Abstract
Our previous studies found that serotonin transporter (SERT) knock-out mice showed increased sensitivity to minor stress and increased anxiety-like behavior but reduced locomotor activity. These mice also showed decreased density of 5-hydroxytryptamine (5-HT1A) receptors in the hypothalamus, amygdala, and dorsal raphe. To evaluate the contribution of hypothalamic 5-HT1A receptors to these phenotypes of SERT knock-out mice, two studies were conducted. Recombinant adenoviruses containing 5-HT1A sense and antisense sequences (Ad-1AP-sense and Ad-1AP-antisense) were used to manipulate 5-HT1A receptors in the hypothalamus. The expression of the 5-HT1A genes is controlled by the 5-HT1A promoter, so that they are only expressed in 5-HT1A receptor-containing cells. (1) Injection of Ad-1AP-sense into the hypothalamus of SERT knock-out mice restored 5-HT1A receptors in the medial hypothalamus; this effect was accompanied by elimination of the exaggerated adrenocorticotropin responses to a saline injection (minor stress) and reduced locomotor activity but not by a change in increased exploratory anxiety-like behavior. (2) To further confirm the observation in SERT-/- mice, Ad-1AP-antisense was injected into the hypothalamus of normal mice. The density and the function of 5-HT1A receptors in the medial hypothalamus were significantly reduced in Ad-1AP-antisense-treated mice. Compared with the control group (injected with Ad-track), Ad-1A-antisense-treated mice showed a significant reduction in locomotor activity, but again no changes in exploratory anxiety-like behaviors, tested by elevated plus-maze and open-field tests. Thus, the present results demonstrate that medial hypothalamic 5-HT1A receptors regulate stress responses and locomotor activity but may not regulate exploratory anxiety-like behaviors.
Keywords: 5-HT1A promoter, ACTH, 125I-MPPI binding, elevated plus maze, open-field test, SERT knock-out
Introduction
The serotonergic [5-hydroxytryptamine (5-HT)] system is essential for regulating several psychophysiological pathways including those involved in emotion, motivation, and stress responses. Among 15 5-HT receptors, 5-HT1A receptors are known to participate in the regulation of anxiety behaviors (Griebel, 1995; Graeff et al., 1996; den Boer et al., 2000). 5-HT1A receptor agonists have been used as anxiolytic drugs. Mice that genetically lack 5-HT1A receptors demonstrate increased anxiety-like behavior (Gingrich and Hen, 2001; Olivier et al., 2001; Groenink et al., 2003). Furthermore, 5-HT1A receptors are involved in the regulation of the hypothalamic-pituitary-adrenal axis (HPA), which plays an important role in stress responses (Carrasco and Van de Kar, 2003). However, the neuronal circuitries and molecular mechanisms mediating these effects of 5-HT1A receptors are still unclear. Understanding these circuitries and mechanisms will help us to develop better approaches for treatment of psychiatric disorders.
Our previous studies showed that lack of serotonin transporter (SERT) in mice increased exploratory anxiety-like behaviors and sensitivity to minor stressors but reduced locomotor activity (Murphy et al., 2001; Holmes et al., 2003b,c). Furthermore, the density of 5-HT1A receptors is reduced in the hypothalamus, amygdala, and dorsal raphe of these mice (Li et al., 1999, 2000). The aim of the present study was to determine whether the behavioral changes in these mice are mediated by the alteration of hypothalamic 5-HT1A receptors. The hypothalamic 5-HT1A receptors are involved in the regulation of the secretion of several stress hormones (Carrasco and Van de Kar, 2003). It is known that the hypothalamus regulates motivated movement (Risold et al., 1997; Swanson, 2000). Although the exploratory anxiety-like behavior is more likely mediated by 5-HT1A receptors in the amygdala (LeDoux, 2003), 5-HT1A receptors in the hypothalamus may be involved in the regulation of this behavior, because the hypothalamus is innervated by the amygdala (Gorman et al., 2000).
The most common approach to study receptor-behavior relationships is using pharmacologic agents to activate the receptors or to block the activation of the receptors. Because most agonists and antagonists have side effects for other receptors, the selectivity of the agents is always a concern. The recent development of transgenic techniques allows for the selective deletion of a target gene. However, compensatory changes induced by the knock-out during development can produce a more complicated system. In the present studies, we used a recombinant adenovirus containing 5-HT1A genes to manipulate the expression of 5-HT1A receptors in the hypothalamus. This technique allows us to selectively change the density of 5-HT1A receptors in adult mice, thus avoiding compensatory developmental changes. We designed the recombinant adenovirus so that the expression of 5-HT1A genes is controlled by the 5-HT1A promoter. Therefore, 5-HT1A genes are only expressed in cells that normally express 5-HT1A receptors. We hypothesized that (1) administration of recombinant adenovirus containing the 5-HT1A sense gene (Ad-1AP-sense) into the hypothalamus of SERT knock-out mice would normalize the altered behaviors in these mice; and (2) injection of recombinant adenovirus containing 5-HT1A antisense gene (Ad-1AP-antisense) into the hypothalamus of normal mice would mimic the behavioral changes observed in SERT knock-out mice.
Materials and Methods
Generation of recombinant adenoviruses containing 5-HT1A receptor genes
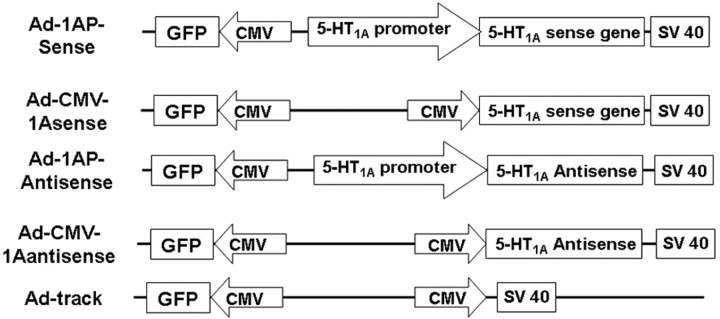
The Ad-Easy adenovirus system (a gift from Dr. Bert Vogelstein, Johns Hopkins University, Baltimore, MD) (He et al., 1998) was used to generate recombinant adenoviruses containing 5-HT1A sense and antisense genes (see Fig. 1). The recombinant adenoviruses were generated using the procedure described by He et al. (1998). Briefly, DNA fragments encoding the 5-HT1A receptor gene and/or 5-HT1A receptor promoter were amplified from mouse genomic DNA by PCR with primers as listed in Table 1. Each primer contained a specific restriction enzyme site for future insertion of 5-HT1A genes into an adenovirus shuttle vector, Ad-track or cytomegalovirus promoter (CMV)-Ad-track. PCR products were inserted into a PCR II-Topo TA vector (Invitrogen, Carlsbad, CA) and transferred into DH 10B Escherichia coli cells. The correct colony was selected by restriction enzyme followed by sequencing. After the mini-preparation of selected clones, the plasmids were digested by restriction enzymes that correspond to the restriction enzyme sites in the primers (Table 1). The digested 5-HT1A promoter and/or 5-HT1A gene fragments were separated from the rest of plasmid by 1% agarose gel and extracted from the gel using a gel extraction kit (Q-Biogene, Carlsbad, CA). The fragments were ligated to the Ad-track vector that predigested with the same restriction enzymes. The plasmids were then transferred into DH 10B cells. Correct colonies were selected by antibiotics followed by restriction enzyme digestion. After a miniprep, the plasmids were linearized using the restriction enzymes PmeI (1A promoter and 1A antisense gene), EcoRI (1A sense gene), or Bst1107 I (1A promoter and 1A gene). To generate recombinant adenovirus, the linearized plasmids were transferred into BJ 5183 cells that contained pretransferred Ad-Easy-1 vector (an adenovirus vector). After 20-24 hr incubation in the 1.5% agar plate containing 50 μg/ml kanomycin, the smallest colonies were picked for minipreps. The plasmids were run in 0.7% agarose gel. The plasmids that do not contain <10 kb bands were identified as potentially correct colonies. The potential correct plasmids were further transformed into DH 10B cell to generate more plasmids. The correct recombinant adenovirus was identified using restriction enzymes and PCR with corresponding primers. The recombinant adenovirus DNA was then linearized by PacI and transfected into human embryonic kidney (HEK) 293 cells using lipofactamine (Invitrogen). The infection of the cells was estimated by observation of green fluorescence protein (GFP; contained in the Ad-track and CMV-Ad-track vectors) expression under a fluorescence microscope. Ten to fourteen days after the transfection, the infected HEK293 cells were collected when ∼50% cells flowed, and all the cells became round. The cells were repeat frozen, thawed, and vortexed four times. The cell lysate was used to further infect 15-20 flasks of HEK293 cells. Three to five days later, the HEK293 cells were collected, and high-titer adenovirus was produced (∼1011 virus/ml) and stored as described by He et al. (1998). Before use, the high-titer recombinant adenovirus (∼30 μl) was transferred into a mini-dialysis device (MC 10,000; Pierce, Rockford, IL) and dialyzed against 1 l saline for >30 min at 4°C. The dialyzed viral solution was then diluted with 1 vol of saline. The adenoviral solution was kept in ice for no longer than 4 hr before injection.
Figure 1.
Scheme of constructs of 5-HT1A genes in the recombinant adenovirus. The arrow indicates the direction of transcription. Ad-1AP-sense, Recombinant adenovirus containing 5-HT1A sense sequence controlled under 5-HT1A promoter; Ad-CMV-1AS sense recombinant adenovirus containing 5-HT1A sense sequence controlled under CMV promoter; Ad-1AP-antisense, recombinant adenovirus containing 5-HT1A antisense sequence controlled under 5-HT1A promoter; Ad-CMV-1A antisense, recombinant adenovirus containing 5-HT1A antisense sequence controlled under CMV promoter.
Table 1.
Primers for 5-HT1A promoter and genes
|
DNA fragment |
Primer |
Primer sequence |
Restriction enzyme |
Encodinga |
|---|---|---|---|---|
| P1 | 5′-GCGCGGTACCTTTGAGATTAGACTGTTTTA-3′ | KpnI | (−1180)-(−1160) | |
| 5-HT1A promoter | P2 | 5′-GCGCGCGGCCGCAGACTGAACATATCCACCT-3′ | NotI | (+17)-(−4) |
| P1 | 5′-GCGCGGTACCTTTGAGATTAGACTGTTTTA-3′ | KpnI | (−1180)-(−1160) | |
| 5-HT1A promoter plus gene | S2 | 5′-GCGCAAGCTTCCCCGAAGGCTGCAAACT | HindIII | 2382-2362 |
| S | 5′-GCGCCTCGAGGCAGGCATGGATATGTTC-3′ | XhoI | (−6)-(+15) | |
| 5-HT1A sense gene | S2 | 5′-GCGCAAGCTTCCCCGAAGGCTGCAAACT | HindIII | 2382-2362 |
| S | 5′-GCGCCTCGAGGCAGGCATGGATATGTTC-3′ | XhoI | (−6)-(+15) | |
| 5-HT1A antisense |
AS |
5′-GCGCGATATCGCGGCCGCATCATCAGCGAAGAACTT-3′ |
EcoRV-NotI |
1273-1254 |
P1, Sense prime for 5-HT1A promoter; P2, antisense prime for 5-HT1A promoter; S, sense prime for sense sequence of 5-HT1A receptor; S2, antisense prime for sense sequence of 5-HT1A receptor; AS, antisense prime for antisense sequence of 5-HT1A receptor.
Animal studies
Animals. Normal C57BL/6 mice and SERT+/+ and SERT-/- mice were used in the present studies. Female mice were used in the present studies, because the density of 5-HT1A receptors was reduced more extensively in female than in male SERT mice (Li et al., 2000). C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). To reduce complications arising from a mixed genetic background, SERT-/- mice were generated and backcrossed onto a congenic C57BL/6J genetic background for 12 generations, as described previously (Bengel et al., 1998). To minimize potential effects of differences in maternal behavior, SERT knock-out (i.e., homozygous null mutant; SERT-/-) and their wild-type littermate (SERT+/+) controls were obtained from breedings between heterozygous null mutant parents (SERT+/-). The animals were 5-8 months of age, with body weights of 25-35 gm. The mice were housed in groups of four to five per cage in a light-(12 hr light/dark cycle; lights on at 6 A.M.), humidity-, and temperature-controlled room. Food and water were available ad libitum. All animal procedures were approved by the National Institute of Mental Health Animal Care and Use Committee.
Injection of recombinant adenovirus into the mouse brain. Mice were anesthetized using 2.5% Avertin [1 gm of tribromoethylethanol (Aldrich, Milwaukee, WI) in 1 ml of t-Amyl alcohol (Sigma, St. Louis, MO); 375 mg/kg, 15 ml/kg, i.p.] and were securely placed in a stereotaxic frame. After removing the fur on the top of head and cleaning the skin with betadine, a ∼1.5 cm incision was made on the scalp. For injection into the hypothalamus, single holes or two holes (one per side, as indicated in the procedures below) were drilled on the skull using a fine drill, according to the following coordinates: anteroposterior (AP) = -0.7 mm and lateral (L) = 0.4 mm from bregma (Franklin and Paxinos, 1997). A single or double internal cannula (31 gauge; Plastics One, Roanoke, VA) was connected to a 25 μl syringe(s) that was controlled by a microinjection pump. After filling with ∼5 μl of dialyzed and diluted recombinant adenoviral solution (kept in ice), the cannula was inserted into the hole in the skull and placed at the following coordinates: AP = -0.7 mm; L = ±0.4 mm; and dorsoventral (DV) = -5.5 mm from bregma. One microliter of the viral solution was injected at a rate of 0.5 μl/min. The cannula was kept in position for an additional 20 min to prevent back-flow of the solution. After surgery, mice were allowed to recover in individual cages until testing.
Behavioral tests. The elevated plus-maze test was conducted as described previously (Holmes et al., 2000, 2003a). The elevated plus-maze apparatus (San Diego Instruments, San Diego, CA) was comprised of two open arms (30 × 5 × 0.5 cm) and two enclosed arms (30 × 5 × 15 cm) that extended from a common central platform (5 × 5 cm). A small raised lip (0.5 cm) around the perimeter of the open arms prevented the mouse from falling. The apparatus was constructed from polypropylene and Plexiglas, with a white floor and clear walls, and elevated to a height of 38 cm above floor level. Mice were individually placed on the center square, facing an open arm, and allowed to freely explore the apparatus under even overhead fluorescent lighting (200 lux) for 5 min. Open and closed arm entries (all four paws in an arm), time spent in the open arms, and rears within the closed arms were scored by an experienced observer using behavioral scoring software (Hindsight; Scientific Programming Services, Wokingham, UK). Time spent (in seconds; percentage of total testing time) and entries (number and percentage of total entries) into the open arms were used as parameters for exploratory anxiety-like behavior. The longer the time spent in open arms, the less anxious is the animal. The entries to the enclosed arms and rearing were the references for locomotor activity.
The open-field test was conducted as described previously (Holmes et al., 2000, 2003a). The open field was a square arena (40 × 40 × 35 cm) with clear Plexiglas walls and floor, evenly illuminated by white overhead fluorescent lighting. Mice were individually placed in the center of the open field and left to freely explore for 15 min. Activity was measured by a computer-assisted Digiscan optical animal activity system (RXYZCM; Omnitech Electronics, Accuscan, Columbus, OH). Eight photocell beams were located on each of the two sides of the arena, at right angles to one another, forming a grid of 64 equally sized squares. To detect vertical movements, a third set of eight photocell beams was located above the square grid. The number of horizontal and vertical beam breaks was taken as measures of horizontal and vertical activity, respectively. Time spent in a peripheral area (20 cm from the edge of the box) of the open field was automatically recorded as margin time.
Autoradiography of 5-HT1A receptor binding
Autoradiography of 125I-MPPI (4-(2′-methoxyphenyl)-1-[2′-[N-(2“pyridinyl-)-iodo-benzamido]ethyl]piperazine) binding for the density of 5-HT1A receptors was conducted as described previously (Li et al., 2000). Mice were killed by rapid cervical dislocation. Brains were removed and frozen immediately in dry, ice-cold 2-methylbutane (isopentane) for 10 sec, transferred to dry ice for 10 min until completely frozen, and then stored at -80°C. Brains were cut into 16-μm-thick coronal sections in a cryostat. The sections containing hypothalamus (bregma, 0.7 to ∼1.94 mm) were thaw mounted onto chromalum-gelatin-coated glass slides and stored at -80°C. The sections were preincubated for 30 min in an assay buffer (50 mm Tris-HCl, pH 7.4, containing 200 nm MgCl2) and were then incubated with 125I-MPPI (0.14 nm in the assay buffer) for 2 hr at room temperature. Nonspecific binding was defined in the presence of 10-5 m 5-HT. Slides were then washed twice with the assay buffer at 4°C for 15 min and rinsed with cold, double-distilled H2O. After being air blow dried, the slides were exposed to Kodak (Rochester, NY) Biomax MR film. The films were exposed in -80°C for 3 d. A set of 125I microscales (Amersham Biosciences, Piscataway, NJ) was exposed with the slides to calibrate the optic density into femtomoles per milligram of tissue equivalent. After exposure, brain images were digitized and analyzed using AIS image software (Imaging Research, St. Catharines, Ontario, Canada). The gray-scale density readings were calibrated to femtomoles per milligram of tissue equivalent using the 125I microscale. Specific 125I-MPPI binding in each brain region was determined by subtracting the nonspecific binding sites from the total binding sites in each region. Data for each individual subject and brain region are the mean of four adjacent sections.
Observation of GFP expression
The GFP in the brain was used to indicate the adenoviral expression. To determine correlation between the viral expression and the alteration in the density of 5-HT1A receptors induced by administration of adenovirus containing 5-HT1A sense or antisense sequence, GFP was observed directly from the fresh-frozen brain sections under a fluorescence microscope. The GFP pictures were taken by a digital camera on the microscope. The GFP in the fresh-frozen brain sections represents the anatomical location of the adenoviral expression in the brain. However, it is not possible to reveal the distribution of GFP at the cellular level. The mice without GFP expression were excluded from the study.
Radioimmunoassay for oxytocin, adrenocorticotropin, and corticosterone
Plasma oxytocin and adrenocorticotropin (ACTH) were examined as described previously (Li et al., 1999). Two hundred fifty and 20 μl of plasma were used for oxytocin and ACTH assays, respectively. All the assays were conduced in triplicate for standards and samples.
Characterization of expression of recombinant adenovirus containing 5-HT1A genes in the brain
Specific expression of 5-HT1A receptors controlled by 5-HT1A promoter. To determine whether the 5-HT1A promoter is able to control the expression of 5-HT1A receptors, so that 5-HT1A receptors are only expressed in the cells those normally express 5-HT1A receptors, the high-titer recombinant adenoviruses containing 5-HT1A sense controlled by CMV promoter (Ad-CMV-1Asense) or by 5-HT1A promoter were unilaterally injected into the striatum (AP = 0.7 mm; mediolateral (ML) = -2 mm; and DV = 4 mm from bregma) or cerebellum (AP = -5.8 mm; ML = -2 mm; and DV = 3 mm from bregma) (Franklin and Paxinos, 1997) of normal mice. Five days after the injection, mice were decapitated, and the brains were collected. The brains were sectioned into 16 μm slices and were thaw mounted onto chromalum-gelatin-coated glass slides and stored at -80°C. The GFP in the fresh-frozen section was observed to evaluate the adenoviral expression. 5-HT1A in situ hybridization, 3H-8-OH-DPAT (8-hydroxy-2(di-n-propylamino)tetralin) binding, and 8-OH-DPAT-induced 35S-GTP-γ-S binding (Li et al., 2000) were used to determine the mRNA, density, and function of 5-HT1A receptors, respectively.
Time course of the expression of recombinant adenovirus. To determine how long the recombinant adenovirus can be expressed in the brain, high-titer recombinant adenoviral solutions were unilaterally injected into various brain regions. Mice were decapitated 2 and 5 d, 2, 4, 6, 9, and 15 weeks, and 6 months after the injection. The brain was sectioned as described above. GFP in the fresh-frozen brain sections was observed under a fluorescence microscope.
Determination of optimal conditions for adenoviral injection. To determine the conditions that produce minimal toxicity and highest efficiency of infection of the recombinant adenovirus, two parameters were tested: dialysis time and dilution of the high-titer adenoviral solution after the dialysis. In the first test, high-titer recombinant adenovirus containing 5-HT1A gene was dialyzed for 20 or 40 min with 1 l of saline, respectively. In the second test, a 40 min dialyzed viral solution was diluted one, two, four, and eight times with saline. One microliter of these adenoviral solutions was unilaterally injected into the hypothalamus (AP = -1.0; ML = -0.4; and DV = -5.5 mm from bregma), as described above. Seven days after the injection, mice were decapitated, and the brains were collected. The frozen brain sections were used for 125I-MPPI binding, Nissl staining, and GFP observation.
Procedure for study 1: effect of injection of Ad-1AP-sense into the hypothalamus of SERT knock-out mice on anxiety-like behaviors
Female SERT-/- and SERT+/+ mice were injected bilaterally with recombinant adenovirus containing 5-HT1A promoter and sense gene or Ad-track (a control) into the hypothalamus, as described above. Seven days after the injection, mice were tested on the elevated plus maze for 5 min. On the day after the test, mice were injected with saline (5 ml/kg, s.c.) as a minor stressor. Fifteen minutes after the injection, mice were decapitated, and the trunk blood was collected for ACTH assay. The brains were collected for autoradiograph of 125I-MPPI binding and observation of GFP.
Procedure for study 2: effect of injection of Ad-1AP-antisense into the hypothalamus of normal mice on anxiety-like behaviors
Female C57/B6 mice were injected bilaterally with Ad-1AP-antisense or Ad-track into the hypothalamus, as described above. Seven days after the surgery, the mice were tested using the elevated plus maze. Then, open-field testing was conducted on the next day. Nine days after the surgery, the mice were injected with saline or 8-OH-DPAT (0.2 mg/kg, s.c.) and were decapitated 15 min after the injection. The trunk blood was collected for plasma hormonal assays. The brains of the mice were collected for autoradiographic 125I-MPPI binding and observation of GFP.
Data analysis and statistics
The data are presented as group means ± SEM for 10-15 mice, unless otherwise noted. All of the data were analyzed by one-way or two-way ANOVA. In the case when a significant difference was detected, a Student-Newman-Keuls post hoc test was used to evaluate the differences between the groups. A computer program, StatView (Abacus Concepts, Berkeley, CA), was used in all statistical analysis.
Results
Characterization of expression of recombinant adenovirus containing 5-HT1A genes in the brain
5-HT1A promoter controls the expression of 5-HT1A receptors To verify the ability of the 5-HT1A promoter to control the expression of 5-HT1A receptors in a cell-specific manner, recombinant adenoviruses containing a 5-HT1A sense sequence controlled by the 5-HT1A promoter or the CMV promoter (Fig. 1), respectively, were injected into the striatum and cerebellum. The striatum and cerebellum do not normally express 5-HT1A receptors. As shown in Figure 2, GFP was observed in the fresh-frozen brain sections injected with either adenovirus, indicating expression of the adenovirus. In the mice that were injected with Ad-CMV-1Asense, 5-HT1A mRNA binding sites and 8-OH-DPAT-stimulated GTP-γ-S binding sites were correlated with GFP distribution in the striatum and cerebellum. However, in the mice injected with Ad-1AP-sense, neither 5-HT1A receptor binding sites nor 8-OH-DPAT-stimulated GTP-γ-S binding were detected in the striatum and cerebellum, although some 5-HT1A mRNA was observed. These data suggest that the 5-HT1A promoter in the adenoviral vector is able to control 5-HT1A expression.
Figure 2.
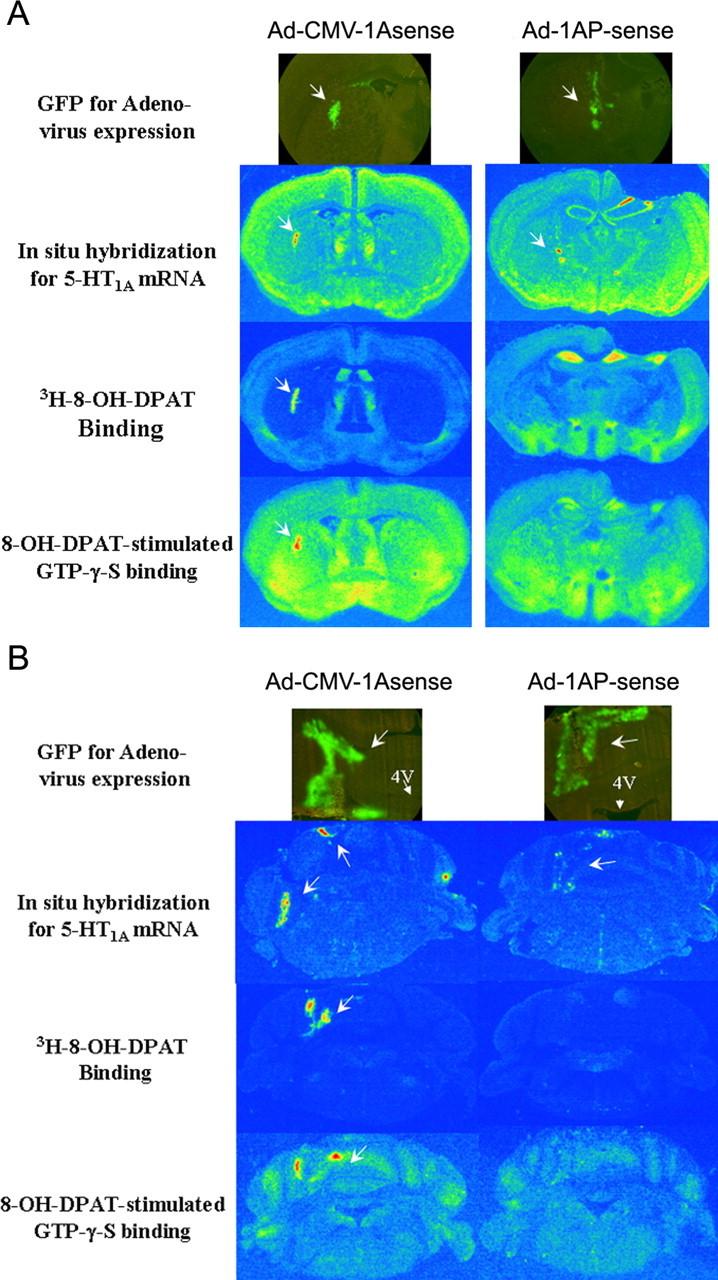
5-HT1A promoter controls the expression of 5-HT1A receptors. Recombinant adenoviruses containing 5-HT1A sense sequence controlled under CMV promoter (Ad-CMV-1Asense) or 5-HT1A promoter (Ad-1AP-sense) were injected into the striatum (A) or cerebellum (B) as described in Materials and Methods. Arrows indicate the expression of adenovirus (GFP) or 5-HT1A receptors and their function (see Materials and Methods and Results). 4V, Fourth ventricle.
Time course of the expression of recombinant adenovirus
One of the drawbacks for recombinant adenovirus is its short infection time, because the replication domain in the viral vector was deleted. In the peripheral tissue, the expression of recombinant adenovirus usually lasts ∼2 weeks. Therefore, we ascertained the time course of the viral expression in the brain by observation of GFP expression. The GFP expression in the brain was identified by 2 d after the injection. The peak of expression was observed between 5 and 10 d. The expression lasted up to 6 months after the injection.
Determination of the optimal condition for adenoviral injection
We sought to ensure that the use of the adenovirus was not associated with neural toxicity. High-titer adenovirus is stored in a high-salt solution containing CsCl, which can cause tissue damage. On the other hand, the adenovirus may produce toxicity to the tissue, although the toxicity has been greatly reduced in the recombinant adenovirus. To remove the CsCl and other salt, the high-titer viral solution needs to be dialyzed before injection. It is important to test the optimal time for the dialysis, because the prolonged dialysis will reduce the infective viral particles. The results showed that 1 μl of a 5-HT1A receptor sense adenoviral solution that was dialyzed for 20 min caused tissue necrosis, and no 5-HT1A receptors were expressed when injected into the hypothalamus. In contrast, tissue damage was not observed in the mice injected with the same viral solution that was dialyzed for 40 min. These results suggest that high-salt solution is the major factor to cause tissue damage. Because viral titer is reduced exponentially, whereas salt solution is diluted linearly, diluting viral solution a few times might not affect the viral efficiency of infection but could reduce the salt concentration remarkably. To ensure that the salt concentration in the viral solution is low enough to avoid tissue damage, we further diluted the adenoviral solution after the dialysis. The results suggest that twice diluting the high-titer adenovirus did not significantly reduce the expression of adenovirus, whereas diluting four or eight times slightly reduced the degree of expression (data not shown). Therefore, in all subsequent studies, the viral solution was 1:1 diluted after at least 30 min of dialysis.
Together, the present data suggest that recombinant adenovirus is a useful tool for manipulation of gene expression in the CNS.
Effect of injection of Ad-1AP-sense into the hypothalamus of SERT+/+ and SERT-/- mice
The density of 5-HT1A receptors is reduced in the hypothalamus of SERT-/- mice. Injection of Ad-1AP-sense into their hypothalamus increased the expression of 5-HT1A receptors in both SERT+/+ and SERT-/- mice. As a result, the density of 5-HT1A receptors in the medial region of the hypothalamus, such as anterior hypothalamic nucleus, paraventricular nucleus, dorsomedial nucleus, and ventromedial nucleus, was significantly increased in the SERT+/+ mice and partially restored in the SERT-/- mice (Fig. 3).
Figure 3.
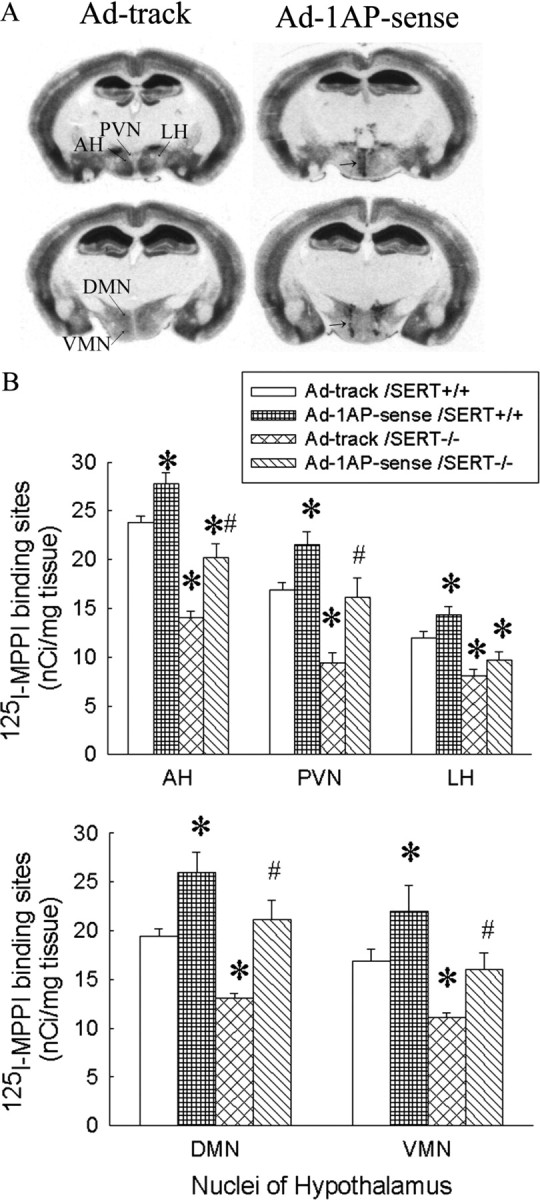
125I-MPPI-labeled 5-HT1A receptors in SERT knock-out mice injected with Ad-1AP-sense in the hypothalamus. A, Autoradiography of 125I-MPPI binding in the hypothalamus of mice that were injected with a recombinant adenovirus-containing control (left column) or 5-HT1A sense (right column). B, 125I-MPPI-labeled 5-HT1A receptors in the hypothalamus of mice injected with a recombinant adenovirus containing a control vector or 5-HT1A sense. AH, Anterior hypothalamus; DMN, dorsomedial hypothalamus; LH, lateral hypothalamus; PVN, paraventricular hypothalamus; VMN, ventromedial hypothalamus. Data are presented as mean ± SEM for 9-11 mice. Two-way ANOVA for AH: main effect of adenoviral treatment, F(1,37) = 26.3, p < 0.0001; main effect of genotype, F(1,37) = 76.8, p < 0.0001; interaction of adenoviral treatment and genotype, F(1,37) = 1.2, p = 0.27. Two-way ANOVA for PVN: main effect of adenoviral treatment, F(1,37) = 18.9, p = 0.0001; main effect of genotype, F(1,37) = 24.1, p < 0.0001; interaction of adenoviral treatment and genotype, F(1,37) = 0.63, p = 0.43. Two-way ANOVA for LH: main effect of adenoviral treatment, F(1,37) = 8.5, p < 0.006; main effect of genotype, F(1,37) = 40.6, p < 0.0001; interaction of adenoviral treatment and genotype, F(1,37) = 0.18, p = 0.67. Two-way ANOVA for DMN: main effect of adenoviral treatment, F(1,37) = 26.4, p < 0.0001; main effect of genotype, F(1,37) = 15.4, p = 0.0004; interaction of adenoviral treatment and genotype, F(1,37) = 0.24, p = 0.63. Two-way ANOVA for VMN: main effect of adenoviral treatment, F(1,37) = 10.2, p = 0.0028; main effect of genotype, F(1,37) = 14.6, p= 0.0005; interaction of adenoviral treatment and genotype, F(1,37) = 0.003, p = 0.96. *Significant difference from Ad-track-injected SERT+/+ mice (p < 0.05). #Significant difference from Ad-track-injected SERT-/- mice (p < 0.05) by Student-Newman-Keuls post hoc test.
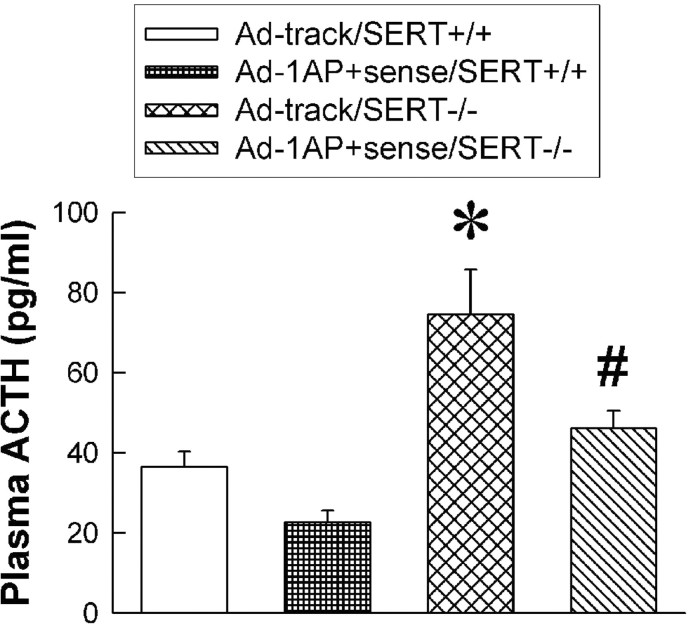
Consistent with our previous observations, a minor stressor, saline injection, significantly increased ACTH secretion in Ad-track-treated SERT-/- mice compared with control SERT+/+ mice (Fig. 4). The ACTH response to saline injection was significantly attenuated in the SERT-/- mice injected with Ad-1AP-sense (Fig. 4). Although the density of 5-HT1A receptors in the hypothalamus was increased in SERT+/+ mice injected with Ad-1AP-sense, the ACTH responses to saline injection were not significantly altered in these mice.
Figure 4.
ACTH response to saline injection (a minor stress) in the Ad-1AP-sense- and Ad-track-treated SERT+/+ and SERT-/- mice. The mice were injected with saline (5 ml/kg, s.c.) 15 min before decapitation. Data are presented as the mean ± SEM for 10-11 mice. Two-way ANOVA: main effect of adenoviral treatment, F(1,37) = 9.7, p = 0.0036; main effect of genotype, F(1,37) = 24.4, p < 0.0001; interaction of adenoviral treatment and genotype, F(1,37) = 1.2, p = 0.29. *Significant difference from Ad-track-injected SERT+/+ mice (p < 0.05). #Significant difference from Ad-track-injected SERT-/- mice (p < 0.05) by Student-Newman-Keuls post hoc test.
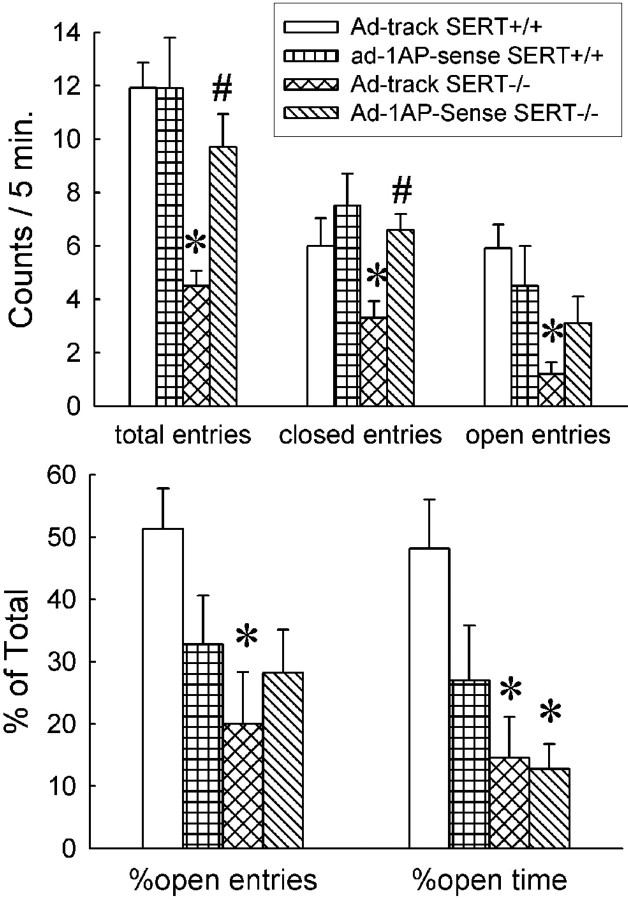
In the elevated plus-maze test, SERT-/- mice injected with Ad-track showed a significant reduction in locomotor activity (decreases in total entries, closed entries, and open entries) compared with Ad-track-injected SERT+/+ mice (Fig. 5). SERT-/- mice that received Ad-1AP-sense showed significantly increased locomotor activity relative to Ad-track-treated SERT-/- mice, although it is still lower than that in control SERT+/+ mice (Fig. 5). However, injection with Ad-1AP-sense in the SERT+/+ mice did not alter their locomotor activity. On the other hand, an increase in the exploratory anxiety-like behavior (decreases in open entries and open time) was observed in the Ad-track-injected SERT-/- mice compared with SERT+/+ mice. Treatment with Ad-1AP-sense did not alter the exploratory anxiety-like behavior in either SERT+/+ or SERT-/- mice (Fig. 5).
Figure 5.
Elevated plus-maze test on Ad-1AP-sense-injected SERT knock-out mice. Top, The parameters indicate locomotor activity. Bottom, The parameters indicate exploratory anxiety-like behavior. Data are presented as mean ± SEM for 9-12 mice. Two-way ANOVA for total entries: main effect of adenoviral treatment, F(1,39) = 3.7, p = 0.06; main effect of genotype, F(1,39) = 17.1, p = 0.0002; interaction of adenoviral treatment and genotype, F(1,39) = 5.3, p = 0.03. Two-way ANOVA for close entries: main effect of adenoviral treatment, F(1,39) = 5.5, p = 0.02; main effect of genotype, F(1,39) = 4.6, p = 0.04; interaction of adenoviral treatment and genotype, F(1,39) = 1.3, p = 0.25. Two-way ANOVA for open entries: main effect of adenoviral treatment, F(1,39) = 0.04, p = 0.85; main effect of genotype, F(1,39) = 8.9, p = 0.005; interaction of adenoviral treatment and genotype, F(1,39) = 2.8, p = 0.1. Two-way ANOVA for percentage open entries: main effect of adenoviral treatment, F(1,39) = 0.31, p = 0.58; main effect of genotype, F(1,39) = 5.0, p = 0.03; interaction of adenoviral treatment and genotype, F(1,39) = 2.6, p = 0.1. Two-way ANOVA for percentage open time: main effect of adenoviral treatment, F(1,39) = 2.0, p = 0.16; main effect of genotype, F(1,39) = 9.5, p = 0.004; interaction of adenoviral treatment and genotype, F(1,39) = 1.3, p = 0.25. *Significant difference from Ad-track-injected SERT+/+ mice. #Significant difference from Ad-track-injected SERT-/- mice (p < 0.05) by Student-Newman-Keuls post hoc test.
Effect of injection of Ad-1AP-antisense into the hypothalamus of SERT normal mice
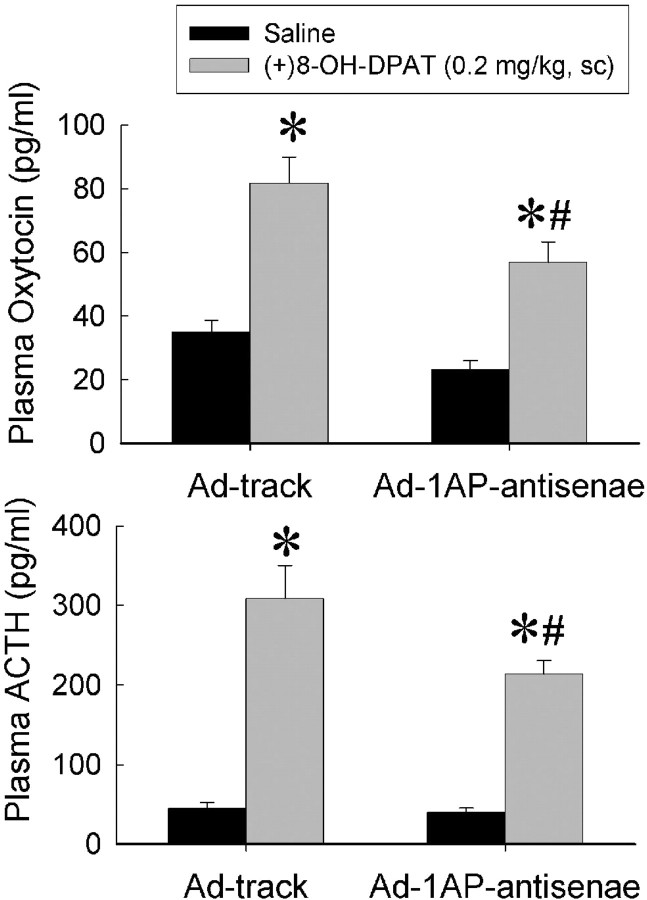
As can be seen in Figure 6, hypothalamic injection of Ad-1AP-antisense efficiently suppressed the expression of 5-HT1A receptors in the medial hypothalamus. The density of 5-HT1A receptors was significantly attenuated in all four nuclei of the medial hypothalamus but not the lateral hypothalamus of Ad-1AP-antisense-treated mice (Fig. 6B). The Nissl staining showed that no detectable tissue damage was observed (Fig. 6A). To assess the functional effect of Ad-1AP-antisense on the expression of 5-HT1A receptors in the medial hypothalamus, we measured plasma ACTH and oxytocin responses to a systemic injection of a 5-HT1A receptor agonist, 8-OH-DPAT. As shown in Figure 7, ACTH and oxytocin responses to 8-OH-DPAT were significantly reduced in Ad-1AP-antisense-treated mice compared with the control group (Ad-track injection), suggesting a decrease in the function of 5-HT1A receptors in the hypothalamus.
Figure 6.
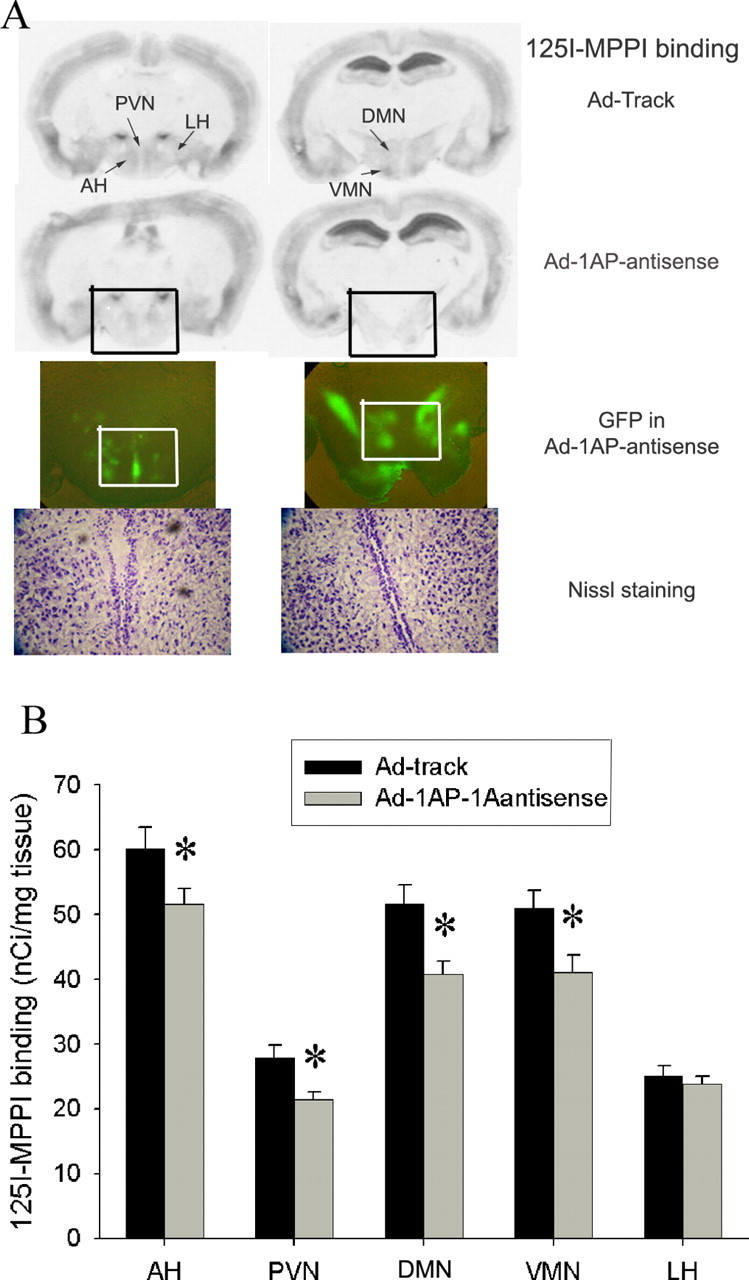
125I-MPPI binding for 5-HT1A receptors in the hypothalamic Ad-1AP-antisense-injected SERT normal mice. A, Photography of 5-HT1A binding, GFP expression, and Nissl staining of hypothalamic sections that received Ad-track and Ad-1AP-antisense adenovirus. GFP and Nissl staining sections were adjacent sections with 125I-MPPI binding of Ad-1AP-antisense. The black box indicates the area presented in the photograph of GFP expression. The white box represents the area in the Nissl staining. 3V, Third ventricle; AH, anterior hypothalamus; DMN, dorsomedial hypothalamus; LH, lateral hypothalamus; PVN, paraventricular hypothalamus; VMN, ventromedial hypothalamus. B, 125I-MPPI binding in the hypothalamus of Ad-1AP-antisense- and Ad-track-injected mice. Data are presented as mean ± SEM for 14-22 mice. One-way ANOVA for AH: F(1,34) = 4.8, p = 0.03; for PVN: F(1,34) = 8.0, p = 0.008; for DMN: F(1,34) = 9.5, p = 0.004; for VMN: F(1,34) = 6.0, p = 0.02; for LH: F(1,34) = 0.13, p = 0.58. *Significant difference from Ad-track-injected mice (p < 0.05) by Student-Newman-Keuls post hoc test.
Figure 7.
Hormonal responses to 8-OH-DPAT in Ad-1AP-antisense- and Ad-track-injected mice. Data are presented as mean ± SEM for 8-10 mice. Top, Oxytocin response to 8-OH-DPAT. Two-way ANOVA: main effect of adenoviral treatment, F(1,32) = 11.8, p = 0.017; main effect of 8-OH-DPAT injection, F(1,32) = 56.7, p < 0.0001; interaction of adenoviral and 8-OH-DPAT treatment, F(1,32) = 1.5, p = 0.23. Bottom, ACTH response to 8-OH-DPAT. Two-way ANOVA: main effect of adenoviral treatment, F(1,35) = 6.07, p = 0.019; main effect of 8-OH-DPAT injection, F(1,35) = 117.9, p < 0.0001; interaction of adenoviral and 8-OH-DPAT treatment, F(1,35) = 4.94, p = 0.03. *Significant difference from saline-treated group with same treatment of adenovirus (p < 0.05). #Significant difference from Ad-track-treated mice with same challenge treatment (p < 0.05) by Student-Newman-Keuls post hoc test.
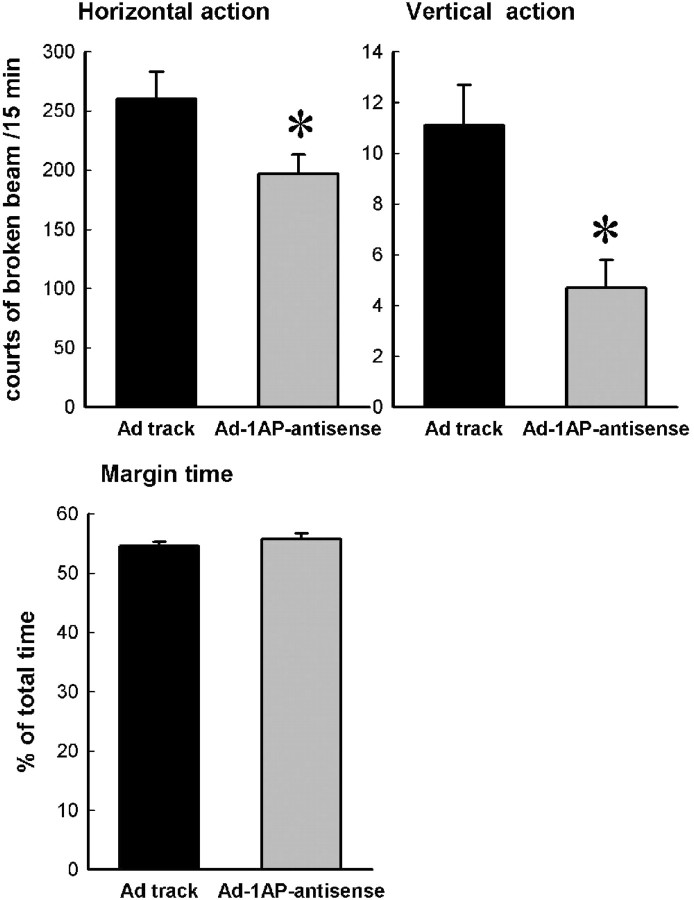
To assess the behavioral effects of Ad-1AP-antisense treatment in the hypothalamus, mice were tested in the elevated plus-maze and open-field tests. In the elevated plus-maze test, Ad-1AP-antisense-treated mice showed significant reductions in measures of locomotor activity (total entries, closed entries, and rears) but not exploratory anxiety-related measures (percentage open entries and percentage open time) relative to control mice (Fig. 8, Ad-track injected). This pattern of results was replicated in the open-field test. Ad-1AP-antisense-treated mice showed significant reductions in locomotor activity (horizontal activity and vertical activity), but not in exploratory anxiety-like behavior (margin time), compared with control mice (Fig. 9, Ad-track injected).
Figure 8.
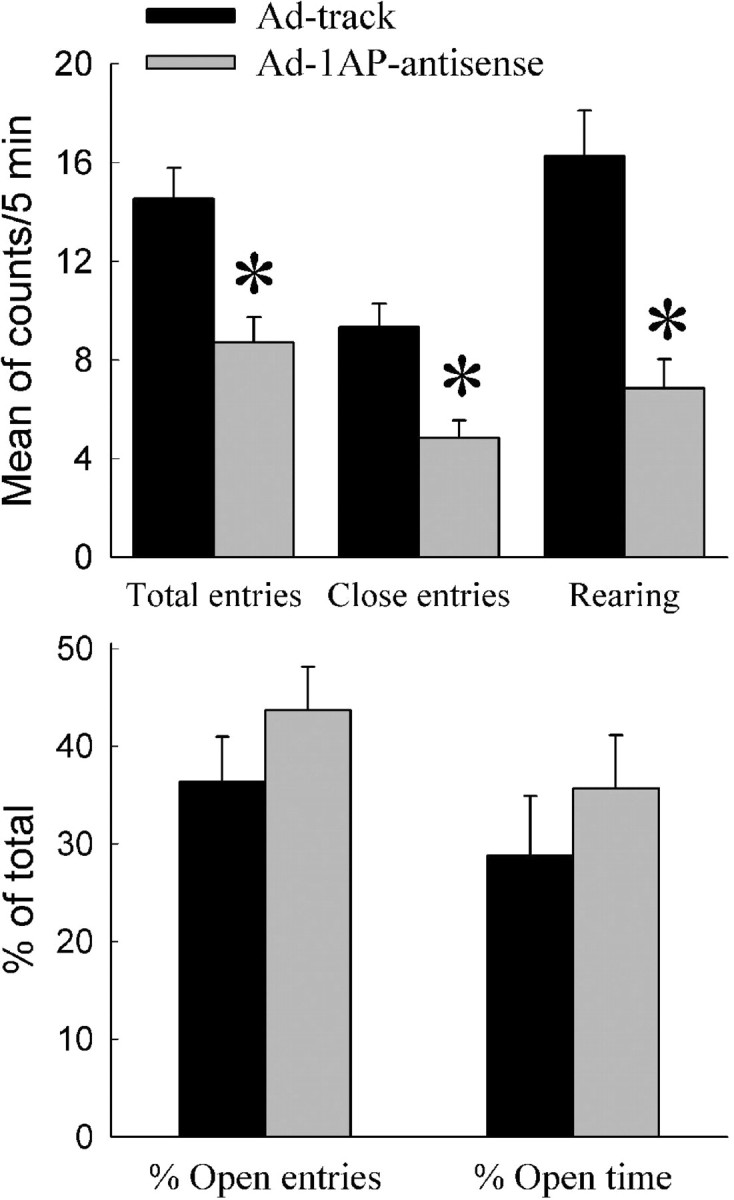
Elevated plus-maze test on the Ad-1AP-antisense-injected mice. Top, The parameters indicate locomotor activity. Bottom, The parameters indicate exploratory anxiety-like behavior. Data are presented as mean ± SEM for 15-20 mice. One-way ANOVA for total entries: F(1,33) = 13.4, p = 0.0009; for close entries: F(1,33) = 15.5, p = 0.0004; for rearing: F(1,33) = 20.4, p < 0.0001; for percentage open entries: F(1,33) = 1.28, p = 0.27; for percentage open time: F(1,33) = 0.68, p = 0.41. *Significant difference from Ad-track-injected mice (p < 0.05) by Student-Newman-Keuls post hoc test.
Figure 9.
Open-field test on Ad-1AP-antisense-injected mice. Data are presented as mean ± SEM for 14-21 mice. One-way ANOVA for horizontal activity: F(1,33) = 5.4, p = 0.03; for vertical activity: F(1,33) = 11.8, p = 0.0016; for margin time: F(1,33) = 0.88, p = 0.36. *Significant difference from Ad-track-injected mice (p < 0.05) by Student-Newman-Keuls post hoc test.
Discussion
The present results suggest that 5-HT1A receptors in the medial hypothalamus are related to the regulation of stress-induced hormonal responses and locomotor activity, tested on the elevated plus maze and open field. Normalization of reduced 5-HT1A receptors in the medial hypothalamus reversed the increased sensitivity to a minor stressor and reduced locomotor activity in SERT-/- mice. A decrease in the density of 5-HT1A receptors in the medial hypothalamus reduced locomotor activity. On the other hand, 5-HT1A receptors in the medial hypothalamus may not play a major role in the regulation of exploratory anxiety-like behaviors in mice, because alterations in the density of hypothalamic 5-HT1A receptors does not change anxiety-like behaviors measured in elevated plus-maze or open-field tests.
In the present study, 5-HT1A receptors in the periventricular and medial hypothalamus were altered using recombinant adenoviruses containing 5-HT1A genes. Among the nuclei in this region, the paraventricular nucleus controls the secretion of several hormones, such as ACTH, corticosterone, and oxytocin (Carrasco and Van de Kar, 2003), and it plays a major role in stress responses. The medial hypothalamus, including the medial preoptic, anterior hypothalamic, ventromedial hypothalamic, and dorsal premammilliary nuclei, receive strong inputs from the amygdala and moderate inputs from the septum and frontal cortex, which are the brain regions that highly relate to fear and anxiety behaviors. On the other hand, the medial hypothalamus strongly projects to the septum, amygdala, and the bed nucleus of stria terminalis. Importantly, the targets of the medial hypothalamic nuclei in the brainstem are periaqueductal gray and the precommissural nucleus neurons (Swanson, 1988; Canteras, 2002). The periaqueductal gray is the key site for defensive behaviors. Therefore, these medial hypothalamic nuclei may be a mediator that links the amygdala and periaqueductal gray to transfer the fear signal to defensive behavior. Finally, the medial hypothalamic nuclei also innervate each other and innervate other nuclei in the hypothalamus (Thompson and Swanson, 2003). These innervations may be involved in the interaction between the neuronal pathways.
An interesting finding in the present study was that restoration of hypothalamic 5-HT1A receptors in SERT-/- mice reduces the increased sensitivity to minor stressors in these mice. Our previous studies demonstrated that SERT+/- and SERT-/- mice are more sensitive to minor stressors, such as handling and saline injection, relative to their SERT+/+ littermates (Li et al., 1999, 2003). The present results showed that restoring the hypothalamic 5-HT1A receptors normalizes the increased sensitivity to a minor stressor (Fig. 4). These data suggest that the hypothalamic 5-HT1A receptors are involved in the regulation of stress-induced ACTH secretion. Evidence has been reported that activation of 5-HT1A receptors inhibits certain stressor-induced increase in ACTH and/or corticosterone secretion (Rittenhouse et al., 1992; Malmkvist et al., 2003). A systemic injection of 8-OH-DPAT, a 5-HT1A receptor agonist, reduced the elevated plus maze-induced increase of ACTH secretion (data not shown). Consistently, blockade of 5-HT1A receptors by its antagonist potentiated stress-induced increase in ACTH secretion (Groenink et al., 1996). The present study demonstrated, for the first time, that the hypothalamic 5-HT1A receptors may mediate the regulation of stress-induced increase in plasma ACTH and corticosterone concentrations. The effect of the hypothalamic 5-HT1A receptors could be mediated by direct or indirect mechanisms. 5-HT1A receptors are located on CRF neurons in the paraventricular nucleus of the hypothalamus (Zhang et al., 2004). Because 5-HT1A receptors are coupled to Gi proteins and produce an inhibitory effect, they could suppress the stress-induced increase in CRF release. However, this hypothesis cannot explain how activation of 5-HT1A receptors in the hypothalamus during resting states increases the secretion of ACTH and corticosterone. Furthermore, 5-HT1A agonists suppress the ACTH and corticosterone responses to certain stressors, such as conditioned fear, but not others, such as immobilization (Rittenhouse et al., 1992). These data suggest that 5-HT1A receptors selectively inhibit certain stress pathways but may not directly act on CRF neurons. Alternatively, the effect of hypothalamic 5-HT1A receptors on the inhibition of stress-induced hormone secretion could be mediated by indirect mechanisms. For example, 5-HT1A receptors could be located on interneurons, such as GABA neurons, which innervate CRF neurons and inhibit their activity. More studies are required to understand the mechanisms underlying the effect of hypothalamic 5-HT1A receptors on the regulation of stress hormones.
The presently observed alterations in the open field and elevated plus maze suggest involvement of hypothalamic 5-HT1A receptors in the regulation of locomotor activity. However, there is little evidence that the medial hypothalamus directly controls motor function. In contrast, there is extensive literature demonstrating a major role for the hypothalamus in the regulation of rodent defensive behaviors, as measured by locomotor responses such as flight, in tests including the open field (Brandao et al., 1986; Schmitt et al., 1986; Blanchard et al., 2001, 2003). 5-HT1A receptors appear to play a major role in the modulation of anxiety-related and defensive behaviors. Several studies have shown that systemic administration of 5-HT1A receptor agonists and 5-HT1A receptor antagonists alter defensive behaviors in rats and mice (Griebel et al., 1995, 1999; Blanchard et al., 2001). Demonstrating a possible role for hypothalamic 5-HT1A receptors in these effects, a recent study reported that activation of 5-HT1A receptors in the medial hypothalamus suppressed defensive rage behaviors in cats induced by electric stimulation of periaqueductal gray (Hassanain et al., 2003). In the context of these findings, it can be speculated that the presently observed behavioral alterations after manipulation of hypothalamic 5-HT1A receptors is more related to changes in defensive behavior than locomotor activity per se. Additional studies will be needed to more directly assess this hypothesis.
The hypothalamus is a component of a corticolimbic neurocircuit thought to subserve anxiety. However, there have been few studies examining the specific role of this brain region in anxiety. The present study found little evidence of changes in exploratory anxiety-like behaviors after hypothalamic 5-HT1A receptor manipulation in either normal mice or SERT-/- mice, suggesting a minor role of hypothalamic 5-HT1A receptors in these behaviors. Together, these data support the concept that anxiety-like behaviors and defensive behaviors are mediated by different serotonergic pathways. Anxiety-like behaviors may be mediated by a dorsal raphe-amygdala pathway, whereas the defensive behaviors may be controlled by a dorsal raphe-medial hypothalamus pathway (Graeff et al., 1996).
Recombinant adenoviruses containing 5-HT1A sense and antisense sequence were used, for the first time, to manipulate the expression of 5-HT1A receptors. Our results suggest that the 5-HT1A promoter is able to region-specifically control the expression of 5-HT1A sequences. In addition, the adenoviral expression in the brain lasted at least 6 months, much longer than in peripheral tissues. This is possibly attributable to the lack of replication of neurons. One of the concerns in using the recombinant adenovirus approach is the inflammatory response to the viral infection, although toxicity of adenovirus infection has been reduced in the Ad-Easy system (Thomas et al., 2001; Lowenstein, 2002). Inflammation increases cytokine release, and it is known that cytokines can regulate the HPA axis and its receptors; therefore, if the inflammatory responses were different between the control and testing groups, the results could reflect a nonspecific effect of inflammation. To minimize the inflammatory response, we determined optimal doses and dilutions of the viral preparation in the present studies. As a result, no significant cell loss was observed by Nissl staining. Also, we used a similar titer of control viral preparation that contained the same adenoviral vector, so that the inflammatory responses between the groups should be comparable. However, we cannot absolutely rule out the possibility that the inflammatory response to the viral infection plays a role in the alterations observed in the present studies.
In conclusion, the present studies demonstrate, for the first time, that medial hypothalamic 5-HT1A receptors may be involved in the regulation of stress responses and defense-related changes in locomotor activity. On the other hand, medial hypothalamic 5-HT1A receptors do not appear to contribute to exploratory anxiety-like behaviors.
Footnotes
This work was supported in part by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award to Q.L. and United States Public Health Service Grant NS34153 to L.D.V.d.K. We thank Dr. Bert Vogelstein of the Howard Hughes Medical Institute and The Sidney Kimmel Comprehensive Cancer Center and The Johns Hopkins University Medical Institutions for kindly providing adenoviral vectors. We also thank Drs. Tong-Chuan He and Jayaprakash D. Karkera for their excellent technical advice in the generation of recombinant adenovirus. We dedicate this manuscript to the memory of Dr. Louis D. Van de Kar, who passed away recently, for his contribution to neuroscience.
Correspondence should be addressed to Dr. Qian Li, Department of Psychiatry and Behavioral Sciences, University Texas of Medical Branch, 5.232 Mary Moody Northen Pavilion, 300 University Boulevard, Galveston, TX 77555-0431. E-mail: qili@utmb.edu.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2410868-10$15.00/0
Deceased, September 4, 2004.
References
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP (1998) Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol 53: 649-655. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ (2001) Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev 25: 205-218. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ (2003) The mouse defense test battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol 463: 97-116. [DOI] [PubMed] [Google Scholar]
- Brandao ML, Discala G, Bouchet MJ, Schmitt P (1986) Escape behavior produced by the blockade of glutamic-acid decarboxylase (Gad) in mesencephalic central gray or medial hypothalamus. Pharmacol Biochem Behav 24: 497-501. [DOI] [PubMed] [Google Scholar]
- Canteras NS (2002) The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav 71: 481-491. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463: 235-272. [DOI] [PubMed] [Google Scholar]
- den Boer JA, Bosker FJ, Slaap BR (2000) Serotonergic drugs in the treatment of depressive and anxiety disorders. Hum Psychopharmacol 15: 315-336. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. New York: Academic.
- Gingrich JA, Hen R (2001) Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology 155: 1-10. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD (2000) Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 157: 493-505. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF (1996) Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 54: 129-141. [DOI] [PubMed] [Google Scholar]
- Griebel G (1995) 5-Hydroxytryptamine-interacting drugs in animal models of anxiety disorders: more than 30 years of research. Pharmacol Ther 65: 319-395. [DOI] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Jung A, Masuda CK, Blanchard RJ (1995) 5-HT1A agonists modulate mouse antipredator defensive behavior differently from the 5-HT2A antagonist pirenperone. Pharmacol Biochem Behav 51: 235-244. [DOI] [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ (1999) Behavioural profiles in the mouse defence test battery suggest anxiolytic potential of 5-HT1A receptor antagonists. Psychopharmacology 144: 121-130. [DOI] [PubMed] [Google Scholar]
- Groenink L, Mos J, Van der Gugten J, Olivier B (1996) The 5-HT1A receptor is not involved in emotional stress-induced rises in stress hormones. Pharmacol Biochem Behav 55: 303-308. [DOI] [PubMed] [Google Scholar]
- Groenink L, Pattij T, de Jongh R, Van der Guten J, Oosting RS, Dirks A, Olivier B (2003) 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol 463: 185-197. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Siegel A (2003) Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1A and 5-HT2 receptors. Brain Res 981: 201-209. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou SB, da Costa LT, Yu J, Kinzler KW, Vogelstein B (1998) A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ (2000) Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol Behav 71: 509-516. [DOI] [PubMed] [Google Scholar]
- Holmes A, Kinney JW, Wrenn CC, Li Q, Yang RJ, Ma L, Vishwanath J, Saavedra MC, Innerfield CE, Jacoby AS, Shine J, Iismaa TP, Crawley JN (2003a) Galanin GAL-R1 receptor null mutant mice display increased anxiety-like behavior specific to the elevated plus-maze. Neuropsychopharmacology 28: 1031-1044. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN (2003b) Abnormal anxiety-related behaviour in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav 2: 365-380. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Lesch KP, Crawley JN, Murphy DL (2003c) Mice lacking the serotonin transporter exhibit 5-HT1A receptor-mediated abnormalities in tests for anxiety-like behavior. Neuropsychopharmacology 28: 2077-2088. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2003) The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van de Kar LD, Lesch KP, Murphy DL (1999) Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther 291: 999-1007. [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Lesch KP, Murphy DL (2000) Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci 20: 7888-7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wichems CH, Ma L, Van de Kar LD, Garcia F, Murphy DL (2003) Brain region-specific alterations of 5-HT2A and 5-HT2C receptors in serotonin transporter knockout mice. J Neurochem 84: 1256-1265. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR (2002) Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol 23: 23-30. [DOI] [PubMed] [Google Scholar]
- Malmkvist J, Hansen SW, Damgaard BM (2003) Effect of the serotonin agonist buspirone on behaviour and hypothalamic-pituitary-adrenal axis in confident and fearful mink. Physiol Behav 78: 229-240. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Li Q, Engel S, Wichems C, Andrews A, Lesch KP, Uhl G (2001) Genetic perspectives on the serotonin transporter. Brain Res Bull 56: 487-494. [DOI] [PubMed] [Google Scholar]
- Olivier B, Pattij T, Wood SJ, Oosting R, Sarnyai Z, Toth M (2001) The 5-HT1A receptor knockout mouse and anxiety. Behav Pharmacol 12: 439-450. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW (1997) The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Brain Res Rev 24: 197-254. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Bakkum EA, O'Connor PA, Carnes M, Bethea CL, Van de Kar LD (1992) Comparison of neuroendocrine and behavioral effects of ipsapirone, a 5-HT1A agonist, in three stress paradigms: immobilization, forced swim and conditioned fear. Brain Res 580: 205-214. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Carrive P, Discala G, Jenck F, Brandao M, Bagri A, Moreau JL, Sandner G (1986) A neuropharmacological study of the periventricular neural substrate involved in flight. Behav Brain Res 22: 181-190. [DOI] [PubMed] [Google Scholar]
- Swanson LW (1988) The neural basis of motivated behavior. Acta Morphol Neerl Scand 26: 165-176. [PubMed] [Google Scholar]
- Swanson LW (2000) Cerebral hemisphere regulation of motivated behavior(1). Brain Res 886: 113-164. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR (2001) Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther 3: 36-46. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW (2003) Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev 41: 153-202. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gray TS, D'Souza DN, Carrasco GA, Damjanoska KJ, Dudas B, Garcia F, Zainelli GM, Sullivan Hanley NR, Battaglia G, Muma NA, Van de Kar LD (2004) Desensitization of 5-HT1A receptors by 5-HT2A receptors in neuroendocrine neurons in vivo. J Pharmacol Exp Ther 310: 59-66. [DOI] [PubMed] [Google Scholar]