9: 871–874. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD (1999) The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci 19: 4337–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Wu J, Rowan MJ, Anwyl R (2002) Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high-frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro J Neurosci 22: 6121–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage MJ, Guo Y-G, Ciallella JR, Flood DG, Scott RW (2002) Activation of c-Jun N-terminal kinase and p38 in an Alzheimer's disease model is associated with amyloid deposition. J Neurosci 22: 3376–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Smith DW, Bowery BJ, Easter A, Reynolds T, Fitzjohn SM, Morton RA, Zheng H, Dawson GR, Sirinathsinghji DJ, Davies CH, Collingridge GL, Hill RG (1999) Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 38: 349–359. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD (2003) A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn Mem 10: 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ (2002) Alzheimer's disease is a synaptic failure. Science 298: 789–791. [DOI] [PubMed] [Google Scholar]
- Shoji M, Iwakami N, Takeuchi S, Waragai M, Suzuki M, Kanazawa I, Lippa CF, Ono S, Okazawa H (2000) JNK activation is associated with intracellular β-amyloid accumulation. Brain Res Mol Brain Res 85: 221–233. [DOI] [PubMed] [Google Scholar]
- Small DH, Mok SS, Bornstein JC (2001) Alzheimer's disease and Aβ toxicity: from top to bottom. Nat Rev Neurosci 8: 595–598. [DOI] [PubMed] [Google Scholar]
- Stephan A, Laroche S, Davis S (2001) Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci 21: 5703–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD (2001) The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem 76: 1–10. [DOI] [PubMed] [Google Scholar]
- Town T, Zolton J, Shaffner R, Schnell B, Crescentini R, Wu Y, Zeng J, Delle-Donne A, Obregon D, Tan J, Mullan M (2002) p35/Cdk5 pathway mediates soluble amyloid-β peptide-induced tau phosphorylation in vitro J Neurosci Res 69: 362–372. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabaccji SA, Xu Z, Maraney AC, Connors TJ, Shelanski ML, Greene LA (2001) β-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem 77: 157–164. [DOI] [PubMed] [Google Scholar]
- Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M (2002) Amyloid β-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA 99: 13217–13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB (1997) Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem 272: 22364–22372. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB (1999) Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274: 25945–25952. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ (2000) Detection of intracellular oligomers of amyloid β-protein in cells derived from human brain. Biochemistry 39: 10831–10839. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubuin I, Fadeeva J, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of the Alzheimer amyloid β-protein potently inhibit long-term potentiation in vivo Nature 416: 535–539. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Zaki PA, O'Dell TJ (2000) Coactivation of β-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci 20: 5924–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder DG, Martin KC, Muzzio IA, Rohrer D, Chuschinski AC, Kobilka B, Kandel ER (1999) ERK plays a regulatory role in induction of LTP by theta frequency stimulation and its modulation by β-adrenergic receptors. Neuron 24: 715–726. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Smith MA (2001a) Activation and redistribution of c-Jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer's disease. J Neurochem 76: 435–441. [DOI] [PubMed] [Google Scholar]
- Zhu X, Rottkamp CA, Hartzler A, Sun Z, Takeda A, Boux H, Shimohama S, Perry G, Smith MA (2001b) Activation of MKK6, an upstream activator of p38, in Alzheimer's disease. J Neurochem 79: 311–318. [DOI] [PubMed] [Google Scholar]