Abstract
Calcium channels of the P/Q subtype mediate transmitter release at the neuromuscular junction and at many central synapses, such as the calyx of Held. Transgenic mice in which α1A channels are ablated provide a powerful tool with which to test compensatory mechanisms at the synapse and to explore mechanisms of presynaptic regulation associated with expression of P/Q channels. Using the calyx of Held preparation from the knock-out (KO) mice, we show here that N-type channels functionally compensate for the absence of P/Q subunits at the calyx and evoke giant synaptic currents [approximately two-thirds of the magnitude of wild-type (WT) responses]. However, although evoked paired-pulse facilitation is prominent in WT, this facilitation is greatly diminished in the KO. In addition, direct recording of presynaptic calcium currents revealed that the major functional difference was the absence of calcium-dependent facilitation at the calyx in the P/Q KO animals. We conclude that one physiological function of P/Q channels is to provide additional facilitatory drive, so contributing to maintenance of transmission as vesicles are depleted during high throughput synaptic transmission.
Keywords: synaptic transmission, knock-out mice, calyx of Held, calcium currents, P/Q channels, facilitation
Introduction
Voltage-gated Ca2+ channels play a crucial role in neurotransmitter release at central and peripheral synapses, where exocytosis is frequently mediated by P/Q-type Ca2+ channels (Katz et al., 1997; Iwasaki et al., 2000). Genetic ablation [knock-out (KO)] of the Cav2.1 (α1A) pore-forming subunit of the P/Q-type Ca2+ channel in mice induces a progressive neurological syndrome characterized by ataxia and early death at approximately postnatal day 20. Recent studies of transmission at the neuromuscular junction (NMJ) in these mice showed that N- and R-type Ca2+ channels replaced the deleted presynaptic P/Q-type channels so that the synapse then exhibited lower quantal output and an absence of paired-pulse facilitation (Urbano et al., 2003). Because of the inaccessibility of the presynaptic nerve terminal at the NMJ, we have refocused our studies on the calyx of Held. This giant synapse forms in the medial nucleus of the trapezoid body (MNTB) and functions as a relay in the binaural auditory brainstem computing sound source localization. Each MNTB principal neuron receives a somatic input from a single presynaptic calyx, where like in the NMJ, P/Q Ca2+ channels also predominantly trigger neurotransmitter release (Forsythe et al., 1998; Iwasaki and Takahashi, 1998). Our aim therefore is twofold: first to determine which channel subtypes compensate for P/Q channels at a central synapse and then to consider how mechanisms of facilitation are altered by this substitution.
Facilitation is an activity-dependent form of synaptic enhancement, which has been observed at invertebrate and vertebrate PNS and CNS synapses (for review, see Zucker and Regehr, 2002). Several models have been proposed to account for short-term facilitation, including summation of residual calcium with repetitive stimulation (Katz and Miledi, 1968; Felmy et al., 2003), local saturation of calcium buffers (Blatow et al., 2003), and an independent facilitating Ca2+ sensor (Tsujimoto et al., 2002). One of the downstream effects of residual Ca2+ is the facilitation of Ca2+ currents. Facilitation by brief depolarizations has been reported at the calyx of Held (Borst and Sakmann, 1998; Cuttle et al., 1998; Forsythe et al., 1998), which despite its modest magnitude is of physiological relevance, because of the supralinear dependence of transmitter release on Ca2+ influx.
To determine how different types of presynaptic Ca2+ channels influence synaptic transmission and plasticity, we have made direct presynaptic recordings from the calyx of Held and from EPSCs in the soma of the MNTB neurons and compared results obtained from wild-type (WT) and KO mice.
Our results demonstrate that P/Q-type channels are replaced by N-type channels in the calyx of Held of the mutant mice. However, whereas presynaptic Ca2+ currents (ICa) and evoked transmitter release facilitate with short-interval paired pulses in WT, paired-pulse facilitation of ICa and evoked transmitter release is greatly diminished in the KO, emphasizing the importance of presynaptic calcium current activation in short-term synaptic plasticity.
Materials and Methods
Preparation of brainstem slices. Mice of 11-15 d of age were killed by decapitation, and their brains were removed rapidly and placed into an ice-cold low-sodium artificial CSF (aCSF). The brainstem was mounted in the Peltier chamber of an Integraslice 7550PSDS (Campden Instruments, Loughborough, UK) vibrating microslicer. Transverse slices containing MNTB were cut sequentially and transferred to an incubation chamber containing normal aCSF with low calcium (0.1 mm CaCl2 and 2.9 mm MgCl2) at 37°C for 1 hr. After incubation, the chamber was allowed to return to room temperature. Slices of 150-200 μm thickness were used for presynaptic Ca2+ current recordings, and 300-μm-thick slices were used for EPSC recordings. Normal aCSF contained (in mm): 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 10 glucose, 0.5 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 1 MgCl2, and 2 CaCl2. Low-sodium aCSF was as above, but NaCl was replaced by 250 mm sucrose, and MgCl2 and CaCl2 concentrations were 2.9 and 0.1 mm, respectively. The pH was 7.4 when gassed with 95% O2 and 5% CO2.
Electrophysiology. Slices were transferred to an experimental chamber that was perfused with normal aCSF at 25°C. MNTB neurons were visualized using Nomarski optics on a BX50WI (Olympus, Tokyo, Japan) microscope. Whole-cell voltage clamp recordings were made with patch pipettes pulled from thin-walled borosilicate glass (GC150F-15; Harvard Apparatus, Holliston, MA). Electrodes had resistances of 3.5-4.5 MΩ when filled with internal solution. Patch pipette solutions contained (in mm): 110 CsCl, 40 HEPES, 10 TEA-Cl, 12 phosphocreatine, 0.5-2 EGTA, 2 MgATP, 0.5 LiGTP, and 1 MgCl2. The pH was adjusted to 7.3 with CsOH. Lucifer yellow was included for visualizing presynaptic terminals. Patch-clamp recordings were made using an Axopatch 200B (Axon Instruments, Union City, CA) amplifier, a Digidata 1200 (Axon Instruments), and pClamp 8.1 software (Axon Instruments). Data were sampled at 5-20 kHz and filtered at 4-6 kHz (low-pass bessel). Series resistances ranged from 10 to 20 MΩ and were compensated by 50-60%. Whole-cell membrane capacitance (15-40 pF) was noted from the amplifier after compensation of the transient generated by a 10 msec voltage step. Ca2+ currents were recorded in the presence of 1 μm TTX and 10 mm TEA-Cl. Activation curves were obtained from tail currents recorded at the holding potential after depolarizing pulses and were fitted by a Boltzmann's function of the form: I(V) = Imax/(1 + exp(V1/2 - V)/k). EPSCs were recorded after addition of strychnine (10 μm, aCSF) to block inhibitory glycinergic synaptic inputs and QX314 (10 mm) in the patch pipette solution to block Na+ currents. Calyx of Held EPSCs were evoked by stimulating the globular bushy cell axons in the trapezoid body at the midline using a bipolar platinum stimulating electrode and an isolated stimulator. Stimuli of 0.1 msec duration were applied every 10-30 sec.
Average data are expressed and plotted as mean ± SEM. Statistical significance was determined using Student's t test.
Results
Evoked EPSCs show reduced amplitude in KO mice
Calyx of Held synaptic responses were evoked in both WT and KO mice, showed synchronous release, and displayed all or nothing behavior and an amplitude (when above threshold) independent of stimulus intensity. Figure 1A shows EPSCs recorded from the soma of an MNTB neuron under voltage-clamp conditions at a holding potential of -70 mV. The mean EPSC amplitude was 5.8 ± 0.6 nA (n = 7) in WT and 3.9 ± 0.4 nA (n = 7) in KO. This suggests a reduction of synaptic current magnitude of 32.6% in the KO compared with WT mice, whereas the mean 10-90% rise time showed no significant difference: 0.28 ± 0.02 msec for WT and 0.34 ± 0.03 msec for KO (n = 7). The EPSC decay time was fit by a double exponential function with similar fast (τ1) and slow (τ2) time constants (and relative amplitudes) in control and transgenic mice: for WT, τ1 = 0.91 ± 0.09 msec (23.5 ± 0.6%), τ2 = 5.5 ± 0.4 msec (76.5 ± 0.5%); and for KO, τ1 = 0.69 ± 0.07 msec (23.2 ± 1.2%), τ2 = 6.5 ± 0.6 msec (76.8 ± 1.2%). The similar time course suggests that loss of P/Q-type channels does not profoundly change the expression of postsynaptic glutamate receptors.
Figure 1.

P/Q KO mice have reduced evoked postsynaptic current (EPSC) and greatly diminished paired-pulse facilitation. EPSCs were evoked in MNTB neurons by stimulation with a bipolar electrode placed at the midline of the brainstem slice. A, Stimuli of 0.1 msec duration and 3-10 V amplitude were applied every 10-30 sec. Currents were recorded under whole-cell voltage-clamp conditions at a holding potential of -70 mV. The EPSC mean amplitude is 5.8 ± 0.6 nA (n = 7) for WT and 3.9 ± 0.4 nA (n = 7) for the KO, giving a 33% reduction in the synaptic current from KO mice with respect to WT. Differences in the 10-90% rise time and decaying time constants are not statistically significant (see Results). B, A pair of stimuli (as in A) was applied with a short interval (10 msec). Left, In low external Ca2+ concentration (0.6 mm) and high external Mg2+ concentration (2 mm), the WT EPSC evoked by the second stimulus is facilitated with respect to the first EPSC. In contrast, no facilitation is observed in KO cells. Right, Mean magnitude of facilitation is 25 ± 5% (n = 5) for WT and -6.2 ± 6.8% (n = 6) for the KO. C, When the release probability is further attenuated by increasing the external Mg2+ concentration to 3 mm, a small facilitation can be measured in KO mice. Left, Sample recordings of EPSCs from WT and KO mice. Right, Mean magnitude of facilitation between the second and first pulse is 26.9 ± 4% in WT mice (n = 3) and 7.8 ± 4.3% in KO (n = 3). Between the third and first pulse, the magnitude of facilitation is 55 ± 7% for WT and 23 ± 9% for KO.
Paired-pulse facilitation of EPSCs is greatly diminished in KO mice
Paired-pulse facilitation is a form of short-term synaptic plasticity evoked using a double pulse protocol; suprathreshold stimuli were applied to the trapezoid axons with a short interstimulus interval (10 msec) and the postsynaptic responses recorded under voltage clamp. In normal aCSF (2 mm Ca2+ and 1 mm Mg2+), the release probability and hence EPSC magnitude were too large, and the dominant feature of the second EPSC was depression (Wong et al., 2003). These studies were therefore conducted in aCSF containing low Ca2+ (0.6 mm) and high Mg2+ (2 mm), in which the paired EPSC was clearly facilitated in the WT mouse with respect to the first conditioning evoked response (25 ± 5%; n = 5). After increasing the interstimulus interval, the EPSC facilitation decayed with a time constant of 19.3 ± 5.6 msec (n = 6). Under the same conditions, EPSCs in KO mice showed no facilitation: -6.2 ± 6.8%, (n = 6) (Fig. 1B). Because the degree of facilitation increases as release probability is reduced, lowering release probability still further (by increasing extracellular Mg2+ to 3 mm) unmasked a small facilitation of 7.8 ± 4.3% (n = 3) in the KO compared with a much greater response of 27 ± 4% (n = 2) in WT (Fig. 1C), indicating that increases in transmitter release are nevertheless possible in the KO animal.
Presynaptic Ca2+ currents characterization at the calyx of Held
To learn more about the underlying mechanisms responsible for the differences in the KO calyx of Held-MNTB-evoked EPSC, the pharmacological and electrophysiological characteristics of presynaptic ICa were studied. Presynaptic Ca2+ currents were elicited by 30 msec depolarizing pulses from a holding potential of -70 to -10 mV under whole-cell voltage-clamp conditions. Lucifer yellow was used to visually confirm the presynaptic recording.
Current density and pharmacological characterization
The average presynaptic ICa density was 39.4 ± 2.5 pA/pF (n = 11) in WT and 26.0 ± 3.2 pA/pF (n = 7) in KO mice, so the KO has approximately one-third less voltage-gated divalent current than a WT terminal.
To identify which Ca2+ channel subtypes were involved, we made long-duration recordings of peak currents after sequential application of selective N, P/Q, L, and R-type Ca2+ channel antagonists: ω-conotoxin GVIA (2 μm), ω-agatoxin IVA (200 nm), nitrendipine (10 μm), and nickel (50 μm), respectively (Fig. 2A). In WT there was a high contribution of P/Q Ca2+ channels, with ω-agatoxin IVA blocking 80.8 ± 2.4% of the Ca2+ currents and 11.5 ± 1.6% of N-type component (n = 4). Tissue from the KO animal showed no sensitivity toω-agatoxin IVA, indicating that P/Q Ca2+ channels were absent, whereas the N-type contribution was 92.7 ± 1.3% (Fig. 2B) of the total divalent current (n = 4).
Figure 2.
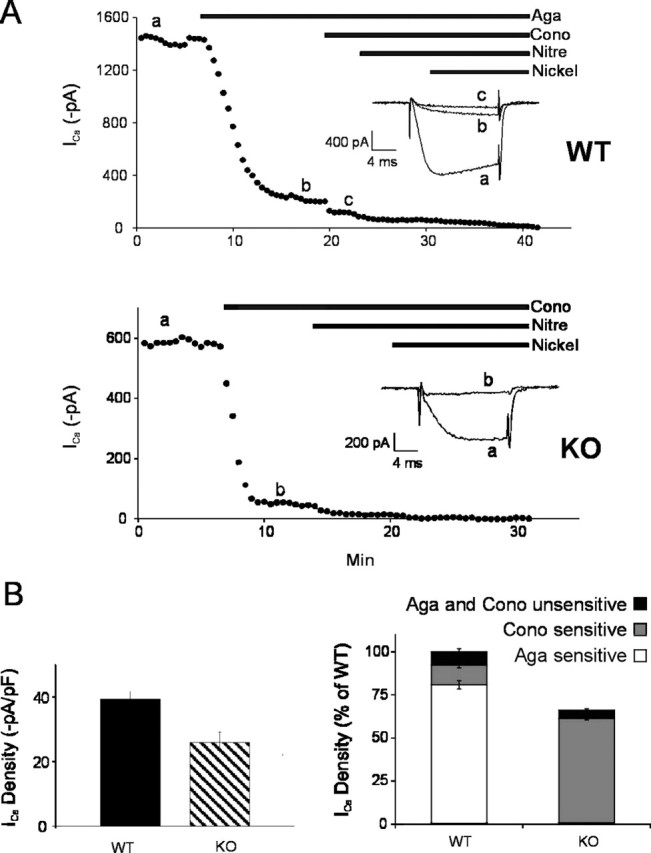
N-type channels replace P/Q-type channels at the calyx of Held presynaptic nerve terminal. Pharmacological dissection of whole-cell presynaptic Ca2+ current subtypes. Calcium currents were evoked by 30 msec step depolarization to -10 mV from a holding potential of -70 mV. A, A plot of calcium current amplitude against time (top, WT; bottom, KO), illustrating the time course of current block by sequential application of selective N, P/Q, L, and R Ca2+ channel antagonists: ω-conotoxin-GVIA (Cono; 2 μm), ω-agatoxin IVA (Aga; 200 nm), nitrendipine (Nitre; 10 μm), and nickel (50 μm), respectively. Horizontal bars indicate the time of drug application. Inset, Representative curve traces during the step to -10 mV elicited at the time points indicated by lowercase letters. The lack of effect of ω-agatoxin IVA on KO mice presynaptic Ca2+ currents was demonstrated in other experiments. B, Left, Average presynaptic ICa density is 39.4 ± 2.5 pA/pF (n = 11) in WT and 26.0 ± 3.2 pA/pF (n = 7) in KO; p = 0.0022. Right, Fractional contribution of each channel type to the total calcium current determined by subtracting current amplitudes before and after application of specific blockers. The P/Q component was 80.8 ± 2.4% (n = 4) in WT mice, whereas in KO mice, this channel was absent. The N-type component was 11.5 ± 1.6% (n = 4) in WT mice and 92.7 ± 1.3% (n = 4) in KO mice.
Voltage dependence and activation kinetics
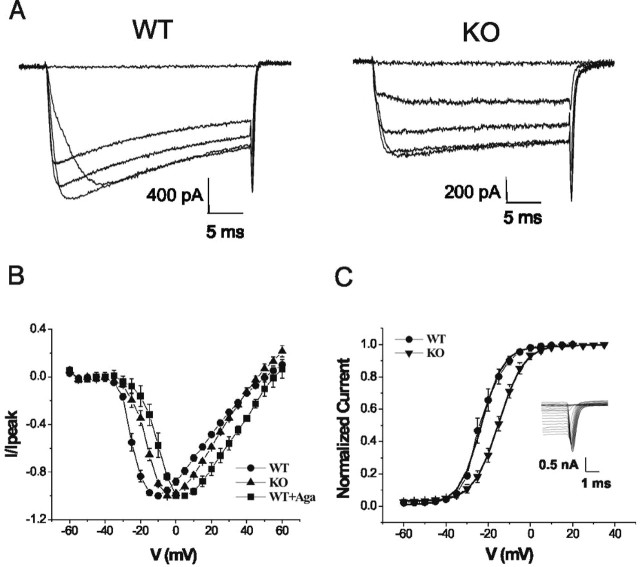
To examine the current-voltage (I-V) relationship of the presynaptic calcium current, terminals were depolarized from a holding potential of -70 mV to potentials ranging from -60 to +60 mV (5 mV increments, duration of 30 msec) (Fig. 3A). Ca2+ currents activated at approximately -35 mV and showed an apparent reversal potential of ∼45-50 mV. The peak inward current was at -10 mV for WT (n = 11) and at -5 mV in KO mice (n = 7). Current-voltage curves in Figure 3B have been normalized to the peak current amplitude. When P/Q Ca2+ channels were blocked in WT mice using ω-agatoxin IVA, the voltage dependence of the remaining calcium current was shifted to positive potentials as for the N-type currents measured in KO mice.
Figure 3.
Positive shift of presynaptic Ca2+ current activation in KO and for presynaptic N-type currents in WT mice. A, Sample traces of presynaptic ICa evoked by 30 msec depolarizing pulses between -60 and 60 mV (5 mV steps) from a holding potential of -70 mV. Left, WT; right, KO. B, Current-voltage relationship from WT, KO, and WT presynaptic terminals after application of 200 nm ω-agatoxin-IVA (Aga). C, ICa activation curves: tail currents are normalized to the maximum peak amplitude, plotted against voltage, and fitted by a Boltzmann's distribution function. The half-activation voltage in KO mice (-14.98 ± 0.06 mV; n = 7) is 8 mV more positive than that in WT mice (-23.3 ± 0.3 mV; n = 11). Slope factors are similar: 5.87 ± 0.06 mV for WT and 5.17 ± 0.26 mV for KO. Inset, Sample traces of tail current elicited by repolarization of the presynaptic terminal to the holding potential of -70 mV from the different depolarizing potentials specified above.
The same shift toward depolarized potentials in the KO with respect to WT is seen for activation curves measured from tail currents (Fig. 3C). Half-activation (V1/2) voltages in KO mice were 8 mV more positive than in WT mice: V1/2 =-14.98 ± 0.06 mV for KO (n = 7) and -23.3 ± 0.3 mV for WT (n = 11), but the slope factors were similar: k = 5.87 ± 0.06 and 5.17 ± 0.26 mV, respectively.
Presynaptic Ca2+ currents undergo facilitation in WT and depression in KO
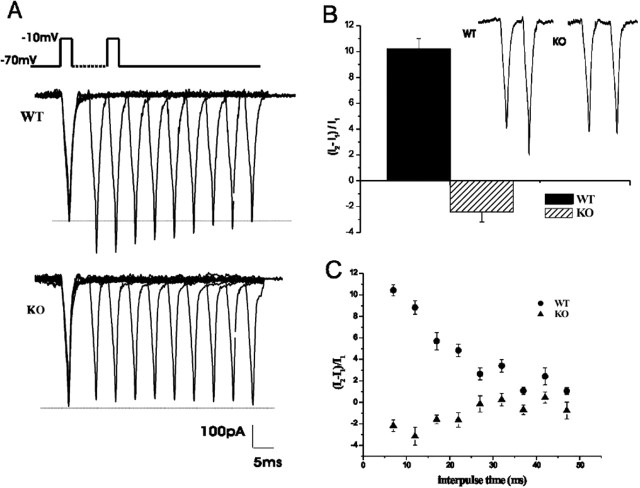
It has been reported that presynaptic calcium currents at the calyx of Held display Ca2+-dependent facilitation (Borst et al., 1998; Cuttle et al., 1998; Forsythe et al., 1998). Considering the reduced EPSC facilitation observed in the KO, this could result from an altered sensitivity during facilitating stimuli. To examine this possibility, pairs of brief depolarizing pulses (2 msec from -70 to -10 mV) were applied at short interpulse intervals (5-50 msec) to the presynaptic calyx of Held. In measurements from WT mice, the second current showed clear facilitation with respect to the first; with maximum facilitation (measured with a 5-10 msec interval) decreasing with longer interpulse intervals with a time constant of 16.9 ± 5.1 msec (n = 6) (Fig. 4A,C). At 2 mm extracellular Ca2+ and 2 mm EGTA in the internal solution, the second ICa showed 10.2 ± 0.8% facilitation (n = 14). In contrast, ICa from KO mice showed a mean depression of -2.4 ± 1% under the same conditions (n = 9) (Fig. 4A,B). The dependence of the mean magnitude of facilitation with the interpulse time interval is plotted in Figure 4C.
Figure 4.
Presynaptic Ca2+ current facilitation is absent in KO nerve terminals. Ca2+ currents were generated by a pair of 2 msec depolarizing to -10 mV from a holding potential of -70 mV, with different interpulse time intervals between 5 and 45 msec. A, Paired-pulse voltage protocol (broken line indicates that time between pulses is variable between 5 and 45 msec) and sample traces (superimposed for all interpulse intervals) showing facilitation in WT and a small depression in KO. B, Mean percentage of facilitation at a 5 msec interpulse interval: 10.2 ± 0.8% (n = 14) for WT and -2.4 ± 0.8% (n = 9) for KO. Inset, Paired-pulse sample traces. C, Dependence of paired-pulse facilitation with the time interval between pulses, for WT and KO.
Discussion
These results clearly show that, after deletion of the α1A subunit, P/Q-type calcium channels are replaced predominantly by N-type channels at the calyx of Held presynaptic terminal. This compensation results in functional synaptic transmission but altered short-term plasticity mediated by calcium-dependent modulation of the presynaptic channels. These data suggest that one special physiological role of P/Q-type channels over N-type channels is to provide a short-term facilitatory mechanism at fast-transmitting synapses.
In newborn WT mice, N-type channels are normally expressed at presynaptic nerve terminals, but their contribution decreases during development, being replaced by P/Q-type channels at approximately the age when functional hearing starts (on opening of the auditory canal, postnatal day 11). The fact that N-type channels compensate for the lack of P/Q channels is interesting, because R-type channels mediate transmitter release at fast synapses (Brenowitz and Regehr, 2003), are present at the calyx during development (Wu et al., 1998), and are known to participate in transmission. The more positive activation voltage of N-type Ca2+ currents (half-activation 8 mV more depolarized) seems a distinctive property of N-type channels compared with P/Q channels (Ishikawa et al., 2003). The same shift was observed in the N-type currents in the calyx of WT mice after incubation with the P/Q-type channel blocker (Fig. 3) (Currie and Fox, 1997). Differences in the activation kinetics could result from the expression of different α subunit isoforms or different auxiliary subunits.
The fact that P/Q-type currents activate at more negative potentials supports the idea that more Ca2+ will flow into the terminal during an action potential in the WT, resulting in more efficient synaptic transmission. Influx of Ca2+ into the calyx via N-type channels in the early stages of development triggers neurotransmitter release less effectively than via P/Q channels because of their more distant localization from the release sites (Wu et al., 1999). This could be a general phenomenon, because N-type replacement of the P/Q-channels at the α1A KO neuromuscular junction is also located more distantly from the release site (Urbano et al., 2003).
Two groups have independently reported presynaptic calcium current facilitation elicited by brief paired depolarizations with short intervals at the calyx of Held (Borst et al., 1998; Cuttle et al., 1998). This activity-dependent facilitation of Ca2+ currents results from a Ca2+-dependent increase in activation kinetics (Cuttle et al., 1998). In the present work, paired-pulse facilitation was present in the WT but not in the KO mice. In every single KO experiment, calcium currents failed to show facilitation, independently of the control current magnitude. In contrast, calcium currents from WT mice displayed a clear facilitation in the amplitude of the second current. These results suggest that the lack of facilitation is a particular feature of N-type currents in the presynaptic nerve terminal and is not caused by the reduced current magnitude. The fact that neither P/Q-type nor N-type currents in WT mice MNTB display paired-pulse facilitation of postsynaptic somatic Ca2+ currents (our unpublished observation) suggests that presynaptic facilitation depends on the expression of particular Ca2+ channel isoforms combined with local interactions with other molecules, such as NCS-1 Ca2+-binding proteins (Tsujimoto et al., 2002).
In normal Ca-Mg concentrations, high-frequency stimulation triggers EPSCs that may facilitate (Wong et al., 2003) but then exhibit profound depression at the same rate in the WT and KO mice. This depression is caused by the combined effects of vesicle depletion and postsynaptic AMPA receptor desensitization (Schneggenburger et al., 2002; Wong et al., 2003). When quantal output was reduced in our experiments, WT EPSCs displayed a strong facilitation, whereas KO EPSCs showed depression or significantly smaller facilitation.
Facilitation of transmitter release has been mainly attributed to residual Ca2+ (Katz and Miledi, 1968; Zucker and Regehr, 2002). In invertebrates and lizard synapses, the contribution of presynaptic currents to this phenomenon was not supported by experimental evidence (Charlton et al., 1982; David et al., 1997). However, at the calyx of Held, presynaptic Ca2+ currents facilitate and may contribute to short-term facilitation of transmitter release (Borst et al., 1998; Cuttle et al., 1998). Considering the nonlinear power relationship between transmitter release and presynaptic calcium current, the lack of a 10-15% facilitation of the KO presynaptic calcium current may fully explain the reduced facilitation of transmitter release observed under our recording conditions. This interpretation is supported by the similar decay time course of both types of facilitation. However, the lack of information on Ca2+ concentration at the vesicle release site, the degree of vesicle depletion, and the degree of postsynaptic glutamate receptor desensitization after the first pulse preclude more definitive calculation. Further reduction of the release probability at the KO MNTB synapse unmasked EPSC facilitation, indicating that other factors may also contribute. In fact, a large calcium current-independent component of synaptic facilitation has been recently postulated (Felmy et al., 2003).
The fact that the KO calyx EPSC facilitation as well as presynaptic calcium current facilitation are both diminished demonstrates a clear role of activity-dependent calcium current facilitation in the generation of this short-term synaptic plasticity in this giant synapse. This interpretation might be extended to other synapses, such as the NMJ, where in normal animals the P/Q channel almost exclusively mediates transmitter release and where the KO animal also lacks paired-pulse facilitation (Urbano et al., 2003). In contrast, facilitation persists in hippocampal CA1 synapses (R. Pisnoy and O. D. Uchitel, unpublished observations) and in granular-Purkinje cell synapses of the KO animal (Miyazaki et al., 2004). This difference could be related to the fact that at these synapses, transmitter release normally depends on more than one type of Ca2+ channel (Wu and Saggau, 1994; Sabatini and Regehr, 1997).
Footnotes
This work was supported by Wellcome Trust Grant 068941/Z/02/Z, Agencia Nacional de Promoción Científica y Tecnológica Grant 6220, and Universidad de Buenos Aires Ciencia y Téchnica Grant X171. We thank Drs. Brian Billups and Fernando Marengo for advice and Silvina Estevez for technical help.
Correspondence should be addressed to Dr. O. D. Uchitel, Instituto de Fisiología, Biología Molecular y Neurociencias, Universidad de Buenos Aires, Pabellon II, Piso 2, Ciudad Universitaria, Capital Federal, Buenos Aires 1428, Argentina. E-mail: odu@fbmc.fcen.uba.ar.
Copyright © 2004 Society for Neuroscience 0270-6474/04/2410379-05$15.00/0
References
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A (2003) Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 38: 79-88. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B (1998) Facilitation of presynaptic calcium currents in the rat brainstem. J Physiol (Lond) 513: 149-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG (2003) “Resistant” channels reluctantly reveal their roles. Neuron 39: 391-394. [DOI] [PubMed] [Google Scholar]
- Charlton MP, Smith SJ, Zucker RS (1982) Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol (Lond) 323: 173-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie KP, Fox AP (1997) Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J Neurosci 17: 4570-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T (1998) Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol (Lond) 512: 723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett JN, Barrett EF (1997) Stimulation-induced changes in [Ca2+] in lizard motor nerve terminals. J Physiol (Lond) 504: 83-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R (2003) Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron 37: 801-811. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T (1998) Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron 20: 797-807. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Hee-Sup S, Takahashi T (2003) Distinct and common properties of N-type and P/Q-type calcium currents at the calyx of Held presynaptic terminal. J Physiol (Lond) 551P: C28. [Google Scholar]
- Iwasaki S, Takahashi T (1998) Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J Physiol (Lond) 509: 419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T (2000) Developmental changes in calcium channel types mediating central synaptic transmission. J Neurosci 20: 59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R (1968) The role of calcium in neuromuscular facilitation. J Physiol (Lond) 195: 481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E, Protti DA, Ferro PA, Rosato Siri MD, Uchitel OD (1997) Effects of Ca2+ channel blocker neurotoxins on transmitter release and presynaptic currents at the mouse neuromuscular junction. Br J Pharmacol 121: 1531-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto K, Shin HS, Kano M, Watanabe M (2004) P/Q-type Ca2+ channel α1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci 24: 1734-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG (1997) Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci 17: 3425-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E (2002) Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci 25: 206-212. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Jeromin A, Saitoh N, Roder JC, Takahashi T (2002) Neuronal calcium sensor 1 and activity-dependent facilitation of P/Q-type calcium currents at presynaptic nerve terminals. Science 295: 2276-2279. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW (2003) Altered properties of quantal neurotransmitter release at end-plates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci USA 100: 3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID (2003) Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci 23: 4868-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Saggau P (1994) Pharmacological identification of two types of presynaptic voltage-dependent calcium channels at CA3-CA1 synapses of the hippocampus. J Neurosci 14: 5613-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JG, Sakmann B (1998) R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA 95: 4720-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B (1999) Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci 19: 726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64: 355-405. [DOI] [PubMed] [Google Scholar]