Abstract
Background
With the continued demand for new, effective, and safe endodontic therapies, the aim of this study was assessment of efficiency of the ethyl acetate (EthOAc) extract of Tanacetum vulgare (L.) against Candida albicans.
Material/Methods
The antifungal effectiveness of the EthOAc extract of T. vulgare was determined using the agar disk diffusion method. The inhibition zones induced by the EthOAc extract were compared after 5 minutes, 60 minutes, and 24 hours to those induced by standard solutions (2% chlorhexidine, saturated calcium hydroxide, and 2% sodium hypochlorite). Statistical analysis of the results was performed using the Kruskal-Wallis test and one-way ANOVA.
Results
The inhibition zone of chlorhexidine against C. albicans was 30.3–19.3 mm, but in combination with EthOAc extract (100 mg/mL) of T. vulgare, this inhibition was from 32.7–30 mm, indicating that this combination exerted a marked synergistic effect against C. albicans. The inhibition zone of sodium hypochlorite (69.7–65 mm) was higher than the inhibition zone of EthOAc extract and chlorhexidine. The combination of EthOAc extract with sodium hypochlorite resulted in a loss of antifungal activity. Furthermore, the activity of the EthOAc extract against C. albicans was decreased after mixing the extract with dentine at a concentration of 25 mg/50 μL (30.3–20.7 mm). The EthOAc extract did not show a genotoxic effect on lymphocyte cells.
Conclusions
The EthOAc extract of T. vulgare may be a useful tool to discover natural bioactive agents that have antifungal activity against C. albicans and could be used as endodontic therapies.
MeSH Keywords: Antifungal Agents, Candida albicans, Endodontics, Tanacetum
Background
The purpose of root canal treatment is the removal of diseased tissue, eradication of microorganisms within the root canal and the prevention of recontamination [1]. Instrumentation of root canal should be followed by irrigation, which enables removal of necrotic tissue and microorganisms [2]. Endodontic irrigants are applied to increase the antimicrobial effects of cleaning and shaping in endodontics. Due to close contact with the periodontal tissue, root canal irrigant must be biocompatible.
Sodium hypochlorite is an irrigant which is commonly used in endodontics, because of its ability to destroy a large number of microorganisms, but it is unable to remove the smear layer [3]. It also has undesirable features that include allergic potential, tissue toxicity, and a bad taste [4]. Chlorhexidine (CHX) is another irrigant that confers antimicrobial activity and biocompatibility, but has no tissue dissolving capabilities [5]. Calcium hydroxide saturated in water possesses antimicrobial activity and creates a hard tissue barrier, making it a good choice as an intercanal medication. A small part of the flora survives after treatment with root canal medication and there is evidence of the detected fungi in the root canal system [6]. Candida albicans has been observed within root canal and periapical region associated with persistent infections [7,8].
The increased prevalence of resistant strains and the side effects caused by synthetic drugs have challenged researchers to search for herbal therapeutic alternatives. The advantages of plant extracts in healing and treating diseases are well-established. The therapeutic properties of plants are due to their composition being rich in bioactive compounds such as essential oils, polyphenols, flavonoids, or tannins [9]. The essential oils extracted from plants contain terpenes and it is the terpene subclasses, monoterpenes and sesquiterpenes, which have antibacterial, antiseptic, and anti-inflammatory properties [10–12].
Tanacetum vulgare L. is a perennial herb of the Asteraceae family [13]. Studies on rats and mice [14,15] have found that T. vulgare extracts have anti-inflammatory properties. The volatile oil obtained from parts of T vulgare have been shown to have inhibitory effect on germ tube formation in C. albicans [16]. Flavonoids present in this plant may be responsible for these biological effects [15,17]. A few studies have reported the antimicrobial activity of herbal root canal irrigants. The aim of this study was to evaluate T. vulgare as a potential antifungal agent in the inhibition of C. albicans in comparison to sodium hypochlorite, CHX, and calcium hydroxide, and to evaluate its genotoxic effects.
Material and Methods
This paper has been approved by the Ethics Commission (Nr. 2417) and for this study, we used 100 extracted human single-rooted teeth, that were extracted for periodontal and orthodontic reasons. The teeth were stored in 1% Sodium hypochlorite prior to the experiment, and then rinsed and autoclaved at 121°C for 15 minutes. The teeth crowns were removed with a diamond saw (Smart Cut 4002, UKAM, Valencia, CA, USA). After endodontic access, the pulp tissue was removed with barbed broaches. Root canals were enlarged with Endostar E5 (Poldent Co., Warsaw, Poland) according to the manufacturer’s instructions to obtain dentine powder. Powdered dentine was suspended in distilled water to 28 mg per 50-μL aliquot.
Medicaments
The medicaments used in this investigation were calcium hydroxide saturated in water, 2% sodium hypochlorite (ChloraXD, Cerkamed, StatowaWola, Poland), and 2% CHX (Gluco-Chex, Cerkamed, StatowaWola, Poland), ethyl acetate (Sigma Aldrich, Switzerland) and dimethyl formamide (DMF; Sigma Aldrich, Switzerland). Sterile water was used as a negative control.
Plant materials
T. vulgare plants were collected in a Kosovo field (Sharri Mountains) in September 2018. A voucher specimen was deposited in the first author’s laboratory (AK1/2018). The aerial part of T. vulgare was air-dried and finely ground and powdered with a blender. The dried powder was vacuum-packed and stored at −20°C until use. The dried T. vulgare powder (10 g) was extracted twice with 100 mL of ethyl acetate (EthOAc) for 48 hours at room temperature. The extracted suspensions were filtered through Whatman No. 1 filter paper and the filtrates were concentrated to dryness using a rotary evaporator, and then stored at −20°C until further use. For the antibacterial activity assays, the extracts were dissolved in N,N-dimethylformamide (DMF) at a concentration of 100 mg/mL and stored at 4°C as stock solutions.
Bacterial strains and inoculum preparation
The antifungal potency of the plant extract, the endodontic irrigant and the combination of plant extract with endodontic irrigants was evaluated using C. albicans (ATTC 10231, Liofilchem, Roseto degli Abruzzi, Italy).
The antifungal activities of the Soxhlet extracts of plants and the antifungal activities of endodontic irrigants were analyzed by a disk diffusion assay. The suspension of C. albicans was cultured for 48 hours in 1 mL of sterile Sabouraud dextrose broth (Liofilchem, Roseto degli Abruzzi, Italy) at 37°C, then adjusted to a turbidity of 0.5 on the McFarland scale (1.5×108 cells/mL). This culture was used throughout the experiments. Mueller-Hinton agar (10 mL) was poured into Petri dishes, which were then inoculated with strains of yeast by the addition of 0.1 mL of cell culture media.
Sterile filter paper disks loaded with endodontic irrigants, plant extracts, and a combination of endodontic irrigants and plant extracts (10 mg/mL) were placed on top of Mueller–Hilton agar plates. These plates were incubated at 5°C for 2 hours to permit plant extract diffusion, and then they were incubated at 35°C for 24 hours. Disks that were only impregnated with extract solvent were used as a control. Inhibition zones, considered indicative of antibacterial activity, were measured using Vernier calipers and recorded.
Growth inhibition assay to determinate the antifungal activity of the medicament on dentine powder
Aliquots of 50 μL of dentine powder suspension in water were mixed and incubated with 50 μL of the medicaments in sealed test tubes at 37°C for 1 hour or 24 hours, before the addition of yeast. Control groups consisted of 50 μL of sterile water instead of dentine powder. The total volume of the test and control mixtures was 150 μL. All mixtures were incubated at 37°C. Dentine powder/medicament/yeast suspension were mixed with a sterile pipette twice (before the 1-hour sample) or 3 times (before the 24-hour sample) during the incubation. Samples for bacterial culturing (10 μL per sample) were taken from the experimental and control suspensions at 5 minutes, 1 hour, and 24 hours after the addition of yeast. The plates were observed after 5 minutes, 60 minutes, and 24 hours, according to Bulacio et al. [18] (Figure 1). The results were recorded based on the standard disk method according to the Clinical and Laboratory Standards Institute (CLSI, USA) and the European Committee for Antibiotic Sensitivity Testing (EUCAST).
Figure 1.

The diameter of inhibition zone of agents used in the study.
Genotoxicity assay of the medicaments
An in vitro genotoxicity assay was performed in cultured lymphocytes. To prepare the lymphocyte cells, blood (12 mL) was taken from 30 human volunteers. For EthOAc extracts of T. vulgaris (0.5 mL), 4 tests were performed. The time duration of the treatment was 72 hours. For the positive control group, we added glyphosate herbicide (36%) and for the negative control group we added no agent. Blood (1 mL) from human volunteers was taken from heparinized venous blood vessels and transferred into 15-mL tubes containing peripheral blood medium (Sigma) for lymphocyte cultivation. Then, the tubes were incubated for 72 hours at 37°C. After 44 hours of incubation, 3 μg/mL of cytochalasin B was added according to method of Fenech and Morley [19–21], which blocks cytokine production but not karyokinesis and produces binuclear cells within the “parent” cell membranes. All procedures were performed under sterile conditions. After incubation for 72 hours at 37°C, cell cultures were centrifuged for 10 minutes at 1000 rpm. The supernatant was then discarded and the pellet containing lymphocytes was resuspended in 5 mL of hypotonic KCl solution (0.074 M), prewarmed to 37°C, subjecting the cells to hypotonic shock for 10 minutes at room temperature. The cultures were then centrifuged for 10 minutes at 1000 rpm. Again, the supernatant was removed, and the cells were fixed for 20 minutes with fixative solution (3: 1 ratio of absolute ethanol: glacial acetic acid) precooled at 4°C. After centrifuging for 10 minutes at 1000 rpm, the supernatant was removed and further centrifuged until the cell pellet became opaque.
After centrifugation and fixation, the cells were finally resuspended in 1 mL of fresh fixation solution. Six extract preparations were placed onto pre-chilled glass slides, which was sufficient for the counting of 500 LBN-Binuclear lymphocytes and were dried at room temperature for several hours. The preparations were stained with 10% Giemsa solution for 20 minutes, then rinsed with distilled water.
Statistical analysis
Data were processed using the SPSS package 22.0. Testing of quantitative data between groups with a normal distribution was performed with one-way ANOVA, while testing of groups with no normal distribution was performed with the Kruskal-Wallis test. A value of P<0.05 was considered statistically significant.
Results
The mean values of fungal growth inhibition produced by EthOAc extract of T. vulgare, CHX, sodium hypochlorite and calcium hydroxide after 5 minutes, 60 minutes, and 24 hours are shown in Table 1. All of the experimental groups were statistically different (P<0.05) for the microorganism tested. After 5 minutes, when the EthOAc extract was used in combination with sodium hypochlorite in the dentin powder-free solution there was no inhibition zone, whereas when the EthOAc extract was used alone there was a 38.7 mm inhibition zone and when sodium hypochlorite was used alone there was a large inhibition zone of 69.7 mm. At this timepoint, there was a statistically significant difference in the growth of C. albicans depending on the material used. After 60 minutes, when the EthOAc extract was used in combination with sodium hypochlorite there was no increase in the inhibition zone, when the extract was used alone there was a decrease in the inhibition zone to 38 mm, and when sodium hypochlorite was used alone there was a decrease in the inhibition zone to 68.3 mm. At this timepoint, there was a statistically significant difference in the growth of C. albicans depending on the preparation used (P=0.018). After 24 hours, when the EthOAc extract was used in combination with sodium hypochlorite in the dentin powder-free solution there was no inhibition zone, when the extract was used alone there was a 25.0 mm inhibition zone, when sodium hypochlorite was used alone there was a smaller inhibition zone of 65.0 mm, and when the EthOAc extract was used in combination with Ca(OH)2 there was no C. albicans inhibition zone. At this timepoint, there was a statistically significant difference in the growth of C. albicans depending on the preparation used (P=0.018).
Table 1.
Influence of EthOAc extract of Tanacetum vulgare with and without chemicalsmMedicaments (chlorhexidine, calcium hydroxide, sodium hypochlorite) in the growth of Candida albicans without dentine powder (values are in mm).
| Number of samples (N=5) | 5 min. | 60 min. | 24 hours | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| EthOAc extract | 38.7 | 0.6 | 38 | 39 | 38.0 | 1.0 | 37 | 39 | 25.0 | 0.0 | 25 | 25 |
| Ca(OH)2-EthOAc extract | 22.3 | 0.6 | 22 | 23 | 14.7 | 0.6 | 14 | 15 | 0.0 | 0.0 | 0 | 0 |
| CHX-EthOAc extract | 32.7 | 1.2 | 32 | 34 | 30.7 | 0.6 | 30 | 31 | 30.0 | 0.0 | 30 | 30 |
| NaOCl-EthOAc extract | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 |
| Ca(OH)2 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 |
| CHX | 30.3 | 0.6 | 30 | 31 | 21.3 | 3.2 | 19 | 25 | 19.3 | 0.6 | 19 | 20 |
| DMF | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 |
| NaOCl | 69.7 | 0.6 | 69 | 70 | 68.3 | 0.6 | 68 | 69 | 65.0 | 0.0 | 65 | 65 |
| Kruskal Wallis test | KW=22.8, P=0.018 | KW=22.8, P=0.018 | KW=22.9, P=0.018 | |||||||||
EthOAc – ethyl acetate; Ca(OH)2 – calcium hydroxide; CHX – chlorhexidine; NAOCl – sodium hypochlorite; DMF – dimethyl formamide; KW – Kruskal Wallis.
With the exception of the combination of EthOAc extract with Ca(OH)2 solution, which showed no C. albicans inhibition zone in dentin powder solution, for all other combinations showed slight differences after 60 minutes and 24 hours (Table 2). After 5 minutes planting in the dentin powder, when EthOAc extract was used in combination with sodium hypochlorite there was no inhibition zone, when the extract was used alone there was a 30.3 mm inhibition zone, and when sodium hypochlorite was used alone there was a large inhibition zone of 60.3 mm. There was a statistically significant difference in the growth of C. albicans 5 minutes after planting in dentine powder solution depending on the preparation used (P=0.018). After 60 minutes, when the herbal EthOAc extract was used in combination with sodium hypochlorite there was no inhibition zone, when the extract was used alone there was a 24.3 mm inhibition zone, and when sodium hypochlorite was used alone there was an inhibition zone of 55.0 mm. There was a statistically significant difference in the growth of C. albicans 60 minutes after planting in dentine powder solution depending on the preparation used (P=0.018). After 24 hours of planting in the dentin powder solution, when the herbal EthOAc extract was used in combination with sodium hypochlorite there was no inhibition zone, when the extract was used alone there was a 20.7 mm inhibition zone, when sodium hypochlorite was used alone there was a large inhibition zone of 40.7 mm, whereas there was no C. albicans inhibition zone when the herbal extract was used in combination with Ca(OH)2. There was a statistically significant difference in the growth of C. albicans 24 hours after planting in dentine powder solution depending on the preparation used (P=0.018).
Table 2.
Inhibitory effect of dentine in the antifungal activity (against Candida albicans) of EthOAc extract of Tanacetum vulgare with and without chemicals/medicaments (chlorhexidine, calcium hydroxide, sodium hypochlorite), (values are in mm)
| Number of samples (N=5) | 5 min. | 60 min. | 24 hours | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| EthOAc extract | 30.3 | 0.6 | 30 | 31 | 24.3 | 0.6 | 24 | 25 | 20.7 | 0.6 | 20 | 21 |
| Ca(OH)2-EthOAc extract | 18.7 | 0.6 | 18 | 19 | 13.7 | 1.2 | 13 | 15 | 0.0 | 0.0 | 0 | 0 |
| CHX-EthOAc extract | 29.7 | 1.2 | 29 | 31 | 29.3 | 0.6 | 29 | 30 | 28.3 | 0.6 | 28 | 29 |
| NaOCl-EthOAc extract | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 |
| Ca(OH)2 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 |
| CHX | 25.7 | 0.6 | 26 | 26 | 19.0 | 1.0 | 18 | 20 | 18.0 | 1.0 | 17 | 19 |
| DMF | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 | 0.0 | 0.0 | 0 | 0 |
| NaOCl | 60.3 | 0.6 | 60 | 61 | 55.0 | 1.0 | 54 | 56 | 40.7 | 0.6 | 40 | 41 |
| Kruskal Wallis test | KW=22.8, P=0.018 | KW=22.8, P=0.018 | KW=22.9, P=0.018 | |||||||||
EthOAc – ethyl acetate; Ca(OH)2 – calcium hydroxide; CHX – chlorhexidine; NAOCl – sodium hypochlorite; DMF – dimethyl formamide; KW – Kruskal Wallis.
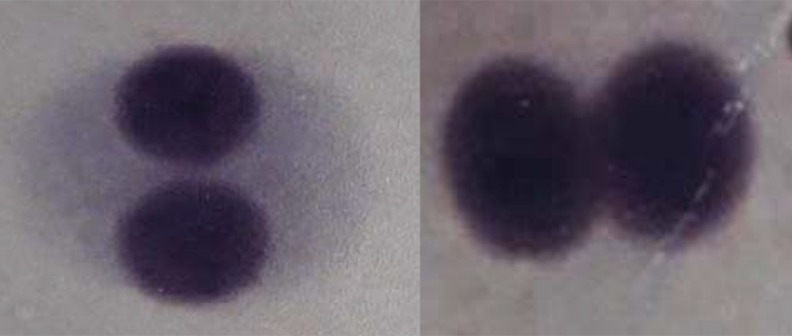
The genotoxic effect of EthOAc extracts of T. vulgare (Figure 2) and glyphosate herbicide as a control positive agent was evaluated by observing the occurrence of micronuclei in 500 lymphocytes. Testing of the occurrence of micronuclei showed significant difference between glyphosate herbicide and EthOAc extracts (P=0.000027) (Table 3).
Figure 2.
Binuclear lymphocytes without micronucleus, tested with ethyl acetate (EthOAc) extract of Tanacetum vulgare.
Table 3.
Genotoxic effects of EthOAc extract of Tanacetum vulgare and glyphosate herbicide assessed by induction of micronuclei in human lymphocytes: percentage of micronuclei observed in 500 lymphocytes (no of individuals=30).
| Agents | (Mean%±SD) | One-way ANOVA |
|---|---|---|
| EthOAc extract | 0 | |
| Glyphosate herbicide | 3.00±1.42 | P=0.000027 |
| No agent | 0 |
EthOAc – ethyl acetate.
Discussion
C. albicans was selected for this study because it is the commonly isolated fungus in infected root canals and can survive chemical/mechanical procedures and root canal medications [22,23]. The disk diffusion method was chosen to evaluate the antimicrobial activity of materials, because it is simple and fast method. The priority of this method is that it provides the direct comparison of different materials and indicates their potential to eliminate bacteria within the root canal system. Dentine powder was used because it can be easily and rapidly mixed with microorganisms to give a homogenous mixture [24].
Local and systemic antibiotics, as well as prolonged endodontic treatment, contribute in the colonization of the root canal by C. albicans. Many herbs family are widely used in treatment of oral diseases such as candidiasis, dental caries and periodontal diseases. Antifungal activity of green propolis has been reported against Candida albicans morphotypes: yeast, pseudohyphae, and hyphae[25]. Turmeric has shown strong antifungal activity against C. albicans [26], and EndoPamhas been reported to have potential as an herbal alternative in root canal irrigation [27]. There are in fact many examples in the literature of herb extracts and phytochemicals with antibacterial properties may have potential applications in endodontics [28,29]. Findings have shown that propolis is a promising endodontic drug with comparable antimicrobial efficacy to sodium hypochlorite and CHX against C. albicans, even when it is present the smear layer. The effects of propolis on such a resistant microorganism suggest that it could be beneficial in root canal treatment [30].
Most alcohol extracts of T. vulgare are reported to have biological effects including anti-inflammatory, antioxidant and antibacterial properties. Furthermore, in vitro experiments showed the promising antifungal activity of ethanolic extract of Praxelisclematidea (Asteraceae family) against C. albicans [31,32].
Based on these findings, we decided to use ethyl acetate to extract the metabolites of T. vulgare (L.) (Asteraceae family), and to test this organic extract for antifungal activity against C. albicans. The EthOAc extract from T. vulgaris demonstrated the strong antifungal activity against C. albicans. We also used different medicaments as standards and these included CHX 2%, sodium hypochlorite 2% and a saturated solution of calcium hydroxide. Sena et al. [33] evaluated the effect of liquid and gel forms of 2% CHX against biofilms of C. albicans on cellulose nitrate membranes. They found that a 2% solution of CHX had high antifungal activity against C. albicans. The contact time required to achieve negative cultures ranged between 30 seconds to 30 minutes. In our experiment, we found that EthOAc extract (38 mm inhibition zone) had higher antifungal activity against C. albicans than 2% CHX (30 mm), which is currently used in endodontic therapy. The antifungal activity of the combination of EthOAc and CHX (32 mm) was higher than that of CHX alone (30 mm).
Studies have shown that sodium hypochlorite is a potent killing agent of C. albicans, whereas this organism is resistant to calcium hydroxide. According to Mohammadi et al., saturated calcium hydroxide showed no antifungal effects, while the best antifungal effects were shown by sodium hypochlorite and 2% CHX [34], and 0.5–5.25% sodium hypochlorite completely eliminated C. albicans in a study by Vianna et al. [35]. Waltimo et al. [36] showed that both 5% and 0.5% concentrations of sodium hypochlorite caused complete eradication of C. albicans cells after 30 second. In our study, 2% sodium hypochlorite had higher antifungal activity against C. albicans (70 mm inhibition zone) compared with all other medicaments that we have used (EthOAc extract, 2% CHX and a saturated solution of calcium hydroxide). However, when a mixture of EthOAc extract and 2% sodium hypochlorite was applied, the antifungal activity of extracts and sodium hypochlorite was lost. We postulate that the sodium hypochlorite may be “spent” by oxidating the bioactive compounds which are present in the EthOAc extract and that this may be the reason why the antifungal activity of sodium hypochlorite and the EthOAc extract is lost.
Studies by Ballal et al. [37] and Ferguson et al. [38] demonstrated that calcium hydroxide had no detectable antifungal effect. It has been reported that C. albicans cells are highly resistant to calcium hydroxide and that aqueous calcium hydroxide had no antifungal activity when in direct contact with C. albicans cells [38].Turk et al. and Pacios et al. reported that Enterococcus faecalis and C. albicans were not inhibited by calcium hydroxide mixed with distilled water [39,40]. Our results were consistent with these findings. In our study, the saturated solution of calcium hydroxide did not show any antifungal activity against C. albicans. Furthermore, the mixture of EthOAc extract with calcium hydroxide solution had lower (22.3 mm) antifungal activity against C. albicans compared with the individual antifungal activity of EthOAc extract (38 mm). It was therefore clear that calcium hydroxide decreased the antifungal activity of EthOAc extract of T. vulgare against C. albicans.
Dentine powder had an inhibitory effect on all tested medicaments. This was similar with the results of Haapasalo et al. [24]. The experiment with dentine powder resulted to be an efficient method with which, it may be evaluated the interactions between root canal agents, dentine and microorganisms [24]. Haapasalo et al. (2000) investigated the inhibitory effect of dentine against some commonly used root canal irrigants. The tested medicaments were calcium hydroxide statured in water, 1% sodium hypochlorite, 0.5% and 0.05% chlorhexidine acetate, and 2–4% and 0.2–0.4% iodine potassium iodide. As a microbe was tested E. faecalis. The aforementioned authors reported that the dentine powder had an inhibitory effect on all tested medicaments against E. faecalis. The inhibitory effect was dependent on the concentration of the medicament and the duration of preincubation time with dentine powder before the addition of bacteria. Based on this, we tested the antifungal activity of EthOAc extract of T. vulgaris and other medicaments after mixing with dentine powder. We found that the dentine powder also had an inhibitory effect on all of the tested medicaments: EthOAc extract, CHX, and sodium hypochlorite.
Based on our preliminary investigations, the results of the agar-diffusion tests showed that 2% CHX and 2% sodium hypochlorite solutions from containers opened several days previously produced smaller inhibition zones than solutions from newly opened containers. This was the reason why we tested the antifungal activity of the EthOAc extract and medicaments after 1 hour and 24 hours of the containers being opened, against C. albicans. The antifungal activity of EthOAc extract decreased with time, with inhibition zones decreasing from 38.7 mm (5 minutes) to 25 mm (after 24 hours). The same trend was seen for CHX and sodium hypochlorite. The saturated solution of calcium hydroxide did not show antifungal activity in any of our experimental tests.
In an in vitro study of Roselli et al. [41] the investigated subspecies of T. vulgare showed no variability in chemical composition and could be classified into belonging to the eudesmanolide chemotype. According to the same study, all components showed cytotoxic activity against cultured cancer and healthy cell lines. In contrast, we found that EthOAc extracts of T. vulgare did not show any genotoxic effects in lymphocyte cells in vitro. Glyphosate herbicide (36%, 0.5 mL) was used as a positive control for this experiment and showed genotoxic effect, where the average of micronuclei formed was 15, or 3.00±1.42% of the 500 lymphocytes observed.
Because endodontic infections involve diverse microbes, drugs that are effective against a microorganism in vitro may not be effective in vivo. Therefore, studies in vivo are required to confirm the effects of calcium hydroxide, CHX, sodium hypochlorite, and EthOAc extract of T. vulgare against C. albicans. Further studies on suitable medicinal plants, their use and dosage are required to gain more information about their toxicity and possible side effects.
Conclusions
During this research, we evaluated the antifungal activity of the EthOAc extract of T. vulgare against C. albicans. The EthOAc extract from T. vulgare has been shown to have high antifungal activity compared with commercial irrigant such as CHX, and may be used as an effective, safe and natural source of antifungal agent. This EthOAc extract in combination with the medicament CHX showed an additive effect on the antifungal activity against C. albicans. The results of the present investigation will contribute to the development of an alternative phytotherapeutic agent to treat infection with C. albicans.
Acknowledgements
We thank Kate Fox, DPhil, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Source of support: Departmental sources
References
- 1.Calt S, Serper A. Time dependent effects of EDTA on dentine structures. J Endod. 2002;28(1):459–61. doi: 10.1097/00004770-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y, Stojicic S, Qian W, et al. The synergistic antimicrobial effect by mechanical agitation and two chlorhexidine preparations on biofilm bacteria. J Endod. 2010;36:100–4. doi: 10.1016/j.joen.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Khademi AA, Babagoli J, et al. Effects of MTAD on the surface of instrumented root canals. J Endod. 2003;29:170–75. [Google Scholar]
- 4.Spanberg L, Engstrom B, Langeland K. Biological effects of dental materials. III Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1973;36:856–71. doi: 10.1016/0030-4220(73)90338-1. [DOI] [PubMed] [Google Scholar]
- 5.Kanisavaran ZM. Chlorhexidine gluconate in endodontics: An update review. Int Dent J. 2008;58:247–57. doi: 10.1111/j.1875-595x.2008.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 6.Gomes BPFA, Lilley JD, Drucker DB. Variations in the susceptibilities of components of the endodontic microflora to biomechanical procedures. Int Endod J. 1996;29:235–41. doi: 10.1111/j.1365-2591.1996.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 7.Sen BH, Piskin B, Dimirci T. Observations of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod Dent Traumatol. 1995;11:6–9. doi: 10.1111/j.1600-9657.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 8.Waltimo TM, Siren EK, Orstavik D, Haapasalo MP. Susceptibility of oral candida species to calcium hydroxide in vitro. Int Endod J. 1999;32:94–98. doi: 10.1046/j.1365-2591.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 9.Muresan ML. Antimicrobial effects of the ethanolic extracts and essential oils of Tanacetum vulgare L from Romania. Acta Universitatis Cibiniensis Series E: Food Technology. 2015;XIX:75–80. [Google Scholar]
- 10.Bagci E, Kocak A. Essential oil composition of two endemic Tanacetum (T. Nitens (Boiss.&Noe) Grierson and T. Argentum (Lam.) Willd. Subsp.argentum) (Asteraceae) taxa, growing wild in Turkey. Industrial Crops and Products. 2010;31:542–45. [Google Scholar]
- 11.Benedec D, Vlase L, Oniga I, et al. Polyphenolic composition, antioxidant and antibacterial activities for two Romanian subspecies of Achilleadistans Waldst. Et Kit. Ex Willd. Molecules. 2013;18(8):8725–39. doi: 10.3390/molecules18088725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Şandru DM. Antimicrobial effect of Escherichia coli on essential oils derived from Romanian aromatic plants. Acta Universitatis Cibiniensis Series E: Food Technology. 2015;XIX(1) [Google Scholar]
- 13.Cote H, Boucher M-A, Pichette A, Legault J. Anti-inflammatory, antioxidant, antibiotic, and cytotoxic activities of Tanacetum vulgare L. essential oil and its constituents. Medicines (Basel) 2017;4(2):34. doi: 10.3390/medicines4020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mordujovich-Buschiazzo P, Rosella M, Schinella G. Anti-inflammatory activity of Tanacetum vulgare. Fitoterapia. 1996;67:319–22. [Google Scholar]
- 15.Schinella GR, Giner RM, Recio MC, et al. Anti-inflammatory effects of South American Tanacetum vulgare. J Pharm Pharmacol. 1998;50:1069–74. doi: 10.1111/j.2042-7158.1998.tb06924.x. [DOI] [PubMed] [Google Scholar]
- 16.Piras A, Falconieri D, Bagdonaitec E, et al. Chemical composition and antifungal activity of supercritical extract and essential oil of Tanacetum vulgare growing wild in Lithuania. Nat Prod Res. 2014;28(21):1906–9. doi: 10.1080/14786419.2014.939085. [DOI] [PubMed] [Google Scholar]
- 17.Williams CA, Harborne JB, Geiger H, Hoult JR. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties. Phytochemistry. 1999;51:417–23. doi: 10.1016/s0031-9422(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 18.Bulacio M, Cecilia M, Cangemi R, Raigen G. In vitro antibacterial effect of different irrigating solutions on Enterococcus faecalis. Acta Odontol Latinoam. 2006;19(2):75–80. [PubMed] [Google Scholar]
- 19.Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutat Res. 1985;147(1–2):29–36. doi: 10.1016/0165-1161(85)90015-9. [DOI] [PubMed] [Google Scholar]
- 20.Fenech M, Morley AA. Cytokinesis-block micronucleus method in human lymphocytes: Effect of in vivo ageing and low dose X-irradiation. Mutat Res. 1986;161(2):193–98. doi: 10.1016/0027-5107(86)90010-2. [DOI] [PubMed] [Google Scholar]
- 21.Fenech M. The cytokinesis-block micronucleus technique: A detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285(1):35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 22.White RR, Hays GL, Janer LR. Residual antimicrobial activity after canal irrigation with chlorhexidine. J Endod. 1997;23:29–31. doi: 10.1016/S0099-2399(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 23.Heling I, Sommer M, Steinberg D, et al. Microbiological evaluation of the efficacy of chlorhexidine in a sustained release device for dentin sterilization. Int Endod J. 1992;25:15–19. doi: 10.1111/j.1365-2591.1992.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 24.Haapasalo HK, Siren EK, Waltimo TM, et al. Inactivation of local root canal medicaments by dentine: An in vitro study. Int Endod J. 2000;33:126–31. doi: 10.1046/j.1365-2591.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 25.Berretta AA, Castro PA, Cavalheiro AH, et al. Evaluation of mucoadhesive gels with propolis (EPP-AF) in preclinical treatment of candidiasis vulvovaginal infection. Evidence Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/641480. 641480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegde V, Kesaria DH. Comparative evaluation of antimicrobial activity of neem, propolis, turmeric, liquorice and sodium hypochlorite as root canal irrigants against E. faecalis and C. albicans – an in vitro study. Endodontology. 2013;25:38–45. [Google Scholar]
- 27.Mathew J, Pathrose S, Kottoor J, et al. Evaluation of an indigenously prepared herbal extract (EndoPam) as an antimicrobial endodontic irrigant: An ex vivo study. J Int Oral Health. 2015;7:88–91. [PMC free article] [PubMed] [Google Scholar]
- 28.Assiri AM, Hassanien MF. Bioactive lipids, radical scavenging potential and antimicrobial properties of cold pressed clove (Syzygium aromaticum) oil. J Med Food. 2013;16:1046–56. doi: 10.1089/jmf.2012.0288. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: Investigation of its bactericidal activity. J Nanosci Nanotechnol. 2013;13:114–22. doi: 10.1166/jnn.2013.6701. [DOI] [PubMed] [Google Scholar]
- 30.Santos VR, Oliveira CF, Abreu Rosa FH, et al. A new red propolismuco adhesive gel: antimicrobial activity against some oral pathogen. Alternative and Integrative Medicine. 2017;4(3):129. [Google Scholar]
- 31.Lubian CT, Texeira JM, Lund RG, et al. [Ativida de antifúngica do extratoaquoso de Arctium minus (Hill) Bernh. (Asteraceae) Sobreespéciesorais de Candida]. Revista Brasileira de Plantas Medicinais. 2010;12(2):157–62. [in Portuguese] [Google Scholar]
- 32.Oliveira-Filho AA, Sousa JP, Fernandes HMB, et al. Antibacterial and antifungal potential of the ethanolic extract of praxelisclematidea R.M. King & Robinson. International Journal of Pharmacognosy and Phytochemical Research. 2013;5(3):183–85. [Google Scholar]
- 33.Sena NT, Gomes BP, Vianna ME, et al. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int Endod J. 2006;39(11):8788–95. doi: 10.1111/j.1365-2591.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 35.Vianna ME, Gomes BP. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 36.Waltimo TM, Orstavik D, Siren EK, Haapasalo MP. In vitro susceptibility of Candida albicans to four disinfectants and their combinations. Int Endod J. 1999;32(6):421–29. doi: 10.1046/j.1365-2591.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 37.Ballal V, Kundabala M, Acharya S, Ballal M. Antimicrobial action of calcium hydroxide, chlorhexidine and their combination on endodontic pathogens. Aust Dent J. 2007;52:118–21. doi: 10.1111/j.1834-7819.2007.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson JW, Hatton JF, Gillespie MJ. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J Endod. 2002;28:68–71. doi: 10.1097/00004770-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Turk BT, Sen BH, Ozturk T. In vitro antimicrobial activity of calcium hydroxide mixed with different vehicles against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:297–301. doi: 10.1016/j.tripleo.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Pacios MG, Silva C, López ME, Cecilia M. Antibacterial action of calcium hydroxide vehicles and calcium hydroxide pastes. J Investig Clin Dent. 2012;3:264–70. doi: 10.1111/j.2041-1626.2012.00147.x. [DOI] [PubMed] [Google Scholar]
- 41.Rosselli S, Bruno M, Maria Raimondo F, et al. Cytotoxic effect of eudesmanolides isolated from flowers of Tanacetum vulgare ssp. siculum. Molecules. 2012;17:8186–95. doi: 10.3390/molecules17078186. [DOI] [PMC free article] [PubMed] [Google Scholar]