Abstract
The environmental context previously associated with opiate use plays an important role in human relapse, but the neuronal mechanisms involved in context-induced drug relapse are not known. Using a rat relapse model, we determined the effect of a group II metabotropic glutamate receptor agonist [LY379268 ((-)-2-oxa-4-aminobicylco hexane-4,6-dicarboxylic acid)] on contextual cue-induced reinstatement of heroin seeking. LY379268, which acts centrally to reduce evoked glutamate release, was injected systemically or directly into the ventral tegmental area (VTA), a brain area involved in opiate reward and conditioned drug effects. Rats were trained to self-administer intravenous heroin for 12 d; drug infusions were paired with a discrete tone-light cue. Subsequently, lever pressing was extinguished in the presence of the discrete cue in a context that differed from the drug self-administration context in terms of visual, auditory, tactile, and circadian cues. After extinction of lever responding, LY379268 was injected systemically or into the VTA, and nonreinforced responding was determined in the extinction context or the drug context. Exposure to the heroin-associated context induced robust reinstatement of drug seeking, and this effect was attenuated by systemic or intra-VTA injections of LY379268. Results indicate that glutamate transmission in the VTA plays an important role in contextual cue-induced relapse to heroin seeking.
Keywords: drug environment, extinction, opiate self-administration, reinstatement, renewal, relapse, substantia nigra
Introduction
Exposure to environmental stimuli previously associated with opiate intake can provoke drug relapse in humans (O'Brien et al., 1992). In laboratory animals, discrete conditioned stimuli (CSs) (e.g., tone and light) that are explicitly paired with opiate injections (Fuchs and See, 2002) or discriminative stimuli (DSs) (e.g., specific odors) that become predictors of opiate availability during discrimination training (McFarland and Ettenberg, 1997) reinstate opiate seeking after extinction of the drug-taking behavior in the absence of these cues. However, although there is evidence that discrete CSs or DSs can reinstate drug seeking in a preclinical relapse model, little is known about the role of contextual stimuli (e.g., the physical characteristics of the drug environment, time of day) in drug relapse (Shalev et al., 2002). This issue is important for the understanding of processes underlying drug relapse, because the environmental context can profoundly influence extinction and reinstatement of learned behaviors (Bouton, 2002).
Recently, we adapted a “renewal” procedure (Bouton and Bolles, 1979) to study the role of the environmental context in reinstatement of drug seeking. We found that reexposing rats to a drug-associated context, after extinction in a different context, reinstates cocaine or speedball (a heroin-cocaine mixture) seeking (Crombag and Shaham, 2002; Crombag et al., 2002). Here, we studied the role of the drug context in reinstatement of heroin seeking. Additionally, we evaluated the effects of systemic or intra-ventral tegmental area (intra-VTA) injections of the metabotropic glutamate receptor 2/3 (mGluR2/3) agonist LY379268 [(-)-2-oxa-4-aminobicylco hexane-4,6-dicarboxylic acid] on context-induced reinstatement of heroin seeking. LY379268 is a selective agonist of group II metabotropic glutamate receptors (mGluR2 and mGluR3 subtypes) that acts centrally to reduce evoked glutamate release (Schoepp, 2001). Previous studies found that systemic injections of LY379268 or other mGluR2/3 agonists attenuate several behavioral effects of abused drugs (Kenny and Markou, 2004), including opiate withdrawal symptoms (Vandergriff and Rasmussen, 1999), amphetamine-induced locomotor sensitization (Vezina, 2004), and discriminative cue-induced reinstatement of cocaine seeking (Baptista et al., 2004).
We studied the role of mGluR2/3 in the VTA (the cell body region of the mesolimbic dopamine system) because this brain area is involved in opiate reward (Wise, 2004) and reinstatement (Stewart et al., 1984) and conditioned drug effects (Everitt and Wolf, 2002). Dopaminergic activity within the VTA is controlled in part by glutamate afferents from several brain areas (Sesack et al., 2003), and previous studies indicate a role of glutamate in this brain area in opiate reinforcement, as measured in the conditioned place-preference (Carlezon et al., 1997; Byrne et al., 2003) and self-administration (Xi and Stein, 2002) procedures.
Here, we first established a procedure to study the role of the drug context in reinstatement of heroin seeking. We then determined whether systemic or intra-VTA injections of LY379268 would attenuate context-induced reinstatement. To determine anatomical specificity, we examined the effect of LY379268 injections into the nearby substantia nigra (SN).
Materials and Methods
Subjects
Male Long-Evans rats (Charles River, Raleigh, NC) (n = 159) weighing 350-400 gm were used. After surgery, rats were housed in the animal facility under a reverse 12 hr light/dark cycle (lights off at 9:00 A.M.). Food and water were available in the home cage ad libitum. Experimental procedures followed the guidelines of the Principles of Laboratory Animal Care (National Institutes of Health publication 86-23). Fifty-four of the 159 subjects were excluded because of catheter failure (n = 19), poor health (n = 14), misplaced cannulas (n = 8), or failure to extinguish lever responding after 18-20 sessions (n = 13).
Intracranial and intravenous surgery
Rats were anesthetized with mixtures of either xylazine plus ketamine (10 and 100 mg/kg, i.p.) or sodium pentobarbital plus chloral hydrate (60 and 225 mg/kg, i.p.), and guide cannulas (23 gauge; Plastics One, Roanoke, VA) were implanted bilaterally 1 mm above the VTA or SN. The coordinates (Paxinos and Watson, 1998) for the VTA and SN (10° angle) were anteroposterior -5.2 and 5.2, mediolateral ±1.8 and 2.8, and dorsoventral -7.5 and 7.5 mm, respectively. The intravenous catheters were implanted as described previously (Crombag et al., 2002). Buprenorphine (0.1 mg/kg, s.c.) was given after surgery, and rats were allowed to recover for 5-10 d.
Systemic and intracranial injections
LY379268 was dissolved in 1.2 m NaOH solution, pH adjusted to 7.4 (Kim and Vezina, 2002), and was injected either 30 (systemic) or 5-10 (intracranial) min before testing. Intracranial infusions were made using 30 gauge injectors (Plastics One). Infusions (0.5 μl) were made over the course of 1 min, and the injectors were left in place for 1 min. After testing, rats were anesthetized and were infused with 2% pontamine sky blue (0.5 μl). Rats were then decapitated, and the brains were removed. Coronal sections were sliced on a cryostat and stained with cresyl violet.
Apparatus
The self-administration chambers (Med Associates, Georgia, VT) were equipped with two levers located 9 cm above the grid floor. Responding on the active retractable lever activated the infusion pump. Responding on the inactive nonretractable lever was also recorded. The two contexts differed in terms of session onset time (9:00 A.M. or 3:00 P.M.) and their tactile (type of grid floors), visual (houselight on or off), and auditory (fan on or off) background stimuli (Crombag and Shaham, 2002; Crombag et al., 2002). The contexts are labeled as A (training) and B (extinction), and their physical characteristics were counterbalanced.
Procedures
Experiment 1. Context-induced reinstatement
The purpose of experiment 1 (Exp. 1) was to establish a procedure to study context-induced reinstatement in heroin-trained rats. Rats were assigned to one of four groups (n = 9-11): “renewal,” “novel,” “control,” and “no extinction” (Table 1). In the renewal group, rats were trained to self-administer heroin in A, lever responding was extinguished in B, and rats were tested for reinstatement in A (the contexts for training, extinction, and reinstatement are abbreviated A-B-A). In the novel group, the conditions for training, extinction, and reinstatement were A-A-B. In the control group, the conditions for training, extinction, and reinstatement were either A-A-A or A-B-B. In the no-extinction group, rats were trained in A and tested in this context 12 d later without undergoing extinction (A-A).
Table 1.
Summary of the experimental groups in experiment 1
|
Group |
Training |
Extinction |
Test |
n |
|---|---|---|---|---|
| Control | A | A | A | 6 |
| A | B | B | 5 | |
| Novel | A | A | B | 11 |
| No extinction | A | None | A | 9 |
| Renewal |
A |
B |
A |
11 |
Training and extinction. Rats were trained to self-administer heroin HCl for 3 hr/d for 12 d under a fixed ratio 1 schedule. Heroin was dissolved in saline and infused over the course of 2.3 sec at a dose of 0.1 (first six sessions) or 0.05 (last six sessions) mg/kg per injection. The heroin training doses are based on previous studies (Shalev et al., 2002), and the unit dose was halved after the sixth day of training to verify that the rats acquired drug self-administration, as indicated by the increase in responding for the lower dose. During training, heroin infusions were paired with a tone (2900 Hz; 20 dB above background)-light (a 7.5 W white light) cue for 2.3 sec. Sessions began with the introduction of the tone-light cue for 2.3 sec and the insertion of the active lever. Depending on the context, either the houselight was illuminated or the ventilation fan was turned on. During the extinction phase (12-20 d), procedures were identical to those of training, except that the drug syringes were removed. For the novel group (A-A-B) and one of the control groups (A-A-A), extinction occurred in the training context. For the renewal group (A-B-A) and the other control group (A-B-B), extinction occurred in a different (nondrug) context. Rats that did not meet an extinction criterion (≤25 active lever responses during the last three extinction sessions) within 18-20 sessions were excluded.
Tests for reinstatement. Rats were tested after exposure to the context previously paired with drug intake (renewal and no-extinction groups), the context previously paired with extinction (control group), or a novel context (novel group). Three daily 1 hr reinstatement tests [which were shorter than the training and extinction sessions (3 hr)] were performed every 5 d; four 3 hr extinction sessions were conducted during the intervening days between these tests. However, although responding in the last two tests in the renewal and the no-extinction groups was significantly higher than that of the control group, it was lower during these tests (data not shown) than during the first test (see Fig. 1C).
Figure 1.
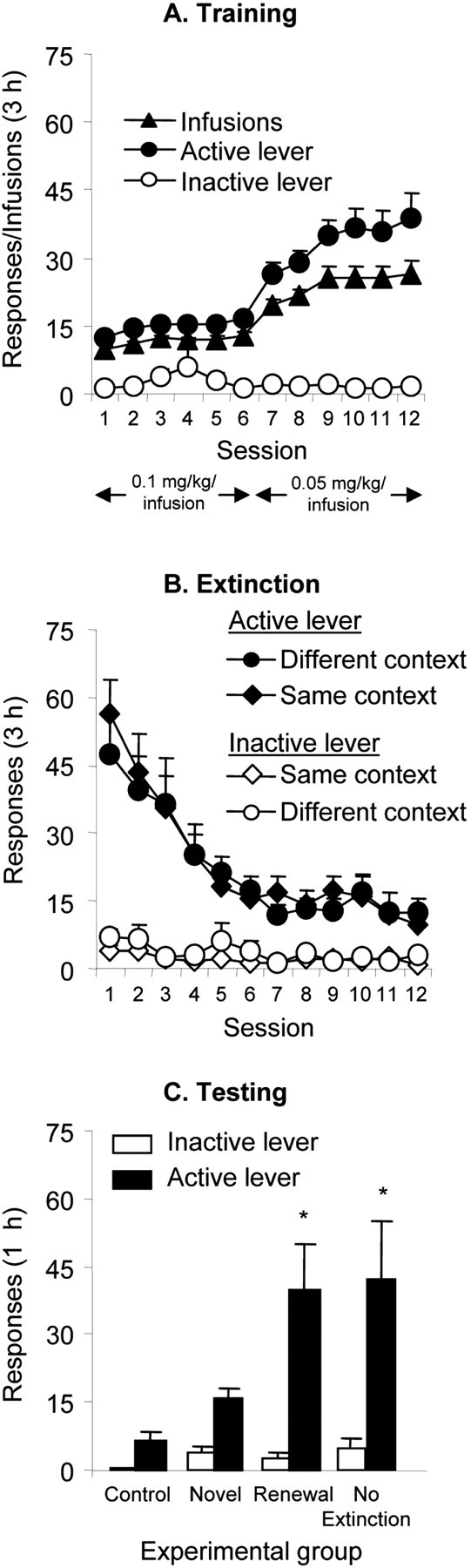
Context-induced reinstatement of heroin seeking. A, Training, Mean ± SEM infusions and responses on the active and inactive levers during the 12 d of training. Data are from all of the rats (n = 42). B, Extinction, Mean responses on the previously active lever and on the inactive lever during the 3 hr extinction sessions, conducted in the presence of the heroin-associated discrete tone-light cue. There were no differences in lever responding between rats that underwent extinction in the same (A-A-A and A-A-B) or different (A-B-B and A-B-A) context as the training context. C, Testing, Mean active and inactive lever responses during the 1 hr reinstatement test session in the four groups of Exp. 1. *p < 0.05 indicates different from control and novel groups; n = 9-11 per group.
Experiment 2. Effects of LY379268 on context-induced reinstatement
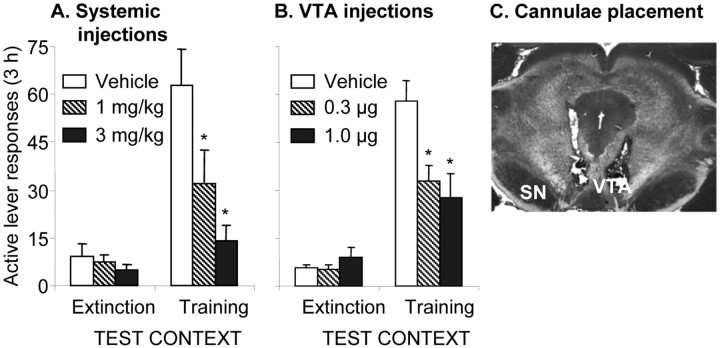
Exp. 2 consisted of three phases: self-administration training (12 d), extinction training (13-18 d), and tests for the effects of systemic or intracranial LY379268 injections on context-induced reinstatement (2 d). Based on the results of the repeated testing (Exp. 1), we used a mixed design with LY379268 dose as the between-subjects factor and test context [extinction (B) or training (A)] as the within-subjects factor. The experimental conditions were the same as those described for the renewal group of Exp. 1. The systemic doses of LY379268 were based on previous reports (Kim and Vezina, 2002; Baptista et al., 2004) and on a pilot study in which we found that doses of 3 mg/kg or lower have no effect on high rates of responding for oral sucrose, whereas a dose of 6 mg/kg decreases responding. LY379268 was not previously injected into discrete brain areas using the methods used here. Thus, the intracranial doses were based on previous studies in which intracranial doses for drugs that are systemically effective at doses of 1-10 mg/kg are typically given at doses of ∼0.1-10 μg/site.
Rats were trained to self-administer heroin and underwent extinction as described above for the renewal group. Tests started after a minimum of 13 daily extinction sessions. Eight groups of rats (n = 7-9) were used. LY379268 was injected systemically (0, 1, or 3 mg/kg; three groups) or intracranially into the VTA (0, 0.3, or 1.0 μg; three groups) or SN (0 or 1.0 μg; two groups).
Statistical analyses
Data were analyzed separately for total (nonreinforced) active and inactive lever responses, using the appropriate within- and between-subjects factors. Significant effects were followed by post hoc Fisher PLSD tests (two-tailed). Inactive lever analyses are not reported because responding on this lever during testing was low (five or less responses per session) and because no significant group differences were observed. Also, the rats assigned to the different LY379268 dose conditions were matched for active lever responding during the training and extinction phases. Thus, the nonsignificant group differences during these phases are not reported.
Results
Experiment 1. Context-induced reinstatement
Figure 1A shows the mean ± SEM number of heroin infusions and responses on the active and inactive levers for all of the rats that participated in experiment 1. The rats demonstrated reliable heroin self-administration, as indicated by the increase in lever responding when the heroin dose was decreased from 0.1 to 0.05 mg/kg per infusion. Figure 1B shows the mean number of responses on the active and inactive levers during the first 12 d of extinction for the rats that were extinguished in the same (novel A-A-B and control A-A-A) or different (renewal A-B-A and control A-B-B) context as their training context. No significant differences were observed for lever responding across the experimental groups during the extinction phase. Figure 1C shows that, during the tests for context-induced reinstatement, active lever pressing in the renewal group was higher than that in the novel or the control groups, but similar to that in the no-extinction group. Analysis revealed a significant effect of experimental group (F(3,38) = 5.9; p < 0.01).
Experiment 2. Effect of LY379268 on context-induced reinstatement
The mean numbers of active lever responding per 3 hr (data collapsed from the eight groups) on the last training day and on the first and last extinction days were 32.8 ± 3.3, 38.7 ± 3.4, and 8.4 ± 0.8, respectively. Figure 2 shows that both systemic and intra-VTA injections of LY379268 attenuated lever responding when rats were exposed to the training context after extinction in the different context. LY379268 had no effect on lever responding in the extinction context. Analyses revealed significant effects of test context (F(1,19) = 35.7 and F(1,23) = 113.6; p < 0.01; for systemic and intra-VTA injections, respectively), LY379268 dose (F(2,19) = 6.5 and F(2,23) = 4.2; p < 0.05), and LY379268 dose by test context (F(2,19) = 6.8 and F(2,23) = 11.0; p < 0.01). Intra-SN injections of the high dose of LY379268 (1 μg) had no effect on context-induced reinstatement. The mean number of responses after intra-SN vehicle or LY379268 injections were 8.5 ± 1.8 and 34.6 ± 2.9 or 12.4 ± 5.7 and 39.6 ± 7.0 for the extinction (B) and the training (A) contexts, respectively. Analysis revealed a significant effect of test context (F(1,11) = 34.3; p < 0.01) but not of LY379268 dose or LY379268 dose by test context (p > 0.05).
Figure 2.
Effects of LY379268 on context-induced reinstatement of heroin seeking. Mean ± SEM responses on the active lever (3 hr sessions) after systemic (A) or intra-VTA (B) injections of the vehicle or LY379268. C, Apictograph of VTA cannula placements. Rats were tested in both the extinction (context B) and the training (context A) context. *p < 0.05 indicates different from vehicle; n = 8-10 per dose.
Discussion
The present experiments yielded three main findings. First, exposure to the heroin-associated context, after extinction of lever responding in the presence of the discrete tone-light heroin-infusion cue in a nondrug context, reinstated drug seeking to levels similar to those of rats that did not undergo extinction training (Fig. 1C). These data extend our findings of context-induced reinstatement of cocaine and speedball seeking (Crombag and Shaham, 2002; Crombag et al., 2002) and are in agreement with results from previous studies using nondrug reinforcers (Bouton, 2002). Second, systemic injections of the mGluR2/3 agonist LY379268 attenuated context-induced reinstatement of heroin seeking. These data are in agreement with those of Baptista et al. (2004) on the effect of LY379268 on discriminative cue-induced reinstatement of cocaine seeking. LY379268 had no effect on responding in the extinction context (Fig. 2A), and, at the doses used here, this drug had no effect on responding for oral sucrose (our unpublished data) or sweetened condensed milk (Baptista et al., 2004), suggesting that the effect of LY379268 on context-induced reinstatement is not caused by motor deficits. The third and most important finding is that intra-VTA injections of LY379268 mimicked the effect of the systemic injections of the drug on context-induced reinstatement. As in the case of the systemic drug injections, it is unlikely that the effects of intra-VTA infusions of LY379268 on context-induced reinstatement are caused by motor deficits. These VTA infusions did not decrease responding in the extinction context (Fig. 2B). Also, infusions of the high dose of the drug into the SN, which is involved in motor performance, had no effect on context-induced reinstatement. Furthermore, Kenny et al. (2003) found that intra-VTA infusions of a different mGluR2/3 agonist (LY314582) to naive rats had no effect on responding for intracranial brain stimulation reward, a dopamine-dependent behavior that is susceptible to motor disruption. Together, the present results indicate that glutamate transmission in the VTA plays an important role in contextual cue-induced relapse to heroin seeking.
Reinstatement of heroin seeking by contextual cues
Several behavioral mechanisms may underlie context-induced reinstatement of drug seeking. The resumption of responding in the renewal group may be caused by a context switch after extinction, resulting in increased arousal and, subsequently, nondirected behavioral activity. This possibility is unlikely, because a context switch and exposure to a novel environment, which increase arousal and behavioral activation (Piazza et al., 1989), had minimal effects on responding during testing (novel group).
Based on results from the learning literature (Bouton, 2002), we suggested that the occasion-setter properties of the drug context underlie its effects on reinstatement of drug seeking (Crombag and Shaham, 2002). Occasion-setter cues are different from traditional CSs in that they do not elicit behavior but rather modulate the ability of other CSs to provoke learned responses. However, under our experimental conditions, we found that reinstatement of heroin seeking induced by reexposure to a discrete tone-light cue after extinction of lever responding in the drug context in the absence of this cue is much weaker (9.4 ± 2.0 active lever responses per 1 hr; n = 7) than reinstatement induced by reexposure to the contextual cues (40.0 ± 9.4) (Fig. 1C). These data suggest that a strict information-processing (nonmotivational) account of context-induced reinstatement of drug seeking is not likely. That is, if the context only functions as an occasion setter, the discrete tone-light cue should have reinstated responding (after extinction of lever responding in the drug-associated context in the absence of this cue) to levels similar to that induced by exposure to the heroin-associated context.
Finally, it is possible that the drug context induces heroin seeking by acquiring motivational properties (Stewart et al., 1984) via its direct association with heroin reward during training, independent of its learned associations with the discrete CSs. This motivational account of context-induced reinstatement of heroin seeking is supported by the finding that this effect involves the VTA, a brain area involved in the incentive motivational effects of drug and nondrug reinforcers (Wise, 2004).
Role of VTA glutamate in reinstatement of heroin seeking
The most important finding in this report is that intra-VTA, but not intra-SN, injections of LY379268 mimicked the effect of the systemic injections of the drug on context-induced reinstatement. These data provide the first evidence for the involvement of VTA glutamate in heroin relapse and extend previous findings on the role of glutamate in this brain area in opiate reinforcement (Carlezon et al., 1997; Xi and Stein, 2002; Byrne et al., 2003). The present data also extend those demonstrating a role of the VTA-accumbens dopamine projections in reward seeking controlled by cues associated with cocaine (Phillips et al., 2003; Di Ciano and Everitt, 2004) or sucrose (Yun et al., 2004).
The origin of the glutamatergic neurons that mediate the effects of LY379268 in the VTA on context-induced reinstatement is not known. The glutamate projections to the VTA originate from the prefrontal cortex, subthalamic nucleus, pedunculopontine nucleus, and the bed nucleus of stria terminalis (Georges and Aston-Jones, 2002; Sesack et al., 2003). Potentially relevant to the present data are the findings that lesions of the pedunculopontine nucleus attenuate the conditioned reinforcing effects of opiate drugs (Bechara et al., 1998). Thus, we speculate that the pedunculopontine-VTA glutamate projection is likely to be involved in the effect of contextual cues on reinstatement of heroin seeking. Another issue to consider concerning the present data is the cellular site of action of LY379368 in the VTA. Pharmacological effects of LY379268 can be mediated by glutamatergic and GABAergic mGluR2 presynaptic sites, nonglutamatergic mGluR2 and mGluR3 postsynaptic sites, or glial cell mGluR3 sites (Schoepp, 2001). Based on studies on the anatomical distribution and function of mGluR2 and mGluR3 in midbrain dopamine areas (Rouse et al., 2000), however, it is likely that glutamatergic presynaptic mGluR2 receptors mediate the effect of intra-VTA infusions of LY379268 on context-induced reinstatement of heroin seeking.
As mentioned, dopaminergic activity within the VTA is controlled in part by glutamate afferents from several brain areas (Sesack et al., 2003). Furthermore, previous studies found that dopamine receptor antagonists block reinstatement of cocaine seeking induced by contextual (Crombag et al., 2002) or discriminative (Weiss et al., 2001) cues. Therefore, a possible mechanism for the effect of LY379268 on context-induced reinstatement of heroin seeking is that it indirectly decreased the activation of the VTA-accumbens dopamine projections by the contextual cues, resulting in decreased responding during testing. Alternatively, intra-VTA injections of LY379268 may have influenced dopamine-independent VTA systems that are also involved in opiate reward under certain conditions (Bechara et al., 1998). Potential support for this dopamine-independent account of context-induced reinstatement is the finding that reinstatement of heroin seeking by discriminative cues in the runway model is not altered by a dopamine receptor antagonist (McFarland and Ettenberg, 1997).
Clinical implications
Based on preclinical studies, it has been suggested the mGluR2/3 agonists can serve as novel therapeutic agents in the treatment of anxiety, schizophrenia, and neurodegeneration (Schoepp, 2001). Recently, Kim and Vezina (2002) reported that systemic injections of LY379268 attenuate psychostimulant sensitization, a phenomenon associated with reinstatement of drug seeking (De Vries et al., 2002; Vezina, 2004). Other studies have shown that mGluR2/3 agonists attenuate opiate withdrawal symptoms (Vandergriff and Rasmussen, 1999) and other behavioral effects of abused drugs (Kenny and Markou, 2004). The present data and those of Baptista et al. (2004) demonstrate that activation of mGluR2/3 attenuates drug seeking induced by discriminative or contextual cues in a rat relapse model (Shaham et al., 2003). Together, these findings and results from other studies on the role of mesocorticolimbic glutamate in drug relapse (Kalivas, 2004) suggest that pharmacological manipulations that target the group II metabotropic glutamate receptors should be considered in the treatment of drug addiction.
Footnotes
We thank Jack Dempsey and Sean Sheffler-Collins for their help in conducting this study, Dr. Hans Crombag for helpful comments, and Eli Lilly (Indianapolis, IN) for providing LY379268.
Correspondence should be addressed to Dr.Yavin Shaham, Behavioral Neuroscience Branch, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, 5500 Nathan Shock Drive, Baltimore, MD 21224. E-mail: yshaham@intra.nida.nih.gov.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2410726-05$15.00/0
References
- Baptista MA, Martin-Fardon R, Weiss F (2004) Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci 24: 4723-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Nader K, van der Kooy D (1998) A two-separate-motivational-systems hypothesis of opioid addiction. Pharmacol Biochem Behav 59: 1-17. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2002) Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52: 976-986. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC (1979) Contextual control of the extinction of conditioned fear. Learn Motiv 10: 445-466. [Google Scholar]
- Byrne R, Harris G, Aston-Jones G (2003) Glutamate input to the ventral tegmental area is necessary for both learning and expression of morphine place preference. Soc Neurosci Abstr 29: 110.1. [Google Scholar]
- Carlezon Jr WA, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ (1997) Sensitization to morphine induced by viral-mediated gene transfer. Science 277: 812-814. [DOI] [PubMed] [Google Scholar]
- Crombag H, Grimm JW, Shaham Y (2002) Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27: 1007-1016. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116: 169-173. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raasø H, Vanderschuren LJ (2002) Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology 26: 18-26. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ (2004) Contribution of the ventral tegmental area to cocaine-seeking maintained by a drug-paired conditioned stimulus in rats. Eur J Neurosci 19: 1661-1667. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22: 3312-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, See RE (2002) Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 160: 425-433. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G (2002) Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22: 5173-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2004) Glutamate systems in cocaine addiction. Curr Opin Pharmacol 4: 23-29. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A (2004) The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol 25: 265-272. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A (2003) Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther 306: 1068-1076. [DOI] [PubMed] [Google Scholar]
- Kim JH, Vezina P (2002) The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav 73: 333-337. [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A (1997) Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology 131: 86-92. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan TA, Ehrman R (1992) Classical conditioning in drug dependent humans. Ann NY Acad Sci 654: 400-415. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, Ed 4. San Diego: Academic.
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422: 614-618. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245: 1511-1513. [DOI] [PubMed] [Google Scholar]
- Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ (2000) Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson's disease and related disorders. Pharmacol Ther 88: 427-435. [DOI] [PubMed] [Google Scholar]
- Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12-20. [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A (2003) Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003: 36-52. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168: 3-20. [DOI] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1-42. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R (1984) Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev 91: 251-268. [PubMed] [Google Scholar]
- Vandergriff J, Rasmussen K (1999) The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropharmacology 38: 217-222. [DOI] [PubMed] [Google Scholar]
- Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychostimulant drugs. Neurosci Biobehav Rev 27: 827-839. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, Ben Shahar O (2001) Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology 25: 361-372. [DOI] [PubMed] [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5: 483-494. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA (2002) Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology 164: 144-150. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM (2004) The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci 24: 2923-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]