Abstract
In rats, feeding can be triggered experimentally using many approaches. Included among these are (1) food deprivation and (2) acute microinjection of the neurotransmitter l-glutamate (Glu) or its receptor agonist NMDA into the lateral hypothalamic area (LHA). Under both paradigms, the NMDA receptor (NMDA-R) within the LHA appears critically involved in transferring signals encoded by Glu to stimulate feeding. However, the intracellular mechanisms underlying this signal transfer are unknown. Because protein-tyrosine kinases (PTKs) participate in NMDA-R signaling mechanisms, we determined PTK involvement in LHA mechanisms underlying both types of feeding stimulation through food intake and biochemical measurements. LHA injections of PTK inhibitors significantly suppressed feeding elicited by LHA NMDA injection (up to 69%) but only mildly suppressed deprivation feeding (24%), suggesting that PTKs may be less critical for signals underlying this feeding behavior. Conversely, food deprivation but not NMDA injection produced marked increases in apparent activity for Src PTKs and in the expression of Pyk2, an Src-activating PTK. When considered together, the behavioral and biochemical results demonstrate that, although it is easier to suppress NMDA-elicited feeding by PTK inhibitors, food deprivation readily drives PTK activity in vivo. The latter result may reflect greater PTK recruitment by neurotransmitter receptors, distinct from the NMDA-R, that are activated during deprivation-elicited but not NMDA-elicited feeding. These results also demonstrate how the use of only one feeding stimulation paradigm may fail to reveal the true contributions of signaling molecules to pathways underlying feeding behavior in vivo.
Keywords: eating, feeding, lateral hypothalamic area, glutamate, NMDA, NR2A, NR2B, Src, Pyk2, tyrosine phosphorylation, signal transduction
Introduction
In recent years, molecular and cellular techniques have helped identify novel macromolecules involved in feeding control and energy homeostasis. Ironically, however, the molecular and cellular mechanisms underlying the actions of these agents remain poorly understood in the freely behaving animal. Identifying such mechanisms is important, especially for neurons within various hypothalamic nuclei, where neuropeptides and small neurotransmitters implicated in feeding control may act on common neural substrates. In such neurons, a few intracellular effectors could integrate multiple signals encoding information important for feeding control. Identifying such effectors could reveal novel targets for treating certain eating and metabolic disorders or cases of drug dependency (Carr, 1996, 2002; Chiesi et al., 2001; DiLeone et al., 2003). Despite progress in identifying mammalian feeding control circuits, the intracellular pathways they use remain virtually unknown in vivo, especially for feeding stimulation.
This is partly attributable to limitations in methods. In the rat, which has the best delineated feeding control neural circuitry but for which a recently developed gene knock-out strategy (Zan et al., 2003) has yet to gain widespread currency (Rossant, 2003), approaches in vivo have primarily involved (1) administering central and peripheral agents that target signaling molecules to alter feeding behavior and (2) manipulating the behavioral state (e.g., food deprivation) to change signaling molecule activity. The former approach poorly reflects the concerted physiological activity of neurochemical systems in vivo, and the latter rarely identifies unambiguously the causal role of a particular signaling molecule or pathway. However, combining these approaches could mitigate the limitations attendant with each, revealing more accurately the intracellular mechanisms engaged by various feeding-related neurochemical systems.
One such system is that for the amino acid l-glutamate (Glu) in the lateral hypothalamic area (LHA) (Stanley, 1996). In satiated rats, LHA-administered Glu or its receptor agonists (kainate and NMDA), elicit powerful, transient feeding behaviors that are stereochemically, anatomically, and behaviorally specific (Stanley et al., 1993b,c; Duva et al., 2001, 2002). Conversely, LHA-injected NMDA receptor (NMDA-R) antagonists suppress eating elicited by NMDA, food deprivation, or the onset of the nocturnal period and reduce body weight when given chronically (Stanley et al., 1996, 1997; Khan et al., 1999). During fasting, endogenous Glu is apparently released within the LHA during meal onset, sharply declining while rats are still eating (Rada et al., 1997, 2003). The orexigen hypocretin/orexin (H/O) requires Glu signals to depolarize H/O-containing neurons within the LHA (Li et al., 2002), and neuropeptide Y requires functional LHA NMDA-Rs to elicit feeding (Lee and Stanley, 2002). Thus, LHA signaling mechanisms underlying feeding behavior are probably driven by multiple neurotransmitters and their receptors, including Glu and its NMDA-R subtype.
Accordingly, we wanted to identify LHA signaling pathways activated during the feeding stimulated either by NMDA injection, or more generally, by food deprivation. Here we present evidence suggesting that feeding stimulation is associated with the robust activation of LHA protein-tyrosine kinases (PTKs), which are also required for the full expression of feeding behavior in vivo.
Parts of this paper have been published previously in preliminary form (Khan et al., 1996, 2000a, 2001; Khan, 2002).
Materials and Methods
Central injection studies
Reagents and solutions for central injection studies
Genistein [4′,5,7-trihydroxyisoflavone; molecular weight (MW) 270.2], daidzein (4′,7-dihydroxyisoflavone; MW 254.2), and tyrphostin A48 [also known as AG112, 2-amino-4-(4′-hydroxyphenyl)-1,1,3-tricyanobuta-1,3-diene; MW 236.2] were purchased from Calbiochem (La Jolla, CA). PP1 [4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; MW 281.4] was obtained from Biomol (Plymouth Meeting, PA). The PTK inhibitors were dissolved in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO), a vehicle recently validated as useful in studies of chemically elicited feeding in the hypothalamus (Blevins et al., 2002). The light-sensitive genistein and daidzein solutions were prepared in dim light, and vials of working solutions were covered with aluminum foil. NMDA and kainic acid (KA) were also purchased from Sigma and were dissolved in artificial CSF (aCSF) just before central injection. Sterile aCSF (in mm: 147 Na+, 154.2 Cl-, 3.0 K+, 1.2 Ca2+, and 0.9 Mg2+, pH 7.4) was prepared using double-distilled water and subsequently filtered through 0.22 μm GS-type or 0.45 μm HV-type filters (Millipore, Bedford, MA). For the experiments using PP1, the aCSF was also buffered with 1.0 mm potassium phosphate. Materials for histological verification of cannula placement were purchased from Sigma.
Central injection methods
Subjects. Adult male Sprague Dawley rats (n = 127; ∼350-500 gm), descended from Charles River Laboratories (Wilmington, MA) animals, were single-housed in a temperature-controlled vivarium with a 12 hr light/dark photoperiod and ad libitum access to food and water, unless otherwise stated. During testing, animals were maintained on a mash diet consisting of 46% laboratory rodent meal (LabDiet; PMI Nutrition International), 37% sucrose, and 17% evaporated milk. Although we used this mash diet instead of chow pellets because it minimizes spillage and therefore helps us measure intake more accurately, rats given LHA NMDA injections will also readily eat normal chow pellets (A. Khan, unpublished observations). Except for food deprivation studies, animals were fed freshly prepared mash at least 1.5 hr before testing to maximize satiety.
Stereotaxic surgery. Animals in central injection studies were stereotaxically implanted under methoxyflurane inhalant (Metofane; Schering-Plough, Kenilworth, NJ) or pentobarbital anesthesia (50 mg/kg, i.p.) with chronic unilateral or bilateral 26 gauge stainless steel in-dwelling guide cannulas (Small Parts, Miami Lakes, FL) targeted toward the LHA. With the incisor bar 3.3 mm below the ear bars, stereotaxic coordinates were 6.1-6.4 mm anterior to the interaural line, 1.8 mm lateral to the midsagittal sinus, and 8.2 mm ventral to the surface of the skull (Paxinos and Watson, 1998, their Figs. 30, 31). Cannulas were secured to the skull by six stainless steel hex screws and dental acrylic, and a plastic guard was placed around the exposed portion of the cannula. A 33 gauge stainless steel obturator was inserted into each cannula to maintain patency. Animals were allowed 5-7 d of postoperative recovery before testing, during which time they were repeatedly handled and mock-injected to adapt them to the testing procedures.
Behavioral testing. Tests were conducted in the midportion of the light phase of the rat circadian cycle and consisted of either acute bilateral injections (one injection per cannula in quick succession) or acute unilateral injections in series 10 min apart (vehicle, tyrphostin A48 or PP1, followed by vehicle or NMDA) or 30 min apart (vehicle, genistein or daidzein, followed by vehicle, NMDA, or KA). Solutions (300 nl volume) were delivered through 33 gauge injectors, each inserted through the guide cannula and projecting exactly 1.0 mm beyond the end of the cannula, directly into the LHA. Doses of NMDA (10 nmol) and KA (1 nmol) were selected on the basis of their efficacy in eliciting eating in previous studies (Stanley et al., 1993b,c; Khan et al., 1999). Food intake was recorded at 0.5, 1, 2, and 4 hr after the final injection by weighing each subject's food bowl to the nearest 0.1 gm and subtracting the resulting weight from the weight of the bowl recorded before injection. Animals received treatments in counterbalanced order (Latin square design) during a series of test days, with each animal receiving each treatment by the end of the experiment. Treatment days were separated by at least 1 d of recovery, during which no procedures were performed.
Testing the effects of genistein on food intake
Experiments 1a and 1b: does genistein suppress eating elicited by NMDA? To determine whether NMDA-elicited eating can be attenuated by the broad-spectrum PTK inhibitor genistein, 24 animals were unilaterally injected in experiment 1a with genistein (240 nmol), its “inactive” analog daidzein (240 nmol), or DMSO vehicle, followed 30 min later by a unilateral injection of NMDA (10 nmol) or aCSF vehicle. In experiment 1b, 18 naive animals were tested in a similar manner, except that three doses of genistein were used (2.4, 24, and 240 nmol), and daidzein was not included as a treatment.
Experiment 2: does genistein suppress eating elicited by kainic acid? As with NMDA injection, a unilateral KA injection into the LHA of satiated rats elicits a robust feeding response (Stanley et al., 1993b,c). To test whether genistein could also suppress eating elicited by KA, 15 naive rats received unilateral LHA injections of 240 nmol of genistein or DMSO vehicle, followed 30 min later by LHA injections of either KA (1 nmol) or aCSF vehicle.
Experiment 3. does genistein suppress eating elicited by food deprivation? To investigate whether genistein can affect natural eating elicited by food deprivation, 12-15 naive animals with ad libitum access to water were food-deprived for ∼20 hr and then given bilateral LHA injections of either genistein (240 nmol/side) or DMSO vehicle. Genistein was given bilaterally in this experiment so that it might block the presumed bilateral activation of the LHA caused by food deprivation. Freshly prepared food was presented 10 min after the injections, and food intake was measured treating the time of final injection as t = 0.
Testing the effects of tyrphostin A48 on food intake
Experiment 4: does tyrphostin A48 suppress eating elicited by NMDA? To determine the effect of the PTK inhibitor tyrphostin A48 on NMDA-elicited eating, 13 naive animals received unilateral LHA injections of one of three doses of tyrphostin A48 (2.1, 21.1, or 211.7 nmol) or DMSO vehicle, followed 10 min later by a unilateral LHA injection of NMDA (10 nmol) or aCSF vehicle.
Testing the effects of PP1 on food intake
Experiments 5a and 5b: does PP1 suppress eating elicited by NMDA? To help determine the involvement of members of the Src family of PTKs in mechanisms underlying NMDA-elicited eating, 15 naive animals were unilaterally injected in experiment 5a with one of three doses of the Src family-selective inhibitor PP1 (0.2, 2.1, or 21.2 nmol) or DMSO vehicle, followed 10 min later by a unilateral LHA injection of NMDA (10 nmol) or aCSF vehicle. In experiment 5b, this study was replicated using 14 naive rats, except that the 0.2 nmol dose of PP1 was not included.
Experiment 6: does PP1 suppress eating elicited by food deprivation? To determine whether Src family PTK activity is important for eating triggered by food deprivation, 13 naive animals were food-deprived under conditions identical to those described in experiment 4 (above), except that the inhibitor, injected bilaterally into the LHA, was PP1 (21.2 nmol/side).
Statistical and histological analyses for behavioral studies
After completion of behavioral testing, subjects were killed by CO2 inhalation and then perfused transcardially with 10% formalin. Using a cryotome, a subset of brains was sectioned into 100-μm-thick sections through the extent of the cannula track. Sections were air-dried on gelatin- or gelatin- and chromate-subbed slides and stained with thionin. Injection sites were localized by tracing their projected images onto size-matched figures adapted from the atlas of Paxinos and Watson (1998). The appearance of the tissue was similar to that of subjects in previous studies (data not shown), the histological photomicrographs of which have been published (Stanley et al., 1993c).
Food intake values were analyzed by one- or two-way general linear model ANOVA. Where overall effects were significant, multiple comparisons were performed using the Student-Newman-Keuls test with α set at 0.05. Nonparametric tests (Mann-Whitney rank sum tests and Kruskal-Wallis ANOVA on ranks) were performed, where appropriate, for scores that were not normally distributed. In such cases, multiple comparisons were performed using Dunn's test, with α set at 0.05. All food intake scores are expressed as mean intakes (in grams) ± SEM.
Immunohistochemistry (experiment 7)
Reagents and solutions
The primary polyclonal Src antibody was raised against the gene products of the Rous sarcoma virus, which includes Src kinase (Biomol). Biotinylated goat anti-rabbit IgG (heavy and light chains) and avidin-HRP solution were from Vector Laboratories (Burlingame, CA). All other materials, including bovine serum albumin, hydrogen peroxide, and 3,3′-diaminobenzidine (DAB) tetrachloride tablets were purchased from Sigma, Vector Laboratories, or local suppliers.
Tissue preparation
As described previously (Khan et al., 1999, 2000b), adult male Sprague Dawley rats (325-475 gm; n = 11) were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and perfused through the ascending aorta with at least 100 ml of cold, heparinized PBS (0.01 m sodium phosphate buffer in 0.9% saline, containing 200 U/l heparin), followed by at least 300 ml of ice-cold 4% p-formaldehyde in 0.1 m sodium phosphate buffer, pH 7.4. The brain was quickly dissected, and a razor blade was used to obtain a block of tissue containing the LHA. These blocks were placed in a 30% sucrose-PBS solution at 4°C for at least 36 hr without an intervening postfixation step. They were then mounted onto the freezing stage of a sliding microtome using Tissue-Tek or Histomount (Fisher Scientific, Houston, TX), frozen using powdered dry ice, and sliced into 30 μm coronal sections. The sections were then transferred to wells containing cold PBS.
Freely floating sections were reacted with 0.1% sodium borohydride for 10 min at room temperature (RT), followed by 0.1% hydrogen peroxide in PBS for 10 min at RT, and then rinsed for 10 min in PBS before the blocking step. Blocking involved incubating the sections for 1 hr at RT in 5-10% normal goat serum (NGS) diluted in 0.3% Triton X-100-containing PBS, pH 7.4 (T-PBS).
Immunodetection
Primary and secondary antibody reactions. Sections were then incubated with the rabbit anti-Src antibody for at least 24 hr at 4°C. Initial experiments were performed using dilutions of 1:500 and 1:1000. Optimal visualization was obtained at a dilution of 1:750. The primary antibody was dissolved in T-PBS containing 0.2% BSA and 1% NGS. Sections were then reacted with a biotinylated goat anti-rabbit IgG (diluted 1:200 in T-PBS) for 1-2 hr at RT.
Immunoperoxidase detection. Sections were then treated for 1 hr at RT in avidin-HRP solution (1:200 in T-PBS). They were transferred briefly to a 0.05 m Tris-HCl solution containing 0.9% NaCl, pH 7.4, and preincubated in a DAB chromogen solution (20 mg of DAB/100 ml of Tris-HCl) for 5-10 min at RT before being reacted at RT with 1-4 μl of 30% H2O2 in 10 ml of DAB solution. Nickel chloride (0.15 gm/100 ml of DAB solution) was added to the DAB solution to intensify the stain, and reactions were stopped with cold Tris-HCl. The optimized time in the DAB/H2O2 solution was 4 min.
Immunohistochemical controls
For a subset of brain sections in all experiments, a solution containing only Triton X-100, serum, and BSA was substituted for the primary antibody.
Tissue mounting, observation, and photomicrography
Sections were mounted onto gelatin- and chromate-subbed slides and air-dried. The slides were dehydrated in ascending concentrations of ethanol, cleared in 100% xylene, and coverslipped with DPX. Brain regions were then examined under bright-field illumination using an Olympus Optical (Tokyo, Japan) BH-2 microscope (with an accompanying Olympus EMM-7 light meter) or a Nikon (Melville, NY) Microphot FX-A microscope. In some cases, camera lucida drawings of immunoreactive cells were created by observing sections under oil immersion at 100× magnification. Photomicrographs of immunoreactive regions were prepared using Eastman Kodak (Rochester, NY) Technical Pan film 5612 (ASA 25) or Kodak Gold film (ASA 200). The former was developed in the University of California, Riverside, darkroom facility, where prints were also prepared using Kodak F4 or F5 paper.
Biochemical identification of tyrosine-phosphorylated LHA proteins
Experiment 8a refers to biochemical studies performed on tissue from rats receiving injections of NMDA (and their aCSF-injected controls), and experiment 8b refers to similar studies performed on tissue from food-deprived rats (and their ad libitum-fed controls). Unless otherwise noted by experiment number, all procedures described below apply to both experiments.
Subjects, housing, and treatments
Adult male Sprague Dawley rats (n = 60), housed under identical conditions as animals used in behavioral experiments, were maintained on a standard pellet diet (LabDiet) with ad libitum access to water at all times, unless otherwise noted.
Experiment 8a: is an acute injection of NMDA into the LHA, which normally triggers a transient eating response, also sufficient to affect Tyr-P of proteins, PTK expression, and PTK activity in LHA-containing tissue? For this experiment, which was conducted between 7 P.M. and 4 A.M. (between the end of the light phase and the beginning of the dark phase), rats (n = 20) each received ∼1.0 ml of sodium pentobarbital anesthesia (50 mg/ml, i.p.) to minimize potential trans-synaptic or sensorimotor feedback effects on tyrosine phosphorylation. When whole-body movements were no longer elicited by a brief tail pinch, rats received central bilateral LHA microinjections of either NMDA (10 nmol; n = 10) or aCSF (n = 10). Parameters for central microinjections (volume and method of delivery) were identical to those described for the behavioral experiments (experiments 1-6). Ten to 15 min after the bilateral LHA injections, each rat was transferred to a cold room for tissue harvesting.
Experiment 8b: is prolonged feeding stimulation, as produced by food deprivation, sufficient to affect Tyr-P of proteins, PTK expression, and PTK activity in LHA-containing tissue? For these experiments, which were conducted between 7 P.M. and 12 A.M. (toward the end of the diurnal phase), four separate tissue harvests were conducted, each on 10 rats, for a total of 40 rats. In each group of 10, 5 rats were food-deprived for 48 hr immediately before the tissue harvest, whereas 5 had ad libitum access to the pellet diet and served as controls. As in experiment 8a, rats received ∼1.0 ml of sodium pentobarbital anesthesia (50 mg/ml, i.p.) just before tissue harvest.
Tissue harvest
Each rat was weighed to the nearest 0.1 gm, transferred to a cold room (10°C), and decapitated, and its brain was quickly dissected and immediately immersed in freshly prepared, ice-cold protease inhibitor mixture (PIM), pH 7.4, for 10-15 sec. The PIM contained 0.32 m sucrose, 50 mm Tris, 0.2 mm sodium orthovanadate (Calbiochem), 20 mm β-glycerophosphate, 1 mm NaF, 0.1 mm phenylmethylsulfonyl fluoride (PMSF), and 5 μg/ml each antipain, leupeptin, and aprotinin (Sigma), all dissolved in double-distilled water. The brain was quickly placed ventral side-up within a chilled stainless steel rat brain mold (Activational Systems) containing grooves 1 mm apart for producing coronal transections. Chilled PIM was immediately poured over the brain, and coronal transections were made at the level of the optic chiasm and midway along the raised surface of the median eminence. These blocks of brain tissue contained virtually all of the LHA and also likely portions of the preoptic and ventral tegmental areas. Each tissue block was placed flat, with the posterior side facing up, onto a chilled microscope slide, and fresh chilled PIM was again poured over it.
With the aid of a dissecting stereoscope (Nikon SMZ-2B) and the atlas of Palkovits and Brownstein (1988), a 14 gauge stainless steel spinal tap catheter was used to core approximately bilaterally symmetrical tissue samples containing the LHA, using the fornix, optic tract, and internal capsule as reference boundaries. Samples were immediately placed in 1.5 ml Eppendorf microcentrifuge tubes containing 1.0 ml of PIM, and tissue wet weight was determined by weighing tared vials to the nearest 0.1 mg. Samples were immediately placed on ice in the cold room and then transferred to a -70°C freezer. They were then shipped overnight, with dry ice, to the University of Toronto at Scarborough, where they were again stored at -70°C until used for immunoprecipitation and immunoblotting.
Immunoprecipitation (experiment 8b only)
As described previously (Takagi et al., 1997; Cheung et al., 2000), NR2A and NR2B antibodies used for immunoprecipitation (IP) were generated against polyhistidine fusion proteins containing the C-terminal region of each subunit corresponding to amino acid residues 934-1203 for NR2A and 935-1455 for NR2B. Antibodies were precoated on Protein A/G PLUS-agarose (Santa Cruz Biotechnology; Santa Cruz, CA) by incubating 0.003 or 0.005 ml of immune serum with 0.02 ml of protein A/G PLUS-agarose for 45 min-1 hr at 4°C in 0.5 ml of 50 mm Tris-HCl buffer, pH 7.4, containing 1% (v/v) NP-40, 0.25% (w/v) sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 0.1 mm each PMSF and sodium orthovanadate, 1 mm NaF, and 5 μg/ml each antipain, leupeptin, and aprotinin (IP buffer). Three hundred micrograms of LHA-containing tissue that had been solubilized by boiling in 100 μl of 1% SDS containing 1% β-mercaptoethanol were diluted 10-fold with binding buffer. Diluted samples were incubated with 0.02 ml of Protein A/G PLUS-agarose for 1 hr at 40°C and centrifuged, and the supernatant was added to the precoated Protein A/G PLUS-agarose and incubated at 4°C for 16 hr. The immunoprecipitates were isolated by centrifugation and washed five times with IP buffer, and proteins were eluted by heating at 100°C in 62.5 mm Tris-HCl, pH 6.8, containing 2% SDS, 1% β-mercaptoethanol, and 5% glycerol (sample buffer). In some cases, eluted immunoprecipitates were divided into equal halves for immunoblotting analysis.
Immunoblotting
For immunoblotting, SDS-solubilized samples (10 μg) were separated on 8% polyacrylamide gels and transferred to nitrocellulose as described previously (Gurd et al., 1992), and protein blots were reacted with specific antibodies as below. Individual proteins were detected by Western immunoblotting using antibodies against the following: phosphotyrosine (clone 4G10) and c-Src (clone GD11) from Upstate Biotechnology (Lake Placid, NY); NR2A [polyclonal antibody as described by Takagi et al. (1997)], NR2B (clone 13), and Pyk2 (clone 11) from Transduction Laboratories (Lexington, KY); and the phosphoryl group on Tyr527 of Src family PTKs (Biosource, Camarillo, CA). For some experiments, a rabbit polyclonal antibody specific to phosphorylated Tyr1472 of NR2B was used, which was generously provided by Dr. Tadashi Yamamoto (Department of Oncology, Institute of Medical Science, University of Tokyo, Tokyo, Japan). In some cases, the first antibody was removed by incubation of the blot in 0.1 m glycine, pH 2.7, at 55°C for 30 min before reaction with a second antibody.
Immunoprecipitation and immunoblotting controls
Controls used during blotting runs of IP samples involved either reacting antibodies to lanes loaded only with Protein A/G (to examine nonspecific binding) or to react blots loaded with hippocampal homogenates immunoprecipitated only with preimmune serum. [NMDA-R subunits are robustly expressed in the hippocampus (Petralia et al., 1994a,b).] An antibody selective for Tyr1472 of the NR2B subunit (Nakazawa et al., 2001) was included in our experiments to independently validate staining obtained with the phosphotyrosine (α-pY) antibody. Also, to examine the specificity of the α-pY antibody, nitrocellulose blots containing proteins from LHA-containing tissue were preincubated with a protein-tyrosine phosphatase (PTPase) to remove the phosphotyrosine residues on all proteins and then incubated with the α-pY antibody. Briefly, the nitrocellulose blots were incubated for 40 min at RT in 2 ml of reaction mixture (as per the supplier's guidelines; Calbiochem), which contained 50 mm Tris, pH 7.5, 100 mm NaCl, 2 mm EDTA, 5 mm DTT, 0.01% Brij 35, 1 mg/ml BSA, 5 μg/ml each antipain, leupeptin, and aprotinin, 0.1 mm PMSF, and 50 U/ml recombinant Yersinia enterocolitica PTPase [designated Yop51⋆ by Zhang et al. (1992), with a Cys→ Arg mutation at residue 235]. The nitrocellulose was then washed 3-5 min with TBS containing Triton X-100 and then subjected to immunoblotting as described above.
Samples, detection, visualization, and analysis
Within each treatment group (NMDA- or aCSF-injected in experiment 8a and fasted or ad libitum-fed in experiment 8b), a single sample contained tissue homogenized from LHA-containing punches pooled from at least two rats (i.e., a pool of at least four punches because the punches were bilateral). This was done to ensure that there was enough protein per sample to allow reliable detection. Bound antibodies were detected by enhanced chemoluminescence (Super Signal; Pierce, Rockford, IL). To obtain optical density (OD) values, exposed x-ray film was scanned using a Bio-Rad (Hercules, CA) GS 700 gel scanner as described previously (Gurd et al., 1992). Because tissue was pooled from subjects within treatment groups as described, each OD score is a quantitation from at least two rats. (For example, a set of five OD scores for a particular band would be derived from tissue samples harvested from a total of at least 10 rats undergoing the same experimental manipulation.) Once OD values were obtained, a mean OD value ± SEM was calculated for the group. Mean scores were analyzed by an unpaired t test or for, nonparametric analysis, by a Mann-Whitney rank sum test, with α set at 0.05.
Results
Genistein suppressed NMDA-elicited eating in a behaviorally selective manner (experiments 1-3)
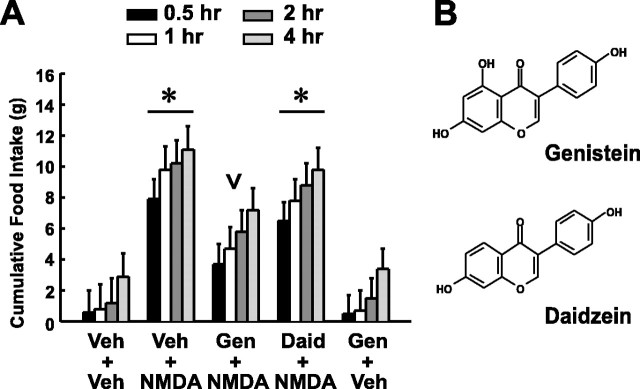
Figure 1A shows the results of experiment 1a. As previously reported (Stanley et al., 1993b,c, 1996, 1997; Khan et al., 1999), a unilateral LHA microinjection of NMDA significantly stimulated food intake at all times (7.9 ± 1.3 gm after NMDA compared with 0.6 ± 1.4 gm after vehicle at 0.5 hr; p < 0.05 by one-way ANOVAs). A new finding was that pretreatment with LHA injection of the PTK inhibitor genistein (chemical structure, Fig. 1B) markedly reduced NMDA-elicited eating, with a maximal suppression of 53% to 3.7 ± 1.3 gm at 0.5 hr after injection. Two-way ANOVA of the genistein and NMDA versus the vehicle and NMDA treatments revealed a significant effect of treatment (F(1,172) = 8.9; p < 0.01) but no effect of time after injection or their interaction.
Figure 1.
Genistein suppresses NMDA-elicited eating in satiated rats (experiment 1a). A, Mean cumulative food intake ± SEM (in grams) after double injections, spaced 30 min apart, of (1) vehicle (Veh), genistein (Gen; 240 nmol), or daidzein (Daid; 240 nmol) and (2) vehicle or NMDA (10 nmol). Bars in the graph are shaded according to the postinjection time that intake was measured. Asterisks indicate intakes significantly different from Veh + Veh treatment; the inverted carat indicates intakes significantly lower overall than those elicited by NMDA alone (Veh + NMDA); n = 24. B, The structures of genistein and daidzein differ by only one hydroxyl group.
Illustrating the chemical specificity of this effect, an equimolar dose of daidzein, an analog of genistein that reportedly lacks effects on PTKs (chemical structure, Fig. 1B), did not suppress NMDA-elicited eating at any postinjection time (e.g., 6.5 ± 1.2 gm at 0.5 hr after injection) (Fig. 1A). This finding suggests that the effects of genistein on NMDA-elicited eating are attributable to its chemical actions as a PTK inhibitor. Although genistein is known to have effects at non-PTK targets that can be mimicked by daidzein (Paillart et al., 1997; Kuiper et al., 1998; Huang et al., 1999; Obayashi et al., 1999; Huang and Dillon, 2000; Patisaul et al., 2001), the effectiveness of genistein but not daidzein in our experiment suggests that these non-PTK effects do not account for the observed suppression of NMDA-elicited feeding. However, other effects of genistein have been shown on some targets, which daidzein does not mimic (Vera et al., 1996; Washizuka et al., 1997, 1998; Molokanova et al., 1999, 2000) or for which the effects of daidzein are unknown (Huang et al., 1992; Illek et al., 1995; Polkowski and Mazurek, 2000). Because our data do not rule out mediation by these latter effects, we tested more selective PTK inhibitors against NMDA-elicited eating (see experiments 4-6).
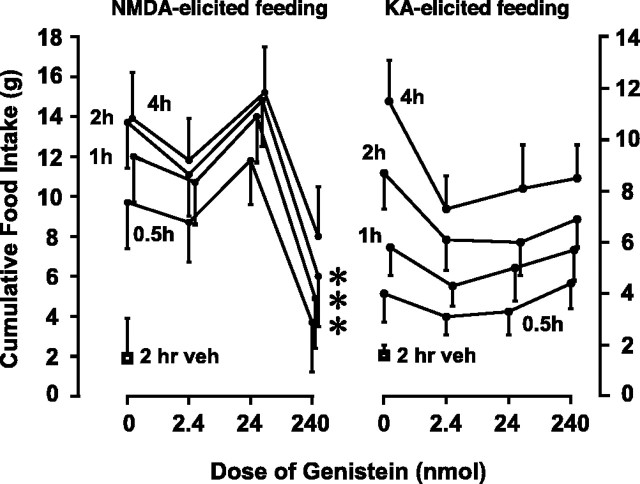
That genistein reliably suppressed NMDA-elicited eating (experiment 1a) was confirmed in experiment 1b (Fig. 2). Significant effects of treatment at each time after injection were noted (e.g., at 0.5 hr after injection, Kruskal-Wallis one-way ANOVAs on ranks, H = 18.3 with 4 df; p < 0.01). NMDA alone elicited 9.7 ± 2.4 gm of intake at 0.5 hr after injection, and this was suppressed to 3.7 ± 1.5 gm by 240 nmol of genistein at 0.5, 1, and 2 hr (p < 0.05) but not 4 hr after injection (Fig. 2, left graph). Lower doses of genistein were ineffective.
Figure 2.
Genistein has differential effects on NMDA- and KA-elicited eating (experiments 1b and 2). Mean ± SEM cumulative food intakes (in grams) at various postinjection times for rats receiving varying doses of genistein are shown. NMDA-elicited (left graph) and KA-elicited (right graph) food intakes are plotted as functions of genistein dose. For simplicity, only the 2 hr intakes from vehicle-injected rats are shown. Asterisks indicate intakes significantly lower than those elicited by NMDA injected alone; n = 18 (left); n = 15 (right).
Figure 2, right graph, shows the results of experiment 2. As shown previously (Stanley et al., 1993b,c; Khan et al., 1999), unilateral LHA injection of (KA, 1 nmol) elicited significantly more eating (4.0 ± 1.1 gm) than vehicle (0.9 ± 0.3 gm) at 0.5 hr. Two-way ANOVA (excluding “vehicle-only” scores) showed small but statistically significant overall effects of treatment on food intake (F(3,204) = 2.7; p = 0.044) and postinjection time on food intake (F(3,204) = 13.3; p < 0.0001), with no significant interaction. In contrast, multiple comparisons revealed no significant effect of genistein at any time after injection. Visual inspection suggests that the slight overall effect was primarily attributable to a delayed suppression (i.e., at 4 hr) produced by the 2.4 nmol dose of genistein. Most importantly, the 240 nmol dose of genistein, which produced 53 and 62% suppressions of NMDA-elicited eating 30 min after injection in experiments 1 and 2, respectively, had no effect on KA-elicited eating at that time.
Finally, in contrast to its effects on NMDA-elicited feeding, genistein did not suppress deprivation-elicited eating (experiment 3) at any postinjection time measured (e.g., at 0.5 hr after injection, vehicle- and genistein-injected rats ate 17.1 ± 1.4 and 16.5 ± 1.6 gm, respectively; at 4 hr, they ate 21.7 ± 1.4 and 22.1 ± 1.5 gm, respectively). That genistein did not suppress KA- or deprivation-elicited eating argues that its suppressive effects on NMDA-elicited eating were not caused by the induction of nausea or malaise because rats injected with genistein were still capable of robust eating responses and, in the case of KA-elicited eating, especially during the time when absolute KA-elicited intake was the largest (i.e., the first 30 min). Critically, the data also suggest that PTKs may not be essential in all LHA mechanisms of eating stimulation. They further suggest that the mechanisms underlying eating attributable to NMDA-R and KA-R activation in the LHA are distinct, a conclusion supported by a recent study of KA-R-delimited signaling (Rozas et al., 2003).
Tyrphostin A48 suppressed NMDA-elicited feeding (experiment 4)
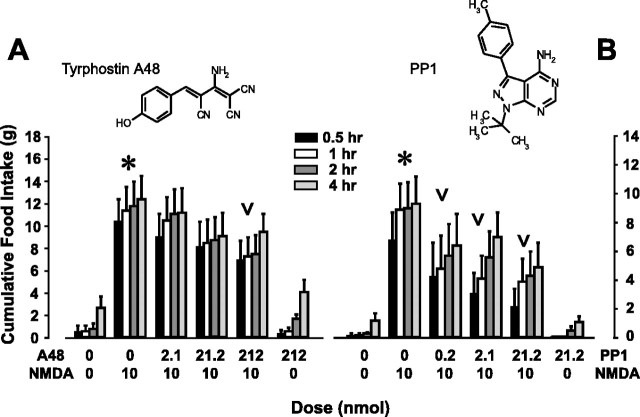
Although they can affect at least one non-PTK target (Martin, 1998), the tyrphostins are reportedly more selective than genistein at inhibiting PTKs because they act at the substrate-binding site in addition to or instead of the ATP-binding site of the PTK (Levitzki, 1992). Tyrphostin A48 (chemical structure, Fig. 3A), also suppressed NMDA-elicited feeding (F(3,152) = 3.0; p < 0.05 by two-way ANOVA excluding vehicle), although the effect was weaker than that produced by genistein. Multiple comparisons revealed that only the highest dose of Tyr A48 (212 nmol) significantly suppressed NMDA-elicited food intake (e.g., from 10.5 ± 1.9 to 6.9 ± 1.8 gm at 0.5 hr after injection) (Fig. 3A).
Figure 3.
Tyrphostin A48 and PP1 suppress NMDA-elicited eating (experiments 4 and 5a). A, Mean ± SEM cumulative food intake (in grams) after double injections, spaced 10 min apart, of (1) vehicle or tyrphostin A48 and (2) vehicle or NMDA. Intake is overall significantly greater after NMDA injection (asterisk) and is significantly suppressed by the highest dose of tyrphostin A48 (inverted carat). The chemical structure of tyrphostin A48 is shown above the graph; n = 13. B, Mean ± SEM cumulative food intake (in grams) after LHA microinjection of vehicle or varying doses of PP1, followed 10 min later by vehicle or NMDA. NMDA-elicited intakes are significantly greater than those after vehicle injection (asterisk) and are suppressed by PP1 pretreatment (inverted carats). The chemical structure of PP1 is shown above the graph; n = 15. Bars in both graphs are shaded according to the postinjection time that intake was measured; these times and their corresponding shading are noted in the key between the graphs.
PP1, an Src family-selective PTK inhibitor, robustly suppressed NMDA-elicited feeding (experiment 5)
PP1 (chemical structure, Fig. 3B) is a selective pyrazolopyrimidine class of PTK inhibitor that acts as an ATP analog. It is orders of magnitude more potent of an inhibitor of Src family PTKs than of other PTK targets (IC50 values, 0.005-0.17 vs 50-100 μm, respectively) and only minimally affects other kinase targets (Hanke et al., 1996, Liu et al., 1999; Waltenberger et al., 1999; Susa et al., 2000). Additionally, PP1 is two orders of magnitude more potent than genistein at inhibiting Src PTKs (cf. Akiyama and Ogawara, 1991; Hanke et al., 1996; Waltenberger et al., 1999; Susa et al., 2000). In experiment 5a, as shown in Figure 3B, LHA-administered NMDA again elicited significant food intake at all postinjection times measured (e.g., at 0.5 hr, NMDA elicited 6.8 ± 1.9 gm of food intake versus 0.2 ± 0.1 gm for vehicle; p < 0.05 by one-way ANOVAs for each time point). A new finding is that PP1 produced a strong dose-dependent suppression of the NMDA-elicited response (Fig. 3B), with 0.2, 2.1, and 21.2 nmol of PP1 suppressing NMDA-elicited feeding by 37% (4.3 ± 2.3 gm), 54% (3.1 ± 1.4 gm), and 69% (2.2 ± 1.2 gm), respectively (F(3,148) = 4.6; p < 0.01 by two-way ANOVA excluding vehicle- and inhibitor-only treatments; p < 0.05 by the Student-Neuman-Keuls method).
In experiment 5b (data not shown), the suppression of NMDA-elicited eating by PP1 was reproduced in a naive group of rats using only the 2.1 and 21.2 nmol doses of PP1. A two-way ANOVA (excluding the vehicle-only treatment) again revealed a significant effect of treatment on food intake (F(2,135) = 3.3; p < 0.05) but not of time after injection or their interaction. The 21.2 nmol dose of PP1 significantly reduced NMDA-elicited eating by up to 41% (e.g., from 10.5 ± 2.3 to 6.2 ± 1.6 gm at 0.5 hr; p < 0.05 by the Student-Neuman-Keuls method). The apparent 16% reduction caused by the lower dose of PP1 (2.1 nmol), in this case, did not achieve statistical significance.
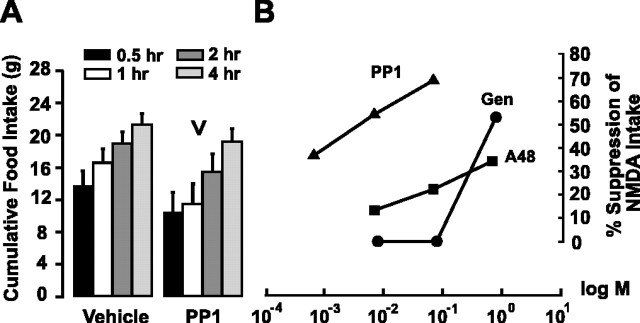
PP1 suppressed feeding triggered by food deprivation (experiment 6)
To help determine the contribution of Src family PTKs to feeding behavior caused by food deprivation, we bilaterally injected PP1 into the LHA of rats deprived of food for ∼24 hr. As shown in Figure 4A, vehicle-injected rats ate 13.8 ± 1.8 gm at 0.5 hr after injection, a score that was suppressed by ∼24% in PP1-injected animals (10.5 ± 2.4 gm). A two-way ANOVA revealed significant effects of both treatment (F(1,68) = 6.7; p < 0.05) and time after injection (F(3,68) = 7.1; p < 0.001) on food intake scores but not their interaction (p = 0.88).
Figure 4.
PP1 suppresses eating triggered by an overnight fast (experiment 6) and is the most powerful and potent PTK inhibitor tested against NMDA-elicited eating. A, Mean ± SEM cumulative food intake scores (in grams) at various postinjection times in 24 hr-fasted rats after bilateral LHA microinjections of either vehicle or PP1. PP1 significantly reduces deprivation-elicited eating (inverted carat); n = 13. B, Concentration-response relationships for PTK inhibitors (experiments 1, 4, and 5a). The percentage of suppression of NMDA-elicited food intake is plotted as a function of inhibitor concentration (expressed as log M) for the three PTK inhibitors. NMDA-elicited intakes were not significantly different across these studies and were treated as the 100% values from which the individual efficacies of genistein (Gen), tyrphostin A48 (A48), and PP1 were compared, respectively.
PTK inhibitors differed in their power and potency to suppress NMDA-elicited eating
We compared the efficacy of all three PTK inhibitors to suppress NMDA-elicited eating behavior across the three sets of studies. Figure 4B shows that PP1 produced the largest suppression of NMDA-elicited feeding (69% at the highest dose tested), followed by genistein (53% at the highest dose tested) and Tyr A48 (34%). PP1 was also the most potent inhibitor. Although it was three orders of magnitude lower in concentration, the lowest effective dose of PP1 produced a suppression similar in magnitude to that produced by the highest dose of Tyr A48 (Fig. 4B).
Src-like immunoreactivity was localized within lateral hypothalamic neurons (experiment 7)
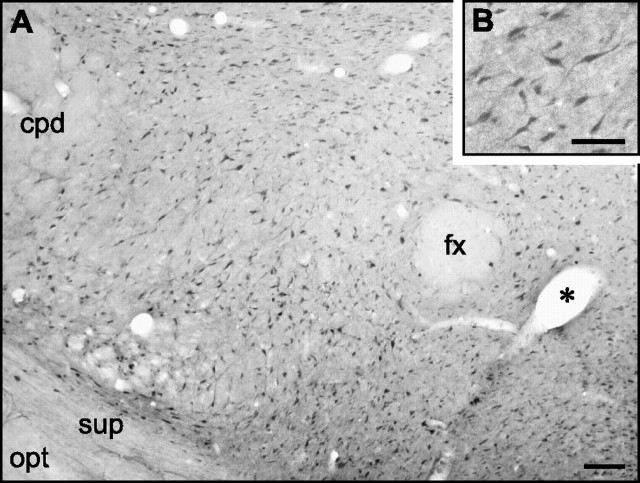
To determine whether Src PTK exists in LHA cells, we performed immunohistochemistry using a polyclonal antibody that recognizes Src. Figure 5A shows that subpopulations of LHA neurons displaying robust Src-like immunoreactivity were present in an area ranging from the perifornical area to the margin of the optic tract. Immunoreactive cells were identified as neurons on the basis of their similarity to LHA neurons described in a Golgi study (Millhouse, 1979). Staining was observed in both somata and neurites (Fig. 5B), and high-power visualization under oil immersion revealed that some of these neurites were probably dendrites, as multiple neurites were often seen extending from a single cell body. Staining was also observed in the nuclei of these cells, which was absent in control sections processed without primary antibody, suggesting that Src-like immunoreactivity is detectable in multiple cellular compartments. Our results support and supplement those of Ross et al. (1988) and Sugrue et al. (1990), who provided brief textual and photographic descriptions, respectively, of Src isoforms in the LHA as part of larger anatomical surveys.
Figure 5.
Src-like immunoreactivity in perifornical LHA neurons (experiment 7). A, Low-power view of Src-immunoreactive neurons in the field of the LHA. cpd, Cerebral peduncle; fx, fornix; opt, optic tract; sup, supraoptic decussation. The asterisk marks a blood vessel in cross section. B, Profiles of some Src-immunoreactive neurons in the LHA. Note that the tissues shown in A and B are from two separate animals. Scale bars: A, 100 μm; B, 50 μm.
In contrast to the robust immunostaining we observed using this antibody, control sections processed in its absence displayed virtually no immunoreactivity (data not shown). Moreover, because the “background staining” observed in the sections treated with the primary antibody was eliminated in the control sections, some of this staining might be from cut immunoreactive neurites out of the plane of section of the tissue.
Food deprivation but not LHA NMDA injection was associated with altered protein-tyrosine phosphorylation of several proteins in LHA-containing tissue
Because some PTK inhibitors were able to suppress feeding triggered not only by NMDA injection but also by food deprivation, we next determined the effects of these feeding conditions on protein-tyrosine phosphorylation in LHA-containing tissue. In experiment 8a, micropunched LHA-containing tissue was removed from rats receiving bilateral injections of aCSF vehicle or NMDA. Figure 6A shows that prominent tyrosine-phosphorylated (Tyr-P) protein components were observed in the 50-200 kDa molecular weight range, including bands of ∼60, 95, 115, and 180-190 kDa. We focused our quantitative analysis on the p60 and p180-190 bands because Src family PTKs are ∼60 kDa, and NR2 subunits of the NMDA-R, which are targets of Tyr-P, are ∼180 kDa. However, this analysis revealed no significant differences in the Tyr-P levels of proteins p60 and p180-190 between the two treatment conditions (Fig. 6D).
Figure 6.
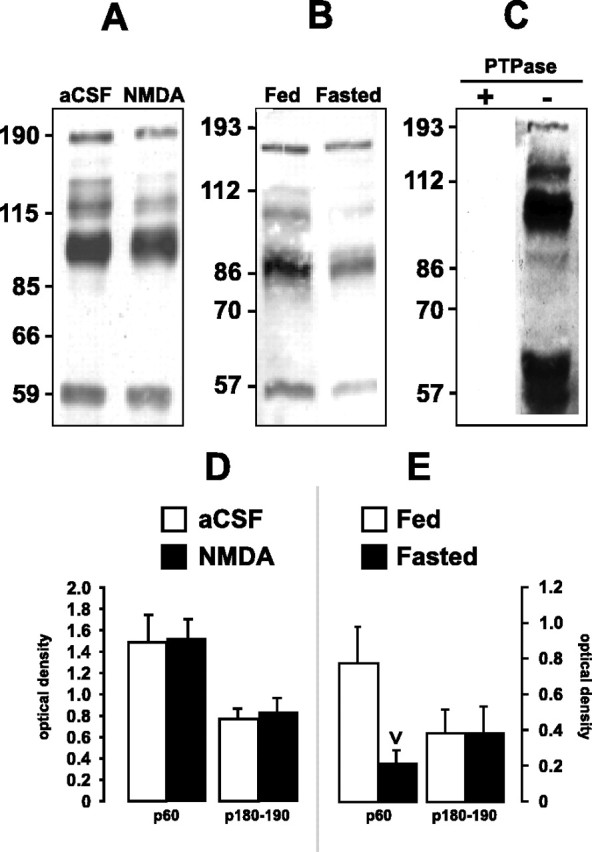
Tyrosine-phosphorylated proteins are present within total protein extracts of homogenized LHA-containing tissue. A, B, Total protein within LHA-containing tissue from aCSF- and NMDA-injected rats (A) or ad libitum-fed or 48 hr-fasted rats (B) was probed with an α-pY antibody. Positions of molecular weight standards are along the left of the blots (in kilodaltons). Note the prominent ∼60, ∼95, and ∼180-190 kDa bands. Data are representative of four experiments, each performed using tissue obtained from one or two animals. D, E, Tyr-P levels are expressed as OD units ± SEM for the 60 and 180-190 kDa bands in tissue from aCSF- and NMDA-injected rats (D) or ad libitum-fed and 48 hr-fasted rats (E). Compared with controls, fasted rats had significantly decreased Tyr-P for the 60 kDa band (inverted carat; p < 0.05). C, Specificity of the α-pY antibody. The α-pY immunoreactivity for total protein from LHA-containing tissue was abolished with (+) but not without (-) tyrosine phosphatase pretreatment.
In contrast, LHA-containing tissue homogenates from animals fasted for 48 hr (experiment 8b) displayed prominent bands with similar molecular weights as those in experiment 8a (Fig. 6B) but with significant differences in the Tyr-P of p60 relative to ad libitum-fed controls. Specifically, the mean OD for the p60 band in ad libitum-fed rats was 0.78 ± 0.20 (11 observations), whereas for 48 hr-fasted rats, the mean OD was significantly lower (0.21 ± 0.07; 11 observations; p < 0.05 by t test) (Fig. 6E). Other bands in the 75-110 kDa range from fasted rats also displayed apparent changes in optical density; these, however, were not quantitatively analyzed. In contrast, there were no significant differences in the Tyr-P levels of p180-190 between the two groups.
To verify the specificity of our phosphotyrosine antibody, we performed a control experiment in which we first exposed the immunoblot to a PTPase and then reacted it with the phosphotyrosine antibody. As shown in Figure 6C, pretreating the blot with PTPase eliminated phosphotyrosine immunostaining of all separated proteins.
Food deprivation but not LHA NMDA injection was associated with altered phosphorylation of Src and altered expression of Pyk2
We next examined whether Src and Pyk2 PTK expression and the phosphorylation of Src family PTKs at Tyr527 differed between aCSF and NMDA or fed and fasted groups. The phosphorylation of Src family PTKs at Tyr527 was examined because dephosphorylation of this regulatory site is a well known mechanism that relieves Src family PTKs from their autoinhibitory confirmation and facilitates their increased activation (Cooper et al., 1986; Thomas and Brugge, 1997; Xu et al., 1999). Thus, the phosphorylation state of this site provides one important index of apparent catalytic activity (Fig. 7C) (for details, see Discussion). Figure 7A shows that no statistically significant differences were observed in the phosphorylation levels of Src family PTKs at Tyr527, total Src protein, or total Pyk2 protein between aCSF- and NMDA-injected rats. Although such changes could have occurred in a subset of LHA neurons but that remained undetectable within the larger micropunched samples we assayed, these results suggest that NMDA injection is not sufficient to drive such changes in vivo. We cannot, however, rule out mechanisms that activate Src PTKs independent of its dephosphorylation at Tyr527 (Ma et al., 2000) or the possibility that the anesthesia might have masked effects that would otherwise have been apparent.
Figure 7.
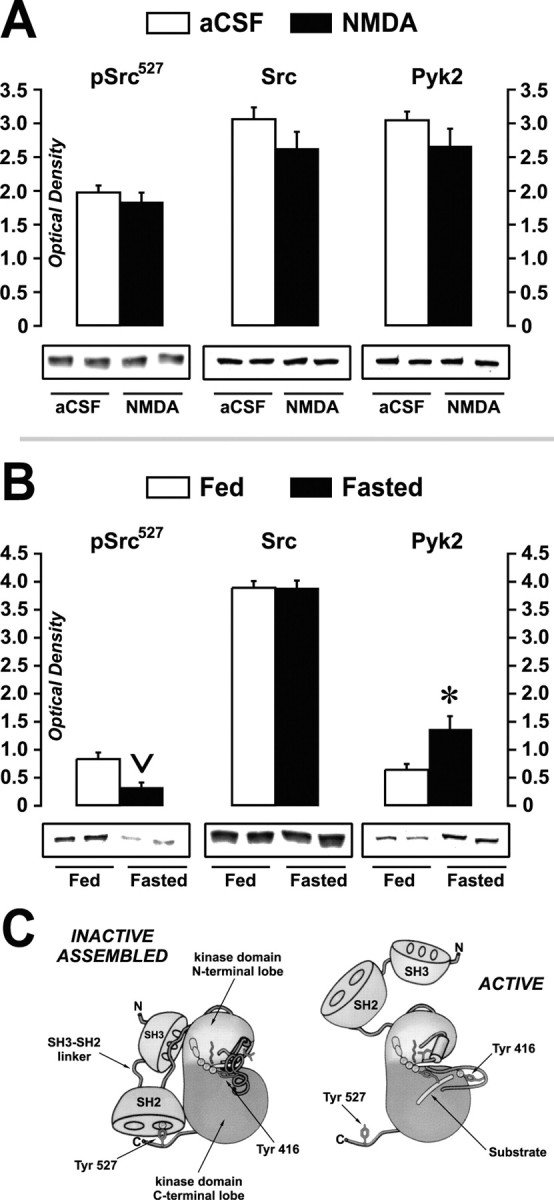
Apparent Src activity and Src and Pyk2 expression in LHA-containing tissue after NMDA injection or food deprivation. Immunoblots of LHA-containing tissue homogenates from aCSF- and NMDA-injected rats (A) or from 48 hr-food-deprived (Fasted) and ad libitum-fed (Fed) rats (B) are shown. Two lanes are shown for each treatment to help illustrate the uniformity of immunostaining within treatment groups. Blots were probed with antibodies directed against the negative regulatory phosphorylation site on Src family kinases at Tyr527 (pSrc527), Src kinase, or Pyk2 kinase, and immunostaining was quantitatively measured as mean optical density values (all ordinate scales) ± SEM. For each antigen, blots are representative of two or three measures. C, Illustration of inactive versus active Src kinase (modified and reproduced with permission from Young et al., 2001). In the INACTIVE ASSEMBLED conformation, note the hypothesized interaction of the phosphoryl group at Tyr527 with an Src homology 2 (SH2) domain binding site. Dephosphorylation at Tyr527 is thought to loosen an inducible snap lock produced by the SH2-SH3 linker domain, disinhibiting the kinase and facilitating greater exposure of the SH2 and SH3 domains to protein-protein binding interactions with other macromolecules (ACTIVE conformation). From this mechanism, the fasting-induced decrease in phosphorylation of Src at Tyr527 (B) suggests that Src is more catalytically active during food deprivation.
In experiment 8b, identical measures were made on LHA-containing tissue rats fasted for 48 hr or their ad libitum-fed controls. Figure 7B shows that, in contrast to NMDA injection, food deprivation significantly changed two of these three end points. Specifically, there was a marked decrease in the mean OD value for immunoreactive pSrc527 in LHA-containing tissue of the fasted rats relative to controls (0.3 ± 0.1 compared with 0.8 ± 0.1, respectively; p < 0.01 by t test) (Fig. 7B). However, the total expression of Src remained unchanged between groups (Fig. 7B). Also, levels of the Src-activating PTK Pyk2 were robustly elevated in LHA-containing tissue from fasted rats (Fig. 7B) (1.4 ± 0.2 compared with 0.6 ± 0.1, respectively; p < 0.05 by t test). We examined Pyk2 because of recent studies demonstrating it is a critical participant within signaling pathways that use NMDA-R activation and Src activity (Huang et al., 2001).
Tyrosine phosphorylation of LHA NMDA-R subunits did not change in association with NMDA injection or fasting
PTKs such as Src can enhance NMDA-R activity (Wang and Salter, 1994; Chen and Leonard, 1996; Köhr and Seeburg, 1996; Yu et al., 1997; Lancaster and Rogers, 1998; Liao et al., 2000). This enhancement may occur as a result of NMDA-R subunit phosphorylation by Src, although this awaits empirical demonstration (Salter and Kalia, 2004). NR2A and NR2B subunits, which can be tyrosine-phosphorylated (Moon et al., 1994; Lau and Huganir, 1995), might also be within LHA NMDA-Rs active during feeding stimulation (Petralia et al., 1994a,b; Khan et al., 1999, 2000b). Accordingly, we hypothesized that, by phosphorylating these subunits in LHA neurons, PTKs could contribute to changes in food intake and body weight by enhancing LHA NMDA-R activity (Khan et al., 1999) (A. M. Khan, E. R. Gillard, B. G. Stanley, Phosphorylation of Hypothalamic NMDA Receptors as a Possible Mechanism Contributing to Changes in Food Intake and Body Weight, in Matters Arising, International Society for Neurochemistry, available on-line). In such a case, feeding stimulation should increase the Tyr-P of LHA NR2A and NR2B subunits.
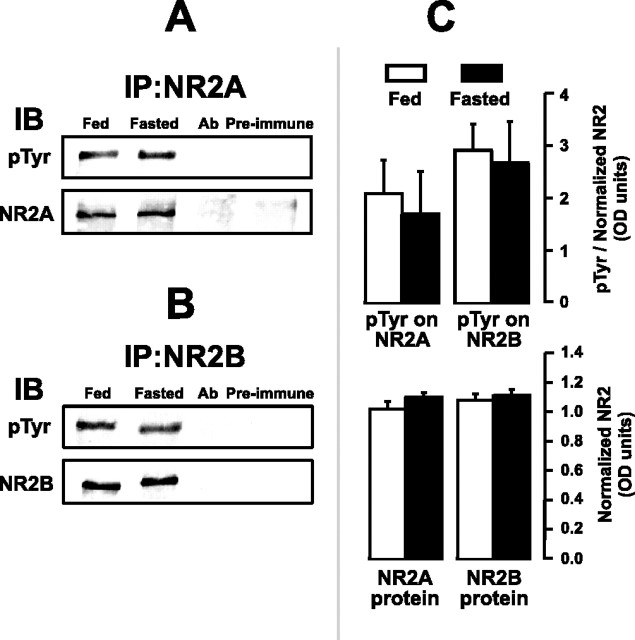
However, we found that NMDA injection did not appreciably change the phosphorylation of NR2B at Tyr1472 (data not shown). Additionally, Figure 8 shows that fasting did not alter levels of protein or Tyr-P for NR2A and NR2B subunits immunoprecipitated from LHA-containing tissue (mean OD scores, 2.09 ± 0.64 and 1.69 ± 0.82, respectively; p = 0.54 by Mann-Whitney rank sum test). Nor did fasting significantly enhance Tyr-P of NR2B at Tyr1472 (data not shown) (mean OD scores, 2.91 ± 0.51 and 2.67 ± 0.79; p = 0.80 by t test). Thus, neither NMDA injection nor food deprivation appears to be associated with changes in the Tyr-P levels of the NR2A and NR2B NMDA-R subunits, although such changes may occur in only subpopulations of LHA neurons and, therefore, at levels that escaped detection by our methods. However, these results do confirm reports of NR2A and NR2B NMDA-R subunits in the LHA (Khan et al., 1999, 2000b) and also provide the first evidence that these subunits are tyrosine-phosphorylated in this region.
Figure 8.
Acute or prolonged feeding stimulation does not alter tyrosine phosphorylation or protein expression levels of NR2A and NR2B NMDA-R subunits immunoprecipitated in LHA-containing tissue. Immunoprecipitated NR2A (A) and NR2B (B) subunits from 48 hr-food-deprived rats (Fasted) and their ad libitum-fed controls (Fed) were probed with antibodies directed against either phosphotyrosine (pTyr) or the subunits themselves (NR2A or NR2B). Lanes marked Ab were loaded with only Protein A/G and were still reacted with antibodies to assess background staining. Lanes marked Preimmune were loaded with naive hippocampal homogenates immunoprecipitated only with preimmune serum. Each Fed or Fasted band includes bilateral LHA-containing homogenized tissue from two rats (4 tissue micropunches), although, in some instances, at least three animals were used. Data are representative of two experiments, each using 9 or 10 rats per feeding condition; total n = 38 rats. C, Mean levels ± SEM in OD units of tyrosine phosphorylation (top graph) and protein expression (bottom graph), respectively, for the NR2A and NR2B subunits immunoprecipitated in A and B. Mean scores were obtained using all data from both experimental runs (for details, see Materials and Methods). IP, Immunoprecipitated protein; IB, immunoblotted protein; pTyr, phosphotyrosine immunostaining.
Discussion
The present study provides four lines of evidence suggesting that Src family PTKs and Tyr-P participate in LHA signaling mechanisms underlying feeding stimulation: (1) NMDA-elicited feeding is powerfully suppressed by structurally distinct LHA-administered PTK inhibitors, including one selective for Src family PTKs; (2) deprivation-induced feeding is also significantly suppressed by the Src family-selective inhibitor; (3) Src is robustly expressed in LHA neurons and, along with Pyk2 PTK and various Tyr-P proteins, is detectable in LHA-containing tissue; and (4) food deprivation is associated with altered Tyr-P of various LHA proteins, an increase in the apparent activity of one or more Src family PTKs, and a robust increase in Pyk2 expression.
PTK inhibitors suppress eating
LHA administration of the PTK inhibitor genistein markedly suppressed NMDA-elicited eating. Because genistein can affect non-PTK targets, we evaluated its chemical and behavioral specificity for producing these effects. First, daidzein, a genistein analog that does not inhibit PTKs, did not suppress NMDA-elicited eating, suggesting that the effects of genistein are specific to its actions as a PTK inhibitor. Second, KA-injected rats given genistein still ate robustly, making it unlikely that the suppression of NMDA-elicited eating by genistein was attributable to drug-induced nausea or malaise. Along with genistein, two other PTK inhibitors, tyrphostin A48 and PP1, also suppressed feeding, suggesting that all three might target a common neural substrate, such as one or more PTK activities.
Clear differences existed in the power and potency of these inhibitors to suppress feeding, which may be partly attributable to differences in their selectivity and efficacy for target PTKs. Unlike the broad-spectrum inhibitors genistein and tyrphostin A48, PP1 is highly selective for Src family PTKs and, compared across similar but not identical assay conditions, apparently inhibits Src family PTKs with a potency at least two orders of magnitude greater than that for genistein (Susa et al. 2000). Although our data do not eliminate the possibility of effects on other targets, these facts, along with the observation that PP1 produced the most potent and powerful suppression of NMDA-elicited feeding, strongly implicate Src family PTKs as the neural substrates most sensitive to signals triggering such feeding. In support of this, PP1 but not genistein suppressed deprivation-elicited feeding, underscoring that perhaps Src PTKs are involved. Thus, our pharmacological studies suggest that PTKs, particularly Src family PTKs, are involved in mechanisms underlying feeding stimulation.
Src, Pyk2, and various other tyrosine-phosphorylated proteins exist in the LHA
Our quantitative immunochemical data also support a role for PTK signaling within LHA neurons, revealing robust expression of Src, Pyk2, and several as yet unidentified tyrosinephosphorylated proteins in LHA-containing tissue. Our Pyk2 detection confirms, at the protein level, studies localizing transcripts for Pyk2 variants in the hypothalamus (Xiong et al., 1998; Menegon et al., 1999). Also, Src was immunolocalized to selected perifornical LHA neurons, which contain neural substrates sensitive to the feeding stimulation effects of neuropeptide Y, hypocretin/orexin, or Glu (Stanley et al., 1992, 1993a,b,c; Khan et al., 2000b; Marcus et al., 2001; Backberg et al., 2002; Lee and Stanley, 2002; Wolak et al., 2003), suggesting that Src can interact with signaling components of these neural substrates to influence feeding or other behaviors.
Effects of NMDA injection or food deprivation on LHA tyrosine phosphorylation, PTK activity, and PTK expression
The first major finding from the biochemistry was that deprivation-induced feeding but not NMDA-elicited feeding was associated with a marked decrease in the Tyr-P of the p60 band. Second, because Src family PTKs (EC 2.7.1.112) are themselves regulated by Tyr-P and are ∼60 kDa (Bjorge et al., 2000; Tatosyan and Mizenina, 2000), we asked whether the decreased Tyr-P of p60 might reflect decreased Tyr-P of Src family PTKs. Accordingly, we reacted immunoblots of micropunched LHA-containing tissue with a phospho-specific Src family PTK antibody directed against Tyr527, located near the C-terminus. Tyr-P of this regulatory site inhibits Src catalytic activity and is a well studied mechanism of Src regulation (Fig. 7C) (Cooper et al., 1986; Sicheri and Kuriyan, 1997; Hubbard and Till, 2000; Wang et al., 2001; Young et al., 2001). This antibody revealed markedly reduced Tyr-P at this site in food-deprived rats, suggesting that food deprivation markedly disinhibits and perhaps activates one or more Src family PTKs. This suggestion is supported by the suppression of deprivation feeding we observed using the Src family-selective PTK inhibitor PP1. Food-deprived rats also displayed elevated expression of Pyk2, a soluble PTK known to interact with Src in some neural systems (Ali and Salter, 2001; Huang et al., 2001; Salter and Kalia, 2004). In contrast, NMDA-elicited feeding stimulation was not associated with elevated Src family activity or Pyk2 expression. Collectively, these results strongly implicate Src family PTKs in LHA signaling mechanisms underlying feeding stimulation produced by food deprivation.
Interpreting the differential effects of NMDA-elicited and deprivation-induced feeding stimulation on PTK activity and expression
Our behavioral data demonstrate that LHA injections of PTK inhibitors, including one selective for the Src family, are more effective in suppressing NMDA-elicited feeding than food deprivation-induced feeding. In contrast, the biochemical data reveal that food deprivation is more effective than NMDA injection in driving detectable changes in the activity and expression of PTKs in LHA-containing tissue. Thus, food deprivation is sufficient to drive changes in PTK activity, but PTKs are not so critical for deprivation feeding to occur. Conversely, NMDA-elicited feeding stimulation is not sufficient to increase PTK activity, but PTKs appear to be quite critical for the full magnitude of the NMDA-elicited feeding response.
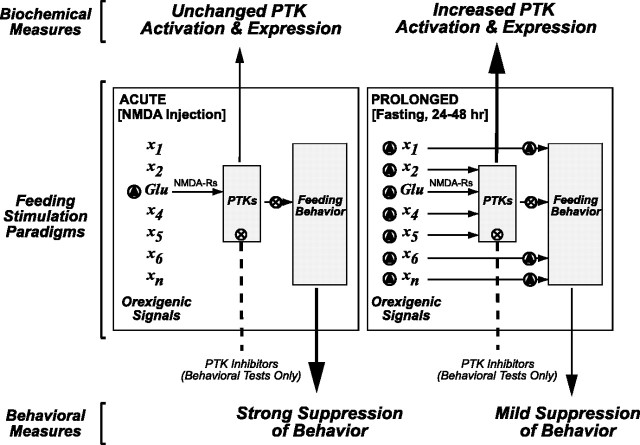
These biochemical and behavioral data together form the basis of the model depicted in Figure 9. The model posits that deprivation feeding will activate more distinct types of neural substrate than feeding driven by LHA NMDA injection (see Orexigenic Signals in both panels of Fig. 9). This model is an elaboration of the prevailing view that food deprivation engages multiple neural circuits and chemical messengers (Berthoud, 2002; Saper et al., 2002) and adds to that formulation the suggestion that food deprivation recruits a greater variety of signaling pathways to elicit feeding than does acute NMDA injection. From this, the model predicts that food deprivation, by engaging many signaling pathways to elicit feeding, would be suppressed to only a minor extent with PTK inhibitors but would still markedly shift the activity and expression of PTKs in the LHA (Fig. 9, right panel). In contrast, although acute NMDA injection triggers feeding behavior that is more easily suppressed by PTK inhibitors and that, therefore, may rely more heavily on PTK signaling mechanisms, such stimulation is not sufficient to alter PTK activity or expression as measured by the indices we used. This is perhaps because the NMDA-R was the only signal, of many possible PTK-linked signals, that was engaged by our NMDA injection (Fig. 9, compare left, right panels).
Figure 9.
Hypothesized participation of PTKs in neural circuits during acute versus prolonged feeding stimulation. Left panel, During acute feeding stimulation, elicited in this case by LHA NMDA injection, only a narrow subset of feeding signals are mobilized (triangle), which require PTK activity. Such a narrow level of stimulation is not sufficient to significantly change PTK activation or expression as measured biochemically and produces a feeding response that is easily suppressed by PTK inhibitors (× symbols). Right panel, In contrast, prolonged feeding stimulation, brought about by food deprivation, mobilizes several orexigenic pathways, including the glutamatergic path that can be activated by NMDA injection. The coordinated actions of these orexigenic signals are sufficient to robustly elevate PTK activation and expression. However, because PTK-mediated feeding signals are but one component of the signals contributing to the feeding response, PTK inhibitors only mildly suppress this feeding behavior. For a full description of this model, see Discussion.
Using in vivo approaches to identify intracellular effectors is challenging but is clearly important for understanding their function in the context of behavior (Dumont et al., 2002). The present work supports the argument that such an understanding is more likely to be achieved in the intact animal by using multiple stimulation paradigms and by using a combination of biochemistry and behavioral analysis to help sharpen and constrain the interpretations that might otherwise be drawn when using only one of these approaches.
Src family PTKs in LHA neurons may integrate feeding signals
Src family PTKs can be activated by G-protein-coupled receptors (GPCRs) or receptors coupled to Tyr-P mechanisms (Ma et al., 2000; Ram and Iyengar, 2001); activation of either receptor class could mobilize Src family PTKs in LHA neurons during food deprivation. For example, LHA afferents from the arcuate nucleus (Elias et al., 1998) could release NPY, AgRP, and α-MSH to activate GPCRs. In contrast, Tyr-P-linked activation of a leptin receptor isoform (Li and Friedman, 1999; Hegyi et al., 2004) described in the LHA (Fei et al., 1997; Elmquist et al., 1998; Iqbal et al., 2001) could trigger downstream effectors that potentially interact with Src (Chaturvedi et al., 1998; Hung and Elliott, 2001). Additionally, serine-threonine protein kinases implicated in feeding stimulation (Gillard et al., 1997, 1998a,b; Sheriff et al., 1997) could cross-signal with LHA PTKs (Nijholt et al., 2000; Schmitt and Stork, 2002). How Src family PTKs help transfer feeding signals within LHA neurons is unclear, but our results suggest that some of this transfer involves Pyk2 and Src recruitment without significant alterations in NMDA-R subunit Tyr-P.
Concluding remarks
These studies constitute the first description of PTK involvement in LHA mechanisms underlying feeding stimulation. Specifically, Src family PTKs, which are robustly expressed within LHA neurons, are apparently activated after food deprivation and appear critical for ensuring the full magnitude of food intake that occurs during acute and prolonged feeding stimulation. That soluble PTKs such as Src mediate signals to help control vital behaviors such as eating underscores the importance of these enzymes for the survival of multicellular organisms, which, in contrast to their unicellular relatives, may be unique in having these versatile proteins encoded within their genomes (Hanks and Hunter, 1995; Darnell, 1997).
Footnotes
This work was supported by an Eli Lilly award (B.G.S.), a grant from the Natural Sciences and Engineering Research Council of Canada (J.W.G.), a University of California, Riverside (UCR), dissertation research grant (A.M.K.), and UCR student research mini grants (J.A.P.). We thank J. C. Bosze, O. Hamzeinejad, A. Khan, A. Nguyen, J. Nikpur, P. Pir, and C. S. Ton for technical assistance, T. Yamamoto for providing a phosphospecific NR2B antibody, and A. G. Watts for critically reading a draft of this manuscript. We also acknowledge the guidance and support of our friend and mentor, Dr. John Ashe, who died while this manuscript was under revision.
Correspondence should be addressed to Dr. Arshad M. Khan, Department of Biological Sciences, Neuroscience Program, University of Southern California, Hedco Neuroscience Building, MC 2520, 3641 Watt Way, Los Angeles, CA 90089-2520. E-mail: arshadk@usc.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2410603-13$15.00/0
References
- Akiyama T, Ogawara H (1991) Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol 201: 362-370. [DOI] [PubMed] [Google Scholar]
- Ali D, Salter MW (2001) NMDA receptor regulation by Src kinase signaling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol 11: 336-342. [DOI] [PubMed] [Google Scholar]
- Backberg M, Hervieu G, Wilson S, Meister B (2002) Orexin receptor-1 (OX R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci 15: 315-328. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R (2002) Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26: 393-428. [DOI] [PubMed] [Google Scholar]
- Bjorge JD, Jakymiw A, Fujita DJ (2000) Selected glimpses into the activation and function of Src kinase. Oncogene 19: 5620-5635. [DOI] [PubMed] [Google Scholar]
- Blevins JE, Stanley BG, Reidelberger RD (2002) DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav 71: 277-282. [DOI] [PubMed] [Google Scholar]
- Carr KD (1996) Feeding, drug abuse, and the sensitization of reward by metabolic need. Neurochem Res 21: 1455-1467. [DOI] [PubMed] [Google Scholar]
- Carr KD (2002) Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76: 353-364. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Reddy MV, Reddy EP (1998) Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene 16: 1749-1758. [DOI] [PubMed] [Google Scholar]
- Chen S-J, Leonard JP (1996) Protein tyrosine kinase-mediated potentiation of currents from cloned NMDA receptors. J Neurochem 67: 194-200. [DOI] [PubMed] [Google Scholar]
- Cheung HH, Takagi N, Teves L, Logan R, Wallace MC, Gurd JW (2000) Altered association of protein tyrosine kinases with postsynaptic densities after transient cerebral ischemia in the rat brain. J Cereb Blood Flow Metab 20: 505-512. [DOI] [PubMed] [Google Scholar]
- Chiesi M, Huppertz C, Hofbauer KG (2001) Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol Sci 22: 247-254. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Gould KL, Cartwright CA, Hunter T (1986) Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science 231: 1431-1434. [DOI] [PubMed] [Google Scholar]
- Darnell Jr JE (1997) Phosphotyrosine signaling and the single cell: metazoan boundary. Proc Natl Acad Sci USA 94: 11767-11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73: 759-768. [DOI] [PubMed] [Google Scholar]
- Dumont JE, Dremier S, Pirson I, Maenhaut C (2002) Cross signaling, cell specificity, and physiology. Am J Physiol 283: C2-C28. [DOI] [PubMed] [Google Scholar]
- Duva MA, Tomkins EM, Moranda LM, Kaplan R, Sukhaseum A, Jiminez A, Stanley BG (2001) Reverse microdialysis of N-methyl-d-aspartic acid into the lateral hypothalamus of rats: effects on eating and other behaviors. Brain Res 921: 122-132. [DOI] [PubMed] [Google Scholar]
- Duva MA, Tomkins EM, Moranda LM, Kaplan R, Sukhaseum A, Bernardo JP, Stanley BG (2002) Regional differences in feeding and other behaviors elicited by N-methyl-d-aspartic acid in the rodent hypothalamus: a reverse microdialysis mapping study. Brain Res 925: 141-147. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, Sakurai T, Yanagisawa M, Elmquist JK (1998) Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol 402: 442-459. [PubMed] [Google Scholar]
- Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB (1998) Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395: 535-547. [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee G-H, Zhao C, Darnell R, Friedman JM (1997) Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA 94: 7001-7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard ER, Khan AM, Haq AU, Grewal RS, Mouradi B, Stanley BG (1997) Stimulation of eating by the second messenger cAMP in the perifornical and lateral hypothalamus. Am J Physiol 273: R107-R112. [DOI] [PubMed] [Google Scholar]
- Gillard ER, Khan AM, Grewal RS, Mouradi B, Wolfsohn SD, Stanley BG (1998a) The second messenger cyclic AMP elicits eating by an anatomically specific action in the perifornical hypothalamus. J Neurosci 18: 2646-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard ER, Khan AM, Mouradi B, Nalamwar O, Stanley BG (1998b) Eating induced by perifornical hypothalamic cAMP is behaviorally selective and involves protein kinase activity. Am J Physiol 275: R647-R653. [DOI] [PubMed] [Google Scholar]
- Gurd JW, Bissoon N, Soulliere J (1992) Procedures for analyzing the tyrosine phosphorylation of synaptic glycoproteins. Neuroprotocols 1: 207-214. [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Chengelian PS, Brissette WH, Weringer EJ, Pollok K, Connelly PA (1996) Discovery of a novel, potent, and Src-family selective tyrosine kinase inhibitor: study of Lck-dependent and Fyn-dependent T-cell activation. J Biol Chem 271: 695-701. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576-596. [PubMed] [Google Scholar]
- Hegyi K, Fulop K, Kovacs K, Toth S, Falus A (2004) Leptin-induced signal transduction pathways. Cell Biol Int 28: 159-169. [DOI] [PubMed] [Google Scholar]
- Huang J, Nasr M, Kim Y, Matthews HR (1992) Genistein inhibits protein histidine kinase. J Biol Chem 267: 15511-15515. [PubMed] [Google Scholar]
- Huang R-Q, Dillon GH (2000) Direct inhibition of glycine receptors by genistein, a tyrosine kinase inhibitor. Neuropharmacology 39: 2195-2204. [DOI] [PubMed] [Google Scholar]
- Huang R-Q, Fang M-J, Dillon GH (1999) The tyrosine kinase inhibitor genistein directly inhibits GABAA receptors. Mol Brain Res 67: 177-183. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF (2001) CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron 29: 485-496. [DOI] [PubMed] [Google Scholar]
- Hubbard SR, Till JH (2000) Protein tyrosine kinase structure and function. Annu Rev Biochem 69: 373-398. [DOI] [PubMed] [Google Scholar]
- Hung W, Elliott B (2001) Co-operative effect of c-Src tyrosine kinase and Stat3 in activation of hepatocyte growth factor expression in mammary carcinoma cells. J Biol Chem 276: 12395-12403. [DOI] [PubMed] [Google Scholar]
- Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW (1995) cAMP-independent activation of CFTR Cl channels by the protein tyrosine kinase inhibitor genistein. Am J Physiol 268: C886-C893. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Pompolo S, Murakami T, Grouzmann E, Sakurai T, Meister B, Clarke IJ (2001) Immunohistochemical characterization of long-form leptin receptor (OB-Rb) in neurochemically defined cells in the ovine hypothalamus. Brain Res 920: 55-64. [DOI] [PubMed] [Google Scholar]
- Khan AM (2002) Lateral hypothalamic NMDA receptor subunits, tyrosine kinases and tyrosine phosphorylation in the central stimulation of eating. PhD thesis, University of California, Riverside.
- Khan AM, Welsbie DS, Khan AM, Ton CS, Nikpur J, Gillard ER, Pir PP, Stanley BG (1996) Feeding elicited by N-methyl-d-aspartate (NMDA) in the lateral hypothalamus (LH) is suppressed by a tyrosine kinase inhibitor. Soc Neurosci Abstr 23: 555.12. [Google Scholar]
- Khan AM, Currás MC, Dao J, Jamal FA, Turkowski CA, Goel RK, Gillard ER, Wolfsohn SD, Stanley BG (1999) Lateral hypothalamic NMDA receptor subunits NR2A and/or NR2B mediate eating: immunochemical/behavioral evidence. Am J Physiol 276: R880-R891. [DOI] [PubMed] [Google Scholar]
- Khan AM, Palarca JA, Bosze JC, Stanley BG (2000a) PP1, a selective inhibitor of the Src family of tyrosine kinases, suppresses eating elicited by NMDA in the lateral hypothalamus. Soc Neurosci Abstr 27: 370.7. [Google Scholar]
- Khan AM, Stanley BG, Bozzetti L, Chin C, Stivers C, Currás-Collazo MC (2000b) N-methyl-d-aspartate receptor subunit NR2B is widely expressed throughout the rat diencephalon: an immunohistochemical study. J Comp Neurol 428: 428-449. [PubMed] [Google Scholar]
- Khan AM, Cheung HH, Gillard ER, Gurd JW, Stanley BG (2001) Evidence for Src protein tyrosine kinase in lateral hypothalamic cells and its decreased tyrosine phosphorylation during food deprivation. Soc Neurosci Abstr 28: 946.1. [Google Scholar]
- Köhr G, Seeburg PH (1996) Subtype-specific regulation of recombinant rat and mouse NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol (Lond) 492: 445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, van der Burg B, Gustafsson J-Å (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 139: 4252-4263. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Rogers MV (1998) A peptide activator of endogenous tyrosine kinase enhances synaptic currents mediated by NMDA receptors. Eur J Neurosci 10: 2302-2308. [DOI] [PubMed] [Google Scholar]
- Lau L-F, Huganir RL (1995) Differential tyrosine phosphorylation of N-methyl-d-aspartate receptor subunits. J Biol Chem 270: 20036-20041. [DOI] [PubMed] [Google Scholar]
- Lee SW, Stanley BG (2002) Evidence that neuropeptide Y-elicited feeding depends upon activation of NMDA receptors in the lateral and perifornical hypothalamus. Soc Neurosci Abstr 29: 775.14. [Google Scholar]
- Levitzki A (1992) Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. FASEB J 6: 3275-3282. [DOI] [PubMed] [Google Scholar]
- Li C, Friedman JM (1999) Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci USA 96: 9677-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN (2002) Hypocretin/orexin excites hypocretin neurons via a local glutamate interneuron: a potential mechanism for orchestrating the hypothalamic arousal system. Neuron 36: 1169-1181. [DOI] [PubMed] [Google Scholar]
- Liao G-Y, Kreitzer MA, Sweetman BJ, Leonard JP (2000) The postsynaptic density protein PSD-95 differentially regulates insulin- and Src-mediated current modulation of mouse NMDA receptors expressed in Xenopus oocytes. J Neurochem 75: 282-287. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bishop A, Witucki L, Kraybill B, Shimizu E, Tsien J, Ubersax J, Blethrow J, Morgan DO, Shokat KM (1999) Structural basis for selective inhibition of Src family kinases by PP1. Chem Biol 6: 671-678. [DOI] [PubMed] [Google Scholar]
- Ma Y-C, Huang J, Ali S, Lowry W, Huang X-Y (2000) Src tyrosine kinase is a novel direct effector of G proteins. Cell 102: 635-646. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6-25. [DOI] [PubMed] [Google Scholar]
- Martin BL (1998) Inhibition of calcineurin by the tyrphostin class of tyrosine kinase inhibitors. Biochem Pharmacol 56: 483-488. [DOI] [PubMed] [Google Scholar]
- Menegon A, Burgaya F, Baudot P, Dunlap DD, Girault J-A, Valtorta F (1999) FAK+ and PYK2/CAKβ, two related tyrosine kinases highly expressed in the central nervous system: similarities and differences in the expression pattern. Eur J Neurosci 11: 3777-3788. [DOI] [PubMed] [Google Scholar]
- Millhouse OE (1979) A Golgi anatomy of the rodent hypothalamus. In: Handbook of the hypothalamus, Vol 1, Anatomy of the hypothalamus (Morgane PJ, Panskepp J, eds), pp 221-265. New York: Dekker.
- Molokanova E, Savchenko A, Kramer RH (1999) Noncatalytic inhibition of cyclic nucleotide-gated channels by tyrosine kinase induced by genistein. J Gen Physiol 113: 45-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molokanova E, Savchenko A, Kramer RH (2000) Interaction of cyclic nucleotide-gated channel subunits and protein tyrosine kinase probed with genistein. J Gen Physiol 115: 685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon IS, Apperson ML, Kennedy MB (1994) The major tyrosine phosphorylated protein in the postsynaptic density fraction is N-methyl-d-aspartate receptor subunit 2B. Proc Natl Acad Sci USA 91: 3954-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T (2001) Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRϵ2 (NR2B) subunit of the N-methyl-d-aspartate receptor. J Biol Chem 276: 693-699. [DOI] [PubMed] [Google Scholar]
- Nijholt I, Blank T, Liu A, Kugler H, Spiess J (2000) Modulation of hypothalamic NMDA receptor function by cyclic AMP-dependent protein kinase and phosphatases. J Neurochem 75: 749-754. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Horie M, Washizuka T, Nishimoto T, Sasayama S (1999) On the mechanism of genistein-induced activation of protein kinase A-dependent Cl- conductance in cardiac myocytes. Pflügers Arch 438: 269-277. [DOI] [PubMed] [Google Scholar]
- Paillart C, Carlier E, Guedin D, Dargent B, Courand F (1997) Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J Pharmacol Exp Ther 280: 521-526. [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ (1988) Maps and guide to microdissection of the rat brain. New York: Elsevier.
- Patisaul HB, Dindo M, Whitten PL, Young LJ (2001) Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor α- and β-dependent gene expression in the brain. Endocrinology 142: 2946-2952. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson J (1998) The rat brain in stereotaxic coordinates, Ed 4. San Diego: Academic.
- Petralia RS, Yokotani N, Wenthold RJ (1994a) Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci 14: 667-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang Y-X, Wenthold RJ (1994b) The NMDA receptor subunits NR2A and NR2B show histological and ultrastructural localization patterns similar to those of NR1. J Neurosci 14: 6102-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkowski K, Mazurek AP (2000) Biological properties of genistein: a review of in vitro and in vivo data. Acta Pol Pharm 57: 135-155. [PubMed] [Google Scholar]
- Rada P, Tucci S, Murzi E, Hernández L (1997) Extracellular glutamate increases in the lateral hypothalamus and decreases in the nucleus accumbens during feeding. Brain Res 768: 338-340. [DOI] [PubMed] [Google Scholar]
- Rada P, Mendialdua A, Hernandez L, Hoebel BG (2003) Extracellular glutamate increases in the lateral hypothalamus during meal initiation, and GABA peaks during satiation: microdialysis measurements every 30 s. Behav Neurosci 117: 222-227. [DOI] [PubMed] [Google Scholar]
- Ram PT, Iyengar R (2001) G protein coupled receptor signaling through the Src and Stat3 pathway: role in proliferation and transformation. Oncogene 20: 1601-1606. [DOI] [PubMed] [Google Scholar]
- Ross CA, Wright GE, Resh MD, Pearson RC, Snyder SH (1988) Brain-specific src oncogene mRNA mapped in rat brain by in situ hybridization. Proc Natl Acad Sci USA 85: 9831-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J (2003) Targeting mammalian genes: rats join in and mice move ahead. Nat Biotechnol 21: 625-627. [DOI] [PubMed] [Google Scholar]
- Rozas JL, Paternain AV, Lerma J (2003) Noncanonical signaling by ionotropic kainate receptors. Neuron 39: 543-553. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5: 317-328. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199-211. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Stork PJS (2002) PKA phosphorylation of Src mediates cAMP's inhibition of cell growth via Rap1. Mol Cell 9: 85-94. [DOI] [PubMed] [Google Scholar]
- Sheriff S, Chance WT, Fischer JE, Balasubramaniam A (1997) Neuropeptide Y treatment and food deprivation increase cyclic AMP response element-binding in rat hypothalamus. Mol Pharmacol 51: 597-604. [DOI] [PubMed] [Google Scholar]
- Sicheri F, Kuriyan J (1997) Structures of Src-family tyrosine kinases. Curr Opin Struct Biol 7: 777-785. [DOI] [PubMed] [Google Scholar]
- Stanley BG (1996) Glutamate and its receptors in lateral hypothalamic stimulation of eating. In: Drug receptor subtypes and ingestive behaviour (Cooper SJ, Clifton PJ, eds), pp 301-322. London: Academic.
- Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF (1992) Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 13: 581-587. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF (1993a) The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res 604: 304-317. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Ha LH, Spears LC, Dee II MG (1993b) Lateral hypothalamic injections of glutamate, kainic acid, d,l-α-amino-3-hydroxy-5-methylisoxazole propionic acid or N-methyl-d-aspartic acid rapidly elicit intense transient eating in rats. Brain Res 613: 88-95. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett III VL, Donias HW, Ha LH, Spears LC (1993c) The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res 630: 41-49. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Willett III VL, Donias HW, Dee II MG, Duva MA (1996) Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol 270: R443-R449. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Butterfield BS, Grewal RS (1997) NMDA receptor coagonist glycine site: evidence for a role in lateral hypothalamic stimulation of feeding. Am J Physiol 273: R790-R796. [DOI] [PubMed] [Google Scholar]
- Sugrue MM, Brugge JS, Marshak DR, Greengard P, Gustafson EL (1990) Immunocytochemical localization of the neuron-specific form of the c-src gene product, pp60c-src(+), in rat brain. J Neurosci 10: 2513-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susa M, Missbach M, Green J (2000) Src inhibitors: drugs for the treatment of osteoporosis, cancer or both? Trends Pharmacol Sci 21: 489-495. [DOI] [PubMed] [Google Scholar]
- Takagi N, Shinno K, Teves L, Bissoon N, Wallace MC, Gurd JW (1997) Transient ischemia differentially increases tyrosine phosphorylation of NMDA receptor subunits 2A and 2B. J Neurochem 69: 1060-1065. [DOI] [PubMed] [Google Scholar]
- Tatosyan AG, Mizenina OA (2000) Kinases of the Src family: structure and functions. Biochemistry (Mosc) 65: 49-58. [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513-609. [DOI] [PubMed] [Google Scholar]
- Vera JC, Reyes AM, Cárcamo JG, Velásquez FV, Rivas CI, Zhang RH, Strobel P, Iribarren R, Scher HI, Slebe JC, Gold DW (1996) Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J Biol Chem 271: 8719-8724. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Uecker A, Kroll J, Frank H, Mayr U, Bjorge JD, Fujita D, Gazit A, Hombach V, Levitzki A, Böhmer F-D (1999) A dual inhibitor of platelet-derived growth factor β-receptor and Src kinase activity potently interferes with motogenic and mitogenic responses to PDGF in vascular smooth muscle cells: a novel candidate for prevention of vascular remodeling. Circ Res 85: 12-22. [DOI] [PubMed] [Google Scholar]
- Wang D, Huang X-Y, Cole PA (2001) Molecular determinants for Cskcatalyzed tyrosine phosphorylation of the Src tail. Biochemistry 40: 2004-2010. [DOI] [PubMed] [Google Scholar]
- Wang YT, Salter MW (1994) Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369: 233-235. [DOI] [PubMed] [Google Scholar]
- Washizuka T, Horie M, Obayashi K, Sasayama S (1997) Does tyrosine kinase modulate delayed-rectifier K channels in guinea pig ventricular cells? Heart Vessels [Suppl] 12: 173-174. [PubMed] [Google Scholar]
- Washizuka T, Horie M, Obayashi K, Sasayama S (1998) Genistein inhibits slow component delayed-rectifier K currents via a tyrosine kinase-independent pathway. J Mol Cell Cardiol 30: 2577-2590. [DOI] [PubMed] [Google Scholar]
- Wolak ML, DeJoseph MR, Cator AD, Mokashi AS, Brownfield MS, Urban JH (2003) Comparative distribution of neuropeptide Y Y1 and Y5 receptors in the rat brain by using immunohistochemistry. J Comp Neurol 464: 285-311. [DOI] [PubMed] [Google Scholar]
- Xiong W-C, Macklem M, Parsons JT (1998) Expression and splice variants of PYK2, a focal adhesion kinase-related protein. J Cell Sci 111: 1981-1991. [DOI] [PubMed] [Google Scholar]
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC (1999) Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell 3: 629-638. [DOI] [PubMed] [Google Scholar]
- Young MA, Gonfloni S, Superti-Furga G, Roux B, Kuriyan J (2001) Dynamic coupling between the SH2 and SH3 domains of c-Src and Hck underlies their inactivation by C-terminal tyrosine phosphorylation. Cell 105: 115-126. [DOI] [PubMed] [Google Scholar]
- Yu X-M, Askalan R, Keil II GJ, Salter MW (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275: 674-678. [DOI] [PubMed] [Google Scholar]
- Zan Y, Haag JD, Chen K-S, Shepel LA, Wigington D, Wang Y-R, Hu R, Lopez-Guajardo CC, Brose HL, Porter KI, Leonard RA, Hitt AA, Schommer SL, Elegbede AF, Gould MN (2003) Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol 21: 645-651. [DOI] [PubMed] [Google Scholar]
- Zhang Z-Y, Clemens JC, Schubert HL, Stuckey JA, Fischer MWF, Hume DM, Saper MA, Dixon JE (1992) Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J Biol Chem 267: 23759-23766. [PubMed] [Google Scholar]