Abstract
The mechanism by which agonist binding triggers pore opening in ligand-gated ion channels is poorly understood. Here, we used unnatural amino acid mutagenesis to introduce subtle changes to the side chains of tyrosine residues (Tyr141, Tyr143, Tyr153, and Tyr234), which dominate the 5-HT3 receptor binding site. Heterologous expression in oocytes, combined with radioligand binding data and a model of 5-HT (serotonin) computationally docked into the binding site, has allowed us to determine which of these residues are responsible for binding and/or gating. We have shown that Tyr 143 forms a hydrogen bond that is essential for receptor gating but does not affect binding, whereas a hydrogen bond formed by Tyr153 is involved in both binding and gating of the receptor. The aromatic group of Tyr234 is essential for binding and gating, whereas its hydroxyl does not affect binding but plays a steric role in receptor gating. Tyr141 is not involved in agonist binding or receptor gating but is important for antagonist interactions. These data, combined with a new model of the nonliganded 5-HT3 receptor, lead to a mechanistic explanation of the interactions that initiate the conformational change leading to channel opening. Thus, we suggest that agonist entry into the binding pocket may displace Tyr143 and Tyr153 and results in their forming new hydrogen bonds. These bonds may form part of the network of bond rearrangements that trigger the conformational change leading to channel opening. Similar rearrangements may initiate gating in all Cys-loop receptors.
Keywords: ligand-gated ion channel, Cys-loop receptor, 5-HT3 receptor binding site, hydrogen bond, unnatural amino acids, activation mechanism, serotonin
Introduction
The 5-HT3 receptor is a member of the Cys-loop family of ligand-gated ion channels, which includes nicotinic ACh (nACh), GABAA, and glycine receptors (Reeves and Lummis, 2002). These receptors are pentameric, with each subunit having a large extracellular domain and four transmembrane domains (M1-M4). No x-ray crystal structures have yet been published for any of these proteins, so the molecular details of ligand binding and channel opening have to be inferred from lower resolution images, homologous proteins, and experimental evidence (Miyazawa et al., 2003). The determination of the structure of the ACh binding protein (AChBP) (Brejc et al., 2001), which is homologous to the extracellular domain of the nACh receptor (nAChR), has had a particularly significant impact on this field and has been used to create homology-based models of the nACh, GABAA, and 5-HT3 receptors (Cromer et al., 2002; Le Novere et al., 2002; Schapira et al., 2002; Reeves et al., 2003). Cryo-electron microscope images have also contributed significantly to our understanding of the structure of these receptors and have led to a model of channel opening: agonist binding to the extracellular domain causes a rotation, which is then transduced via the M2-M3 loop to the pore-lining domain, M2. A twist in this region both removes the hydrophobic residues lining the channel and widens the pore, thus allowing ions to flow (Miyazawa et al., 2003). Although this is a plausible model, more experimental data are required both to substantiate it and to determine the molecular details of these processes.
The homology model of the 5-HT3 receptor (Reeves et al., 2003) reveals a ligand-binding pocket containing a large proportion of aromatic residues. Two of these residues are tryptophans, one of which (Trp183) forms a cation-π interaction with agonist (Beene et al., 2002). There is also a phenylalanine (Phe226), but most of the aromatic character is provided by tyrosines (Tyr141, Tyr143, Tyr153, and Tyr234). Previous data (Venkataraman et al., 2002; Price and Lummis, 2004) have shown the importance of these residues in the binding and/or function of the receptor, but it has not been possible to define their exact roles. Here, we combined conventional mutagenesis with non-sense suppression methodology to incorporate both natural and unnatural amino acids into positions 141, 143, 153, and 234. The latter has allowed us to examine the effects on function and ligand binding of subtle changes to these tyrosine residues (see Fig. 1) and, thus, precisely define their roles. Assimilating these data with the homology model of the 5-HT3 receptor binding site, into which 5-HT has been computationally docked, has allowed us not only to confirm the orientation of 5-HT in the agonist binding pocket but also to determine which bonds are essential for receptor binding and/or gating. Combining this information with a new model of the ligand binding site in the closed state has led us to propose a mechanism by which agonist binding could trigger the series of conformational changes leading to channel opening.
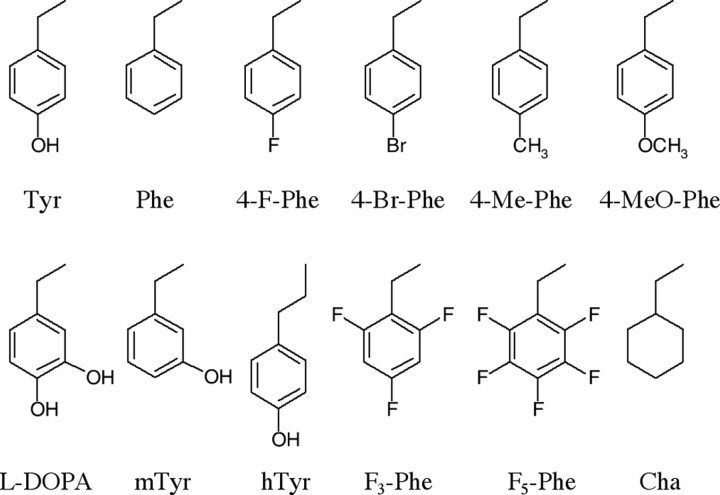
Figure 1.
Structures of the side chains of the unnatural amino acids used in this study.
Materials and Methods
Mutagenesis and preparation of cRNA and oocytes. Mutant 5-HT3A receptor subunits were developed using pcDNA3.1 (Invitrogen, Abingdon, UK) containing the complete coding sequence for the 5-HT3A(b) subunit (GenBank accession number Ay605711) from N1E-115 cells as described previously (Hargreaves et al., 1996). For non-sense suppression, the tyrosine codons were replaced individually by the non-sense codon TAG as described previously (Beene et al., 2002). Mutagenesis reactions were performed using the Kunkel method (Kunkel, 1985) and confirmed by DNA sequencing. Wild-type and mutant receptor subunit coding sequences were then subcloned into pGEMHE (Liman et al., 1992). This was linearized with NheI (New England Biolabs, Beverly, MA) and cRNA synthesized using the T7 mMESSAGE mMACHINE kit (Ambion, Austin, TX). This was then injected into oocytes from Xenopus laevis, which were prepared and maintained as described previously (Beene et al., 2002). Experiments were performed 18-36 hr after injection.
Synthesis of tRNA and dCA amino acids. Unnatural amino acids (Fig. 1) were chemically synthesized as nitroveratryloxycarbonyl-protected cyanomethyl esters and coupled to the dinucleotide dCA, which was then enzymatically ligated to 74-mer THG73 tRNACUA as detailed previously (Nowak et al., 1998). Immediately before coinjection with mRNA, tRNA-aa was deprotected by photolysis (Kearney et al., 1996). Typically, 5 ng of mRNA and 25 ng of tRNA-aa were injected into stage V-VI oocytes in a total volume of 50 nl. For control experiments, mRNA was injected, first, in the absence of tRNA and, second, with the THG73 74-mer tRNA.
Characterization of mutant receptors. 5-HT-induced currents were recorded from individual oocytes using two-voltage electrode clamp with either a GeneClamp 500 amplifier or an OpusXpress system (Axon Instruments, Union City, CA). All experiments were performed at 22-25°C. Serotonin (creatinine sulfate complex; Sigma, St. Louis, MO) was stored as 25 mm aliquots at -80°C, diluted in calcium-free ND96, and delivered to cells via computer-controlled perfusion systems. Glass microelectrodes were backfilled with 3 m KCl and had a resistance of ∼1 MΩ. The holding potential was -60 mV unless otherwise specified. To determine EC50 values, 5-HT concentration-response data were fitted to the Hill equation, I = [Imax(A)n]/[EC50n + (A)n], where Imax is the maximal peak current, [A] is the concentration of agonist, and n is the Hill coefficient.
Results
Validation of the non-sense suppression method, reintroduction of tyrosine
As an initial test of the validity of the non-sense suppression method, tyrosine was incorporated via acylated tRNA at each of the four positions (141, 143, 153, and 234). For the non-sense suppression methodology to give reliable results, the EC50 values and Hill coefficients for activation by 5-HT must be indistinguishable from each other and from those of the wild-type 5-HT3A homopentamer expressed in Xenopus oocytes.
All mutant mRNAs, when coinjected into Xenopus oocytes with tRNA-Tyr molecules, produced functional receptors that responded to application of 5-HT with an inward current that desensitized in response to maintained 5-HT. The results shown in Tables 1, 2, 3, 4 establish that the EC50 values and Hill coefficients obtained from 141-Tyr, 143-Tyr, 153-Tyr, and 234-Tyr and wild-type receptors were indeed similar to each other. This indicates that the wild-type phenotype was successfully “rescued” by the delivery of tRNA-Tyr molecules. For each receptor, maximal 5-HT-induced currents (Imax) were typically 0.8-2 μA at a holding potential of -60 mV (Fig. 2). This suggests that the pGEMHE vector can yield levels of full-length, correctly folded protein at least as high as the original pAMV-PA vector (Nowak et al., 1998). The efficiency of non-sense suppression has been estimated at ∼10% (Nowak et al., 1995), and comparison of Imax values for the wild-type 5-HT3A receptor expressed in oocytes using pGEMHE to rescued TAG mutant receptors suggests a similar efficiency here.
Table 1.
EC50 values (μm) and Hill coefficients for 5-HT activation of 141-Tyr mutant receptors (n = 3-6) (A) and [3H]granisetron binding affinities of Tyr mutants (B)
|
A |
|
|
|---|---|---|
| 141- |
EC50 ± SE |
nH ± SE |
| Ala | 2.73 ± 0.15 | 2.68 ± 0.40 |
| Ser | 4.70 ± 0.38 | 2.02 ± 0.31 |
| Cha | 3.14 ± 0.40 | 1.92 ± 0.21 |
| Phe | 0.92 ± 0.06 | 2.80 ± 0.44 |
| 4-F-Phe | 1.32 ± 0.05 | 2.47 ± 0.17 |
| F5-Phe | 1.38 ± 0.08 | 1.89 ± 0.17 |
| Tyr (WT) | 1.16 ± 0.04 | 2.76 ± 0.20 |
| mTyr | 1.69 ± 0.06 | 3.24 ± 0.29 |
| 4-Me0-Phe |
3.36 ± 0.14 |
2.17 ± 0.16 |
| B |
|
|
|---|---|---|
| 141- |
Kd (nm) |
n
|
| Ala | 8.97 ± 2.44* | 4 |
| Ser | NB | 8 |
| Phe | 0.98 ± 0.15 | 4 |
| Tyr (WT) |
0.32 ± 0.035 |
7 |
The asterisk indicates a significant difference (p<0.05) to Tyr. Substitutions have been approximately ordered by increasing size. NB, No binding; WT, wild type. The asterisk data in B are from Price and Lummis (2004).
Table 2.
EC50 values (μm) and Hill coefficients for 5-HT activation of 143-Tyr mutant receptors (A) and [3H]granisetron binding affinities of Tyr mutants (B)
|
A |
|
|
|---|---|---|
| 143- |
EC50 ± SE |
nH ± SE |
| Ala | 354 ± 29.0* | 2.62 ± 0.53 |
| Ser | 472 ± 47.9* | 2.68 ± 0.62 |
| Phe | 78.6 ± 2.86* | 2.73 ± 0.21 |
| 4-F-Phe | NR | NR |
| Tyr (WT) | 1.15 ± 0.03 | 2.98 ± 0.19 |
| mTyr | NR | NR |
| 4-MeO-Phe | NR | NR |
| hTyr | 33.24 ± 2.60* | 1.80 ± 0.22* |
|
l-DOPA |
7.16 ± 0.90*
|
2.34 ± 0.54 |
| B |
|
|
|---|---|---|
| 143- |
Kd (nm) |
n
|
| Ala | 1.2 ± 0.24 | 8 |
| Ser | 1.13 ± 0.23 | 7 |
| Phe | 0.53 ± 0.10 | 3 |
| Tyr (WT) |
0.32 ± 0.035 |
7 |
Asterisks indicate a significant difference (p<0.05) to Tyr (n = 3-6). Data in B are from Price and Lummis (2004). Substitutions have been approximately ordered by increasing size. NR, No response to concentrations of 5-HT up to 1 mm; NB, no binding; WT, wild type.
Table 3.
EC50 values (μm) and Hill coefficients for 5-HT activation of 153-Tyr mutant receptors (n = 3-6) (A) and [3H]granisetron binding affinities of Tyr mutants (B)
|
A |
|
|
|---|---|---|
| 153- |
EC50 ± SE |
nH ± SE |
| Ala | 120 ± 9.43* | 2.43 ± 0.38 |
| Ser | 84.1 ± 5.60* | 2.54 ± 0.37 |
| Phe | 22.5 ± 0.43* | 2.60 ± 0.11 |
| 4-F-Phe | 19.7 ± 1.22* | 1.82 ± 0.14* |
| F5-Phe | (>500) | |
| Tyr (WT) | 1.15 ± 0.04 | 2.78 ± 0.20 |
| mTyr | (>500) | |
| 4-Me-Phe | 18.2 ± 0.48* | 2.48 ± 0.11 |
| 4-MeO-Phe |
6.65 ± 0.27*
|
1.98 ± 0.12*
|
| B |
|
|
|---|---|---|
| 153- |
Kd (nm) |
n
|
| Ala | 3.62 ± 1.75* | 3 |
| Ser | NB | 6 |
| Phe | 0.90 ± 0.20 | 5 |
| Tyr (WT) |
0.32 ± 0.035 |
7 |
Data in B are from Price and Lummis (2004). Substitutions have been approximately ordered by increasing size. The asterisk indicates a significant difference (p<0.05) to Tyr. NB, No binding; WT, wild type.
Table 4.
EC50 values (μm) and Hill coefficients for 5-HT activation of 234-Tyr mutant receptors using non-sense suppression (A) and [3H]granisetron binding affinities of Tyr mutants (B)
|
A |
|
|
|---|---|---|
| 234- |
EC50 ± SE |
nH ± SE |
| Ala | 120 ± 9.43* | 2.43 ± 0.38 |
| Ser | 84.1 ± 5.60* | 2.54 ± 0.37 |
| Phe | 10.22 ± 0.80* | 1.92 ± 0.26 |
| Cha | NR | NR |
| 4-F-Phe | 4.11 ± 0.20* | 2.36 ± 0.19 |
| F3-Phe | NR | NR |
| F5-Phe | NR | NR |
| Tyr (WT) | 1.24 ± 0.05 | 2.89 ± 0.28 |
| mTyr | 7.42 ± 0.32* | 2.83 ± 0.32 |
| 4-Br-Phe | 1.78 ± 0.08 | 2.79 ± 0.32 |
| 4-Me-Phe |
1.13 ± 0.05 |
2.49 ± 0.22 |
| B |
|
|
|---|---|---|
| 234 |
Kd (nm) |
n
|
| Phe | 1.3 ± 0.36 | 4 |
| Ala | NB | 5 |
| Ser | NB | 5 |
| Tyr (WT) |
0.32 ± 0.035 |
7 |
Asterisks indicate a significant difference (p<0.05) to Tyr (n = 3.6). Data in B are from Price and Lummis (2004). Substitutions have been approximately ordered by increasing size. NR, No response to concentrations of 5-HT up to 1 mm; NB, no binding; WT, wild type.
Figure 2.
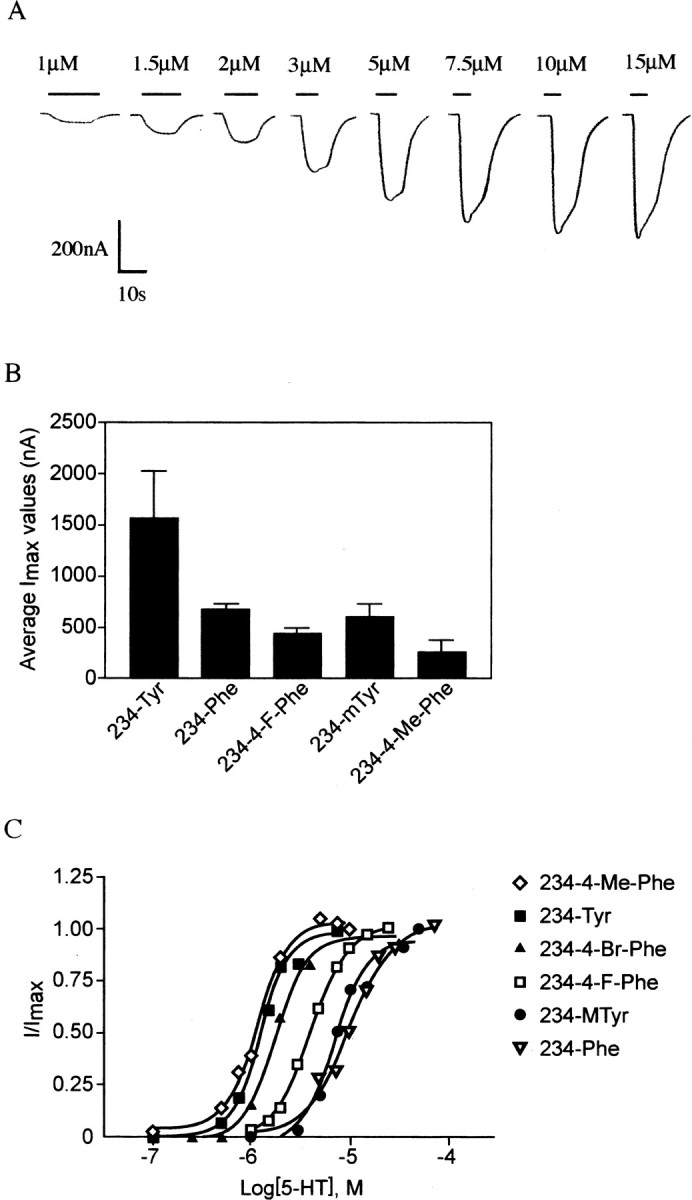
A, Exemplar concentration-response data obtained from two-electrode voltage-clamp recordings from a single Xenopus oocyte expressing the 234-4-F-Phe mutant. B, Average readings of maximal current (Imax) obtained for receptors with substitutions of Tyr234. The wild-type Tyr residue produced generally larger currents than Phe or the unnatural side chains, but all residues studied gave responses adequate for systematic studies. C, Concentration-response curves showing the effects of unnatural and natural aromatic amino acid residue incorporation at position 234. Error bars (<10%) removed for clarity.
No currents in response to application of high concentrations of 5-HT (1 mm) were detected from oocytes injected with mRNA alone or with mRNA and tRNA not ligated to dCA-aa (THG73 74-mer tRNA). It therefore appears that transcripts, which have been truncated by the inclusion of a stop codon at the position of the mutated tyrosine residue, cannot produce functional receptors. This is to be expected, because these truncated receptors would contain none of the transmembrane regions required for ion channel activity. We can also conclude that any amino acid that is incorporated into these four sites of the mutant receptor is specifically introduced by the injected tRNA-aa and not by the endogenous tRNA population of the oocyte. Finally, the lack of response from oocytes injected with mRNA and unacylated tRNA shows that there is no detectable reacylation of tRNA with naturally occurring amino acids in vivo by endogenous synthetases.
Incorporation of unnatural amino acids
A subset of the unnatural aromatic amino acids shown in Figure 1 was selected for testing at each of the mutant positions. These compounds were previously incorporated into the nAChR (Nowak et al., 1995, 1998) and are therefore compatible with the oocyte expression system. In addition to the naturally occurring amino acids tyrosine and phenylalanine, the amino acids tested included 3-OH-phenylalanine (subsequently referred to as metatyrosine or mTyr), homotyrosine (hTyr), l-DOPA, 4-methyl-phenylalanine (4-Me-Phe), 4-fluoro-phenylalanine (4-F-Phe), F3-phenylalanine (F3-Phe), F5-phenylalanine (F5-Phe), 4-methoxy-phenylalanine (4-MeO-Phe), and cyclohexylalanine (Cha).
Data from a typical concentration-response experiment (4-FPhe at position 234) are shown in Figure 2. Although there were changes in the EC50 values and Hill coefficients for some mutant receptors (see below), activation and desensitization kinetics were similar to wild type for all receptors incorporating unnatural amino acids that responded to 5-HT.
The data however did show that receptors containing any residue apart from the natural tyrosine residues at any of the positions tested display lower Imax values; a typical example with some of the position 234 mutants is shown in Figure 2. Because this effect was observed for both incorporation of phenylalanine and the unnatural amino acids, it probably did not arise because the oocyte cellular machinery processes unnatural amino acids inefficiently and may be caused by lower levels of expression of some mutant 5-HT3 receptors.
Tyr141 is not critical for function but may play a role in antagonist binding and receptor assembly
Incorporation of Phe, mTyr, 4-F-Phe, 4-MeOPhe, F5-Phe, and Cha using non-sense suppression mutagenesis and substitution with the natural amino acids Ala, Ser, and Phe using standard mutagenesis techniques produced receptors that responded to 5-HT in a manner very similar to wild-type 5-HT3A receptors (Fig. 3; Table 1). These data therefore show that neither the aromatic nor the hydroxyl groups are important for agonist binding or receptor gating. However, this residue has been implicated in antagonist binding because changes in affinity of 5-HT3 receptor antagonists have been observed when this residue is exchanged for a nonaromatic (Venkataraman et al., 2002; Price and Lummis, 2004). Thus, Tyr141Ala receptors have decreased [3H]granisetron, d-tubocurarine, and lerisetron binding affinity compared with wild-type receptors, whereas Tyr141Ser receptors do not bind [3H]granisetron at all. The model of the 5-HT3 receptor binding site (Fig. 4) [based on model 4 of Reeves et al. (2003)] supports these data, because Tyr141 could contact these larger antagonists but is not close enough to be in direct contact with a bound 5-HT molecule.
Figure 3.
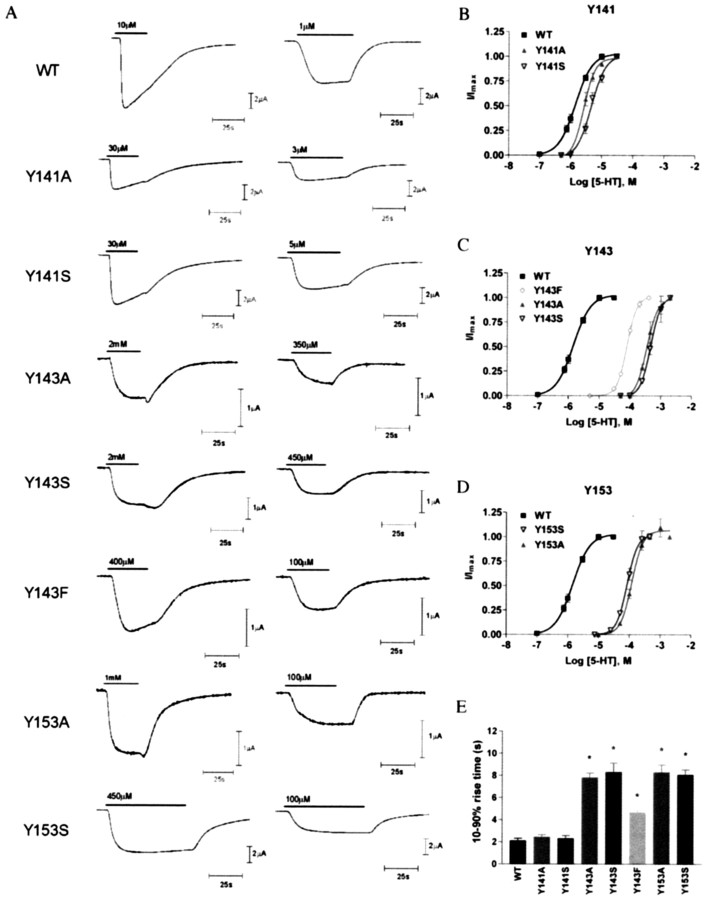
A, Typical response waveforms to 5-HT at approximately Imax and EC50 concentrations for wild type (WT) and each mutant receptor that responded to 5-HT. Bars indicate 5-HT application. B, Concentration-response curves showing the effect of replacing Tyr141, Tyr143, and Tyr153 with alanine, serine, and (for Tyr143 only) phenylalanine. No responses were detected from oocytes injected with Tyr234Ala or Tyr234Ser RNA.
Figure 4.
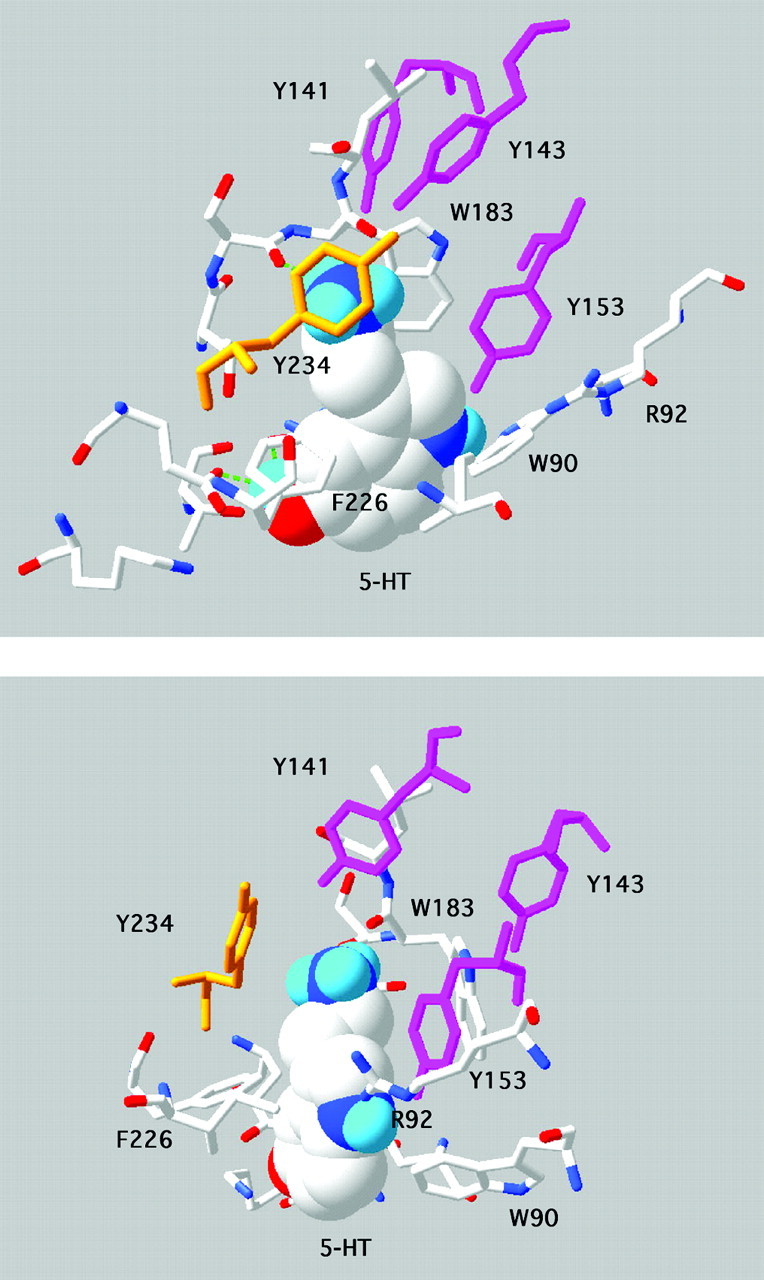
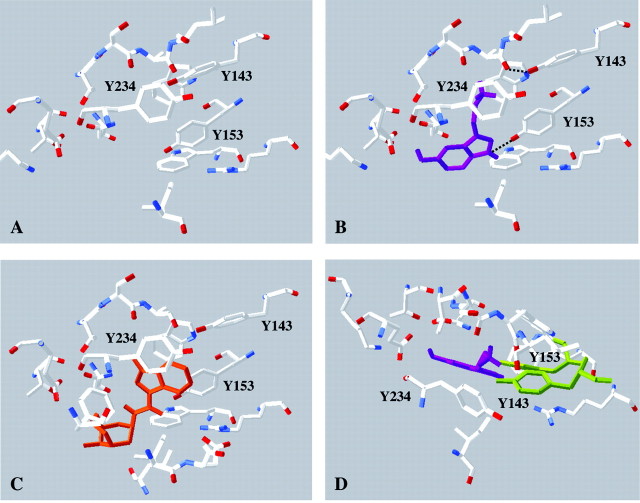
Model showing two views of 5-HT docked into the 5-HT3A receptor binding site. This is based on model 4 of Reeves et al. (2003), which differs from model 5 by a small rotation of the primary amine; all other models had a different orientation of 5-HT and are not supported by the data. Tyrosine residues contributed from loops E (141, 143, 153, purple) and C (234, orange) are indicated. The primary amine group of 5-HT is sandwiched between Trp183, where there is a cation-π interaction, and Tyr234. Tyr153 abuts the indole N.
In previous studies (Venkataraman et al., 2002; Price and Lummis, 2004), Tyr141Ala and Tyr141Ser receptors did not function when expressed in human embryonic kidney 293 cells. However, our data show they have similar characteristics to wild-type receptors when expressed in oocytes. Oocytes are generally more tolerant than mammalian cells to expression of ion channel proteins, which may require longer periods to fold correctly, the difference perhaps arising from the fact that oocytes are incubated at lower temperatures, which would favor complex multi-subunit assemblies (Denning et al., 1992). These data therefore suggest a role for this residue in correct receptor folding and/or assembly, perhaps locally in the binding site region.
Tyr143 forms a hydrogen bond that is essential for efficient receptor gating
Data from Tyr143 mutant receptors suggest that an aromatic ring with a hydroxyl group at the 4 position is essential for efficient receptor function: DOPA, which has these groups, was the only Tyr143 substitute that produced receptors with a <10-fold increase in EC50. Incorporation of even subtle changes such as 4-F-Phe, 4-MeO-Phe, and mTyr resulted in receptors that did not respond to 5-HT (Table 2). DOPA also has a hydroxyl at the 3 position, and the increase in EC50 observed with this compound (approximately sixfold) suggests that bulk here is not desirable. An increased chain length or removal of the hydroxyl, however, is more deleterious: Phe and hTyr caused 30- to 40-fold increases in EC50. Removal of the aromatic group and/or further displacement of the hydroxyl group, as in replacement with Ala or Ser, resulted in even more severe changes: >100-fold EC50 increases and changes in apparent activation and desensitization rates, 10-90% rise times were twofold to fourfold slower than wild-type receptors (Fig. 3E), and the receptors displayed little or no desensitization. These data demonstrate the importance of both the aromatic and the hydroxyl group of Tyr143, and in particular suggest that the hydroxyl forms a hydrogen bond that is essential for efficient receptor gating.
To confirm the presence of such a bond would require x-ray crystal structural data, which unfortunately is not available. However, mutagenesis data alone are usually considered sufficient, providing there is some supporting structural data such as a homology model (Grutter et al., 2003). Indeed, the unnatural amino acid methodology that we used here is a much more powerful probe for this type of bond than conventional mutagenesis, because we can not only introduce subtle changes that minimally perturb the global protein structure but also design the amino acid to determine whether the residue acts as a hydrogen bond donor or acceptor. Thus, using conventional mutagenesis, we can remove the hydroxyl at position 143 (in Phe) but not replace it (Ser is unsatisfactory as a replacement, because hydrogen bonds depend critically on the distance between donor and acceptor atoms, and the hydroxyls in these two residues are in quite distinct locations). Unnatural amino acid substitution can surmount this problem; DOPA has a similarly placed hydroxyl to Tyr, whereas 4-Me-O Phe places an O atom in the same location, and the hydroxyl of hTyr is only subtly displaced from that of Tyr. Data from these substitutions support the proposal of a hydrogen bond at Tyr143 and indeed further suggest that the hydroxyl here acts as a hydrogen bond donor.
The model of 5-HT docked into the 5-HT3A receptor extracellular domain (Fig. 4) shows that the hydroxyl of Tyr143 has the potential to hydrogen bond with one of a number of residues on the opposite side of the binding pocket. These are the hydroxyl or the π ring of Tyr234 and the carbonyl oxygens of Trp183 or Leu184, all of which are <3 Å away. The fact that 4-Me-Phe could substitute satisfactorily for Tyr234 suggests that there is no such bond between the hydroxyls of Tyr143 and Tyr234, but as yet we cannot exclude any of the other possibilities. Of course the accuracy of the inter-residue distances must be viewed with caution, both because of the problems inherent with using a model based on a homologous structure and because, in the docking procedure, the protein side chains remain rigid, which would not be the case in vivo. Nevertheless, the modeling data suggest that the most probable hydrogen bonding partner of Tyr143 is the backbone carbonyl of Trp183, an interaction that has also been previously suggested by Maksay et al. (2003).
The presence of either an aromatic or a hydroxyl at position 143, however, is not essential for antagonist interactions: alanine, serine, and phenylalanine mutants of Tyr143 bound [3H]granisetron with an affinity similar to wild-type receptors (Table 2) (Venkataraman et al., 2002; Price and Lummis, 2004). Competitive antagonists such as granisetron bind in the binding pocket but cannot trigger the conversion to the open state. These data, combined with the modeling data, thus suggest that neither an aromatic group nor a hydrogen bond is required for ligand binding, but both are essential for efficient receptor gating.
A hydrogen bond formed by Tyr153 is involved in both binding and gating of the receptor
Large increases in EC50 values (Table 3), modifications in receptor kinetics (Fig. 3), and changes in [3H]granisetron binding affinity (Table 3) in mutant receptors suggest that Tyr153 plays a role in both binding and gating of the receptor. The data show that both an aromatic ring and a hydroxyl group in the 4 position are required for correct receptor function. Therefore, removal of the aromatic ring (Ala, F5Phe, Ser) resulted in ≥100-fold EC50 increases, whereas deleting the tyrosine hydroxyl group (Phe) or replacing it (4-F-Phe or 4-Me-Phe) led to ∼20-fold increases in EC50, and relocating it (mTyr and Ser) resulted in ≥100-fold EC50 increases. However, 4-MeO-Phe increased EC50 only sixfold, indicating that the importance of the hydroxyl is via its O atom. These data strongly indicate the presence of a hydrogen bond, and the hydroxyl of Tyr153 acts as a hydrogen bond acceptor. This hydroxyl is located such that it could participate in a hydrogen bond interaction either with the 5-HT indole nitrogen, 2.9 Å away, or with Arg92 (3.1 Å). Arg92, like Tyr153, is located on the subunit “inner loop,” which is proposed to move relative to the “outer loop” after receptor activation (Unwin et al., 2002). Because the relative movement of the two residues during channel opening would therefore be negligible, it is unlikely that removing a hydrogen bond here would result in the changes that we observed. We therefore propose that Tyr153 hydrogen bonds to 5-HT.
The aromatic and hydroxyl groups of Tyr234 play distinct roles
Replacement of tyrosine at this position with Phe, 4-F-Phe, mTyr, and 4-Me-Phe produced receptors for which EC50 values for 5-HT activation were either unchanged or increased <10-fold compared with wild-type receptors. Thus, the hydroxyl group of Tyr234 is not essential for correct receptor function. Indeed, the equivalent residue here in the guinea pig 5-HT3 receptor is Phe, which, as we would have expected, has a slightly lowered EC50 value compared with other species (Lankiewicz et al., 1998). An aromatic group here, however, is important. A single F substituent (4-F-Phe) caused an increase in EC50, whereas multiple F substituents or removal of the aromatic ring (Cha, Ala, or Ser) resulted in nonfunctional receptors. F is small and nonbulky and therefore does not change the bulk of the aromatic ring; however, it significantly affects its electronic structure, making it “less aromatic” as the number of Fs increase.
The data also suggest that a cation-π interaction at Tyr234 is unlikely (the electrostatic potential of the aromatic rings in tyrosine, Phe, and 4-F-Phe is Phe ≈ Tyr >4-F-Phe, but the EC50 values were Tyr <4-F-Phe < Phe). This is interesting because in the model (Fig. 4), this residue has the potential to interact with the primary amine of 5-HT. It may be that the strength of the cation-π interaction between this group of 5-HT and Trp183 (estimated to be 4 kcal/mol) (Beene et al., 2002) renders an interaction with Tyr234 superfluous.
The pattern of EC50 values for unnatural amino acid substitution is consistent with bulk at the 4 position of the aromatic ring being required for correct receptor function. Those residues with a substituent at the 4 position (4-Me-Phe, 4-Br-Phe, and 4-F-Phe) give lower EC50 values than those without (Phe, mTyr). Such a finding was reported for the aligning residue (αTyr198) in the nACh receptor (Kearney et al., 1996), where a larger number of unnatural residues were tested. The size of this substituent also appeared to be important, with larger substituents not being tolerated and smaller ones also being less favored, suggesting a steric role.
A new model of the 5-HT binding pocket
We previously docked 5-HT into a model of the 5-HT3 receptor and used this model to further examine the data we report here (Fig. 4). The new data strongly support our previous suggestion that the correct orientation of 5-HT in the binding pocket is with the amino group located between Trp183 and Tyr234 and are also consistent with hydrogen bonds between Tyr143 and Trp183, and Tyr153 and 5-HT (Fig. 5). We also created a new “closed state” model based on the data of Unwin et al. (2002). These data indicate differences in the binding pocket between the closed state of the nACh receptor and AChBP (which is considered to be closer to the open state). In the closed state model, Tyr143 and 153 are 3 Å closer to the center of the pocket, and clashes between these residues and 5-HT indicate that binding of this agonist in the orientation supported by the experimental data is not possible (Fig. 5). Antagonist binding, however, would not be affected. Thus, if we assume that similar movements in the binding pocket occur in the nACh and 5-HT3 receptors, and that our new model provides a reasonable approximation of the closed state of the receptor, then Tyrs 143 and 153 would need to be displaced to allow 5-HT to dock into the binding site (Fig. 5B). We await high-resolution structural data to test this hypothesis.
Figure 5.
A, Model of the 5-HT3 receptor binding site in the closed state; Tyr143 and Tyr153 are 3 Å closer to the center of the pocket (created using SwissPdb viewer). B, Model of the 5-HT3 receptor binding site in the open state with 5-HT (purple) docked (as in Fig. 4), showing potential new hydrogen bonds formed by Tyr143 and Tyr153. Other hydrogen bonds may also form when the agonist binds (e.g., with Glu236), as discussed previously (Maksay et al., 2003; Reeves et al., 2003). C, Model of the 5-HT3 receptor binding site in the open state with granisetron (orange) docked using AUTODOCK based on the orientation favored by Maksay et al. (2003). Granisetron, however, is most likely to bind to the closed state and, thus, there may be some inaccuracies in this model. Nevertheless, granisetron in this orientation would fit into the closed state model (A), where it would be within 3Å of Tyr153 but further from Tyr143. D, Rotated model A with 5-HT (purple) docked in the same position as in B. Steric interference with Tyr143 and Tyr153 (green) would not allow 5-HT to be docked in this position.
Discussion
The in vivo non-sense suppression method of unnatural amino acid incorporation is a powerful tool for the investigation of receptor structure-function relationships. Using a selection of modified tyrosine and phenylalanine residues, we show that tyrosines located in or close to the receptor binding pocket each play a different role in receptor function and do not simply provide a featureless extended aromatic environment. Thus, a tyrosine at position 141 is not critical, but for efficient function the residues at position 143 and 153 form hydrogen bonds, and the residue at position 234 should be an aromatic with an appropriately sized substituent at the 4 position. These data, combined with previous data that have shown that Trp183 makes a cation-π interaction with the amine group of 5-HT (Beene et al., 2002), have allowed us to define the orientation of 5-HT in a homology model of the binding site and, combined with a new closed state model, lead us to propose that a series of bond rearrangements occur in the binding site that are required for gating and thus may be the trigger for the conformational changes that result in channel opening.
Roles of 5-HT3 receptor binding site tyrosine residues
Tyr141 does not play a critical role in 5-HT3 receptor agonist binding or gating, but it may be involved in antagonist binding and receptor assembly. The recent structure of AChBP bound to agonists (Celie et al., 2004) shows that the residue equivalent to Tyr141 (Leu102) hydrogen bonds to nicotine via a water molecule. In our model of the 5-HT3 receptor, Tyr141 does not contact 5-HT (and indeed in AChBP, residue Leu102 does not contact carbachol) but does not preclude it interacting with larger antagonists and/or to another residue in the binding pocket, perhaps during subunit folding, to assist its correct formation.
Tyr143 is a sensitive residue in that it can only be replaced by a limited number of alternative amino acids to form functional receptors. Data from these mutant receptors (no change in [3H]granisetron binding affinity and a large change in EC50) combined with the modeling data (no interaction with 5-HT) strongly suggest that Tyr143 forms a hydrogen bond between two regions of the receptor that is essential for receptor gating. Our data further suggest that this bond may be between the hydroxyl group and the backbone carbonyl group of Trp183, which has been previously suggested by Maksay et al. (2003). Previous data have also shown the importance of this residue in 5-HT3 receptor function (Venkataraman et al., 2002), and the equivalent residue in AChBP (Arg104) has been shown to make contact with carbachol (Celie et al., 2004).
The data also strongly suggest that Tyr153 forms a hydrogen bond, and that this bond plays a role in both binding and gating. The equivalent residue in AChBP (Met114) makes contact with both carbachol and nicotine (Celie et al., 2004), and data from other studies also suggest that it is involved in gating. Venkataraman et al. (2002) have observed that Tyr153Ala mutant receptors display unusual response and desensitization kinetics, and mutation of the aligning residue in the GABAA receptor γ2 subunit (Thr142) resulted in the antagonist flumazenil acting as a partial agonist (Mihic et al., 1994). Interestingly, the equivalent residue in the 5-HT3B receptor is histidine (Hanna et al., 2000). Thus, heteromeric (A plus B) rodent receptors would have this residue at the binding site. This provides support for our suggestion that the hydroxyl at Tyr153 acts as a hydrogen bond acceptor, because histidine is one of relatively few amino acids for which side chains can perform this function. The small shift in EC50 observed in heteromeric receptors, however, suggests this bond is less optimal.
Thus, our data show that the hydrogen bonds formed by Tyr143 and Tyr153 are critical for correct receptor function, suggesting these bonds play an important role in the conformational change leading to gating. This could be because they stabilize the bound state of the receptor and/or provide the energy required for protein rearrangement.
An aromatic residue at position 234 is essential for 5-HT3 receptor function. Indeed conservation of an aromatic residue at the aligning position among all 5-HT3 receptor subunits, and in fact all Cys-loop receptors, indicates the importance of this aromatic group, which appears to play a vital role as part of the “aromatic box” proposed to be critical for agonist binding in all members of this ligand-gated ion channel family (Mu et al., 2003). However, a substituent at the 4 position appears to be important solely for gating, because antagonist binding is unaffected by removal of the Tyr234 hydroxyl, and the docking data suggest that there is no interaction between this group and 5-HT (Reeves et al., 2003). The effect is steric, because hydroxyl and bromine, which have similar sizes, yield optimum gating efficiency, whereas smaller ones such as fluorine and hydrogen function less effectively.
A model for initiating gating
The differences observed between AChBP x-ray crystal data and nACh receptor cryoelectron microscopy data suggest that AChBP better represents the open rather than the closed state of the receptor (Unwin et al., 2002). Thus, the docking of 5-HT into the homology model of the 5-HT3 receptor binding site, as shown in Figure 4, is likely to be broadly accurate, whereas removal of 5-HT from this structure would not be a good representation of the closed state. Data from Unwin et al. (2002) show that in this region, the residues equivalent to Tyr143 and Tyr153 are closer to the residues equivalent to Trp183 and Tyr234 in the unbound (closed state) nACh receptor compared with AChBP (open-like state). These changes in distance are likely to be only a few angstroms, because the binding site is close to the point around which the inner and outer loops of the subunit pivot. Therefore, we created a 5-HT3 receptor binding site closed state model based on these data, which is shown in Figure 5A. Here, both Tyr143 and Tyr153 are 3 Å closer to the center of the binding pocket. We propose that 5-HT entering the pocket forms a cation-π bond with Trp183 and, in doing so, displaces Tyr143. The hydroxyl of Trp234 ensures that Tyr143 moves toward and subsequently hydrogen bonds with Trp183 and not with another E loop residue, as might be the case if formation of the alternative rotamer was not prevented by the group located here. Tyr153 is also relocated as 5-HT enters the pocket; assisted by the hydrogen bond, it forms with the tertiary amine on this molecule. These two residues are located on separate β sheets linked by a turn and, therefore, their combined movement could provide considerable torsional force. Combined with energy provided by the formation of hydrogen and other bonds, this could initiate the twist that triggers the conformational change.
Some support for this hypothesis comes from docking studies with the 5-HT3 receptor antagonist granisetron, which is not expected to cause a conformational change when bound. A recent report by Maksay et al. (2003) suggests that granisetron docked into a homology model of the 5-HT3 receptor binding site does not approach Tyr143 but does come relatively close to Tyr153 and interacts strongly with the aromatic group of Tyr234. Docking studies that we performed (K. L. Price and S. C. R. Lummis, unpublished observations) similarly reveal potential interactions of granisetron with Tyr234 and Tyr153 but not with Tyr143. One orientation of granisetron that we observed, which is similar to that reported by Maksay et al., (2003), is shown in Figure 5C. Granisetron in this orientation would also fit comfortably into the closed state model, and indeed it may hydrogen bond with the hydroxyl of Tyr153 (<3 Å distant) in this model.
Additional support comes from reports of movement in the nACh receptor binding site (Miyazawa et al. 2003; Unwin et al., 2002; Chakrapani et al., 2004) and in the GABAA receptor (Wagner and Czajkowski, 2001), which is likely to be similar in all Cys-loop receptors. However this has not yet been confirmed as no ligand-free atomic resolution structure of AChBP has yet been resolved. Thus we await with interest a high resolution structure of an unbound ligand-gated ion channel binding region to test the mechanistic aspects of our hypothesis.
In summary, the data obtained from introducing subtle changes to tyrosine residues in the 5-HT3 receptor binding site, combined with those obtained from models of the binding pocket, have shown distinct roles of each of these residues in the binding site. Combining these data with those from a model of the binding pocket has allowed us to generate a hypothesis of the mechanism that triggers the conformational change leading to channel opening. The data therefore show the power of unnatural amino acid mutagenesis in providing high-precision information that is highly complementary to modeling efforts. In addition, given the structural and functional similarity of ligand-gated ion channels, we believe the proposed mechanism of conformation change (agonist-stimulated movement of the binding loops combined with the formation of novel bonds) will be broadly similar in all members of the family.
Footnotes
This work was supported by The Wellcome Trust (S.C.R.L. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Science), the Medical Research Council (a studentship to K.L.P.), and the National Institutes of Health (Grants NS11756 and NS34407). We thank Dr. Nigel Unwin for helpful discussions.
Correspondence should be addressed to Sarah C. R. Lummis, Department of Biochemistry, Tennis Court Road, Cambridge CB2 1QW, UK. E-mail: sl120@cam.ac.uk.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249097-08$15.00/0
D.L.B. and K.L.P. contributed equally to this work.
References
- Beene DL, Brandt GS, Zhong W, Zacharias NM, Lester HA, Dougherty DA (2002) Cation-π interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: the anomalous binding properties of nicotine. Biochemistry 41: 10262-10269. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411: 269-276. [DOI] [PubMed] [Google Scholar]
- Celie PHN, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK (2004) Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 41: 907-914. [DOI] [PubMed] [Google Scholar]
- Chakrapani S, Bailey TD, Auerbach A (2004) Gating dynamics of the acetylcholine receptor extracellular domain. J Gen Physiol 123: 341-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer BA, Morton CJ, Parker MW (2002) Anxiety over GABAA receptor structure relieved by AChBP. Trends Biochem Sci 27: 280-287. [DOI] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ (1992) Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358: 761-764. [DOI] [PubMed] [Google Scholar]
- Grutter T, Prado de Carvalho L, Le Novere N, Corringer PJ, Edelstein S, Changeux JP (2003) An H-bond between two residues from different loops of the acetylcholine binding site contributes to the activation mechanism of nicotinic receptors. EMBO J 22: 1990-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna MC, Davies PA, Hales TG, Kirkness EF (2000) Evidence for expression of heteromeric serotonin 5-HT(3) receptors in rodents. J Neurochem 75: 240-247. [DOI] [PubMed] [Google Scholar]
- Hargreaves AC, Gunthorpe MJ, Taylor CW, Lummis SC (1996) Direct inhibition of 5-hydroxytryptamine3 receptors by antagonists of L-type Ca2+ channels. Mol Pharmacol 50: 1284-1294. [PubMed] [Google Scholar]
- Kearney PC, Nowak MW, Zhong W, Silverman SK, Lester HA, Dougherty DA (1996) Dose-response relations for unnatural amino acids at the agonist binding site of the nicotinic acetylcholine receptor: tests with novel side chains and with several agonists. Mol Pharmacol 50: 1401-1412. [PubMed] [Google Scholar]
- Kunkel TA (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 82: 488-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankiewicz S, Lobitz N, Wetzel CH, Rupprecht R, Gisselmann G, Hatt H (1998) Molecular cloning, functional expression, and pharmacological characterization of 5-hydroxytryptamine3 receptor cDNA and its splice variants from guinea pig. Mol Pharmacol 53: 202-212. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Grutter T, Changeux JP (2002) Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc Natl Acad Sci USA 99: 3210-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P (1992) Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861-871. [DOI] [PubMed] [Google Scholar]
- Maksay G, Bikadi Z, Simonyi M (2003) Binding interactions of antagonists with 5-hydroxytryptamine3A receptor models. J Recept Signal Transduct Res 23: 255-270. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Klein RL, Wafford KA, Harris RA (1994) A single amino acid of the human γ-aminobutyric acid type A receptor γ2 subunit determines benzodiazepine efficacy. J Biol Chem 269: 32768-32773. [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N (2003) Structure and gating mechanism of the acetylcholine receptor pore. Nature 424: 949-955. [DOI] [PubMed] [Google Scholar]
- Mu TW, Lester HA, Dougherty DA (2003) Different binding orientations for the same agonist at homologous receptors: a lock and key or a simple wedge? J Am Chem Soc 125: 6850-6851. [DOI] [PubMed] [Google Scholar]
- Nowak MW, Kearney PC, Sampson JR, Saks ME, Labarca CG, Silverman SK, Zhong W, Thorson J, Abelson JN, Davidson N, Schultz PG, Dougherty DA, Lester HA (1995) Nicotinic receptor binding site probed with unnatural amino acid incorporation in intact cells. Science 268: 439-442. [DOI] [PubMed] [Google Scholar]
- Nowak MW, Gallivan JP, Silverman SK, Labarca CG, Dougherty DA, Lester HA (1998) In vivo incorporation of unnatural amino acids into ion channels in Xenopus oocyte expression system. Methods Enzymol 293: 504-529. [DOI] [PubMed] [Google Scholar]
- Price K, Lummis SC (2004) The role of tyrosine residues in the extracellular domain of the 5-HT3 receptor. J Biol Chem 279: 23294-23301. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Lummis SC (2002) The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review). Mol Membr Biol 19: 11-26. [DOI] [PubMed] [Google Scholar]
- Reeves DC, Sayed MF, Chau PL, Price KL, Lummis SC (2003) Prediction of 5-HT3 receptor agonist-binding residues using homology modeling. Biophys J 84: 2338-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M, Abagyan R, Totrov M (2002) Structural model of nicotinic acetylcholine receptor isotypes bound to acetylcholine and nicotine. BMC Struct Biol 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N, Miyazawa A, Li J, Fujiyoshi Y (2002) Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the α subunits. J Mol Biol 319: 1165-1176. [DOI] [PubMed] [Google Scholar]
- Venkataraman P, Venkatachalan SP, Joshi PR, Muthalagi M, Schulte MK (2002) Identification of critical residues in loop E in the 5-HT3ASR binding site. BMC Biochem 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C (2001) Structure and dynamics of the GABA binding pocket: a narrowing cleft that constricts during activation. J Neurosci 21: 67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]