Abstract
Dehydration causes an increase in the sodium (Na) concentration and osmolarity of body fluid. For Na homeostasis of the body, controls of Na and water intake and excretion are of prime importance. However, the system for sensing the Na level within the brain that is responsible for the control of Na- and water-intake behavior remains to be elucidated. We reported previously that the Nax channel is preferentially expressed in the circumventricular organs (CVOs) in the brain and that Nax knock-out mice ingest saline in excess under dehydrated conditions. Subsequently, we demonstrated that Nax is a Na-level-sensitive Na channel. Here we show that the subfornical organ (SFO) is the principal site for the control of salt-intake behavior, where the Nax channel is the Na-level sensor. Infusion of a hypertonic Na solution into the cerebral ventricle induced extensive water intake and aversion to saline in wild-type animals but not in the knock-out mice. Importantly, the aversion to salt was not induced by the infusion of a hyperosmotic mannitol solution with physiological Na concentration in either genotype of mice. When Nax cDNA was introduced into the brain of the knock-out mice with an adenoviral expression vector, only animals that received a transduction of the Nax gene into the SFO among the CVOs recovered salt-avoiding behavior under dehydrated conditions. These results clearly show that the SFO is the center of the control of salt-intake behavior in the brain, where the Na-level-sensitive Nax channel is involved in sensing the physiological increase in the Na level of body fluids.
Keywords: sodium channel, circumventricular organs, salt-intake behavior, SFO, OVLT, CSF
Introduction
Sodium (Na) is the major ionic component of extracellular fluids, and sodium homeostasis is inseparably linked with body-fluid control (Andersson, 1978). Thus, the dynamic regulation of Na and water intake is essential to life. When an animal is dehydrated, the Na concentration in body fluids, and accordingly plasma osmolarity, increases by 5-10% (Wakerley et al., 1978; Nose et al., 1992). A line of experimental evidence indicates that an Na increase in body fluids is detected by a putative Na sensor within the brain that is distinct from the osmosensor (McKinley et al., 1978; Denton et al., 1996).
The Nax channel has been classified as a subfamily of voltagegated Na channels (Goldin et al., 2000), although the consensus amino acid sequences essential for voltage sensitivity and channel inactivation are not well conserved (Goldin, 2001). The gene encoding Nax is designated SCN7A [Online Mendelian Inheritance in Man number 182392]. We generated Nax-gene knock-out mice by inserting the lacZ gene and identified Nax-producing cells by examining the lacZ expression (Watanabe et al., 2000). Besides alveolar type II cells in the lung and dorsal root ganglia and nonmyelinating Schwann cells in the peripheral nervous system (Watanabe et al., 2002), only limited areas of the CNS, including the subfornical organ (SFO), organum vasculosum of the lamina terminalis (OVLT), posterior pituitary, and median eminence, express Nax (Watanabe et al., 2000).
These loci, generically named circumventricular organs (CVOs), are midline structures situated in the walls of the ventricle and are accessed by the CSF via a single layer of specialized ependymal cells. In addition, they have characteristic structures of unusual dense and permeable capillary networks that facilitate tissue penetration of circulating substances (Johnson and Gross, 1993). SFO and OVLT harbor neuronal cell bodies with efferent neural connections to many other brain regions and are called the sensory CVOs (McKinley et al., 2003). The anterior wall of the third ventricle has been suggested to be involved in Na sensing (Andersson, 1978; Denton et al., 1996; Johnson et al., 2003). The Nax-gene knock-out mice showed hyperactivity of neurons in SFO and OVLT under dehydrated conditions (Watanabe et al., 2000).
We previously performed behavioral studies with the Nax-gene knock-out mice. Under dehydrated conditions, wild-type mice reduce salt ingestion. In contrast, the knock-out mice ingest excessive salt, despite their normal taste reception (Watanabe et al., 2000). We demonstrated subsequently that Nax is a Na-level-sensitive Na channel with a physiological threshold (Hiyama et al., 2002). When a series of sodium solutions higher than the physiological level were applied to Nax-positive cells isolated from the SFO, persistent inward Na currents appeared. The threshold value for the [Na+]o was 157 mm. Based on these findings, we have proposed that Nax is the bona fide Na-level sensor involved in the control of Na intake.
To identify the primary locus where the putative Na sensor for the regulation of Na and water intake is situated and verify the hypothesis that Nax functions as the endogenous sensor, we now performed behavioral studies by intracerebroventricular infusion of hypertonic solutions and site-directed expression of Nax by adenoviral transfer. These studies demonstrated that the SFO primarily detects the Na-level increase in the CSF through the function of the Nax channel and controls the Na-intake behavior.
Materials and Methods
Animals and housing. All animal experiments were conducted in accordance with the guidelines of the National Institute for Basic Biology (Okazaki, Japan). For all tests, male mice at 8-12 weeks of age were used. Both Nax knock-out and wild-type mice were congenic, having been backcrossed for 10-12 generations (N10-N12) to the C57BL/6J line. We have confirmed that the knock-out mice and wild-type mice are individually congenic enough to show the respective salt-intake behaviors uniformly.
All mice were adapted to the experimental room for at least 1 week before experimentation, with ad libitum access to water and food (0.32% sodium; CLEA Rodent Diet CE-2; CLEA Japan, Tokyo, Japan). They were housed individually in plastic basket cages under a constant room temperature (23°C) with a 12 hr light/dark cycle (incandescent lights on at 7:00 A.M.; the brightness of the room was adjusted to 100 lux). All experiments were started at 7:00 P.M., and the room was kept dark during the experiments. Human activity in the room was restricted to the light period as were all experimental manipulations. For any surgical procedure, mice were anesthetized with sodium pentobarbital (50 mg/kg body weight, i.p. injection), and the operation was conducted under aseptic conditions.
Water- and salt-intake measurements. Preference-aversion behavior was measured with a 12 hr preference test. Mice were isolated in individual cages 1 week before the experiment. To prepare dehydrated conditions, mice were deprived of water for 48 hr with feeding. Just before the start of the experiment, mice were presented with a choice of distilled water or a 0.3 m NaCl solution. The trial lasted for 12 hr in cages set in the circadian-rhythm measuring system (Neuroscience, Tokyo, Japan). During the experiment, the system was operated from outside of the room. When the two genotypes were to be compared, both genotypes were always tested in pairs (see Figs. 1, 2). To monitor the intake volume, a dropper was inserted in the tube supplying each solution, and the number of drops was counted with an optical based counter. Data on the drop number were collected in 10 min bins by the AB System (Neuroscience). The preference ratio was defined as the ratio of volumes of saline intake and total fluid intake.
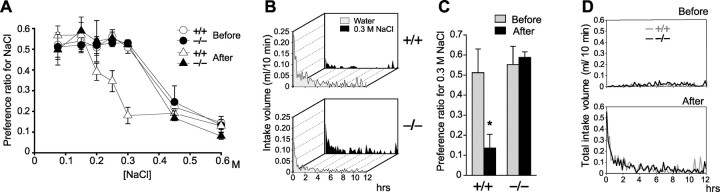
Figure 1.
Nax knock-out mice exhibit abnormal salt-intake behavior under dehydrated conditions. A, Preference-aversion function for various saline concentrations. To avoid differences in the cumulative effect depending on the salt-intake history of mice, 10 mice were used freshly for each concentration (accordingly, a total of 70 mice for each genotype), and feeding was stopped for 12 hr during the test. *p < 0.01 by one-tailed Mann-Whitney tests; mean ± SE; n = 10. B, Averaged time course of water and saline (0.3 m NaCl) intake in wild-type (+/+) and knock-out (-/-) mice during the dark phase immediately after 48 hr dehydration. Each point shows the average quantity per 10 min period. n = 10. C, Preference ratio for the 0.3 m NaCl solution for 12 hr before and after 48 hr dehydration. The data were obtained with the volumes of water and saline consumed during the period of 12 hr. *p < 0.01 by one-tailed Mann-Whitney tests; mean ± SE; n = 10. D, Averaged time course of total intake of water and saline in wild-type (+/+) and knock-out (-/-) mice during 12 hr in the dark phase immediately after 48 hr dehydration. Each point shows the average of six mice for 10 min bins of data.
Figure 2.
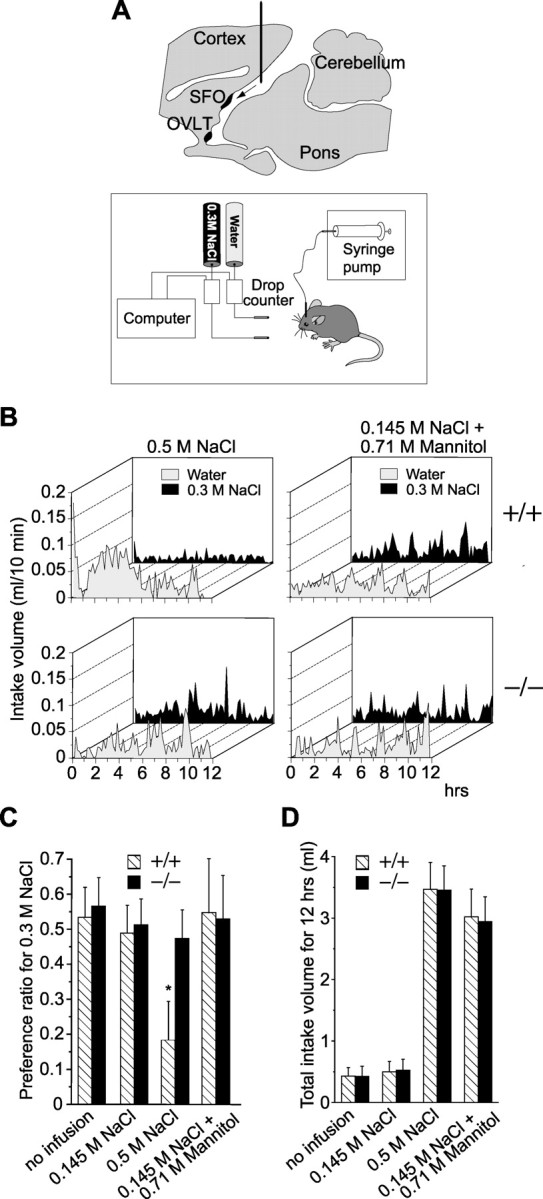
Nax knock-out mice are insensitive to increases of the Na level in the CSF. A, Top, Location of the cannula for intracerebroventricular microinfusions. The tip of the cannula was positioned at the lateral ventricle. Bottom, A schematic representation of the experimental setup for the two-bottle test. Two drinking tubes were presented to free-moving mice infused with sodium solutions into the cerebral ventricle for 12 hr. B, Averaged time course of water and saline (0.3 m NaCl) intake in wild-type (+/+) and knock-out (-/-) mice during intracerebroventricular infusions of a hypertonic (0.5 m) NaCl solution or hypertonic mannitol (0.145 m NaCl plus 0.71 m mannitol) solution. Each point shows the average quantity per 10 min period. n = 10. C, Preference ratio for the 0.3 m NaCl solution for 12 hr during intracerebroventricular infusions of test solutions. *p < 0.01 by one-tailed Mann-Whitney tests; mean ± SE; n = 10. D, Total intake volume for 12 hr during intracerebroventricular infusions of test solutions. Mean ± SE; n = 10.
Intracerebroventricular microinfusion. Na solutions were infused into the cerebral ventricle via a chronically implanted cannula, and the preference of the animal for the 0.3 m NaCl solution was examined using a 12 hr two-bottle test. Mice were first implanted with a guide stainless steel cannula (26 gauge) stereotaxically aiming at the lateral ventricle (antero-posterior, -0.22 mm; lateral, +1.0 mm; ventral, +1.5 mm; relative to bregma). The coordinates were determined from an atlas (Paxinos and Franklin, 2001), and the placement of the cannula in the lateral ventricle was confirmed by the injection of Evans blue (2 μl) in several test mice. The cannulas were positioned in place with acrylic dental cement and secured with skull screws. A stylus was placed in the guide cannula to prevent clogging. Animals were allowed 1 week to recuperate. Subsequently, the stylus was removed, and then a 32 gauge injection cannula was inserted into the guide cannula. The injection cannula was connected via PE20 tubing to a microsyringe driven by a microinfusion pump. The isotonic (0.145 m NaCl), hypertonic (0.5 m NaCl) saline, or hypertonic mannitol (0.145 m NaCl plus 0.71 m mannitol) solution was infused at a rate of 0.013 μl/min into the lateral ventricle of the brain in free-moving mice for 12 hr.
Construction of adenoviral expression vectors and infection. For construction of the adenoviral Nax expression vector, a 6.1 kb mNav2.3 cDNA fragment including 0.3 kb of 5′ noncoding, 5.0 kb of coding, and 0.8 kb of 3′ noncoding regions (GenBank accession L36179) was used. The adenoviral vectors were constructed as described previously (Miyake et al., 1996). An adenoviral vector carrying the Nax gene was coinjected with that carrying the egfp gene into the brain of 102 mice with microglass pipettes. The tip of the pipette was aimed at the anterior wall of the third ventricle, especially at the SFO or OVLT. Separately, the egfp adenoral vector was singly injected into the SFO of 12 mice. The adenoviral concentration used in our experiments was ∼2 × 106 pfu/ml. We could not detect any change either in the cytoarchitecture or in the electrophysiological properties of cells in the SFO in the control adenoviral infection experiments.
All the mice were allowed to recuperate for 1 week and then subjected to the two-bottle preference tests for 0.3 m NaCl against pure water under various conditions. Subsequently, animals were perfused transcardially first with PBS and then with 10% formaldehyde under deep pentobarbital anesthesia. The fixed brains were cut coronally 100-μm-thick with a microtome (VT1000S; Leica, Nussloch, Germany). The images of the slices were captured by a cooled CCD camera (Cool Snap; Roper Scientific, Duluth, GA) with a stereoscopic microscope (MZ Apo; Leica). The site of adenoviral infection was examined afterward by the fluorescence of enhanced green fluorescent protein (EGFP), and the infected regions were identified by the atlas (Paxinos and Franklin, 2001). Among 102 mice that received the two adenoviral vectors carrying egfp and Nax, 43 mice were successfully infected in the parenchyma of the brain at 20 loci: the cingulate cortex, corpus callosum, lateral septal nucleus, triangular septal nucleus, dorsal fornix, septofimbrial nucleus, ventral hippocampal commissure, fimbrial hippocampus, SFO, choroid plexus, paracentral thalamic nucleus, paraventricular thalamic nucleus, parataenial thalamic nucleus, stria medullaris of thalamus, fornix, OVLT, median preoptic nucleus, ventromedial preoptic nucleus, medial septal nucleus, and ependymal cells.
Results
Nax knock-out mice do not stop ingesting salt when dehydrated
To analyze the daily pattern of water and salt intake in a free-moving state, we developed a drinking-behavior monitoring system for mice. Using this system, we measured the amount drank by individual mice. Then the preference ratios, defined as the volume ratio of saline intake divided by saline plus pure water intake in 12 hr, was calculated (Fig. 1A). When fully satiated with water, both the Nax knock-out and wild-type mice showed a progressive aversion at concentrations above 0.3 M. The preference-aversion curves for a series of NaCl solutions was nearly identical between the two genotypes, confirming that the Na- and water-intake behavior of the knock-out mice is normal in the hydrated condition (Watanabe et al., 2000). After dehydration for 2 d, however, the behavioral difference between the two genotypes became apparent. The preference-aversion curve of wild-type mice shifted to lower concentrations; that is, aversion to saline became more sensitive. In contrast, the curve of the knock-out mice was not changed at all by the dehydration. The difference between the two genotypes was greatest at 0.3 M in the dehydrated condition. We determined to use 0.3 M NaCl in the subsequent preference assays.
Using the same system, we monitored the time course of in-take of pure water and 0.3 M NaCl independently. After dehydration, animals of both genotypes rushed to drink fluids in large amounts, and subsequently, the drinking rate decreased gradually (Fig. 1B). When dehydrated, wild-type mice preferred pure water and avoided the hypertonic saline, and as a result, the preference ratio for 0.3 M NaCl was markedly reduced. In contrast, the knock-out mice took both pure water and 0.3 M NaCl equally, and the preference ratio for 0.3 M NaCl was not changed by dehydration (Fig. 1C). Despite this difference, the time course profiles of the total volume drank (the total amount of pure water plus 0.3 M NaCl) were not significantly different between the two genotypes not only before but also after the water deprivation (Fig. 1D).
Nax knock-out mice showed abnormal salt-intake behavior during microinjection of hypertonic NaCl into the cerebral ventricle
In dehydrated animals, the Na concentration of body fluids is obviously inclined to rise all over the body. Next, we examined the effects of the direct stimulation of CVOs with hypertonic Na solutions from the cerebral ventricle on drinking behavior in the two-bottle test (Fig. 2A). When the isotonic saline (0.145 M NaCl) was continuously infused into the ventricle, both genotypes took 0.3 m NaCl and water equivalently (Fig. 2C), indicating that neither the operation nor injection itself affected the drinking behavior. When the hypertonic saline (0.5 M NaCl) was infused into the cerebral ventricle, however, wild-type animals clearly avoided the salt solution (Fig. 2C). In contrast to the wild type, the knock-out mice did not show such an aversion to the salt solution (Fig. 2C). The time course of the drinking behavior is shown in Figure 2B. Notably, the initial prompt water-drink response in wild-type mice was not observed in the knock-out mice.
On the other hand, when both genotypes were infused with a hypertonic mannitol solution in isotonic saline (0.145 M NaCl plus 0.71 M mannitol; the osmotic pressure being ∼0.5 M NaCl), there was no significant difference in the preference ratios at ∼0.5 between the two genotypes (Fig. 2B,C). Importantly, the total intake volume was increased equally in both genotypes when mice were infused with the hypertonic saline solution and hypertonic mannitol solution (Fig. 2D), suggesting that the increase in total intake is regulated by the osmolarity. These results indicate that Nax is involved in sensing an increase in the level of Na in the CSF and is specifically relevant to the control of Na intake. On the other hand, the control of the total intake is independent of Nax.
Abnormal salt-intake behavior of Nax knock-out mice was rescued by transduction of the Nax gene into the SFO
To specify the brain locus from which the difference in Na- and water-intake behavior in the two genotypes originates, the Nax gene was locally introduced into the brain of the knock-out mice with an adenoviral expression vector carrying Nax under the control of the cytomegalovirus promoter. An adenoviral expression vector encoding the egfp gene was coinjected to identify the infected site after the experiments. Except for the cases in which the vectors leaked into the cerebral ventricle, 43 mice were finely infected in the parenchyma of the brain, although individual loci showed subtle differences. One week after the injection, the mice were subjected to the behavioral tests to examine whether Na-aversion behavior was rescued. When the preference ratio for 0.3 m NaCl was lower than 0.2 in the preference test under dehydrated conditions, we concluded that the salt-aversion behavior was completely restored.
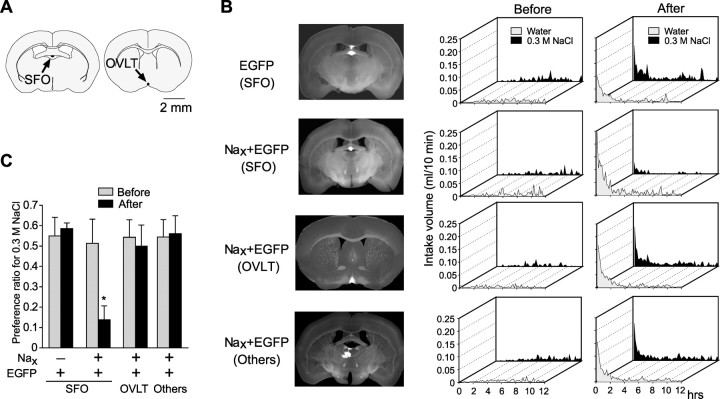
Among 102 knock-out mice that received the Nax gene together with the egfp gene, six showed the Na aversion as in the wild-type mice when dehydrated. Here, the intake of 0.3 m NaCl was decreased and that of water was increased by the Nax gene transduction, the total intake again being constant. We then analyzed the infected regions in these mice. Of note is that all of the six mice that were conferred with the Na-aversion behavior showed a common infection finely in the SFO (Fig. 3B,C; Nax plus EGFP, SFO). Some mice with partial restoration of the Na-aversion behavior (the preference ratio is 0.5-0.2) showed a weak infection in the SFO along with the other main loci of infection (data not shown). The Nax channel expression in the SFO thus appeared to be essential and sufficient for the control of salt-intake behavior.
Figure 3.
Abnormal salt-intake behavior of Nax knock-out mice was rescued by introduction of the Nax gene to SFO. A, The location of the SFO and OVLT in a coronal section of the mouse brain. B, The coronal sections of the brain showing the loci infected by the expression of EGFP (left column). Time course of water and saline (0.3 m NaCl) intake by the infected mice before and after 48 hr dehydration (middle and right columns, respectively). Behavioral data are the average of six mice that were successfully infected in a specific site in the brain by an adenoviral vector encoding egfp (EGFP) or by vectors encoding Nax and egfp (Nax and EGFP). C, Preference ratio for the 0.3 m NaCl solution. *p < 0.01 by one-tailed Mann-Whitney test; mean ± SE; n = 6.
We then prepared the knock-out mice (n = 6) such that the adenoviral vector encoding the egfp gene was singly injected in the SFO. Their preference for saline was unchanged in the dehydrated condition, notwithstanding that the EGFP was successfully expressed in the SFO [Fig. 3B,C; EGFP (SFO)]. This control experiment indicates that the operation and introduction into the SFO of the adenoviral vectors themselves did not affect the salt-intake behavior.
Next, we analyzed the behavioral data of six mice that had received the Nax and egfp genes in the OVLT to verify that they were not behaviorally rescued [Fig. 3B,C; Nax plus EGFP (OVLT)]. For additional confirmation, another group of six mice who had received both genes at loci other than the SFO and OVLT were analyzed [Fig. 3B,C; Nax plus EGFP (Others)]. In addition, when the Nax-adenoviral vector was injected into the ventricle, a small number of ependymal cells were sparsely infected; however, this transduction of Nax was not effective in the behavioral rescue of the knock-out mice (data not shown). Thus, the Na-aversion behavior of the knock-out mice under dehydrated conditions was recovered only when the adenoviral vector carrying Nax was successfully introduced into the SFO. This strongly indicates that the defects of the knock-out mice in detecting the systemic Na concentration and control of Na-intake behavior is mainly attributable to the deficiency in Nax channel expression in the SFO.
Discussion
Since Bengt Andersson hypothesized the existence of a sodium receptor three decades ago (Andersson, 1971), the molecular entity of the Na-specific sensor and the precise location of the Na-sensing cells have been a major interest of physiologists. In this report, we demonstrated that SFO is the primary site of Na-level sensing for the control of Na-intake behavior, where Na-level-sensitive channel Nax plays an essential role in the sensing.
Abnormal salt intake of Nax knock-out mice is attributed to brain defects and not to tasting
As we reported (Watanabe et al., 2000), Nax knock-out mice have a normal tasting ability, including the ability to taste salt. This is clear also from the experimental result that the sensitivity to saline is normal in the knock-out mice: the two genotypes showed an equal aversion to saline solutions over 0.3 M (Fig. 1A) under water-satiated conditions. Dehydrated wild-type animals show a shift in concentration sensitivity to the lower side (Fig. 1A). Here, the threshold for the concentration of salt that is judged to be harmful to the animal is thought to change from 0.3 to 0.15 M. Accordingly, dehydrated wild-type mice exhibited an aversion to saline at concentrations over 0.15 M, more than the physiological level. On the other hand, the knock-out mice appear to be deficient in this resetting ability of the threshold in the brain that is required when dehydrated.
Na-level sensing and osmosensing in the brain
For body-fluid homeostasis, neuroendocrine and behavioral regulatory systems must respond to fluctuations in the Na and water balance in body fluids. Because dehydration is reflected in the loss of free water, an osmotic increase is usually accompanied by proportional rises in the Na level in the plasma and CSF. For this reason, the existence and necessity of a Na-specific receptor distinct from osmoreceptors was long controversial (Johnson and Edwards, 1990). In this report, we injected a hypertonic mannitol solution in physiological saline to investigate the effect of hyperosmolarity independent of the Na level on Na and water intake and found that the response to hyperosmolarity is normal in the knock-out mice, suggesting the existence of an osmosensing system(s) distinct from the Na-level sensing system. In the SFO, the vanilloid receptor-related channel, activated osmotically, has been reported as a candidate for the vertebrate osmoreceptor (Liedtke et al., 2000). However, this channel is gated in response to hypotonicity, and the molecule for detecting hypertonicity remains to be elucidated.
It is well recognized that circulating angiotensin II is a stimulator of Na and water intake. Intracerebroventricular infusion of losartan, an angiotensin II type I receptor (AT1) antagonist, has been reported to reduce Na and water intake in several species, including mice (Blair-West et al., 1994). AT1 is preferentially expressed in the SFO and OVLT (McKinley et al., 2003), and angiotensin II induces c-fos expression at these loci (Denton et al., 1996). Intravenous injection of angiotensin II induced both Na and water intake (Buggy and Fisher, 1974). Our results showed that the Na aversion under dehydrated conditions was impaired in the knock-out mice, but the total intake of water and saline did not differ between the two genotypes, suggesting that the abnormal behavior in the knock-out mice is not directly relevant to the angiotensin system. This view is supported by a previous report showing that different populations of neurons are excited by hypertonic saline and angiotensin II in both the SFO and OVLT (Denton et al., 1996). The total intake of water and saline is speculated to be regulated through distinct mechanisms by signals from osmoreceptors and angiotensin receptors, but salt intake in dehydrated animals is specifically controlled by the Nax signal.
Intracerebroventricular injection suggested a role for Nax in water-intake control
When the hypertonic Na solution (0.5 M NaCl) was infused into the cerebral ventricle, wild-type mice took a large quantity of water immediately in response to the infusion and then stopped drinking within 1 hr (Fig. 2B). The knock-out mice did not show such a response, suggesting that Nax is involved in this water intake by sensing a rise in the Na concentration in the CSF. When the hypertonic mannitol solution was infused, neither genotype of mice showed this prompt and robust water drinking behavior (Fig. 2B), in contrast to the dehydrated mice (Fig. 1B). This indicates that osmotic increase in the CSF is not so effective as to immediately induce thirst in animals, in contrast to Na-level increase.
The pause in drinking mentioned above is considered a feedback suppression by neural inputs to the brain from the oropharynx and/or hepatic portal vein (Stricker and Sved, 2000). However, the mice subsequently began to drink water again, but not the salt solution, during the experiment. Such a delayed phase of water intake, which was not observed in the dehydrated animals (Fig. 1B), was probably induced by the continuous infusion of the hypertonic saline. Because of the persistent infusion of the hypertonic saline, the Na concentration in the CSF presumably kept elevating and was not compensated merely by a robust water intake.
Nax in the SFO but not OVLT is the primary sodium-level-sensing site
When Nax cDNA was introduced into the brain of the knock-out mice with adenoviral expression vector, animals that received transduction of the Nax gene into the SFO among the CVOs regained the salt-avoiding behavior under dehydrated conditions. This strongly indicates that Nax in the SFO is the primary site of sodium-level sensing and for the control of salt-intake behavior. It is known that the SFO has efferents to integrative and effector motor regions in the brain, including the amygdaloid nucleus (Johnson et al., 1999; McKinley et al., 2003). These neural pathways would be directly responsible for the control of the Naand water-intake behavior. On the other hand, transduction of the Nax gene into the OVLT could not rescue the abnormal salt-intake behavior. There exist efferent and afferent projections between the SFO and OVLT (McKinley et al., 2003), and both regions are selectively hyperactived in the knock-out mice under dehydrated conditions (Watanabe et al., 2000). Despite close similarities, there must be functional differences between the two sensory loci. Many neurons in the dorsal cap of the OVLT (and to a lesser extent in the SFO) send efferent projections to the supraoptic nucleus (SON) (McKinley et al., 1994). The neurons in the OVLT are considered to drive the neurons in the SON to secrete vasopressin in response to dehydration.
In summary, this study showed for the first time that the Nax sodium channel in the SFO is the primary sodium-level sensor for the control of salt-intake behavior. To know the functional differences among Nax channels distributed in the several CVOs, additional investigation based on the identification of Nax-positive cells and neural networks will be essential.
Footnotes
This research was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Japan Science and Technology Agency (Core Research for Evolutional Science and Technology and Precursory Research for Embryonic Science and Technology), The Asahi Glass Foundation, The Mitsubishi Foundation, and The Japan Science Society. We are very grateful to Dr. Michael M. Tamkun for mouse Nax cDNA, Mie Yasuda and Satsuki Unou for technical assistance, and Akiko Kodama for secretarial assistance.
Correspondence should be addressed to Dr. Masaharu Noda, Division of Molecular Neurobiology, National Institute for Basic Biology, 5-1 Higashiyama, Myodaiji-cho, Okazaki, Aichi 444-8787, Japan. E-mail: madon@nibb.ac.jp.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249276-06$15.00/0
References
- Andersson B (1971) Thirst-and brain control of water balance. Am Sci 59: 408-415. [PubMed] [Google Scholar]
- Andersson B (1978) Regulation of water intake. Physiol Rev 58: 582-603. [DOI] [PubMed] [Google Scholar]
- Blair-West JR, Burns P, Denton DA, Ferraro T, McBurnie MI, Tarjan E, Weisinger RS (1994) Thirst induced by increasing brain sodium concentration is mediated by brain angiotensin. Brain Res 637: 335-338. [DOI] [PubMed] [Google Scholar]
- Buggy J, Fisher AE (1974) Evidence for a dual central role for angiotensin in water and sodium intake. Nature 250: 733-735. [DOI] [PubMed] [Google Scholar]
- Denton DA, McKinley MJ, Weisinger RS (1996) Hypothalamic integration of body fluid regulation. Proc Natl Acad Sci USA 93: 7397-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL (2001) Resurgence of sodium channel research. Annu Rev Physiol 63: 871-894. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA (2000) Nomenclature of voltage-gated sodium channels. Neuron 28: 365-368. [DOI] [PubMed] [Google Scholar]
- Hiyama TY, Watanabe E, Ono K, Inenaga K, Tamkun MM, Yoshida S, Noda M (2002) Nax channel involved in CNS sodium-level sensing. Nat Neurosci 5: 511-512. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Edwards GL (1990) The neuroendocrinology of thirst: afferent signaling and mechanisms of central integration. Curr Top Neuroendocrinol 10: 151-190. [Google Scholar]
- Johnson AK, Gross PM (1993) Sensory circumventricular organs and brain homeostatic pathways. FASEB J 7: 678-686. [DOI] [PubMed] [Google Scholar]
- Johnson AK, de Olmos J, Pastsuskovas CV, Zardetto-Smith AM, Vivas L (1999) The extended amygdala and salt appetite. Ann NY Acad Sci 877: 258-280. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Beltz TG, Thunhorst RL, Johnson AK (2003) Investigations on the physiological controls of water and saline intake in C57BL/6 mice. Am J Physiol Regul Integr Comp Physiol 285: R394-R403. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Denton DA, Weisinger RS (1978) Sensors for antidiuresis and thirst-osmoreceptors or CSF sodium detectors? Brain Res 141: 89-103. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Hards DK, Oldfield BJ (1994) Identification of neural pathways activated in dehydrated rats by means of Fos-immunohistochemistry and neural tracing. Brain Res 653: 305-314. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ (2003) The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: III-XII, 1-122. [DOI] [PubMed] [Google Scholar]
- Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I (1996) Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA 93: 1320-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose H, Doi Y, Usui S, Kubota T, Fujimoto M, Morimoto T (1992) Continuous measurement of Na concentration in CSF during gastric water infusion in dehydrated rats. J Appl Physiol 73: 1419-1424. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, Ed 2. New York: Academic.
- Stricker EM, Sved AF (2000) Thirst. Nutrition 16: 821-826. [DOI] [PubMed] [Google Scholar]
- Wakerley JB, Poulain DA, Brown D (1978) Comparison of firing patterns in oxytocin- and vasopressin-releasing neurones during progressive dehydration. Brain Res 148: 425-440. [DOI] [PubMed] [Google Scholar]
- Watanabe E, Fujikawa A, Matsunaga H, Yasoshima Y, Sako N, Yamamoto T, Saegusa C, Noda M (2000) Nav2/NaG channel is involved in control of salt-intake behavior in the CNS. J Neurosci 20: 7743-7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E, Hiyama TY, Kodama R, Noda M (2002) Nax sodium channel is expressed in non-myelinating Schwann cells and alveolar type II cells in mice. Neurosci Lett 330: 109-113. [DOI] [PubMed] [Google Scholar]