Abstract
Neuronal transmission relies on signals transmitted through a vast array of excitatory and inhibitory neuronal synaptic connections. How do axons communicate with dendrites to build synapses, and what molecules regulate this interaction? There is a wealth of evidence suggesting that cell adhesion molecules (CAMs) provide much of the information required for synapse formation. This review highlights the molecular mechanisms used by CAMs to regulate presynaptic and postsynaptic differentiation.
Keywords: adhesion, axon terminal, dendrite, neuron, synapse, synaptogenesis
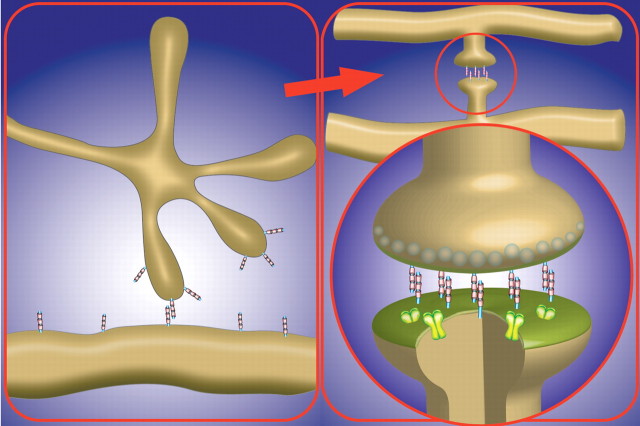
In the nervous system, information is exchanged between neurons at sites of contact known as synapses. Before becoming a chemical synapse, a physical contact is typically formed between an axon and a dendrite. Contact and synapse formation is commonly thought to occur between an axonal filopodium (often from a growth cone) and a dendritic shaft (Ahmari et al., 2000; Jontes et al., 2000). However, dendritic filopodia have also been seen to play an active role in synapse formation (Ziv and Smith, 1996; Jontes et al., 2000). Initial contact establishment is followed by spatially and temporally controlled changes in morphology and molecular content to form a mature synapse characterized by the specific accumulation of synaptic vesicles and active zone components within the axon, in close apposition to a dendritic membrane studded with receptors (Fig. 1), which are held in place by a submembranous scaffold (Sheng and Kim, 2002).
Figure 1.
Diagram depicting the morphological transformation of an axodendritic contact to a mature synapse. Contact between an axonal growth cone filopodia and a dendrite via the homophilic adhesion molecule SynCAM 1 (blue-pink) develops to form a stereotypical excitatory synapse. SynCAM, an example of one of several synaptically localized adhesion molecules, remains at the synapse to hold the presynaptic and postsynaptic terminals together. The presynaptic terminal is filled with synaptic vesicles (white spheres), and neurotransmitter receptors (green) are recruited to the postsynaptic membrane.
Recent advances in subcellular fluorescence microscopy have revealed that the transformation of a contact site to a synapse involves the rapid recruitment and stabilization of both presynaptic and postsynaptic elements. These studies have shown that major components of the synaptic vesicle and active zone machinery travel in clusters together with other presynaptic proteins, such as calcium channels, and are rapidly recruited to new sites of contact (Ahmari et al., 2000; Zhai et al., 2001; Washbourne et al., 2002). On the postsynaptic side, receptor subunits and components of the scaffold or PSD (postsynaptic density) are recruited separately and with distinct time courses within minutes to hours after initial contact (Friedman et al., 2000; Bresler et al., 2001, 2004; Washbourne et al., 2002).
These studies, however, have not shed light on key factors that control this process. Elucidation of the molecular mechanisms by which a transient contact between axonal growth cone and dendrite is transformed into a synapse is crucial for understanding the synaptic deficits that ultimately underlie disorders ranging from autism to schizophrenia (Zoghbi, 2003). In early studies using myoballs and motoneurons, it was apparent that adhesion played a strong role in setting up a communicative link between two cells (Chow and Poo, 1985). Although adhesion molecules had long been surmised to play a role in at least holding the contact together, it was not until very recently that membrane-bound CAMs were discovered to be major players in triggering synapse formation (Scheiffele et al., 2000; Biederer et al., 2002; Sytnyk et al., 2002; Fu et al., 2003) and thus acting as signal transducers. Furthermore, studies have shown that the action of adhesion molecules is not limited to initial contact formation but is also involved in specific target recognition (Yamagata et al., 2002; Shen and Bargmann, 2003; Shen et al., 2004) and regulation of synaptic size and strength (for review, see Scheiffele, 2003; Yamagata et al., 2003).
Because of the asymmetric nature of the synapse, the formation of the initial point of contact requires different responses in the presynaptic and postsynaptic structures (Fig. 1). Cell contact mediated by the heterophilic interaction between neuroligin and β-neurexin is the best understood mechanism for setting up the asymmetry at the synapse. However, the action of several other CAMs, including protocadherins and synaptic cell adhesion molecule 1 (SynCAM 1), are known to be or likely to be mediated by homophilic interactions (Obata et al., 1995; Biederer et al., 2002). It remains unclear whether these separate adhesive interactions may differentially modulate synaptic specificity and whether all act in concert to produce the huge variety of possible synaptic connections and strengths.
Here, we summarize recent findings on the roles of the following cell adhesion molecules in establishing synapses: neural cell adhesion molecule (NCAM), neuroligin/β-neurexin, SynCAM 1, and γ-protocadherins. We also discuss the recent discovery that thrombospondins (TSPs), adhesive extracellular matrix (ECM) molecules, are novel glia-released factors involved in this process.
Synapse formation mediated by NCAM
NCAM was one of the first membrane proteins implicated in adhesion between neurons. NCAM belongs to the Ig superfamily of CAMs and is involved in homophilic and heterophilic interactions with recognition molecules. Many biological functions of NCAM are mediated by the negatively charged carbohydrate polysialic acid, which appears to be exclusively carried by this molecule and is synthesized in a developmentally, cell type-, and activity-dependent manner (Muller et al., 1996; Eckhardt et al., 2000; Kleene and Schachner, 2004). Previous studies suggested functions for NCAM in fasciculation of axons to form bundles, such as the retinotectal and mossy fiber projections (Rutishauser, 1985; Cremer et al., 1997). More recent data suggest that NCAM is involved in both early synaptogenesis and subsequent synaptic maturation. Studies on developing hippocampal neurons maintained for 2-3 d in culture have shown that clusters of NCAM at the cell surface are linked via spectrin to trans-Golgi-derived organelles (Sytnyk et al., 2002). These NCAM-organelle complexes translocate along neurites to sites of contacts within minutes after initial contact formation. Experiments in heterogenotypic cocultures of NCAM-deficient and wild-type neurons provide evidence that both presynaptic and postsynaptic NCAM is required to anchor trans-Golgi-derived organelles at nascent synapses (Sytnyk et al., 2002). At later stages (4-7 d in vitro), the relative levels of postsynaptic but not presynaptic NCAM expression regulates both the number of synapses and strength of excitatory synaptic connections in an NMDA receptor-dependent manner (Dityatev et al., 2000). This mechanism involves the interaction between the polysialylated form of NCAM and heparan sulfate proteoglycans and requires fibroblast growth factor receptor (FGFR)-mediated signaling (Dityatev et al., 2004). Strikingly, a peptide corresponding to the binding site of NCAM to FGFR promotes synapse formation and memory (Cambon et al., 2004), indicating that CAM-derived compounds have potential therapeutic value. The importance of mammalian NCAM for synapse dynamics is further supported by the following: (1) activity-dependent expression of NCAM (Schuster et al., 1998); (2) impaired long-term potentiation and depression in mice deficient in NCAM or associated polysialic acid (Muller et al., 1996; Eckhardt et al., 2000; Bukalo et al., 2004); (3) alterations in transmission, vesicle dynamics, and transmitter release machinery at NCAM-deficient neuromuscular junctions (Polo-Parada et al., 2004); and (4) pivotal roles of NCAM homologs, fasciclin II and Aplysia cell adhesion molecule (apCAM), in formation of synapses in Drosophila and Aplysia (for review, see Packard et al., 2003).
Role for neuroligin and β-neurexin in excitatory synapse formation
The heterotypic cell adhesion “couple” β-neurexin and neuroligin was proposed as a potential trigger of synaptogenesis when it was discovered that neuroligin, a transmembrane protein identified as an interacting protein of β-neurexin [a splice variant of the latrotoxin receptor α-neurexin (Ichtchenko et al., 1995)], was able to induce the formation of presynaptic terminals onto nonneuronal cells (Scheiffele et al., 2000; Fu et al., 2003). Further characterization of the presynaptic side of this heterotypic system established that it is indeed the clustering of β-neurexin that brings about the recruitment of synaptic vesicles (Dean et al., 2003).
Previously, it had been established that neuroligin contained a PDZ (postsynaptic density 95/Discs large/zona occludens 1) binding domain that could bind PSD-95 (Irie et al., 1997) and could therefore establish a link between the presynaptic cell and the postsynaptic density. It was further determined that neuroligin-mediated adhesion promotes the functional recruitment of postsynaptic NMDA receptors probably via the interaction with PSD-95 when transiently expressed in nonneuronal cells (Fu et al., 2003). Increasing neuroligin-mediated adhesion by overexpression stimulates recruitment of postsynaptic scaffolding molecules such as PSD-95 and Homer and synaptic recruitment of NMDA receptors (B. Chih and P. Scheiffele, unpublished observations). This indicates that β-neurexin-neuroligin adhesion complexes promote synapse assembly in a bidirectional manner and provide a platform for postsynaptic neurotransmitter receptor recruitment. Suppression of neuroligin-1, -2, and -3 expression by RNA interference leads to a loss of synapses and dendritic spines in hippocampal neurons (Chih and Scheiffele, unpublished observations). Interestingly, also a loss of inhibitory terminals formed on cells with reduced neuroligin-1, -2, and -3 expression was observed. Therefore, the function of neuroligins in synaptogenesis might not be restricted to excitatory synapses, as anticipated previously. This is consistent with the observation that endogenous neurexins are concentrated at excitatory and inhibitory presynaptic terminals (Ullrich et al., 1995). These in vitro studies suggest that the β-neurexin-neuroligin adhesion system is essential for the appropriate assembly of synaptic circuits. Such an essential role is further supported by the recent realization that mutations in neuroligin-3 and -4 genes are associated with mental retardation and autism (Philibert et al., 2000; Jamain et al., 2003; Laumonnier et al., 2004), two neurodevelopmental disorders that are characterized by aberrant spine morphogenesis and defects in dendritic arbor growth. All presently identified neuroligin-3 and -4 mutations result in a loss of function, either attributable to truncation or a point mutation of the cholinesterase domain, which result in intracellular retention (Chih et al., 2004; Comoletti et al., 2004). Furthermore, it appears that point mutations and chromosomal rearrangements in regions of the genome containing the genes for neuroligin-2 and PSD-95 are associated with autism (Mariner et al., 1986; Risch et al., 1999).
New work from Prange et al. (2004) demonstrates that concerted actions of cell adhesion molecules and scaffolding proteins regulate the morphology, number, and type of synapses formed. For example, the presence of PSD-95 dictates what kinds of synapses are induced by neuroligin-1: excitatory or inhibitory. PSD-95 restricts neuroligin-1 to excitatory synapses, and manipulations that alter endogenous levels of these proteins result in an overall change in the ratio of excitatory to inhibitory synaptic contacts. Therefore, improper expression and/or targeting of molecules that control synaptic specificity may result in formation of aberrant synapses and a change in the balance of neuronal excitation-inhibition that underlie complex psychiatric disorders (Rubenstein and Merzenich, 2003).
SynCAM
SynCAM 1 and neuroligins are the only two known CAMs that are sufficient to drive formation of presynaptic terminals (Scheiffele et al., 2000; Biederer et al., 2002; Fu et al., 2003) (Y. Sara, T. Biederer, D. Atasoy, M. G. Mozhayeva, T. C. Sudhof, and E. T. Kavalali, unpublished observation). SynCAM 1 is a vertebrate-specific Ig superfamily member with homophilic binding properties. It is localized to both sides of the synaptic cleft (Fig. 1) and shares similarities with the invertebrate molecules fasciclin II and apCAM (Biederer et al., 2002). Like neuroligin, when SynCAM 1 is expressed in nonneuronal cells and cocultured with neurons, the formation of presynaptic terminals is induced. In such a coculture system, the activities of SynCAM 1 and neuroligin-1 are highly comparable with respect to the properties of synaptic vesicle recycling in the induced presynaptic terminals. Furthermore, recent results show that either SynCAM 1 or neuroligin-1 can be used to reconstitute both evoked release and spontaneous miniature events (minis) (Biederer et al., 2002; Fu et al., 2003) (Sara, Biederer, Atasoy, Mozhayeva, Sudhof, and Kavalali, unpublished observation). This is probably attributable to the fact that both adhesion molecules may share the same presynaptic signaling pathways. The known protein interaction motifs in the cytosolic tails of SynCAM 1 and β-neurexins are highly conserved. They therefore presumably bind the same adaptor proteins at the presynaptic terminal. However, binding proteins, such as CASK (calcium/calmodulin-dependent serine kinase) have so far only been characterized in vitro (Biederer and Südhof, 2001; Biederer et al., 2002), and the downstream partners of SynCAM 1 and β-neurexin in vivo remain to be identified. Studies with dominant-negative constructs strongly indicate that PDZ domain containing adaptor proteins are involved in this process (Biederer et al., 2002). Via this presynaptic signaling, both SynCAM 1 and neuroligins are capable of inducing presynaptic terminals with a complete physiological complement.
SynCAM 1 overexpression in hippocampal neurons specifically promotes excitatory synaptic transmission and increases the frequency of minis (Biederer et al., 2002). This activity of SynCAM 1 depends on its cytosolic sequence, highlighting the importance of finding the downstream signaling partners to understand the function of synaptic adhesion molecules (Sara, Biederer, Atasoy, Mozhayeva, Sudhof, and Kavalali, unpublished observation). SynCAM 1 is expressed throughout the CNS, and its developmentally controlled expression and glycosylation pattern (see below) strongly suggests that it exerts its synaptogenic activity predominantly during early development (Biederer et al., 2002). However, a role for SynCAM 1 may still be determined at adult synapses.
Protocadherins
In addition to the requirement for homotypic and heterotypic CAM interactions for the general formation of synaptic contacts, CAM interactions have the potential to provide molecular mechanisms for determining synaptic partners. This could be considered a corollary to Sperry's classic “chemoaffinity hypothesis” (Sperry, 1963) in which molecular cues present on presynaptic and postsynaptic neurons determine synaptic specificity in a “lock-and-key” manner. Because of the vast complexity of synaptic connections, such a mechanism would require great combinatorial diversity; the discovery of the α-, β-, and γ-protocadherin genes (Kohmura et al., 1998; Wu and Maniatis, 1999) has provided one potential source of such diversity. Over 50 alternative exons, each encoding the extracellular, transmembrane, and proximal cytoplasmic domains of a particular protocadherin isoform, are arrayed in tandem on a single chromosome in the mouse and human genomes (Wu and Maniatis, 1999; Wu et al., 2001). Each such “variable” exon is, in the α and γ clusters, spliced to three “constant” exons that encode a common C-terminal domain. Multiple α- and γ-protocadherin genes are highly expressed by many neurons, and the proteins are concentrated at synapses (Kohmura et al., 1998; Wang et al., 2002a,b; Phillips et al., 2003). Thus, the protocadherins have the potential to channel a great multiplicity of presynaptic and postsynaptic specificities into a common signal transduction pathway.
Evidence that protocadherins are important molecules in synaptic development was obtained recently from studies by J. A. Weiner, X. Wang (Northwestern University, Evanston, IL), and J. R. Sanes (Harvard University, Cambridge, MA) on the analysis of mice mutant for the 22 γ-protocadherins. Mice lacking the entire locus (Pcdh-γdel/del) die at birth, exhibiting little voluntary movement or reflex action (Wang et al., 2002b). This is attributable to massive late embryonic apoptosis of spinal interneurons and a concomitant neurodegeneration and loss of spinal synaptic density. To differentiate between defects associated with neurodegeneration and synapse loss, apoptosis was blocked by crossing the Pcdh-γdel/del mice with mice lacking the gene encoding Bax, a pro-cell death member of the Bcl-2 family (Deckwerth et al., 1996). As expected, the neurodegenerative phenotype is completely rescued in the resulting double mutants; however, the mice still die at birth with reduced motor activity and exhibit a reduction in spinal synaptic density similar to that of the Pcdh- γdel/del single mutants. Similar synaptic defects (and neonatal lethality) in the absence of neurodegeneration are also observed in a hypomorphic mouse mutant in which the common Pcdh-γ C terminus is truncated. This result confirms the phenotype in the Pcdh-γ/Bax double mutants and suggests that synapse loss in the double mutants is unlikely to reflect simply aberrant neuronal phenotypes attributable to artificial blocking of the cell death program (J. A. Weiner, X. Wang, J. C. Tapia, and J. R. Sanes, in preparation).
Together, these results confirm that γ-protocadherins are required for normal synaptic development. Initial electrophysiological analyses of mutant spinal neurons in vitro have revealed reductions in the amplitude of both EPSCs and IPSCs, corresponding to reductions in size and number of immunostained synaptic puncta. However, it remains unclear whether these defects reflect primarily a disruption of synapse formation, specificity, or maturation. Examination of the dynamics of synapse assembly in Pcdh-γ mutant neurons can be addressed in spinal neuron cultures, but analysis of synaptic specificity will likely require further genetic manipulations in vivo of particular neuronal subtypes with defined projection patterns.
Thrombospondins
Very little is known on how synaptic adhesion and synapse formation is modulated. Surprisingly, recent evidence suggests that synapse formation may be regulated by key secreted factors that are produced by astrocytes. Consistent with an important role for glia in synapse formation, the bulk of CNS synapse formation takes place only after astrocytes develop. The discovery that CNS synapse number is profoundly enhanced by signals secreted by astrocytes (Ullian et al., 2001) prompted the search for soluble factors involved in this process. Recently, TSPs were discovered as soluble factors that are released from astrocytes. TSPs are necessary and sufficient for synapse formation and are capable of inducing ultrastructurally normal synapses that are presynaptically active but postsynaptically silent (K. S. Christopherson, E. M. Ullian, and B. A. Barres, unpublished observation). In vivo, TSPs are concentrated at astrocyte processes and synapses throughout the developing brain. Furthermore, mice deficient in two of the TSP isomers expressed in the CNS (TSP1 and TSP2) show a significant decrease in synapse number in cortex and superior colliculus (Christopherson, Ullian, and Barres, unpublished observation).
How do TSPs induce synaptogenesis? TSPs are large oligomeric ECM molecules that act as multifunctional regulators of cell-cell and cell-ECM adhesive interactions (Adams and Tucker, 2000; Lawler, 2000; Adams, 2001; Bornstein, 2001). There are five TSP family members that form either homotrimers or homopentamers. TSP1 and TSP2, the closely related trimeric TSPs, share the same domain structure and are both able to promote synapse formation. Many of the known effects of TSPs in other systems are mediated by direct or indirect modulation of integrin function, although other TSP-interacting molecules have been identified, including CD36, LRP (lipoprotein receptor-related protein), calreticulin, heparan and chondroitin sulfate proteoglycans, and heparan-binding growth factors such as TGF-β1. Although little is known about how TSPs could be functioning in the CNS (Iruela-Arispe et al., 1993; Scott-Drew and ffrench-Constant, 1997; Adams and Tucker, 2000), it is plausible that they promote synapse formation by aligning or maintaining adhesion of presynaptic and postsynaptic specializations. This idea is supported by the observation that treatment of neurons with TSP-depleted astrocyte conditioned medium results in the induction of presynaptic and postsynaptic puncta that are no longer colocalized (Christopherson, Ullian, and Barres, unpublished observation). Alternatively or concurrently, TSPs could be mediating certain intracellular signaling pathways by binding to one of their known cell surface receptors. To elucidate the cellular and molecular mechanism by which TSPs induce synaptogenesis, the B. A. Barres laboratory at Stanford University (Stanford, CA) is investigating the domain(s) of TSP responsible for promoting synaptogenesis. Together, these findings show that TSP1 and TSP2 promote CNS synaptogenesis and add to the growing evidence that astrocytes are active participants in CNS synaptogenesis in vivo.
Concluding remarks
In light of the remarkable heterogeneity of CNS synapses, it is not surprising that the factors that govern the diversity of synaptic contacts have been elusive. Recently, functional studies of individual adhesion molecules have begun to provide a wealth of information on their role in synapse assembly, spine morphogenesis, and synaptic plasticity. Although the disparate adhesion systems share the ability to mediate adhesive interactions, they individually control specific aspects of synapse formation. Because it appears that multiple systems cooperate at individual synapses, it is of great interest to determine whether separate CAM systems act in a parallel or in a hierarchical manner. Cadherins, neurexins, and several other recently identified synaptic adhesion proteins are coupled to common cytoplasmic scaffolds, and their functions might be integrated at that level, with molecules of one class triggering recruitment of adhesive factors from a different family. In contrast, the strong synaptogenic activity displayed by SynCAM and β-neurexin-neuroligin suggests that these may be positioned upstream of other CAMs. It could be envisioned that these “early” CAMs are required for the initial synaptogenic event and that “late” CAMs are then required to decide what type of synapse it becomes or whether the synapse is stabilized and matures. It is conceivable that certain CAMs may also regulate the size of specific neuronal populations. The requirement for γ-protocadherins in both synapse development and neuronal survival suggests that they may provide a molecular link between the processes of neuronal apoptosis and synaptogenesis during development.
Another outstanding question is how the activity of a particular CAM may be regulated either developmentally or in response to activity. For instance, palmitoylation and polysialylation drastically modify adhesive properties, binding partners, and signaling mediated by NCAM (Rutishauser and Landmesser, 1996; Niethammer et al., 2002). Interestingly, the SynCAM 1 glycoform primarily expressed during the peak of synaptogenesis is the one that displays the strongest homophilic binding (A. Fogel and Biederer, unpublished observation), and it is tempting to speculate that controlled N-glycosylation regulates both SynCAM 1 binding and synapse-inducing activity. Another possibility may be activity-dependent alternative splicing of CAM transcripts, which may be used to modulate function and/or expression levels, as has been described for NCAM and NMDA receptors (Doherty et al., 1992; Mu et al., 2003). Understanding the mechanisms that govern the synthesis and degradation of cell adhesion molecules and their subsynaptic distribution and signaling will be relevant for understanding how these events contribute to the regulation of synapse stability and number.
Footnotes
We thank A. Kimberley McAllister and Pamela Arstikaitis for critically reading this manuscript and Andrea Draper for providing artwork.
Correspondence should be addressed to Dr. Philip Washbourne, Institute of Neuroscience, University of Oregon, 1254 University of Oregon, Eugene, OR 97403-1254. E-mail: pwash@uoneuro.uoregon.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249244-06$15.00/0
References
- Adams JC (2001) Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol 17: 25-51. [DOI] [PubMed] [Google Scholar]
- Adams JC, Tucker RP (2000) The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn 218: 280-299. [DOI] [PubMed] [Google Scholar]
- Ahmari SE, Buchanan J, Smith SJ (2000) Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci 3: 445-451. [DOI] [PubMed] [Google Scholar]
- Biederer T, Südhof TC (2001) Cask and protein 4.1 support F-actin nucleation on neurexins. J Biol Chem 276: 47869-47876. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC (2002) SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297: 1525-1531. [DOI] [PubMed] [Google Scholar]
- Bornstein P (2001) Thrombospondins as matricellular modulators of cell function. J Clin Invest 107: 929-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresler T, Ramati Y, Zamorano PL, Zhai R, Garner CC, Ziv NE (2001) The dynamics of SAP90/PSD-95 recruitment to new synaptic junctions. Mol Cell Neurosci 18: 149-167. [DOI] [PubMed] [Google Scholar]
- Bresler T, Shapira M, Boeckers T, Dresbach T, Futter M, Garner CC, Rosenblum K, Gundelfinger ED, Ziv NE (2004) Postsynaptic density assembly is fundamentally different from presynaptic active zone assembly. J Neurosci 24: 1507-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Fentrop N, Lee A, Salmen B, Law JWS, Wotjak CT, Schweizer M, Dityatev A, Schachner M (2004) Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci 24: 1565-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K, Hansen SM, Venero C, Herrero AI, Skibo G, Berezin V, Bock E, Sandi C (2004) A synthetic neural cell adhesion molecule mimetic peptide promotes synaptogenesis, enhances presynaptic function, and facilitates memory consolidation. J Neurosci 24: 4197-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Afridi SK, Clark L, Scheiffele P (2004) Disorder-associated mutations lead to functional inactivation of neuroligins. Hum Mol Genet 13: 1471-1477. [DOI] [PubMed] [Google Scholar]
- Chow I, Poo MM (1985) Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci 5: 1076-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoletti D, De Jaco A, Jennings LL, Flynn RE, Gaietta G, Tsigelny I, Ellisman MH, Taylor P (2004) The Arg451Cys-neuroligin-3 mutation associated with autism reveals a defect in protein processing. J Neurosci 24: 4889-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Goridis C, Represa A (1997) NCAM is essential for axonal growth and faciculation in the hippocampus. Mol Cell Neurosci 8: 323-335. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P (2003) Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6: 708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckwerth TL, Elliott JL, Knudson CM, Johnson Jr EM, Snider WD, Korsmeyer SJ (1996) BAX is required for neuronal death after trophic deprivation and during development. Neuron 17: 401-411. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Schachner M (2000) Synaptic strength as a function of post- versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron 26: 207-217. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, Muller D, Schachner M (2004) Polysialylated neural cell adhesion molecule PSA-NCAM promotes formation and remodeling of hippocampal synapses. J Neurosci, in press. [DOI] [PMC free article] [PubMed]
- Doherty P, Moolenaar CECK, Ashton SV, Michalides RJAM, Walsh FS (1992) The VASE exon downregulates the neurite growth promoting activity of NCAM 140. Nature 356: 791-793. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Bukalo O, Chazal G, Wang L, Goridis C, Schachner M, Gerardy-Schahn R, Cremer H, Dityatev A (2000) Mice deficient in the polysialyl-transferase ST8SialV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci 20: 5234-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE (2000) Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron 27: 57-69. [DOI] [PubMed] [Google Scholar]
- Fu Z-Y, Washbourne P, Ortinski P, Vicini S (2003) Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol 90: 3950-3957. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC (1995) Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell 81: 435-443. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC (1997) Binding of neuroligins to PSD-95. Science 277: 1511-1515. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P (1993) Differential expression of thrombospondin 1, 2 and 3 during murine development. Dev Dyn 197: 40-56. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T, Study PARIS (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34: 27-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jontes JD, Buchanan J, Smith SJ (2000) Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat Neurosci 3: 231-237. [DOI] [PubMed] [Google Scholar]
- Kleene R, Schachner M (2004) Glycans and neural cell interactions. Nat Rev Neurosci 5: 195-208. [DOI] [PubMed] [Google Scholar]
- Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda RP, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T (1998) Diversity revealed buy a novel family of cadherins expressed in neurons at a synaptic complex. Neuron 20: 1137-1151. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns JP, Ropers HH, Hamel BC, Andres C, Barthelemy C, Moraine C, Briault S (2004) A-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 74: 552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J (2000) The functions of thrombospondin-1 and -2. Curr Opin Cell Biol 12: 634-640. [DOI] [PubMed] [Google Scholar]
- Mariner R, Jackson AWI, Levitas A, Hagerman RJ, Braden M, McBogg PM, Smith AC, Berry R (1986) Autism, mental retardation, and chromosomal abnormalities. J Autism Dev Disord 16: 425-440. [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD (2003) Activity-dependent mRNA splicing controls ER export and delivery of NMDA receptors. Neuron 40: 581-594. [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ (1996) PSA-NCAM is required for activity-induced synaptic plasticity. Neuron 17: 413-422. [DOI] [PubMed] [Google Scholar]
- Niethammer P, Delling M, Sytnyk V, Dityatev A, Fukami K, Schachner M (2002) Co-signaling via lipid raft associated kinases and the FGF receptor is required for NCAM-mediated neuritogenesis. J Cell Biol 157: 521-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata S, Sago H, Mori N, Rochelle JM, Seldin MF, Davidson M, St. John T, Taketami S, Suzuki ST (1995) Protocadherin Pcdh2 shows properties similar to, but distinct from, those of classical cadherins. J Cell Sci 108: 3765-3773. [DOI] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V (2003) FASt remodeling of synapses in Drosophila Curr Opin Neurobiol 13: 527-534. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Winfield SL, Sandhu HK, Martin BM, Ginns EI (2000) The structure and expression of the human neuroligin-3 gene. Gene 246: 303-310. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Tanaka H, Frank M, Elste A, Fidler L, Benson DL, Colman DR (2003) Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci 23: 5096-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Parada L, Bose CM, Plattner F, Landmesser LT (2004) Distinct roles of different neural cell adhesion molecule (NCAM) isoforms in synaptic maturation revealed by analysis of NCAM 180 kDa isoform-deficient mice. J Neurosci 24: 1852-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Wong T-P, Gerrow K, Wang Y-T, El-Husseini A (2004) A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci USA 101: 13915-13920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeich D, Nouri N, Hinds D, Hallmayer J, Kalaydjieva L, McCague P, Dimiceli S, Pitts T, Nguyen L, Yang J, Harper C, Thorpe D, Vermeer S, Young H, Hebert J, Lin A, Ferguson J, Chiotti C, et al. (1999) A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet 65: 493-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2: 255-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U (1985) Influences of the neural cell adhesion molecule on axon growth and guidance. J Neurosci Res 13: 123-131. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Landmesser L (1996) Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci 19: 422-427. [DOI] [PubMed] [Google Scholar]
- Scheiffele P (2003) Cell-cell signalling during synapse formation in the CNS. Annu Rev Neurosci 26: 485-508. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101: 657-669. [DOI] [PubMed] [Google Scholar]
- Schuster T, Krug M, Hassan H, Schachner M (1998) Increase in proportion of hippocampal spine synapses expressing neural cell adhesion molecule NCAM180 following long-term potentiation. J Neurobiol 37: 359-372. [DOI] [PubMed] [Google Scholar]
- Scott-Drew S, ffrench-Constant C (1997) Expression and function of thrombospondin-1 in myelinating glial cells of the central nervous system. J Neurosci Res 50: 202-214. [DOI] [PubMed] [Google Scholar]
- Shen K, Bargmann CI (2003) The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans Cell 112: 619-630. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI (2004) Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell 116: 869-881. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ (2002) Postsynaptic signaling and plasticity mechanisms. Science 298: 776-780. [DOI] [PubMed] [Google Scholar]
- Sperry R (1963) Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA 50: 703-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytnyk V, Leshchyns'ka I, Delling M, Dityateva G, Dityatev A, Schachner M (2002) Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts. J Cell Biol 159: 649-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science 291: 657-661. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC (1995) Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14: 497-507. [DOI] [PubMed] [Google Scholar]
- Wang X, Su H, Bradley A (2002a) Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev 16: 1890-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Weiner JA, Levi S, Craig AM, Bradley A, Sanes JR (2002b) Gamma protocadherins are required for survival of spinal interneurons. Neuron 36: 843-854. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK (2002) Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci 5: 751-759. [DOI] [PubMed] [Google Scholar]
- Wu Q, Maniatis T (1999) A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97: 779-790. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang T, Cheng JF, Kim Y, Grimwood J, Schmutz J, Dickson M, Noonan JP, Zhang MQ, Myers RM, Maniatis T (2001) Comparative DNA sequence analysis of mouse and human protocadherin gene clusters. Genome Res 11: 389-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR (2002) Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell 110: 649-660. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA (2003) Synaptic adhesion molecules. Curr Opin Cell Biol 15: 621-632. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC (2001) Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron 29: 131-143. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ (1996) Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17: 91-102. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY (2003) Postnatal neurodevelopmental disorders: meeting at the synapse? Science 302: 826-830. [DOI] [PubMed] [Google Scholar]