Abstract
The hypothalamic-pituitary-adrenal axis regulates mammalian stress responses by secreting glucocorticoids. The magnitude of the response is in part determined by gender, for in response to a given stressor, circulating glucocorticoids reach higher levels in female rats than in males. This gender difference could result from estrogen regulation of the corticotropin-releasing hormone (CRH) promoter via either of its receptors: estrogen receptor (ER)α or ERβ. Immunocytochemistry revealed that a subset (12%) of medial parvocellular CRH neurons in the rat hypothalamus contain ERβ but not ERα. To determine whether ERs could regulate CRH promoter activity, we cotransfected cells with a CRH promoter construct and either ERα or individual ERβ isoforms. ERα weakly stimulated CRH promoter transcriptional activity in a ligand-independent manner. Conversely, all ERβ isoforms tested stimulated CRH promoter activity with different ligand profiles. ERβ1 and ERβ2δ3 displayed constitutive activity (ERβ1 more than ERβ2δ3). Ligand-dependent activity of β isoforms 1 and 2 was altered by an Exon3 splice variant (δ3) or by the additional 18 amino acids in the ligand-binding domain of ERβ2 isoforms. Lastly, we suggest that ER regulation of CRH takes place through an alternate pathway, one that requires protein-protein interactions with other transcription factors or their associated complexes. However, a pure ER-activator protein-1 alternate pathway does not appear to be involved.
Keywords: estrogen receptor α, estrogen receptor β, corticotropin-releasing hormone, estradiol, tamoxifen, stress
Introduction
Although a consensus opinion does not exist as to the definition of “stress,” it is accepted that a stimulus that activates the hypothalamic-pituitary-adrenal axis (HPAA) is a stressor (Pacak and Palkovits, 2001). HPAA activation leads to adrenal glucocorticoid secretion, which in turn downregulates the axis. States in which glucocorticoids are chronically elevated or the axis is inappropriately downregulated are pathologic. For example, the inability of dexamethasone to downregulate the HPAA in humans correlates with depression (Gold et al., 1986a), and HPAA dys-regulation is associated with anorexia nervosa (Gold et al., 1986b). Both of these disorders have a female preponderance (Brotman, 2001; Wulsin, 2001). HPAA gender differences are also present in the rat: females secrete higher levels of adrenocorticotropin and glucocorticoids in response to a stressor than males, and this difference tracks to circulating estrogens (Burgess and Handa, 1992). Therefore, estrogens may interfere with glucocorticoid-mediated downregulation and/or facilitate HPAA activation.
Hypothalamic paraventricular parvocellular neurons integrate sensory and hormonal inputs. These neurons synthesize and secrete corticotropin-releasing hormone (CRH) (Vale et al., 1981) in response to numerous stimuli (for review, see Pacak and Palkovits, 2001). Because CRH neurons contain glucocorticoid receptors (GR) (Cintra et al., 1987; Liposits et al., 1987; Uht et al., 1988), and because glucocorticoids downregulate cAMP-activated CRH transcription (Guardiola-Diaz et al., 1996), it may be that a component of downregulation occurs at the level of CRH transcription.
The estrogen receptor (ER) immunocytochemical status of CRH neurons has not been well established. At the level of mRNA, however, ERα and ERβ have strikingly different distributions (Shughrue et al., 1997). In contrast to ERα, ERβ mRNA is abundant in the rat paraventricular nucleus of the hypothalamus (PVH). Also, immunoreactive (IR) ERβ is present in mouse parvocellular neurons (Mitra et al., 2003). Because ERβ mRNA has been detected in rat IR CRH neurons (LaFlamme et al., 1998), and IR ERβ has been reported in mouse paraventricular nuclei (PVNs) (Mitra et al., 2003), we first asked whether IR ERβ colocalized with IR CRH in the rat PVH.
ERβ exists in several splice variants (see Fig. 1B) (Petersen et al., 1998; Price et al., 2000), with ERβ1 being the first described. ERβ2 contains 18 additional amino acids in the ligand-binding domain (LBD) (Petersen et al., 1998). δ3 isoforms lack the DNA-binding domain (DBD) second zinc finger (see Fig. 1B) (Petersen et al., 1998). Because multiple ERβ isoforms are present in the rat PVH (Price et al., 2000), differential expression could permit a spectrum of estrogen responses.
Figure 1.
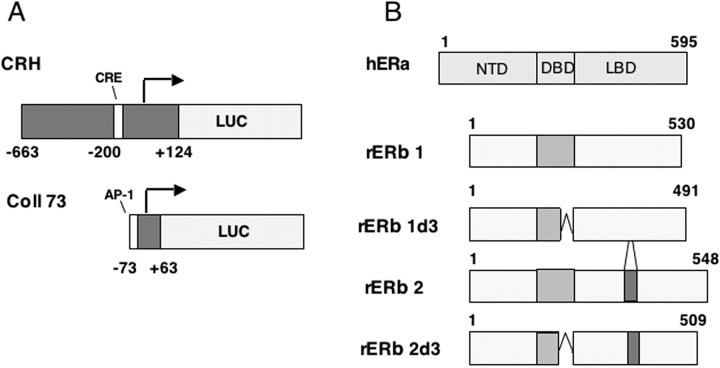
ER structures and reporter constructs. A, CRH and Coll73 promoter constructs used for transient transfections. The human CRH promoter fragment is 787 nt long, 124 of which extend 3′ to the start site (indicated by the arrow). The human collagenase promoter is a highly truncated collagenase promoter that has been used extensively to study ER regulation through the activator protein-1 complex. B, Comparison of ERα and ERβ isoforms. All ERβ isoforms are shorter than ERα as a result of a truncated N-terminal domain (NTD). All ERs depicted occur naturally in the rat CNS at the level of mRNA. h, Human; r, rat.
Agonist-bound ERαs and ERβs bind palindromic estrogen response elements (EREs) and then activate transcription. Paradoxically, none of these full EREs are present in the CRH promoter, although ERE half-sites are present (Vamvakopoulos and Chrousos, 1993). ERs also regulate transcription via alternate pathways. For example, ERα and ERβ1 stimulate activator protein-1 (AP-1) activity (Gaub et al., 1990; Umayahara et al., 1994; Webb et al., 1995; Paech et al., 1997) but with different ligand profiles: agonist- and antagonist-bound ERα stimulate AP-1 transcriptional activity, whereas only antagonist-bound ERβ stimulates this activity (Paech et al., 1997). Given the importance of alternate pathways and the diversity of ERβ variants, we asked whether ERβ and its isoforms could differentially regulate CRH promoter transcriptional activity.
Materials and Methods
Animals
Animal protocols were approved by the Animal Care and Use Committee of Colorado State University (Fort Collins, CO). Five young adult female Sprague Dawley rats (200-250 gm) were purchased from Charles River Laboratories (Portage, MI). Animals were acclimated to laboratory conditions (12 hr light/dark cycle) with food and water available ad libitum. For immunocytochemical studies, animals were anesthetized with sodium pentobarbital (50 mg/kg) and placed into a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Colchicine (10 μl of a 10 μg/μl solution) was infused into the third ventricle through a Hamilton syringe over a period of 4-5 min. Animals were kept alive for another 18-24 hr and were then perfused with 0.9% saline followed by 10% neutral buffered formalin. For PCR analysis, animals were female Sprague Dawley rats (200-250 gm) purchased from Charles River Breeding Laboratories. Animals were bilaterally ovariectomized 3 d before they were killed. This procedure was used to upregulate the expression of ERβ in the PVH, because previous studies have shown that ERβ mRNA is downregulated after estrogen treatment (Tx) (Osterlund et al., 1998; Patisaul et al., 1999; Suzuki and Handa, 2004).
Immunocytochemistry
Tissue preparation. Brains were postfixed for 2 hr at room temperature, placed in 5% neutral buffered formalin-15% sucrose in 0.1 m PBS overnight at 4°C, and then transferred to 30% sucrose in 0.1 m PBS at 4°C until permeated. This fixation procedure was found to provide the most robust ERβ immunostaining when compared with 4% paraformaldehyde and 2% acrolein fixation. Thus, this procedure was used throughout. Subsequently, the tissues were cut on a cryostat at 35 μm and saved in 0.1 m PBS- 0.1% sodium azide at 4°C until processed, as described above.
Double-label immunohistochemistry. The following antibodies and dilutions were used: ERβ (Z8P; 1:4000; Zymed Laboratories, San Francisco, CA), CRH (1:25,000; from Dr. Wylie Vale, The Salk Institute, La Jolla, CA), and ERα (C1355; 1:10,000; from Dr. M. Shupnik, University of Virginia, Charlottesville, VA).
Tissue sections were processed as described previously (Kerr et al., 1995). Tissue was washed in 0.1 m PBS with 0.1% Triton X-100 (TX) and incubated with 0.3% H2O2 in 0.1 m PBS with 0.1% TX to quench endogenous peroxidase activity. After washes, sections were incubated in 6% normal goat serum (NGS) in 0.1 m PBS with 0.1% TX to block nonspecific binding and then incubated for 48 hr at 4°C with ERα or ERβ antiserum in 0.1 m PBS with TX and 2% NGS. Next, the tissue was washed and incubated with biotinylated goat anti-rabbit IgG (1:500; Vector Laboratories, Burlingame, CA) in PBS with TX and 2% NGS for 2 hr at room temperature. Sections were processed according to the ABC procedure (1:500; Vector Laboratories). The tissue was rinsed in 0.1 m Tris-buffered saline and then developed with nickel-intensified 3,3′-diaminobenzidine (DAB) (0.5 mg/ml; Sigma, St. Louis, MO) in 0.1 m Tris-buffered saline containing 0.03% hydrogen peroxide. The reaction was stopped by washes in 0.1 m PBS.
Subsequently, the tissue was processed for CRH immunoreactivity. The procedure was as above, except that primary antibody incubation was for 72 hr at 4°C. Sections were developed with DAB (0.5 mg/ml with 0.03% hydrogen peroxide) to produce a brown reaction product. Samples were examined with a Zeiss (Thornwood, NY) Axioplan 2 microscope. Double-labeled neurons had a dark blue-black nucleus (IR ERβ) and brown cytoplasm and nerve fibers (CRH immunoreactivity).
Methods for counting cells. Tissue sections were analyzed using a Zeiss Axioplan 2 imaging universal microscope, and images were captured with a Zeiss AxioCam digital camera. From three rat brains, tissue sections (35 μm thick) corresponding to bregma level -1.53 were chosen for analysis. At this level, CRH immunoreactivity was mainly seen in the medial parvocellular part, and ERβ immunoreactivity was strong. The percentage of colocalization was determined by manually counting the number of cells positively labeled for ERβ and CRH. Double-labeled neurons were identified as cells containing a dark blue nucleus (ERβ) and a brown cytoplasm and fibers (CRH). Peptide-positive, ERβ-negative neurons were identified as having a brown cytoplasm and a pale or unstained nucleus.
Amplification of hypothalamic ERβ isoforms
Total RNA isolation. For PVH microdissection, a micropunch technique was used that was similar to the protocol used by Palkovits et al. (1985), as modified by Price et al. (2000). Brain sections were cut frozen on a cryostat, thaw mounted onto glass slides, and kept at -80°C until micropunching. Micropunching was accomplished with a blunted needle with a 0.5 mm diameter. Isolation of total RNA was performed according to the protocol of Chomczynski and Sacchi (1987). Punch-dissected tissues were pooled from two animals and immediately homogenized in a centrifuge tube containing 250 μl of guanidinium isothiocyanate buffer (4 m guanidinium isothiocyanate, 0.5% sarcosyl, 25 mm sodium citrate, pH 7.0, and 0.1 m β-mercaptoethanol). After phenol chloroform-isoamyl alcohol extraction and ethanol precipitation, the RNA was reconstituted in RNase-free water, and concentration was measured with a spectrophotometer. Only samples with a 260:280 ratio of >1.6 were used.
Reverse transcription. Equal amounts of total RNA (0.5 μg) were reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Rockville, MD) in the presence of oligo-dT primers, deoxyNTPs (100 mm each), first strand buffer [containing (in mm): 100 Tris HCl, 900 KCl, 1 MgCl2], and 2.5 mm dithiothreitol. The reverse transcription (RT) reaction was performed at room temperature for 10 min followed by a 10 min incubation at 44.2°C. The reaction was terminated by denaturing reverse transcriptase at 95°C for 5 min. RT-generated cDNA samples were stored at -20°C until PCR amplification.
ERβ primers. PCR primers for rat ERβ were designed using commercially available software (Oligo, version 6.1; Molecular Biology Insights, Cascade, CO). Twenty-one base primers were developed on the basis of established GenBank sequence (accession number U57439). The ERβ primers spanned the known splice variants and thus could identify individual ERβ splice variants in a single reaction. The forward primer position began at nucleotide 455, and the reverse primer position began at nucleotide 1570. The predicted PCR product size was 1190 nt for ERβ2, 1136 nt for ERβ1, 1073 nt for ERβ2δ3, and 1019 nt for ERβ1δ3.
Real-time PCR amplification. Real-time PCR was performed according to the protocols of Solum and Handa (2002). Briefly, hot-start PCR was performed using the LightCycler DNA Master SYBR Green mix (Roche Molecular Biochemicals, Indianapolis, IN). Samples were subjected to an initial melting step at 95°C for 2 min and amplified at 40 cycles (∼5-10 cycles beyond the beginning of the linear phase of amplification) of a 95°C melting step (2 sec), a 66°C annealing step (7 sec), and a 72°C elongation step (48 sec). Samples without template were used as negative controls. After PCR amplification, samples were separated on a 1.5% agarose gel with an appropriate molecular weight size marker (Invitrogen) to ensure specificity of the PCR products. The agarose gel was stained using ethidium bromide and visualized under UV light.
Transcription experiments
Plasmids. All plasmids have been described previously (Fig. 1 A). Reporter constructs were as follows: [(-663) - (+124)CRH]:luciferase (LUC) (Guardiola-Diaz et al., 1994) and human collagenase promoter (Coll73):LUC for AP-1 activity (Webb et al., 1995) (Fig. 1 A); ER expression vectors were ERα (Webb et al., 1995), ERβ, and ERβ isoforms (Fig. 1 B) (Price et al., 2001). Plasmids used to correct for efficiency of transfection were actin β-galactosidase or pJ3 (Uht et al., 1997).
Cells, cell culture, transfections, and treatments. HeLa cells were maintained in culture as described previously (Uht et al., 1997). Briefly, they were kept in phenol red-free DMEM (Sigma) supplemented with newborn calf serum (10%) tested for low estrogenic activity (catalog #100-504; Gemini Bioproducts, Woodland, CA), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Invitrogen). A total of 1,500,000 cells were transiently cotransfected with either 5 or 10 μg of CRH:LUC and a range of ERα and ERβ expression vector quantities by electroporation, as described previously (Paech et al., 1997). β-Galactosidase plasmids were cotransfected to correct for the efficiency of transfection. Immediately after plating, cells were treated with ethanolic vehicle (EtOH), estradiol (E2) (Sigma), or tamoxifen (Tmx) (Sigma) at 10-7 or 5 × 10-6 m, respectively. On the day of harvest, each well was visually inspected to determine whether estradiol and/or tamoxifen had a marked effect on cell number or morphology. No discernable change in either was present. Cells were harvested 40-48 hr after treatment.
Luciferase and β-galactosidase assays. Cells were lysed with cell lysis buffer (Promega, Madison, WI). Samples were analyzed for luciferase activity (Promega) and β-galactosidase activity via a light-emitting assay (Tropix, Bedford, MA). An MGM Optocomp II luminometer (MGM Instruments, Hamden, CT) was used to detect light emission.
Transfection data analysis. ER titration plus or minus ligand experiments: each receptor titration experiment was performed a minimum of three times. Txs were performed in triplicate or quadruplicate. To permit pooling data across experiments that would reflect both an effect of treatment and an effect of amount transfected, data values are expressed as a fold of the 0 ng point of a given treatment. In other words, the effect of treatment at a given amount of transfected ER was expressed relative to the effect of the treatment at 0 ng ER [Tx(x ng)/Tx(0 ng)].
All receptor titration data (see Figs. 3, 4, 5) were analyzed by two-way ANOVAs. Unless otherwise specified, Bonferroni (all pairwise) multiple-comparison tests (Bonferroni tests) were performed to determine a difference within groups (Tx or nanograms of plasmid). The other test was a Fisher's least significant difference (LSD) multiple-comparison test. For the planned comparison tests, α was adjusted for the number of tests performed; data were deemed significant at α < 0.050. The data were analyzed twice: after the SD was calculated for all individual groups, points that were ≥2 SD from the mean were discarded. ANOVAs and pairwise analyses were performed on the remaining data.
Figure 3.
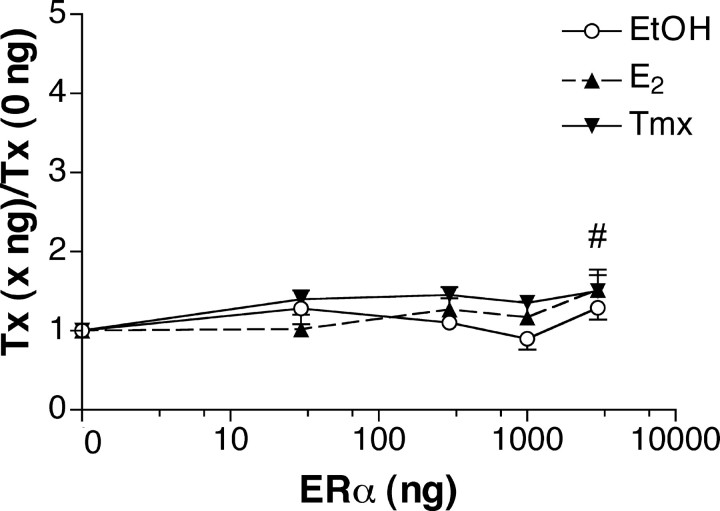
ERα modestly regulates the CRH promoter in a manner that is both ligand dependent and dependent on the amount of transfected ERα plasmid. Cells were treated with ethanolic vehicle, E2 (10-7 m), or Tmx (5 × 10-6 m) immediately after plating for 40-45 hr. The y-axis represents relative light units elicited by luciferase, divided by relative light units for β-galactosidase (to correct for efficiency of transfection). Data are expressed as a fold of the response to a given treatment at 0 ng of transfected ERα. Thus, the response to transfection with empty expression vector for each treatment is 1. The data represent the average of individual points from multiple experiments (total, n = 197 determinations). Error bars represent the SEM. High levels of transfected ERα display a small degree of increased activity. The n-group was 22-46 per amount of ERα plasmid transfected. # indicates that the effect of transfecting 3000 ng of ERα was greater than the effect of transfecting 0 ng (1.43 vs 1.00). ○, EtOH; ▴, E2; ▾, Tmx.
Figure 4.
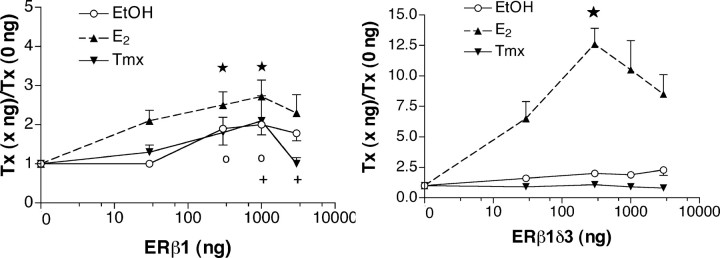
ERβ1 displays constitutive activity, and ERβ1δ3 activity is ligand dependent. Data are analyzed and depicted as described in the legend to Figure 3. A, ERβ1 activates the CRH promoter in the presence of E2. The total number of points analyzed was 176 (EtOH, n = 68; E2, n = 62; Tmx, n = 46). ★, E2 at 300 ng (n = 16) and 1000 ng (n = 12) differs from all treatments at 0 ng; the 1000 ng point differs from EtOH at 30 ng as well (p < 0.050). ○, +, Fisher's LSD indicated a significant difference between individual points p < 0.050). ○, EtOH treatment at 300 and 1000 ng both differed from E2 at 0 and 30 ng. +, Tmx at 1000 ng differed from Tmx at 0 and 3000 ng; Tmx at 3000 ng also differed from E2 at 3000 ng and Tmx at 1000 ng. B, The ERβ1δ3 isoform displays strong E2 responsiveness. ★, E2 at 300 ng (n = 8) was 12.6-fold that of the E2 treatment at 0 ng (n = 15) and 6.4-fold that of the EtOH treatment (n = 8) at 300 ng. Error bars represent SEM.
Figure 5.
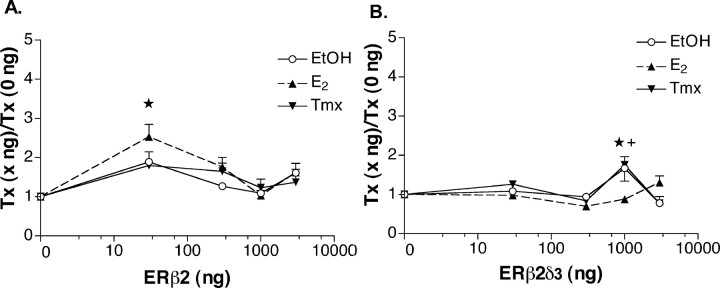
ERβ2 displays ligand-dependent activity, whereas ERβ2δ3 displays constitutive activity. Data are analyzed and depicted as described in the legend to Figure 3. A, ★, E2 at 30 ng (n = 12) differed from EtOH at 0, 300, and 1000 ng, from E2 at 1000 ng, and from Tmx at 0, 1000, and 3000 ng. n for these points ranged from 10-16 per group. B, ERβ2δ3 displays constitutive activity and ligand effects. The EtOH point at 1000 ng (n = 16) differs from the EtOH point at 0 ng (n = 20). ★, E2 at 1000 (n = 14) differed from both EtOH (n = 16) and Tmx (n = 11) at 1000 ng. +, Tmx treatment at 1000 ng (n = 11) differed from all EtOH treatment points (n = 85) except that at 30 ng (n = 19), all E2 points (n = 86) except that at 3000 ng (n = 16), and all other Tmx treatment points (0 ng, n = 16; 300 ng, n = 11; 3000 ng, n = 11) except that at 30 ng (n = 15). Error bars represent SEM.
To compare CRH and Coll73 promoter responses to treatment in the presence of ERβ1 (see Fig. 6) and ERβ2 (data not shown), four tests of normality were first performed: skewness, kurtosis, omnibus normality of residuals, and a modified-Levene equal-variance test. If the data met normality criteria by three of the four tests, they were then analyzed by a one-way ANOVA, and pairwise comparisons were made using the Bonferroni test. If the data failed to meet normality criteria by two or more of the four tests, they were analyzed by a Kruskal-Wallis one-way ANOVA on ranks followed by the Kruskal-Wallis multiple-comparison Z value test. All data analyses were performed using the Number Cruncher Statistical System program (NCSS, Kaysville, UT). Data are presented as means ± SEM.
Figure 6.
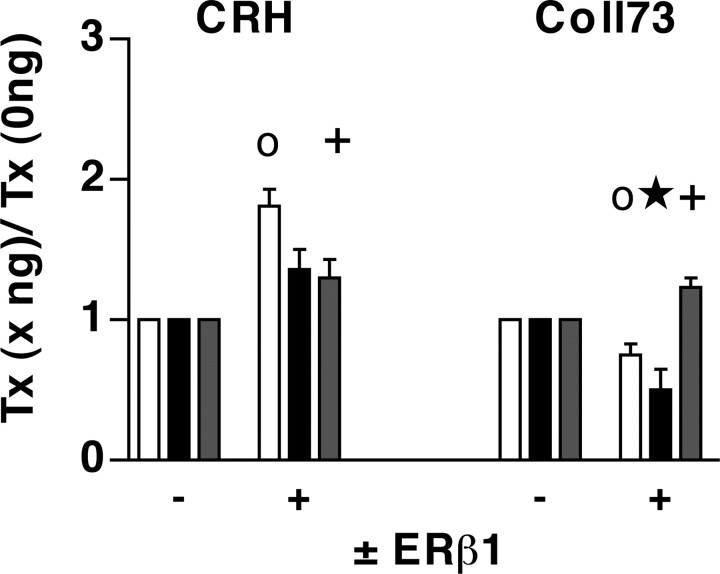
Ligand profiles of ERβ1 at the CRH versus those at an AP-1 site. ERβ1 was cotransfected with either the CRH (1 μg of ER) or AP-1 (5 μg of ER) promoter constructs depicted in Figure 1 A. The same passage of HeLa cells was transfected with either CRH or Coll73 along with ERβ1. ○, At the CRH promoter (left), in the presence of ERβ1, there was an 81% increase in the EtOH group over the EtOH group in the absence of ERβ1 (n = 12 for this and all EtOH groups). +, Tmx significantly reduced the effect seen in the presence of EtOH (n = 12). ○, At the Coll73 promoter (right), there was a 25% reduction of basal activity in the presence of ERβ1 without ligand (EtOH; n = 12). ★, E2 was 50% of the E2 activity in the absence of ER (1.0). +, Tmx treatment increased activity by 24% compared with the Tmx activity in the absence of ER (1.0). □, EtOH; ▪, E2; ⊡, Tmx. Error bars represent SEM.
Results
ERβ colocalizes with CRH in parvocellular neurons of the PVH
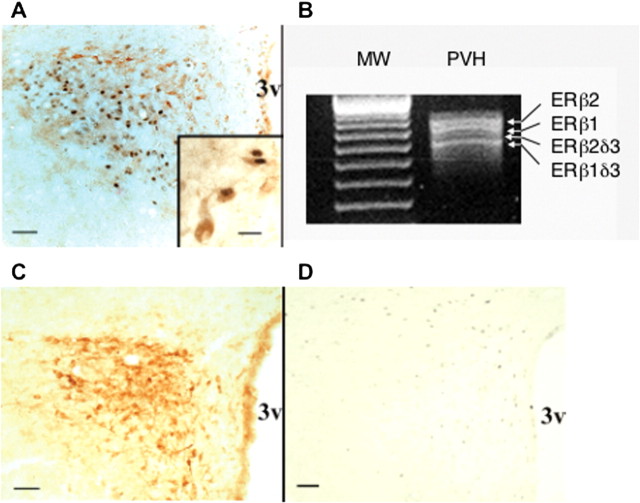
We sought to determine whether ER protein colocalizes with CRH in medial parvocellular neurons using dual-label immunocytochemistry for ERβ and CRH. Hypothalamic paraventricular parvocellular neurons have been shown to contain ERβ immunoreactivity in the mouse (Mitra et al., 2003). The PVH contained dual-labeled cells localized in the medial parvocellular region (Fig. 2 A). Colocalization of CRH with ERβ (Fig. 2A, inset) was found in 12% ± 2 of CRH neurons. Such colocalization suggests that the receptor may play a role in regulating CRH transcription in certain physiologic or pathophysiologic states, at least in a subset of CRH-expressing neurons.
Figure 2.
IR ERβ colocalizes with CRH immunoreactivity in the rat PVH. A, Colocalization of IR ERβ (nuclear) and IR CRH (brown; cytoplasmic) in parvocellular neurons of colchicine-treated female rats. Scale bars: A, 50 μm; inset, 15 μm. B, Gel electrophoretic analysis of RT-PCR products showing ERβ isoforms in total RNA taken from micropunched PVH of ovariectomized female rats. Arrows point to the different ERβ splice variant mRNAs. C, Single-labeling study showing CRH distribution in the PVH. D, The distribution of CRH immunoreactivity does not overlap that of IR ERα nuclei, which is weak and found predominantly in neurons adjacent to the PVH. Scale bars, C, D, 50 μm. 3v, Third ventricle; MW, molecular weight markers.
To determine whether the PVH contained ERβ isoforms, the Palkovits punch technique was used to obtain mRNA from ovariectomized rats. Amplification by RT-PCR revealed that ERβ1 and ERβ2 and their δ isoforms mRNAs were present (Fig. 2B). Amplified ERβ2δ3 was consistently detected, albeit at low levels compared with the other three ERβ isoforms. Given that antibodies specific to each isoform have not yet been successfully generated, we do not know whether they are also present at the protein level. However, the presence of their mRNA suggests that they may be.
To compare the localization of paraventricular ERβ to ERα in our experimental conditions, we performed CRH and ERα immunocytochemistry. IR ERα was found only in a few hypothalamic paraventricular neurons scattered in the dorsal PVH; its distribution did not overlap that of CRH or ERβ (Fig. 2, compare D with A,C). Together, these anatomical data indicate that ERβ is the predominant ER in CRH-positive parvocellular neurons in the rat PVH.
ERα exerts modest effects on CRH promoter transcription
The original study of ER regulation of the CRH promoter (Vamvakopoulos and Chrousos, 1993) was reported before the discovery of ERβ (Kuiper et al., 1996). Using a 2.4 kb CRH promoter, the authors concluded that ERα stimulated the CRH promoter by twofold to threefold. Because ERα could be present in the CRH parvocellular neurons examined above but below the level of detection by the immunocytochemical techniques used, we asked whether ERα could regulate transcription of the CRH promoter in our conditions. We cotransfected HeLa cells with the CRH promoter construct (Fig. 1) and increasing amounts of the ERα expression vector. After plating, cells were treated with vehicle (EtOH), E2 (10-7 m), or Tmx (5 × 10-6 m). A two-way ANOVA revealed a small effect of the amount of ERα transfected (F(4,182) = 3.18; p = 0.0150) (Fig. 3). There was no effect of treatment (F(2,182) = 2.25; p = 0.4543) or an interaction between the two (F(8,182) = 0.60; p = 0.2726). The 3000 ng amount significantly differed from the 0 ng group (p < 0.050), because at 3000 ng of transfected ERα, the mean was 43% greater than that at 0 ng (set to 1; p < 0.050). As a control, ERα was transfected either with the CRH promoter:reporter construct or with the empty vector. Although the CRH promoter construct supported a small degree of activation, the corresponding signals for the vector control were all at the level of the mock transfection (data not shown). Thus, the effects we report here are mediated through the CRH promoter. The relatively small effects of ERα at the CRH promoter are in accord with the data reported by Vamvakopoulos and Chrousos (1993). They showed a twofold to threefold increase in transcriptional activity elicited by E2 (Vamvakopoulos and Chrousos, 1993). Their data, together with the ERα data presented here, suggest that ERα plays a small but significant role in regulating the CRH promoter. Whether ERα activation of the CRH promoter is ligand dependent or ligand independent likely depends on cell and promoter context.
ERβ isoforms elicit different patterns of CRH promoter regulation
ERβ1 and ERβ1δ3
In contrast to ERα, transfection of ERβ1 activated the CRH promoter (Fig. 4A). A two-way ANOVA revealed an effect of treatment (F(2,165) = 6.41; p = 0.0021) and amount transfected (F(4,165) = 7.85; p < 0.0001) but no interaction (F(8,165) = 0.86; p = 0.5541). There was a difference between treatment groups (p < 0.050). The E2 group differed from EtOH and Tmx; however, there was no difference between EtOH and Tmx groups. For amounts, the 0 ng group differed from the 300, 1000, and 3000 ng groups (p < 0.050). The 30 ng group differed only from the 1000 ng group. Importantly, this isoform displayed a strong tendency to display constitutive activity; compare EtOH at 0 ng with EtOH at 1000 ng (Fig. 4A). Although the difference between these two points was not significant using the Bonferroni test (p < 0.050), it was using Fisher's LSD test (p < 0.050). As a control, ERβ1 was transfected either with the CRH promoter:reporter construct or with the empty vector, as were all the other ERβ expression vectors examined. The CRH promoter construct supported activation. As above, the corresponding signals for the vector control were all at the level of the mock transfection (data not shown). Thus, the effects we report here are mediated through the CRH promoter. Together, the data demonstrate a very different pattern of CRH transcriptional activation by ERβ1 compared with ERα. Thus, the degree to which ERβ1 is expressed in a cell could determine the net response of the CRH gene to ERβ1 in the absence of ligand or in the presence of a ligand, E2, or a selective estrogen receptor modulator (SERM) such as Tmx.
In contrast to ERβ1, ERβ1δ3 supported a strong ligand effect at the CRH promoter (Fig. 4B). A two-way ANOVA revealed a strong and highly significant effect of treatment (F(2,162) = 82.33; p < 0.0001), effects of amounts (F(4,162) = 9.26; p < 0.0001), and an interaction between the two (F(8,162) = 7.15; p < 0.0001). Pairwise analysis indicated that the E2 group differed from both the EtOH and Tmx groups; however, the Tmx and EtOH groups did not differ (p < 0.050). All amounts of transfected ERβ1δ3 differed from the 0 ng group. Except for this, none differed from each other (p < 0.050). The E2 effect is striking: at 300 ng, the E2 treatment was 12.6-fold of E2 at 0 ng and approximately sixfold the EtOH treatment at 300 ng (note the difference in the scale of the y-axis in this graph compared with all others). This point differed from all other EtOH and Tmx points and from E2 at 0 and 30 ng (p < 0.050). The response elicited was greater than those of all other ERs examined, ERα and the other three ERβ isoforms. In summary, the ERβ1δ3 isoform is a strong, E2-dependent activator of CRH transcription.
ERβ2 and ERβ2δ3
ERβ2 isoforms contain an additional 18 amino acids in their LBDs (Fig. 1B). One would think that additional amino acids in this domain might compromise ligand-dependent ER regulation. Instead, ERβ2 supported an E2 response (Fig. 5A). A two-way ANOVA revealed that only the amount group contained significantly different points (F(4,180) = 11.63; p < 0.0001). There was no effect of treatment (F(2,180) = 1.45; p < 0.2377) and no interaction between treatment and amount (F(8,180) = 1.02; p < 0.4202). Analysis of pairs revealed a significant difference between the 30 ng group and all other amounts (p < 0.050). Furthermore, only the E2 point at 30 ng differed from other points, specifically: EtOH at 0, 300, 1000, and 3000 ng; Tmx at 0, 1000, and 3000 ng; and E2 at 0 and 1000 ng. Thus, E2 activation of the CRH promoter when bound to ERβ2 is ligand dependent only at a small amount of transfected plasmid. These data suggest that E2-bound ERβ2 only activates CRH at restricted levels of receptor.
As was the case for ERβ1 and ERβ1δ3, lack of amino acids corresponding to the Exon3 coding region changed the profile of ERβ2. Here, it conferred a degree of constitutive activity. A twoway ANOVA revealed no effect of treatment (F(2,220) = 1.95; p = 0.1450), a highly significant effect of amount of receptor transfected (F(4,220) = 9.30; p < 0.0001), and an interaction between the two (F(8,220) = 4.80; p < 0.0001). The 1000 ng group differed from all others, none of which differed from any group other than the 1000 ng group (p < 0.050). E2 restrained CRH transcriptional activity. This was most evident at 1000 ng, at which it was 52% of EtOH treatment (p < 0.050). E2 treatment also differed from Tmx treatment at this point. Tmx permitted increased transcription by 75% of the E2 response at 1000 ng and 75% of Tmx at 0 ng. It did not differ from the EtOH response at 1000 ng. Thus, ERβ1δ3 was a phenotypic hybrid of ERα and ERβ1 at the CRH promoter. Like ERα, ligand effects were small. Like ERβ1, ERβ2δ3 displayed constitutive activity; compare the EtOH response at 0 to 1000 ng for each receptor (Figs. 4A,5B). Together, these data reveal a complex regulation of ERβ activity by the amino acids coded for by Exon3 and the additional 18 amino acids found in ERβ2 isoforms.
ERβ isoforms regulate transcription differently at CRH and AP-1
It has been reported previously that the CRH promoter lacks palindromic EREs and also suggested that ERα stimulates CRH activity via ERE half-sites (Vamvakopoulos and Chrousos, 1993). Another possibility is that ERs could be regulating CRH through an alternate pathway that involves a different transcription factor (e.g., through AP-1 sites). Because the CRH promoter contains several AP-1 and AP-1-like sites [Transcription Factor Database (TRANSFAC) analysis; data not shown], we sought to determine whether the behavior of ERβ1 and ERβ2 at CRH was different from those at an AP-1 site. We compared ERβ behaviors at the CRH promoter with that of a highly studied AP-1-regulated promoter, Coll73 (Kushner et al., 2000). We did so in the same experiment using cells from the same passage. It was shown above that E2-dependent activation of the CRH promoter by ERβ1 is modest (Fig. 4A). Furthermore, at low doses, the more robust constitutive activity of ERβ1 is not apparent. To elicit the more robust activity, we transfected cells with 1000 ng of ERβ1. A one-way ANOVA revealed a significant effect of treatment (F(2,34) = 4.68; p < 0.0165). A one-way ANOVA for effect of receptor in the presence of EtOH (in the presence vs the absence of ERβ1) revealed significant constitutive activity (EtOH-treated groups, F(3,44) = 40.40; p < 0.0001), as above; compare the EtOH group in the presence of receptor with the absence of receptor in Figure 6 to the EtOH group at 0 and 1000 ng in Figure 4A. The pattern of Coll73 regulation by ERβ1 was as reported previously (Paech et al., 1997; Price et al., 2000). A one-way ANOVA revealed a highly significant effect of treatment (F(2,31); p < 0.0001). E2 repressed activity seen in the presence of EtOH by 33%, whereas Tmx increased it by 66%. ERβ2 tended to act in a manner similar to ERβ1 at these two promoters; however, the ligand effects were not significant (data not shown). This likely reflects the difficulty of optimizing the response of two stress-responsive promoters to two different ERβ isoforms simultaneously. Together, the data indicate that the dominant functional element targeted by ERβ in the CRH promoter [(-663) - (+124)] is not an AP-1 site.
Discussion
Together, the data presented here corroborate the hypothesis that ERs regulate CRH in the PVH of the hypothalamus. ERβ immunoreactivity is present in 12% of IR CRH neurons in the parvocellular region. Additionally, all four ERβ isoforms are present in the PVH at the level of mRNA (Fig. 2). We do not know the extent to which ERβs play a role in CRH transcriptional regulation in vivo; 12% is a modest proportion. However, these animals were treated with colchicine, and it may be that in other conditions (e.g., ovariectomy combined with adrenalectomy) that the number of neurons labeled with both ERβ and CRH would increase. Regardless, the finding of even a small population of neurons displaying ERβ and CRH, and an absence of ERα staining in the medial parvocellular region, suggests that ERβ, rather than ERα, is poised to contribute to CRH transcriptional regulation involved in the stress response.
In keeping with our anatomical data, we found that ERβ isoforms, rather than ERα, exert substantial regulation of the CRH promoter. The constitutive activity of ERα described here differs from that reported previously. Previously, ERα was found to stimulate CRH to a small extent (two to three times) in the presence of E2 (Vamvakopoulos and Chrousos, 1993). The use of different promoters (-2.4 kb-1) and cell lines (CV-1s) (Vamvakopoulos and Chrousos, 1993) may explain these differences. It is also possible, however, that the regulation reported previously and our observation of a small effect of ERα are attributable to the fact that ERα is an inherently weak transcriptional activator at the CRH promoter. Together with our colocalization findings, our data suggest that the physiologically important ER(s) in CRH parvocellular neurons of the PVH are the ERβ isoforms rather than the first identified ER, ERα.
We do not yet know which ERβ isoform(s) are in CRH neurons, because available ERβ antibodies cannot distinguish between them. However, neurons of the PVH express a number of ERβ mRNA splice variants, as determined by PCR (Fig. 2B) (Price et al., 2000). Therefore, a given CRH neuron could express one or multiple isoforms of ERβ. The possibility of multiple isoform expression is relevant in that ERβ isoforms have been shown to heterodimerize with themselves and with ERα (for review, see Pettersson and Gustafsson, 2001). This is a possible mechanism of CRH regulation that, although we have not yet studied, suggests the potential for a broad array of genomic responses in a hypothalamic paraventricular subpopulation of IR ERβ CRH parvocellular neurons.
CRH parvocellular neurons that regulate the stress response reside in an extremely complicated brain nucleus, the PVH. In fact, the parvocellular area alone has at least three subnuclei: medial, dorsal, and ventral (Swanson and Kuypers, 1980). Medial parvocellular neurons project to the median eminence (Merchenthaler et al., 1983; Swanson et al., 1983). This region contains CRH neurons that trigger the HPAA. The dorsal and ventral neurons project to autonomic nuclei in the brainstem (Swanson and Sawchenko, 1980). The neurons expressing both CRH and ERβ (Fig. 2A, insert) are in the region of the medial parvocellular division; however, without combined retrograde tracing and immunocytochemistry, we cannot say with absolute certainty that they are the neuroendocrine motor neurons of the HPAA. Regardless, we believe that the finding of ERβ in CRH parvocellular neurons combined with our data from transfection-reporter gene studies suggests that ERβ isoforms play a role in maintaining homeostasis, be it through regulating the HPAA or the CNS component of the autonomic nervous system.
Our data bear on two aspects of ER-regulated CRH transcription: (1) structure-function correlates of ERβ and (2) the nature of the ERβ regulatory pathway. With respect to structure-function correlates, absence of the region corresponding to Exon3 (the δ3 isoforms) had a striking effect on ERβ1 and ERβ2 phenotypes. Exon3 codes for the second ER zinc-binding domain of the DBD. The DBD plays an important role in ERE-mediated and alternate pathways (Umayahara et al., 1994; Webb et al., 1995; Jakacka et al., 2001; Price et al., 2001; Bjornstrom and Sjoberg, 2002; Uht et al., 2004). At the CRH promoter, the δ3 splice variants substantially changed ligand responsiveness of ERβ1 and ERβ2 (Figs. 4, 5). The δ3 deletion converts ERβ1 from a receptor that exhibits constitutive activity (Fig. 4A) to one with activity that is predominantly ligand dependent (Fig. 4B). In the context of ERβ2, the receptor is converted from a ligand-dependent transcription factor (Fig. 5A) to one that exhibited weak ligand responses and modest constitutive activity (Fig. 5B). Thus, the region encoded by ERβ Exon3 plays a critical role in the ability of ERβ1 and ERβ2 to respond to ligand, as well as to display constitutive activity at the CRH promoter.
In considering pathways by which ERβ could regulate the CRH promoter, an alternate (nonclassic) pathway of transcription can be invoked, because the CRH promoter does not contain full, palindromic EREs (Vamvakopoulos and Chrousos, 1993) [TRANSFAC analysis (Wingender et al., 1996); data not shown)]. It does contain AP-1 and AP-1-like sites. However, the observed ligand profiles for both ERα and ERβ isoforms fail to uniformly match previously established patterns of ERβ-regulated AP-1 activity (Paech et al., 1997; Price et al., 2001). In general, ERα stimulates the ER-AP-1 pathway in the presence of both estrogens and SERMs (e.g., Tmx). At the CRH promoter used here, there was no effect of treatment. ERβ1 and ERβ2 inhibit AP-1-activated transcription in the presence of estrogens and stimulate it in the presence of SERMs (Paech et al., 1997; Price et al., 2000). Here, E2 modestly stimulated transcription (Figs. 4A,5A). In the presence of Tmx, the effects of ERβ1 and ERβ2 were minimal and inhibitory, when significant (Figs. 4A, 5A). ERβ1 and two δ3 isoforms have been shown to reverse the profile of E2 and Tmx responses at an AP-1 site: E2 stimulates, and Tmx tends to inhibit, but not significantly so. As at an AP-1 site, the ER β1δ3 isoform enhanced E2-stimulated CRH transcription and tended to inhibit CRH in the presence of Tmx (Fig. 4B). The ER β2δ3 isoform here, however, led to E2 restraint of transcriptional activity and no effect of Tmx. Together, the reported data (Price et al., 2000), our titration data, and the side-by-side comparison of the CRH promoter and the Coll73 promoter (Fig. 6) indicate that the ERβ isoforms studied do not regulate the CRH gene through a mechanism solely or predominantly mediated by an AP-1 site.
In addition to ER regulation mediated through AP-1 sites, ERα and ERβ also stimulate transcription through nonconsensus cAMP response elements (CREs), as shown in the CyclinD1 promoter (Liu et al., 2002). Like CyclinD1, the CRH promoter contains CRE and CRE-like elements. One in particular has been implicated consistently in CRH promoter regulation: that at position -232 to -215 (in human CRH) (Fig. 2A). This CRE mediates cAMP-stimulated transcription (Guardiola-Diaz et al., 1994) that is blocked by glucocorticoids (Guardiola-Diaz et al., 1996). Thus, it could be a target for estrogen regulation and/or a site of integration of information conveyed by the steroid hormone milieu.
The observations that the amplitude of the stress response is gender specific, that this specificity is estrogen mediated, and that disorders of HPAA regulation have a female preponderance raise the following question: could the opposing effects of estrogens and glucocorticoids on the HPAA be explained on a molecular basis, one that involves integration of these signals through the CRH promoter? It appears that the answer is yes. It has been shown that ERs and GRs modulate the effects of each other both through classic, ERE-mediated pathways (Meyer et al., 1989) and ER-AP-1 pathways (Uht et al., 1997). Furthermore, AP-1 family members and the CRE binding protein (CREB) share a common coactivator, the CREB binding protein (CBP) (Kwok et al., 1994; Vo and Goodman, 2001). We have evidence that ER transcriptional activation mediated through CBP (Webb et al., 1998; Kushner et al., 2000) is attenuated by GR (R. M. Uht, unpublished observations). Thus, it is quite possible that the CRE in the CRH promoter (-232 to -215) is a node of integration for estrogen, glucocorticoid, and cAMP-regulated CRH expression. Thus, the findings presented here may shed light on mechanisms of an aspect of gender differences in the stress response and pathogenesis of HPAA disorders.
Footnotes
This work was funded by National Institutes of Health Grant R01 NS39951 (R.H., R.M.U.) and by a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression to R.M.U., a 2003 Lieber Investigator. CRH:LUC was a generous gift from Audrey Seasholtz (University of Michigan, Ann Arbor, MI). Emilie Rissman assisted with statistical analysis.
Correspondence should be addressed to Dr. Rosalie M. Uht, Department of Pathology, MR5, Room 3123, 415 Lane Road, University of Virginia School of Medicine, P.O. Box 800904, Charlottesville, VA 22908-0904. E-mail: ruht@virginia.edu.
W. J. S. Miller's present address: University of Virginia School of Medicine, Charlottesville, VA 22908-0904.
S. Suzuki's present address: University of California Davis, Davis, CA 95616.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2410628-08$15.00/0
References
- Bjornstrom L, Sjoberg M (2002) Mutations in the estrogen receptor DNA-binding domain discriminate between the classical mechanism of action and cross-talk with Stat5b and activating protein 1 (AP-1). J Biol Chem 277: 48479-48483. [DOI] [PubMed] [Google Scholar]
- Brotman AW (2001) Eating disorders. In: Psychiatric secrets, Ed 2 (Jacobson JL, Jacobson AM, eds), p 137. Philadelphia: Hanley and Belfus.
- Burgess LH, Handa RJ (1992) Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology 131: 1261-1269. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single step method of RNA isolation by acid guanidinium thiocyanate-phenol chloroform extraction. Anal Biochem 162: 156-159. [DOI] [PubMed] [Google Scholar]
- Cintra A, Fuxe K, Harfstrand A, Agnati LF, Wikstrom AC, Okret S, Vale W, Gustafsson JA (1987) Presence of glucocorticoid receptor immunoreactivity in corticotrophin releasing factor and in growth hormone releasing factor immunoreactive neurons of the rat di- and telencephalon. Neurosci Lett 77: 25-30. [DOI] [PubMed] [Google Scholar]
- Gaub MP, Bellard M, Scheuer I, Chambon P, Sassone-Corsi P (1990) Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell 63: 1267-1276. [DOI] [PubMed] [Google Scholar]
- Gold P, Loriaux D, Roy A, Kling M, Calabrese J, Kellner C, Nieman L, Post R, Pickar D, Gallucci W, Avgerinos P, Paul S, Oldfield E, Cutler Jr G, Chrousos G (1986a) Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing's disease. N Engl J Med 314: 1329-1335. [DOI] [PubMed] [Google Scholar]
- Gold PW, Gwirtsman H, Avgerinos PC, Nieman LK, Gallucci WT, Kaye W, Jimerson D, Ebert M, Rittmaster R, Loriaux DL (1986b) Abnormal hypothalamic-pituitary-adrenal function in anorexia nervosa. Pathophysiologic mechanisms in underweight and weight-corrected patients. N Engl J Med 314: 1335-1342. [DOI] [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Boswell C, Seasholtz AF (1994) The cAMP-responsive element in the corticotropin-releasing hormone gene mediates transcriptional regulation by depolarization. J Biol Chem 269: 14784-14791. [PubMed] [Google Scholar]
- Guardiola-Diaz HM, Kolinske JS, Gates LH, Seasholtz AF (1996) Negative glucocorticoid regulation of cyclic adenosine 3′,5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol Endocrinol 10: 317-329. [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL (2001) Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276: 13615-13621. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ (1995) Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology 136: 3213-3221. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93: 5925-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P (2000) Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74: 311-317. [DOI] [PubMed] [Google Scholar]
- Kwok RP, Lundblad JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SG, Green MR, Goodman RH (1994) Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 370: 223-226. [DOI] [PubMed] [Google Scholar]
- LaFlamme N, Nappi RE, Drolet G, Labrie C, Rivest S (1998) Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36: 357-378. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Uht R, Harrison R, Gibbs F, Paull W, Bohn M (1987) Ultrastructural localization of glucocorticoid receptor (GR) in hypothalamic paraventricular neurons synthesizing corticotropin-releasing factor (CRF). Histochemistry 87: 407-412. [DOI] [PubMed] [Google Scholar]
- Liu MM, Albanese C, Anderson CM, Hilty K, Webb P, Uht RM, Price Jr RH, Pestell RG, Kushner PJ (2002) Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem 277: 24353-24360. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Vigh S, Petrusz P, Schally A (1983) The paraventriculoinfundibular corticotropin releasing factor (CRF) pathway as revealed by immunocytochemistry in long-term hypophysectomized or adrenalectomized rats. Regul Pept 5: 295-305. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P (1989) Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57: 433-442. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE (2003) Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 144: 2055-2067. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper G, Gustafsson J, Hurd Y (1998) Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res 54: 175-180. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22: 502-548. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS (1997) Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277: 1508-1510. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ, Vale W (1985) Distribution of corticotrophin releasing factor in rat brain. Fed Proc 44: 215-219. [PubMed] [Google Scholar]
- Patisaul HB, Whitten PL, Young LJ (1999) Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17-beta estradiol and the phytoestrogen, coumestrol. Brain Res Mol Brain Res 67: 165-171. [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA (1998) Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology 139: 1082-1092. [DOI] [PubMed] [Google Scholar]
- Petersson K, Gustafsson JA (2001) Role of estrogen receptor beta in estrogen action. Annu Rev Physiol 63: 165-192. [DOI] [PubMed] [Google Scholar]
- Price Jr RH, Lorenzon N, Handa RJ (2000) Differential expression of estrogen receptor beta splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Res Mol Brain Res 80: 260-268. [DOI] [PubMed] [Google Scholar]
- Price Jr RH, Butler C, Webb P, Uht R, Kushner P, Handa RJ (2001) A splice variant of estrogen receptor beta missing exon 3 displays altered subnuclear localization and capacity of transcriptional activation. Endocrinology 142: 2039-2049. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I (1997) Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388: 507-525. [DOI] [PubMed] [Google Scholar]
- Solum D, Handa R (2002) Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci 22: 2650-2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ (2004) Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology 145: 3658-3670. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG (1980) The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555-570. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE (1980) Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 31: 410-417. [DOI] [PubMed] [Google Scholar]
- Swanson L, Sawchenko P, Rivier J, Vale W (1983) Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165-186. [DOI] [PubMed] [Google Scholar]
- Uht RM, McKelvy JF, Harrison RW, Bohn MC (1988) Demonstration of glucocorticoid receptor-like immunoreactivity in glucocorticoid-sensitive vasopressin and corticotropin-releasing factor neurons in the hypothalamic paraventricular nucleus. J Neurosci Res 19: 405-411. [DOI] [PubMed] [Google Scholar]
- Uht RM, Anderson CM, Webb P, Kushner PJ (1997) Transcriptional activities of estrogen and glucocorticoid receptors are functionally integrated at the AP-1 response element. Endocrinology 138: 2900-2908. [DOI] [PubMed] [Google Scholar]
- Uht RM, Webb P, Nguyen P, Price Jr RH, Valentine C, Favre H, Kushner PJ (2004) A conserved lysine in the estrogen receptor DNA binding domain regulates ligand activation profiles at AP-1 sites, possibly by controlling interactions with a modulating repressor. Nucl Recept 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T (1994) Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem 269: 16433-16442. [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J (1981) Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science 213: 1394-1397. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP (1993) Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. J Clin Invest 92: 1896-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo N, Goodman R (2001) CREB-binding protein and p300 in transcriptional regulation. J Biol Chem 276: 13505-13508. [DOI] [PubMed] [Google Scholar]
- Webb P, Lopez GN, Uht RM, Kushner PJ (1995) Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9: 443-456. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stall-cup MR, Kushner PJ (1998) Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol 12: 1605-1618. [DOI] [PubMed] [Google Scholar]
- Wingender E, Dietze P, Karas H, Knuppel R (1996) TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res 24: 238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin LR (2001) Depressive disorders. In: Psychiatric secrets, Ed 2 (Jacobson JL, Jacobson AM, eds), p 71. Philadelphia: Hanley and Belfus.