Abstract
Patient: Female, 48
Final Diagnosis: Plummer-Vinson syndrome
Symptoms: Chest pain • fatigue • palpitation
Medication: —
Clinical Procedure: Esophagogastroduodenoscopy (EGD) • colonoscopy
Specialty: General and Internal Medicine
Objective:
Rare disease
Background:
Plummer-Vinson syndrome (PVS) is a rare disorder composed of the triad of dysphagia, iron-deficiency anemia (IDA), and esophageal webs. It is most prevalent in middle-aged white women, and the dysphagia often improves when the anemia is treated. It is well established that chronic hypertension can lead to congestive heart failure (CHF). While IDA is frequently found concomitantly with CHF, there have been no reported cases of new-onset CHF with anemia presenting as PVS.
Case Report:
We present the case of a 48-year-old African American woman with symptomatic anemia and new-onset congestive heart failure secondary to hypertension, who presented with the classic symptoms of PVS.
Conclusions:
CHF with accompanying IDA may be an independent risk factor for the development of PVS. At the very least, there is an association between CHF-induced IDA and PVS. Patients presenting with CHF with symptoms of dysphagia should be considered at risk for the syndrome, and endoscopy may be warranted. Treatment for PVS includes iron replacement, and in some cases requires mechanical dilation.
MeSH Keywords: Anemia, Iron-Deficiency; Heart Failure; Plummer-Vinson Syndrome
Background
Plummer-Vinson syndrome (PVS) is a rare clinical disorder characterized by dysphagia, iron-deficiency anemia (IDA), and post-cricoid esophageal webs [1,2]. It is typically a disease of middle-aged white women and usually presents with painless, progressive dysphagia limited to solids [1–3]. Although PVS has been described from all parts of the world, the literature is still restricted to single case reports and small case series [4]. The anemia frequently associated with CHF may be related to multiple factors, including increased cytokine production, iron deficiency, or renal insufficiency. While the etiology remains unclear, several theories have been proposed regarding the underlying cause, including malnutrition, genetic predisposition, autoimmune processes, and other dietary deficiencies [2]. While correcting the IDA has reproducibly been shown to alleviate dysphagia in most patients, the extreme rarity of the condition in individuals of African descent suggests a genetic component as well. Nonetheless, a significant percentage of patients with CHF also have anemia, which is the antecedent risk factor for PVS. We present the case of a middle-aged African American woman with new-onset CHF and the classic symptoms of PVS who had improvement in her dysphagia after iron replacement therapy and treatment of CHF.
Case Report
A 48-year-old African American woman with past medical history only significant for uncontrolled essential hypertension presented to the Emergency Department with a chief complaint of palpitations. She also reported associated fatigue and shortness of breath. Over the past month, she described an intermittent sensation of an irregular heartbeat without any specific triggers. Her symptoms progressed to left-sided chest discomfort with associated left upper-extremity tingling. Additionally, she reported black spots in her vision, as well as dysphagia primarily to solids, which also had been worsening over the past few weeks. She had no history of similar symptoms in the past. She was not on any medications.
On admission, vital signs were significant for a blood pressure of 166/92 mmHg and heart rate of 109 beats/minute. A physical exam was significant for conjunctival pallor, without evidence of glossitis or koilonychia. In the Emergency Department, a complete metabolic panel was unremarkable including a serum creatinine of 0.56 mg/dL [0.60–1.20 mg/dL]. An electrocardiogram showed sinus tachycardia with occasional premature ventricular complexes. Troponins were within normal limits. Hemoglobin on admission was 5.7 g/dL [11.9–15.1 g/dL] with a mean corpuscular volume of 65.3 fL [80.0–96.0 fL]. Her serum iron on admission was 12 ug/dL [50–212 ug/dL], iron saturation was 3% [20–50%], and ferritin was 3.1 ng/mL [11.0–306.8 ng/mL]. The fecal immunochemical test (FIT) result was negative. Upon further questioning, the patient stated that she still menstruated, but did not describe menorrhagia. She denied hematemesis, hematochezia, or melena. She was transfused with 2 units of packed red blood cells in the Emergency Department and was started on 1400 mg of intravenous iron dextran complex infusions.
The patient was unable to sit for an esophagogram to evaluate for dysphagia secondary to nausea and vomiting while attempting to drink the contrast agent. Gastroenterology was consulted, and the patient underwent esophagogastroduodenoscopy (EGD) and colonoscopy on day 6 of her hospitalization. Colonoscopy results were within normal limits. The EGD revealed a few intrinsic stenoses in the upper esophagus, with the narrowest measuring 9 millimeters (Figure 1). Biopsies were obtained from the proximal and distal esophagus with cold forceps for suspected eosinophilic esophagitis versus Plummer-Vinson syndrome, based on gross appearance. A pathology exam of the esophagus showed squamous-only mucosa, with mild chronic inactive inflammation (Figure 2). No eosinophilia was seen. Given the clinical, laboratory, and endoscopic findings, a diagnosis of Plummer-Vinson syndrome was made. Her dysphagia improved over the next 9 days in the hospital after iron infusions and blood transfusions, and she was able to tolerate a regular diet by eating slowly with small bites.
Figure 1.
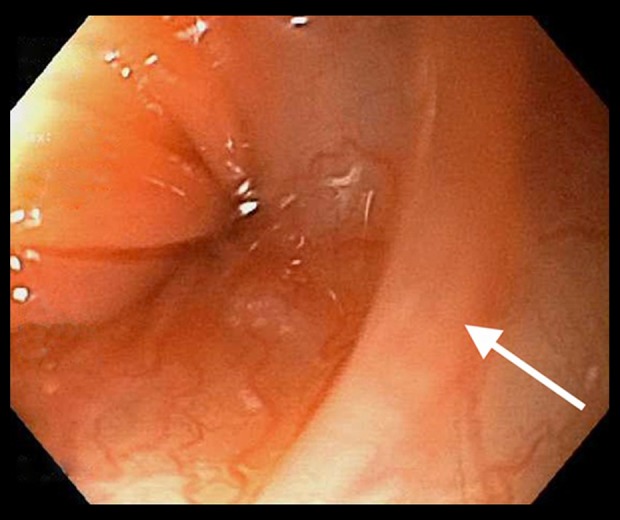
Esophageal web located in the upper esophagus.
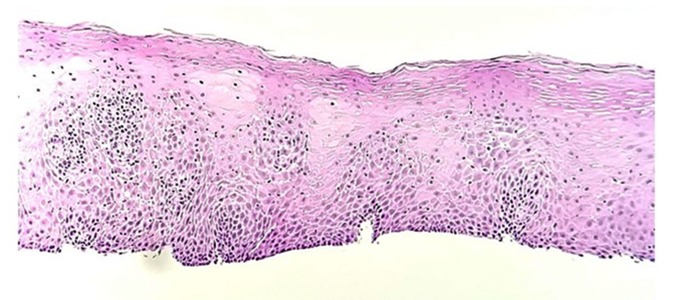
Figure 2.
Proximal esophagus biopsy shows squamous mucosa with mild chronic inactive inflammation (hematoxylin and eosin stain; original magnification ×100).
She underwent a cardiac workup to assess the palpitations, including an echocardiogram, which revealed an ejection fraction of 30–34%, with global hypokinesis and without valvular pathology or pulmonary hypertension. A nuclear stress test was negative for myocardial ischemia. A new diagnosis of congestive heart failure with reduced ejection fraction was made. Cardiology was consulted and she was started on carvedilol, lisinopril, spironolactone, and hydralazine. She was given a Life Vest in the hospital at discharge.
Approximately 6 months after her discharge, a follow-up phone call was made to the patient. She stated that she had been compliant with all of her medications and her dysphagia had resolved. At the time of the phone call, she was tolerating a regular diet without any dietary restrictions.
Discussion
Plummer-Vinson syndrome is a rare clinical disorder seldom seen in African American patients, and the presenting symptoms may reflect a disease process aggravated by anemia of varying etiologies. Our patient’s new-onset congestive heart failure secondary to long-standing hypertension may have been a precipitating factor leading to anemia and ultimately the development of PVS. The exact etiopathogenesis of how the triad of anemia, dysphagia, and esophageal webs develops remains speculative. However, it has been well established that PVS is most prevalent in white women ages 40–70 years old [1–3]. In the African American population, which has relatively high rates of IDA, cases of PVS are fairly uncommon, making our case particularly unique [5]. Additionally, there are several known comorbidities associated with PVS, including Celiac disease and menorrhagia, which may be underlying causes of IDA. However, to the best of our knowledge, there have been no reported cases of PVS with new-onset congestive heart failure, despite CHF being another well-established disorder associated with IDA [6]. The causes of Plummer-Vinson syndrome and of web formation remain unknown. The most widely accepted theory of how PVS develops is that IDA plays a role in the development of dysphagia and esophageal web formation. It is hypothesized that IDA causes dysfunction of iron-dependent enzymes in the esophagus, which in turn causes oxidative stress and damages cellular DNA. This is thought to cause degeneration and ultimately mucosal web formation [1]. The fact that dysphagia symptoms are often ameliorated by iron replacement therapy supports this theory. There are few recently published cases on PVS, likely due to improvements in nutritional status, but its recognition is important as it identifies a group of patients at increased risk of developing squamous cell carcinoma of the pharynx and the esophagus. Analysis of previous case reports showed that among patients with Plummer-Vinson syndrome, virtually all patients had IDA. Among patients with PVS, patients presented with a mean hemoglobin value of 8.2 g/dL [5]. In comparison, our patient had a much lower value of 5.7 g/dL. This may be explained by normal deviation from the mean, or by the patient’s anemia being caused by an underlying source different from those of previously reported cases, which in this case we believe to be new-onset congestive heart failure.
Up to 50% of patients with congestive heart failure with reduced ejection fraction (CHFrEF) have been diagnosed with IDA [7]. Moreover, IDA has been determined to be an independent predictor of morbidity and mortality in these patients [7]. One of the proposed etiologies of IDA in CHFrEF is malabsorption from increased inflammatory markers such as hepcidin and intestinal wall edema [8]. Given the inflammatory component in CHFrEF, a higher threshold of inflammatory markers is required to diagnose IDA in CHFrEF. Ferritin less than 100 ug/L and iron saturation <20% are used to diagnose IDA in CHFrEF, and this was noted in our patient [6,7]. Iron infusion is an effective treatment for IDA in both PVS and CHFrEF [7]. IDA in CHFrEF and IDA in PVS is well established in the literature. However, there are no previous case reports showing an association between PVS and CHFrEF. While our conjecture remains speculative as the first report of its kind, it is possible that in our patient’s case, CHFrEF-induced IDA led to the development of her PVS.
Additionally, Plummer-Vinson syndrome is a documented risk factor for developing squamous cell carcinoma in approximately 3–15% of patients [5]. The prognosis is especially poor in cases with an associated upper-gastrointestinal squamous cell carcinoma [9]. The diagnosis relies on a thorough workup that includes a full medical history and physical examination, laboratory investigation for anemia, and imaging that should include a barium swallow test. Additional endoscopic examinations may be used for direct visualization of the esophagus, with the opportunity for histopathologic analysis of biopsied tissue [5,6]. Iron supplementation alone can resolve dysphagia in many patients [1]. When the webs produce significant luminal stenosis causing dysphagia refractory to iron therapy, mechanical dilation of the webs can be performed [5]. In our case, the patient was treated with iron supplementation and her dysphagia improved over the course of her hospitalization.
In clinical workups of African American women presenting with symptomatic iron-deficiency anemia and dysphagia without signs of overt gastrointestinal bleeding, an endoscopic examination may be a useful diagnostic tool to detect esophageal webs as part of this rare syndrome. Additionally, in the setting of CHFrEF, providers should be aware of the possibility of resulting IDA and the importance of iron replacement. This would not only improve morbidity and mortality in these patients, but also may prevent the possibility of patients developing rare diseases induced by IDA, such as PVS.
Conclusions
Plummer-Vinson syndrome is likely an under-recognized disease process that is rare in African Americans. Our case demonstrates a previously undescribed cause of anemia in PVS patients – new-onset CHF – that may have ultimately led to the development of the patient’s symptomatology. Special efforts should be made to identify patients with CHF, who may have concomitant anemia presenting as PVS. Symptoms of dysphagia in the setting of IDA should prompt an appropriate workup for PVS, including possible barium swallow or endoscopic evaluation, as these patients are at increased risk for developing squamous cell carcinoma.
Footnotes
Conflict of interest
None.
References:
- 1.Verma S, Mukherjee S. Plummer-Vinson syndrome. StatPearls. 2019. https://www.ncbi.nlm.nih.gov/books/NBK538306/ [PubMed]
- 2.Goel A, Bakshi SS, Soni N, Chhavi N. Iron deficiency anemia and Plummer-Vinson syndrome: Current insights. J Blood Med. 2017;8:175–84. doi: 10.2147/JBM.S127801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann RM, Jaffe PE. Plummer-Vinson syndrome: A case report and literature review. Arch Intern Med. 1995;155:2008–111. doi: 10.1001/archinte.155.18.2008. [DOI] [PubMed] [Google Scholar]
- 4.Masri O, Sharara AI. Plummer-Vinson syndrome. Clin Gastroenterol Hepatol. 2013;11(12):e85. doi: 10.1016/j.cgh.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Novacek G. Plummer-Vinson syndrome. Orphanet J Rare Dis. 2006;1:36. doi: 10.1186/1750-1172-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah R, Agarwal AK. Anemia associated with chronic heart failure: Current concepts. Clin Interv Aging. 2013;8:111–22. doi: 10.2147/CIA.S27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Haehling S, Ebner N, Evertz R, et al. Iron deficiency in heart failure: An overview. JACC Heart Fail. 2019;7(1):36–46. doi: 10.1016/j.jchf.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Cunha GJL, Rocha BML, Menezes Falcão L. Iron deficiency in chronic and acute heart failure: A contemporary review on intertwined conditions. Eur J Intern Med. 2018;52:1–7. doi: 10.1016/j.ejim.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Messmann H. Squamous cell cancer of the oesophagus. Best Pract Res Clin Gastroenterol. 2001;15(2):249–65. doi: 10.1053/bega.2000.0172. [DOI] [PubMed] [Google Scholar]