It has been said that people with autism suffer from a lack of “central coherence,” the cognitive ability to bind together a jumble of separate features into a single, coherent object or concept (Frith, 1989). Ironically, the same can be said of the field of autism research, which all too often seems a fragmented tapestry stitched from differing analytical threads and theoretical patterns. Defined and diagnosed by purely behavioral criteria, autism was first described and investigated using the tools of behavioral psychology. More recent years have added brain anatomy and physiology, genetics, and biochemistry, but results from these new domains have not been fully integrated with what is known about autistic behavior. The unification of these many levels of analysis will not only provide therapeutic targets for prevention and remediation of autism but can also provide a test case for theories of normal brain and cognitive development. Autism research therefore has much to learn from and much to offer to the broader neuroscience community.
Clinical features
Clinically, autism is defined by a “triad” of deficits comprising impaired social interaction, impaired communication, restricted interests, and repetitive behaviors. Although in some cases speech never develops fully or never develops at all, in other cases, speech may be present but so inflexible and unresponsive to context that it is unusable in normally paced conversation; often, speech is limited to echolalia or confined to narrow topics of expertise in which discourse can proceed without conversational interplay. The communicative impairment extends also to nonverbal signals such as gaze, facial expression, and gesture. Social behaviors, too, are beset by a lack of flexibility and rapid coordination: children with autism do not coordinate attention between objects of mutual interest and the other people who may be interested in them, often engage in “parallel play” at the edge of a group rather than joining in cooperative play, and do not engage in pretend play. Intense and narrowly focused interests tend to concentrate on systems (Baron-Cohen, 2002) that operate deterministically and repeatably according to tractable sets of rules, whether these are abstract and complex systems such as computers or role-playing games or very concrete and simple systems such as toilets or washing machines. Critical to identifying the causal factors of autism, and key to its relevance to normal development, is the recognition that autism is actually the extreme of a spectrum of abnormalities. Milder phenotypes on this spectrum include Asperger syndrome (Wing, 1981) in which language is relatively unimpaired, and the “Broader Autism Phenotype” in which characteristic cognitive traits are present subclinically (Dawson et al., 2002). The combination of this broad variation of phenotypes and a 60-90% concordance rate in identical twins (Bailey et al., 1995) suggests a large number of genetic and environmental biasing factors (Muhle et al., 2004).
A basis in neural connectivity?
In addition to the central coherence paradigm, autism has been variously characterized as a deficit of executive function (Ozonoff et al., 1991), complex information processing (Minshew et al., 1997), theory of mind (Baron-Cohen et al., 1985), and empathy (Baron-Cohen, 2002). Each of these theories is a valid description of many aspects of the autistic syndrome but each, in answering unsolved questions at one level of explanation, introduces them at another. Recent attempts at a theoretical synthesis have focused on abnormal neural connectivity, and, superficially, there seems some disagreement as to whether this abnormality involves a surfeit (Rubenstein and Merzenich, 2003; Belmonte et al., 2004) or a deficit (Brock et al., 2002; Just et al., 2004) of connectivity. The picture is complicated by the fact that the term “connectivity” admits more than a single meaning. Conceptually, we can differentiate local connectivity within neural assemblies from long-range connectivity between functional brain regions. On another axis, we can also separate the physical connectivity associated with synapses and tracts from the computational connectivity associated with information transfer. Physically, in the autistic brain, high local connectivity may develop in tandem with low long-range connectivity (Just et al., 2004), perhaps as a consequence of widespread alterations in synapse elimination and/or formation (Sporns et al., 2000). Furthermore, indiscriminately high physical connectivity and low computational connectivity may reinforce each other by failing to differentiate signal from noise (Rubenstein and Merzenich, 2003; Belmonte et al., 2004) (Fig. 1). This model is consistent not only with the impairments in higher-order cognition described by the diagnostic triad but also with impairments of motor coordination (Teitelbaum et al., 1998), perceptual abnormalities such as high visual motion coherence thresholds (Milne et al., 2002) and broad tuning of auditory filters (Plaisted et al., 2003), abnormal growth within regions of local but not long-range white-matter projections (Herbert et al., 2004), and the substantial comorbidity of epilepsy with autism (Ballaban-Gil and Tuchman, 2000).
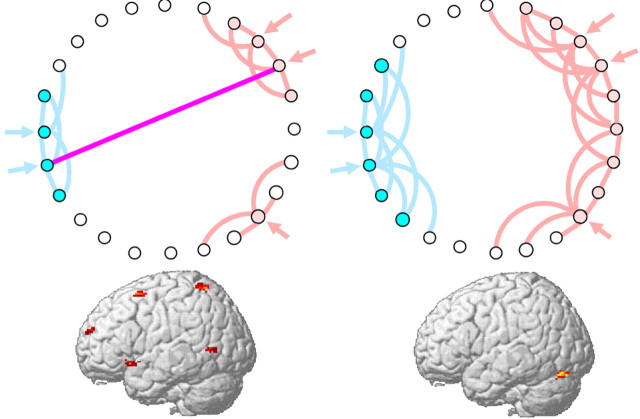
Figure 1.
Potential effects of network connectivity patterns on brain activation. In the network on the left, a combination of strong local connectivity within delimited groups of neural units and selective long-range connectivity between local groups constitutes a computational structure within which information can be efficiently represented and efficiently propagated. Inputs (double arrows) evoke representations that are easily differentiable from noise (single arrow) and can be linked across regions, yielding high computational connectivity. In the network on the right, strongly connected subregions are not appropriately delimited and differentiated, and computationally meaningful long-range connections fail to develop. The brain images at bottom, from a visual attention task, display distributed patterns of functional activation in the normal brain (left) and abnormally intense and regionally localized activation in the autistic brain (right), a pattern that may stem from such differences at the network level.
Functional anatomy in an abnormally wired brain
How can we test and refine this model of reduced information transfer as a consequence of local overconnectivity and long-range underconnectivity? One experimental approach is the physiological study of attention in autism using methods such as functional magnetic resonance imaging (fMRI) and evoked potentials. In an overconnected network, sensory inputs should evoke abnormally large activations for attended and unattended stimuli alike, giving rise within sensory regions to an overall increase in activation but a reduction in the selectivity of this activation, and potentially incurring a high load at later stages of perceptual processing as distractors are differentiated from targets. Conversely, brain regions subserving integrative functions will be cut off from their normal inputs and should therefore manifest reductions in activation and in functional correlations with sensory regions. A combination of EEG (Belmonte, 2000) and fMRI (Belmonte and Yurgelun-Todd, 2003) measures in a task of visual spatial attention demonstrates exactly this pattern. Furthermore, new data suggest abnormally strong activation in parietal cortex during suppression of distractors, at the same time as integrative regions in prefrontal and medial temporal cortices are abnormally quiescent (Belmonte and Baron-Cohen, 2004). Non-autistic brothers of people with autism seem to share the prefrontal and medial temporal hypoactivation but not the posterior hyperactivation, suggesting that low activity in integrative brain regions may be an endophenotype reflecting familial patterns of brain organization that may place individuals at heightened risk for autism.
The cerebellum and development of abnormal connectivity
Particularly implicated in deficits of long-range connectivity and coordination of cognitive functions is the cerebellum, one of the most common sites of anatomic abnormality in autism (Courchesne, 1997; Courchesne and Pierce, 2002). MRI morphometry reveals hypoplasia of the cerebellar vermis and hemispheres, and autopsy studies report reductions in numbers of cerebellar Purkinje cells. Moreover, recent genetic (Gharani et al., 2004) and MRI-behavior correlation (Akshoomoff et al., 2004; Kates et al., 2004) studies suggest that cerebellar abnormality may play a more central role in autism than previously thought. Neurobehavioral studies have shown associations between cerebellar anatomic abnormality and certain motor, cognitive, and social deficits (Haas et al., 1996; Harris et al., 1999; Townsend et al., 1999; Pierce and Courchesne, 2001).
Functionally, in autism, cerebellar activation is abnormally low during a task of selective attention (Allen and Courchesne, 2003) and abnormally high during a simple motor task (Allen et al., 2004). Both of these functional abnormalities correlate significantly with reduced size of cerebellar subregions, and it seems likely that this structure-function correspondence extends to the microscopic level and in particular to the reduction in Purkinje cell numbers. Such a reduction would release the deep cerebellar nuclei from inhibition, producing abnormally strong physical connectivity and potentially abnormally weak computational connectivity along the cerebello-thalamocortical circuit. This altered pattern of cortical excitation may produce aberrant activity-dependent patterning and may thus be related to findings of abnormal individual variability in cortical maps for motor function (Müller et al., 2001) and face processing (Pierce et al., 2001) and to abnormal overgrowth in frontal lobes (Carper and Courchesne, 2000).
Coordinated brain activity and the development of temporal binding
fMRI can capture inter-regional correlations on a timescale of seconds, but what about neural connectivity on a shorter timescale? Brock et al. (2002) proposed that underconnectivity between separate functional brain regions in autism might be reflected in a lack of EEG synchrony in the gamma band (30-80 Hz). In normal subjects, gamma activity is modulated by a variety of integrative processes, including feature binding (Tallon-Baudry et al., 1998), top-down feature selection (Hermann and Mecklinger, 2001), attention (Müller et al., 2000), face processing (Keil et al., 1999; Rodriguez et al., 1999), emotional arousal (Keil et al., 2001), and memory rehearsal (Tallon-Baudry et al., 1998, 1999). We are in the process of testing Brock's hypothesis using a delayed match-to-sample task, and preliminary data suggest abnormality of gamma activity over frontal cortex and visual processing areas during both stimulus encoding and memory rehearsal. Decreased and/or delayed gamma activation would suggest disrupted neural signaling and would support the hypothesis of abnormal regional activation patterns.
Linking altered structure and function with altered neural development
Neuropathological studies of cerebral cortex in autism indicate abnormalities of synaptic and columnar structure (Williams et al., 1980; Casanova et al., 2002) and of neuronal migration (Bailey et al., 1998a). MRI morphometry in young children with autism reveals excessive volume of cerebrum or cerebral white matter (Courchesne et al., 2001; Sparks et al., 2002; Herbert et al., 2003) or increased total brain volume (Piven et al., 1995; Aylward et al., 2002). The absence of such a volume difference in adults (Courchesne et al., 2001; Aylward et al., 2002) suggests that early hyperplasia in autism is followed by a plateau during which brain growth in normal subjects catches up. Retrospective analyses of head circumference measurements suggest that much of the overgrowth occurs postnatally within the first 6-14 months (Courchesne et al., 2003), coinciding with what is normally a period of exuberant synaptogenesis, dendritic arborization, and ongoing myelination. Regionally, frontal lobes show the greatest degree of enlargement and occipital lobes the least (Carper et al., 2002; Piven, 2004), and, within the frontal lobe, the dorsolateral convexity shows significant overgrowth, whereas precentral gyrus and orbital cortex are not robustly affected (Carper and Courchesne, 2004). Thus, the cortical areas most affected are precisely those broadly projecting, phylogenetically and ontogenetically late-developing regions that are essential to complex cognitive functions such as attention, social behavior, and language.
Fragile X syndrome and autism: are there common mechanisms in the development of synaptic connectivity?
The early developmental timing of brain overgrowth in autism, the neuropathological indications of altered synaptic structure, and the considerable dependence on genetics are especially interesting in light of the presence of autistic behavior in fragile X syndrome (FXS), a disorder with a known genetic cause and substantial symptomatic overlap with autism. Approximately one-quarter to one-third of people with FXS show the symptoms of autism (Bailey et al., 1998b; Rogers et al., 2001). FXS is caused by the silencing of a single gene (FMR1) (Pieretti et al., 1991) that codes for the fragile X mental retardation protein (FMRP), an RNA binding protein (Ashley et al. 1993) whose absence presumably alters expression of the genes associated with its mRNA cargoes. Thus, although FXS is in one sense a single-gene disorder, it is more proximally the result of disruption of complex patterns of expression of many genes, genes that may likewise be abnormally expressed in autism. Examinations of gross neuroanatomy as well as neuronal morphology in FXS have revealed specific structural alterations (for review, see Beckel-Mitchener and Greenough, 2004). Dendritic spines in specific cortical regions are present at high density and are abnormally long and thin, suggesting an immature morphology that may produce overconnectivity. Although large-scale, parallel studies with autistic brains are lacking, decreased dendritic branching in the hippocampi of two postmortem autistic brains (ages 7 and 9) (Raymond et al., 1996) suggests a reduction in connectivity. Additional work in autism is necessary to characterize neural structure across anatomical regions and developmental periods and to evaluate the possible roles of FMRP-associated genes.
Immune signaling in normal brain development and plasticity: implications for autism
One possible point of convergence between genetic and environmental causal factors in autism is immunological challenge. Autism and the immune system have been linked genetically and symptomatically (Warren et al., 1996; van Gent et al., 1997; Krause et al., 2002). Recent studies have shown that normal neurons in developing and adult brains express proteins of the major histocompatibility complex (MHC) class I, known for their role in the immune system (Corriveau et al., 1998; Huh et al., 2000). Furthermore, these immune proteins are required for specific forms of developmental and functional plasticity, demonstrating that changes in MHC expression can lead to neurodevelopmental defects. Interestingly, maternal viral infection at midpregnancy has been called “the principal nongenetic cause of autism” (Ciaranello and Ciaranello, 1995). Cerebellar Purkinje cells, which are reduced in autism, are a site of striking MHC class I expression. Decreased expression of MHC class I impairs the pruning of inappropriate synaptic connections (Huh et al., 2000), an effect that may explain the early developmental increase in brain volume in autism and the symptomatic overlap with FXS. A possibility currently being investigated is that specifically timed changes in neuronal MHC class I expression contribute to the development and/or expression of autism.
Conclusion
We have presented abnormal neural connectivity as an explanatory framework within which genetic and neuropathological findings on autism may be unified with neuroanatomy, neurophysiology, and behavior. Communication between these levels of analysis promises a greater understanding of mechanisms underlying both normal and pathological development of neural and cognitive systems and has the potential to render a multiplicity of experimental and theoretical approaches more coherent.
Footnotes
M.K.B. and S.J.W. were supported by grants from Cure Autism Now. L.M.B. received support from the Harvard Society of Fellows.
Correspondence should be addressed to Matthew K. Belmonte, Autism Research Centre, University of Cambridge, Douglas House, 18b Trumpington Road, Cambridge CB2 2AH, UK. E-mail: belmonte@mit.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249228-04$15.00/0
References
- Akshoomoff N, Lord C, Lincoln AJ, Courchesne RY, Carper RA, Townsend J, Courchesne E (2004) Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry 43: 349-357. [DOI] [PubMed] [Google Scholar]
- Allen G, Courchesne E (2003) Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am J Psychiatry 160: 262-273. [DOI] [PubMed] [Google Scholar]
- Allen G, Müller R-A, Courchesne E (2004) Cerebellar function in autism: functional magnetic resonance image activation during a simple motor task. Biol Psychiatry 56: 269-278. [DOI] [PubMed] [Google Scholar]
- Ashley Jr CT, Wilkinson KD, Reines D, Warren ST (1993) FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262: 563-566. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N (2002) Effects of age on brain volume and head circumference in autism. Neurology 59: 175-183. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25: 63-77. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P (1998a) A clinicopathological study of autism. Brain 121: 889-905. [DOI] [PubMed] [Google Scholar]
- Bailey Jr DB, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L (1998b) Autistic behavior in young boys with fragile X syndrome. J Autism Dev Disord 28: 499-508. [DOI] [PubMed] [Google Scholar]
- Ballaban-Gil K, Tuchman R (2000) Epilepsy and epileptiform EEG: association with autism and language disorders. Ment Retard Dev Disabil Res Rev 6: 300-308. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S (2002) The extreme male brain theory of autism. Trends Cogn Sci 6: 248-254. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U (1985) Does the autistic child have a “theory of mind”? Cognition 21: 37-46. [DOI] [PubMed] [Google Scholar]
- Beckel-Mitchener A, Greenough WT (2004) Correlates across the structural, functional, and molecular phenotypes of fragile X syndrome. Ment Retard Dev Disabil Res Rev 10: 53-59. [DOI] [PubMed] [Google Scholar]
- Belmonte MK (2000) Abnormal attention in autism shown by steady-state visual evoked potentials. Autism 4: 269-285. [Google Scholar]
- Belmonte MK, Baron-Cohen S (2004) Normal sibs of children with autism share negative frontal but not positive sensory abnormalities: preliminary evidence from fMRI during processing of visual distractors. Soc Neurosci Abstr 30: 582.10. [Google Scholar]
- Belmonte MK, Yurgelun-Todd DA (2003) Functional anatomy of impaired selective attention and compensatory processing in autism. Cognit Brain Res 17: 651-664. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook Jr EH, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang Y, DeLorey TM, Tierney E (2004) Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry 9:646-663. Unabridged edition available at http://www.cureautismnow.org/media/3915.pdf. [DOI] [PubMed]
- Brock J, Brown CC, Boucher J, Rippon G (2002) The temporal binding deficit hypothesis of autism. Dev Psychopathol 14: 209-224. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E (2000) Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain 123: 836-844. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E (2004) Localized enlargement of the frontal lobe in early autism. Biol Psychiatry, in press. [DOI] [PubMed]
- Carper RA, Moses P, Tigue ZD, Courchesne E (2002) Cerebral lobes in autism: early hyperplasia and abnormal age effects. NeuroImage 16: 1038-1051. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E (2002) Minicolumnar pathology in autism. Neurology 58: 428-432. [DOI] [PubMed] [Google Scholar]
- Ciaranello AL, Ciaranello RD (1995) The neurobiology of infantile autism. Annu Rev Neurosci 18: 101-128. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ (1998) Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21: 505-520. [DOI] [PubMed] [Google Scholar]
- Courchesne E (1997) Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol 7: 269-278. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K (2002) Autism. In: Encyclopedia of the human brain (Ramachandran VS, ed), pp 321-342. San Diego: Academic.
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY (2001) Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57: 245-254. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper RA, Akshoomoff NA (2003) Evidence of brain overgrowth in the first year of life in autism. JAMA 290: 337-344. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T (2002) Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol 14: 581-611. [DOI] [PubMed] [Google Scholar]
- Frith U (1989) Autism: explaining the enigma. Oxford: Blackwell.
- Gharani N, Benayed R, Mancuso V, Brzustowicz LM, Millonig JH (2004) Association of the homeobox transcription factor, ENGRAILED 2, with autism spectrum disorder. Mol Psychiatry 9: 474-484. [DOI] [PubMed] [Google Scholar]
- Haas RH, Townsend J, Courchesne E, Lincoln AJ, Schreibman L, Yeung-Courchesne R (1996) Neurologic abnormalities in infantile autism. J Child Neurol 11: 84-92. [DOI] [PubMed] [Google Scholar]
- Harris NS, Courchesne E, Townsend J, Carper R, Lord C (1999) Neuroanatomic contributions to slowed orienting of attention in children with autism. Cogn Brain Res 8: 61-71. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O'Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness Jr VS (2003) Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 126: 1182-1192. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness Jr VS (2004) Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol 55: 530-540. [DOI] [PubMed] [Google Scholar]
- Hermann C, Mecklinger A (2001) Gamma activity in human EEG is related to high-speed memory comparisons during object selective attention. Vis Cogn 8: 593-608. [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ (2000) Functional requirement for class I MHC in CNS development and plasticity. Science 290: 2155-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ (2004) Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127: 1811-1821. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, Reiss AL, Pearlson GD (2004) Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry 161: 539-546. [DOI] [PubMed] [Google Scholar]
- Keil A, Mueller M, Gruber T, Wienbruch C, Elbert T (1999) Human large-scale oscillatory brain activity during an operant shaping procedure. Cognit Brain Res 12: 397-407. [DOI] [PubMed] [Google Scholar]
- Keil A, Muller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T (2001) Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol 112: 2057-2068. [DOI] [PubMed] [Google Scholar]
- Krause I, He X-S, Gershwin ME, Shoenfeld Y (2002) Brief report: immune factors in autism: a critical review. J Autism Dev Disord 32: 337-345. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K (2002) High motion coherence thresholds in children with autism. J Child Psychol Psychiatry 43: 255-263. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Siegel DJ (1997) Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc 3: 303-316. [PubMed] [Google Scholar]
- Muhle R, Trentacoste SV, Rapin I (2004) The genetics of autism. Pediatrics 113: e472-e486. [DOI] [PubMed] [Google Scholar]
- Müller MM, Gruber T, Keil A (2000) Modulation of induced gamma band activity in the human EEG by attention and visual information processing. Int J Psychophysiol 38: 283-299. [DOI] [PubMed] [Google Scholar]
- Müller R-A, Pierce K, Ambrose JB, Allen G, Courchesne E (2001) Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance imaging study. Biol Psychiatry 49: 665-676. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington B, Rogers SJ (1991) Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J Child Psychol Psychiatry 32: 1081-1105. [DOI] [PubMed] [Google Scholar]
- Pierce K, Courchesne E (2001) Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry 49: 655-664. [DOI] [PubMed] [Google Scholar]
- Pierce K, Müller R-A, Ambrose J, Allen G, Courchesne E (2001) Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain 124: 2059-2073. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, Nelson DL (1991) Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 23: 817-822. [DOI] [PubMed] [Google Scholar]
- Piven J (2004) Longitudinal MRI study of 18-35 month olds with autism. Abstract presented at International Meeting for Autism Research, Sacramento, CA, May.
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P (1995) An MRI study of brain size in autism. Am J Psychiatry 152: 1145-1149. [DOI] [PubMed] [Google Scholar]
- Plaisted K, Saksida L, Alcantara JI, Weisblatt EJL (2003) Towards an understanding of the mechanisms of weak central coherence effects: experiments in visual configural learning and auditory perception. Philos Trans R Soc London B Biol Sci 358: 375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond GV, Bauman ML, Kemper TL (1996) Hippocampus in autism: a Golgi analysis. Acta Neuropathol 91: 117-119. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ (1999) Perception's shadow: long-distance synchronization of human brain activity. Nature 397: 430-433. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R (2001) The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr 22: 409-417. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2: 255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR (2002) Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59: 184-192. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM (2000) Theoretical neuroanatomy: relating anatomical and functional connectivity in graphs and cortical connection matrices. Cereb Cortex 10: 127-141. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J (1998) Induced gamma band activity during the delay of a visual short-term memory task in humans. J Neurosci 18: 4244-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Kreiter A, Bertrand O (1999) Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci 16: 449-459. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P, Teitelbaum O, Nye J, Fryman J, Maurer RG (1998) Movement analysis in infancy may be useful for early diagnosis of autism. Proc Natl Acad Sci USA 95: 13982-13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, Lowry TP, Press GA (1999) Spatial attention deficits in patients with acquired or developmental cerebellar abnormality. J Neurosci 19: 5632-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent T, Heijnen CJ, Treffers PD (1997) Autism and the immune system. J Child Psychol Psychiatry 38: 337-349. [DOI] [PubMed] [Google Scholar]
- Warren RP, Singh VK, Averett RE, Odell JD, Maciulis A, Burger RA, Daniels WW, Warren WL (1996) Immunogenetic studies in autism and related disorders. Mol Chem Neuropathol 28: 77-81. [DOI] [PubMed] [Google Scholar]
- Williams RS, Hauser SL, Purpura DP, DeLong GR, Swisher CN (1980) Autism and mental retardation: neuropathologic studies performed in four retarded persons with autistic behavior. Arch Neurol 37: 749-753. [DOI] [PubMed] [Google Scholar]
- Wing L (1981) Asperger's syndrome: a clinical account. Psychol Med 11: 115-129. [DOI] [PubMed] [Google Scholar]